Abstract
Background:
Esophageal remodeling is a factor in disease progression and symptom severity for patients with eosinophilic esophagitis (EoE). Remodeling can begin early in children, resulting in stricture and food impaction. Detection of esophageal remodeling often depends on endoscopy and is appreciated only in its later stages.
Objective:
We sought to determine whether luminal eosinophil-associated and remodeling proteins captured by the esophageal string test (EST) correlate with measures of esophageal remodeling and biomarkers of the epithelial–mesenchymal transition (EMT).
Methods:
Patients with EoE (7–18 years old) were enrolled from 2 pediatric hospitals. Participants performed the EST and underwent endoscopy. Histology, distensibility measured by endoluminal functional lumen imaging probe, and symptoms were assessed. Protein quantitation by ELISAwas performed on mucosal biopsy and EST samples. Tissue sections were evaluated for EMT. Outcome measures were summarized, and Spearman ρ was used to assess bivariate correlations.
Results:
Forty patients (68% male) were enrolled (mean age, 12.5 years). Twenty-four (60%) had active disease (≥15 eosinophils per high-power field). EST-captured eotaxin-3, major basic protein 1, EDN, eosinophil peroxidase, and Charcot-Leyden crystal protein/galectin-10 showed significant correlations with peak eosinophils per high-power field (ρ 0.53–0.68, P < .001). Luminal proteins positively correlated with endoscopic features and markers of EMT, and negatively with esophageal distensibility. Periostin was captured by the EST and correlated with eosinophil density, basal zone hyperplasia, endoscopic appearance, and markers of EMT.
Conclusion:
Luminal markers of esophageal remodeling in addition to biomarkers of eosinophilic inflammation correlate with epithelial and functional remodeling in EoE.
Keywords: Eosinophilic esophagitis, esophageal string test, remodeling, epithelial mesenchymal transition, EndoFLIP, distensibility, basal zone hyperplasia
Graphical Abstract
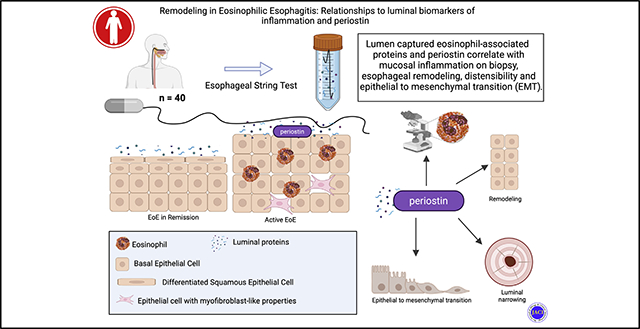
Esophageal remodeling underlies disease progression and symptom severity for patients with eosinophilic esophagitis (EoE) and results in esophageal stricture, food impaction, and worse patient outcomes.1–3 However, the mainstay of disease assessment has largely remained histologic inflammation and eosinophil density.4 Recognizing that this inflammatory measure does not always accurately reflect disease severity or symptoms, several measures have been developed to provide additional disease assessment including histologic scoring,5 endoscopic reference scores,6 esophageal distensibility7,8 and symptom indices.9,10 Several of these metrics provide a measurement of fibrostenotic remodeling such as esophageal rings and luminal narrowing. At the same time, an ongoing clinical challenge is balancing the need to evaluate disease severity as well as the extent of tissue remodeling and inflammation while minimizing the number of endoscopies. Efforts are being made to lessen the burden of invasive endoscopies with transnasal endoscopy11 and the development of less invasive luminal sampling techniques, such as the esophageal string test12,13 and the cytosponge.14
Several tissue-based factors associated with eosinophilic inflammation have been implicated in the progression of esophageal remodeling and fibrosis in EoE. For example, the cytokines TGF-β, IL-13, and TNF-α can stimulate myofibroblast differentiation, promote synthesis/contraction of extracellular matrix proteins,15–20 and induce epithelial–mesenchymal transition (EMT), all leading to subepithelial fibrosis.15,21,22 Periostin is upregulated in EoE,23 is induced by TGF-β1 and IL-13, is associated with eosinophil recruitment and adhesion,24 and promotes myofibroblast differentiation and collagen production.25 Eosinophil granule cationic proteins, particularly major basic protein 1 (MBP-1) and EDN, induce expression of tissue remodeling factors by epithelial cells and fibroblasts.26 While we understand that these effectors are driving remodeling, the extent to which the inflammatory milieu in EoE may serve as a surrogate for remodeling and disease activity is unknown.
The esophageal string test (EST), a swallowed capsule containing a woven nylon string, allows for the sampling of esophageal luminal secretions and sloughed epithelial and inflammatory cells. Eosinophil-associated proteins (EAPs) measured from the string eluate correlate strongly with EAPs measured in extracts of mucosal biopsy samples as well as with eosinophil density on histology.12,13
In the current study, we sought to evaluate if EAPs captured by the EST correlate with clinical measures of remodeling and biomarkers of EMT. We also assessed if periostin, a secreted protein implicated in fibrotic remodeling, could be captured by the EST and if it correlated with other measures of inflammation or esophageal remodeling. We measured EAPs along with periostin from mucosal biopsy samples and luminal eluate and compared them to clinical and histologic markers of remodeling.
METHODS
Patient selection
Patients were recruited prospectively at Children’s Hospital Colorado and Children’s Hospital of Philadelphia. Subjects 7 to 18 years of age who had a confirmed or suspected diagnosis of EoE and who were undergoing clinical upper endoscopy were eligible for participation. Patients were excluded if they had a known esophageal stricture that might preclude their swallowing the EST capsule, or if they had a diagnosed connective tissue disease, inflammatory bowel disease or other comorbid non-EoE eosinophilic gastrointestinal diseases, gelatin allergy, history of esophageal surgery or injury, or achalasia. Receipt of orally or intravenously provided steroids in the 60 days before the procedure was an additional exclusion criterion. Included subjects had a confirmed diagnosis of EoE based on a history of esophageal eosinophilia of ≥15 eosinophils per high-power field (eos/hpf) and esophageal symptoms, with other causes for these findings excluded. All subjects and their parents provided informed consent and assent as appropriate. This study was approved by the local institutional review boards at each institution.
