Methods that physically separate cells of interest on the basis of measurable characteristics have numerous uses in research and clinical applications, including cellular therapies. Microfluidics, filters, and centrifugation can be used to identify and physically separate cells from a heterogeneous population according to intrinsic characteristics such as size, shape, and deformability. Cells can also be sorted on the basis of signals from extrinsic probes.
Fluorescence-activated cell sorting (FACS) is the most popular means of separating a population of cells into subsets according to the total amount of key biomarkers that are expressed by each cell. These biomarkers are typically detected with fluorochrome-labeled antibodies. Schraivogel and colleagues1 have recently reported an exciting system that sorts cells on the basis of the appearance of biomarkers (i.e., their morphologic properties), not just the amount of each biomarker, at rates of up to 15,000 cells per second — the speed of conventional FACS.
The prototype instrument for this new means of sorting is an adaptation of the BD FACSMelody cell sorter (BD Biosciences), which is considered the workhorse of FACS. Although this prototype instrument has only a few fluorescence channels (complex FACS systems have many more), it sorts cells according to the spatial pattern of fluorescence within each cell. (In contrast, conventional FACS measures only the total fluorescence signal in each cell.) The system relies on fast fluorescence imaging that uses radiofrequency-tagged emission and specialized low-latency signal processing and sorting electronics, a clever approach that provides spatial information for each signal. The sensitivity of the prototype matches that of conventional FACS, but it does not use slow and expensive cameras, an advance that is the most recent in a series of instruments2–4 that allow physical sorting according to morphologic and localization phenotypes. This system is faster and has more sort bins than these predecessors.
