Abstract
Epidemiological data have shown a positive correlation between lipid levels and tumor occurrence, such as the correlation between tumor frequency and aggressiveness, and cardiovascular disease, obesity, type 2 diabetes mellitus, and hyperinsulinemia. Therefore, reducing fat accumulation or weakening lipid metabolism may affect the carcinogenic processes of cells. Many studies have shown that traditional Chinese Medicine (TCM) has obvious advantages over traditional therapies in terms of fewer side effects, lower toxicity, and lower economic burden. This paper reviews the mechanism by which TCM regulates lipid metabolism and its antitumor effect through this regulation, with the aim of elucidating the bioactive compounds in TCM with good efficacy and few side effects that can provide promising therapeutic drugs for targeting lipid metabolism reprogramming in cancer.
Keywords: Traditional Chinese medicine, TCM, tumor, lipid metabolism reprogramming, cancer therapy
Introduction
Metabolic reprogramming refers to the metabolic changes that cells undergo in response to various stressors 1. Metabolic reprogramming is a widespread phenomenon in a variety of diseases, including metabolic pathways such as glucose, lipid, and amino acid metabolism, which are closely related to the occurrence and development of diseases 2. Unlike normal cells, tumors rely primarily on glycolysis rather than mitochondrial oxidative phosphorylation for energy 3. Because the energy supply of tumor cells through the glycolytic pathway is very inefficient, the energy required by tumor cells to maintain their rapid proliferation can be provided by increasing lipid metabolism in addition to increasing glucose intake and consumption 4. Increasing epidemiological data show a positive correlation between lipid levels and tumor occurrence, such as the correlation between tumor frequency and aggressiveness, and cardiovascular disease, obesity, type 2 diabetes mellitus, and hyperinsulinemia 5. Several statins that regulate lipid levels, such as simvastatin, lovastatin, and mevastatin, have been shown to inhibit tumor growth 6-8. Therefore, reducing fat accumulation or weakening lipid metabolism may affect the carcinogenic processes of cells.
Traditional Chinese medicine (TCM) has been widely used alone or as a complementary approach for cancer treatment in East Asia for hundreds of years 9. A large amount of evidence has shown that TCM has obvious advantages over traditional therapies in terms of fewer side effects, lower toxicity, and lower economic burden 9-11. Although previous studies have summarized the progress of the antitumor effect of TCM through the regulation of metabolic pathway reprogramming 12-14, the specific mechanism by which TCM regulates lipid metabolism and mediates antitumor effects has not been systematically summarized. This paper reviews the mechanism by which TCM regulates lipid metabolism and its antitumor effect through this regulation, with the aim of elucidating the bioactive compounds in TCM with good efficacy and few side effects that can provide promising therapeutic drugs for targeting lipid metabolism reprogramming in cancer.
Biology of lipid metabolism
Lipid metabolism in normal cells
Lipids is the general term for triacylglycerol and lipids, which also include sterol, sterol lipids, phospholipids, and glycolipids. Lipids play an important role in the biological processes of energy supply, biofilm formation, energy storage, and generation of signaling molecules 15. Lipid metabolism refers to the emulsification of most of the fat ingested by the human body into small particles by bile, and the hydrolysis of fatty acids in fat into free fatty acids and monoglycerides (and occasionally complete hydrolysis into glycerol and fatty acids) by lipases in the pancreas and small intestine. Hydrolyzed small molecules such as glycerol and short- and medium-chain fatty acids are absorbed into the bloodstream by the small intestine. After monolipids and long-chain fatty acids are absorbed, triglycerides are first resynthesized in small intestinal cells, together with phospholipids, cholesterol, and proteins, to form chylomicrons, which enter the blood circulation from the lymphatic system 16. Fatty acids are important components of various lipids that play a vital role in cells 17. Raw materials for fatty acid synthesis are catalyzed by adenosine-triphosphate (ATP)-citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), and fatty acid synthase (FASN) to synthesize fatty acids from de facto. Some fatty acids are used in the synthesis of triacylglycerols and are stored in tissues as an energy supply 18. When the body requires energy, the triacylglycerol stored in the cells is broken down into glycerol and fatty acids under the action of lipase. Glycerol is decomposed by the glycolic pathway or glucose is generated by the gluconeogenic pathway to provide energy for cells, whereas fatty acids are decomposed into acetyl- CoA under sufficient oxygen supply and are thoroughly oxidized into CO2 and H2O, releasing a large amount of energy 19. Most tissues can oxidize fatty acids, except the brain tissues, because fatty acids cannot pass through the blood-brain barrier 20. Other fatty acids are used to form biofilms and produce lipid signaling molecules that meet the needs of cell division, proliferation, and signal transduction 21. In the normal body, total fatty acids are mainly derived from exogenous fatty acids obtained from food, and the proportion of fatty acids produced through de novo synthesis is very small 22.
Cholesterol can be synthesized in almost all tissues of the body; the liver is the main site, and synthesis of cholesterol is primarily carried out in the cytosol and endoplasmic reticulum 23. Acetyl-CoA is the building block for cholesterol synthesis and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) is a restriction enzyme in cholesterol synthesis that is regulated by the level of cellular free cholesterol 24. Cholesterol can be converted into bile acids, sterol hormones, or 7-dehydrogenated cholesterol in the body, among which the transformation into bile acids is the main route of cholesterol metabolism 23.