EST and mucosal biopsy collection
The EnteroTracker string test device (EnteroTrack, Aurora Colo) was used to capture a sample, via liquid biopsy, that contained esophageal luminal secretions as well as sloughed epithelial and inflammatory cells as previously described.12,13 The EnteroTracker device consists of a weighted gelatin capsule containing 90 cm of woven nylon string. The capsule is swallowed, and the proximal end of the string is taped to the subject’s cheek. The string is removed by pulling it out through the mouth 1 hour after being swallowed. The esophageal segment, identified by pH or distance measurements, is placed in EST elution buffer and the eluate frozen for later quantitation of EAPs and other biomarkers as previously described.12,13 We performed the ESTwithin 2 days of the patient’s scheduled endoscopy, and no treatment changes occurred during this period. Pinch biopsy samples were obtained during endoscopy from the distal esophagus and either snap frozen or placed in buffered formalin and stored at room temperature until analysis. Both string and biopsy samples were stored at −80°C until eluted and extracted as previously described.12,13
Histology slides of mucosal biopsy samples were assessed for peak eos/hpf (surface area 0.26 mm2) and for grade and stage according to the validated EoE Histologic Scoring System (EoEHSS).5 Eight features of esophageal biopsy samples were defined and evaluated: eosinophilic inflammation, basal zone hyperplasia (BZH), eosinophil abscess, eosinophil surface layering, intracellular spacing, surface epithelial alteration, dyskeratotic epithelial cells, and lamina propria fibrosis. To calculate the EoEHSS expressed as a ratio, features were first scored from 0 to 24 for grade (severity) and from 0 to 24 for stage (extent), then divided by the maximum possible value of EoEHSS that could be obtained on the basis of the features available.
Protein quantitation
EST samples and mucosal biopsy samples were processed for quantitation of EAPs as described elsewhere.12,13 EAP concentrations in biopsy extracts were normalized to total extracted protein and are reported as ng biomarker/mg protein. EAP concentrations in the EST samples are reported as ng/mL of EST supernatant. The selected EAPs were proteins previously shown to be captured by the EST and associated with eosinophils and EoE disease activity.12,13 Measured EAPs included the eosinophil granule–associated proteins: eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPX), and MBP-1, the eosinophil cytosolic protein Charcot-Leyden crystal protein/galectin-10 (CLC/Gal-10), and eotaxin-3 (Eot-3, CCL26). Periostin was selected as a protein known to be increased in EoE and mechanistically associated with fibrotic remodeling. ELISAs were performed on biopsy extracts and EST string samples using either commercially available or in-house tests as follows: EDN (catalog no. 7630, MBL International, Woburn, Mass), EPX (L-F7094, LSBio, Seattle Wash), MBP-1 and CLC/Gal-10 (Ackerman Laboratory, in-house assays), and Eot-3/CCL26 (DY346, DuoSet, R&D Systems, Minneapolis, Minn) as previously described,12,13 and periostin (AG-45B-0004-KI01, AdipoGen Life Sciences, San Diego, Calif). Other proteins associated with epithelial remodeling were assessed (TGF-β1, filaggrin, and PAI-1), but they were not consistently detected, and methods to capture these proteins by the EST or in biopsy extracts were not further optimized.
Evaluation of EMT
Tissue sections from mucosal biopsy samples were stained and evaluated for the presence of EMT as previously described.22,27 Briefly, sections 4 mm thick of formalin-fixed, paraffin-embedded biopsy samples were cut at the Research Histology and Tissue Imaging Core facility at the University of Illinois at Chicago. Slides were labeled by analysts unaware of EoE disease status. Slides were sequentially stained for E-cadherin and vimentin using anti–E-cadherin primary antibody clone 4A2 (1:50, 14472) and anti-vimentin primary antibody clone D21H3 (1:100, 5741) from Cell Signaling Technologies (Danvers, Mass). Alexa Fluor 488– and Alexa Fluor 555–labeled secondary antibodies (Cell Signaling Technologies) were used for the detection of E-cadherin and vimentin, respectively. Sections were counterstained with 4′,6-diamidino-2-phenylindole. Immunostaining was performed on a Leica BOND RX autostainer (Leica Biosystems, Buffalo Grove, Ill) in batches that included both positive and negative controls for EMT. Slides were scanned at 200-fold magnification using a Vectra 3 multispectral imaging system (Akoya Biosciences, Waltham, Mass). The percentage of epithelial cells positive for vimentin, total vimentin expression per cell, and mean and total expression of E-cadherin across pixels in each cell membrane were quantified. The ratio of total vimentin to total E-cadherin (Vim/Ecad) expression per cell was computed. A total of 477,267 epithelial cells across 45 slides, an average of 10,606 epithelial cells per biopsy sample, were analyzed in total.
Endoscopic procedures and symptom assessment
The endoscopist calculated the EoE Endoscopic Reference Score (EREFS) at the time of endoscopy. This validated tool captures the presence and severity of esophageal edema, rings, exudates, furrows, and strictures.6 The presence of edema, white plaques, and/or linear furrows was categorized as inflammatory endoscopic features; the presence of rings and/or narrowing with or without inflammatory features was categorized as fibrostenotic features. EREFS score from the proximal, mid, and distal esophagus were summed for a total EREFS score (range, 0–27).