When Schraivogel et al. first tested their system using cells that expressed fluorescent proteins targeted to specific organelles (Fig. 1), the investigators could detect changes in localization induced by various drugs. They then screened the effects that resulted from ablation of specific genetic loci across the human genome (using the Cas9 enzyme and guide RNA) to identify the regulators of the translocation of a fluorescence-tagged protein (RelA) into the nucleus when the cell was stimulated by tumor necrosis factor α (TNF-α). RelA is a member of the NF-κB family of transcription factors, which are implicated in a wide array of disease, most notably immune dysfunction and cancer. It is typically localized to the cytoplasm of cells; when activated by various environmental stressors, it translocates to the nucleus.
Figure 1. Image-Based Localization Phenotypes Detectable in Cells in Suspension.
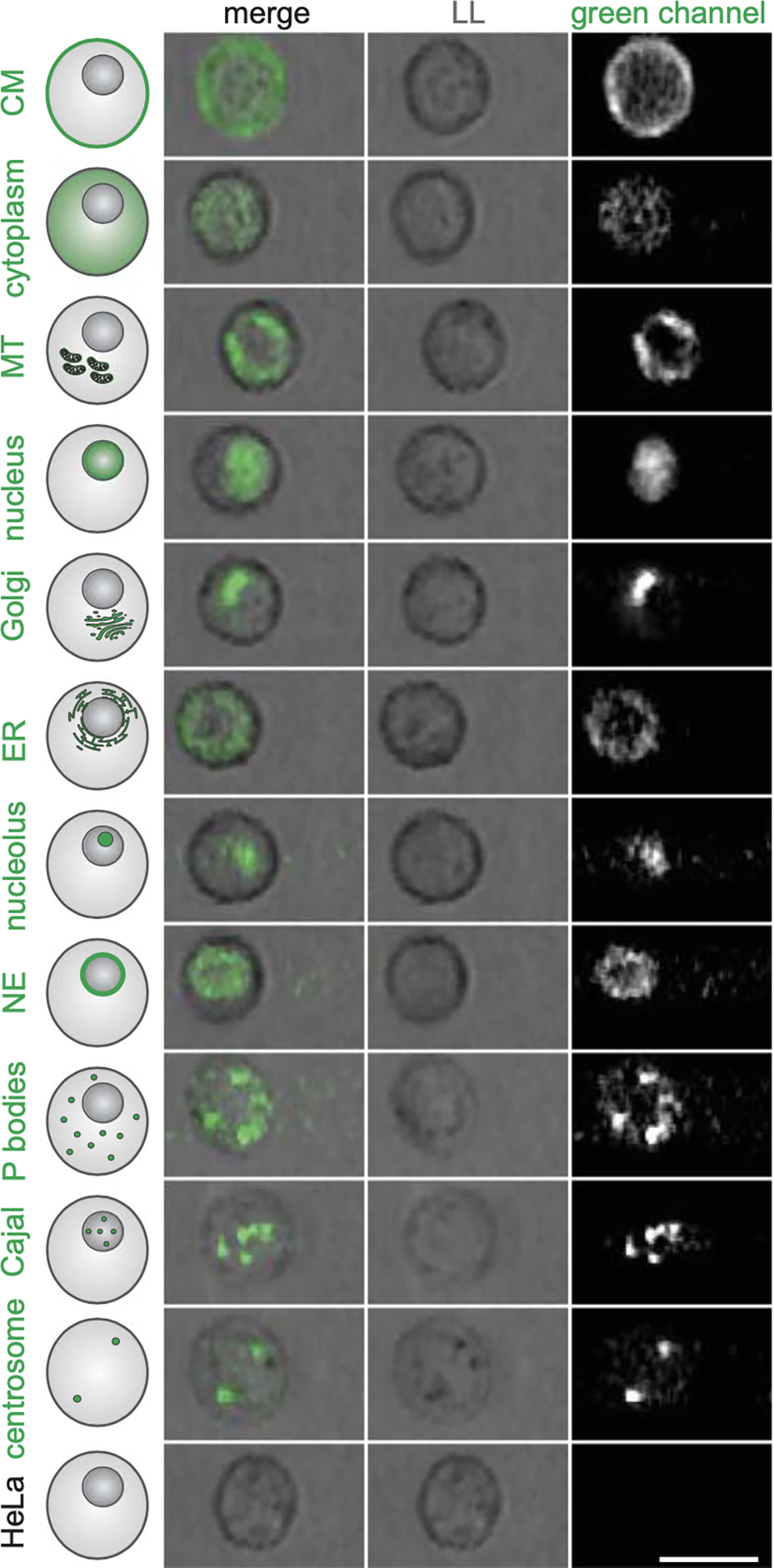
A variety of protein localizations can be detected, classified, and sorted with the use of an image-based cell sorter. Light loss imaging is similar to traditional light field microscopy. (Adapted from Schraivogel et al.1 with permission from the American Association for the Advancement of Science.)
The sorter physically separated the cells that had nuclear expression of RelA after 9 hours of run time at a sorting rate of 14 million cells per hour. The investigators could then identify specific loci, the ablation of which blocked the nuclear translocation of RelA. They did this by sequencing unique guide RNAs that served as barcodes in the affected cells (Fig. 2). Overall, it appears that this system can aptly sort cells on the basis of prespecified complex morphologic characteristics.
Figure 2. Genomewide Genetic Perturbation Screening.
