Abstract
Background
There is considerable variation in reported chronic obstructive pulmonary disease (COPD) prevalence internationally, partly due to differing definitions in use. Accurate estimates of disease prevalence are important for allocation of health-care resources, yet UK estimates of COPD prevalence have not been updated for a decade. We calculated yearly COPD prevalence in England between 2000 and 2019 using different definitions of COPD.
Methods
We used routinely collected primary care electronic healthcare record (EHR) data from the Clinical Practice Research Datalink (CPRD) Aurum database linked with secondary care data from the Hospital Episode Statistics (HES) Admitted Patient Care (APC) database. Mid-year point prevalence was calculated yearly from 2000 to 2019 in English adults aged ≥40 years using 5 definitions: (i) validated COPD, (ii) Quality and Outcomes Framework (QOF) COPD, (iii) COPD symptoms, inhaler prescription, and no asthma diagnosis, (iv) hospitalisation with COPD as any diagnosis, (v) hospitalisation with COPD as primary or secondary diagnosis. Prevalence was further stratified by gender, age group, and region.
Results
A total of 12,745,793 people were included over the 20-year period. Annual cohort sizes ranged from 4,373,538 in 2000 to 6,159,496 in 2019. Estimates of COPD prevalence increased every year from 2000 and the difference in estimated prevalence between the validated and QOF definitions has grown over time. In 2019, a COPD prevalence of 4.9% was found using validated events in either primary or secondary care (definition 1 or definition 5). Additionally, including potentially undiagnosed cases (definition 3) in the COPD definition produced an increased prevalence of 6.7%.
Conclusion
Common definitions of COPD (eg, QOF codes), may underestimate the true prevalence. The extent of this underestimate has increased over time and could lead to under-allocation of resources where need is estimated based on these definitions. Standardisation of COPD coding in routine EHRs and metrics such as spirometry is key to accurate disease monitoring.
Keywords: prevalence, England, chronic obstructive pulmonary disease, COPD, clinical practice research datalink, CPRD
Background
Chronic obstructive pulmonary disease (COPD) is a chronic condition characterised by progressive airflow obstruction, which is not completely reversible.1,2 Airflow limitation may precede the development of significant symptoms of COPD by many years and its progression is directly linked to the continuing exposure to risk factors, particularly tobacco smoking. As COPD is difficult to diagnose clinically (without spirometry) in its milder forms, it is often diagnosed late - the average age at diagnosis of COPD in the UK is 67 years.2 Widespread use of spirometry to allow early detection of airflow obstruction has been increasingly advocated as it enables early management of COPD.3
There is considerable variation in the reported prevalence of COPD internationally. One reason for this is the differing definitions in use. The British Thoracic Society (BTS) criteria are based on the post bronchodilator values of forced expiratory volume in 1 second (FEV1) and the forced vital capacity (FVC), ie, FEV1/ FVC <0.70 and FEV1 <80% predicted, using British reference values derived from the Health Survey for England (HSfE). The National Institute for Health and Care Excellence (NICE) COPD guideline,4 originally developed using the BTS criteria and last updated in 2019, states that COPD should be diagnosed in people with clinical symptoms confirmed by airflow obstruction, defined as a reduced FEV1/FVC ratio less than 0.7.
There is no consensus regarding using a fixed threshold to define airflow obstruction versus using the lower limit of normal (LLN) adjusted for age.5 Accurate estimates of disease prevalence are important for allocation of health-care resources, yet estimates of COPD prevalence in the UK have not been updated for a decade.6 Therefore, using routinely collected electronic healthcare record (EHR) data, we calculated yearly prevalence of COPD in England between 2000 and 2019 using multiple definitions of COPD. We then used validated definitions to calculate COPD and potentially undiagnosed COPD prevalence, stratified by age, gender, and region.
Methods
Population/Database
We used data from the May 2021 build7 of Clinical Practice Research Datalink (CPRD) Aurum,8,9 a large UK primary care electronic healthcare record (EHR) database containing current data for approximately 20% of the UK population along with historic data for almost 40 million patients.7 CPRD Aurum is considered representative of the English population in terms of age, gender, deprivation, and regional distribution.9 These primary care data were linked10,11 with 2015 English Index of Multiple Deprivation (IMD) data12,13 and secondary care data from NHS England’s Hospital Episode Statistics (HES) Admitted Patient Care (APC) database14,15 to generate annual cohorts of English primary care patients ≥40 years old for each calendar year from 2000 to 2019. An age of at least 40 years was required as previous estimates of COPD prevalence found very few people under 40 years diagnosed with COPD6 and people under 40 years are more likely to have alpha‑1 antitrypsin deficiency.4 Patients ineligible for linkage11 to HES were excluded from the cohorts. The population was restricted to male and female patients only due to low numbers of patients with indeterminate or unknown gender information.