Lipid metabolism in tumor cells
Fatty acid metabolism
In rapidly proliferating cells, fatty acid synthesis is accelerated, providing large amounts of lipids for cell membrane components and facilitating β-oxidation of proteins and fatty acyl modification. Therefore, increased fatty acid synthesis plays an important role in highly proliferating cancer cells 25. In normal cells, exogenous fatty acids taken from food are mainly used, and de novo fatty acid synthesis is inhibited; but, the enhancement of de novo fatty acid synthesis pathway in tumor cells promotes the synthesis of tumor biofilms and increases membrane lipid saturation, thereby affecting signal transduction, gene expression, and other basic life processes 26. The enhancement of de facto fatty acid synthesis pathway is the main manifestation of lipid metabolic reprogramming in tumor cells, which involves a variety of key enzymes, including increased expression of ACLY, ACC, and FASN 27, 28. ACLY is the first key enzyme in de novo synthesis of fatty acids, which also links glycolysis and lipid metabolism pathways. Multiple studies have shown that ACLY is highly expressed in tumors, including gastric cancer 29, non-cellular lung cancer 30, breast cancer (BC) 31, and ovarian cancer 32, and is associated with poor prognosis. Further studies have found that ACLY inhibitors inhibit tumor growth, further supporting the role of ACLY in promoting cancer 33-35. Notably, miRNAs have been found to inhibit de novo lipogenesis by downregulating ACLY expression, thus inhibiting tumor growth and metastasis 36, 37. ACC is a key enzyme that catalyzes the production of acetyl-CoA and malonyl-CoA and participates in the de novo synthesis of fatty acids. ACC overexpression has been detected in early BC, ductal carcinoma in situ (DCIS), and lobular carcinoma in situ; further, the phosphorylation levels of ACC are closely associated with BC and lung cancer metastasis 38. In addition, it has been found that increased ACC expression is accompanied by increased FASN and ACLY expression in prostate and hepatocellular carcinoma 39, indicating that ACC may play a synergistic role with FASN and ACLY to promote tumor growth. Liu et al. 40 showed that ACC depletion suppresses de novo fatty acid synthesis and mitochondrial beta-oxidation in the synthesis of acetyl-Coa carboxylase castration-resistant prostate cancer cells. Moreover, many studies have found that regulating fatty acid levels by interfering with ACC expression can help inhibit tumor growth 41-43. Therefore, ACC1 inhibition has become an appealing choice for antitumor therapy 43, 44. Encouraging results indicate that some specific tumor types may respond to ACC1 inhibition. It may be one of the hot spots for future studies to expand the clinical indications of ACC1 inhibitors. ACC catalyzes the formation of malonyl-CoA and gradually synthesizes fatty acids through the action of FASN 45. FASN upregulation is a common feature of human cancers and their precancerous lesions and is closely associated with chemotherapy resistance, tumor metastasis, and poor patient prognosis 46. Overexpression of FASN in tumors is dependent on the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) signal transduction pathway and the transcriptional control of the solid alcohol modulator, junction egg white. Activated PI3K/AKT activates sterol regulatory element binding protein-1c (SREBP-1c) and promotes its entry into the nucleus, thereby inducing the expression of adipose-synthesis-related genes 47. In addition, adenosine monophosphate-activated protein kinase (AMPK)/mammalian target of Rapamycin (mTOR) 48 and signal transducer and activator of transcription 3 (STAT3) 49 signaling pathways have also been reported to mediate the regulation of FASN in tumor proliferation and metastasis. It is worth noting that inhibitors of FASN, similar to the two abovementioned enzymes, also inhibit tumor progression 50-52.
In tumor cells, with fatty acid removal, enhanced head group synthesis occurs together with enhanced fatty acid oxidation (FAO) 53. Carnitine palmitoyltransferase 1 (CPT1) is a key enzyme in FAO. Fatty acids are first activated into fatty acyl coenzyme A and then transported to the mitochondria by CPT1 for FAO 54. After dehydrogenation, water addition, re-dehydrogenation, and thiohydrolysis, acetyl-coenzyme A is generated, which enters the tricarboxylic acid cycle. This process not only generates ATP to supply energy to cells, but also prevents lipid toxicity caused by excessive lipid accumulation 54. The resulting acetyl-CoA enters the cytoplasm and participates in the metabolic response to generate NADPH, which generates large amounts of NADPH to support cellular redox homeostasis, thereby preventing oxidative damage in tumor cells 55. FAO plays a key role in tumor cell proliferation and resistance to chemotherapy. Inhibition of FAO in the mitochondria can affect the production of NADPH and increase the production of reactive oxygen species, leading to ATP depletion in glioblastoma cells and cell death 56. Targeting CPT1 enhances the effect of radiotherapy in patients with nasopharyngeal carcinoma 57. In addition, studies have shown that mitochondrial FAO reprogramming is enhanced in breast cancer, and CPT1A expression is elevated in recurrent breast cancer, which is associated with poor prognosis in patients with breast cancer 58. The de facto fatty acid synthesis pathway is enhanced in tumor cells, and a large number of synthesized fatty acids can supply energy to tumor cells, while FAO is also significantly enhanced 59. Both are in dynamic equilibrium to a certain extent, under which FAO provides energy, and lipid toxicity caused by excessive accumulation of fatty acids is prevented, creating favorable conditions for tumor progression.
Cholesterol metabolism
Abnormal activation of the cholesterol anabolic pathway is one of the signs of several tumors, which helps in the rapid growth of tumor cells to synthesize cell membranes, required lipids, and conduct necessary signals. It is characterized by the activation of cholesterol synthesis signaling SREBP-1 60 and the inhibition of cholesterol efflux signaling liver X receptors (LXRs) 61. The activation of SREBP-1 is regulated by negative feedback of intracellular cholesterol concentration, but tumor cells can bypass this regulation in several ways, allowing continued activation. Normal p53 can promote the transcription of ATP-binding cassette transporter A1 (ABCA1), a cholesterol efflux protein, thereby inhibiting the maturation of SREBPs precursors. The deletion of p53 leads to decreased expression of ABCA1 and upregulated expression of SREBP-1 62. Additionally, sustained activation of protein kinase B in liver cancer phosphorylates phosphoenolpyruvate carboxykinase 1 (PCK1) in the cytoplasm, and phosphorylated PCK1 promotes SREBP-1 to leave the endoplasmic reticulum and activate SREBP-1, thereby promoting tumor growth 63.
Mechanism of TCM in regulating lipid metabolism
TCM regulates fatty acid metabolism
It has been concluded in the above chapters that increased expression of ACLY, ACC, and FASN is the main manifestation of the reprogramming of lipid metabolism in tumor cells. Honeysuckle is a well-known TCM that has been widely used for several years. Its extract has been reported to inhibit the expression of ACLY and ACC1 64. In addition, Qingfei oral liquid, a TCM formulation with clinically proven anti-inflammatory properties, decreases ACLY expression by activating AKT signaling, thereby controlling fatty acid synthesis 65. These results indicate that the extracts or formulations of TCM could regulate the activity of ACLY and affect the synthesis of fatty acids.
