Abstract
Background:
Interferon-gamma release assays (IGRAs) are approved for children ≥2 years old to aid in diagnosis of Mycobacterium tuberculosis (TB) infection and disease. Tuberculin skin tests (TSTs) continue to be the recommended method for diagnosis of TB infection in children <2 years, in part due to limited data and concern for high rates of uninterpretable results.
Methods:
We performed a retrospective cohort study of IGRA use in patients <2 years old in two large Boston healthcare systems. The primary outcome was the proportion of valid versus invalid/indeterminate IGRA results. Secondary outcomes included concordance of IGRAs with paired TSTs and trends in IGRA usage over time.
Results:
A total of 321 IGRA results were analyzed; 308 tests (96%) were valid and 13 (4%) were invalid/indeterminate. Thirty-seven IGRAs were obtained in immunocompromised patients; the proportion of invalid/indeterminate results was significantly higher among immunocompromised (27%) compared with immunocompetent (1%) patients (P <0.001). Paired IGRAs and TSTs had a concordance rate of 64%, with most discordant results in bacille Calmette-Guérin (BCG)-vaccinated patients. The proportion of total TB tests that were IGRAs increased over the study period (Pearson correlation coefficient 0.85, P <0.001).
Conclusion:
The high proportion of valid IGRA test results in patients <2 years of age in a low TB prevalence setting in combination with the known logistical and interpretation challenges associated with TSTs support the adoption of IGRAs for this age group in certain clinical scenarios. Interpretation of IGRAs, particularly in immunocompromised patients, should involve consideration of the broader clinical context.
Background
Infants and young children are at higher risk of progression from Mycobacterium tuberculosis (TB) infection to disease compared to older children and adults. Specifically, children <2 years old with TB infection have a 25–50% risk of developing TB disease within one year of initial infection if not treated.1–3 Those with disease experience increased morbidity because of higher rates of extrapulmonary disease and less robust immune systems compared to older patients.
Accurate diagnosis of (“latent”) TB infection in young children is therefore crucial but poses challenges. Tuberculin skin tests (TSTs) and interferon gamma release assays (IGRAs) are indirect immunologic tests for TB infection. TSTs are recommended for use in children <2 years old but have several limitations. TSTs can be falsely positive in the setting of bacille Calmette-Guérin (BCG) vaccination or non-tuberculous mycobacterial infection. False negative tests can arise if patients are unable to mount an immune response to tuberculin, or if the test was improperly administered or read.4–8 Furthermore, TSTs require application and interpretation by a trained provider, and the sequential health care visits needed to administer and read TSTs are opportunities for loss to follow-up. Such follow-up may be particularly challenging for socio-demographically disadvantaged children who are often at higher risk of TB infection.8,9
IGRAs measure T cell response in blood to Mycobacterium tuberculosis complex-specific antigens. In contrast to TSTs, IGRAs do not react to BCG vaccine antigens,4,7 do not require sequential visits, and are not subject to variability in interpreter experience. In 2016, the Infectious Diseases Society of America recommended use of IGRAs instead of TSTs to test for TB infection for individuals ≥5 years who are at low risk for progression to TB disease; these recommendations have not yet been expanded to children <5 years old.10 In 2018, the American Academy of Pediatrics recommended expanding IGRA use from ≥5 years to ≥2 years, supported by reports of comparable sensitivity of IGRAs to TSTs in low prevalence settings.3,11,12 Although some pediatric TB experts use IGRAs in younger age groups,3,11 both the Infectious Diseases Society of America and the American Academy of Pediatrics continue to recommend TSTs for detection of TB infection in patients <2 years old.
Use of IGRAs for this age group has been historically limited by lack of clinical data and concern that younger patients may not mount appropriate immune responses for an adequate positive control leading to higher proportions of invalid/indeterminate results.13–15 IGRAs can yield invalid/indeterminate results if the positive or negative control fails because of patient characteristics or technical issues.3,12 More recent studies have reported feasibility and lower rates of invalid/indeterminate IGRAs in children <2 years old, but these studies primarily included immunocompetent patients.9,16 Additional real-world evidence, particularly among immunocompromised patients and those with medical complexity, is needed to better understand use and limitations of these tests among children <2 years old.
In this cohort study of children <2 years old tested for TB infection or disease using IGRAs in a low-prevalence setting, we evaluated rates of valid and invalid/indeterminate IGRA results and concordance with paired TSTs. We also investigated trends in IGRA usage over time among all patients <2 years old tested for TB.
Methods
Study design and data sources:
We conducted a retrospective cohort study including children <2 years old who had IGRA testing between October 1, 2015 and January 31, 2021 in two large Boston healthcare systems (System 1 and System 2). Two commercially available IGRAs were utilized at these institutions: the T-SPOT.TB test (T-SPOT) (Oxford Immunotec; used preferentially in System 1) and the QuantiFERON-TB Gold test (QFT) (Qiagen; used preferentially in System 2, which switched from QFT-In Tube [QFT-IT] to QFT-Plus in December 2018).