Variable Definitions
All SNOMED CT (primary care) and ICD-10 (secondary care) codes used to define the variables used in this study can be found at https://github.com/NHLI-Respiratory-Epi/COPD_prevalence.
COPD Population
We determined COPD using 3 methods in primary care and 2 methods in the linked secondary care data:
Primary care: a COPD definition validated for use in primary care EHR databases.16
Primary care: the Quality and Outcomes Framework (QOF) COPD business rules definition. The QOF is a primary care pay-for-performance scheme: general practices receive points for completing specific items of care within a certain timeframe. The proportion of maximum possible points received in a year determines the size of financial bonus received at the end of the year.17
Primary care: to find potentially undiagnosed COPD we looked for a history of smoking, at least 1 respiratory symptom (cough, dyspnoea, or sputum production), a prescription for an inhaler, and no diagnosis of asthma.
Secondary care: an admission with COPD or emphysema as a primary, secondary, or comorbidity diagnosis (any diagnosis position in the HES record).
Secondary care: an admission with COPD or emphysema as the primary or secondary diagnosis only.
People with a diagnosis of COPD (Definition 1 or Definition 2) before their 40th birthday were removed from both the numerator and denominator of the cohort. Definitions 1 and 5 combined were used to define a comprehensive COPD population for all subsequent analyses to ensure diagnoses were found in either primary or secondary care EHRs.
Stratification/Composition Variables
The UK population sample was stratified by gender, age (split into 10-year bands), and English region to compare COPD prevalence in each of these groups. Where data were not provided for the whole UK population sample, but only for the COPD population, the proportion of patients with the variable of interest within the COPD cohort was calculated. This was done for 2015 English IMD (by quintile), ethnicity (white, South Asian, black, other, mixed, or unknown), smoking status (current, ex, or never), exacerbations in last year (0, 1, or 2 or more), inhaled therapy (dual therapy [long-acting beta-agonist (LABA) and long-acting muscarinic agonist (LAMA)] or triple therapy [LABA, LAMA, and inhaled corticosteroid (ICS)]), and for measures of COPD severity (Global Initiative for Obstructive Lung Disease (GOLD) stage, MRC grade, and pulmonary rehabilitation (PR) status [referred, commenced, or completed]).
The 2015 English IMD is a rank of relative deprivation between English Lower-layer Super Output Areas (LSOAs), which have an average population of 1500 residents, that takes into consideration income, employment, education, health, crime, housing, and environment in each LSOA.12 Ethnicity was defined using a validated definition created by Mathur18,19 based on the 5 broad ethnicity categories used in the National Census. Previous COPD exacerbations were determined using a validated definition described by Rothnie et al,20 and GOLD stage was defined based on post-bronchodilator FEV1, as described by GOLD.21 Smoking status, inhaled therapy, MRC grade, and PR status were defined based on a SNOMED CT codelist created by us and available at https://github.com/NHLI-Respiratory-Epi/COPD_prevalence.
Statistical Analysis
Using each COPD definition, the mid-year (1st July) point prevalence was calculated for each annual cohort using the following equation:
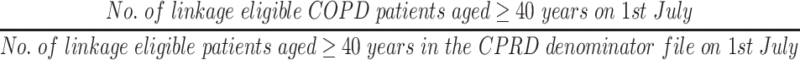 |
Prevalence was also calculated stratified by gender, age (split into 10-year bands), and geographic region to compare COPD prevalence in each of these groups for the comprehensive COPD definition (Definition 1 OR Definition 5 [note that OR is used as the logical OR operator in this context]) and the potentially undiagnosed COPD definition (Definition 3 excluding any individuals that also fall in to Definition 1).
Where data were not provided for the whole denominator population (only for the COPD numerator population), making stratification impossible, the demographics of the COPD cohort were instead presented. This was done for English IMD, ethnicity, smoking status, exacerbations in the last year, inhaled therapy, and for measures of COPD severity (GOLD stage, MRC grade, and PR status). Analyses were completed using Stata MP 17 and graphs were generated using the “white_tableau” scheme by Naqvi.22
Ethical Approval
CPRD has NHS Health Research Authority (HRA) Research Ethics Committee (REC) approval to allow the collection and release of anonymised primary care data for observational research [NHS HRA REC reference number: 05/MRE04/87].23 Each year CPRD obtains Section 251 regulatory support through the HRA Confidentiality Advisory Group (CAG), to enable patient identifiers, without accompanying clinical data, to flow from CPRD contributing GP practices in England to NHS Digital, for the purposes of data linkage [CAG reference number: 21/CAG/0008].23 The protocol for this research was approved by CPRD’s Research Data Governance (RDG) Process24,25 (protocol number: 21_000596) and the approved protocol is available upon request. Linked pseudonymised data was provided for this study by CPRD. Data is linked by NHS Digital, the statutory trusted third party for linking data, using identifiable data held only by NHS Digital. Select general practices consent to this process at a practice level with individual patients having the right to opt-out.
