Abstract
We report a patient presenting with unique neuroophthalmological features of contraversive ocular tilt reaction and concomitant contralesional pseudo-abducens palsy. Magnetic resonance imaging confirmed the presence of an acute infarct in the right thalamomesencephalic region. We discuss the clinical topography of these unique neuroophthalmological findings.
Keywords: Ocular motility, Stroke, Vertical gaze, Midbrain, Ophthalmoplegia, Oculomotor palsy, Interstitial nucleus of Cajal
Introduction
The nucleus prepositus hypoglossi and medial vestibular nuclei located at the caudal pons and dorsal rostral medulla facilitate neural integration for horizontal gaze holding [1, 2]. Similarly, the interstitial nucleus of Cajal (INC) located at the rostral midbrain caudal to the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) is the primary neural center for the integration (gaze holding) of vertical saccadic and torsional conjugate eye movements and eye-head coordination [1, 2].
Both riMLF and INC project – via the posterior commissure – to the contralateral trochlear nucleus and oculomotor neuron subnuclei for elevation, as well as to their contralateral riMLF and INC. The riMLF harbors most of the excitatory burst neurons for vertical saccades and projects bilaterally to the oculomotor subnuclei for eye elevation but only unilaterally to the nuclei for eye depression (superior oblique nucleus, trochlear nucleus) and inferior rectus subnucleus (oculomotor nucleus). Clinically, an INC lesion may be suspected only by the presence of a vertical and rotatory nystagmus of the ipsilateral eye [2]. Besides the INC, cerebellum and several cerebral cortical areas (parietal, occipital, and temporal lobes) also contribute to ocular motor integration [3, 4].
We describe the clinico-neuroradiological correlation of a patient with a right paramedian thalamomesencephalic lesion affecting the ipsilateral INC presenting with a contralateral head tilt and concomitant contralesional pseudo-abducens palsy, which is a unique neuroophthalmological association. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000531085).
Case Presentation
A 56-year-old man presented with a 4-day history of sudden-onset binocular diplopia, vertigo, and tendency to fall to the left side. He had a history of untreated hypertension and smoked for several years when he was young. He was on warfarin as a treatment for atrial fibrillation but was not compliant to his therapy. On admission, neurological examination showed an alert patient with intact cognitive functions. The oculomotor disturbances described below were observed. There was no motor or sensory deficit. There was no dysmetria or diadochokinesis. He was unable to sit straight and fell toward the left without support. He was able to walk with minimal support but swayed toward the left. General examination revealed an afebrile patient with a blood pressure of 141/89 mm Hg, a regular pulse rate of 82/min, and oxygen saturation of 99%. No bruits were heard over the arteries, and findings on cardiopulmonary examination were normal.
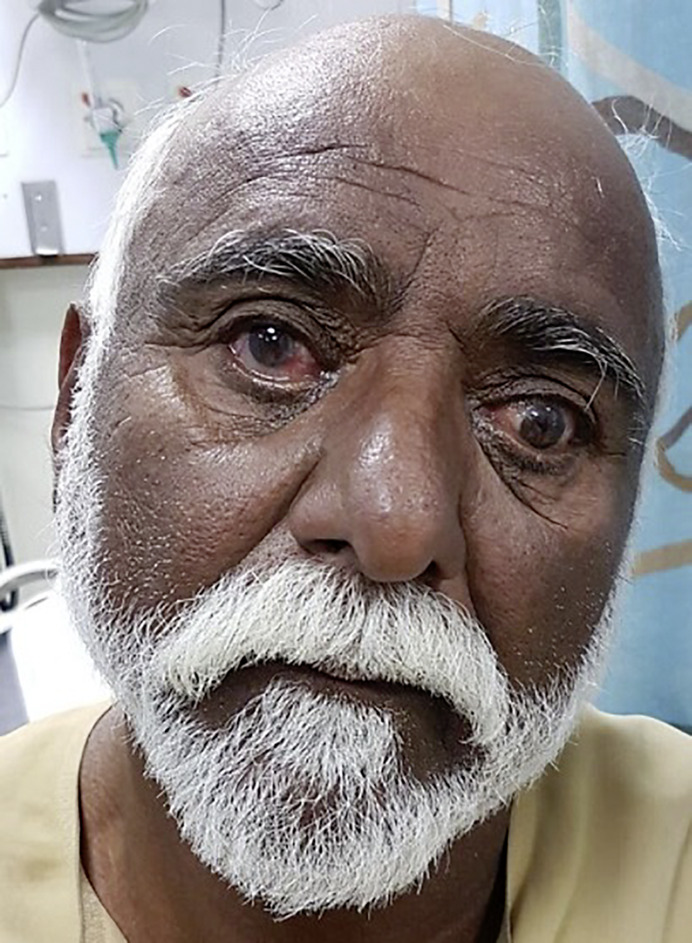
Neuroophthalmological examination revealed visual acuity 6/6 bilaterally and intact visual fields on confrontation. There was a head tilt toward the left (shown in Fig. 1). Pupillary responses were symmetric and reactive to light. Skew deviation with right-eye hypertropia and the left-eye abducted and down was noted on primary gaze straight ahead (Fig. 1) with rotatory nystagmus toward the left (clockwise torsion of both eyes). Horizontal voluntary saccades and smooth pursuit were preserved, but there was limited abduction of the left eye. Vertical saccades were slowed, downward more than upward, and vertical oculomotor range was limited (upward and downward about 15 deg), but vertical vestibulo-ocular reflex elicited a full range of vertical eye movements. Vertical pursuit was moderately saccadic, more for downward than upward pursuit.