All patients <2 years old who had IGRA and/or TST documented in the electronic health record (EHR) within the study period were identified using an automated EHR review of laboratory ordering and result records. For this study, the main cohort consisted of all patients tested with an IGRA. The number of TSTs obtained during the study period was also recorded. IGRAs that were collected but not resulted (e.g., from unsuccessful lab draw, insufficient blood quantity drawn, or test unable to be performed without additional information provided) were documented but excluded from further analysis. Additionally, if a single patient had multiple IGRAs within the study period, repeat tests were noted but excluded from primary analysis.
Outcomes:
Possible T-SPOT results included positive, negative, borderline, and invalid. QFT results included positive, negative, and indeterminate. Our primary outcome was a “valid” IGRA test. We defined “valid” results as those that were positive, negative, or borderline. Borderline T-SPOTs pass the positive and negative controls and are thus clinically interpretable. We defined “non-interpretable” results as those that did not pass the positive or negative control, including invalid T-SPOTs and indeterminate QFTs. Test results were defined by the standard parameters determined by each assay type.17–19
Our secondary outcome was concordance of IGRA results with paired TST results when both tests were obtained within 4 weeks. Because specific measurement of TST induration was not routinely recorded in the EHR, we accepted TST interpretations documented by a clinician in the medical record. Concordance was defined as both tests being positive or negative. IGRAs that were invalid or indeterminate were classified as discordant when paired with a TST.
Finally, we reviewed records for documentation of TB disease by the date of the most recent clinical note in the EHR.
Covariates
For all patients who had an IGRA, we abstracted patient demographics (i.e., age, sex, country of birth), BCG vaccination status, recent reported travel/immigration, location of testing (inpatient, outpatient primary care, outpatient subspecialty clinic), and indication for testing.
Indication for testing was categorized as recent travel/immigration, high-risk domestic exposure, symptoms of TB disease (such as fever, cough, persistent lymphadenopathy), employment/school screening, impending immunocompromise, or other. Impending immunocompromise was defined as undergoing evaluation for bone marrow or solid organ transplant, or diagnosis of inflammatory condition with plan to start new immunomodulatory medication.
Additional data obtained from the EHR included comorbidities at the time of testing. Current immunocompromise was defined as hematologic malignancy not in remission, malignancy up to 6 months after completion of chemotherapy, primary immunodeficiency, asplenia, HIV infection (regardless of virologic suppression), auto-inflammatory conditions currently requiring immunomodulatory therapy, or any other administration of immunosuppressive medication at time of testing. If patients were receiving immunosuppressive medication, the type of medication (categorized as steroids, T cell depleting agents, B cell depleting agents, tumor necrosis factor [TNF]-alpha inhibitors, or interleukin inhibitors), dose of medication, and duration of time receiving the medication were recorded.
Statistical analysis:
We used proportions and descriptive statistics to summarize test results, patient characteristics, and testing locations, as well as to determine paired test concordance. We compared proportions of valid and indeterminate/invalid IGRA testing using Fisher’s exact test. We used the Pearson correlation coefficient to assess month of test and the proportion of IGRA tests over time. Analyses were conducted using Stata v17 and Microsoft Excel 365 v2202.
Ethics:
The Boston Children’s Hospital and MassGeneral Brigham institutional review boards approved this study. Informed consent was waived.
Results
We identified orders for 372 IGRAs, of which 339 were attempted to be collected as part of clinical care; there was no additional information available about the 33 tests that were ordered but not collected. Of these 339 attempted IGRAs, 8 did not yield a test result, and an additional 10 were repeat tests. After excluding these tests, 321 IGRA tests were included in the main analysis. Figure 1 displays the cohort selection and test results. Table 1 displays the demographic and clinical characteristics of included patients. Median age was 16 months (interquartile range [IQR] 1—20 months). Supplemental Table 1 describes characteristics of the 37 immunocompromised patients included in this study.
Figure 1.
