Abstract
Objective:
To examine risk factors for gestational diabetes mellitus (GDM) and factors associated with breastfeeding patterns among women with GDM from different racial/ethnic groups.
Methods:
We used data from Phase 8 (2016-2018) of the Pregnancy Risk Assessment Monitoring Surveillance. We used logistic regression to estimate factors associated with GDM and with breastfeeding initiation, and conducted survival analysis using Kaplan Meier curves, and Cox proportional hazards regression to analyze duration of breastfeeding.
Results:
Among American Indian and Alaska Native (AI/AN) women, higher education reduced odds (aOR=0.33; 95% CI:0.19-0.59) and being married increased odds (aOR=1.35; 95% CI: 1.02-1.79) of GDM. AI/AN women who received WIC benefits had lower odds of initiating breastfeeding (aOR=0.70; 95% CI: 0.51-0.95). While there was no association between GDM and initiation of breastfeeding, only a third of AI/AN women with GDM were still breastfeeding by 36 weeks postpartum, compared to more than half of non-Hispanic white and Hispanic women.
Conclusions for Practice:
Efforts to reduce GDM among those most at risk are needed, especially among racial and ethnic minorities. Increasing support for women with GDM to continue to breastfeed may improve maternal and child health outcomes and reduce health disparities, particularly among AI/AN women.
Keywords: PRAMS, pregnancy, gestational diabetes, American Indian, Alaska Native, breastfeeding
INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common pregnancy complication in the US, affecting approximately 6% of all women who give birth annually (Deputy, Kim, Conrey, & Bullard, 2018). GDM can propel a series of negative health trajectories, increasing risk for poor health outcomes extending from the prenatal period through postpartum for both women and their infants. Among pregnant women, GDM increases the risk of pre-eclampsia (Weissgerber & Mudd, 2015), preterm birth (Anderson, Spicer, & Peercy, 2016; Domanski et al., 2018), and cesarean section (Catalano, 2010). Among infants, GDM increases the risk of macrosomia, and breastfeeding problems. Previous GDM diagnosis places both mothers and children at higher risk of subsequent development of Type 2 Diabetes (David J Pettitt & Jovanovic, 2007).
GDM disproportionately affects American Indian and Alaska Native (AI/AN) women. A 2012 systematic review focusing on diabetes during pregnancy among Indigenous populations found that 65% of studies reported higher prevalence of GDM among Indigenous groups compared to referent groups (Porter, Skinner, & Ellis, 2012). More than 9% of AI/AN women have GDM during pregnancy, second highest of any group behind non-Hispanic Black (NHB) women (11%) and nearly twice as high as NHW women (5%) (Deputy et al., 2018). GDM is particularly troubling as it can have both short-term and long terms impacts for two generations. Interventions to address GDM in AI/ANs are urgently needed.
Breastfeeding has myriad short and long-term benefits (Horta, Loret De Mola, & Victora, 2015; Nguyen, Pham, Chu, Van Duong, & Van Do, 2019; Victora et al., 2016), and can mitigate the risk of type 2 diabetes for both mothers (Aune, Norat, Romundstad, & Vatten, 2014; Chowdhury et al., 2015) and their offspring (Horta et al., 2015). However, breastfeeding initiation and duration are not constant across race/ethnicity or diabetes status (Oza-frank, 2014). The United States Department of Health and Human Services Healthy People 2020 Initiative set nationwide goals of 82% of all infants being ever breastfed and 60% still breastfed at 6 months (U.S. Department of Health and Human Services, 2012). These goals were not met by all racial and ethnic groups equally, as only 68% of AI/AN women initiate any breastfeeding, the second lowest only to non-Hispanic Black women (66%) (Louis-Jacques, Deubel, Taylor, & Stuebe, 2017). Some evidence suggests that breastfeeding can be particularly effective among Indigenous populations following GDM diagnosis. A large study conducted in Manitoba comparing type 2 diabetes incidence found an 18% reduction in risk of developing diabetes among First Nations women who had previously had GDM and breastfed (compared to just an 11% reduction among First Nations women who had not had GDM and breastfed) (Martens et al., 2016). Breastfeeding is one approach to reduce risk of advancing to type 2 diabetes following GDM. At present, no known study has focused on the potential role of breastfeeding in shaping the burden of diabetes among AI/AN women who have had GDM.
Despite the well-established benefits associated with breastfeeding, including reduction of risk of type 2 diabetes, women who have GDM are frequently less likely to breastfeed than other groups of women. A 2019 systematic review found women who have GDM are less likely to “exclusively/predominantly” breastfeed their infant and to exclusively/predominantly breastfeed for a shorter duration compared to women who do not have GDM (Nguyen et al., 2019). Of the 11 U.S. based studies included in this review, none focused on AI/AN women (Nguyen et al., 2019). This lack of representation of AI/AN women in research on breastfeeding and gestational diabetes is alarming, as breastfeeding in itself represents a low-cost and traditional intervention to reduce the risks of developing type 2 diabetes (Capriccioso, n.d.; Gunderson et al., 2018). It remains unknown, therefore, the degree to which the association between GDM and breastfeeding is present among AI/AN women and women of different races and ethnicities. Examining these associations is a first step to develop an intervention aimed at increasing breastfeeding among AI/AN women with GDM.
To bolster the evidence base for breastfeeding among AI/AN women with GDM specifically, and in relation to other groups of women, the purpose of this study is to examine risk factors for GDM and factors associated with breastfeeding patterns among women with GDM from different racial/ethnic groups in five states (Alaska, Oklahoma, New Mexico, South Dakota, and Washington) using the Pregnancy Risk Assessment Monitoring Surveillance (PRAMS). We have selected these states because they have the highest number of AI/AN births. In the 5 states included in our study (Alaska, New Mexico, Oklahoma, South Dakota, and Washington) we aim to 1) Evaluate levels of GDM and associations with risk and protective factors among AI/AN, NHW, NHB and Hispanic women; and 2) Evaluate breastfeeding initiation, duration, and associations with risk and protective factors, particularly GDM, among AI/AN, NHW, NHB, and Hispanic women.
METHODS
Overview of PRAMS Methodology
Initiated in 1987, PRAMS is an ongoing collaborative annual surveillance project between the CDC and individual state and tribal-based health departments to track progress on health indicators (Shulman, D’Angelo, Harrison, Smith, & Warner, 2018). PRAMS uses a sampling frame drawn from state-issued birth certificates to sample women who have recently had a live birth. Women are sampled and initially contacted to complete the survey between 2 and 6 months of giving birth. PRAMS oversamples women from underrepresented groups, including racial and ethnic minorities, in order to produce reliable estimates among women and infants who are at both normal and high risk for maternal, neonatal and postnatal health complications. PRAMS uses a standard set of measures for all participating states, including pregnancy, pre-conception health care, prenatal care, participation in Medicaid and WIC, breastfeeding initiation and duration, substance use before and during the most recent pregnancy, health insurance coverage, safety and intimate partner violence, contraceptive use, maternal stress, economic status, obstetric history and infant growth, health and development. Beyond these core measures, states can incorporate additional measures appropriate for that state context. PRAMS survey responses are linked to birth certificates in order to include the respondent’s demographic and medical information. More details about PRAMS can be found elsewhere (Shulman et al., 2018).
Outcome Measures
The core PRAMS survey instrument includes one question about prevalence of GDM, asking respondents “During your most recent pregnancy, did you have any of the following health conditions? (Gestational diabetes).“ This question was used to estimate prevalence of GDM among respondents. Respondents are also asked “Did you ever breastfeed or pump breast milk to feed your new baby, even for a short period of time”, which captures initiation of breastfeeding. Additionally, three states, Alaska, Oklahoma and South Dakota include two additional breastfeeding questions to capture duration of breastfeeding by asking first “Are you currently breastfeeding or feeding pumped milk to your new baby?” and “How many weeks or months did you breastfeed or feed pumped milk to your baby?” Since the American Academy of Pediatricians recommends exclusive breastfeeding for infants until 6 months of age, with continued breastfeeding until 12 months (Eidelman & Schanler, 2012), we considered no longer breastfeeding or feeding pumped milk to one’s baby at the time of survey as “early cessation.”