Concerning ACC, Dang et al. found that Ling-gui-zhu-gan decoction markedly inhibited the activity of ACC, SREBP-1, and HMGCR, resulting in decreased lipid synthesis in the liver 66. Another Chinese medicine, Jinlida, ameliorates high-fat diet-induced insulin resistance in rats by reducing lipid accumulation and increasing AMPK and ACC phosphorylation in skeletal muscles 67. In addition, Polygonum multiflorum 68, Abrus mollis 69, danthron 70 and other TCM have been reported to inhibit the expression of ACC and FASN to regulate lipid metabolism. Therefore, TCM can regulate lipid metabolism by regulating these three key enzymes.
TCM regulates cholesterol metabolism
Inhibition of cholesterol absorption in the intestine
Cholesterol is an important component of cell membranes and can be obtained through resynthesis from acetyl-CoA or from diet. There is a positive correlation between circulating low-density lipoprotein cholesterol (LDL-C) levels, which can damage arteries and cholesterol absorption. Studies have shown that TCM reduces cholesterol absorption. Water-soluble polysaccharides (WSP) from Cassia seeds bind to bile acids and reduce the amount of absorbable cholesterol 71. In addition, Wang et al. determined that berberine (BBR), a principal bioactive compound in Coptis chinensis and many other medicinal plants, decreases cholesterol levels in rats through multiple mechanisms, including the inhibition of cholesterol absorption 72. In the process of exploring this mechanism, the role of acetyl-CoA cholesterol acyltransferase (ACAT) (an enzyme that catalyzes cholesterol esterification in the cell and accelerates intestinal absorption) in mediating the regulation of cholesterol absorption by TCM has attracted the attention of researchers 73. Hawthorn (Crataegus pinnatifida) is an edible fruit used in Chinese medicine to lower blood lipid levels. Interestingly, Lin et al. found that hawthorn extract inhibited ACAT activity in Caco-2 cells. They further constructed an animal model demonstrating that triterpenic acids present in hawthorn extract reduced plasma cholesterol by inhibiting intestinal ACAT activity in hamsters 74.
Inhibition of endogenous cholesterol synthesis
Only one-third of cholesterol in the body comes from the food supply, with the rest coming from endogenous synthesis. Moriarty et al. evaluated the effect of Xuezhikang (XZK), an extract of fermented red yeast rice with lipid-lowering properties, on blood lipids in subjects with dyslipidemia but without coronary heart disease. The results of this multicenter, randomized, placebo-controlled study showed that daily administration of XZK 1200 mg and 2400 mg for 4-12 weeks resulted in statistically significant and clinically meaningful decrease in non-HDL-C and LDL-C levels compared to placebo 75. Notably, XZK contains a naturally occurring statin (monacolin K) that is identical to lovastatin. Importantly, statins reduce intracellular cholesterol synthesis primarily by competitively inhibiting HMGCR 76. These results suggested that XZK can restrict endogenous cholesterol synthesis by inhibiting HMGCR activity.
Promotion of cholesterol excretion in the liver
Cholesterol that is transported into the liver and endogenously synthesized is lost from the body via biliary secretion after conversion to bile acids. Cholesterol 7-alpha hydroxylase (CYP7A1) is the first rate-limiting enzyme in the neutral pathway of bile acid synthesis and is the main route for cholesterol removal from the body 77. An increasing number of studies have explored the role of TCM in promoting cholesterol excretion by the liver. For example, the coptis alkaloids extract, Jiang-Zhi-Ning (JZN), columbamine from Rhizoma coptidis (RC), RC alkaloids, and Heshouwu, regulate lipids associated with increased cholesterol conversion into bile acids by upregulating CYP7A1 mRNA level 78-82. In addition to the above Chinese herbal extracts or monomers, which can affect the excretion of cholesterol in liver by regulating the activity of CYP7A1, Chinese herbal compounds also play a role. Guo et al. developed Fufang Zhenzhu Tiao Zhi (FTZ), which comprises eight types of quality-maintaining Chinese herbs. By upregulating the expression and activity of the CYP7A1 gene, FTZ promotes the transformation of cholesterol into bile acids, thus reducing serum cholesterol in hyperlipidemic rats 83. However, the specific components that play a role in this compound are unclear and require further analysis. This mechanism is illustrated in Fig. 1.
Figure 1.
Promotion of cholesterol excretion in the liver by TCM. TCM including coptis alkaloids extract, JZN, Columbamine from RC, RC alkaloids, Heshouwu, and FTZ regulates the activity of CYP7A1 to promote the excretion of liver cholesterol into the intestine.
TCM regulates tumor lipid metabolism
Upregulation of FA anabolism is an important characteristic of tumor cell metabolism. Previous reviews have indicated that natural products derived from TCM suppress fatty acid biosynthetic pathways by targeting metabolic enzymes and are regarded as promising inhibitors for cancer treatment. Fatty acid-binding protein (FABP), an intracellular fatty acid transporter, is upregulated in many tumors. Liu et al. 84 isolated the novel natural triterpene GL22 from Ganoderma leucocontextum and showed that GL22 significantly inhibits the growth of liver cancer cell lines and tumor xenografts in vivo. Importantly, this study demonstrated that GL22 treatment decreased the expression of FABPs, which likely underlies the loss of cardiolipin, mitochondrial dysfunction, and cell death 84. In addition, Ganoderma tsugae (GT) has been reported to reduce the levels of fatty acids and lipids in prostate cancer cells by inhibiting the expression of SREBP-1, a key transcriptional regulator controlling lipogenesis, thereby inhibiting the growth of prostate cancer cells 85. In addition, oridonin, a diterpenoid isolated from Rabdosia rubescens 86 and Zhiheshouwu 87, has been reported to interfere with SREBP-1. Oridonin reduced the expression of SREBP-1 mRNA and protein in colorectal cancer cells, whereas Zhiheshouwu extract reduced fatty acid production via inhibiting SREBP-1 and its downstream factor stearyl-CoA dehydrogenase1 (SCD1) in hepatocellular carcinoma (HCC) cells thereby affecting fatty acid formation in tumors 86, 87. As a key enzyme in fatty acid synthesis, FASN plays an important role in tumor progression. Quercetin induced apoptosis of human HCC cells by inhibiting FASN activity and downregulating FASN expression 88. This indicates that TCM can inhibit tumor growth by interfering with FASN activity and affecting fatty acid production. Identifying potential targets for intervention may help improve efficacy and avoid drug resistance. Lin et al. found that in human epidermal growth factor receptor 2 (HER2)-overexpressed BC cells, the AKT/mTOR pathway mediated the inhibition of FASN expression by Osthole 89. In another study, demethoxycurcumin (DMC) derived from the rhizomes of turmeric decreased the activity and/or expression of FASN through AMPK activation in triple-negative breast cancer (TNBC) cells 90.