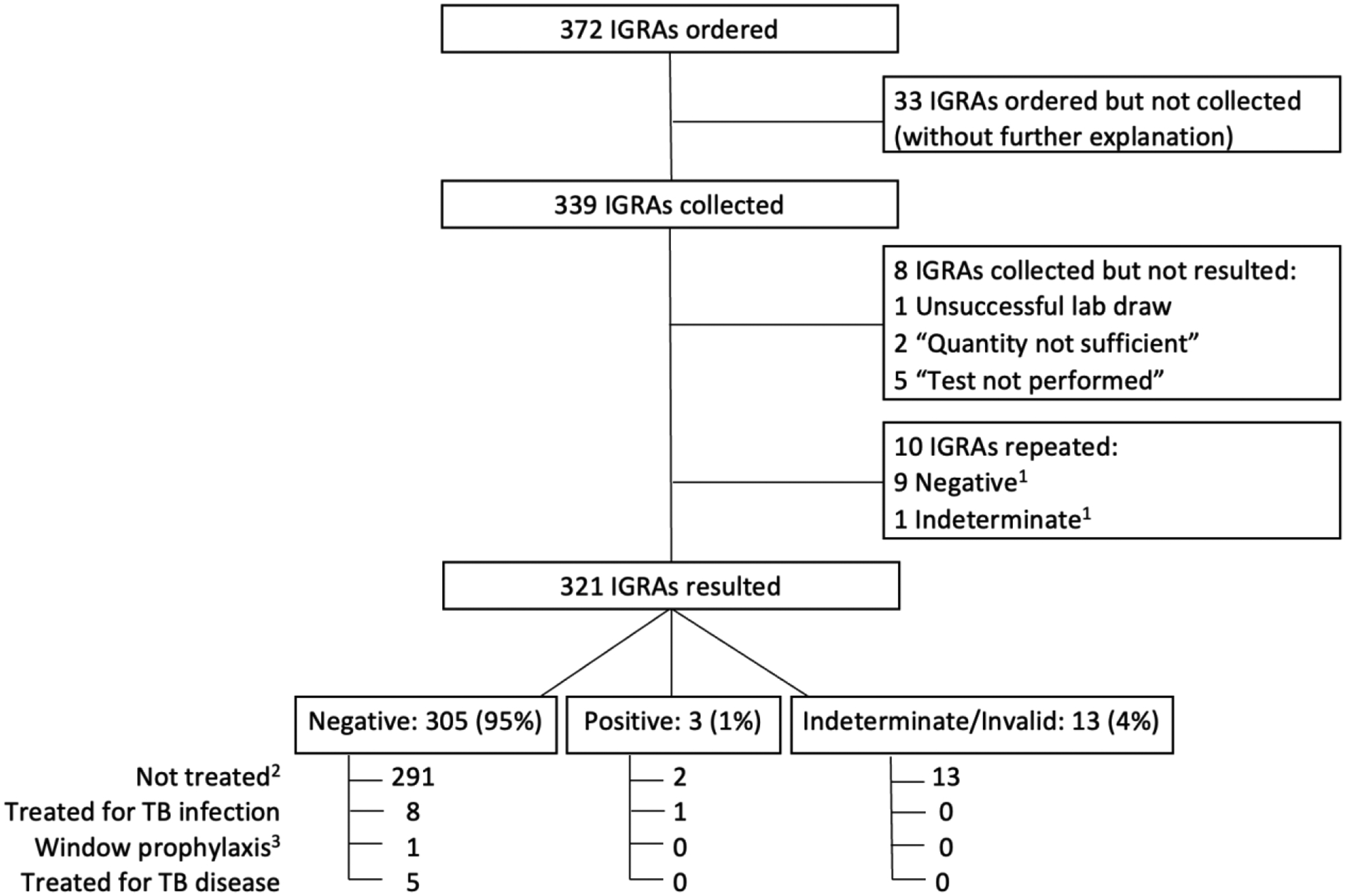
IGRA tests, results, and associated treatment among children younger than 2 years of age. There were no borderline T-SPOT results.
1A total of 10 tests were repeated among 9 patients. Three negative repeat tests followed an initial invalid IGRA. Two negative repeat IGRAs followed an initial positive IGRA. Four negative repeat tests followed initial negative IGRAs. The one indeterminate repeat test followed an initial negative IGRA result.
2 Two patients with positive IGRA results did not receive treatment. One patient had received a bone marrow transplant and it was suspected that the donor T cells were sensitized resulting in positive IGRA. The other patient was diagnosed with a primary immunodeficiency with initial positive T-SPOT and two subsequent negative T-SPOT tests. The first positive T-SPOT was thought to be a false positive result.