During endoscopy, an endoluminal functional lumen imaging probe (FLIP; EndoFLIP, Medtronic, Minneapolis, Minn) was used to measure esophageal distensibility. The catheter was placed 2 cm across the esophagogastric junction and inflated in 10 mL increments, pausing for 10 seconds between inflations.8 Inflation continued until a pressure of 50 mm Hg or a balloon volume of 60 mL was reached, whichever occurred first. The balloon was then deflated and removed from the esophagus. All recorded FLIP studies were individually reviewed and only lumen electrodes included. To determine the distensibility of the esophageal lumen, raw FLIP data were downloaded and filtered for analysis as previously described.8 Distensibility was defined as the minimal lumen diameter at a mean intrabag pressure of 40 mm Hg. Data were filtered and analyzed by StataSE v16 (StataCorp, College Station, Tex).
Subject- and parent-reported EoE-related symptoms were captured by Pediatric Eosinophilic Esophagitis Symptoms Scores (PEESS v2.0).9 PEESS is an age-specific validated index measuring EoE symptomatology and includes a frequency score and severity score. Scores range from 0 to 100, with a higher score indicating more frequent and/or severe symptoms.
Statistical analyses
Statistical analyses were performed by SAS v9.4 (SAS Institute, Cary, NC). Participants’ characteristics and outcome measures were summarized using percentages for categorical variables and means and SDs or medians and interquartile ranges, as appropriate, for continuous variables.
An EoEScore was calculated for each individual in the study using the equation EoEScore 5 (Probability of eos/hpf ≥ 15) 5 Exp(XB)/(1 + XB), where XB = [−0.9247 + (0.00179 × Eot-3) + (0.000775 × MBP-1)].13 Validity of this original model was assessed using the C statistic and Brier score. C statistics of the original model and C statistic of the EoE score for the validation data were compared by z approximation.
Spearman ρ, which is robust to outliers, was used to assess bivariate correlation. Following the Cohen analysis, strength of correlation was interpreted as small, medium, and large, respectively, for 0.1 < |ρ| < 0.3, 0.3 < |ρ| < 0.5, and | ρ| > 0.5. P values for testing |ρ| = 0 are reported. P < .05 was considered statistically significant. No adjustment for type I error was used for multiple outcomes.
RESULTS
Study subjects
Forty EoE patients (68% male) were enrolled and met the eligibility criteria (Table I). Mean age was 12.5 years (median, 12 years; interquartile range, 10–15 years). Twenty-four (60%) had active disease (≥15 eos/hpf). Most were receiving proton pump inhibitor therapy at time of study participation (73%). Patients could receive more than 1 treatment. Fourteen (38%) were prescribed swallowed or topical corticosteroids, and 24 patients (60%) underwent a food-allergen elimination diet. The majority had at least 1 endoscopic inflammatory feature (edema, white plaques, linear furrows). One participant had fibrostenotic features assessed by EREFS. A full description of outcome measures by disease activity is shown in Table E1 in this article’s Online Repository (available at www.jacionline.org).
TABLE I.
Characteristics and clinical features of 40 subjects
| Characteristic | Value |
|---|---|
|
| |
| Age (years) | 12.45 ± 2.88 |
| Male | 27 (67.5) |
| Hispanic/Latino | 6 (15.0) |
| White | 35 (87.5) |
| Proton pump inhibitor treatment | 27 (73.0) |
| Swallowed steroid treatment | 14 (37.85) |
| Dietary elimination | 24 (60.0) |
| Active EoE (≥15 eos/hpf) | 24 (60.0) |
| Edema | 11 (27.5) |
| Rings | 1 (2.5) |
| Exudates | 12 (23.3) |
| Furrows | 21 (52.5) |
| Stricture | 1 (2.5) |
| Distensibility (mm) (N = 35) | 15.55 ± 2.55 |
Data are presented as nos. (%) or means ± SDs.
Luminal captured EAPs correlate with EAPs in biopsy tissue, mucosal eosinophils, and endoscopic features
In this study, we validated previous findings12,13 demonstrating that measurements of luminal EAPs correlate with the same EAPs measured in extracts of mucosal biopsy samples and with peak eos/hpf. Correlation between luminal EAP and corresponding mucosal biopsy EAP measurements had large effects (ρ 0.66–0.77, P < .001) (see Fig E1 in the Online Repository available at www.jacionline.org). Luminal EAPs had differential expression based on inactive and active disease (Fig E1). Measurement of luminal Eot-3, MBP-1, EDN, EPX and CLC/Gal-10 by ESTall showed significant correlations with peak eosinophil counts, ranging from ρ 0.53 to ρ 0.68 (P < .001) (Fig 1). In addition, luminal EAPs significantly and positively correlated with total EREFS. EREFS scores ranged from 0 to 15 (median, 1.0; interquartile range, 0–6; mean ± SD, 3.41 ± 4.3) for the study cohort. Eot-3 was the most highly correlated, with r 0.61 (P <.01), while other EAPs ranged ρ 0.53 to ρ 0.36 (P <.01).
FIG 1.

String eosinophil protein levels correlate with mucosal eosinophilia and endoscopic findings. Spearman analysis correlating EST-EAPs with peak eos/hpf and total endoscopic reference score. Analyzed here is the sum of proximal, mid, and distal esophagus scores (0–27). Spearman rank correlation ρ coefficients are shown. *P < .05; #P < .01.
Symptoms were evaluated by PEESS 2.0. Studies to date have not demonstrated strong correlation between peak eosinophil count and symptoms.9,10 Similarly, we found that EAPs and symptom scores did not correlate (by Spearman) in this cohort (see Table E2 in the Online Repository available at www.jacionline.org).
It has been previously reported that the combined measurement of Eot-3 and MBP-1 (EoEScore) is the most sensitive and specific for monitoring disease activity in EoE defined by peak eosinophil density.13 A combined scoring for Eot-3 plus MBP-1 in this validation data set had acceptable discriminating ability, with an area under the curve C statistic of 0.74 (95% confidence interval, 0.59–0.89) (see Fig E2 in the Online Repository available at www.jacionline.org). Although a lower area under the curve for this cohort was observed compared to the training data set13 (0.74 vs 0.83, respectively), the difference was not statistically significant.
