Abstract
Purpose
The 2016–2020 Utah Comprehensive Cancer Prevention and Control Plan prioritized strategies to address cancer survivorship experiences. In this paper we present estimates for nine indicators evaluating these priorities, trends over time, and assess disparities in survivorship experiences across demographic subgroups.
Methods
We surveyed a representative sample of Utah cancer survivors diagnosed between 2012–2019 with any reportable cancer diagnosis. We calculated weighted percentages and 95% confidence intervals (CI) for each indicator. We assessed change over time using a test for trend across survey years in a logistic regression model and used Rao-Scott F-adjusted chi-square tests to test the association between demographic characteristics and each survivorship indicator.
Results
Most of the 1,793 respondents (93.5%) reported their pain was under control, 85.7% rated their overall health as good, very good, or excellent, but 46.5% experienced physical, mental, or emotional limitations. Only 1.7% of survivors aged 75 or older were current smokers, compared to 5.8% of 65–74-year-olds and 7.9% of survivors aged 55–74 (p<0.006). No regular physical activity was reported by 20.6% and varied by survivor age and education level. The proportion who received a survivorship care plan increased from 34.6% in 2018 to 43.0% in 2021 (p=0.025). However, survivors under age 55 were significantly less likely to receive a care plan than older survivors.
Conclusion
This representative survey of cancer survivors fills a gap in understanding of the cancer survivorship experience in Utah. Results can be used to evaluate and plan additional interventions to improve survivorship quality of life.
Keywords: cancer survivor, cancer control, quality of life, survivorship care plans, health disparities, cancer registries
Introduction
As cancer screening and treatment have improved, the cancer survivorship population has been growing [1–3]. In 2019 there were nearly 17 million cancer survivors living in the United States [4]. Cancer survivors face numerous challenges, not only just the short-term effects of the disease. Cancer and its treatment may have long-term effects on health-related quality of life including pain and functional status limitations, health behaviors such as decreased physical activity, and on access to and utilization of health services. Accordingly, nationwide efforts such as the Healthy People 2020 and Healthy People 2030 initiatives include objectives of improving health-related quality of life for cancer survivors. Additionally, supporting cancer survivors and their caregivers is a priority for the Centers for Disease Control and Prevention’s National Comprehensive Cancer Control Program [5, 6].
The Utah Comprehensive Cancer Control Program and leaders of the Utah Cancer Action Network, a diverse coalition of stakeholders including public health professionals, healthcare workers, community organizations, and patient/survivor advocates, [7] collaborated to develop the 2016 – 2020 Utah Comprehensive Cancer Prevention and Control Plan (State Cancer Plan). The State Cancer Plan is created and disseminated every five years by the Utah Cancer Action Network to serve as a guide for those involved in the planning, implementation, and evaluation of cancer control efforts in Utah [8]. One of the priority areas of focus they highlighted in the 2016–2020 State Cancer Plan was survivorship health and quality of life. The plan included a variety of strategies and activities for coalition stakeholders to prioritize in their efforts to address survivorship health and quality of life.
The Utah Cancer Control Program and Utah Cancer Action Network leaders identified nine survivorship indicators that could be used to assess the coalition’s progress in carrying out these strategies. Many of these nine indicators were modeled on the Healthy People 2020 goals [9]. These included indicators of two health behaviors: cigarette smoking and physical activity, and four health-related quality of life indicators: pain control, overall health, life dissatisfaction, and functional limitations. Three cancer-specific indicators of health services were also included: insurance coverage for cancer treatment, clinical trial participation as part of cancer treatment, and receipt of a survivorship care plan. The Utah Comprehensive Cancer Control Program collaborated with the Utah Cancer Registry to develop and implement a survey to collect data for these indicators directly from a representative sample of cancer survivors. The purpose of this paper is to: 1) present estimates and evaluate trends over time for each of the nine survivorship indicators included in the Utah State Cancer Plan and 2) assess disparities in these indicators across demographic subgroups.
Methods
Procedures and participants
The sample frame for this study was derived from records from Utah Cancer Registry, a population-based central cancer registry that collects and maintains information on all reportable cancer diagnoses in Utah. Reportable diagnoses include all primary invasive and in situ cancers (with certain exceptions). Benign tumors are not reportable unless they occur in the brain or other areas of the central nervous system, in which case they are reportable. Utah Cancer Registry data meet quality and completeness standards established by the U.S. National Cancer Institute Surveillance, Epidemiology, and End Results program and the U.S. Centers for Disease Control and Prevention National Program of Cancer Registries.
We used a stratified random sample to oversample survivors based on two variables: Hispanic ethnicity and residing in an area of Utah with higher proportions of uninsured residents. The area-level unit used was the Utah “Small Health Statistical Areas,” geographic units developed by the Utah Department of Health which combine neighboring ZIP codes into areas comprised of relatively equal numbers of residents for purposes of analyzing health statistics at the community level [10]. To measure the proportion uninsured in each Small Health Statistical Area, we utilized data from the Utah Behavioral Risk Factors Surveillance System survey (BRFSS) [11] to calculate the proportion of residents in each Small Health Statistical Area who are uninsured. We classified each area as “high proportion uninsured” or “low proportion uninsured” using the median proportion of residents without health insurance.
Eligibility criteria for the survey entailed being a living cancer survivor diagnosed in the years 2012 through 2019, age 18 or older at time of diagnosis, and a Utah resident at time of diagnosis and at the time of the survey. The survey was conducted annually from 2018–2021, using a new sample of survivors each year of the survey. The sample frame for the survey conducted in 2018 included cancer cases diagnosed in 2012 through 2016, the sample frame for the 2019 survey included cases diagnosed in 2013 through 2017, etc. The sample frame included all reportable, invasive cancer diagnoses. Cancers of any site diagnosed at the in situ stage were excluded from the sample frame. In 2018, the sample frame included reportable benign brain or central nervous system tumor diagnoses, but these diagnoses were excluded from the 2019–2021 samples.