3 Window prophylaxis was defined as treatment for TB infection for close contact exposure despite negative TB testing.
Table 1:
Characteristics of patients younger than 2 years old who underwent IGRA testing (N=321)
| Characteristic | N (%) | Positive, N (%) | Negative, N (%) | Indeterminate/Invalid, N (%) | |
|---|---|---|---|---|---|
| Age (months) | 0 – <6 | 21 (7) | 0 (0) | 19 (90) | 2 (10) |
| 6 – <12 | 62 (19) | 1 (2) | 56 (90) | 5 (8) | |
| 12 – <18 | 94 (29) | 2 (2) | 88 (94) | 4 (4) | |
| 18 – <24 | 144 (45) | 0 (0) | 142 (99) | 2 (1) | |
| Sex at birth | Female | 141 (44) | 1 (1) | 132 (94) | 8 (6) |
| Male | 180 (56) | 2 (1) | 173 (96) | 5 (3) | |
| BCG vaccinated | Vaccinated | 47 (15) | 1 (2) | 45 (96) | 1 (2) |
| Unvaccinated | 190 (59) | 1 (1) | 177 (93) | 12 (6) | |
| Unknown/not documented | 84 (26) | 1 (1) | 83 (99) | 0 (0) | |
| Immunocompromised status | Immunocompromised | 37 (12) | 2 (5) | 25 (68) | 10 (27) |
| Immunocompetent | 284 (88) | 1 (0.4) | 280 (99) | 3 (1) | |
| Foreign born status | Born outside the United States | 64 (20) | 2 (3) | 59 (92) | 3 (5) |
| Born in the United States | 191 (60) | 1 (1) | 180 (94) | 10 (5) | |
| Unknown/not documented | 66 (21) | 0 (0) | 66 (100) | 0 (0) | |
| Location of testing | Primary care clinic | 143 (45) | 1 (1) | 141 (99) | 1 (1) |
| Subspecialty clinic | 60 (19) | 0 (0) | 57 (95) | 3 (5) | |
| Unknown clinic | 29 (9) | 0 (0) | 29 (100) | 0 (0) | |
| Inpatient | 86 (27) | 2 (2) | 75 (87) | 9 (10) | |
| Unknown | 3 (1) | 0 (0) | 3 (100) | 0 (0) | |
| Indication for TB testing1 | Post travel/new immigrant | 110 (33) | |||
| Impending immunocompromise2 | 92 (27) | ||||
| Symptoms of TB disease | 51 (15) | ||||
| High-risk domestic exposure | 9 (3) | ||||
| Employment/school screening | 3 (1) | ||||
| Other3 | 25 (7) | ||||
| Unknown/Not documented | 47 (14) | ||||
| Total | 337 | ||||
| Reason for ordering IGRA (vs TST) | Both IGRA and TST ordered at same time | 23 (7) | |||
| Ordered as follow up to positive TST | 11 (3) | ||||
| Patient was BCG vaccinated | 11 (3) | ||||
| Ease of obtaining IGRA relative to TST | 10 (3) | ||||
| Other4 | 7 (2) | ||||
| Unknown/not documented | 259 (81) | ||||
| Total | 321 | ||||
Several patients had multiple indications for testing. Therefore, these numbers sum to greater than total number of tests.
Impending immunocompromise included impending solid organ transplant (N=57, 17%), inflammatory disorder with plan to start new immunosuppressant medication (N=23, 7%), impending bone marrow transplant (N=6, 2%), and other (N=6, 2%). “Other” impending immunocompromise included patients without inflammatory disorders with plan to start immunomodulating medication.
“Other” indications for TB testing included “routine screening,” accidental test ordering, patients undergoing workup for disorder that could require treatment with immunosuppressants, new patient to practice (not immigrant), patient considered at risk (parents or family member born outside the United States, living in shelter, family member with positive TST), or required for travel visa.
“Other” reasons for ordering IGRA include concern that patient would not mount response to TST due to immunosuppression, recent live vaccination, and patient reportedly had a previous negative TST.
IGRA results
Of the 321 included IGRA tests, 308 (96%) test results were “valid,” and 13 (4%) tests were invalid (12 T-SPOT) or indeterminate (1 QFT-IT). Proportions of indeterminate results for combined QFTs (QFT-IT and QFT-Plus) and invalid results for T-SPOTs were similar (P = 0.47) (Supplemental Table 2). No T-SPOTs were borderline.
Of the 37 tests performed among immunocompromised patients, 27 (73%) tests were valid (2 positive, 25 negative), and 10 (27%) were indeterminate/invalid. Of the 284 tests performed in immunocompetent patients, 281 (99%) were valid (1 positive, 280 negative), and 3 (1%) were indeterminate/invalid. The proportion of patients with indeterminate/invalid results was significantly higher among immunocompromised patients (27%) compared with immunocompetent patients (1%) (P <0.001). Characteristics of immunocompromised patients with indeterminate/invalid test results are detailed in Supplemental Table 3. Of the 10 immunocompromised patients who had indeterminate/invalid results, 3 had repeat IGRAs within 1 month, all of which were negative.
Concordance with paired TSTs
Among the 321 patients who had IGRAs collected, 47 were paired with TSTs that had results documented within a 4-week period (an additional 12 TSTs were planted but not read, and one was planted >4 weeks after IGRA collection). Overall concordance of paired test results was 64% (Table 2a). Of all discordant results, 76% occurred among BCG-vaccinated patients (Table 2b).
Table 2.
a) Concordance of paired IGRA and TST results (N=47). Bolded numbers represent concordant results. b) BCG vaccination status among paired TST/IGRA results.
| IGRA+ | IGRA− | IGRA Indeterminate/Invalid | |
|---|---|---|---|
| TST+ | 0 | 14 | 0 |
| TST− | 1 | 30 | 2 |
| BCG vaccinated N (%) |
BCG unvaccinated N (%) |
Unknown N (%) |
||
|---|---|---|---|---|
| Concordant | TST−/IGRA− | 4 (13) | 20 (67) | 6 (20) |
| Discordant | TST+/IGRA− | 11 (79) | 3 (21)1 | 0 (0) |
| TST−/IGRA+ | 1 (100) | 0 (0) | 0 (0) | |
| TST−/IGRA indeterminate/invalid | 1 (50) | 1 (50) | 0 (0) |
No patients in this group were considered immunocompromised. Two patients were treated for TB infection, and one patient was ultimately diagnosed with M. genavense.
