Abstract
Although riboswitches have long been known to regulate translation initiation and transcription termination, a growing body of evidence indicates that they can also control bacterial RNA lifetimes by acting directly to hasten or impede RNA degradation. Ligand binding to the aptamer domain of a riboswitch can accelerate RNA decay by triggering a conformational change that exposes sites to endonucleolytic cleavage or by catalyzing the self-cleavage of a prefolded ribozyme. Alternatively, the conformational change induced by ligand binding can protect RNA from degradation by blocking access to an RNA terminus or internal region that would otherwise be susceptible to attack by an exonuclease or an endonuclease. Such changes in RNA longevity often accompany a parallel effect of the same riboswitch on translation or transcription. Consequently, a single riboswitch aptamer may govern the function of multiple effector elements (expression platforms) that are co-resident within a transcript and act independently of one another.
Keywords: lysC, yitJ, glmS, tfoY, sugE, Vc2, SAM-I, S-box, guanidine-III, c-di-GMP-I, GlcN6P, glucosamine-6-phosphate, lysine, RNase E, RNase Y, RNA degradosome
Riboswitches are an ancient mechanism for controlling gene expression in response to the cellular concentration of a metabolite or ion without the involvement of a specialized protein receptor. Found primarily in bacteria and consisting solely of RNA, these cis-acting regulatory elements comprise an aptamer domain able to selectively bind a particular metabolite or ion and an overlapping effector domain, known as the expression platform, that governs gene expression (Serganov & Nudler, 2013). These structurally interdependent domains constitute an integrated unit that can adopt either of two base-paired conformations, only one of which is conducive to gene expression. Typically, ligand binding causes the aptamer to undergo a conformational change that alters the structure of the expression platform in a way that enables or prevents ribosome binding (i.e., translation initiation) or premature transcription termination (Figure 1). Dozens of distinct classes of riboswitches with diverse ligand specificities have been identified (McCown et al., 2017).
Figure 1. Regulatory mechanisms of riboswitches.

In the absence of its ligand, the aptamer domain (red line) of most riboswitches is unfolded or only partially folded. Binding of the ligand (blue circle) causes the aptamer to adopt a fully folded structure that alters the base-paired conformation of an overlapping expression platform (represented in the unliganded state as a stem-loop), thereby enabling or inhibiting translation initiation (top), transcription termination (middle), RNA degradation (bottom), or (infrequently) RNA splicing (not shown). In each case, only activation or repression is depicted. The conformational change induced by ligand binding to actual riboswitches is typically more complex than portrayed in these simplified pictorial representations and can upregulate or downregulate gene expression by causing a loss or gain of base pairing in the expression platform. White rectangle, beginning of the protein-coding region. Black rectangle, Shine-Dalgarno element (SD). Gray line and rectangle, unsynthesized RNA segment downstream of a transcription terminator. Scissors, ribonuclease.
Less well studied are the mechanisms by which riboswitches regulate genes by altering rates of RNA decay. In bacteria, mRNA half-lives range from seconds to almost an hour due to differences in the vulnerability of transcripts to degradation by a combination of cytoplasmic endonucleases and exonucleases, which often assemble as multiprotein complexes (Hui et al., 2014, Trinquier et al., 2020). In contrast to eukaryotes, where most of the key mRNA-degrading enzymes are widely conserved, it is not uncommon for unrelated bacterial species to use distinct sets of ribonucleases for mRNA degradation. For example, while all bacteria employ one or more 3′ exonucleases as scavengers for decay intermediates, Gammaproteobacteria like Escherichia coli rely first on the endonuclease RNase E to fragment full-length transcripts, whereas Bacillus subtilis and countless other Firmicutes degrade mRNA with an entirely different endonuclease (RNase Y) and the 5′ exonuclease RNase J, neither of which is present in E. coli. All of these bacterial enzymes act preferentially on unpaired regions of RNA not masked by bound ribosomes, and two (RNase E and RNase J) are stimulated when the RNA 5′ end is monophosphorylated as a result of prior phosphate removal or internal cleavage.
Interestingly, the lifetimes of specific bacterial mRNAs can increase or decrease in reaction to environmental signals and stresses, a response that is sometimes mediated by ligand binding to a riboswitch (Hui et al., 2014). With one notable exception (see below), riboswitch-dependent changes in RNA stability generally result from ligand-induced structural remodeling that alters the susceptibility of a transcript to ribonuclease attack (Figure 1).