Potential Risk and Protective Factors
Maternal socio-demographic and health information were largely derived from the linked birth certificate data. Race and ethnicity were derived from the birth certificate where women could select multiple racial categories. To maximize representation of AI/AN women, women who selected AI/AN and any other race were categorized as AI/AN in the current analysis. We categorized maternal age using five age groups (<19 years, 20-24 years old, 25-29 years, 30-34 years, 35 years and older). Educational attainment was collapsed into less than high school, high school graduate, some college or Associate’s degree, and college graduate or more. Marital status was dichotomized as currently married or not. Parity prior to the index pregnancy was collapsed into 0, 1-2, or 3 or more previous births. Federal poverty level (FPL) was categorized as 0-99%, 100-199%, and 200 or more of the FPL. Respondents were dichotomized based on whether they were receiving food through the Women, Infants and Children (WIC) program (Aussenberg & Colello, 2012). Maternal urban or rural residence was drawn from each state’s sampling design.
Pre-pregnancy risk factors for GDM included BMI category corresponding to underweight (lower than 18.5), normal weight (18.5-24.9), overweight (25.0-29.9) and obese (30 or higher). For analysis, underweight and normal weight were collapsed as a referent category. Respondents were asked whether, prior to pregnancy, they had experienced depression or anxiety. Smoking status prior to pregnancy was dichotomized based on whether the respondent reported smoking during the three months prior to pregnancy. The survey also asked whether they had experienced intimate partner violence in the year prior to becoming pregnant, from either a current or ex-partner. The survey also asked respondents whether they had experienced violence from a partner or ex-partner while pregnant. For analysis, these questions were consolidated into a single measure of intimate partner violence in the past year. Number of prenatal care appointments attended was categorized as <=8 visits, 9-11 visits, or 12 or more. Timing of the pregnancy was assessed by asking participants to think back to just before they became pregnant, and whether they wanted to become pregnant at that time, later, sooner, never, or if they weren’t sure. For analysis, these categories were collapsed into a measure of pregnancy intendedness, for intended (wanted to become pregnant then), unintended/mistimed (wanted to become pregnant later, sooner, or never) and ambivalent (not sure).
Analysis
Descriptive statistics of women, stratified by racial and ethnic group, were calculated using proportions. We estimated the prevalence of GDM and initiation of breastfeeding for each group. We then estimated the association of potential risk and protective factors for GDM and for initiation of breastfeeding for each racial and ethnic group. Descriptive bivariate analyses were estimated using row percentages, and, within each group of women, variables significant at alpha<0.05 level in bivariate association were included multiple regression models. Multiple logistic regression modeling was performed to determine risk factors for each categorical outcome of interest (i.e., GDM, and initiation of breastfeeding) specific to each group of women, informed by bivariate analyses. Possible collinearity in models was evaluated by inspecting variance inflation factors and eigenvalues. Among those who had initiated breastfeeding, survival analyses using Kaplan Meier curves were used to estimate time in weeks until early cessation of breastfeeding; times after 36 weeks were censored. Cox proportional hazards models were used to estimate the probability of early cessation of breastfeeding during the survey period, among those who had initiated breastfeeding. Cox model selection was performed in the same way as logistic regression modeling. Within each racial and ethnic group, factors significant in bivariate analyses were then included in adjusted models. All analyses were conducted using Stata 15, were weighted and accounted for the complex survey design.
RESULTS
Descriptive statistics describing each racial and ethnic group by sociodemographic and reproductive health characteristics are displayed in Table 1. Every sociodemographic characteristic varied significantly across racial and ethnic group. AI/AN women tended to be significantly younger, have higher parity, be more impoverished, be more likely to live in a rural area, and less likely to be married compared to other groups of women (p<0.0001).
Table 1.
Characteristics of PRAMS respondents, 2018-2019, stratified by race/ethnicity (Alaska, New Mexico, Oklahoma, South Dakota, Washington)
| American Indian/Alaska Native |
non- Hispanic White |
Non- Hispanic Black |
Hispanic | ||
|---|---|---|---|---|---|
| (n=3,068) | (n=6,287) | (n=1,559) | (n=2,458) | p-value | |
| Age | <0.0001 | ||||
| <19 years old | 10.3 | 3.7 | 5.9 | 5.8 | |
| 20-24 years | 27.4 | 17.4 | 23.5 | 24.9 | |
| 25-29 years | 32 | 31 | 29.8 | 29.7 | |
| 30-34 years | 20.6 | 31.7 | 23.7 | 23.9 | |
| 35 years and older | 9.8 | 16.3 | 17.2 | 15.7 | |
| Education | <0.0001 | ||||
| Less than high school | 22.7 | 7.6 | 14.2 | 34.1 | |
| High school graduate | 38.4 | 21.7 | 30.3 | 29.4 | |
| Some college | 30.4 | 32.7 | 32.7 | 25.2 | |
| College or more | 8.5 | 38.1 | 38.1 | 11.3 | |
| Marital status | <0.0001 | ||||
| Married | 32.9 | 70.6 | 46.5 | 53.9 | |
| Not married | 67.1 | 29.4 | 53.5 | 46.1 | |
| Parity | <0.0001 | ||||
| 0 | 31.1 | 39.2 | 35.5 | 30.9 | |
| 1-2 | 45.7 | 49.5 | 46.4 | 51.1 | |
| 3 or more | 23.2 | 11.3 | 18.1 | 18.1 | |
| % of Federal Poverty Level | <0.0001 | ||||
| 0-99% FPL | 61.7 | 24.9 | 47 | 52.5 | |
| 100-199% FPL | 23.2 | 22.4 | 30.9 | 30.1 | |
| 200% or more FPL | 15.1 | 52.7 | 22.1 | 17.4 | |
| Received WIC during pregnancy | <0.0001 | ||||
| Yes | 61.1 | 30 | 55.8 | 64.6 | |
| No | 38.9 | 70 | 44.2 | 35.4 | |
| Residence | <0.0001 | ||||
| Rural | 53.8 | 20.9 | 7.6 | 17.8 | |
| Urban | 46.2 | 79.1 | 92.4 | 82.2 | |
| State | <0.0001 | ||||
| Alaska | 19.4 | 5.9 | 2.7 | 3 | |
| New Mexico | 26.7 | 15.9 | 6.7 | 11.4 | |
| Oklahoma | 30.5 | 20.3 | 37.6 | 19.7 | |
| South Dakota | 10.0 | 5.8 | 3.6 | 1.5 | |
| Washington | 13.5 | 52.2 | 49.3 | 64.4 | |
| Outcomes | |||||
| Had gestational diabetes in most recent pregnancy | 12.2 | 8.5 | 8.6 | 12.3 | <0.0001 |
| Initiated breastfeeding | 85.0 | 93.1 | 87.7 | 92.1 | <0.0001 |
| Currently breastfeeding | 51.4 | 68.6 | 57 | 58.9 | <0.0001 |
Factors Associated with GDM
Bivariate descriptive associations between risk and protective factors and GDM, stratified by race/ethnicity are shown in Table 2. Higher BMI category increased the odds of GDM for every racial/ethnic group, except for NHB women, for whom only obesity increased odds of GDM. For AI/AN women, being overweight doubled (aOR=2.1; 95% CI: 1.46-3.06) and being obese tripled (aOR= 3.02; 95% CI: 2.18-4.18) the odds of GDM, compared to AI/AN women who were of normal weight or were underweight. Increasingly older age was also a risk factor for each group, though at different age categories, relative to women giving birth at 19 or younger. For example, among AI/AN women odds of GDM increased beginning at age 25 and older (aORs 2.3 for 25-29 years and 4.7 for women 35 and older). For AI/AN women, higher education was protective, reducing odds of GDM by 67% (aOR=0.33; 95% CI:0.19-0.59). Compared to unmarried AI/AN women, being married increased risk of GDM by 35% (aOR=1.35; 95% CI: 1.02-1.79).
