Abstract
Epilepsy is a neurological disease affecting about 65 million persons worldwide and characterized by an “enduring predisposition to generate epileptic seizures”. Although the etiology of epilepsy in many individuals is unknown, identified causes are either acquired or linked to genetic mutations. The first-line treatment for epilepsy consists of more than 20 anti-seizure medications, nonetheless, about one-third of patients fail to achieve seizure control. Anti-seizure medications act primarily on neurons and provide symptomatic control of seizures in drug-sensitive patients, but do not interfere with the onset and progression of epilepsy, and cause serious side effects. Thus, medications with new cellular and molecular targets and mechanisms of action are needed. Among the pivotal cellular components of the epileptogenic circuitry, astrocytes raised increasing interest for their contribution to epilepsy. Progress in understanding astrocyte function in health and disease led to new discoveries on how astroglia-mediated changes in brain metabolism and excitability play a role in epilepsy. This review reports our understanding of how dysregulation of key astrocyte-based functions, namely gliotransmission, cell metabolism, and immune properties, play a role in the development and expression of hyperexcitability in epilepsy. Strategies to mitigate astrocyte dysfunctions and methods to monitor cell activation states in vivo will be discussed, with the overarching goal of defining new targets to prevent the initiation and progression of epilepsy.
Introduction
Astrocytes are specialized CNS cells of neuroepithelial origin that critically contribute to tissue homeostasis and neural signaling. There are mainly two types of astrocytes, protoplasmic and fibrous, which differ in morphologic appearance and location (gray and white matter, respectively). Recent studies further highlighted astrocyte diversity based on transcriptomic profiling, immunohistochemistry, and functional read-outs. Thus, astrocytes comprise a heterogeneous population of cells that display brain region- and disease-specific properties1. Among their various interrelated physiological functions, astrocytes play a key role in maintaining blood-brain barrier (BBB) structure and permeability properties2 by directly interacting with endothelial cells and pericytes; they mediate neurovascular coupling3, and are involved in blood flow regulation and energy metabolism2,3, thus providing metabolic support to neurons. As part of the tripartite synapse4, astrocytes regulate extracellular levels of neurotransmitters implicated in seizures (e.g., glutamate and GABA), as well as ions, water, pH, and they release gliotransmitters, thus contributing to synaptic transmission and neuronal excitability5,6.
In healthy conditions, astrocytes are constantly activated in response to physiological stimuli whereas various types of CNS insults and pathologies shift their physiological state to an “activated state” through the process of “reactive astrogliosis”7. The latter involves complex and dynamic cell responses to diverse pathological conditions which involve a spectrum of genetic, epigenetic, molecular, metabolic, morphological and functional changes that are context-dependent and regulated by specific signaling events. Reactive astrogliosis is a hallmark of the epileptic focus both in human and experimental epilepsy5,6 (Fig. 1). Glial fibrillary acidic protein (GFAP), a major constituent of intermediate filaments, has been mostly used as a cellular marker of reactive astrocytes in epilepsy8, although other markers such as vimentin, connexin (Cx) 43, S100β, adenosine kinase (ADK), and glutamine synthase (GS) have also been assessed9–12. A recent study provided evidence for the presence of GFAP/S100β-positive immature astroglia in close apposition with the neurogenic niche in the dentate gyrus of the hippocampus in human mesial temporal lobe epilepsy (MTLE) but not in control tissue, suggesting it represents a pathological phenotype of this type of epilepsy with a functional role in modulating epileptic activity13. In addition, modulation of astrocyte activation in immature rodent brain improved memory outcomes after febrile seizures14.
Figure 1. Astrocytes and their interactions with neurons and blood vessels.

Photomicrographs depict GFAP-positive immunostaining in human astrocytes. A,B: Protoplasmic astrocytes morphology in normal human temporal cortex depicting highly branched bushy processes; cells are widely distributed between neurons. An astrocyte embracing a neuron is shown in B (magnification in inset). Arrows indicate a large astroglia process extending its foot along a blood vessel. C,D: Chronic dense fibrillary gliosis in human TLE sclerotic hippocampus. Arrow in C indicates a surviving CA1 neuron entrapped in dense glial meshwork. Scale bars: 50 μm.