Clinical settings, indication for testing, and usage trends
IGRAs were obtained in outpatient and inpatient settings for a variety of indications (Table 1). Over the course of the study period, the proportion of all ordered TB tests that were IGRAs increased from 0.04 at the start to 0.36 at the end of the study (Pearson correlation coefficient between time and proportion IGRA = 0.85, P<0.001), with maximum proportion of 0.60 in December 2020. We observed increasing numbers of IGRAs and declining numbers of TSTs ordered, particularly after the onset of COVID-19 in March 2020 (Figure 2).
Figure 2.
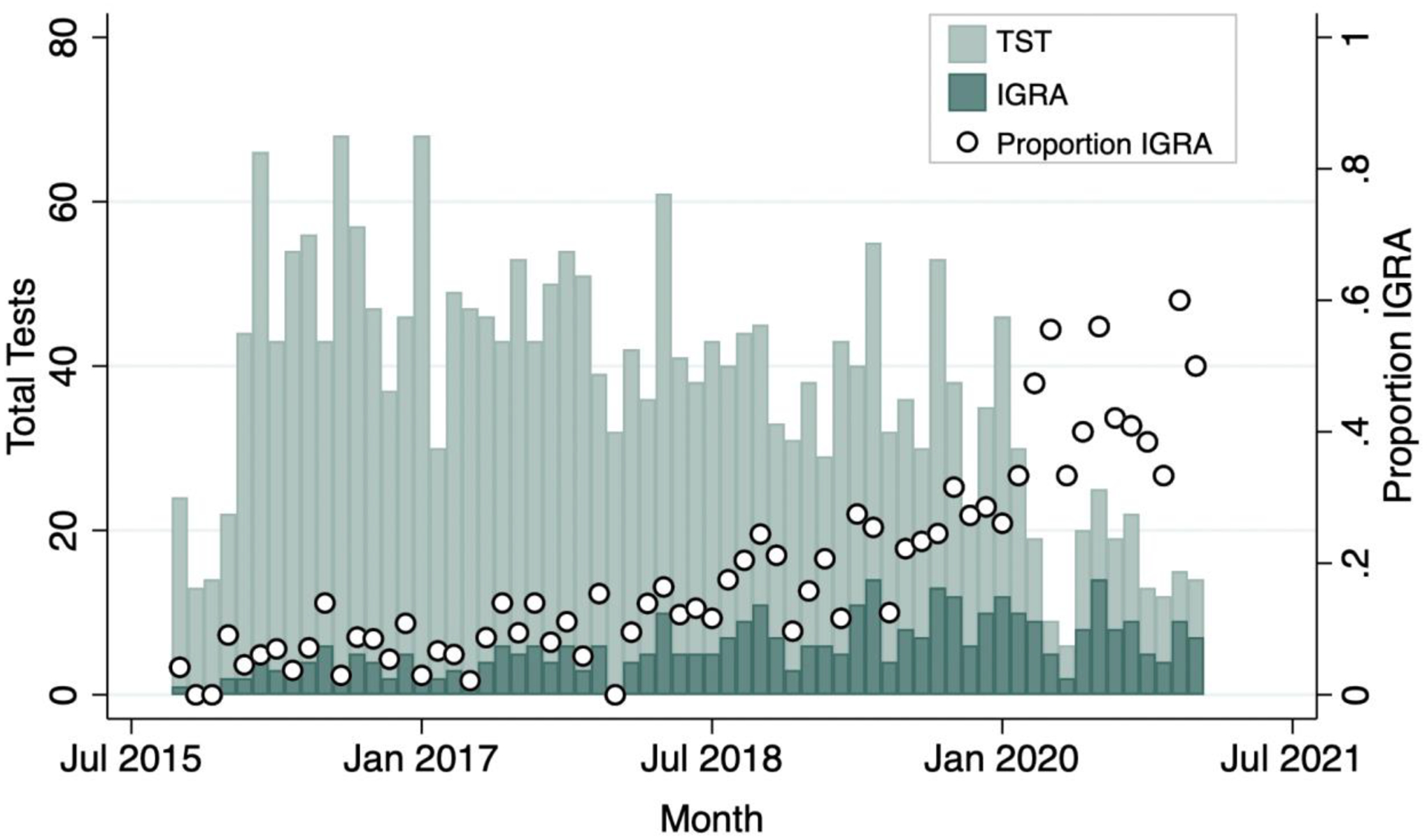
Total number of tests and proportion of obtained tests that were IGRAs by month from October 2015-January 2021.
Clinical outcomes
Treatment for TB disease and infection for included patients is presented in Figure 1. Only 1 patient who received TB infection treatment was considered immunocompromised at time of testing and had a negative IGRA.