Results
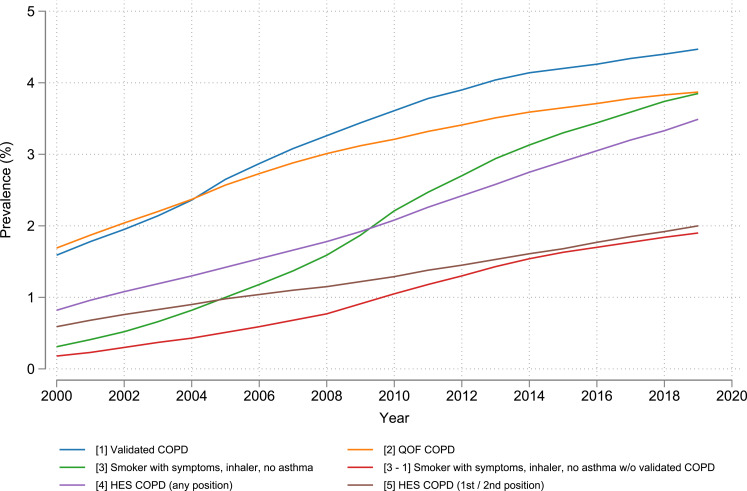
A total of 12,745,793 people were included over the 20-year period, with the annual cohort sizes ranging from 4,373,538 in 2000 to 6,159,496 in 2019 (Table S1). Estimates of COPD prevalence have increased every year since 2000 when using any of the COPD definitions assessed (Figure 1). The difference in estimated COPD prevalence between the validated primary care definition [Definition 1] and the QOF definition [Definition 2] of COPD has grown over time, with both estimating a prevalence of 2.4% in 2004, but by 2019 the gap between the two had grown to 0.6% (absolute difference), with estimates of 4.5% and 3.9%, respectively, for the validated and QOF definitions (Figure 1). The prevalence of COPD was 3.9% in 2019 when using either the QOF definition or a definition based on COPD symptoms, smoking history, inhaler prescriptions, and an absence of an asthma diagnosis [Definition 3]. Defining COPD based on hospital admissions with a COPD ICD-10 code anywhere in the admission record [Definition 4] found a prevalence of 3.5%, but limiting to primary and secondary diagnoses of COPD only [Definition 5], COPD prevalence was 2% in 2019. Potentially undiagnosed COPD (patients with symptoms, smoking history, and an inhaler, but no diagnosis of asthma or COPD) [Definition 3 minus Definition 1] had a similar prevalence to COPD based on a primary or secondary diagnosis only during hospitalisation [Definition 5] at 1.9% in 2019 (Figure 1).
Figure 1.
Prevalence of COPD in English adults ≥40 years old from 2000 to 2019 using different definitions of COPD. Number in brackets “[]” represents the corresponding definition of COPD described in Methods. Note that “-” is used as a minus sign.
Abbreviations: COPD, chronic obstructive pulmonary disease; QOF, Quality and Outcomes Framework; HES, Hospital Episode Statistics.
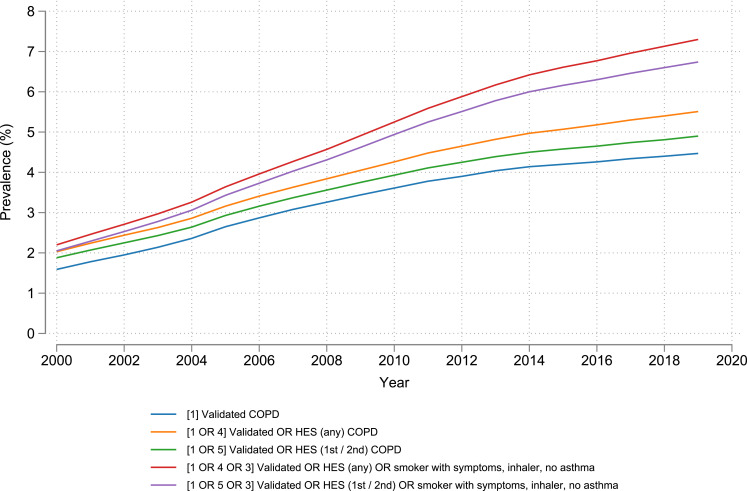
In 2019, a COPD prevalence of 4.9% (301,669 people) was found when using validated events in either primary or secondary care [Definition 1 OR Definition 5] (Figure 2). When additionally including events from secondary care with COPD comorbidity codes [Definition 1 OR Definition 4], prevalence rose to 5.5%. If potentially undiagnosed COPD cases (based on symptoms) were additionally included in the COPD definition, prevalence rose to 6.7% and 7.3%, respectively, in 2019, based on whether the secondary care definition did not [Definition 1 OR Definition 5 OR Definition 3] or did [Definition 1 OR Definition 4 OR Definition 3] include comorbid COPD (Figure 2).