Few riboswitch-containing transcripts have been examined for a ligand-dependent change in half-life. Among those that have been tested, most are destabilized upon ligand binding (Nou & Kadner, 1998, Winkler et al., 2004, Spinelli et al., 2008, Shahbabian et al., 2009, Caron et al., 2012). Three such transcripts stand out as paradigms: E. coli lysC and B. subtilis yitJ and glmS (Table 1). For two of them (lysC and yitJ), the RNA conformational change induced by ligand binding exposes specific sites to endonuclease cleavage. In the case of glmS, ligand binding activates RNA self-cleavage.
Table 1.
Bacterial RNAs whose lifetime is controlled by a riboswitch
| lysC | yitJ | glmS | P1-Vc2 | sugE | |
|---|---|---|---|---|---|
| Species | E. coli | B. subtilis | B. subtilis | V. cholerae | L. pneumophila |
| Riboswitch | Lysine | SAM-I (S-box) | GlmS | c-di-GMP-I | Guanidine-III |
| Ligand | Lysine | S-adenosylmethionine | Glucosamine-6-phosphate | Cyclic di-GMP | Guanidine |
| Effect of ligand binding | Destabilization | Destabilization | Destabilization | Protection | Protection |
| Cleavage activity affected | RNase E | RNase Y | GlmS ribozyme | 3′ exonucleases | RNase E |
| Role of the ligand | Allosteric | Allosteric | Catalytic | Allosteric | Allosteric |
The lysC transcript of the Gram-negative bacterium E. coli encodes an aspartokinase that catalyzes an early step in lysine biosynthesis. Within its 5′ untranslated region (UTR) is a dual-acting riboswitch that senses the cellular concentration of lysine (Sudarsan et al., 2003, Caron et al., 2012). Lysine binding to the aptamer domain of this riboswitch causes the RNA to undergo a conformational change that downregulates gene expression in two ways: by sequestering the Shine-Dalgarno element in a base-paired structure that prevents ribosome binding and by exposing two previously sequestered sites to cleavage by the endonuclease RNase E (Figure 2) (Caron et al., 2012), which cuts RNA at specific locations in single-stranded regions (Kaberdin, 2003). Cleavage at these two adjacent sites, which is independent of translational repression, triggers subsequent decay of the lysC protein-coding region, presumably because the monophosphorylated 5′ end of the initial cleavage products stimulates further degradation by recruiting RNase E via its 5′ monophosphate-binding pocket (Mackie, 1998, Callaghan et al., 2005).
Figure 2. Influence of riboswitches on ribonuclease attack.
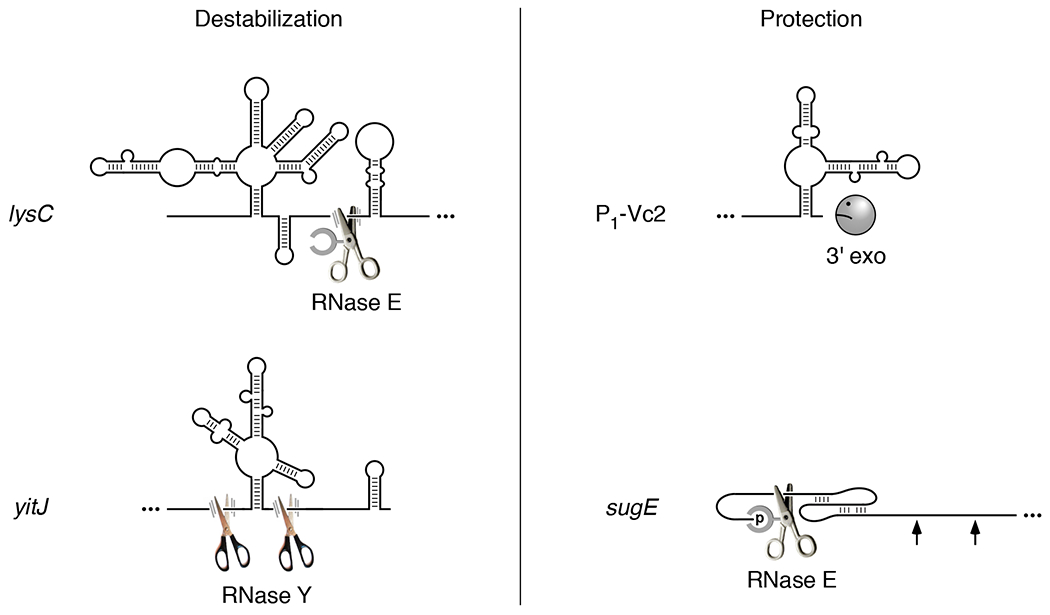
(Left) Ligand-induced RNA destabilization. Ligand binding to the E. coli lysC or B. subtilis yitJ riboswitch results in an RNA conformational change that exposes previously inaccessible cleavage sites for RNase E or RNase Y, respectively, two endonucleases that cut at specific sites in single-stranded regions of RNA. Cleavage at only one of the two adjacent RNase E cleavage sites in the lysC 5′ UTR is shown. (Right) Ligand-induced RNA protection. (Upper right) The conformational change caused by ligand binding to the V. cholerae Vc2 riboswitch is thought to protect P1-Vc2 RNA from 3′-exonucleolytic attack by sequestering its 3′ end in base pairing. (Lower right) Ligand-induced formation of a coaxially