Table 2.
Percentage of PRAMS respondents with gestational diabetes by pre-pregnancy, prenatal, and socio-demographic characteristics, and adjusted odds ratios from multiple logistic regression estimating the association between factors and gestational diabetes in most recent pregnancy, 2018-2019, stratified by race/ethnicity (Alaska, New Mexico, Oklahoma, South Dakota, Washington)
| American Indian/Alaska Native |
non-Hispanic White | Non-Hispanic Black | Hispanic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-pregnancy factors | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | ||||||||
| BMI category | ||||||||||||||||
| Normal or underweight | 5.7 | *** | ref | 4.7 | *** | ref | 4.9 | ** | ref | 6.4 | *** | ref | ||||
| Overweight | 12.5 | 2.11 (1.46-3.06) | *** | 7.9 | 1.63 (1.11-2.38) | * | 8.6 | 1.64 (0.89-3.01) | 13.9 | 2.17 (1.35-3.49) | ** | |||||
| Obese | 17.8 | 3.02 (2.18-4.18) | *** | 16.2 | 4.09 (2.95-5.69) | *** | 11.8 | 1.90 (1.08-3.36) | * | 18.4 | 2.93 (1.82-4.70) | *** | ||||
| Depression | 12.8 | 7.9 | 7.9 | 14.3 | ||||||||||||
| Anxiety | 18.3 | 9 | 6.6 | 14.2 | ||||||||||||
| Smoking | 13.3 | 11.6 | ** | 1.86 (1.29-2.69) | ** | 7.9 | 11.3 | |||||||||
| Past year IPV | 12.3 | 11.2 | 7.3 | 14.7 | ||||||||||||
| Number of prenatal visits | ||||||||||||||||
| <=8 visits | 13.6 | 6.8 | # | 4.3 | ** | ref | 10.3 | |||||||||
| 9-11 visits | 10.1 | 7.4 | 9 | 2.41 (1.20-4.84) | * | 11 | ||||||||||
| 12 or more visits | 12.2 | 9.4 | 10.9 | 2.90 (1.45-5.79) | ** | 13.7 | ||||||||||
| Pregnancy Intendedness | ||||||||||||||||
| Unintended | 13.6 | 8 | 8.4 | 11.8 | ||||||||||||
| Intended | 10.9 | 8.6 | 10.5 | 13.2 | ||||||||||||
| Not sure | 12.1 | 8.6 | 6.6 | 11 | ||||||||||||
| Stressors during pregnancy | ||||||||||||||||
| Experienced IPV | 7.6 | 8.5 | 5.3 | 5.4 | # | |||||||||||
| Age | ||||||||||||||||
| <19 years old | 5.4 | *** | ref | 1.6 | *** | ref | 1.7 | *** | ref | 2.5 | *** | ref | ||||
| 20-24 years | 6.4 | 1.14 (0.57-2.26) | 5.7 | 2.57 (0.58-11.42) | 5 | 3.07 (0.41-23.06) | 8.1 | 3.32 (1.33-8.25) | * | |||||||
| 25-29 years | 13.0 | 2.30 (1.17-4.50) | * | 6.9 | 2.85 (0.65-12.39) | 8.3 | 5.69 (0.79-40.79) | # | 11.3 | 5.15 (2.15-12.37) | *** | |||||
| 30-34 years | 17.4 | 3.36 (1.69-6.69) | ** | 9.6 | 4.37 (0.65-12.39) | * | 8.6 | 4.49 (0.61-33.04) | 13.4 | 5.72 (2.38-13.73) | *** | |||||
| 35 years and older | 22.3 | 4.67 (2.22-9.81) | *** | 13.8 | 7.06 (1.62-30.73) | ** | 16.4 | 10.05 (1.37-73.38) | * | 23.2 | 10.70 (4.43-25.85) | *** | ||||
| Education | ||||||||||||||||
| Less than high school | 10.4 | * | ref | 7.4 | 6 | 14.7 | ||||||||||
| High school graduate | 13.2 | 0.91 (0.64-1.30) | 8.5 | 7 | 11.4 | |||||||||||
| Some college | 13.4 | 0.85 (0.58-1.24) | 9 | 9.7 | 11 | |||||||||||
| College or more | 7.5 | 0.33 (0.19-0.59) | *** | 8 | 10.4 | 10.3 | ||||||||||
| Marital status | ||||||||||||||||
| Married | 15.0 | ** | 1.35 (1.02-1.79) | * | 9.3 | * | 1.60 (1.07-2.38) | 11.2 | ** | 1.37 (0.84-2.23) | 13.7 | |||||
| Not married | 10.7 | ref | 6.4 | ref | 6.3 | ref | 10.8 | |||||||||
| Parity | ||||||||||||||||
| 0 | 7.9 | *** | 7.8 | 8.4 | 12.3 | |||||||||||
| 2-Jan | 12.5 | 8.4 | 9 | 12.2 | ||||||||||||
| 3 or more | 17.4 | 10.7 | 7.9 | 12.8 | ||||||||||||
| % of Federal Poverty Level | ||||||||||||||||
| 0-99% FPL | 12.4 | 7 | 6.5 | * | ref | 11.4 | ||||||||||
| 100-199% FPL | 13.6 | 8.9 | 12.1 | 1.43 (0.83-2.43) | 12.9 | |||||||||||
| 200% or more FPL | 11.5 | 9.2 | 10.7 | 0.91 (0.48-1.73) | 15.1 | |||||||||||
| Received WIC during pregnancy | ||||||||||||||||
| Yes | 11.8 | 7.6 | 7.7 | 12.2 | ||||||||||||
| No | 12.2 | 8.9 | 9.7 | 12 | ||||||||||||
| Residence | ||||||||||||||||
| Rural | 12.3 | 8.1 | 6.1 | 12.1 | ||||||||||||
| Urban | 12.1 | 8.6 | 8.7 | 12.4 | ||||||||||||
p<0.001
p <0.01
p<0.05
Factors Associated with Initiation of Breastfeeding
Bivariate descriptive associations using row percentages and adjusted odds ratios from multiple regression models examining the association between risk or protective factors with initiation of breastfeeding, stratified by race/ethnicity are shown in Table 3. Compared to women who had less than a high school degree, women who had some college or a college degree or more had higher odds of having ever breastfed, for all groups except Hispanic women. For AI/AN women, being married nearly doubled the odds of initiating breastfeeding (aOR=1.99; 95% CI: 1.44-2.76). Higher parity, specifically having 3 or more children prior to the index pregnancy significantly decreased odds of breastfeeding initiation among AI/AN women, NHW women, and NHB women. Compared to AI/AN women who did not receive WIC benefits, AI/AN women who received WIC benefits had lower odds of breastfeeding (aOR=0.70; 95% CI: 0.51-0.95), even after adjusting for federal poverty level.
Table 3.