Cholesterol metabolism is vital for the survival and growth of cancer cells (Figure 2). Emodin, an active component of Chinese herbs, sensitizes HCC cells to the anticancer effects of sorafenib by suppressing cholesterol metabolism. Mechanistically, emodin inhibits the sterol regulatory SREBP-2 transcriptional activity, which suppresses cholesterol biosynthesis and AKT signaling 91. In addition, TCM can affect tumor progression by regulating ACAT. Protopanaxadiol (PPD), a ginseng metabolite generated by gut bacteria, was shown to inhibit FASN and ACAT-2 expression, thereby inducing colorectal cancer cell death 92. In another study, Shim et al. showed that bitter melon extract (BME) treatment inhibited ACAT-1 expression in TNBC cells and reduced tumor growth in TNBC mammospheres implanted into NOD scid gamma mouse (NSG) mice 93. Another BME, momordica anti-HIV protein (MAP30), inhibits ovarian cancer cell progression by reducing glucose transporter (GLUT)-1/3 mediated glucose uptake, lipogenesis, and lipid droplet formation 94. Notably, Actinidia chinensis Planch root (acRoots) extract has been used to treat various types of cancers. A previous study indicated that it inhibits human HCC proliferation by reducing LDL uptake and intracellular cholesterol levels via reducing the expression of LDL receptor. However, the specific mechanism remains unclear and requires further investigation 95. Detailed information regarding the effects of natural products on lipid metabolism is summarized in Table 1.
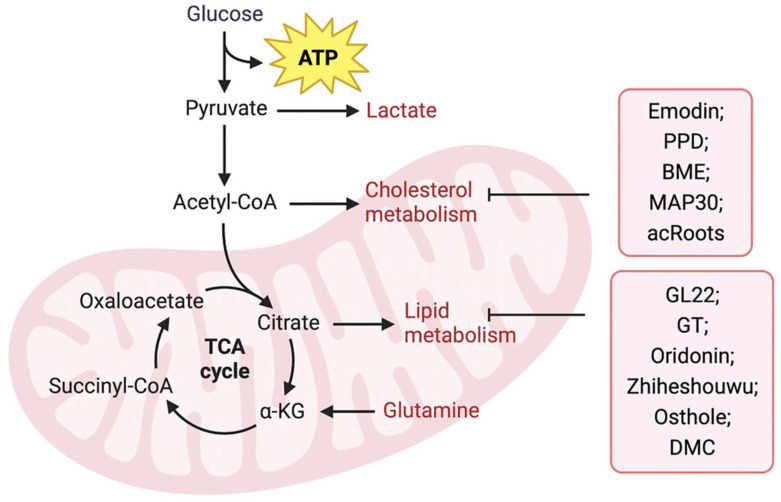
Figure 2.
TCM regulates tumor lipid metabolism. TCM including Emodin, PPD, BME, MAP30, and acRoots, inhibits tumor progression by inhibiting fatty acid metabolism, while GL22, GT, Oridonin, Zhiheshouwu, Osthole, and DMC, inhibit tumor progression by inhibiting cholesterol metabolism in tumor cells.
Table 1.
Regulation of TCM and its bioactive compounds on lipid metabolism
| Bioactive compounds | Chinese herbs | Cancer cells | Potential mechanisms | Ref. |
|---|---|---|---|---|
| GL22 | Ganoderma leucocontextum | Liver cancer cells | Decreasing the expression of FABPs | 84 |
| / | GT | Prostate cancer cells | Inhibiting the expression of SREBP-1 | 85 |
| Oridonin | Rabdosia rubescen | Colorectal cancer cells | Inhibiting the expression of SREBP-1 | 86 |
| / | Zhiheshouwu | HCC cells | Inhibiting SREBP-1 and its downstream factor SCD1 | 87 |
| / | Quercetin | HCC cells | Inhibiting FASN activity and downregulating FASN expression | 88 |
| Osthole | Cnidium monnieri (L.) Cusson | Breast cancer cells | Inhibiting FASN expression | 89 |
| DMC | Rhizomes of turmeric | TNBC cells | Inhibiting FASN expression | 90 |
| Emodin | / | HCC cells | Regulating the transcriptional activity of SREBP-2 | 91 |
| Protopanaxadiol | Ginseng | Colorectal cancer cells | Inhibiting the expression of FASN and ACAT-2 | 92 |
| BME | Bitter melon | TNBC cells | Inhibiting ACAT-1 expression | 93 |
| MAP30 | Bitter melon | Ovarian cancer cells | Reducing GLUT-1/3 expression | 94 |
| acRoots extract | acRoots | HCC cells | Reducing LDL uptake and intracellular cholesterol levels | 95 |
Conclusion and prospects
Reprogramming of lipid metabolism is an important feature of tumor cells. It is essential to explore safer and more effective antitumor treatment strategies by further identifying the dysregulated metabolic processes in tumor cells and understanding the molecular mechanisms related to metabolic reprogramming. Recent studies have shown that single-target inhibitors targeting the reprogramming pathway of lipid metabolism have not achieved ideal efficacy. With increasing studies on antitumor activities of TCM, it has been proven that TCM can effectively intervene in tumor metabolism, inhibit tumor cell proliferation, and promote tumor cell apoptosis through multiple targets and approaches. Combining TCM with current cancer treatment methods may provide ideas and programs for more effective clinical treatment of cancer.