A total of 5 included patients were started on treatment for TB disease, including 2 for extrapulmonary TB (one patient with TB osteomyelitis diagnosed and partially treated prior to testing in our setting, and one patient with presumed TB meningitis). The other 3 patients who initially started treatment for TB disease were ultimately diagnosed with disease from disseminated BCG, M. flavescens, and M. genavense; all had negative IGRAs.
Nine patients were treated for TB infection, and 1 patient received window prophylaxis.
Of 14 patients with positive TST but negative or invalid/indeterminate IGRAs, 7 received treatment for TB infection. No TB disease was identified among the 7 untreated patients over a median 665 days (IQR 53–1,046 days) of clinical follow-up.
Discussion:
While IGRAs for children <2 years old are not formally recommended, our study indicates that they are often used in this age group, and that they usually yield valid results among immunocompetent patients.
Obtaining IGRAs in children <2 years old was feasible in our real-world clinical setting. Only 4% of tests yielded indeterminate/invalid results. Adding the 8 IGRAs that did not yield results, and the 33 tests that were ordered but not obtained, the total proportion of IGRAs ordered without a valid result was 15%; this compares favorably to the 20% of TSTs paired with IGRAs in our cohort that were planted but never read. Two prior studies in patients <2 years old also report feasibility of obtaining IGRA tests: a cohort study of IGRAs in a U.S. primary care setting found that only 3/116 ordered IGRAs failed or were unable to be obtained,9 and a multicenter study in Italy identified only 12/835 incomplete results.16
The 4% overall rate of indeterminate/invalid results in our study was similar to several prior studies. The previously noted study of patients <2 years old in a U.S. primary care setting documented 3 indeterminate tests out of 116 QFTs analyzed (3%).9 The aforementioned study of immunocompetent children in Italy found that 4% of IGRA results were indeterminate.16 In a large-scale study of 2,797 immunocompetent BCG-vaccinated infants, QFTs were obtained at enrollment and at day 336; an overall indeterminate QFT rate of 0.4% was found.20 Other studies of IGRAs have shown higher rates of invalid/indeterminate results among children <2 years old. These studies primarily included a broad age range of pediatric patients and were limited by small sample sizes of patients <2 years old. For example, a study of 269 pediatric patients found high indeterminate rates among 55 immunocompetent (38%) and 11 immunocompromised (64%) children <2 years old.15 A separate study of children with laboratory-confirmed TB disease, including 36 children <2 years old, found that 14% of IGRA results were indeterminate.21
We included patients who received different IGRA types in our analysis. Prior studies have shown comparable performance of available commercial IGRAs in children.22,23 In our study, the proportion of invalid T-SPOTs was not significantly different from the combined proportion of indeterminate QFTs. Prior studies report varying proportions of invalid/indeterminate test results when comparing IGRA assays in pediatric patients.24,25 One review of pediatric studies found overall similar rates of invalid/indeterminate results when comparing T-SPOTs and QFTs, but a lower proportion of invalid results for T-SPOTs compared with QFTs in studies of non-HIV-infected immunocompromised patients.26
We found significantly higher rates of invalid/indeterminate IGRAs among immunocompromised compared with immunocompetent patients. Although immune compromise is generally recognized as a risk factor for indeterminate/invalid results, there are limited data on IGRA testing specifically among immunocompromised children <2 years old. In the previously mentioned study including 11 immunocompromised children <2 years old, 40% of IGRA tests were indeterminate among 5 immunocompromised patients <1 year old, and 83% of IGRAs were indeterminate among 6 immunocompromised patients ages 1–2 years.15 A meta-analysis and meta-regression of pediatric studies with a mean or median age <18 years old identified immunocompromised status as main contributor to indeterminate IGRAs among several variables including IGRA type, median or mean age group, and continent of study.26 To our knowledge, ours is the largest cohort study to date that specifically examines IGRA use among immunocompromised children <2 years old. Our findings contribute further evidence for the limitations of these tests for young immunocompromised patients.
Notably, invalid/indeterminate results may provide some information in the context of immunosuppression that is unavailable in patients tested with TST alone. Assuming the absence of user error, an invalid/indeterminate IGRA in an immunocompromised patient suggests that the patient is unable to mount an immune response. In contrast, a negative TST could indicate that the patient either does not have TB or is unable to mount a response to tuberculin. As a result, IGRAs may still have advantages compared with the use of TSTs alone.
We did not attempt to examine sensitivity and specificity of IGRAs for TB infection or disease in our cohort. Previous studies have documented false negative IGRA results among immunocompromised children diagnosed with TB disease.27 In vitro studies involving addition of immunosuppressing medication to blood assays have shown impaired IGRA performance,28 and some studies have shown a change in IGRA result from positive to negative with addition of these medications.29,30 These findings suggest that a negative IGRA test in an immunocompromised patient should be interpreted within a broader clinical context. Additionally, while immunocompromised status is a risk for invalid/indeterminate and false negative IGRAs, a similar risk of false negative results exists with TSTs in patients without robust immune responses.5
We observed lower concordance between IGRA and TST results (64%) than previous studies which found concordance rates of 91%-93% among patients <2 years old, with higher rates of concordance among BCG-unvaccinated patients.16,21 The lower concordance rates in our study are likely due to a high rate of BCG vaccination, with 76% of discordant tests occurring among BCG vaccinated patients. Additionally, in real-world use, clinicians may have been more likely to order “confirmatory” IGRAs in the setting of positive TSTs but not negative TSTs, thereby increasing the observed number of discordant IGRA tests.