Percentage of PRAMS respondents who initiated breastfeeding by pre-pregnancy, prenatal, and socio-demographic characteristics, and adjusted odds ratios from multiple logistic regression estimating the association between factors and breastfeeding initiation in most recent pregnancy, 2018-2019, stratified by race/ethnicity (Alaska, New Mexico, Oklahoma, South Dakota, Washington)
| American Indian/Alaska Native |
non-Hispanic White | Non-Hispanic Black | Hispanic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | % | aOR (95% CI) | |||||||||
| Had gestational diabetes in most recent pregnancy | 86.0 | 92.9 | 91.0 | 92.3 | ||||||||||||
| BMI category | ||||||||||||||||
| Normal or underweight | 83.3 | ** | ref | 94.6 | *** | ref | 89.3 | 92.6 | ||||||||
| Overweight | 88.9 | 1.38 (0.97-1.98) | # | 93.4 | 0.88 (0.60-1.28) | 87.4 | 93.0 | |||||||||
| Obese | 83.5 | 0.83 (0.59-1.15) | 89.7 | 0.65 (0.45-0.95) | * | 85.4 | 92.1 | |||||||||
| Depression | 84.8 | 92.1 | 78.0 | *** | 1.54 (1.00-2.35) | * | 94.2 | |||||||||
| Anxiety | 92.2 | 96.0 | 95.3 | 96.9 | ||||||||||||
| Smoking | 81.7 | ** | 0.89 (0.67-1.18) | 85.9 | *** | 0.82 (0.57-1.18) | 77.4 | *** | 0.79 (0.55-1.13) | 92.4 | ||||||
| Past year IPV | 84.6 | 89.6 | 77.9 | * | 1.47 (0.61-3.53) | 88.9 | ||||||||||
| Number of prenatal visits | ||||||||||||||||
| <=8 visits | 82.5 | ** | ref | 91.5 | 85.9 | 89.7 | ||||||||||
| 9-11 visits | 83.4 | 0.82 (0.59-1.14) | 93.1 | 86.3 | 91.8 | |||||||||||
| 12 or more visits | 88.2 | 1.22 (0.85-1.74) | 93.3 | 89.5 | 93.1 | |||||||||||
| Pregnancy Intendedness | ||||||||||||||||
| Unintended | 85.3 | # | 91.6 | *** | ref | 85.7 | * | ref | 92.6 | |||||||
| Intended | 86.9 | 95.1 | 1.29 (0.91-1.83) | 91.5 | 1.40 (0.99-1.98) | # | 91.4 | |||||||||
| Not sure | 82.4 | 90.2 | 1.38 (0.90-2.12) | 86.2 | 1.34 (0.88-2.04) | 92.7 | ||||||||||
| Stressors during pregnancy | ||||||||||||||||
| Experienced IPV | 82.3 | 90.2 | 86.0 | 88.6 | ||||||||||||
| Age | ||||||||||||||||
| <19 years old | 82.4 | 81.6 | *** | ref | 76.6 | ** | ref | 90.2 | ||||||||
| 20-24 years | 84.6 | 90.3 | 1.11 (0.58-2.13) | 84.8 | 1.03 (0.54-1.94) | 94.1 | ||||||||||
| 25-29 years | 86.6 | 93.5 | 1.26 (0.63-2.52) | 88.5 | 1.09 (0.55-2.14) | 92.6 | ||||||||||
| 30-34 years | 82.8 | 95.3 | 1.18 (0.55-2.55) | 89.7 | 1.09 (0.51-2.32) | 91 | ||||||||||
| 35 years and older | 87.8 | 93.5 | 0.72 (0.31-1.63) | 91.1 | 0.68 (0.30-1.53) | 90.6 | ||||||||||
| Education | ||||||||||||||||
| Less than high school | 77.5 | *** | ref | 75.1 | *** | ref | 75.5 | *** | ref | 88.6 | *** | ref | ||||
| High school graduate | 82.0 | 1.37 (0.97-1.91) | # | 88.9 | 2.34 (1.50-3.66) | *** | 84.5 | 2.37 (1.54-3.66) | *** | 92.3 | 1.29 (0.79-2.12) | |||||
| Some college | 90.8 | 2.67 (1.77-4.05) | *** | 94.1 | 3.46 (2.16-5.55) | *** | 89.7 | 3.45 (2.18-5.45) | *** | 93.7 | 1.27 (0.74-2.16) | |||||
| College or more | 96.4 | 4.50 (2.13-9.49) | *** | 98.3 | 8.63 (4.37-17.02) | *** | 96.4 | 8.47 (4.33-16.57) | *** | 97.9 | 3.08 (1.00-9.53) | # | ||||
| Marital status | ||||||||||||||||
| Married | 90.9 | *** | 1.99 (1.44-2.76) | *** | 95.4 | *** | 1.29 (0.90-1.86) | 94.7 | *** | 1.27 (0.89-1.82) | 91.7 | |||||
| Not married | 81.9 | ref | 87.5 | ref | 81.3 | ref | 92.6 | |||||||||
| Parity | ||||||||||||||||
| 0 | 86.7 | * | ref | 94.9 | *** | ref | 91.3 | *** | ref | 96.0 | *** | ref | ||||
| 2-Jan | 85.5 | 0.83 (0.58-1.18) | 93.0 | 0.73 (0.50-1.06) | 87.9 | 0.74 (0.51-1.08) | 90.3 | 0.45 (0.26-0.77) | ** | |||||||
| 3 or more | 81.2 | 0.67 (0.46-0.97) | * | 87.0 | 0.52 (0.30-0.89) | * | 80.5 | 0.51 (0.30-0.88) | * | 90.7 | 0.60 (0.32-1.14) | |||||
| % of Federal Poverty Level | ||||||||||||||||
| 0-99% FPL | 81.9 | *** | ref | 86.3 | *** | ref | 79 | *** | ref | 89.1 | *** | ref | ||||
| 100-199% FPL | 88.5 | 1.15 (0.80-1.65) | 93.3 | 1.37 (0.92-2.04) | 92.7 | 1.38 (0.94-2.05) | 94.6 | 1.91 (1.13-3.23) | * | |||||||
| 200% or more FPL | 94.3 | 1.10 (0.67-1.80) | 97.3 | 1.68 (1.02-2.78) | * | 97.8 | 1.86 (1.12-3.09) | * | 96.4 | 2.07 (0.84-5.12) | ||||||
| Received WIC during pregnancy | ||||||||||||||||
| Yes | 82.1 | *** | 0.70 (0.51-0.95) | * | 87.1 | *** | 0.91 (0.63-1.33) | 85.1 | *** | 0.89 (0.61-1.29) | 91.8 | |||||
| No | 89.4 | ref | 95.7 | ref | 90.9 | ref | 93.2 | |||||||||
| Residence | ||||||||||||||||
| Rural | 83.8 | 88.2 | *** | ref | 70.5 | *** | ref | 88.8 | * | ref | ||||||
| Urban | 86.4 | 94.3 | 1.56 (1.13-2.16) | ** | 89.1 | 1.55 (1.12-2.13) | ** | 92.8 | 1.33 (0.84-2.10) | |||||||
p<0.001
p <0.01
p<0.05
Duration of Breastfeeding
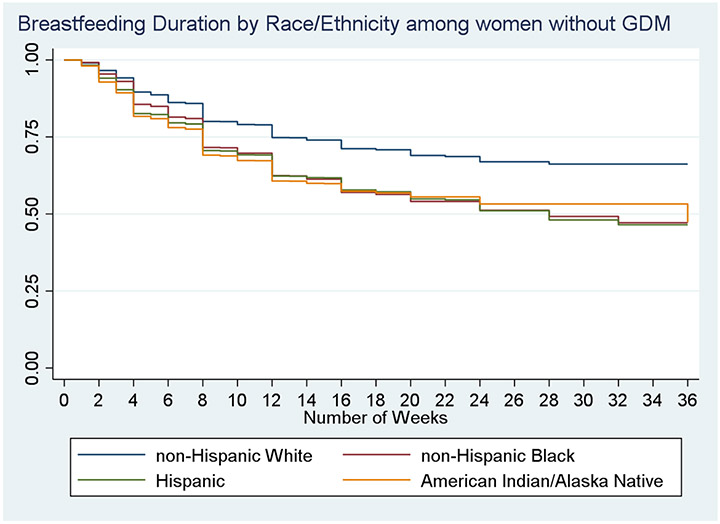
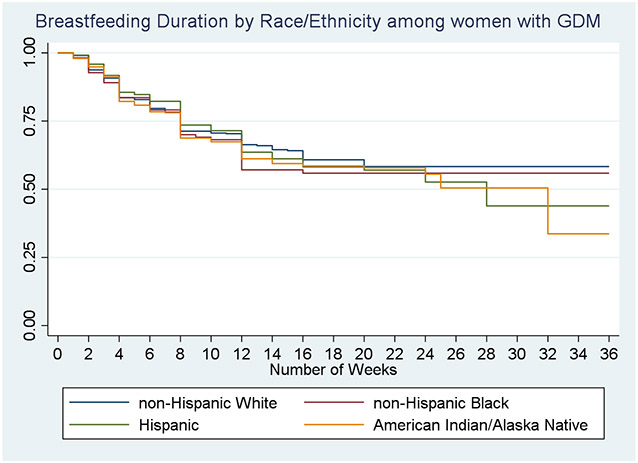
Among those who had initiated breastfeeding, survival curves showing duration of breastfeeding for each racial and ethnic group by GDM status are shown in Figures 1, and 2. Among those without GDM (Figure 1), AI/AN women, NHB women, and Hispanic women had significantly lower survival curves for breastfeeding compared to NHW women (log rank, p<0.0001). For example, at 10 weeks post birth more than three quarters of NHW women without GDM were breastfeeding, compared to approximately 65-68% of women of color. Survival curves for all women with GDM were significantly lower than for those without GDM (curve not shown, log rank, p<0.0009). Among women with GDM (Figure 2), there was no significant difference in survival curves by race/ethnicity. By 36 weeks, after which times were censored, only about a third of AI/AN women with GDM were still breastfeeding, compared to more than half of NHW women and Hispanic women.