Luminal EAPs correlate with a histologic scoring system
EoE involves more changes in the mucosa beyond eosinophilic infiltrate,5,28 including BZH, dilated intercellular spaces, eosinophil layering/microabscesses, and lamina propria fibrosis. These additional histologic parameters have been shown to correlate better with symptoms and are frequently measured in research studies by EoEHSS.5,29,30 We further analyzed the inflammatory components of the histologic scoring system (eosinophilic inflammation, eosinophil abscesses, surface layering, surface epithelia alteration) and the architectural components (BZH, dilated intercellular spaces, dyskeratotic epithelial cells, lamina propria fibrosis). EoEHSS scores including total, inflammatory, and architectural scores positively and significantly correlated with EAPs. Eot-3, MBP-1, and EDN are highly correlated with all histologic scoring system scores (r 0.58–0.74), while EPX and CLC/Gal-10 showed moderate correlation (ρ 0.39–0.67) (Fig 2). Correlation coefficients were not substantially different between stage and grade subscores.
FIG 2.
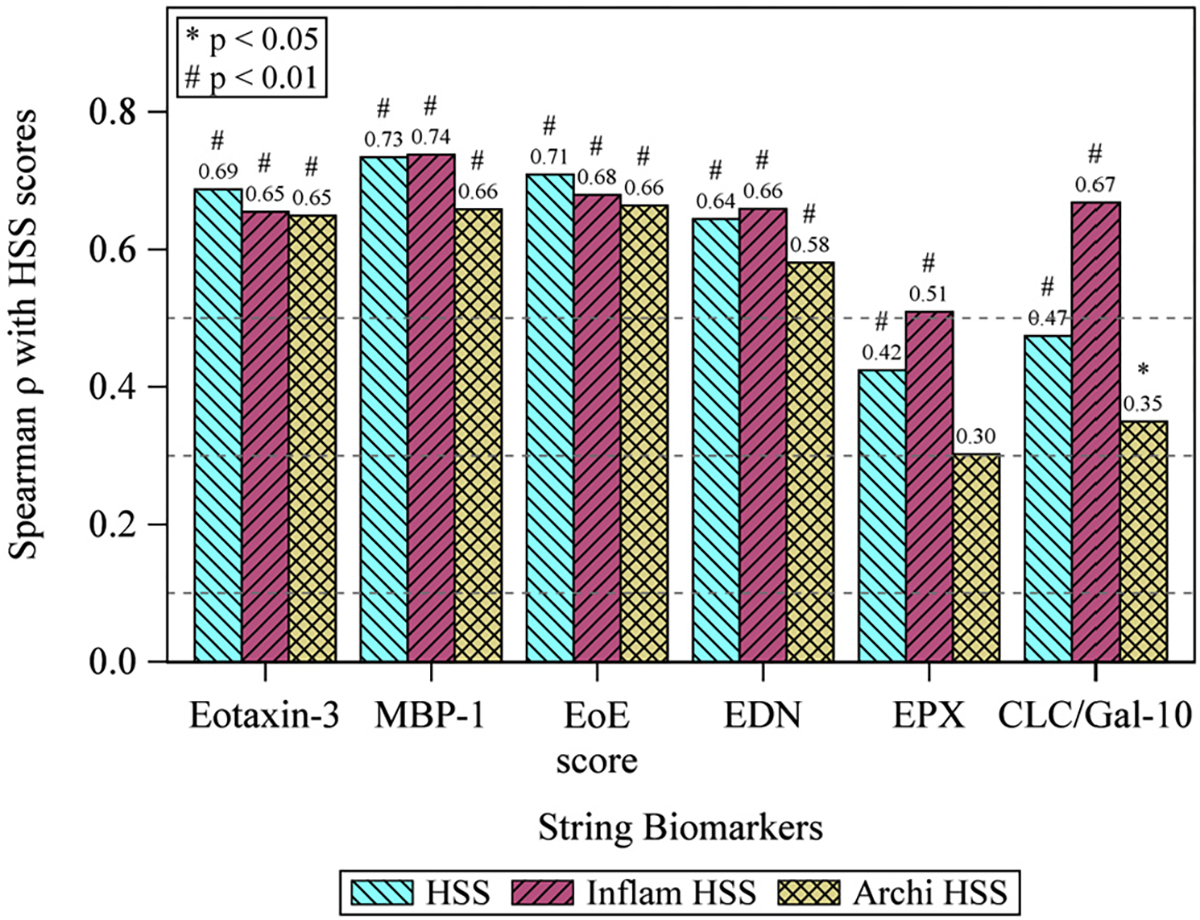
String eosinophil protein level correlates with EoEHSS. Spearman analysis correlating EST EAPs with histologic features of the EoEHSS. The combined EoEScore had the greatest correlation coefficients for both total, inflammatory and architectural features. HSS indicates total histologic scoring system; grade, severity of findings; and stage, coverage of findings. Inflammatory features include eosinophilic inflammation, eosinophil abscesses, surface layering, and surface epithelia alteration. Architectural features include BZH, dilated intercellular spaces, dyskeratotic epithelial cells, and lamina propria fibrosis. *P < .05; #P < .01.
Luminal EAPs correlate with distensibility
Because esophageal remodeling and fibrosis represent major complications of eosinophilic inflammation, we sought to assess how luminal-captured EAPs correlate with esophageal distensibility. FLIP allows for measurement of the luminal diameter and assessment of intraesophageal pressure, or esophageal distensibility. We have previously shown that the distensibility of the pediatric esophagus is significantly decreased in the setting of EoE—and even more so with active disease.8 We again found that distensibility is significantly decreased in patients with active EoE (see Fig E3 in the Online Repository available at www.jacionline.org). Additionally, we found that select EAPs captured by the EST (EDN and CLC/Gal-10) correlate significantly and negatively with esophageal distensibility (ρ −0.44 and ρ −20.47, respectively (P <.01) (Table II).
TABLE II.