However, most existing studies have focused on the regulation of tumor lipid metabolism by TCM monomers and their mechanisms, and relatively few studies have focused on the regulation of tumor metabolism by TCM compounds. TCM formulas have the advantage of being multi-component and multi-target, showing outstanding efficacy in clinical tumor treatment. However, complex drug compositions and various influencing factors may lead to difficulties in research. Therefore, a discussion on the mechanism of TCM formula intervention in tumor lipid metabolism reprogramming may be a direction for future research, which may better explain the scientific nature and reliability of TCM compound therapy for tumors.
Acknowledgments
Funding
This work was supported financially by the National Natural Science Foundation (No. 82060648) and the Natural Science Foundation of Hebei Province (Grant No. H2021406054) and Science and Technology Research Program of Hebei Provincial Department of Education (QN2022114) and Scientific research start-up fund for high-level talents of Chengde Medical University (No. 202209) and Qinghai Provincial Science and Technology Department Project (2021-ZJ-920).
Author contributions
Hui Liu and Xiuming Li wrote the manuscript and created the figures. Yajie Dong and Changhua Zhou collected and prepared the related papers. Caidan Rezeng conceived the final approval of the version to be submitted and provided the funding. All authors read and approved the final manuscript.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Abbreviations
- PI3K
phosphatidylinositol 3 kinase
- AKT
protein kinase B
- AMPK
adenosine monophosphate-activated protein kinase
- mTOR
mammalian target of Rapamycin
- ATP
adenosine-triphosphate
- TCM
traditional Chinese Medicine
- ACLY
ATP-citrate lyase
- FASN
fatty acid synthase
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- BC
breast cancer
- DCIS
ductal carcinoma in situ
- FAO
fatty acid oxidation
- CPT1
carnitine palmitoyl transferase 1
- SREBP-1
sterol regulatory element-binding proteins-1
- LXRs
liver X receptors
- ABCA1
ATP-binding cassette transporter A1
- PCK1
phosphoenolpyruvate carboxykinase 1 in cytoplasm
- LDL-C
low density lipoprotein cholesterol
- WSP
Water-soluble polysaccharides
- BBR
berberine
- ACAT
acetyl-coA cholesterol acyltransferase
- XZK
Xuezhikang
- CYP7A1
cholesterol 7-alpha hydroxylase
- RC
RhizomaCoptidis
- FTZ
Fufang Zhenzhu Tiao Zhi
- FABP
fatty acid binding protein
- GT
Ganoderma tsugae
- SCD1
stearyl-CoA dehydrogenase1
- HCC
hepatocellular carcinoma
- DMC
demethoxycurcumin
- PPD
protopanaxadiol
- BME
bitter melon extract
- acRoots
actinidia chinensis Planch root
References
- 1.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N. et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. doi: 10.1186/s12943-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohshima K, Morii E. Metabolic Reprogramming of Cancer Cells during Tumor Progression and Metastasis. Metabolites. 2021. 11. [DOI] [PMC free article] [PubMed]
- 3.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211–8. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021. 218. [DOI] [PMC free article] [PubMed]
- 5.Jabczyk M, Nowak J, Hudzik B, Zubelewicz-Szkodzinska B. Curcumin in Metabolic Health and Disease. Nutrients. 2021. 13. [DOI] [PMC free article] [PubMed]
- 6.Lee YG, Chou FN, Tung SY, Chou HC, Ko TL, Fann YC, Tumoricidal Activity of Simvastatin in Synergy with RhoA Inactivation in Antimigration of Clear Cell Renal Cell Carcinoma Cells. Int J Mol Sci. 2023. 24. [DOI] [PMC free article] [PubMed]
- 7.Gao J, Hu J, Yu F, Wang C, Sheng D, Liu W. et al. Lovastatin inhibits erythroleukemia progression through KLF2-mediated suppression of MAPK/ERK signaling. BMC Cancer. 2023;23:306. doi: 10.1186/s12885-023-10742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beton K, Wysocki P, Brozek-Pluska B. Mevastatin in colon cancer by spectroscopic and microscopic methods - Raman imaging and AFM studies. Spectrochim Acta A Mol Biomol Spectrosc. 2022;270:120726. doi: 10.1016/j.saa.2021.120726. [DOI] [PubMed] [Google Scholar]
- 9.Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo N. et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends. 2010;4:297–307. [PubMed] [Google Scholar]
- 10.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 11.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–61. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. doi: 10.1016/j.phrs.2021.105728. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Wang F, Kong X, Li Q, Shi H, Zhao S. et al. The role of metabolic reprogramming in cancer metastasis and potential mechanism of traditional Chinese medicine intervention. Biomed Pharmacother. 2022;153:113376. doi: 10.1016/j.biopha.2022.113376. [DOI] [PubMed] [Google Scholar]
- 14.Lai GH, Wang F, Nie DR, Lei SJ, Wu ZJ, Cao JX. et al. Correlation of Glucose Metabolism with Cancer and Intervention with Traditional Chinese Medicine. Evid Based Complement Alternat Med. 2022;2022:2192654. doi: 10.1155/2022/2192654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace M, Metallo CM. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin Cell Dev Biol. 2020;108:65–71. doi: 10.1016/j.semcdb.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Lei Q, Yang J, Li L, Zhao N, Lu C, Lu A. et al. Lipid metabolism and rheumatoid arthritis. Front Immunol. 2023;14:1190607. doi: 10.3389/fimmu.2023.1190607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carvalho C, Caramujo MJ. The Various Roles of Fatty Acids. Molecules. 2018. 23. [DOI] [PMC free article] [PubMed]