There are different splice variants of GFAP (β, γ, δ, κ) including the predominant GFAPα form8. The minor isoforms are increased in subpopulations of astrocytes in focal lesions associated with epilepsy, however, their functional consequences for the epileptic network are unclear.
E: Schematic representation of the shift of astrocytic functional states (in healthy conditions, blue color) to reactive pathologic states (orange color). Reactive astrocytes, due to impairment of their homeostatic functions (blue circles), cause several alterations in surrounding brain tissue (orange shape) that contribute to epilepsy initiation and progression.
Comparative analyses suggested that there is substantial similarity between human and rodent astrocytes with regard to their functional properties and extensive conservation in astrocytic gene expression, which warrants employing mouse or rat models to investigate the involvement of astrocytes in the etiology of human CNS diseases15,16. Animal models of epilepsy mimicking human etiologies17 allowed to characterize the complex and dynamic structural, cellular, molecular alterations occurring in the brain during epilepsy development. Functional and interventional studies showed that some alterations contribute to disease onset and development (epileptogenesis), or to mechanisms of seizure generation and recurrence (ictogenesis). In this context, reactive astrocytes may critically contribute to epilepsy initiation and progression due to impairment of their homeostatic functions or gain of aberrant properties9,11,18. In particular, reactive astrocytes can either initiate the disease (as in the genetic gliopathy Alexander disease19; Box 1) or they might contribute to disease onset and progression, as suggested in acquired epilepsy5,20. For example, the induction of reactive astrocytes by knocking out the β1-integrin gene in transgenic mice, or delivering a specific adenoviral vector in the healthy mouse brain, elicits spontaneous seizures associated with impairment of GABAergic transmission21,22. However, the process of reactive astrogliosis is not uniformly negative since beneficial functions have been described in epilepsy such as defense from oxidative stress, production of anti-inflammatory mediators and neuroprotective molecules. These “resilient astrocytes“7 often co-exist in the epileptic focus with maladaptive astrocytes23–26, or the same cells may express both homeostatic/compensatory and pathologic markers. Overall, the evidence suggests that these compensatory states are likely to be insufficient in epilepsy for contrasting the detrimental consequences of dysfunctional astrocytes27.
BOX 1. Gliopathy in genetic epilepsy.
Epilepsy is a frequent neurological feature of neurodevelopmental disorders associated with gene mutations196. Astrocyte reactivity is commonly observed with a spectrum of complex morphological and functional changes119,188. Thanks to rapid advances in genetic technology, various studies described the astrogliopathology associated with specific genetic mutations, emphasizing the aberrant neuro-glia crosstalk, as well as interglial communication contributing to the clinical phenotype. Examples are provided below.
Tuberous sclerosis complex (TSC) represents the prototypic monogenic disorder of the mammalian target of rapamycin (mTOR) pathway dysregulation. TSC is typically characterized by benign tumors in diverse organs and neurological manifestations that include epilepsy, neurodevelopmental delay and neuropsychiatric disorders197. Astrocytes display a reactive phenotype associated with increased proliferation and enhanced expression of pro-inflammatory mediators in cortical tubers. Moreover, astrocytes are characterized by decreased homeostatic functions related to ion homeostasis and neurotransmitter metabolism119. Understanding mTOR-related astrocyte dysfunction may offer new insights into common neurobiological mechanisms underlying epilepsy as well as the cognitive and behavioral comorbidities that are characteristic of the spectrum of mTOR-associated neurodevelopmental disorders119.
Alexander disease is a fatal disorder of the CNS typically characterized by macrocephaly, rapid neurological deterioration, seizures, spasticity and retarded psychomotor development. It represents a classical example of a primary astrogliopathy caused by a dominant mutation of the GFAP gene, resulting in various pathological features, including changes in cell morphology, and dysfunction198 associated with the activation of cell stress and inflammatory pathways19,198.
Aicardi-Goutieres syndrome is a rare genetically determined infantile encephalopathy characterized by progressive microcephaly, psychomotor retardation, and premature death. Astrocytes are key players in the phenotypic presentations through cell-autonomous and non-cell-autonomous mechanisms199 contributing to the inflammatory response with increased interferon-α production200.
The application of sophisticated research strategies (single nucleus RNA sequencing, spatial transcriptomics, human iPSC-derived astrocytes), represents an ideal approach towards a better identification of human astrocyte subpopulations and elucidation of the phenotypic spectrum of genetic epilepsies.