Figure 1.
Kaplan Meier Curve showing proportion of women still breastfeeding over time, by racial and ethnic group, among women who did not have Gestational Diabetes Mellitus in most recent pregnancy
Figure 2.
Kaplan Meier Curve showing proportion of women still breastfeeding over time, by racial and ethnic group, among women who had Gestational Diabetes Mellitus in most recent pregnancy
Factors Associated with Early Cessation of Breastfeeding
Results from Cox regressions, limited to those who had initiated breastfeeding, showing the unadjusted and adjusted hazard ratios of early cessation of breastfeeding stratified by racial and ethnic group are shown in Table 4. GDM increased hazard of early cessation of breastfeeding for NHW women only (aHR=1.38; 95% CI: 1.09-1.76). Obesity was associated with increased hazard of early cessation for AI/AN women (aHR=1.32; 95% CI: 1.12-1.56), NHW women (aHR=1.84; 95% CI: 1.53-2.23), and NHB women (aHR=1.47; 95% CI: 1.15-1.86), relative to their underweight and normal weight counterparts, as did smoking. Compared to those whose most recent pregnancy was intended, AI/AN women whose pregnancy was unintended had a higher hazard of early breastfeeding cessation (aHR=1.23; 95% CI: 1.03-1.48). No association between pregnancy intendedness was observed for any other group in adjusted models. Across all groups, some higher levels of education were associated with decreased hazard of early breastfeeding association. For AI/AN women this association was the strongest, with a 54% decrease in the hazard compared to women with a high school education or less (aHR=0.46; 95% CI: 0.32-0.67). Marriage was protective against early cessation of breastfeeding for all groups except AI/AN women. Receiving WIC benefits significantly increased the hazard of early breastfeeding cessation for NHW women (aHR=1.28; 95% CI: 1.06-1.54), though this association was only marginal for AI/AN women in adjusted models (aHR=1.18; 95% CI: 0.99-1.40).
Table 4:
Cox proportional hazards model on early breastfeeding cessation by gestational diabetes and demographic characteristics, 2018-2019, stratified by race/ethnicity (Alaska, Oklahoma, South Dakota)
| American Indian/Alaska Native |
non-Hispanic White | Non-Hispanic Black | Hispanic | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Unadjusted Hazard Ratio |
Adjusted Hazard Ratio |
Unadjusted Hazard Ratio |
Adjusted Hazard Ratio |
Unadjusted Hazard Ratio |
Adjusted Hazard Ratio |
Unadjusted Hazard Ratio |
Adjusted Hazard Ratio |
||||||||
| No gestational diabetes | ref | ref | ref | ref | ref | |||||||||||
| Gestational diabetes | .096 (0.78-1.20) | 1.56 (1.24-1.96) | *** | 1.38 (1.09-1.76) | ** | 0.94 (0.66-1.32) | 1.07 (0.81-1.41) | |||||||||
| BMI category | ||||||||||||||||
| Normal or underweight | ref | ref | ref | ref | ref | ref | ||||||||||
| Overweight | 1.09 (0.91-1.31) | 1.22 (0.99-1.50) | # | 1.58 (1.31-1.89) | *** | 1.48 (1.23-1.79) | *** | 1.01 (0.79-1.29) | 0.99 (0.75-1.31) | 0.93 (0.74-1.17) | ||||||
| Obese | 1.32 (1.12-1.56) | *** | 1.53 (1.26-1.85) | *** | 2.28 (1.93-2.70) | *** | 1.84 (1.53-2.23) | *** | 1.33 (1.07-1.66) | * | 1.47 (1.15-1.86) | ** | 1.24 (0.99-1.54) | # | ||
| Depression | 1.33 (1.12-1.58) | ** | 1.18 (0.97-1.44) | # | 1.91 (1.61-2.28) | *** | 1.34 (1.11-1.62) | ** | 1.52 (1.13-2.04) | ** | 1.19 (0.86-1.66) | 1.15 (0.85-1.57) | ||||
| Smoking | 1.44 (1.26-1.66) | *** | 1.27 (1.08-1.49) | ** | 2.51 (2.13-2.94) | *** | 1.44 (1.20-1.72) | *** | 2.48 (1.99-3.08) | *** | 1.56 (1.19-2.04) | ** | 1.79 (1.33-2.42) | *** | 1.41 (0.99-2.00) | # |
| Past year IPV | 1.24 (0.95-1.60) | 1.79 (1.20-2.65) | ** | 0.63 (0.31-1.30) | 1.55 (0.95-2.54) | # | 0.86 (0.53-1.42) | |||||||||
| Number of prenatal visits | ||||||||||||||||
| <=8 visits | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| 9-11 visits | 0.81 (0.68-0.97) | * | 0.86 (0.71-1.06) | 0.68 (0.56-0.84) | *** | 0.79 (0.65-0.98) | * | 0.75 (0.59-0.96) | * | 0.89 (0.69-1.16) | 0.84 (0.66-1.06) | 0.88 (0.67-1.17) | ||||
| 12 or more visits | 0.79 (0.67-0.93) | ** | 0.85 (0.70-1.03) | # | 0.72 (0.60-0.86) | *** | 0.86 (0.71-1.04) | 0.75 (0.59-0.95) | * | 0.91 (0.70-1.18) | 0.77 (0.61-0.97) | * | 0.82 (0.63-1.08) | |||
| Pregnancy Intendedness | ||||||||||||||||
| Unintended | 1.40 (1.19-1.64) | *** | 1.23 (1.03-1.48) | ** | 1.40 (1.19-1.65) | *** | 1.12 (0.94-1.33) | 1.45 (1.16-1.82) | ** | 1.03 (0.79-1.34) | 1.18 (0.97-1.44) | 1.04 (0.82-1.31) | ||||
| Intended | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| Not sure | 1.30 (1.08-1.56) | ** | 1.10 (0.90-1.35) | 1.81 (1.49-2.20) | *** | 1.08 (0.87-1.35) | 1.67 (1.29-2.16) | *** | 1.17 (0.86-1.59) | 1.32 (1.02-1.70) | * | 1.24 (0.93-1.66) | ||||
| Stressors during pregnancy | ||||||||||||||||
| Experienced IPV | 0.82 (0.57-1.18) | 1.74 (1.08-2.81) | * | 1.08 (0.44-2.66) | 1.77 (1.13-2.79) | * | 1.54 (1.04-2.29) | * | 0.92 (0.54-1.58) | |||||||
| Age | ||||||||||||||||
| <20 years | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| 20-24 years | 0.77 (0.61-0.97) | * | 0.98 (0.72-1.33) | 0.78 (0.58-1.05) | 1.25 (0.88-1.78) | 0.74 (0.52-1.06) | 0.96 (0.58-1.60) | 0.68 (0.49-0.94) | * | 0.82 (0.53-1.26) | ||||||
| 25-29 years | 0.65 (0.52-0.82) | *** | 0.89 (0.64-1.24) | 0.51 (0.38-0.68) | *** | 1.02 (0.71-1.47) | 0.47 (0.33-0.67) | *** | 0.79 (0.48-1.32) | 0.53 (0.38-0.73) | *** | 0.83 (0.53-1.31) | ||||
| 30-34 years | 0.62 (0.48-0.79) | *** | 0.90 (0.63-1.30) | 0.40 (0.40-0.54) | *** | 1.02 (0.69-1.50) | 0.35 (0.24-0.51) | *** | 0.55 (0.32-0.95) | * | 0.44 (0.31-0.62) | *** | 0.81 (0.50-1.32) | |||
| 35+ years | 0.57 (0.42-0.79) | ** | 0.93 (0.60-1.43) | 0.35 (0.25-0.49) | *** | 0.91 (0.59-1.39) | 0.37 (0.25-0.56) | *** | 0.79 (0.45-1.38) | 0.46 (0.32-0.68) | *** | 0.84 (0.50-1.40) | ||||
| Education | ||||||||||||||||