The survey was conducted annually from 2018–2021 using a mixed-mode, push-to-web methodology [12] for survivors under age 80, and a paper-only response method for survivors aged 80 or above. We used both postal mailings and phone calls to contact potential participants, including a pre-notification letter with brochure about the registry, a formal invitation letter with either the survey web address or a paper questionnaire and stamped return envelope, a reminder letter, a packet containing a replacement questionnaire or a first paper questionnaire and stamped return envelope, and then a phone call follow-up to reach those who did not respond to mailed contacts. The formal invitation included a $2.00 cash pre-incentive. This study was reviewed by the Utah Department of Health Institutional Review Board. Participants were informed that completing the survey signified consent to participate.
Measures
To measure each of the State Cancer Plan’s nine survivorship indicators, we created a questionnaire containing questions based on well-established items included in leading nationwide heath surveys. The items measuring physical activity were taken from the National Cancer Institute’s Health Information National Trends Survey (HINTS) [13] whereas the rest of our questions were based on those asked on the CDC’s BRFSS survey [14]. Development, testing, and evaluation of these instruments have previously been reported [15–17]. The full study questionnaire, included as Additional File 1, includes the precise wording and response options for each question contained in our survey. All nine survivorship indicators were analyzed in a dichotomous fashion to match how they were defined in the State Cancer Plan. This entailed collapsing responses to some questions, including the measure of overall health (good, very good, excellent vs. fair, poor) and life dissatisfaction (dissatisfied, very dissatisfied vs. other responses). The variable representing no regular physical activity was created using responses to two items representing aerobic exercise and strength training, classifying participants reporting no days of either activity in the last week as having no regular exercise (vs. all other responses). Our measure of receipt of a survivorship care plan was based on affirmative responses to two questions that asked if survivors had received: a) a written summary of the cancer treatments they had received and b) written instructions for when and where to return for follow-up care. We also examined responses to these two items individually.
Most covariates used in our analyses were variables contained within existing registry records. These include participants’ year of cancer diagnosis, age, sex, and rurality, which was a county-level designation of rural (vs. urban) location of residence based on the Rural-Urban Continuum Codes [18]. Other covariates were obtained from questionnaire responses. These included self-reported educational attainment (dichotomized as high school or less or some college or more). When available, we used self-reported race and ethnicity from survey responses, but used cancer registry records when self-reported race and ethnicity were not available.
Data Analysis
Demographic differences in respondents compared to non-respondents were assessed using chi-square tests. Non-responders included cases that actively refused participation when reached by telephone, individuals who did not respond to requests to complete the questionnaire, and those we were unable to contact due to incorrect contact information in registry records. For each survivorship indicator, we calculated weighted percentages with 95% confidence intervals (CI) for the full sample and stratified by demographic subgroups. Percentages were weighted to account for the survey sample design and non-response, and age-adjusted to the Utah adult cancer survivor population. Rao-Scott F-adjusted chi-square tests were calculated to assess the association between demographic characteristics and each survivorship indicator. We assessed change over time in each indicator using a test for trend across survey years in a logistic regression model. Statistical analyses were conducted using SAS 9.4 and figures were constructed in R. Cases with missing data for an item were excluded from analyses with that item, and percentages were based on the number of participants with non-missing values.
Results
Descriptive statistics
The survey obtained 1,793 responses (58.6% weighted response rate). Table 1 documents the demographics of the cancer survivor survey participants with both weighted and unweighted (raw) percentages. Responding survivors were weighted to be 53.1% female, 13.6% rural, and 6.2% Hispanic (of any race). A majority of survivors were over age 65, and 22.7% had a high school education or less. Respondents included cases diagnosed in 2012 through 2019, with the largest number sampled from diagnosis years 2014–2017. The most common cancer sites represented among participants were those that are most commonly diagnosed in Utah, including breast, prostate, melanoma, colorectal, and thyroid cancers. The next most common cancers of participants were endometrial, lymphoma, and kidney cancers.
Table 1.
Demographic characteristics for a survey of Utah cancer survivors, 2018–2021
| Males | Females | Total | |||||
|---|---|---|---|---|---|---|---|
| n | Weighted %a | n | Weighted % a | n | Weighted % a | Raw % b |
|
|
| |||||||
| Sex | |||||||
| Male | - | - | - | - | 828 | 46.9 | 46.2 |
| Female | - | - | - | - | 965 | 53.1 | 53.8 |
| Race and ethnicity | |||||||
| Hispanic, any race | 103 | 5.4 | 163 | 6.9 | 266 | 6.2 | 14.8 |
| Non-Hispanic white | 706 | 90.8 | 782 | 89.9 | 1488 | 90.3 | 83.0 |
| Non-Hispanic, any other race | 19 | 3.9 | 20 | 3.2 | 39 | 3.5 | 2.2 |
| Current age | |||||||
| Under 55 | 97 | 15.6 | 251 | 32.1 | 348 | 24.4 | 19.4 |
| 55–64 | 164 | 18.4 | 275 | 25.0 | 439 | 21.9 | 24.5 |
| 65–74 | 330 | 36.4 | 259 | 23.4 | 589 | 29.5 | 32.9 |
| 75+ | 237 | 29.6 | 180 | 19.4 | 417 | 24.2 | 23.3 |
| Education | |||||||
| High school or less | 183 | 19.1 | 263 | 25.8 | 446 | 22.7 | 25.3 |
| Some college or more | 632 | 80.9 | 684 | 74.2 | 1316 | 77.3 | 74.7 |
| Geography | |||||||
| Urban | 701 | 86.7 | 819 | 86.2 | 1520 | 86.4 | 84.8 |
| Rural | 127 | 13.3 | 146 | 13.8 | 273 | 13.6 | 15.2 |
| Area-level proportion uninsured | |||||||
| Above median | 426 | 40.2 | 469 | 38.0 | 895 | 39.0 | 49.9 |
| Below median | 402 | 59.8 | 495 | 62.0 | 897 | 61.0 | 50.1 |
| Diagnosis year | |||||||
| 2012–2013 | 106 | 13.4 | 119 | 13.3 | 225 | 13.3 | 12.6 |
| 2014–2015 | 307 | 37.8 | 353 | 36.9 | 660 | 37.3 | 36.8 |
| 2016–2017 | 318 | 38.1 | 378 | 40.3 | 696 | 39.3 | 38.8 |
| 2018–2019 | 97 | 10.7 | 115 | 9.5 | 212 | 10.1 | 11.8 |
Percentage weighted to account for survey sample design and nonresponse.