base-paired pseudoknot by the guanidine-III riboswitch of L. pneumophila sugE mRNA blocks access to distal sites by RNase E, which binds the monophosphorylated 5′ end of the transcript and scans linearly from there in search of cleavage sites. All four riboswitches are depicted in the base-paired conformation they adopt upon ligand binding (McDaniel et al., 2003, Kulshina et al., 2009, Smith et al., 2009, Caron et al., 2012, Sherlock & Breaker, 2017). Scissors, endonuclease. Frowning Pac-Man, 3′ exonuclease deterred by a base-paired 3′ end. Arrow, inaccessible cleavage site. Ellipsis, RNA segment not shown. Parallel lines representing motion blur identify endonucleases able to cut lysC and yitJ RNA at the indicated sites. The absence of such lines in the case of sugE signifies a scanning endonuclease (RNase E) that is stalled at a site it cannot cut.
Although the Gram-positive microbe B. subtilis relies on an endonuclease other than RNase E for mRNA degradation, the similar specificity of this enzyme (RNase Y) enables a SAM-I (S-box) riboswitch in the 5′ UTR of the B. subtilis yitJ transcript to have an analogous effect on RNA turnover. Binding of S-adenosylmethionine to the aptamer domain of this riboswitch causes the expression platform to refold as an intrinsic terminator, attenuating transcription upstream of the protein-coding region and thereby blocking production of a homocysteine S-methyltransferase that contributes to methionine biosynthesis (McDaniel et al., 2003). The prematurely terminated transcript containing the riboswitch is then rapidly cleaved at two previously inaccessible sites flanking the ligand-bound aptamer by the endonuclease RNase Y (Figure 2), which like RNase E cuts at specific locations in single-stranded regions of RNA (Shahbabian et al., 2009). The RNA fragments thereby generated are swiftly degraded by the B. subtilis 5′ exonuclease RNase J and the 3′ exonucleases polynucleotide phosphorylase and RNase R. Cleavage at these two sites by RNase Y is inhibited in the absence of the bound ligand, apparently because they are structurally sequestered in the alternative RNA conformation.
Another B. subtilis transcript, glmS, which encodes a glucosamine-6-phosphate (GlcN6P) synthase, contains a unique type of riboswitch in its 5′ UTR: a self-cleaving ribozyme activated by GlcN6P (Winkler et al., 2004). Rather than functioning allosterically like the lysC and yitJ riboswitch ligands, GlcN6P binds to the already fully folded ribozyme and stimulates RNA self-cleavage by acting as a coenzyme that helps to catalyze the reaction without altering the RNA conformation (Cochrane et al., 2007). No longer protected by a 5′ triphosphate, the 3′ cleavage product containing the glmS protein-coding region then undergoes rapid exonucleolytic degradation by RNase J (Collins et al., 2007).
Recent reports have identified two riboswitch-containing RNAs, a 5′-terminal fragment of the Vibrio cholerae tfoY transcript and Legionella pneumophila sugE mRNA, for which ligand binding has the opposite effect, protecting them from degradation (Table 1). In each case, the ligand causes the aptamer domain to adopt a conformation resistant to ribonuclease attack.