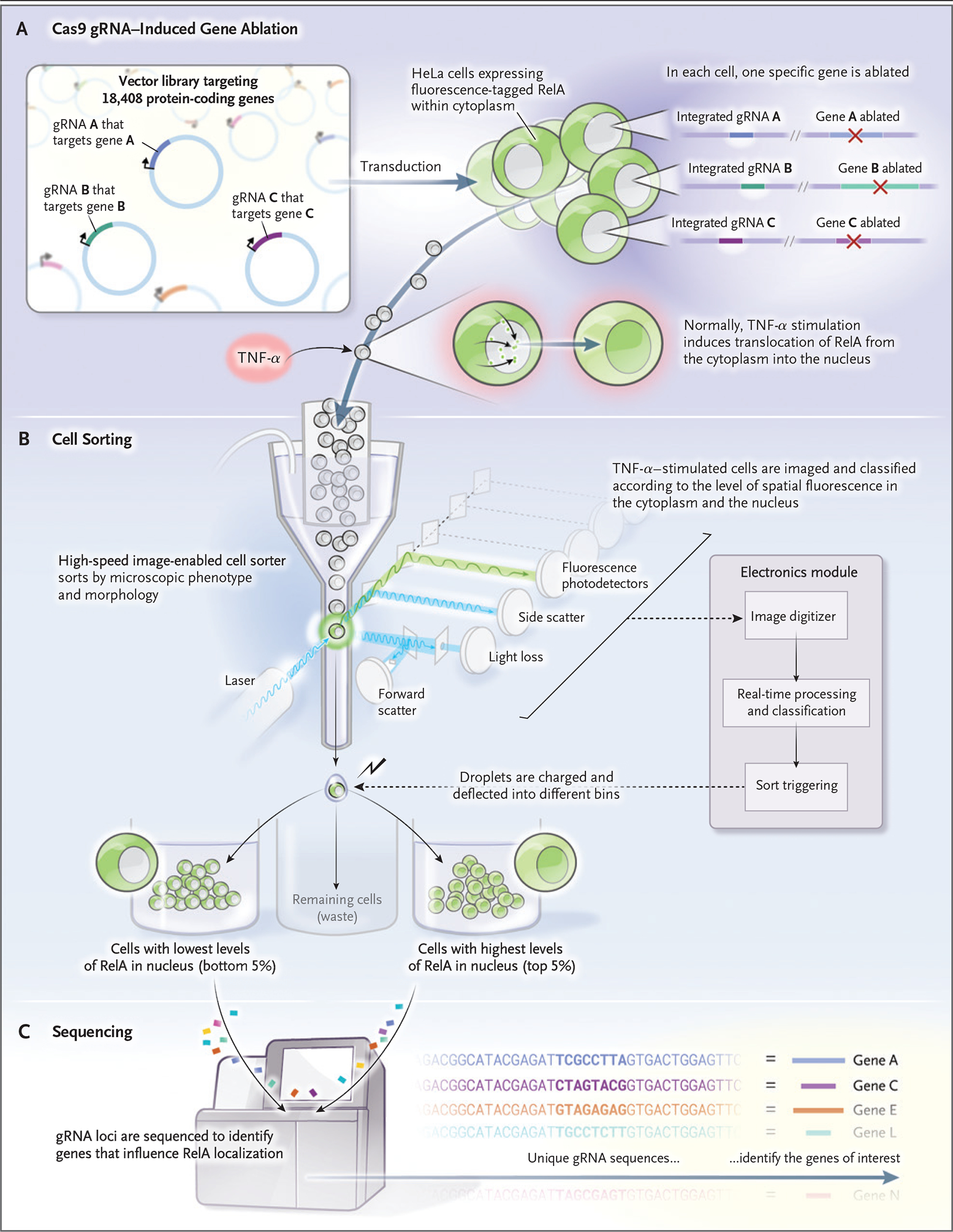
A library of guide RNA (gRNA) is introduced into cells containing Cas9 — in this case, HeLa cells that express a fluorescent protein-tagged RelA. RelA, which normally is located in the cytoplasm, is induced to move into the nucleus when stimulated with tumor necrosis factor α (TNF-α). The stimulated cells are then sorted on the basis of correlation between the RelA intensity signal and a fluorescent nuclear stain. The 5% lowest-correlating and highest-correlating cells are physically separated from the remaining cells. The two cell populations undergo sequencing for detection of gRNA, which serves as a barcode to identify the genes that, when perturbed, affect RelA localization.
Image-based cell-sorting technologies have myriad applications. First, as shown by Schraivogel et al., bar-coded genetic reagents that are present in image-sorted cell populations can be sequenced, which permits powerful genomewide knockdown or overexpression screens. This application expands the number of phenotypes that are amenable to screens to identify genes that underlie disease-related cell phenotypes, a development that can uncover potential therapeutic targets (Fig. 2).