Figure 2.
Prevalence of COPD in English adults ≥40 years old from 2000 to 2019 using combined definitions of COPD. Number in brackets “[]” represents the corresponding definition of COPD described in Methods. Note that “OR” refers to the logical OR operator.
Abbreviations: COPD: chronic obstructive pulmonary disease; HES: Hospital Episode Statistics.
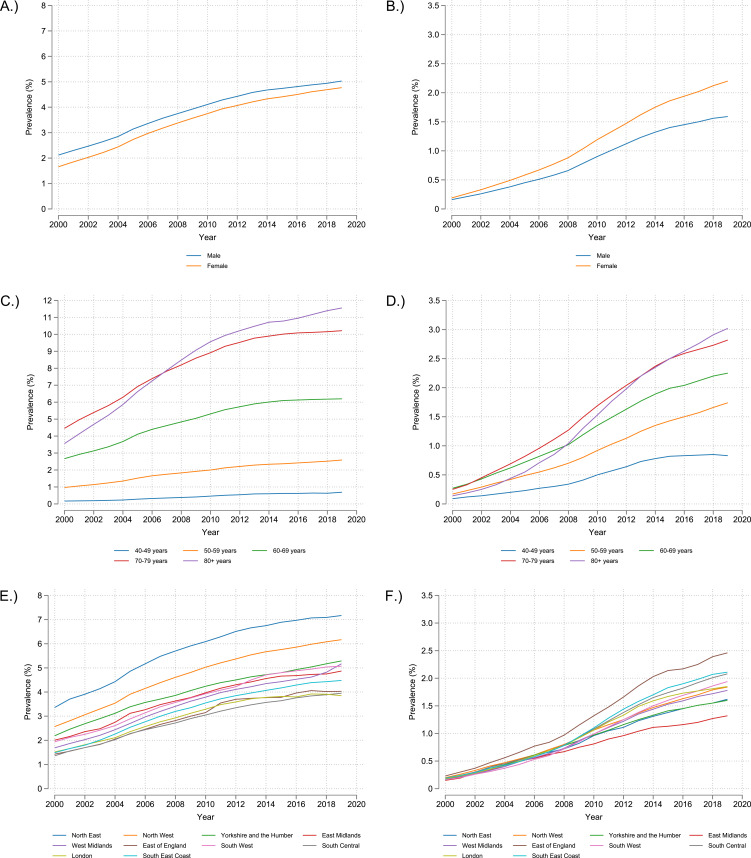
Prevalence of COPD has been higher by approximately 0.3% (absolute difference) in men than women every year from 2000 to 2019 albeit with a slight decrease over time, with the difference being 0.46% in 2000 and 0.26% in 2019 (Figure 3A and Table S2). However, the number of women with potentially undiagnosed COPD has increased at a greater rate than it has for men since 2000, with the difference between the genders being 0.0% in 2000 and 0.6% in 2019 (Figure 3B and Table S3). Prevalence of COPD increases as age increases, with a large increase in prevalence from 50–59 years to 60–69 years (2.6% to 6.2% in 2019) and then 60–69 years to 70–79 years (6.2% to 10.2% in 2019) (Figure 3C and Table S4). Prevalence also appears to have started to level off in each age group around 2014 except in individuals aged ≥80 years, where prevalence still appears to be increasing. Potentially undiagnosed COPD shows a similar pattern of higher prevalence with higher age, however potentially undiagnosed COPD still appears to be increasing over time except for individuals aged 40–49, where there appears to be the beginning of decline in prevalence (0.9% in 2018 to 0.8% in 2019) (Figure 3D and Table S5). The prevalence of COPD in most English regions was within approximately 1% (absolute difference) of each other, falling within a range of approximately 4% to 5% in 2019. London had the lowest prevalence at 3.9% in 2019, however the North West and North East had much higher COPD prevalence at 6.2% and 7.2%, respectively (Figure 3E and Table S6). Undiagnosed COPD prevalence was similar between the regions, however the East Midlands had the lowest undiagnosed COPD prevalence at 1.3% in 2019, while the East of England had the highest undiagnosed COPD at 2.5% (Figure 3F and Table S7). Interestingly, the East of England had one of the lowest diagnosed COPD prevalence at 4.0% in 2019 (Figure 3E and Table S6).
Figure 3.
2000 to 2019 prevalence of COPD (Definition 1 OR Definition 5 as described in Methods) stratified by: (A) Gender, (C) Age, (E) English region; and potentially undiagnosed COPD (Definition 3 excluding any individuals that also fall in to Definition 1) stratified by: (B) Gender, (D) Age, (F) English region.