- 18.Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–61. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159:23–34. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- 20.Doi Y. Glycerol metabolism and its regulation in lactic acid bacteria. Appl Microbiol Biotechnol. 2019;103:5079–93. doi: 10.1007/s00253-019-09830-y. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24:50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korshunov DA, Kondakova IV, Shashova EE. Modern Perspective on Metabolic Reprogramming in Malignant Neoplasms. Biochemistry (Mosc) 2019;84:1129–42. doi: 10.1134/S000629791910002X. [DOI] [PubMed] [Google Scholar]
- 23.Goicoechea L, Conde de la Rosa L, Torres S, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023;61:102643. doi: 10.1016/j.redox.2023.102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albi E, Mandarano M, Cataldi S, Ceccarini MR, Fiorani F, Beccari T, The Effect of Cholesterol in MCF7 Human Breast Cancer Cells. Int J Mol Sci. 2023. 24. [DOI] [PMC free article] [PubMed]
- 25.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–92. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012;72:3709–14. doi: 10.1158/0008-5472.CAN-11-4112. [DOI] [PubMed] [Google Scholar]
- 27.Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–19. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papaevangelou E, Almeida GS, Box C, deSouza NM, Chung YL. The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model. Int J Cancer. 2018;143:992–1002. doi: 10.1002/ijc.31392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian X, Hu J, Zhao J, Chen H. ATP citrate lyase expression is associated with advanced stage and prognosis in gastric adenocarcinoma. Int J Clin Exp Med. 2015;8:7855–60. [PMC free article] [PubMed] [Google Scholar]
- 30.Csanadi A, Kayser C, Donauer M, Gumpp V, Aumann K, Rawluk J. et al. Prognostic Value of Malic Enzyme and ATP-Citrate Lyase in Non-Small Cell Lung Cancer of the Young and the Elderly. PLoS One. 2015;10:e0126357. doi: 10.1371/journal.pone.0126357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Yin L, Wei J, Yang Z, Jiang G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumour Biol. 2017;39:1010428317698338. doi: 10.1177/1010428317698338. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Wang Y, Shen L, Pang Y, Qiao Z, Liu P. Prognostic and therapeutic implications of increased ATP citrate lyase expression in human epithelial ovarian cancer. Oncol Rep. 2012;27:1156–62. doi: 10.3892/or.2012.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ismail A, Mokhlis HA, Sharaky M, Sobhy MH, Hassanein SS, Doghish AS. et al. Hydroxycitric acid reverses tamoxifen resistance through inhibition of ATP citrate lyase. Pathol Res Pract. 2022;240:154211. doi: 10.1016/j.prp.2022.154211. [DOI] [PubMed] [Google Scholar]
- 34.Verrelli D, Dallera L, Stendardo M, Monzani S, Pasqualato S, Giorgio M, Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway. Nutrients. 2022. 14. [DOI] [PMC free article] [PubMed]
- 35.Ismail A, Doghish AS, B EME, Salama SA, Mariee AD. Hydroxycitric acid potentiates the cytotoxic effect of tamoxifen in MCF-7 breast cancer cells through inhibition of ATP citrate lyase. Steroids. 2020;160:108656. doi: 10.1016/j.steroids.2020.108656. [DOI] [PubMed] [Google Scholar]
- 36.Xin M, Qiao Z, Li J, Liu J, Song S, Zhao X. et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget. 2016;7:44252–65. doi: 10.18632/oncotarget.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Huang X, Ye T. MiR-22 down-regulates the proto-oncogene ATP citrate lyase to inhibit the growth and metastasis of breast cancer. Am J Transl Res. 2018;10:659–69. [PMC free article] [PubMed] [Google Scholar]
- 38.Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A. et al. Acetyl-CoA Carboxylase 1-Dependent Protein Acetylation Controls Breast Cancer Metastasis and Recurrence. Cell Metab. 2017;26:842–55. doi: 10.1016/j.cmet.2017.09.018. e5. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Huang T. Recent development in acetyl-CoA carboxylase inhibitors and their potential as novel drugs. Future Med Chem. 2020;12:533–61. doi: 10.4155/fmc-2019-0312. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Lai J, Feng Y, Zhuo Y, Zhang H, Chen Y. et al. Acetyl-CoA carboxylase 1 depletion suppresses de novo fatty acid synthesis and mitochondrial beta-oxidation in castration-resistant prostate cancer cells. J Biol Chem. 2023;299:102720. doi: 10.1016/j.jbc.2022.102720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saisomboon S, Kariya R, Boonnate P, Sawanyawisuth K, Cha'on U, Luvira V, Diminishing acetyl-CoA carboxylase 1 attenuates CCA migration via AMPK-NF-kappaB-snail axis. Biochim Biophys Acta Mol Basis Dis. 2023: 166694. [DOI] [PubMed]
- 42.Zhen L, Pan W. ALKBH5 inhibits the SIRT3/ACC1 axis to regulate fatty acid metabolism via an m6A-IGF2BP1-dependent manner in cervical squamous cell carcinoma. Clin Exp Pharmacol Physiol. 2023;50:380–92. doi: 10.1111/1440-1681.13754. [DOI] [PubMed] [Google Scholar]
- 43.Su YW, Huang WY, Lin HC, Liao PN, Lin CY, Lin XY. et al. Silmitasertib, a casein kinase 2 inhibitor, induces massive lipid droplet accumulation and nonapoptotic cell death in head and neck cancer cells. J Oral Pathol Med. 2023;52:245–54. doi: 10.1111/jop.13378. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Nie Q, Wang Z, Di Y, Chen X, Ren K. Targeting acetyl-CoA carboxylase 1 for cancer therapy. Front Pharmacol. 2023;14:1129010. doi: 10.3389/fphar.2023.1129010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikai A. [Fatty acid synthetase] Tanpakushitsu Kakusan Koso. 1993;38:1100–8. [PubMed] [Google Scholar]
- 46.Gunenc AN, Graf B, Stark H, Chari A. Fatty Acid Synthase: Structure, Function, and Regulation. Subcell Biochem. 2022;99:1–33. doi: 10.1007/978-3-031-00793-4_1. [DOI] [PubMed] [Google Scholar]