V. cholerae produces four tfoY transcripts that all encode the same transcription factor, each with a distinct 5′ end (P1, P2, P3, and P4) (Pursley et al., 2018). Within the 5′ UTR of two of them is a cyclic di-GMP (c-di-GMP-I) riboswitch, Vc2, thought to sequester the ribosome binding site and repress translation upon ligand binding. More abundant than these four protein-coding mRNAs is a noncoding RNA, P1-Vc2, that begins at the P1 transcription initiation site, ends immediately downstream of the riboswitch aptamer, and affects flagellum-based motility (Pursley et al., 2019). Binding of cyclic di-GMP to the riboswitch significantly prolongs the lifetime of this noncoding RNA by a mechanism that remains to be determined but likely involves folding of the aptamer into a base-paired structure that protects the 3′ terminus from digestion by 3′ exonucleases such as polynucleotide phosphorylase, RNase II, and RNase R (Figure 2), which prefer unpaired 3′ ends (Hui et al., 2014).
The guanidine-III riboswitch of the L. pneumophila sugE transcript, which encodes a guanidine efflux pump, uses an entirely different type of mechanism to protect mRNA from degradation while also regulating translation in a more conventional way. Upon binding guanidine, the aptamer domain of this riboswitch folds into a compact H-type pseudoknot (Sherlock & Breaker, 2017). This conformational change is thought to disrupt an overlapping stem-loop, thereby freeing the sugE Shine-Dalgarno element from sequestration and enabling ribosome binding. In addition, the pseudoknot shields downstream cleavage sites by a novel mechanism related to the recent discovery that RNase E accesses such sites in 5′ monophosphorylated RNA by binding the 5′ end and then scanning from there linearly along single-stranded regions of RNA (Richards & Belasco, 2019, Richards & Belasco, 2021). As a result, cleavage by this endonuclease can be impeded by obstacles encountered along the way. RNase E is able to bypass minor impediments such as orthogonally base-paired stem-loops but not large structural discontinuities created by bound proteins or ribosomes or by base pairing that is coaxial with the path from the 5′ monophosphate to a cleavage site. By forming a coaxially base-paired pseudoknot that hinders scanning by RNase E, the ligand-bound guanidine-III aptamer domain is able to protect distal cleavage sites en masse without altering their structure (Figure 2) (Richards & Belasco, 2021). Because the long-range protective effect of this riboswitch depends solely on the conformation of its aptamer domain and not on its sequence context or any parallel effect on translation, a similar shielding mechanism likely contributes to gene regulation by other riboswitch aptamers that fold into a coaxially base-paired pesudoknot upon ligand binding.
In each of these five examples, the riboswitch acts directly to accelerate or impede RNA degradation. Riboswitches that regulate translation initiation are likely also to affect mRNA decay indirectly by altering the protection against ribonuclease attack afforded to transcripts by bound ribosomes (Deana & Belasco, 2005, Caron et al., 2012). In this manner, a reversible downregulation of translation can be made irreversible by triggering the degradation of a message that has become functionally inactive.
The potential for riboswitches to directly regulate rates of RNA degradation by exposing or masking cleavage sites suggests a need to expand the concept of an expression platform and its spatial relationship with the aptamer domain of a riboswitch. Whereas expression platforms that control RNA or protein synthesis contain a remodelable signal for transcription termination or translation initiation, those that directly control RNA degradation comprise one or more cleavage sites and associated structural elements. A single riboswitch may have multiple expression platforms within the same transcript, one for each of its independent regulatory mechanisms. The two expression platforms of a dual-acting riboswitch need not overlap one another, but they must both be responsive to ligand binding by the aptamer. Interestingly, the degradative expression platform of a riboswitch that blocks scanning by RNase E does not even have to overlap the aptamer, which can act at a considerable distance from the distal cleavage sites whose accessibility it governs.
Once poorly appreciated, the importance of riboswitches for controlling rates of bacterial RNA decay is becoming increasingly apparent. The growing realization that these diverse regulatory elements often act directly to shorten or prolong RNA lifetimes can be expected to spur additional studies of their influence on RNA degradation, which is likely to be widespread. Already, several different mechanisms by which they can alter RNA stability have been identified. More such mechanisms can be envisioned and undoubtedly remain to be discovered.