Recording of MRC grade was poor until 2010 after which approximately 80% of COPD patients had an MRC grade recorded. Approximately half of the cohort were classified as either MRC grade 2 or 3 (Figure S1). Recording of ethnicity has improved over time with almost 90% of patients having a record of ethnicity by 2019. The cohort is mostly white with 83.86% of the 2019 cohort having codes indicating they are of a white ethnicity: in keeping with the general population (Figure S2). Recording of FEV1%-predicted has improved over time, however as of 2019 almost 30% of patients still do not have a record of FEV1%-predicted. The largest number of patients fall into GOLD stage 2 as calculated by FEV1%-predicted (Figure S3). By 2019 only 13.8% of the cohort had been referred to pulmonary rehabilitation (Figure S4). Most of the cohort were ex-smokers with the number of current smokers decreasing over time. There was a consistent prevalence of approximately 5.5% never smokers over time (Figure S5). Consistently over time approximately 60% of the cohort had experienced no exacerbations in the last year, while 20% had experienced 1, and the remaining 20% had experienced 2 or more (Figure S6). Comparing the use of dual versus triple therapy over time, it appears that prescription of dual therapy has only started to take off from 2015 onwards (Figure S7). In 2015, by the 2015 measure of IMD, 14.89% of people with COPD were in the 1st (least deprived) quintile of IMD, 17.41% were in the 2nd quintile, 18.91% were in the 3rd quintile, 21.75% were in the 4th quintile, 26.97% were in the 5th (most deprived) quintile, and 0.07% of people with COPD did not have IMD data (data not shown).
Discussion
We have found that a definition of COPD validated16 for use in UK primary care EHR databases gives higher estimates of COPD prevalence than the commonly used QOF definition. The difference in estimate of prevalence that these two definitions give has also increased over time, which raises the question of whether the QOF definition is missing some key codes relating to COPD. Adding information from secondary care to this validated COPD definition led to a further absolute increase of approximately 0.4% to our prevalence estimates over time. Adding further information on symptoms that may describe undiagnosed cases increases prevalence estimates even more to 6.7% in 2019. However, we have not used this as our primary definition of COPD as there could be alternative explanations for those symptoms. We believe the best estimate of diagnosed COPD prevalence in 2019 in people aged 40 years or older in England to be 4.9%. Scaling these definitions up to the population of England, using the 2019 mid-year ONS population estimate,26 would mean that approximately 1.4 million people aged 40 years or over in England have a diagnosis of COPD and appoximately 500,000 are undiagnosed.
Having explored COPD prevalence using five distinct definitions, three within primary care and two within secondary care, we would encourage researchers, the NHS and policy makers going forward to use a definition combining two data sources: a validated primary care diagnosis of COPD or an admission in secondary care with COPD (or emphysema) as the primary or secondary diagnosis to ensure that studies and trials are consistent with definitions to allow us to truly capture changes over time and to ensure that work being undertaken is always measuring the same thing.
Using our primary COPD definition based on the validated16 primary care definition and a primary or secondary diagnosis only in secondary care and then stratifying by gender, it was interesting to see that while there is a small reduction in the difference in prevalence between men and women over time, there is an increasing difference in potentially undiagnosed COPD with women having more undiagnosed COPD. We wonder if this could perhaps be explained by differences in primary care utilisation between men and women.27–29 Stratifying by age, COPD prevalence seems to still be growing in people aged 80 years or over, whilst it appears to be levelling off in the younger age groups. This could be because we tend to use a fixed ratio to make a diagnosis in the UK, rather than LLN,4 which would lead to an overestimation in this age group. Stratifying by region, prevalence appears to be highest in the north of England, although this could perhaps be explained by higher average age in the north of England26.
It has been shown previously that the prevalence of COPD is higher in smokers and in men, and it increases with age.30 Stopping smoking prevents the development of COPD or slows its progression and reduces the risk of hospital admissions.31 Smoking cessation programmes are highly cost-effective, particularly when targeted at individuals with asymptomatic airway obstruction.32 This is because smokers may be motivated to attempt to quit when given a diagnosis of airflow limitation.33 The Finnish National Programme for Chronic Bronchitis and COPD was set up in 1998 to reduce prevalence and improve diagnosis and care. Prevalence remained unchanged, but smoking decreased in males from 30% to 26% and in females from 20% to 17%. They also achieved significant improvements in the quality of spirometry, hospitalisation decreased by 39.7% (p < 0.001), and COPD costs were 88% lower than had been anticipated.34 Exploring similar models of care in England could help to improve COPD care.