- 47.Lupien LE, Dunkley EM, Maloy MJ, Lehner IB, Foisey MG, Ouellette ME. et al. An Inhibitor of Fatty Acid Synthase Thioesterase Domain with Improved Cytotoxicity against Breast Cancer Cells and Stability in Plasma. J Pharmacol Exp Ther. 2019;371:171–85. doi: 10.1124/jpet.119.258947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu T, Sun L, Wang Z, Zhang Y, He Z, Xu C. Fatty acid synthase enhances colorectal cancer cell proliferation and metastasis via regulating AMPK/mTOR pathway. Onco Targets Ther. 2019;12:3339–47. doi: 10.2147/OTT.S199369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J, Zou XQ, She S, Shu F, Tuo H, Ren H. et al. [Fatty acid synthase interacts with signal transducer and activator of transcription 3 to promote migration and invasion in liver cancer cells] Zhonghua Gan Zang Bing Za Zhi. 2019;27:681–6. doi: 10.3760/cma.j.issn.1007-3418.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Tantanate C, Khowawisetsut L, Pattanapanyasat K. Performance Evaluation of Automated Impedance and Optical Fluorescence Platelet Counts Compared With International Reference Method in Patients With Thalassemia. Arch Pathol Lab Med. 2017;141:830–6. doi: 10.5858/arpa.2016-0222-OA. [DOI] [PubMed] [Google Scholar]
- 51.Al-Bahlani S, Al-Lawati H, Al-Adawi M, Al-Abri N, Al-Dhahli B, Al-Adawi K. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Apoptosis. 2017;22:865–76. doi: 10.1007/s10495-017-1366-2. [DOI] [PubMed] [Google Scholar]
- 52.Huang SY, Huang GJ, Hsieh PF, Wu HC, Huang WC. Osajin displays potential antiprostate cancer efficacy via impairment of fatty acid synthase and androgen receptor expression. Prostate. 2019;79:1543–52. doi: 10.1002/pros.23876. [DOI] [PubMed] [Google Scholar]
- 53.De Martino M, Daviaud C, Hajjar E, Vanpouille-Box C. Fatty acid metabolism and radiation-induced anti-tumor immunity. Int Rev Cell Mol Biol. 2023;376:121–41. doi: 10.1016/bs.ircmb.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Rodriguez R, Fosch A, Garcia-Chica J, Zagmutt S, Casals N. Targeting carnitine palmitoyltransferase 1 isoforms in the hypothalamus: A promising strategy to regulate energy balance. J Neuroendocrinol. 2023: e13234. [DOI] [PubMed]
- 55.Garcia JG, Ansorena E, Izal I, Zalba G, de Miguel C, Milagro FI. Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5) J Physiol Biochem. 2023. [DOI] [PMC free article] [PubMed]
- 56.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–32. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan Z, Xiao L, Tang M, Bai F, Li J, Li L. et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329–47. doi: 10.7150/thno.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong Y, Liu Z, Zhao X, Ruan S, Zhang X, Wang S. et al. CPT1A regulates breast cancer-associated lymphangiogenesis via VEGF signaling. Biomed Pharmacother. 2018;106:1–7. doi: 10.1016/j.biopha.2018.05.112. [DOI] [PubMed] [Google Scholar]
- 59.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue L, Qi H, Zhang H, Ding L, Huang Q, Zhao D. et al. Targeting SREBP-2-Regulated Mevalonate Metabolism for Cancer Therapy. Front Oncol. 2020;10:1510. doi: 10.3389/fonc.2020.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pontini L, Marinozzi M. Shedding light on the roles of liver X receptors in cancer by using chemical probes. Br J Pharmacol. 2021;178:3261–76. doi: 10.1111/bph.15200. [DOI] [PubMed] [Google Scholar]
- 62.Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JPt. et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 2019;176:564–80. doi: 10.1016/j.cell.2018.11.011. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y. et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–5. doi: 10.1038/s41586-020-2183-2. [DOI] [PubMed] [Google Scholar]
- 64.Wan J, Jiang CX, Tang Y, Ma GL, Tong YP, Jin ZX. et al. Structurally diverse glycosides of secoiridoid, bisiridoid, and triterpene-bisiridoid conjugates from the flower buds of two Caprifoliaceae plants and their ATP-citrate lyase inhibitory activities. Bioorg Chem. 2022;120:105630. doi: 10.1016/j.bioorg.2022.105630. [DOI] [PubMed] [Google Scholar]
- 65.An L, Lu M, Xu W, Chen H, Feng L, Xie T. et al. Qingfei oral liquid alleviates RSV-induced lung inflammation by promoting fatty-acid-dependent M1/M2 macrophage polarization via the Akt signaling pathway. J Ethnopharmacol. 2022;298:115637. doi: 10.1016/j.jep.2022.115637. [DOI] [PubMed] [Google Scholar]
- 66.Dang Y, Hao S, Zhou W, Zhang L, Ji G. The traditional Chinese formulae Ling-gui-zhu-gan decoction alleviated non-alcoholic fatty liver disease via inhibiting PPP1R3C mediated molecules. BMC Complement Altern Med. 2019;19:8. doi: 10.1186/s12906-018-2424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zang SS, Song A, Liu YX, Wang C, Song GY, Li XL. et al. Chinese medicine Jinlida (JLD) ameliorates high-fat-diet induced insulin resistance in rats by reducing lipid accumulation in skeletal muscle. Int J Clin Exp Med. 2015;8:4620–34. [PMC free article] [PubMed] [Google Scholar]
- 68.Xian Z, Liu Y, Xu W, Duan F, Guo Z, Xiao H. The Anti-hyperlipidemia Effects of Raw Polygonum multiflorum Extract in Vivo. Biol Pharm Bull. 2017;40:1839–45. doi: 10.1248/bpb.b17-00218. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Jiang ZZ, Chen M, Wu MJ, Guo HL, Sun LX. et al. Protective effect of total flavonoid C-glycosides from Abrus mollis extract on lipopolysaccharide-induced lipotoxicity in mice. Chin J Nat Med. 2014;12:461–8. doi: 10.1016/S1875-5364(14)60072-8. [DOI] [PubMed] [Google Scholar]
- 70.Zhou R, Wang L, Xu X, Chen J, Hu LH, Chen LL. et al. Danthron activates AMP-activated protein kinase and regulates lipid and glucose metabolism in vitro. Acta Pharmacol Sin. 2013;34:1061–9. doi: 10.1038/aps.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang YL, Chow CJ, Tsai YH. Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012;134:1967–72. doi: 10.1016/j.foodchem.2012.03.127. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Yi X, Ghanam K, Zhang S, Zhao T, Zhu X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism. 2014;63:1167–77. doi: 10.1016/j.metabol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 73.Bhattacharjee P, Rutland N, Iyer MR. Targeting Sterol O-Acyltransferase/Acyl-CoA:Cholesterol Acyltransferase (ACAT): A Perspective on Small-Molecule Inhibitors and Their Therapeutic Potential. J Med Chem. 2022;65:16062–98. doi: 10.1021/acs.jmedchem.2c01265. [DOI] [PubMed] [Google Scholar]