We identified a trend of increasing use of IGRAs compared with TSTs in this cohort. This trend was largely driven by decreasing numbers of TSTs placed during the early months of the COVID-19 pandemic, potentially from reluctance to use tests requiring multiple follow-up visits, and likely reflecting broader TB and primary care service disruptions related to COVID-19.
Although our study was not designed to follow patients longitudinally, no patients with negative IGRA results had documentation of TB disease in medical records including 7 patients with positive TSTs and negative IGRAs who did not receive TB-directed therapy. Similarly, 2 prior pediatric studies reported no cases of TB disease among untreated patients with TST positive/IGRA negative results, including one study of 146 TST positive/IGRA negative patients <15 years old13 and one study that included 6 TST-positive/IGRA-negative patients <2 years old.9 These studies provide limited but consistent findings that negative IGRAs can be trusted for children <2 years old who have positive TSTs, given the otherwise high rate of TB progression among these young patients.
Our study has several limitations. It was performed in a low TB prevalence setting, so conclusions may not be generalizable to higher prevalence settings where pretest probability of TB is higher and more technical barriers to obtaining IGRAs may exist. Additionally, most IGRA test results were qualitative, so we were unable to perform quantitative analysis of tube values for QFTs or well values for T-SPOTs. Finally, because this study was retrospective and relied on routine clinical data, we were unable to capture complete follow-up information about interval TB diagnoses.
Conclusion:
We observed a high proportion of valid IGRA results among patients <2 years old in a setting with a low TB prevalence. We also observed a significantly higher proportion of indeterminate/invalid results among young immunocompromised patients. Our results add to evidence supporting use of IGRAs for evaluation of TB infection in patients younger than 2 years in specific clinical contexts. However, higher rates of invalid or indeterminate results limit the utility of IGRAs in young immunocompromised children.
Supplementary Material
Funding/Support:
Dr. Campbell was supported by Agency for Healthcare Research and Quality grant number T32 HS000063 as part of the Harvard-wide Pediatric Health Services Research Fellowship Program.
Contributor Information
Mary E. Tabatneck, Department of Pediatrics, Boston Children’s Hospital, Boston, Massachusetts, USA.
Mingwei Sun, Center for Research Information Technology, Boston Children’s Hospital, Boston, Massachusetts, USA.
Wei He, Center for Research Information Science and Computing, Massachusetts General Hospital, Boston, Massachusetts, USA.
Gabriella S. Lamb, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA.
Don Goldmann, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA.
Vishakha Sabharwal, Division of Pediatric Infectious Diseases, Boston Medical Center, Boston, Massachusetts, USA.
Thomas J. Sandora, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA.
Jessica E. Haberer, Center for Global Health, Massachusetts General Hospital, Boston, Massachusetts, USA.
Jeffrey I. Campbell, Division of Infectious Diseases, Boston Children’s Hospital, Boston, Massachusetts, USA; and Division of Pediatric Infectious Diseases, Boston Medical Center, Boston, Massachusetts, USA.