Other approaches to image-based pooled screening exist that may be more suited to specific experiments. Microscope-based approaches are typically slower than flow-based systems, but they permit the detection of morphologic characteristics that are only discernible at higher resolution or in cells that are attached to and spread on a glass surface. Screening with the use of microscopy can involve the selective ablation of cells or the illumination of cells, a process that is followed by dissociation and sorting. Alternatively, microscope-based approaches can detect genetic perturbations in each imaged cell in situ, which permits the reanalysis of the captured images in order to detect other phenotypes and allows for precise, continuous measurements of phenotypes for each genetic perturbation tested (as opposed to sorting technologies that only count how often a cell with a particular characteristic is found in each sort bin).5
Image-based cell sorting can be used to explore the relationship between the sorted, visible cell phenotypes and other characteristics. Cell subpopulations sorted according to morphologic characteristics might subsequently be analyzed for their genome, transcriptome, epigenome, proteome, or additional morphologic properties. In addition, sorted populations can be compared according to the response to perturbations such as drug treatments.
Finally, image-based cell sorting might be used simply to obtain samples of cells that cannot be purified to the same extent with conventional FACS, whether for research or clinical use. For example, one application might be to purify, enrich, and grow cell subtypes, such as circulating tumor cells, ex vivo, for diagnosis and for testing various drugs to guide therapeutic choices. In another potential application, blood products might be purified to eliminate damaged cells that are identified on the basis of morphologic characteristics. Stem cells with favorable properties might be sorted according to differentiation potential or other defined therapeutic goals.
Of course, many downstream uses of sorted cells require that they be free of fixatives or toxic markers, which are required for the detection of many phenotypes. Fortunately, advances in deep learning are beginning to show that label-free bright-field imaging holds promise for use in the identification of many complex and unexpected phenotypes that were previously thought to require specific markers; a computer could be trained to recognize cells from a given disease state (e.g., leukemia6) or to convert unlabeled images into artificially fluorescent ones.7,8 Sorting technologies require the classification of each cell in real time, and classifying cells with complex phenotypes requires machine learning. Therefore, whether cells with complex phenotypes might be successfully sorted remains to be seen. Nevertheless, together these advancements in the process point to a future in which precision cell subpopulations might be rapidly purified and put to good use.
Acknowledgments
We thank Paul Blainey for helpful discussion.
Footnotes
Disclosure forms provided by the authors are available at NEJM.org.
Contributor Information
Andrew Filby, Innovation, Methodology, and Application Research Theme, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
Anne E. Carpenter, Broad Institute of MIT and Harvard, Cambridge, MA
References
- 1.Schraivogel D, Kuhn TM, Rauscher B, et al. High-speed fluorescence image-enabled cell sorting. Science 2022;375:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitta N, Sugimura T, Isozaki A, et al. Intelligent image-activated cell sorting. Cell 2018;175(1):266–276.e13. [DOI] [PubMed] [Google Scholar]
- 3.Isozaki A, Mikami H, Tezuka H, et al. Intelligent image-activated cell sorting 2.0. Lab Chip 2020;20:2263–73. [DOI] [PubMed] [Google Scholar]
- 4.Ota S, Horisaki R, Kawamura Y, et al. Ghost cytometry. Science 2018;360:1246–51. [DOI] [PubMed] [Google Scholar]
- 5.Feldman D, Singh A, Schmid-Burgk JL, et al. Optical pooled screens in human cells. Cell 2019;179(3):787–799.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doan M, Case M, Masic D, et al. Label-free leukemia monitoring by computer vision. Cytometry A 2020;97:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ounkomol C, Seshamani S, Maleckar MM, Collman F, Johnson GR. Label-free prediction of three-dimensional fluorescence images from transmitted-light microscopy. Nat Methods 2018;15:917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen EM, Yang SJ, Ando DM, et al. In silico labeling: predicting fluorescent labels in unlabeled images. Cell 2018;173(3):792–803.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]