Fig. 1.
Left OTR and skew deviation with right-eye hypertropia and the left-eye abducted and down on primary gaze straight ahead.
Oculocephalic movements in the vertical plane were absent and Bell’s phenomenon was bilaterally absent. There was limited abduction of the left eye on horizontal gaze (pseudo-abducens palsy). Convergence was diminished. Lid movements appeared normal. Optokinetic stimuli elicited no horizontal or vertical movements.
Laboratory investigation showed mild normocytic normochromic anemia, elevated glucose (18.83 mmol/L) and creatinine at 217 mmol/L. The international normalized ratio was 1.6 (subtherapeutic). Electrocardiogram showed atrial fibrillation with a ventricular response at 82/min.
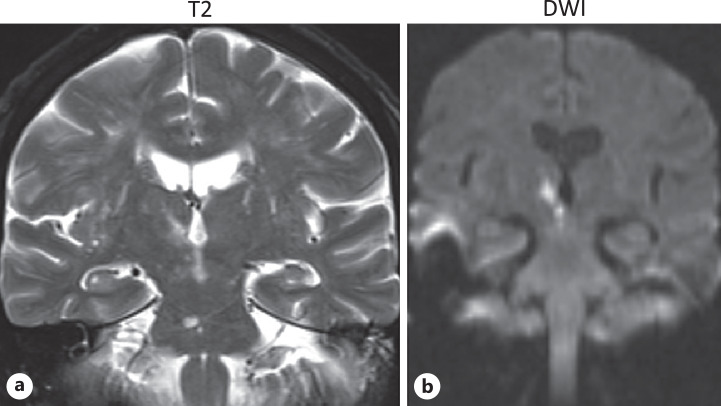
Brain MRI, as seen on T2WI, FLAIR, and DWI, revealed a small paramedian infarct in the right side of the thalamus and rostral midbrain (shown in Fig. 2). On serial imaging, there was evidence of an old pontine infarct. MRA of intracranial vessels revealed a stenotic lesion distally in the left posterior cerebral artery (PCA) and narrowed right P2 segment.
Fig. 2.
Brain MRI. a Coronal T2-weighted image. b Diffusion-weighted image demonstrating the right periventricular mesodiencephalic infarct.
Transthoracic echocardiography showed an ejection fraction of 50% with no significant changes. No cardiac masses or thrombi were observed. Because of difficulties in anticoagulation monitoring, he was started on aspirin. Antiplatelet therapy was started.
Discussion
The triad of ocular torsion, skew deviation, and head tilt is known as the ocular tilt reaction (OTR) [5]. OTR may originate from lesions anywhere along the otolith-ocular pathway, including the labyrinth, vestibular nerve, medial longitudinal fasciculus (MLF), pons, and midbrain.
Skew deviation is a vertical misalignment of the eyes resulting from an imbalance in tonic signals within the otolith-ocular reflex pathways. Patients with a skew deviation complain of binocular vertical diplopia and associated cyclorotation of both eyes with an impression of visual vertical tilt. Complete OTR indicates a unilateral deficit of otolith input or a unilateral lesion in the graviceptive projections – located in the MLF – from the vestibular nuclei to the contralateral INC and riMLF and ultimately arriving in the posterolateral thalamus. The projecting pathway crosses the midline at the pontomedullary level where the vestibular nuclei are localized. Classically, two types of OTR have been described; the ipsiversive type (ipsilateral eye lowermost, ocular torsion and head tilt toward the side of lesion) resulting from unilateral peripheral or pontomedullary lesions involving the medial and/or superior vestibular nuclei below the decussation; and, as in our case, the contraversive type (contralateral eye lowermost, ocular torsion and head tilt away from the side of lesion) resulting from unilateral pontomesencephalic brainstem lesions above the decussation, indicating the involvement of the MLF or INC and riMLF. At the level of the pons and depending on the location of the lesion below or above the decussation, OTR may be either ipsiversive or contraversive [5–8]. Finally, an isolated INC lesion with OTR is contraversive, but a posterolateral thalamic lesion can be ipsiversive or contraversive presumably related to bilateral vestibular input to the thalamus [9].