Correlation of EAPs with distensibility and EMT
| EAP | Spearman ρ with distensibility† | Spearman ρ with Vim/Ecad ratio‡ |
|---|---|---|
|
| ||
| Eot-3 | −0.24 | 0.40* |
| MBP-1 | −0.26 | 0.43** |
| EoEScore§ | −0.23 | 0.39* |
| EDN | −0.40* | 0.43** |
| EPX | −0.12 | 0.22 |
| CLC/Gal-10 | −0.44** | 0.50** |
Distensibility measured by FLIP.
EMT score is measured by a machine learning algorithm of immunofluorescence of vimentin (Vim) and E-cadherin (Ecad) in membrane and cytosol.
EoEScore is the algorithmic probability index for active EoE (≥15 eos/hpf), based on levels of Eot-3 and MBP-1 in EST samples.13
P < .05.
P < .01.
Markers of EMT correlate with EAPs
In the setting of chronic inflammation, epithelial cells take on characteristics of mesenchymal cells (fibroblasts, myofibroblasts), expressing collagens and vimentin and losing their epithelial cadherins (E-cadherin).1,15,21,22 These changes in the epithelium contribute to esophageal remodeling. We hypothesized that luminal measures of eosinophil inflammation would correlate with EMT as assessed by Vim/Ecad ratio from vimentin and E-cadherin immunohistochemistry. As previously shown, EMT markers varied with disease activity (Fig E3). The number of vimentin-positive cells and cytosolic vimentin staining significantly correlated with certain EAPs. Specifically, E-cadherin staining correlated negatively with Eot-3, EDN, and CLC/Gal-10. The Vim/Ecad ratio correlated positively with Eot-3, MBP-1, EDN, and CLC/Gal-10 (Table II, Fig E3). Conversely, the epithelial marker E-cadherin was negatively and significantly correlated with EAPs. Therefore, the Vim/Ecad ratio showed medium to large correlations with EST-captured EAPs (ρ 0.41–0.51, P < .01). Thus, luminal measures of eosinophil inflammation are associated with tissue remodeling observed in the esophageal epithelium (Table II).
Luminal periostin correlates with EMT and histologic remodeling
Periostin is a matricellular protein expressed by epithelial cells that promotes subepithelial fibrotic remodeling.31 Its expression is reported to increase in the esophagus in patients with active EoE.24,32 Periostin was detectable by means of the EST and correlates with periostin expression in biopsy samples (ρ 0.86, P < .001) (Table E1). It was increased in quantity in patients with active EoE (Fig 3, A). Correlations between EST-captured periostin and reported EAPs had a medium effect (ρ 0.29–0.54, P <.01).
FIG 3.
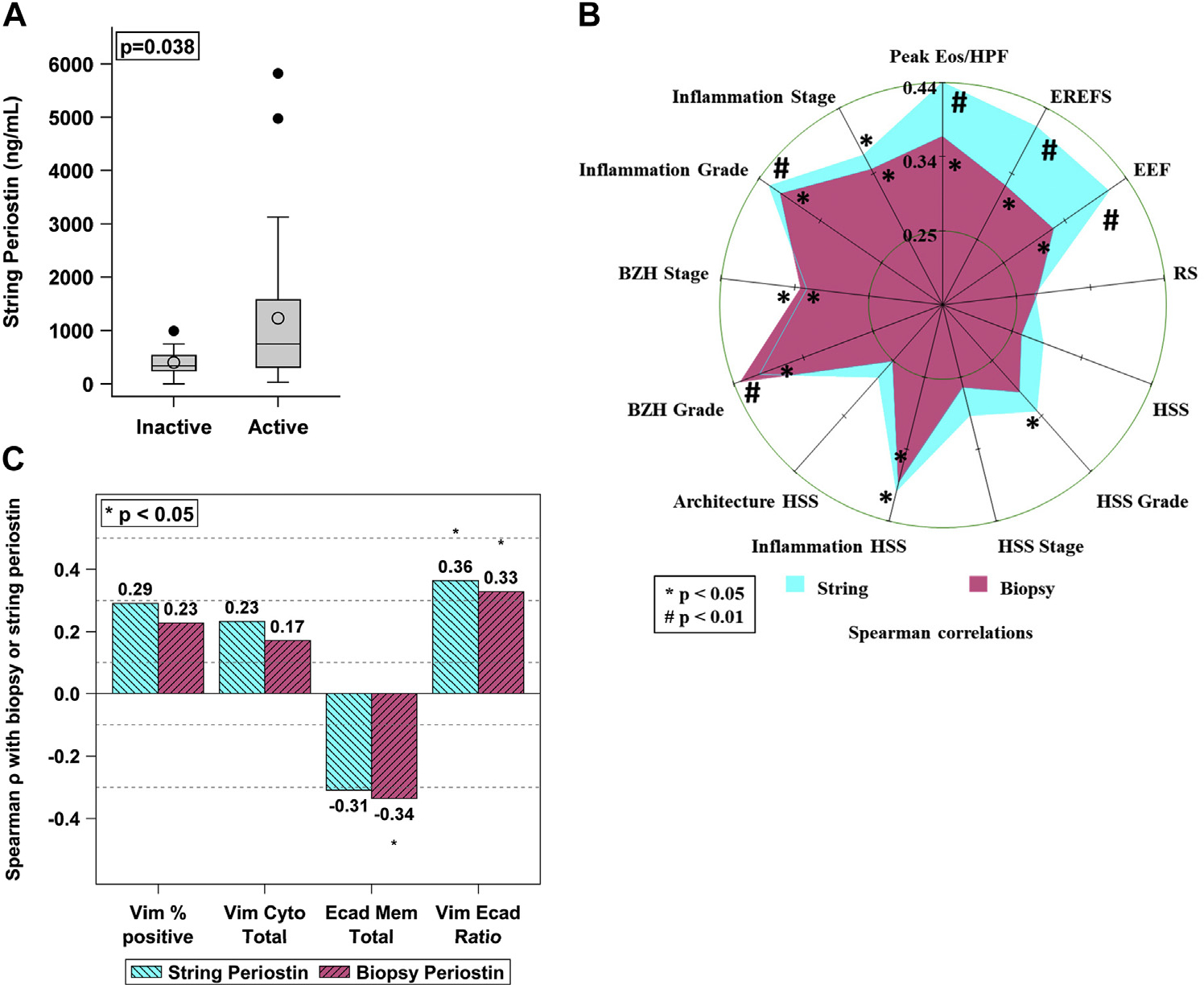
String periostin correlates with histologic and endoscopic findings as well as EMT markers. Periostin detected by the string was increased in active EoE compared to inactive EoE (A). Spearman analysis correlating EST periostin levels with endoscopic and histologic scoring by way of a radar plot (B) and EMT markers (C). Axes of the radar plot (B) are equally scaled from 0 to 0.40. Inner and outer circles indicate, respectively, minimum and maximum Spearman ρ correlation coefficients. The crossing point of a star with an axis is the estimate of the Spearman correlation with P, shown as *P < .05; #P < .01.