| Less than HS | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| High School grad | 0.70 (0.60-0.83) | *** | 0.78 (0.64-0.96) | * | 0.74 (0.59-0.93) | * | 1.05 (0.80-1.39) | 1.18 (0.84-1.65) | 1.11 (0.77-1.60) | 1.14 (0.92-1.42) | 1.18 (0.91-1.54) | |||||
| Some college | 0.51 (0.42-0.62) | *** | 0.58 (0.46-0.73) | *** | 0.47 (0.38-0.59) | *** | 0.74 (0.55-0.99) | * | 0.93 (0.66-1.30) | 1.02 (0.70-1.48) | * | 0.80 (0.63-1.02) | # | 0.82 (0.60-1.11) | ||
| College or more | 0.33 (0.24-0.45) | *** | 0.46 (0.32-0.67) | *** | 0.20 (0.16-0.26) | *** | 0.47 (0.33-0.67) | *** | 0.59 (0.41-0.85) | ** | 1.05 (0.66-1.66) | 0.40 (0.26-0.62) | *** | 0.55 (0.31-0.98) | * | |
| Marital Status | ||||||||||||||||
| Not married | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| Married | 0.73 (0.63-0.85) | *** | 0.94 (0.79-1.13) | 0.37 (0.32-0.43) | *** | 0.59 (0.49-0.71) | *** | 0.34 (0.28-0.41) | *** | 0.44 (0.33-0.57) | *** | 0.60 (0.50-0.71) | *** | 0.78 (0.62-0.98) | * | |
| Parity | ||||||||||||||||
| 0 | ref | ref | ref | ref | ref | ref | ||||||||||
| 1-2 | 0.84 (0.72-0.98) | * | 0.88 (0.71-1.08) | 0.96 (0.83-1.12) | 0.92 (0.75-1.13) | 0.85 (0.69-1.04) | 0.83 (0.63-1.09) | |||||||||
| 3 or more | 0.95 (0.79-1.15) | 0.93 (0.71-1.22) | 0.95 (0.73-1.22) | 0.83 (0.62-1.12) | 0.74 (0.56-0.96) | * | 0.68 (0.46-1.00) | # | ||||||||
| % of Federal Poverty Level | ||||||||||||||||
| 0-99% FPL | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| 100-199% FPL | 0.88 (0.73-1.06) | 1.18 (0.96-1.45) | 0.63 (0.52-0.76) | *** | 0.89 (0.73-1.09) | 0.67 (0.53-0.85) | ** | 0.85 (0.66-1.09) | 0.90 (0.72-1.13) | 0.92 (0.72-1.18) | ||||||
| 200% or more FPL | 0.60 (0.48-0.74) | *** | 1.04 (0.79-1.38) | 0.38 (0.32-0.45) | *** | 0.98 (0.77-1.25) | 0.51 (0.39-0.66) | *** | 0.80 (0.55-1.17) | 0.58 (0.42-0.79) | ** | 0.87 (0.58-1.31) | ||||
| WIC | ||||||||||||||||
| No | ref | ref | ref | ref | ref | ref | ref | ref | ||||||||
| Yes | 1.37 (1.19-1.58) | *** | 1.18 (0.99-1.40) | # | 2.43 (2.10-2.81) | *** | 1.28 (1.06-1.54) | * | 1.50 (1.25-1.82) | *** | 1.00 (0.78-1.29) | 1.44 (1.18-1.75) | *** | 1.21 (0.94-1.55) | ||
| Rural | ref | ref | ref | ref | ref | ref | ||||||||||
| Urban | 0.97 (0.84-1.12) | 0.69 (0.59-0.80) | *** | 0.90 (0.76-1.07) | 0.70 (0.52-0.95) | * | 0.86 (0.63-1.18) | 1.06 (0.86-1.31) | ||||||||
p<0.001
p<0.01
p<0.05
p<0.10
DISCUSSION
Our study sought to examine risk and protective factors for GDM and breastfeeding behaviors among AI/AN, NHW, NHB and Hispanic women. GDM was shown to have risk factors common across groups (obesity, age), risk factors unique to certain groups (marriage for AI/AN women; number of prenatal visits for NHB women), and protective factors (higher education for AI/AN women). The associations found in this analysis regarding marital status and education and risk for GDM here have not been identified previously in the literature for AI/AN women. Neither GDM nor weight category was significantly associated with initiation of breastfeeding for any group of women. Higher education increased odds of breastfeeding initiation, and higher parity decreased odds of breastfeeding initiation, across racial and ethnic groups except for Hispanic women. Among AI/AN women only, receipt of WIC benefits was significantly associated with decreased odds of breastfeeding initiation. Among NHW and NHB women, higher income and urban residence were associated with increased odds of initiation. Results from Cox regression indicate that GDM was associated with higher odds of early breastfeeding cessation for non-Hispanic white women only. Visual inspection of Kaplan Meier survival analyses, however, demonstrate that compared to their counterparts without GDM, fewer women with GDM are still breastfeeding throughout and by 36 weeks postpartum in every racial and ethnic group except NHB women. While both Cox models and survival analyses predict the probability of stopping breastfeeding by 36 weeks, the Kaplan Meier curve demonstrates the overall survival curve and does not adjust for any additional variables, whereas the Cox models allow adjustment for additional factors.
Findings regarding breastfeeding initiation echo national trends observed by race and ethnicity (Louis-Jacques et al., 2017), whereby AI/AN women and NHB women initiate breastfeeding at lower levels compared to NHW and Hispanic women. Contrary to some other studies that found that women diagnosed with GDM are less likely to initiate breastfeeding compared to women without GDM (Finkelstein et al., 2013), no association was found for any racial and ethnic group in this analysis. Previous researchers, conducting analyses using 2009-2011 PRAMS have found no difference in initiation by GDM status but that women with GDM were less likely to continue to breastfeed past 2 months postpartum (Oza-Frank, Chertok, & Bartley, 2015). Our analysis builds on this previous work by stratifying by race/ethnicity, using updated data and extending the window of breastfeeding duration examined to 36 weeks. Smaller studies have also found differences in infant feeding with women with GDM more likely to supplement with formula at earlier stages compared to women without GDM (Oza-Frank, Moreland, McNamara, Geraghty, & Keim, 2016). Findings regarding WIC are particularly troubling given the WIC program’s emphasis on promoting breastfeeding among its participants (National Academies of Science, Engineering, 2016). While previous research has documented disparities in breastfeeding among WIC participants compared to WIC eligible non-participants (Dieke, Zhang, Kissin, Barfield, & Boulet, 2017), none have focused on AI/AN WIC recipients. Future work that considers and addresses infant feeding practices in the context of a mother’s GDM diagnosis, the post-partum food environment and food insecurity may help illustrate the complex health-related, historical, socioeconomic and cultural factors that shape AI/AN mothers’ decision-making around infant feeding and the downstream effects for both mother and baby/child.