Perforating paramedian thalamic arteries arising from the proximal P1 peduncular segment of the PCA supply the paramedian thalamus. These perforating branches also provide blood supply to the riMLF and INC. In our case, the infarction was restricted to the paramedian part of the right thalamus extending in the right rostral part of the midbrain. The MRA showed an absent distal part of the left PCA and a narrowed P2 segment of the right PCA which explained the infarction of the area corresponding to the paramedian thalamomesencephalic artery.
Our patient was ataxic with gait deviation and falling (thalamic astasia) toward the unaffected side during walking despite the lack of motor weakness or pyramidal tract signs. It has been suggested that this balance disorder resulted from involvement of vestibular projections to the INC [6, 10].
Since there was no simultaneous acute microvascular lesion in the contralateral pons, affecting the abducens nerve or its fibers, the abduction deficit observed in our patient was most likely due to pseudo-abducens palsy which is commonly associated with supranuclear vertical gaze palsy [11–13]. These abduction deficits associated with upgaze palsy occur in lesions involving [11, 14] the frontopontine horizontal gaze pathway situated adjacent to the riMLF/INC and projecting to the abducens nucleus [15, 16]; the internuclear fibers from the medial rectus oculomotor subnucleus to the contralateral abducens nucleus producing contralesional abduction deficit [17]; and finally, in lesions affecting the inhibitory convergence pathways traveling to the midbrain “near-response” neurons or injury to pathways or neurons that compose the vergence integrator increased convergence activity [18]. The descending pathways for convergence travel through the paramedian thalamus and inhibit the contralateral premotor vergence neurons in the midbrain. Subsequently, these neurons project to the ipsilateral medial rectus oculomotor subnucleus. In our patient, the right-sided thalamomesencephalic infarct affected this descending inhibitory pathway before decussation and is hence the only one which can account for a contralesional pseudo-abducens palsy. The convergence neurons are located a few millimeters dorsolateral to the oculomotor nucleus, and involvement of these neurons without involvement of the oculomotor nuclei as a cause of abducens palsy is highly unlikely. Convergence spasm was reported in the literature in retrospective studies, correlating the clinical findings of ocular deviation along with a CT finding of thalamic hemorrhage [19, 20].
Conclusion
In conclusion, we presented with unique neuroophthalmological presentation consisting of contralateral head tilt and a concomitant contralesional pseudo-abducens palsy right paramedian thalamomesencephalic lesion caused by an ipsilateral INC lesion.
Acknowledgments
We would like to thank the Department of Neurology and Family Medicine at Hamad Medical Corporation. Open access fees was funded by the Qatar National Library.
Statement of Ethics
The patient has given a written informed consent to publish the case (including publication of images). The case was approved by the Abhath Research Approval Center, under Hamad Medical Corporation (MRC-04-18-236).
Conflict of Interest Statement
The authors report no conflicts of interest.
Funding Sources
No funding was required.
Author Contributions
Mohamad Fateh Dabbagh participated in drafting the manuscript. Lina Okar participated in drafting the manuscript and reviewing the literature. Dirk Deleu participated in drafting the work and critically revising it for important intellectual content. Boulenouar Mesraoua contributed to final approval of the version to be published.