In this data set, luminal captured periostin correlates significantly with eosinophil density (ρ 0.44, P = .004), BZH (BZH grade ρ 0.4, P = .01, and BZH stage ρ 0.33, P = .04), total EREFS (ρ 0.37, P = .017), and EMT (Vim/Ecad ratio; ρ 0.36, P = .02) with medium effect (Fig 3, B) when analyzing all patients. For results analyzed by disease activity (active EoE, treated EoE in remission), there was no significant correlation of EMT and periostin; however, the pattern suggests that the correlation is stronger for patients with active EoE. Periostin showed only a weak nonsignificant correlation with total EoEHSS score and did not correlate with distensibility in this EoE cohort. In this data set, no combined set of predictors including periostin or EAPs was identified to more strongly associate with markers of remodeling. Taken together, these findings demonstrate that a mesenchymal-derived protein is captured in the lumen by the EST and that luminal periostin correlates with markers of both inflammation and cellular markers of esophageal remodeling.
DISCUSSION
To our knowledge, we are the first to show that eosinophil- and fibrosis-associated proteins captured and quantified using the minimally invasive EST correlate with histologic and clinical markers of remodeling including BZH, EMT, and EREFS—and, for several, esophageal distensibility. This provides evidence that luminal captured biomarkers may be able to reflect a global assessment of disease activity. Periostin, a secreted mesenchymal-derived protein expressed by both fibroblasts and epithelial cells, is known to be upregulated in EoE; however, the ability to capture this protein in luminal secretions is novel.
Once treatment is initiated, monitoring of EoE disease activity currently relies on repeat endoscopy and biopsy to provide visual inspection and histologic eosinophil counts, respectively, because symptoms continue to be an unreliable marker of disease activity, as seen in this study.9 Eosinophil count alone, however, does not provide a complete picture of disease activity, in particular the development of esophageal remodeling and the progression of stenosis. Fibrostenosis is important to assess because it has been associated with an increased likelihood of food impaction or a need for esophageal dilation.33,34 This underscores the importance of the pursuit of minimally invasive or noninvasive assessments that can serve as surrogates for both esophageal eosinophil density and epithelial and subepithelial remodeling in EoE.
Epithelial damage and BZH are histologic hallmarks of EoE disease, and incomplete epithelial terminal differentiation and barrier disruption contribute to EoE pathogenesis and symptomatology.28 Beyond this role, the damaged and hyperplastic epithelium may be an effector of subepithelial fibrosis, including loss of distensibility.21 EMT is the process by which epithelial cells take on properties of fibroblasts and myofibroblasts, such as collagen deposition, vimentin expression, and decreased expression of E-cadherin. Notably, after exposure to profibrotic cytokines (TNF-α, TGF-β, IL-1β), it was shown that esophageal epithelial cells in vitro express collagen, migrate, and become contractile.15,21 Previous studies examining esophageal mucosal biopsy samples have shown that EMT contributes to subepithelial fibrosis in EoE and resolves with treatments that decrease eosinophilic inflammation.22 In addition, EMT strongly correlated with epithelial eosinophil counts, EAPs, lamina propria fibrosis, and epithelial levels of TGF-β. Further, biopsy specimens from EoE patients demonstrate that in the EoE epithelium, there is increased expression of mesenchymal genes associated with EMT, including N-cadherin, vimentin, and fibronectin.1,15,21 Epithelial and luminal based markers of fibrostenotic remodeling are of particular importance, as routine evaluation of the lamina propria with biopsy samples is not possible.8,35 Thus, assessment of EMT may lend itself to future prognostic works to evaluate the development of fibrosis in EoE.
Periostin, formerly known as osteoblast-specific factor 2, is an extracellular matrix protein with identified roles in a number of conditions characterized by tissue remodeling, including asthma and pulmonary fibrosis,25,31,36 myocardial infarction and valvular heart disease,37 chronic kidney disease,38 and bone development and regeneration.39 Under physiologic conditions, these actions contribute to tissue healing and regeneration, but under pathologic conditions, they may lead to tissue fibrosis and dysfunction. Recent studies in EoE have shown that periostin enhances eosinophil survival in vitro.40 Periostin increases eosinophil adhesion to fibronectin, and mice lacking periostin are protected from the development of EoE-like disease after exposure to intranasal Aspergillus fumigatus.24 Further, epithelial 3-D culture models have demonstrated enhanced periostin in the setting of desmoglein-1 knockdown.41 Thus, periostin has been shown both in vitro and in vivo to affect allergic inflammation and epithelial integrity. In the current study, we show that esophageal luminal captured levels of periostin are detectable using the EST and are significantly correlated with a number of key parameters of esophageal inflammation and fibrotic remodeling in EoE, including inflammatory EAPs as well as endoscopic (EREFS) and histologic features (BZH, EMT). These data, taken together, show periostin to be expressed in the disrupted EoE epithelium and subepithelium. Now we show that it is detectable in the esophageal lumen, making it ubiquitous in the EoE esophagus. This suggests that periostin may be a biomarker of disease activity and remodeling—and potentially also a therapeutic target. Furthermore, because other epithelial and/or subepithelial proteins might be captured by the EST, an unbiased proteomic analysis of EST samples could identify additional informative biomarkers of esophageal remodeling and fibrosis in EoE.