Unweighted percent of participants.
Race and ethnicity were associated with survey response, with highest participation by non-Hispanic whites (p<0.001, Table 2). Age was also associated with response (p<0.001), with survivors under age 55 being underrepresented among respondents compared to older survivors. Response also differed by cancer site; survivors of melanoma of the skin and prostate cancer had better response rates and colorectal and thyroid cancers lower response (p<0.001).
Table 2.
Respondents compared to nonrespondents: Utah cancer survivors surveyed 2018–2021
| Respondents | Nonrespondents | Weighted response rate | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | p | ||
|
| ||||||
| Total | 1793 | 54.4 | 1503 | 45.6 | -- | 58.6 |
| Sex | ||||||
| Female | 965 | 54.4 | 808 | 45.6 | 0.97a | 58.5 |
| Male | 828 | 54.4 | 695 | 45.6 | 58.7 | |
| Race and ethnicity | ||||||
| Hispanic, any race | 284 | 37.2 | 480 | 62.8 | <0.001a | 38.8 |
| Non-Hispanic white | 1478 | 60.5 | 966 | 39.5 | 60.8 | |
| Non-Hispanic, any other race | 31 | 35.2 | 57 | 64.8 | 35.8 | |
| Current age | ||||||
| <55 | 348 | 40.8 | 506 | 59.3 | <0.001a | 47.7 |
| 55–64 | 439 | 55.2 | 356 | 44.8 | 56.7 | |
| 65–74 | 589 | 62.5 | 354 | 37.5 | 66.8 | |
| 75+ | 417 | 59.2 | 287 | 40.8 | 61.1 | |
| Rural residence | ||||||
| Yes | 273 | 56.3 | 212 | 43.7 | 0.37a | 57.3 |
| No | 1520 | 54.1 | 1291 | 45.9 | 58.8 | |
| Area-level insurance | ||||||
| More uninsured | 895 | 51.2 | 854 | 48.8 | <0.001a | 53.9 |
| Fewer uninsured | 897 | 58.0 | 649 | 42.0 | 61.8 | |
| Cancer site | ||||||
| Breast | 382 | 56.7 | 292 | 43.3 | <0.001a | 59.7 |
| Colorectal | 115 | 48.9 | 120 | 51.1 | 55.8 | |
| Melanoma | 246 | 60.0 | 164 | 40.0 | 60.1 | |
| Prostate | 340 | 60.5 | 222 | 39.5 | 65.1 | |
| Thyroid | 102 | 44.7 | 126 | 55.3 | 52.9 | |
| Other | 608 | 51.2 | 579 | 48.8 | 55.5 | |
| Year of diagnosis | ||||||
| 2012–2013 | 225 | 56.4 | 174 | 43.6 | 0.32a | 59.7 |
| 2014–2015 | 660 | 55.4 | 531 | 44.6 | 58.2 | |
| 2016–2017 | 696 | 54.1 | 591 | 45.9 | 58.9 | |
| 2018–2019 | 212 | 50.7 | 206 | 49.3 | 57.7 | |
Chi-squared test
Aim 1: Estimates and trends over time in cancer survivorship indicators
Survivors’ survey responses regarding health behaviors indicate that in total 5.0% (95% CI 3.8–6.1) of survivors reported being current smokers and 20.6% (CI 18.5–22.7) reported no regular physical activity (Table 3). In responses to the health-related quality of life questions, most survivors (93.5%, CI 92.2–94.7) reported their pain was under control and 85.7% (CI 83.8–87.5) indicated their health was good, very good, or excellent. Only 7.1% (CI 5.7–8.5) reported life dissatisfaction. Nearly half of survivors (46.5%, CI 43.8–49.1) reported experiencing limitations due to physical, mental, or emotional problems.
Table 3.