Funding Statement
No funding was required.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Leigh RJ, Zee DS. The neurology of eye movements. 5th ed.USA, New York: Oxford Univ Press; 2015. [Google Scholar]
- 2. Rowe F. Supranuclear and internuclear control of eye movements: a review. Br Orthopt J. 2003;60:2–9. [Google Scholar]
- 3. Karatas M. Internuclear and supranuclear disorders of eye movements: clinical features and causes. Eur J Neurol. 2009;16(12):1265–77. 10.1111/j.1468-1331.2009.02779.x. [DOI] [PubMed] [Google Scholar]
- 4. Shaikh AG, Ghasia FF. Gaze holding after anterior-inferior temporal lobectomy. Neurol Sci. 2014;35(11):1749–56. 10.1007/s10072-014-1825-2. [DOI] [PubMed] [Google Scholar]
- 5. Halmagyi GM, Brandt T, Dieterich M, Curthoys IS, Stark RJ, Hoyt WF. Tonic contraversive ocular tilt reaction due to unilateral meso-diencephalic lesion. Neurology. 1990;40(10):1503–9. 10.1212/wnl.40.10.1503. [DOI] [PubMed] [Google Scholar]
- 6. Dieterich M, Brandt T. Thalamic infarctions: differential effects on vestibular function in the roll plane (35 patients). Neurology. 1993;43(9):1732–40. 10.1212/wnl.43.9.1732. [DOI] [PubMed] [Google Scholar]
- 7. Brandt T, Dieterich M. Vestibular syndromes in the roll plane: topographic diagnosis from brainstem to cortex. Ann Neurol. 1994;36(3):337–47. 10.1002/ana.410360304. [DOI] [PubMed] [Google Scholar]
- 8. Dieterich M, Brandt T. Wallenberg’s syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann Neurol. 1992;31(4):399–408. 10.1002/ana.410310409. [DOI] [PubMed] [Google Scholar]
- 9. Barth A, Bogousslavsky J, Caplan LR. Thalamic infarcts and hemorrhage. 2nd ed. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. Cambridge University Press; 2001. [Google Scholar]
- 10. Rambold H, Helmchen C, Büttner U. Vestibular influence on the binocular control of vertical-torsional nystagmus after lesions in the interstitial nucleus of Cajal. Neuroreport. 2000;11(4):779–84. 10.1097/00001756-200003200-00025. [DOI] [PubMed] [Google Scholar]
- 11. Pullicino P, Lincoff N, Truax BT. Abnormal vergence with upper brainstem infarcts: pseudoabducens palsy. Neurology. 2000;55(3):352–8. 10.1212/wnl.55.3.352. [DOI] [PubMed] [Google Scholar]
- 12. Thurtell MJ, Leigh RJ, Halmagyi GM. Monocular ophthalmoplegia and partial supranuclear vertical gaze palsy due to unilateral paramedian rostral midbrain infarction. J Neurol. 2009;256(4):664–6. 10.1007/s00415-009-0103-3. [DOI] [PubMed] [Google Scholar]
- 13. Mesraoua B, Deleu D, D’souza A, Imam YZ, Melikyan G. Neurocysticercosis presenting as a vertical one-and-a-half syndrome with associated contralesional horizontal gaze paresis. J Neurol Sci. 2012;323(1–2):250–3. 10.1016/j.jns.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 14. Bender MB. Brain control of conjugate horizontal and vertical eye movements: a survey of the structural and functional correlates. Brain. 1980;103(1):23–69. 10.1093/brain/103.1.23. [DOI] [PubMed] [Google Scholar]
- 15. Masdeu JC, Rosenberg M. Midbrain-diencephalic horizontal gaze paresis. J Clin Neuro Ophthalmol. 1987;7(4):227–34. 10.3109/01658108709007457. [DOI] [PubMed] [Google Scholar]
- 16. Leichnetz GR. The prefrontal cortico-oculomotor trajectories in the monkey. J Neurol Sci. 1981;49(3):387–96. 10.1016/0022-510x(81)90029-0. [DOI] [PubMed] [Google Scholar]
- 17. Clendaniel RA, Mays LE. Characteristics of antidromically identified oculomotor internuclear neurons during vergence and versional eye movements. J Neurophysiol. 1994;71(3):1111–27. 10.1152/jn.1994.71.3.1111. [DOI] [PubMed] [Google Scholar]
- 18. Choi KD, Jung DS, Kim JS. Specificity of “peering at the tip of the nose” for a diagnosis of thalamic hemorrhage. Arch Neurol. 2004;61(3):417–22. 10.1001/archneur.61.3.417. [DOI] [PubMed] [Google Scholar]
- 19. Walshe TM, Davis KR, Fisher CM. Thalamic hemorrhage: a computed tomographic-clinical correlation. Neurology. 1977 Mar;27(3):217–22. 10.1212/wnl.27.3.217. [DOI] [PubMed] [Google Scholar]
- 20. Barraquer-Bordas L, Illa I, Escartin A, Ruscalleda J, Marti-Vilalta JL. Thalamic hemorrhage. A study of 23 patients with diagnosis by computed tomography. Stroke. 1981 Jul-Aug;12(4):524–7. 10.1161/01.str.12.4.524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.