There is no consensus regarding using a fixed threshold to define airflow obstruction versus using LLN adjusted for age.5 The difference between these two definitions is illustrated by the pooled prevalence estimates of an international systematic review and meta-analysis.35 Using the GOLD definition (stage 1: FEV1 ≥ 80% predicted; stage 2: 50% ≤ FEV1 < 80% predicted; stage 3: 30 ≤ FEV1 < 50% predicted; stage 4: FEV1 < 30% predicted4) and including GOLD (stage 1)/FEV1/FVC <0.70, the population prevalence was estimated at 9.8% (95% CIs 5.9–15.8). Including only GOLD (stage 2)/FEV1/FVC <0.70 and FEV1 <80% predicted and worse, the population prevalence was 5.5% (95% CIs 3.3–9.0).
The GOLD definition has also been used in a previous analysis of the 2000 HSfE data by Shahab et al,36 which was used for prevalence estimates by NICE and the COPD National Strategy. This found a prevalence of 13.3% in over 35s. The Department of Health Outcomes Strategy for People with COPD and Asthma in England published in 2011 uses this figure to estimate there are around 835,000 people currently diagnosed with COPD in the UK and an estimated 2,200,000 people with COPD who remain undiagnosed.37 As a result of using only the one spirometry criterion, prevalence estimates from these sources are larger. That study also calculated the prevalence directly from the survey data, where the estimates shown were obtained from the modelled/expected estimates and extrapolated for the population of England for validation purposes. As might be expected, the extrapolated number of cases were somewhat lower.
Although there were improvements in spirometry recorded over time, in 2019, approximately 30% of people with COPD still did not have a record of FEV1% coded in their medical record. While some may have had this entered as free text or scanned into the notes, which we were unable to access, it is still likely that not everyone had spirometry recorded. Spirometry is important not only in making a diagnosis of COPD, but in monitoring the natural history of the disease and there are recommended codes for use in primary care based on the National Asthma and COPD Audit Programme (NACAP).38 Using the BTS definition, the Nacul et al methodology paper39 gave the overall expected prevalence in the English population over 15 years of age of 3.1% (3.9% in men and 2.4% in women). For those over 45 years old, the estimated prevalence was 5.3% (6.8% and 3.9% in men and women, respectively). This corresponds to over 1.3 million people in England with COPD, of whom nearly 800 thousand or 60% are men.
A systematic review of good quality COPD prevalence studies yielded estimates for England of between 4% and 10%.40 The 2004 UK Health Needs Assessment report suggested a prevalence of 5% for men and 3% for women of middle age and upwards.41
There have been many prevalence surveys published, including by Public Health England.42 Most of these have used the international Burden of Obstructive Lung Disease (BOLD) protocol and study design,43 and hence the GOLD definition.21 Unfortunately, relatively few contain data on risk factors other than age, gender, and smoking, but nevertheless some are relevant to the UK. For example, a population-based study of adults, aged ≥40 years, in Maastricht, the Netherlands, found an overall prevalence of COPD of 24%, which was higher for men (28.5%) than for women (19.5%).44 In this study, overall prevalence of current smoking was 23%, and the prevalence of doctor-diagnosed COPD was only 8.8%.44
Observational studies allow understanding of disease management and outcomes using real-world data sources, in this case electronic health data. However, strengths and limitations are associated with this study design. Any changes seen in prevalence over time may be related to differences in the clinical practice of diagnosis and/or coding practices rather than true changes in disease prevalence. So, the low initial prevalence in 2000 and large increase in prevalence since then could potentially largely be explained by improved record keeping by GPs over time. Figure S1 provides some evidence for this where there is a substantial improvement in recording of MRC grade from 2010 onwards. This may suggest that the prevalence estimates prior to 2010 are not as reliable as those from the most recent decade.
It is important to note that some people with COPD simply do not seek advice from a health-care professional and will never receive a formal diagnosis nor have health-care records suggestive of COPD and therefore will not be captured in this study. Changing health-care priorities and financial incentivisation over time may have led to differences in the completeness of recording of data such as smoking status, spirometry, and referral to pulmonary rehabilitation. This may limit the ability to which this study can follow patients in these sub-groups over time. Whilst these are all potential limitations, their exploration is important for policy planning and public health.
Conclusion
Common definitions of COPD, such as QOF clinical codes may underestimate the true prevalence of COPD. The extent of this underestimate has increased over time and could lead to an under-allocation of resources if health-care providers estimate need based on unvalidated definitions of COPD. We therefore recommend defining COPD prevalence using both validated events in primary care and hospital admissions with a primary or secondary diagnosis of COPD or emphysema (ie, Definition 1 OR Definition 5). Standardisation of measurement of COPD from routine electronic health-care records and important metrics such as spirometry is key if we are to continue to monitor changes in the disease and in outcomes.
Acknowledgments
This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. Hospital Episode Statistics (HES) data, copyright © 2023, re-used with the permission of The Health & Social Care Information Centre. All rights reserved.
Funding Statement
Asthma + Lung UK provided funding to Imperial College London on behalf of the Taskforce for Lung Health to undertake this work.