Cancer survivors’ status on Utah State Cancer Plan health indicators and trends over time 2018–2021
| Total |
By Year |
||||||
|---|---|---|---|---|---|---|---|
| Health indicator |
%a |
95% CI | 2018a | 2019a | 2020a | 2021a | p-trendb |
|
| |||||||
| Current cigarette smoker | 5.0 | 3.8–6.1 | 6.0 | 4.6 | 5.5 | 3.8 | 0.30 |
| No regular physical activity | 20.6 | 18.5–22.7 | 21.8 | 23.7 | 17.5 | 19.4 | 0.45 |
| Pain under control | 93.5 | 92.2–94.7 | 92.4 | 92.9 | 93.7 | 94.8 | 0.16 |
| Good, very good, or excellent health | 85.7 | 83.8–87.5 | 86.7 | 83.9 | 85.6 | 86.4 | 0.71 |
| Dissatisfied with life | 7.1 | 5.7–8.5 | 7.2 | 7.8 | 5.9 | 7.5 | 0.86 |
| Experience limitations due to physical, mental, or emotional problems | 46.5 | 43.8–49.1 | 48.4 | 46.6 | 46.0 | 44.8 | 0.46 |
| Cancer treatment was covered in part or full by health insurance | 97.7 | 96.9–98.6 | 97.3 | 98.7 | 96.9 | 98.0 | 0.44 |
| Participated in a clinical trial as part of cancer treatment | 10.4 | 8.6–12.1 | 8.6 | 10.8 | 10.2 | 11.8 | 0.34 |
| Received written survivorship care plan (including summary of both cancer treatment and follow-up care) | 40.4 | 37.0–43.9 | 34.6 | 39.1 | 43.5 | 43.0 | 0.025 |
| Survivorship care plan: cancer treatment only | 51.2 | 47.6–54.8 | 42.4 | 46.2 | 56.0 | 57.8 | <0.001 |
| Survivorship care plan: follow-up instructions only | 68.2 | 65.0–71.3 | 65.5 | 73.3 | 64.7 | 69.7 | 0.88 |
Percentages are weighted to account for survey sample design and nonresponse and are age-adjusted to the Utah cancer survivor population.
P-values for trend were computed using logistic regression.
For the survivorship indicators in the area of health services, nearly all survivors (97.7%, CI 96.9–98.6) had their cancer treatment covered in part or in full by insurance. Approximately one in ten survivors (10.4%, CI 8.6–12.1) reported participating in a clinical trial as part of their cancer treatment. Forty percent of survivors (40.4%, CI 37.0–43.9) received a survivorship care plan that included both a written summary of their cancer treatment and written instructions for future follow-up care.
Across the four years of data collection, 2018–2021, estimates of most survivorship indicators remained relatively stable (Table 3). The data for cigarette smoking among cancer survivors trended downward from 6.0% in 2018 to 3.8% in 2021, but the trend was not statistically significant (p-trend=0.30). The proportion who reported pain was under control increased over time but did not exhibit a significant trend (p-trend=0.16). The percentage of survivors who received a complete survivorship care plan did increase significantly over the four years, from 34.6% in 2018 to 43.0% in 2021 (p-trend=0.025).
When evaluating the two components of a survivorship care plan separately, we observed different patterns. The percentage of survivors who received a summary of their cancer treatment increased significantly over the period time of 2018 through 2021 (p-trend=<0.001). The percentage of survivors who received a written summary of follow-up care instructions did not increase significantly over time (p-trend=0.88).
Aim 2: Demographic disparities in survivorship indicators
We observed some variation in survivorship indicators across demographic subgroups. The percent of survivors who were current smokers varied significantly by age, with only 1.7% of those aged 75 or older being current smokers compared to 7.9% of those aged 55–64 and 5.8% of survivors aged 65–74 (Figure 1a; Additional File 2). More individuals with a high school education or less were current smokers (12.8%, CI 9.0, 16.6) than those with at least some college education (2.9%, CI 1.9, 3.9). More females reported no regular physical activity (24.3%, CI 21.2, 27.3) than males (17.4%, CI 14.5, 20.3), Figure 1b. Reporting no regular physical activity was more common among survivors 75 or older (30.4%, CI 25.6, 35.3) than all younger age groups, including those aged 65–74 (20.5%, CI 17.0, 24.0), and among survivors with a high school education or less (28.3%, CI 23.5, 33.1) compared to those with some college or more (18.2%, CI 15.9, 20.5).
Fig. 1.

Percent of Utah cancer survivors who report being a current smoker (a), getting no regular physical activity (b), whose pain is under control (c), who report good, very good, or excellent health (d), who are dissatisfied with life (e), and who experience limitations due to physical, mental, or emotional problems (f).
The proportion of survivors reporting their pain was under control was high and did not vary significantly across subgroups (Figure 1c). Fewer Hispanic survivors (76.0%, CI 67.8, 84.1) reported being in good, very good, or excellent health compared to non-Hispanic white survivors (86.6%, CI 84.7, 88.4). We observed no significant differences in life dissatisfaction (Figure 1e) by sex, race/ethnicity, age, or education. The percent of survivors reporting experiencing limitations due to physical, mental, or emotional problems was lower for those aged under 55 (37.9%, CI 32.1, 43.7) compared to older age groups for whom the percentage neared 50% (Figure 1f).
There were also some disparities in access to health services. The percent of survivors who had insurance coverage for their cancer treatment was slightly lower among survivors with lower educational attainment (94.3%, CI 91.6, 97.0) than those with some college or more (98.9%, CI 98.3, 99.6), Figure 2a. Hispanic survivors were significantly more likely to have participated in a clinical trial as part of their treatment (19.6%, CI 12.0, 27.3) than non-Hispanic white survivors (9.5%, CI 7.8, 11.2, Figure 2b). Thyroid cancer survivors were less likely to report clinical trial participation (0.1%, CI 0.0, 0.4) than others, but we observed no other differences across other cancer sites (results not shown). Receipt of a survivorship care plan did not vary by sex, ethnicity, or education but was lower among younger survivors under age 55 (29.5%, CI 22.6, 36.4) compared to survivors 55–64 (44.0%, CI 37.0, 51.0) and those aged 65–74 (48.7%, CI 42.8, 54.7), Figure 2c. Receipt of a survivorship care plan did not vary by cancer site (results not shown).
Fig. 2.