This study sought to determine the association of luminal captured biomarkers with inflammation and remodeling in a defined group of children. Because this study enrolled patients from 2 sites, we are confident that our findings are reflective of this population, and we confirm the utility of the EST in providing meaningful samples. However, both of these sites are specialized care centers. Application of the EST in community practice and outpatient allergy offices will need to be evaluated.
This study population had few patients with gross features of fibrotic remodeling (rings, strictures), both because of the study exclusion criteria and the cohort age. Because patients with dysphagia may be reluctant to participate in a swallowing study, we may have selected a population with less fibrostenotic features. Nevertheless, we identified significant associations between EST-captured EAP biomarkers (including periostin) and several parameters of esophageal remodeling and epithelial integrity. This may permit capture of luminal biomarkers directly associated with the early development of esophageal remodeling in EoE, such as periostin, in order to personalize therapeutic approaches. We anticipate finding more accentuated correlations with clinical staging of esophageal remodeling in a more heterogenous population that includes children with more pronounced fibrostenotic features and adults with EoE. In our previously reported pediatric EoE cohort,8 we found 30% of patients had dysphagia, 20% had evidence of lamina propria fibrosis and 20% had signs of fibrosis at endoscopy (rings or narrow caliber), and 20% had a history of impaction. Thus, although less common, a notable proportion of pediatric patients do demonstrate symptomatic, histologic, and/or endoscopic evidence of remodeling, fibrosis, or both. The lower percentage of fibrostenotic features is often attributed to shorter disease duration. Although our study cohort with less fibrostenotic features may be seen as a weakness, we think our younger cohort provides an occasion to assess remodeling before the onset of a fixed stricture and for biomarker discovery in early remodeling.
In summary, we have shown that luminal EAPs captured by the minimally invasive EST, and thereby the degree of esophageal inflammation, correlates with EMT, consequent subepithelial fibrosis, and functional loss of esophageal distensibility. The implication is that it is important to halt mucosal inflammation in EoE in order to reestablish epithelial homeostasis and prevent esophageal remodeling.
Supplementary Material
Clinical implications:
The EST captures biomarkers of esophageal remodeling and associations with active eosinophilic inflammation in EoE.
Acknowledgments
We thank all the patients who participated in the study. We are also grateful to our colleagues and clinical support staff for performing biopsies and for procuring blood samples and clinical data. We would like to thank Tatiana Karakasheva for help making the graphical abstract with the program BioRender.
This study was supported by End Allergies Together (EAT). C.M.K. is supported by the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases K23DK109263. A.B.M. is supported by R01DK124266-01 and R03DK118310. C.M.K. and A.B.M. are supported by R21TR003039-02. S.J.A. was supported in part by R01FD004086. G.T.F. receives support from the La Cache Endowed Chair for GI Allergic and Immunologic Diseases.
Abbreviations used
- BZH
Basal zone hyperplasia
- CLC/Gal-10
Charcot-Leyden crystal protein/galectin-10
- EAP
Eosinophil-associated protein
- EDN
Eosinophil-derived neurotoxin
- EMT
Epithelial–mesenchymal transition
- EoE
Eosinophilic esophagitis
- EoEHSS
EoE Histologic Scoring System
- EoEScore
Algorithmic probability index for active EoE
- eos/hpf
Eosinophils per high-power field
- Eot-3
Eotaxin-3
- EPX
Eosinophil peroxidase
- EREFS
EoE Endoscopic Reference Score
- EST
Esophageal string test
- FLIP
Functional lumen imaging probe
- MBP-1
Major basic protein 1
- PEESS
Pediatric Eosinophilic Esophagitis Symptoms Score
- Vim/Ecad
Ratio of vimentin to E-cadherin
Footnotes
Disclosure of potential conflict of interest: S. J. Ackerman is founder and chief scientific officer of EnteroTrack LLC. G. T. Furuta is founder and chief medical officer of EnteroTrack LLC. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Muir AB, Wang JX, Nakagawa H. Epithelial–stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol 2019;54:10–8. 10.1007/s00535-018-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:577–85.e4. 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, Smout AJPM, Bredenoord AJ. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol 2018;113:836–44. 10.1038/s41395-018-0052-5. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES, Speck O, Woodward K, Covey S, Rusin S, Shaheen NJ, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28:383–90. 10.1038/modpathol.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins MH, Martin LJ, Alexander ES, Todd Boyd J, Sheridan R, He H, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. 10.1111/dote.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013;62:489–95. 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 7.Carlson DA, Lin Z, Hirano I, Gonsalves N, Zalewski A, Pandolfino JE. Evaluation of esophageal distensibility in eosinophilic esophagitis: an update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil 2016;28:1844–53. 10.1111/nmo.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menard-Katcher C, Benitez AJ, Pan Z, Ahmed FN, Wilkins BJ, Capocelli KE, et al. Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am J Gastroenterol 2017;112:1466–73. 10.1038/ajg.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin LJ, Franciosi JP, Collins MH, Abonia JP, Lee JJ, Hommel KA, et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol 2015;135:1519–28.e8. 10.1016/j.jaci.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safroneeva E, Straumann A, Coslovsky M, Zwahlen M, Kuehni CE, Panczak R, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016;150:581–90.e4. 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, et al.Transnasal endoscopy in unsedated children with eosinophilic esophagitis using virtual reality video goggles. Clin Gastroenterol Hepatol 2019;17:2455–62. 10.1016/j.cgh.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013;62:1395–405. 