Disclosure
Dr Philip W Stone reports grants from Asthma + Lung UK, during the conduct of the study; grants from Royal College of Physicians and Gilead Sciences, outside the submitted work. Mr Andrew Ellis, Mrs Rebecca Coaker, and Mr Michael Osen report grants, non-financial support from Amgen Ltd, AstraZeneca, Boehringer Ingelheim, Copley Scientific Limited, Chiesi Ltd, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson Ltd, Merck Sharpe and Dohme Ltd (MSD), Medtronic Limited, Novartis Pharmaceuticals UK Ltd, PARI Medical Ltd, Pfizer, Sanofi, Seqirus, Takeda Pharma, Trudell Medical, Verona Pharma, Vitalograph; non-financial support from Air Liquide Healthcare, Bristol-Myers Squibb, Circassia, My mHealth, Nowus Healthcare, Roche, Spink (BEAMA), Association of the British Pharmaceutical Industry, during the conduct of the study; grants, non-financial support from Amgen Ltd, AstraZeneca, Boehringer Ingelheim, Copley Scientific Limited, Chiesi Ltd, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson Ltd, Merck Sharpe and Dohme, Medtronic Limited, Novartis Pharmaceuticals, PARI Medical Ltd, Pfizer, Sanofi, Seqirus, Takeda Pharma, Trudell Medical, Verona Pharma, Vitalograph; non-financial support from Air Liquide Healthcare, Bristol-Myers Squibb, Circassia, My mHealth, Nowus Healthcare, Roche, Spink (BEAMA), Association of the British Pharmaceutical Industry, outside the submitted work. Professor Jennifer K Quint reports grants from Taskforce through A+L UK, during the conduct of the study; grants and personal fees from MRC, HDR UK, AZ, BI, Insmed, Gilead, GSK, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Halpin DMG, Miravitlles M. Chronic obstructive pulmonary disease: the disease and its burden to society. Proc Am Thorac Soc. 2006;3(7):619–623. doi: 10.1513/pats.200603-093SS [DOI] [PubMed] [Google Scholar]
- 2.Devereux G. ABC of chronic obstructive pulmonary disease: definition, epidemiology, and risk factors. BMJ. 2006;332(7550):1142–1144. doi: 10.1136/bmj.332.7550.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372–377. doi: 10.1164/ajrccm.164.3.2004029 [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE. Available from: https://www.nice.org.uk/guidance/NG115. Accessed January 12, 2023. [PubMed] [Google Scholar]
- 5.Bhatt SP, Sieren JC, Dransfield MT, et al. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax. 2014;69(5):410–415. doi: 10.1136/thoraxjnl-2012-202810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics. Available from: https://statistics.blf.org.uk/copd. Accessed September 27, 2017.
- 7.Clinical Practice Research Datalink. CPRD Aurum May 2021 (Version 2021.05.001). Available from: 10.48329/q2n0-4n14 Accessed November 1, 2022. [DOI]
- 8.Clinical Practice Research Datalink. Primary care data for public health research. Available from: https://www.cprd.com/primary-care-data-public-health-research. Accessed November 1, 2022.
- 9.Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48:1740–1740g. doi: 10.1093/ije/dyz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical Practice Research Datalink. CPRD linked data. Available from: https://www.cprd.com/cprd-linked-data. Accessed November 1, 2022.
- 11.Clinical Practice Research Datalink. CPRD Aurum Source file March 2021 (Version 2021.03.001); 2023. Available from: 10.48329/537a-6q30. Accessed March 07, 2023. [DOI]
- 12.Department for Communities and Local Government. English indices of deprivation 2015. GOV.UK. Available from: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015. Accessed January 16, 2023.
- 13.Clinical Practice Research Datalink. CPRD Aurum Small Area data (patient) March 2021 (Version 2021.03.001); 2023. Available from: 10.48329/n7wz-k681. Accessed March 07, 2023. [DOI]
- 14.NHS Digital. Hospital Episode Statistics (HES). NHS Digital. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics. Accessed November 25, 2019. [Google Scholar]
- 15.Clinical Practice Research Datalink. CPRD Aurum HES APC March 2021 (Version 2021.03.001). Available from: 10.48329/14gk-m942. Accessed 7 March, 2023. [DOI]
- 16.Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi: 10.1136/bmjopen-2014-005540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS Digital. Quality and Outcomes Framework (QOF). NHS Digital. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/quality-outcomes-framework-qof. Accessed December 22, 2022. [Google Scholar]
- 18.Mathur R. Ethnicity codelist. Available from: https://datacompass.lshtm.ac.uk/id/eprint/2102/65/ethnicity_aurum_may20.txt. Accessed December 22, 2022.