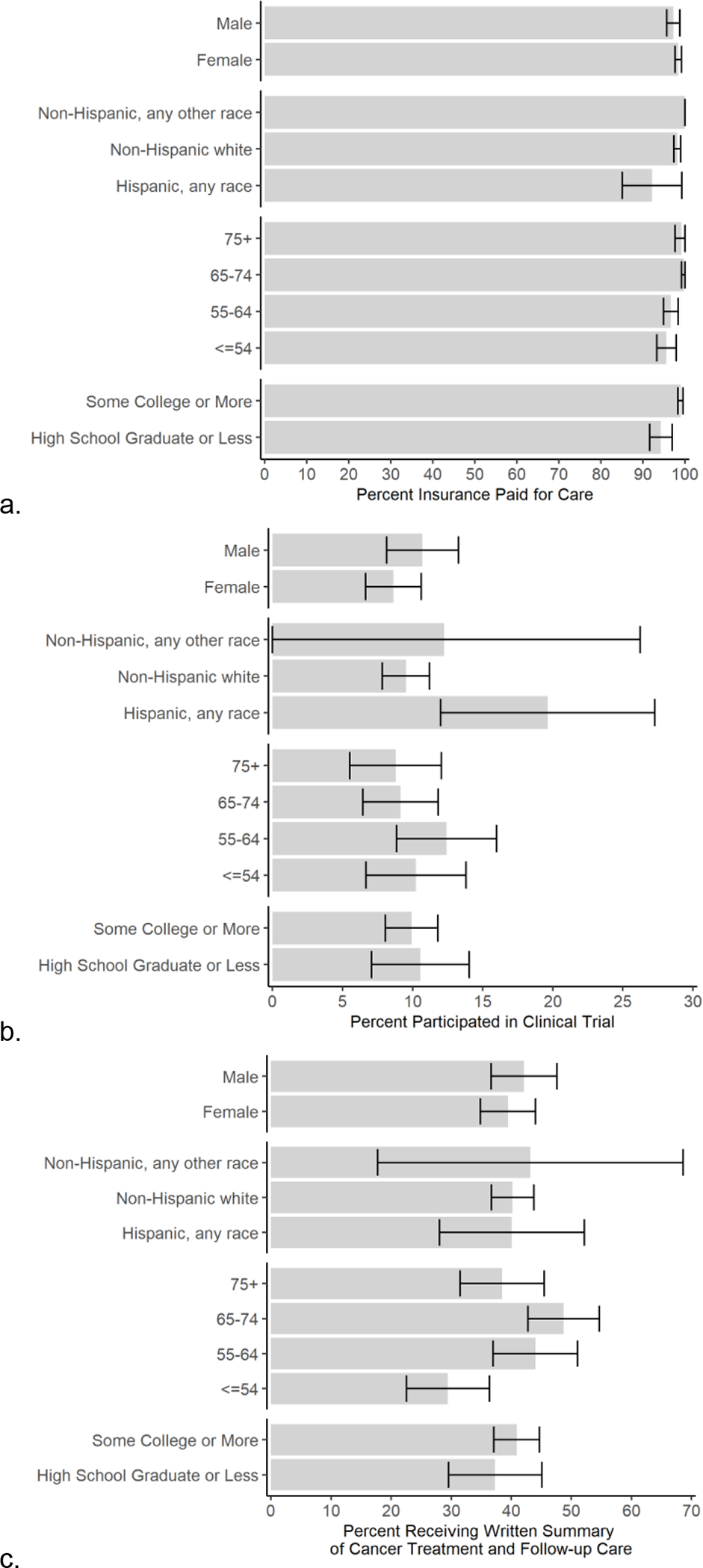
Percent of Utah cancer survivors who report that their cancer treatment was paid for by health insurance (a), who participated in a clinical trial as part of their cancer treatment (b), and who received a survivorship care plan summarizing their treatment and follow-up care instructions (c).
Discussion
In this paper, we present results of an analysis of nine survivorship indicators for a representative sample of Utah cancer survivors. Overall, we found most survivors report their general health is good, very good, or excellent, but many report limitations. Further, our analysis demonstrates disparities that warrant further investigation and targeted interventions.
Five percent of survivors reported being current smokers, with a non-significant trend down to 3.8% in 2021. This prevalence is lower than the estimated 12% of survivors nationwide estimated to be current smokers in 2015 [19], and is in keeping with the low prevalence of smoking in Utah, estimated at 3.5% for the general population aged 65 and older Utah in 2020 [20]. However, consistent with prior research on survivors [19, 21], we found younger survivors had higher smoking prevalence than the older survivors. Individuals with lower educational attainment, an indicator of socioeconomic disparity, were also more likely to be current smokers. Twenty percent of survivors reported no regular physical activity, and this prevalence was even higher among survivors with lower educational attainment. Reports based on large cohorts have found that low physical activity among cancer survivors is associated with poor outcomes in several domains of health-related quality of life [22, 23]. The higher prevalence of smoking and physical inactivity among survivors with lower education should be of concern for those addressing health disparities. The implications of compounding multiple poor health behaviors among survivors who may also be at increased risk for financial instability after cancer treatment [24] highlight the need for more targeted interventions to improve health outcomes among the cancer survivor population.
For health-related quality of life measures in the present study, over 90% of survivors reported their pain is under control. Cancer survivors are nearly twice as likely as those without a prior cancer diagnosis to report experiencing chronic pain [19], making effective pain control an issue of particular relevance to this population. Overall health was described as good, very good, or excellent by 85% of survivors. This is consistent with reports from Utah cancer survivors from the 2009–2010 BRFSS survey [25]. However, disparities were detected, with fewer Hispanic survivors reporting good health. While the general Hispanic or Latino population in the United States experiences less disease than the non-Hispanic White population according to some metrics—e.g., a lower death rate and a lower prevalence of heart disease and cancer—Hispanic or Latino individuals are almost three times as likely to be uninsured [26]. Lack of insurance coverage can prevent access to routine healthcare, with deleterious effects on health outcomes. For example, Hispanic and Latino cancer patients are more likely to be diagnosed at later stage disease for many cancers [27]. Hispanic and Latino cancer survivors are also disproportionately affected by poor social determinants of health that significantly affect access to care and overall health and wellbeing [28]. Such disparities highlight the need for better interventions to address upstream social determinants of health [29].