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackerman SJ, Kagalwalla AF, Hirano I, Gonsalves N, Katcher PM, Gupta S, et al. One-hour esophageal string test: a nonendoscopic minimally invasive test that accurately detects disease activity in eosinophilic esophagitis. Am J Gastroenterol 2019;114:1614–25. 10.14309/ajg.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzka DA, Smyrk TC, Alexander JA, Geno DM, Beitia RMA, Chang AO, et al. Accuracy and safety of the cytosponge for assessing histologic activity in eosinophilic esophagitis: a two-center study. Am J Gastroenterol 2017;112:1538–44. 10.1038/ajg.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir AB, Dods K, Noah Y, Toltzis S, Chandramouleeswaran PM, Lee A, et al. Esophageal epithelial cells acquire functional characteristics of activated myofibroblasts after undergoing an epithelial to mesenchymal transition. Exp Cell Res 2015;330:102–10. 10.1016/j.yexcr.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2012;1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2007;119:206–12. 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-β1–induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. J Allergy Clin Immunol 2014;134:1100–7.e4. 10.1016/j.jaci.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2–inhibited pathway. J Immunol 2010;185:660–9. 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasagi Y, Dods K, Wang JX, Chandramouleeswaran PM, Benitez AJ, Gambanga F, et al. Fibrostenotic eosinophilic esophagitis might reflect epithelial lysyl oxidase induction by fibroblast-derived TNF-α. J Allergy Clin Immunol 2019;144: 171–82. 10.1016/j.jaci.2018.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muir AB, Lim DM, Benitez AJ, Modayur Chandramouleeswaran P, Lee AJ, Ruchelli ED, et al. Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro. Exp Cell Res 2013;319:850–9. 10.1016/j.yexcr.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, et al.Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol 2012;129: 1387–96.e7. 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins MH, Alexander ES, Martin LJ, Grotjan TM, Mukkada VA, Sheil A, et al. Acquired esophageal strictures in children: morphometric and immunohistochemical analyses. Pediatr Dev Pathol. In press. 10.1177/10935266211041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol 2008;1:289–96. 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Dwyer DN, Moore BB. The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci 2017;4305–14. 10.1007/s00018-017-2649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pretolani M, Pégorier S, Wagner LA, Gleich GJ. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol 2006;177:4861–9. 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 27.Gann PH, Deaton RJ, McMahon N, Collins MH, Dellon ES, Hirano I, et al. An anti–IL-13 antibody reverses epithelial–mesenchymal transition biomarkers in eosinophilic esophagitis: phase 2 trial results. J Allergy Clin Immunol 2020;146: 367–76.e3. 10.1016/j.jaci.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Whelan KA, Godwin BC, Wilkins B, Elci OU, Benitez A, DeMarshall M, et al. Persistent basal cell hyperplasia is associated with clinical and endoscopic findings in patients with histologically inactive eosinophilic esophagitis. Clin Gastroenterol Hepatol 2020;18:1475–82.e1. 10.1016/j.cgh.2019.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warners MJ, Ambarus CA, Bredenoord AJ, Verheij J, Lauwers GY, Walsh JC, et al. Reliability of histologic assessment in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2018;47:940–50. 10.1111/apt.14559. [DOI] [PubMed] [Google Scholar]
- 30.Aceves SS, King E, Collins MH, Yang G-Y, Capocelli KE, Abonia JP, et al. Alignment of parent-and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites HHS public access. J Allergy Clin Immunol 2018;142:130–8. 10.1016/j.jaci.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenberg-Riethmacher E, Miehe M, Riethmacher D. Periostin in allergy and inflammation. Front Immunol 2021;12:722170. 10.3389/fimmu.2021.722170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Politi E, Angelakopoulou A, Di Grapsa, Zande M, Stefanaki K, Panagiotou I, et al. Filaggrin and periostin expression is altered in eosinophilic esophagitis and normalized with treatment. J Pediatr Gastroenterol Nutr 2017;65:47–52. 10.1097/MPG.0000000000001419. [DOI] [PubMed] [Google Scholar]
- 33.Chen JW, Pandolfino JE, Lin Z, Ciolino JD, Gonsalves N, Kahrilas PJ, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016;48:794–801. 10.1055/s-0042-107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicodème F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal Distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2013;11:1101–7.e1. 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Park JY, Huang R, Souza RF, Spechler SJ, Cheng E. Obtaining adequate lamina propria for subepithelial fibrosis evaluation in pediatric eosinophilic esophagitis. Gastrointest Endosc 2018;87:1207–14.e3. 10.1016/j.gie.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanri Y, Nunomura S, Terasaki Y, Yoshihara T, Hirano Y, Yokosaki Y, et al. Cross-talk between transforming growth factor-b and periostin can be targeted for pulmonary fibrosis. Am J Respir Cell Mol Biol 2020;62:204–16. 10.1165/rcmb.2019-0245OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon IMC, Landry NM, Rattan SG. Periostin reexpression in heart disease contributes to cardiac interstitial remodeling by supporting the cardiac myofibroblast phenotype. In: Kudo A, editor. Periostin. Singapore: Springer Singapore; 2019. pp. 35–41. 10.1007/978-981-13-6657-4_4. [DOI] [PubMed] [Google Scholar]
- 38.Wallace DP. Periostin in the kidney. In: Kudo A, editor. Periostin. Singapore: Springer; 2019. 10.1007/978-981-13-6657-4_11. [DOI] [Google Scholar]
- 39.Stock M, Schett G. Vitamin K–dependent proteins in skeletal development and disease. Int J Mol Sci 2021;22:9328. 10.3390/ijms22179328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vimalathas P, Farris A, Letner D, Deshpande V, Yajnik V, Shreffler W, et al. Integrin αM activation and upregulation on esophageal eosinophils and periostin-mediated eosinophil survival in eosinophilic esophagitis. Immunol Cell Biol 2018;96:426–38. 10.1111/imcb.12018. [DOI] [PubMed] [Google Scholar]
- 41.Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol 2014;7:718–29. 10.1038/mi.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