This study also provided some interesting findings regarding racial and ethnic specific factors associated with breastfeeding. While previous studies have found postpartum depression to be associated with shorter duration of breastfeeding (Dias & Figueiredo, 2015), we found that previous experience of depression was associated with increased odds of initiation of breastfeeding among NHB women. Furthermore, our study found no association between depression and probability of early cessation of breastfeeding among NHB women. More focused analyses are needed in order to understand and address this important issue. In addition, pregnancy intendedness was associated with early cessation of breastfeeding for AI/AN women only.
This study has a number of limitations. As a secondary data analysis of PRAMS there are additional measures which would be valuable to include if they were available. These include treatment for and management of GDM, and sources of support and behavior change implemented among those who received this diagnosis. Additional socio-economic variables, such as need to return to work and workplace support for breastfeeding would also be worthwhile to include, in order to illustrate the broader context in which breastfeeding decisions are made. In addition, the window of time for breastfeeding duration was short, and limited by the typical window whereby the survey is sent to and completed by recently postpartum window. The PRAMS breastfeeding measure is also somewhat crude, in that it is not possible to discern whether women were breastfeeding exclusively, or also supplementing with formula, which may be related to cessation patterns. Finally, by design we focused on only a few states, which have a large number of births to AI/AN women; within these states, our duration of breastfeeding analysis was further limited, as only three states asked about duration of breastfeeding. Inherent to these data is the possibility of misclassification bias, which is a known problem with birth certificates and is innate to this dataset as maternal race is in many states a stratification variable on which the sampling plan is based (Shulman et al., 2018). Further, in combining races as we have done here, we may have facilitated additional possibility of misclassification bias with regard to AI/ANs, whose risk factors for GDM may differ from those in women of other races. As a secondary analysis we are reliant on PRAMS and the individual states, but it would be worthwhile if more states participating in PRAMS asked additional breastfeeding measures.
Despite these limitations, this study has potentially important implications for interventions and programs to reduce GDM. By using a large dataset and focusing on states with sizable births to AI/AN women, this study focuses on a population at high risk for GDM but who are often lumped into an “other” category that masks both their unique healthcare ecologies and needs. Additionally, though the length of breastfeeding duration available for study not complete, it indicates that realizing longer term goals of supporting breastfeeding among these populations may benefit from early and sustained interventions and breastfeeding supports. Supporting breastfeeding beyond 3 months postpartum has potential to support not only infant health and nutrition but also has been documented to dramatically reduce subsequent diabetes diagnosis among mothers diagnosed with GDM (Ziegler et al., 2012).
With its focus on identifying factors associated with by race and ethnicity, this research suggests areas where targeted intervention may benefit specific groups of women. Seminal research conducted among the Pima Indians indicate that risk of diabetes to offspring is significantly increased among not just those whose mothers were diagnosed with GDM but also those with normal glucose tolerance (Franks et al., 2006). Additional studies with this population has documented significantly lower risk of subsequent childhood diabetes among infants who were breastfed compared to those who were formula fed (30% vs. 44% among those with diabetic mothers) (D J Pettitt & Knowler, 1998). Future studies might build on these results by investigating barriers and exploring supports to breastfeeding continuation among AI/AN diagnosed with GDM women specifically (Houghtaling, Byker Shanks, Ahmed, & Rink, 2018). This is particularly critical given the burden of diabetes among AI/AN populations. Other work among AI/AN communities has found that while perceptions that breast milk is beneficial to the infant are prevalent, greater emphasis on the diabetes-specific related benefits are needed (Eckhardt et al., 2014). Such studies could continue the trajectory from here and further bolster the evidence base for good policy regarding breastfeeding and GDM. Clinical trials currently focusing on gestational diabetes among AI/AN groups may benefit from findings from this study indicating duration of breastfeeding is shorter among AI/AN women diagnosed with GDM. Specifically, supporting women to breastfeed their infants may help empower women and address the sense of “fear, shame, and powerlessness” that may accompany a GDM diagnosis among AI/AN women (Stotz, Charron-Prochownik, Terry, Gonzales, & Moore, 2019). Primary prevention of GDM among AI/AN women in particular is tantamount to interrupting the intergeneration cycle catalyzed by GDM diagnosis. Stopping GDM, a clinical trial adapted and directed for AI/AN adolescent and young women and their mothers specifically aims to address health in the preconception period (Moore et al., 2019; Terry et al., 2020). Findings from our study may complement findings in Stopping GDM to extend the intervention into the prenatal and postpartum periods for those women who are diagnosed with GDM.
Findings from this study add to the growing evidence base regarding GDM and breastfeeding, and how associations vary by race and ethnicity. Additional research that disentangles the specific feeding practices for different groups of women with GDM may help inform efforts to support infant nutrition and mothers’ postpartum health, while also making progress on national breastfeeding targets and potentially reducing the burden of diabetes in the U.S.
Supplementary Material
What is already known on this subject?
Gestational Diabetes is a pressing maternal and child health issue which disproportionately affects American Indian and Alaska Native (AI/AN) women. Despite the robust scientific documentation of the myriad benefits associated with breastfeeding, and the outsized burden of gestational diabetes among AI/AN, AI/AN women are less likely to initiate breastfeeding than other groups of women.
What this study adds?
AI/AN women experience common and unique factors associated with GDM. While this study demonstrates no difference in initiation of breastfeeding by gestational diabetes status among different groups of women, fewer AI/AN women with GDM are breastfeeding by 36 weeks compared to other groups of women.
Acknowledgments
The authors wish to thank the Centers for Disease Control and Prevention and the PRAMS Working Group for the PRAMS data.
Funding:
This research was supported by the Center for American Indian and Alaska Native Diabetes Translation Research, through a grant (P30DK092923) awarded by the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Competing Interests: The authors have no conflicts of interest or competing interests to disclose.
Ethics Approval: Our study was exempt from ethical review because it did not meet the federal definition of human subjects research (45 CFR 46.102).
Consent to participate: This is a secondary data analysis therefore no consent was obtained directly from participants for this analysis, however the original PRAMS surveys from which the data is drawn did obtain informed consent from all participants.
Consent to publish: All authors give their consent to publish this manuscript.
Availability of Data: Data is available upon application to the PRAMS Working Group.