Few survivors (7%) are dissatisfied with life, but nearly half experienced limitations due to their physical, mental, or emotional health. Due to the nature of the survey question used, we were unable to differentiate between these three types of limitations or assess the extent to which physical and mental health may be interrelated. Others have found that physical disability is a key driver of psychological distress among cancer survivors [30]. In general, cancer survivors have been reported to be affected by worse health-related quality of life, including mental health, physical function and other domains, than comparable-age peers without a history of cancer [22, 31–34]. These limitations can have far-reaching implications, with survivors reporting physical or mental health limitations more likely to report low incomes and being unemployed [35]. However, cancer control programs face challenges in identifying appropriate programs and interventions for cancer survivors, as according to Healthy People 2030 [36], quality of life in cancer survivors is “a high-priority public health issue that doesn’t yet have evidence-based interventions developed to address it.”
Estimates for survivorship indicators related to healthcare services also varied across demographic subgroups. Nearly 98% of survivors had at least some of the cost of their cancer treatment covered by health insurance. This is encouraging, but we did observe that individuals with lower educational attainment were less likely to report health insurance coverage for treatment. Employment changes and treatment-related financial toxicity are substantial concerns for cancer survivors [37]. Our findings that those with lower educational attainment are more likely to lack insurance coverage for treatment highlights the vulnerable position of those who may not have access to health insurance through employment in the United States. It is also important to recognize that even insured cancer patients can have substantial out-of-pocket costs that can negatively affect financial stability. Using results from this survey, we recently demonstrated how financial toxicity is a common experience after cancer treatment and is also associated with changes in caregivers’ employment status [38]. Medicaid expansion was fully enacted in Utah in 2020, so lower income survivors may now have more avenues for health insurance coverage. Future research should further ascertain the extent of out-of-pocket costs and impacts on financial stability for cancer survivors with varying insurance coverage.
About 10% of Utah cancer survivors reported participating in a clinical trial as part of their cancer treatment. A 2019 meta-analysis found 8% of cancer patients participated in a trial [39]. The primary barriers to participation included the lack of availability of trials at place of treatment and patients not meeting eligibility criteria. Our study included survivors of all cancer sites and stages, many of whom may not have been candidates for investigational treatments. Hispanic survivors were more likely to report have participated in a clinical trial than non-Hispanic white survivors. This contrasts with prior reports [40, 41] and is a potentially a positive development in addressing the longstanding underrepresentation of Hispanic and Latino patients in clinical research. While our findings do not necessarily imply that Hispanic and Latino cancer patients are proportionally represented in clinical trials in Utah, we do believe this result could be reflective of targeted recruitment efforts in the state aimed at improving the diversity of clinical trial participants.
We found that 40.4% of the survivors received a written survivorship care plan containing both a summary of cancer treatment and instructions for follow-up care. This percentage is similar to findings from a survey of New Jersey cancer survivors which found over half had not received a survivorship care plan [42]. In 2009–2010, just under 30% of Utah cancer survivors reported receiving a summary of their cancer treatment [25]. However, our results varied when examining the two components of the care plan separately. Throughout all years of our study the proportion who reported receiving instructions for follow-up care was higher than the proportion who reported receiving a summary of cancer treatment. This initial disparity in what types of information survivors reported receiving in their care plans might explain why we observed differences in trends across the two survivorship care plan components over the course of our study.
Similar to prior research [43], we found that younger survivors (under age 55) were less likely to report receiving a care plan. This indicator was the only of those we examined that exhibited a significant change over the period of the study, increasing from 34.6% in 2018 to 43.0% in 2021. This is consistent with efforts of a variety of stakeholders during this time to increase use of survivorship care plans. These include a 2016 standard requiring facilities accredited by the American College of Surgeons’ Commission on Cancer to increase the use of survivorship care plans and initiatives by the Utah Cancer Control Program and other stakeholders to increase the use of these plans. However, in 2019 the Commission on Cancer reversed this requirement [44]. There were a variety of barriers to implementation of care plans [45]. Further, many expressed concerns that the plans were not achieving their primary goals of engaging patients and primary care providers in understanding treatments received, risk of potential late effects, and recommendations for screening [44]. These reported barriers to care plan implementation, particularly a lack of resources available to implement care plans, could also in part explain why we observed significant changes over time in implementation of one of the two components of the care plan summary.
This study is subject to limitations. Estimates of health indicators in this study are based on self-report, which could be subject to measurement error and social desirability bias. However, self-report is the most widely used strategy for obtaining information about many health indicators. Further, the breadth of topics represented in the survivorship indicators from the State Cancer Plan prevented us from asking more detailed questions about each topic. Thus, we were unable to ascertain the extent of insurance coverage survivors had for their cancer treatment or distinguish between physical, mental, or emotional limitations survivors reported. Future studies that explore each of these topics in more detail would be valuable. In future surveys, we intend to inquire in more detail about limitations experienced as a result of cancer and its treatment. We also aim to further explore the costs incurred during cancer treatment to assess their relationship to financial toxicity. Also, given our study’s focus on survivors of all cancer sites, small numbers of participants for many cancer sites limited our ability to explore site-specific differences. Additionally, most of the Utah cancer survivor population is non-Hispanic white, which prohibits us from producing reliable estimates for survivors identifying as any other race. Strengths of this study include use of established measures of health indicators, a high response rate, and a probability-based sample representing the Utah cancer survivor population.