- 19.Davidson J, Warren-Gash C, Mcdonald H, et al. LSHTM Data Compass. Codelists for: “Ethnic differences in the incidence of clinically diagnosed influenza: an England population-based cohort study 2008-2018”; 2021. Available from: 10.17037/DATA.00002102. Accessed December 22, 2022. [DOI] [PMC free article] [PubMed]
- 20.Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi: 10.1371/journal.pone.0151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Global Initiative for Chronic Obstructive Lung Disease – GOLD. Pocket guide to COPD diagnosis, management, and prevention: a guide for health care professionals - 2022 edition; 2021. Available from: https://goldcopd.org/2022-gold-reports-2/. Accessed June 20, 2022.
- 22.Naqvi A. SCHEMEPACK v1.4; 2023. Available from: https://github.com/asjadnaqvi/stata-schemepack. Accessed January 18, 2023.
- 23.Clinical Practice Research Datalink. ISAC minutes and annual reports. Available from: https://www.cprd.com/isac-minutes-and-annual-reports. Accessed March 21, 2023.
- 24.Clinical Practice Research Datalink. Research applications. Available from: https://www.cprd.com/research-applications. Accessed March 21, 2023.
- 25.Clinical Practice Research Datalink. Data access. Available from: https://www.cprd.com/data-access. Accessed March 21, 2023.
- 26.Office for National Statistics. Population estimates for the UK, England and Wales, Scotland and Northern Ireland; 2019. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2019estimates. Accessed February 9, 2023. [Google Scholar]
- 27.Nathanson CA. Sex roles as variables in preventive health behavior. J Community Health. 1977;3(2):142–155. doi: 10.1007/BF01674236 [DOI] [PubMed] [Google Scholar]
- 28.Verbrugge LM. Sex differentials in health. Public Health Rep. 1982;97(5):417–437. [PMC free article] [PubMed] [Google Scholar]
- 29.Ladwig KH, Marten-Mittag B, Formanek B, Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16(6):511–518. doi: 10.1023/A:1007629920752 [DOI] [PubMed] [Google Scholar]
- 30.Mindell J, Chaudhury M, Aresu M, Jarvis D. Health survey for England - 2010, respiratory health: chapter 3, lung function in adults. NHS Digital. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2010-respiratory-health. Accessed January 13, 2023. [Google Scholar]
- 31.Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax. 2002;57(11):967–972. doi: 10.1136/thorax.57.11.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233–239. doi: 10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 33.Goérecka D, Bednarek M, Nowinéski A, Pusécinéska E, Goljan-Geremek A, Zielinéski J. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123(6):1916–1923. doi: 10.1378/chest.123.6.1916 [DOI] [PubMed] [Google Scholar]
- 34.Kinnula VL, Vasankari T, Kontula E, Sovijarvi A, Saynajakangas O, Pietinalho A. The 10-year COPD programme in Finland: effects on quality of diagnosis, smoking, prevalence, hospital admissions and mortality. Prim Care Respir Med. 2011;20(2):178–183. doi: 10.4104/pcrj.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605 [DOI] [PubMed] [Google Scholar]
- 36.Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61(12):1043–1047. doi: 10.1136/thx.2006.064410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Department of Health, Medical Directorate, Respiratory Team. An outcomes strategy for people with chronic obstructive pulmonary disease (COPD) and asthma in England. GOV.UK. Available from: https://www.gov.uk/government/publications/an-outcomes-strategy-for-people-with-chronic-obstructive-pulmonary-disease-copd-and-asthma-in-england. Accessed January 13, 2023.
- 38.Royal College of Physicians. Support for service teams: primary care. RCP London. Available from: https://www.rcplondon.ac.uk/projects/outputs/support-service-teams-primary-care. Accessed March 7, 2023. [Google Scholar]
- 39.Nacul LC, Soljak M, Meade T. Model for estimating the population prevalence of chronic obstructive pulmonary disease: cross sectional data from the health survey for England. Popul Health Metr. 2007;5(1):8. doi: 10.1186/1478-7954-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. doi: 10.1378/chest.123.5.1684 [DOI] [PubMed] [Google Scholar]
- 41.Stevens DA, Raftery J, Mant J, Simpson S. Health Care Needs Assessment: The Epidemiologically Based Needs Assessment Reviews. Radcliffe Publishing; 2004. [Google Scholar]
- 42.Office for Health Improvement and Disparities. Modelled prevalence estimates. Available from: https://fingertips.phe.org.uk/profile/prevalence. Accessed May 22, 2023.
- 43.Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2(2):277–283. doi: 10.1081/COPD-57610 [DOI] [PubMed] [Google Scholar]
- 44.Vanfleteren LEGW, Franssen FME, Wesseling G, Wouters EFM. The prevalence of chronic obstructive pulmonary disease in Maastricht, the Netherlands. Respir Med. 2012;106(6):871–874. doi: 10.1016/j.rmed.2012.01.008 [DOI] [PubMed] [Google Scholar]