ACKNOWLEDGMENTS
The support of a research grant from the National Institutes of Health to J.G.B. (R01GM123124) is gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Callaghan AJ, Marcaida MJ, Stead JA, McDowall KJ, Scott WG & Luisi BF, (2005) Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature 437: 1187–1191. [DOI] [PubMed] [Google Scholar]
- Caron MP, Bastet L, Lussier A, Simoneau-Roy M, Massé E & Lafontaine DA, (2012) Dual-acting riboswitch control of translation initiation and mRNA decay. Proc Natl Acad Sci U S A 109: E3444–E3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane JC, Lipchock SV & Strobel SA, (2007) Structural investigation of the GlmS ribozyme bound to Its catalytic cofactor. Chem. Biol 14: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Irnov I, Baker S & Winkler WC, (2007) Mechanism of mRNA destabilization by the glmS ribozyme. Genes Dev. 21: 3356–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana A & Belasco JG, (2005) Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 19: 2526–2533. [DOI] [PubMed] [Google Scholar]
- Hui MP, Foley PL & Belasco JG, (2014) Messenger RNA degradation in bacterial cells. Annu Rev Genet 48: 537–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaberdin VR, (2003) Probing the substrate specificity of Escherichia coli RNase E using a novel oligonucleotide-based assay. Nucleic Acids Res 31: 4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulshina N, Baird NJ & Ferré-D’Amaré AR, (2009) Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nat. Struct. Mol. Biol 16: 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GA, (1998) Ribonuclease E is a 5’-end-dependent endonuclease. Nature 395: 720–723. [DOI] [PubMed] [Google Scholar]
- McCown PJ, Corbino KA, Stav S, Sherlock ME & Breaker RR, (2017) Riboswitch diversity and distribution. RNA 23: 995–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel BA, Grundy FJ, Artsimovitch I & Henkin TM, (2003) Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the leader RNA. Proc Natl Acad Sci U S A 100: 3083–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nou X & Kadner RJ, (1998) Coupled changes in translation and transcription during cobalamin-dependent regulation of btuB expression in Escherichia coli. J. Bacteriol 180: 6719–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursley BR, Fernandez NL, Severin GB & Waters CM, (2019) The Vc2 cyclic di-GMP-dependent riboswitch of Vibrio cholerae regulates expression of an upstream putative small RNA by controlling RNA stability. J. Bacteriol 201: e00293–00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursley BR, Maiden MM, Hsieh ML, Fernandez NL, Severin GB & Waters CM, (2018) Cyclic di-GMP regulates TfoY in Vibrio cholerae to control motility by both transcriptional and posttranscriptional mechanisms. J. Bacteriol 200: e00578–00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J & Belasco JG, (2019) Obstacles to scanning by RNase E govern bacterial mRNA lifetimes by hindering access to distal cleavage sites. Mol. Cell 74: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J & Belasco JG, (2021) Widespread protection of RNA cleavage sites by a riboswitch aptamer that folds as a compact obstacle to scanning by RNase E. Mol. Cell 81: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A & Nudler E, (2013) A decade of riboswitches. Cell 152: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbabian K, Jamalli A, Zig L & Putzer H, (2009) RNase Y, a novel endoribonuclease, initiates riboswitch turnover in Bacillus subtilis. EMBO J. 28: 3523–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock ME & Breaker RR, (2017) Biochemical validation of a third guanidine riboswitch class in bacteria. Biochemistry 56: 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR & Strobel SA, (2009) Structural basis of ligand binding by a c-di-GMP riboswitch. Nat. Struct. Mol. Biol 16: 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli SV, Pontel LB, Garcia Véscovi E & Soncini FC, (2008) Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol. Lett 280: 226–234. [DOI] [PubMed] [Google Scholar]
- Sudarsan N, Wickiser JK, Nakamura S, Ebert MS & Breaker RR, (2003) An mRNA structure in bacteria that controls gene expression by binding lysine. Genes Dev. 17: 2688–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinquier A, Durand S, Braun F & Condon C, (2020) Regulation of RNA processing and degradation in bacteria. Biochim. Biophys. Acta Gene Regul. Mech 1863: 194505. [DOI] [PubMed] [Google Scholar]
- Winkler WC, Nahvi A, Roth A, Collins JA & Breaker RR, (2004) Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428: 281–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