This study was the result of an innovative collaboration between a central cancer registry and state cancer control program. It demonstrates the utility of using a central registry [46], which provides a complete sample frame for obtaining representative samples to assess survivors’ health indicators. Results indicate that the cancer control program’s efforts to increase the use of survivorship care plans was successful. Further, our findings identify disparities, with smoking, lack of physical activity, and access to health services disproportionately affecting survivors with lower educational attainment and poorer overall health among Hispanic survivors. Future surveys will continue to track health indicators and also include new measures to address priorities of the new State Cancer Plan that went into effect in 2021.
An increasing numbers of cancer patients are surviving many years after diagnosis [1, 3]. National and state public health programs recognize cancer survivors as a priority population, and routinely collected data are necessary to monitor and address their unique needs [47, 48]. The present study demonstrates that surveys conducted through central cancer registries are a tool to obtain population-based data, which can be useful for those seeking to evaluate future interventions.
Supplementary Material
Acknowledgement
This project was supported by the U.S. Centers for Disease Control and Prevention’s National Program of Cancer Registries, Cooperative Agreement No. NU58DP006320. The Utah Cancer Registry is also supported by the U.S. National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, Contract No. HHSN261201800016I, with additional support from the University of Utah and Huntsman Cancer Foundation. We thank Kate Hak and Lori Burke of the Utah Cancer Registry for their efforts to recruit participants and collect data for this study.
Funding
This work was supported by the U.S. Centers for Disease Control and Prevention’s National Program of Cancer Registries, Cooperative Agreement No. NU58DP006320. The Utah Cancer Registry is also supported by the U.S. National Cancer Institute’s SEER Program, Contract No. HHSN261201800016I, with additional support from the University of Utah and Huntsman Cancer Foundation.
Footnotes
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
This study was reviewed by the Utah Department of Health Institutional Review Board, which deemed the project exempt from human subjects research approval because it was a program evaluation initiative.
Consent to Participate
Informed consent was obtained from all participants included in this study.
Consent to Publish
Not applicable. This manuscript does not present individual data.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy restrictions.
References
- 1.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.Epi-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9). doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute: Cancer statistics: About cancer. https://www.cancer.gov/about-cancer/understanding/statistics (2020). Accessed 21 January 2022.
- 5.Centers for Disease Control and Prevention: National Comprehensive Cancer Control Program Priorities. https://www.cdc.gov/cancer/ncccp/priorities/index.htm (2021). Accessed 18 March 2022.
- 6.Rohan EA, Miller N, Bonner F 3rd, Fultz-Butts K, Pratt-Chapman ML, Alfano CM, et al. Comprehensive cancer control: promoting survivor health and wellness. Cancer Causes Control. 2018;29(12):1277–85. doi: 10.1007/s10552-018-1107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utah Cancer Action Network: Utah Cancer Action Network home page. https://www.utahcancercoalition.org Accessed 12 December 2022.
- 8.Utah Cancer Action Network. 2016–2020 Utah Comprehensive Cancer Prevention and Control Plan. 2016.
- 9.US Department of Health and Human Services (DHHS): Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives Accessed 27 August 2020.
- 10.Utah Department of Health: Utah Small Health Statistical Areas. https://gis.utah.gov/data/health/health-small-statistical-areas/ (2018). Accessed 17 June 2022.
- 11.Utah Department of Health: Utah Behavioral Risk Factor Surveillance System Data. https://ibis.health.utah.gov/ibisph-view/ (2018). Accessed 17 June 2022.
- 12.Dillman DA. The promise and challenge of pushing respondents to the web in mixed-mode surveys. Survey Methodology. 2017;43(1):Epub https://www150.statcan.gc.ca/n1/pub/12-001-x/2017001/article/14836-eng.htm. [Google Scholar]
- 13.National Cancer Institute: What is HINTS? https://hints.cancer.gov Accessed 7 April 2022.
- 14.Centers for Disease Control and Prevention: Behavioral Risk Factor Surveillance System Survey. https://www.cdc.gov/brfss/index.html (2014). Accessed 18 March 2022.
- 15.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res Methodol. 2013;13(1):49. doi: 10.1186/1471-2288-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks W, Cantor D. Health Information National Trends Survey 4 (HINTS 4) Cycle 1 Cognitive Interviewing Report. Rockville, MD: Westat; 2011. [Google Scholar]
- 17.Cantor D, Coa K, Crystal-Mansour S, Davis T, Dipko S, Sigman R. Health Information National Trends Survey (HINTS) 2007 Final Report. Rockville, MD: Westat; 2009. [Google Scholar]
- 18.U.S. Department of Agriculture: Rural-Urban Continuum Codes. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/ (2013). Accessed 17 June 2022.