- 74.Lin Y, Vermeer MA, Trautwein EA. Triterpenic Acids Present in Hawthorn Lower Plasma Cholesterol by Inhibiting Intestinal ACAT Activity in Hamsters. Evid Based Complement Alternat Med. 2011;2011:801272. doi: 10.1093/ecam/nep007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moriarty PM, Roth EM, Karns A, Ye P, Zhao SP, Liao Y. et al. Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo-controlled study. J Clin Lipidol. 2014;8:568–75. doi: 10.1016/j.jacl.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Fernandes Silva L, Ravi R, Vangipurapu J, Laakso M. Metabolite Signature of Simvastatin Treatment Involves Multiple Metabolic Pathways. Metabolites. 2022. 12. [DOI] [PMC free article] [PubMed]
- 77.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Zhao H, Yang Y, Liu B, Ni J, Wang W. Lipid-lowering and antioxidant activities of Jiang-Zhi-Ning in Traditional Chinese Medicine. J Ethnopharmacol. 2011;134:919–30. doi: 10.1016/j.jep.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 79.Li N, Chen Z, Mao X, Yu J, Zhao R. Effects of lipid regulation using raw and processed radix polygoni multiflori in rats fed a high-fat diet. Evid Based Complement Alternat Med. 2012;2012:329171. doi: 10.1155/2012/329171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He K, Hu Y, Ma H, Zou Z, Xiao Y, Yang Y. et al. Rhizoma Coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochim Biophys Acta. 2016;1862:1696–709. doi: 10.1016/j.bbadis.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Han Y, Chai F, Xiang H, Huang T, Kou S. et al. The antihypercholesterolemic effect of columbamine from Rhizoma Coptidis in HFHC-diet induced hamsters through HNF-4alpha/FTF-mediated CYP7A1 activation. Fitoterapia. 2016;115:111–21. doi: 10.1016/j.fitote.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 82.Cao Y, Bei W, Hu Y, Cao L, Huang L, Wang L. et al. Hypocholesterolemia of Rhizoma Coptidis alkaloids is related to the bile acid by up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine. 2012;19:686–92. doi: 10.1016/j.phymed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Guo J, Bei W, Hu Y, Tang C, He W, Liu X. et al. A new TCM formula FTZ lowers serum cholesterol by regulating HMG-CoA reductase and CYP7A1 in hyperlipidemic rats. J Ethnopharmacol. 2011;135:299–307. doi: 10.1016/j.jep.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Liu G, Wang K, Kuang S, Cao R, Bao L, Liu R. et al. The natural compound GL22, isolated from Ganoderma mushrooms, suppresses tumor growth by altering lipid metabolism and triggering cell death. Cell Death Dis. 2018;9:689. doi: 10.1038/s41419-018-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang SY, Huang GJ, Wu HC, Kao MC, Huang WC. Ganoderma tsugae Inhibits the SREBP-1/AR Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules. 2018. 23. [DOI] [PMC free article] [PubMed]
- 86.Kwan HY, Yang Z, Fong WF, Hu YM, Yu ZL, Hsiao WL. The anticancer effect of oridonin is mediated by fatty acid synthase suppression in human colorectal cancer cells. J Gastroenterol. 2013;48:182–92. doi: 10.1007/s00535-012-0612-1. [DOI] [PubMed] [Google Scholar]
- 87.Li H, Xiang L, Yang N, Cao F, Li C, Chen P. et al. Zhiheshouwu ethanol extract induces intrinsic apoptosis and reduces unsaturated fatty acids via SREBP1 pathway in hepatocellular carcinoma cells. Food Chem Toxicol. 2018;119:169–75. doi: 10.1016/j.fct.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 88.Zhao P, Mao JM, Zhang SY, Zhou ZQ, Tan Y, Zhang Y. Quercetin induces HepG2 cell apoptosis by inhibiting fatty acid biosynthesis. Oncol Lett. 2014;8:765–9. doi: 10.3892/ol.2014.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin VC, Chou CH, Lin YC, Lin JN, Yu CC, Tang CH. et al. Osthole suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt/mTOR pathway. J Agric Food Chem. 2010;58:4786–93. doi: 10.1021/jf100352c. [DOI] [PubMed] [Google Scholar]
- 90.Shieh JM, Chen YC, Lin YC, Lin JN, Chen WC, Chen YY. et al. Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple-negative breast cancer cells. J Agric Food Chem. 2013;61:6366–75. doi: 10.1021/jf4012455. [DOI] [PubMed] [Google Scholar]
- 91.Kim YS, Lee YM, Oh TI, Shin DH, Kim GH, Kan SY, Emodin Sensitizes Hepatocellular Carcinoma Cells to the Anti-Cancer Effect of Sorafenib through Suppression of Cholesterol Metabolism. Int J Mol Sci. 2018. 19. [DOI] [PMC free article] [PubMed]
- 92.Jin HR, Du CH, Wang CZ, Yuan CS, Du W. Ginseng metabolite protopanaxadiol interferes with lipid metabolism and induces endoplasmic reticulum stress and p53 activation to promote cancer cell death. Phytother Res. 2019;33:610–7. doi: 10.1002/ptr.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shim SH, Sur S, Steele R, Albert CJ, Huang C, Ford DA. et al. Disrupting cholesterol esterification by bitter melon suppresses triple-negative breast cancer cell growth. Mol Carcinog. 2018;57:1599–607. doi: 10.1002/mc.22882. [DOI] [PubMed] [Google Scholar]
- 94.Chan DW, Yung MM, Chan YS, Xuan Y, Yang H, Xu D. et al. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol Res. 2020;161:105157. doi: 10.1016/j.phrs.2020.105157. [DOI] [PubMed] [Google Scholar]
- 95.He M, Hou J, Wang L, Zheng M, Fang T, Wang X. et al. Actinidia chinensis Planch root extract inhibits cholesterol metabolism in hepatocellular carcinoma through upregulation of PCSK9. Oncotarget. 2017;8:42136–48. doi: 10.18632/oncotarget.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]