References
- 1.Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367(4):348–61. [DOI] [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med. 2006;173(10):1078–90. [DOI] [PubMed] [Google Scholar]
- 3.Nolt D, Starke JR. Tuberculosis Infection in Children and Adolescents: Testing and Treatment. Pediatrics. 2021;148(6). [DOI] [PubMed] [Google Scholar]
- 4.Ling DI, Zwerling AA, Steingart KR, Pai M. Immune-based diagnostics for TB in children: what is the evidence? Paediatr Respir Rev. 2011;12(1):9–15. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg PJ, Temesgen Z, Banerjee R. Tuberculosis in Children. Pediatr Rev. 2019;40(4):168–178. [DOI] [PubMed] [Google Scholar]
- 6.AM M, A. D. Diagnosis of tuberculosis infection in children. In: JR S, PR D, eds. Handbook of Child & Adolescent Tuberculosis New York, NY: Oxford University Press; 2016:79–96. [Google Scholar]
- 7.Detjen AK, Keil T, Roll S, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45(3):322–8. [DOI] [PubMed] [Google Scholar]
- 8.Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 9.Gaensbauer J, Young J, Harasaki C, Aiona K, Belknap R, Haas MK. Interferon-Gamma Release Assay Testing in Children Younger Than 2 Years in a US-Based Health System. Pediatr Infect Dis J. 2020;39(9):803–807. [DOI] [PubMed] [Google Scholar]
- 10.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. 2017;64(2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatrics AAo. Tuberculosis. In: Kimberlin DW, Brady, et al. , eds. Red Book: 2018 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2018:829–853. [Google Scholar]
- 12.Committee on Infectious Diseases AAoP. Tuberculosis. In: Kimberlin DW, MD, FAAP, Barnett Elizabeth D. M, FAAP, et al. , eds. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd ed.: 2021:786–814. [Google Scholar]
- 13.Grinsdale JA, Islam S, Tran OC, Ho CS, Kawamura LM, Higashi JM. Interferon-Gamma Release Assays and Pediatric Public Health Tuberculosis Screening: The San Francisco Program Experience 2005 to 2008. J Pediatric Infect Dis Soc. 2016;5(2):122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Critselis E, Amanatidou V, Syridou G, et al. The effect of age on whole blood interferon-gamma release assay response among children investigated for latent tuberculosis infection. J Pediatr. 2012;161(4):632–8. [DOI] [PubMed] [Google Scholar]
- 15.Haustein T, Ridout DA, Hartley JC, et al. The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J. 2009;28(8):669–73. [DOI] [PubMed] [Google Scholar]
- 16.Garazzino S, Galli L, Chiappini E, et al. Performance of interferon-γ release assay for the diagnosis of active or latent tuberculosis in children in the first 2 years of age: a multicenter study of the Italian Society of Pediatric Infectious Diseases. Pediatr Infect Dis J. 2014;33(9):e226–31. [DOI] [PubMed] [Google Scholar]
- 17.QIAGEN. QuantiFERON-TB Gold (QFT) Kit. Available at: https://www.qiagen.com/cn/products/diagnostics-and-clinical-research/infectious-disease/quantiferon-tb-gold-test/. Accessed June 8, 2022.
- 18.Inc OI. T-Spot.TB Package Insert. Available at: https://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/Final-File-PI-TB-US-V6.pdf. Accessed June, 8, 2022.
- 19.Diagnostics Q. QuantiFERON TB Gold Plus. Available at: https://www.questdiagnostics.com/healthcare-professionals/clinical-education-center/faq/faq204. Accessed July 8, 2022.
- 20.Andrews JR, Nemes E, Tameris M, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med. 2017;5(4):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay AW, Islam SM, Wendorf K, Westenhouse J, Barry PM. Interferon-γ Release Assay Performance for Tuberculosis in Childhood. Pediatrics. 2018;141(6). [DOI] [PubMed] [Google Scholar]
- 22.Venkatappa TK, Punnoose R, Katz DJ, et al. Comparing QuantiFERON-TB Gold Plus with Other Tests To Diagnose Mycobacterium tuberculosis Infection. J Clin Microbiol. 2019;57(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay AW, DiNardo AR, Dlamini Q, et al. Evaluation of the QuantiFERON-Tuberculosis Gold Plus Assay in Children with Tuberculosis Disease or Following Household Exposure to Tuberculosis. Am J Trop Med Hyg. 2019;100(3):540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connell TG, Ritz N, Paxton GA, Buttery JP, Curtis N, Ranganathan SC. A three-way comparison of tuberculin skin testing, QuantiFERON-TB gold and T-SPOT.TB in children. PLoS One. 2008;3(7):e2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergamini BM, Losi M, Vaienti F, et al. Performance of commercial blood tests for the diagnosis of latent tuberculosis infection in children and adolescents. Pediatrics. 2009;123(3):e419–24. [DOI] [PubMed] [Google Scholar]
- 26.Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M, Ritz N. Risk Factors for Indeterminate Interferon-Gamma Release Assay for the Diagnosis of Tuberculosis in Children-A Systematic Review and Meta-Analysis. Front Pediatr. 2019;7:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noguera-Julian A, Calzada-Hernández J, Brinkmann F, et al. Tuberculosis Disease in Children and Adolescents on Therapy With Antitumor Necrosis Factor-ɑ Agents: A Collaborative, Multicenter Paediatric Tuberculosis Network European Trials Group (ptbnet) Study. Clin Infect Dis. 2020;71(10):2561–2569. [DOI] [PubMed] [Google Scholar]
- 28.Edwards A, Gao Y, Allan RN, et al. Corticosteroids and infliximab impair the performance of interferon-γ release assays used for diagnosis of latent tuberculosis. Thorax. 2017;72(10):946–949. [DOI] [PubMed] [Google Scholar]
- 29.Clifford V, Zufferey C, Germano S, et al. The impact of anti-tuberculous antibiotics and corticosteroids on cytokine production in QuantiFERON-TB Gold In Tube assays. Tuberculosis (Edinb). 2015;95(3):343–9. [DOI] [PubMed] [Google Scholar]
- 30.Barton E, Gao Y, Ball D, et al. Calcineurin Inhibitors and Variation in the Performance of Interferon-γ Release Assays Used to Detect Tuberculosis Infection. Ann Am Thorac Soc. 2019;16(6):771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