REFERENCES
- Anderson KG, Spicer P, & Peercy MT (2016). Obesity, diabetes and birth outcomes among American Indians and Alaska Natives. Maternal Child Health Journal, 20(12), 2548–2556. 10.1007/s10995-016-2080-3.Obesity [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune D, Norat T, Romundstad P, & Vatten LJ (2014). Breastfeeding and the maternal risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Nutrition, Metabolism and Cardiovascular Diseases, 24(2), 107–115. 10.1016/j.numecd.2013.10.028 [DOI] [PubMed] [Google Scholar]
- Aussenberg RA, & Colello KJ (2012). Domestic food assistance: Summary of programs. Food Assistance Programs and Measures of Food Security in the United States, 1–20. [Google Scholar]
- Capriccioso R. (n.d.). Breastfeeding’s Way of Taking Back Native Culture. Retrieved from https://indiancountrytoday.com/archive/breast-feeding-s-role-in-taking-back-native-culture-C1KFoyN0S0qmOd8pIq0nEw [Google Scholar]
- Catalano PM (2010). The impact of gestational diabetes and maternal obesity on the mother and her offspring. Journal of Development Origins of Health and Disease, 1(4), 208–215. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, … Martines J (2015). Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatrica, International Journal of Paediatrics, 104, 96–113. 10.1111/apa.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deputy NP, Kim SY, Conrey EJ, & Bullard KM (2018). Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth — United States, 2012–2016. Morbidity and Mortality Weekly Report. 10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias CC, & Figueiredo B (2015). Breastfeeding and depression: A systematic review of the literature. Journal of Affective Disorders, 171, 142–154. 10.1016/j.jad.2014.09.022 [DOI] [PubMed] [Google Scholar]
- Dieke AC, Zhang Y, Kissin DM, Barfield WD, & Boulet SL (2017). Disparities in Assisted Reproductive Technology Utilization by Race and Ethnicity, United States, 2014: A Commentary. Journal of Women’s Health (2002), 26(6), 605–608. 10.1089/jwh.2017.6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski G, Lange AE, Ittermann T, Allenberg H, Spoo RA, Zygmunt M, & Heckmann M (2018). Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes : a population-based study, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt CL, Lutz T, Karanja N, Jobe JB, Maupomé G, & Ritenbaugh C (2014). Knowledge, Attitudes, and Beliefs that Can Influence Infant Feeding Practices in American Indian Mothers. Journal of the Academy of Nutrition and Dietetics, 114(10), 1587–1593. 10.1016/j.jand.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman AI, & Schanler RJ (2012). Breastfeeding and the use of human milk. Pediatrics, 129(3). 10.1542/peds.2011-3552 [DOI] [Google Scholar]
- Finkelstein SA, Keely E, Feig DS, Tu X, Yasseen AS, & Walker M (2013). Breastfeeding in women with diabetes: Lower rates despite greater rewards. A population-based study. Diabetic Medicine, 30(9), 1094–1101. 10.1111/dme.12238 [DOI] [PubMed] [Google Scholar]
- Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, & Knowler WC (2006). Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes, 55(2), 460–465. 10.2337/diabetes.55.02.06.db05-0823 [DOI] [PubMed] [Google Scholar]
- Gunderson EP, Lewis CE, Lin Y, Sorel M, Gross M, Sidney S, … Quesenberry CP (2018). Lactation duration and progression to diabetes in women across the childbearing years the 30-year CARDIA Study. JAMA Internal Medicine, 178(3), 328–337. 10.1001/jamainternmed.2017.7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta BL, Loret De Mola C, & Victora CG (2015). Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatrica, International Journal of Paediatrics, 104, 30–37. 10.1111/apa.13133 [DOI] [PubMed] [Google Scholar]
- Houghtaling B, Byker Shanks C, Ahmed S, & Rink E (2018). Grandmother and health care professional breastfeeding perspectives provide opportunities for health promotion in an American Indian community. Social Science and Medicine, 208, 80–88. 10.1016/j.socscimed.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis-Jacques A, Deubel TF, Taylor M, & Stuebe AM (2017). Racial and ethnic disparities in U.S. breastfeeding and implications for maternal and child health outcomes. Seminars in Perinatology, 41(5), 299–307. 10.1053/j.semperi.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Martens PJ, Shafer LA, Dean HJ, Sellers EAC, Yamamoto J, Ludwig S, … Shen GX (2016). Breastfeeding Initiation Associated With Reduced Incidence of Diabetes in Mothers and Offspring. Obstetrics and Gynecology, 128(5), 1095–1104. 10.1097/AOG.0000000000001689 [DOI] [PubMed] [Google Scholar]
- Moore K, Stotz S, Nadeau KJ, Terry MA, Garcia-Reyes Y, Gonzales K, & Charron-Prochownik D (2019). Recommendations from American Indian and Alaska Native Adolescent Girls for a Community-Based Gestational Diabetes Risk Reduction and Reproductive Health Education Program. Research Journal of Women’s Health, 6(1), 1. 10.7243/2054-9865-6-1 [DOI] [Google Scholar]
- National Academies of Science, Engineering, and M. (2016). Review of WIC Food Packages: Proposed Framework for Revisions: Interim Report. Review of WIC Food Packages. Washington, DC, US: The National Academies Press. 10.17226/21832 [DOI] [PubMed] [Google Scholar]
- Nguyen PTH, Pham NM, Chu KT, Van Duong D, & Van Do D (2019). Gestational Diabetes and Breastfeeding Outcomes: A Systematic Review. Asia-Pacific Journal of Public Health, 31(3), 183–198. 10.1177/1010539519833497 [DOI] [PubMed] [Google Scholar]
- Oza-Frank R, Chertok I, & Bartley A (2015). Differences in breast-feeding initiation and continuation by maternal diabetes status. Public Health Nutrition, 18(4), 727–735. 10.1017/S1368980014000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza-frank RKR (2014). Differences in Breastfeeding Initiation by Maternal Diabetes Status and Race, Ohio 2006 – 2011, 2226–2235. 10.1007/s10995-014-1472-5 [DOI] [PubMed] [Google Scholar]
- Oza-Frank R, Moreland JJ, McNamara K, Geraghty SR, & Keim SA (2016). Early lactation and infant feeding practices differ by maternal gestational diabetes history. Journal of Human Lactation, 32(4), 658–665. 10.1177/0890334416663196.Early [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt DJ, & Knowler WC (1998). Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care, 21 Suppl 2, B138–41. [PubMed] [Google Scholar]
- Pettitt David J, & Jovanovic L (2007). The vicious cycle of diabetes and pregnancy. Current Diabetes Reports, 7(4), 295–297. 10.1007/s11892-007-0047-x [DOI] [PubMed] [Google Scholar]
- Porter C, Skinner T, & Ellis I (2012). The current state of Indigenous and Aboriginal women with diabetes in pregnancy: A systematic review. Diabetes Research and Clinical Practice, 98(2), 209–225. 10.1016/j.diabres.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Shulman HB, D’Angelo DV, Harrison L, Smith RA, & Warner L (2018). The Pregnancy Risk Assessment Monitoring System (PRAMS): Overview of design and methodology. American Journal of Public Health, 108(10), 1305–1313. 10.2105/AJPH.2018.304563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz S, Charron-Prochownik D, Terry MA, Gonzales K, & Moore K (2019). Reducing Risk for Gestational Diabetes Mellitus (GDM) Through a Preconception Counseling Program for American Indian/Alaska Native Girls: Perceptions From Women With Type 2 Diabetes or a History of GDM. Diabetes Educator, 45(2), 137–145. 10.1177/0145721718821663 [DOI] [PubMed] [Google Scholar]
- Terry MA, Stotz SA, Charron-Prochownik D, Beirne S, Gonzales K, Marshall G, & Moore KR (2020). Recommendations from an expert panel of health professionals regarding a gestational diabetes risk reduction intervention for American Indian/Alaska Native Teens. Pediatric Diabetes, 21(3), 415–421. 10.1111/pedi.12990 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2012). The Surgeon General’s Call to Action to Support Breastfeeding. (Office of the Surgeon General (US), Ed.) (Vol. 37). Washington, D.C.: U.S. Department of Health and Human Services. 10.1097/NMC.0b013e3182370cf1 [DOI] [Google Scholar]
- Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, … Richter L (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet, 387(10017), 475–490. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- Weissgerber TL, & Mudd LM (2015). Preeclampsia and Diabetes. Current Diabetes Reports. 10.1007/s11892-015-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A-G, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachman L, … Hummel S (2012). Long-term protective effect of lactation on the development of Type 2 Diabetes in women with recent Gestational Diabetes Mellitus. Diabetes, 61, 3167–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.