- 19.Gallaway MS, Glover-Kudon R, Momin B, Puckett M, Lunsford NB, Ragan KR, et al. Smoking cessation attitudes and practices among cancer survivors - United States, 2015. J Cancer Surviv. 2019;13(1):66–74. doi: 10.1007/s11764-018-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau of Health Promotion Tobacco Prevention and Control Program: Current cigarette smoking by age group, Utah adults aged 18 and older, 2020. https://ibis.health.utah.gov/ibisph-view/indicator/view/CigSmokAdlt.Age.html (2022). Accessed 5 May 2022. [Google Scholar]
- 21.Ehrenzeller MF, Mayer DK, Goldstein A. Smoking Prevalence and Management Among Cancer Survivors . Oncol Nurs Forum. 2018;45(1):55–68. doi: 10.1188/18.Onf.55-68. [DOI] [PubMed] [Google Scholar]
- 22.Blair CK, Robien K, Inoue-Choi M, Rahn W, Lazovich D. Physical inactivity and risk of poor quality of life among elderly cancer survivors compared to women without cancer: the Iowa Women’s Health Study. J Cancer Surviv. 2016;10(1):103–12. doi: 10.1007/s11764-015-0456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees-Punia E, Patel AV, Nocera JR, Chantaprasopsuk S, Demark-Wahnefried W, Leach CR, et al. Self-reported physical activity, sitting time, and mental and physical health among older cancer survivors compared with adults without a history of cancer. Cancer. 2020;127(1):115–23. doi: 10.1002/cncr.33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight TG, Deal AM, Dusetzina SB, Muss HB, Choi SK, Bensen JT, et al. Financial Toxicity in Adults With Cancer: Adverse Outcomes and Noncompliance. J Oncol Pract. 2018;14(11):e665–e73. doi: 10.1200/jop.18.00120. [DOI] [PubMed] [Google Scholar]
- 25.Fowler B, Ding Q, Pappas L, Wu YP, Linder L, Yancey J, et al. Utah Cancer Survivors: A Comprehensive Comparison of Health-Related Outcomes Between Survivors and Individuals Without a History of Cancer. Journal of cancer education : the official journal of the American Association for Cancer Education. 2018;33(1):214–21. doi: 10.1007/s13187-016-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention: Vital Signs: Hispanic Health. https://www.cdc.gov/vitalsigns/hispanic-health/index.html (2015). Accessed 11 January 2023.
- 27.Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin. 2021;71(6):466–87. doi: 10.3322/caac.21695. [DOI] [PubMed] [Google Scholar]
- 28.Kronenfeld JP, Graves KD, Penedo FJ, Yanez B. Overcoming Disparities in Cancer: A Need for Meaningful Reform for Hispanic and Latino Cancer Survivors. Oncologist. 2021;26(6):443–52. doi: 10.1002/onco.13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharmal N, Pitkin Derose K, Felician M, Weden MM. Understanding the upstream social determinants of health: working paper. RAND Health; 2015. [Google Scholar]
- 30.Joshy G, Thandrayen J, Koczwara B, Butow P, Laidsaar-Powell R, Rankin N, et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Medicine. 2020;18(1):372. doi: 10.1186/s12916-020-01830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underwood JM, Rim SH, Fairley TL, Tai E, Stewart SL. Cervical cancer survivors at increased risk of subsequent tobacco-related malignancies, United States 1992–2008. Cancer Causes & Control. 2012;23(7):1009–16. doi: 10.1007/s10552-012-9957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101(12):860–8. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leach CR, Bellizzi KM, Hurria A, Reeve BB. Is it my cancer or am i just getting older?: Impact of cancer on age-related health conditions of older cancer survivors. Cancer. 2016;122(12):1946–53. doi: 10.1002/cncr.29914. [DOI] [PubMed] [Google Scholar]
- 34.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2108–17. doi: 10.1158/1055-9965.Epi-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89(1):128–36. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- 36.US Department of Health and Human Services (DHHS): Healthy People 2030. https://health.gov/healthypeople Accessed 20 January 2022.
- 37.Mols F, Tomalin B, Pearce A, Kaambwa B, Koczwara B. Financial toxicity and employment status in cancer survivors. A systematic literature review. Support Care Cancer. 2020;28(12):5693–708. doi: 10.1007/s00520-020-05719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warner EL, Millar MM, Orleans B, Edwards SL, Carter ME, Vaca Lopez PL, et al. Cancer survivors’ financial hardship and their caregivers’ employment: results from a statewide survey. J Cancer Surviv. 2022. doi: 10.1007/s11764-022-01203-1. [DOI] [PubMed] [Google Scholar]
- 39.Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation. Journal of the National Cancer Institute. 2019;111(3):245–55. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 41.Perni S, Moy B, Nipp RD. Disparities in phase 1 cancer clinical trial enrollment. Cancer. 2021;127(23):4464–9. doi: 10.1002/cncr.33853. [DOI] [PubMed] [Google Scholar]
- 42.Fong AJ, Evens AM, Bandera EV, Llanos AAM, Devine KA, Hudson SV, et al. Survivorship transition care experiences and preparedness for survivorship among a diverse population of cancer survivors in New Jersey. Eur J Cancer Care (Engl). 2022;31(2):e13553. doi: 10.1111/ecc.13553. [DOI] [PubMed] [Google Scholar]
- 43.Timsina LR, Zarzaur B, Haggstrom DA, Jenkins PC, Lustberg M, Obeng-Gyasi S. Dissemination of cancer survivorship care plans: who is being left out? Support Care Cancer. 2021;29(8):4295–302. doi: 10.1007/s00520-020-05915-x. [DOI] [PubMed] [Google Scholar]
- 44.Blaes AH, Adamson PC, Foxhall L, Bhatia S. Survivorship Care Plans and the Commission on Cancer Standards: The Increasing Need for Better Strategies to Improve the Outcome for Survivors of Cancer. JCO Oncology Practice. 2020;16(8):447–50. doi: 10.1200/jop.19.00801. [DOI] [PubMed] [Google Scholar]
- 45.Dulko D, Pace CM, Dittus KL, Sprague BL, Pollack LA, Hawkins NA, et al. Barriers and facilitators to implementing cancer survivorship care plans. Oncol Nurs Forum. 2013;40(6):575–80. doi: 10.1188/13.Onf.575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White MC, Babcock F, Hayes NS, Mariotto AB, Wong FL, Kohler BA, et al. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer. 2017;123 Suppl 24:4969–76. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L, Deane J, Sharp L. Understanding survivors’ needs and outcomes: the role of routinely collected data. Curr Opin Support Palliat Care. 2018;12(3):254–60. doi: 10.1097/spc.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 48.Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a Quality of Cancer Survivorship Care Framework: Implications for Clinical Care, Research, and Policy. Journal of the National Cancer Institute. 2019;111(11):1120–30. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy restrictions.