The following sections will present key pathophysiological features of astrocytes which are shared by CNS pathologies associated with neuronal network hyperexcitability and seizures. There is growing evidence and consensus that key mechanisms of the epileptogenic process, including the pathogenic role of astrocytes, are shared across a wide spectrum of acquired epilepsies28. Because astrocytes in culture may undergo dramatic changes and often acquire artificial functional properties, we have confined this review to data obtained in situ, after acute isolation or in vivo and explicitly indicated the few cases where culture work was cited.
Gliotransmission
Astrocytic processes are tightly associated with synapses, and it has been estimated that one astrocyte can oversee more than 100,000 synapses29. The close spatial and functional interactions of synapses and astrocytic perisynaptic processes gave rise to the concept of the ‘tripartite synapse’ according to which astrocytes detect and respond to synaptic signals through release of ‘gliotransmitters’ which influence neuronal activity4. Different forms of neuron–glia interaction across brain regions have since been described but are still controversially discussed30,31. However, increasing evidence does suggest that gliotransmission is an important component of normal brain function and that abnormalities in gliotransmission are involved in epilepsy.
Gliotransmission in the healthy brain
The first gliotransmitter shown to modify neuronal activity in cell culture was glutamate32,33. Subsequently, vesicular structures and Ca2+-dependent astroglial glutamate release were identified in situ34. The presence of vesicular glutamate transporters in astrocytes has been questioned but these cells express many components required for vesicular release35. Astrocytic Ca2+ increase and glutamate release by astrocytes triggered by GABA, glutamate, endocannabinoids and ATP entail slow inward currents (SICs) in neurons through extrasynaptic GluN receptors (GluNRs) (Fig. 2), which synchronizes neuronal activity36,37 and increases synaptic strength38,39. Besides glutamate, astrocytes release the GluNR co-agonist D-serine and thereby control long-term potentiation40. Ca2+ elevations and D-serine release from astrocytes influence learning and memory41,42. ATP is also a gliotransmitter, which after its release activates purinergic P2 receptors or, after degradation by ectonucleotidases to adenosine, affects neurotransmitter release through presynaptic A1 receptors and regulates sleep homeostasis43 (Fig. 2). Moreover, astrocytes release GABA, e.g., in the thalamus, where the gliotransmitter provokes outward currents in thalamocortical neurons, in the olfactory bulb, where astroglial GABA inhibits mitral and granule cells, in the hippocampus or in the cerebellum, where GABA release by astrocytes or Bergmann glia mediates tonic inhibition44 (Fig. 2). In addition to Ca2+ dependent exocytosis, several other release mechanisms for gliotransmitters have been proposed, including through Cx43 hemichannels, pannexin-1 (Panx1) channels, volume regulated anion channels, bestrophin1 (Best1) anion channels, two-pore domain K+ (K2P) channels, reversal of amino acid transporters, or through P2X7 receptors (P2X7Rs). Ultimately, however, it is still largely unclear in which brain regions astrocytes are equipped with which transmitter release machinery.
Figure 2. Gliotransmission in the epileptic brain.

(1) Glutamate or ATP derived from neurons, astrocytes or microglia trigger an increase in the intracellular Ca2+ concentration [Ca2+]i in astrocytes, which promotes the release of gliotransmitters. (2) Glutamate released by astrocytes activates extrasynaptic neuronal GluNRs and mGluRs, thereby increasing presynaptic release probability (Pr) and postsynaptic excitability. (3) Astrocytic ATP release evoked by elevated [Ca2+]i levels or activation of cytokine receptors lead to autocrine or paracrine activation of P2YRs, resulting in potentiation and intercellular propagation of astrocytic Ca2+ signals. It also triggers microglial activation and release of pro-inflammatory cytokines through activation of microglial P2X7 and/or P2Y1Rs. (4) Extracellular ATP is rapidly hydrolysed by ectonucleotidases to adenosine, with the latter targeting pre- and postsynaptic A1Rs to decrease neuronal excitability. (5) Epileptic activity triggers astrocytic GABA production via decarboxylation of glutamate by glutamate decarboxylase (GAD) and degradation of putrescine by monoamine oxidase B (MAO-B). Upon release into the extracellular space, GABA activates extrasynaptic GABAARs on excitatory neurons and elicits tonic inhibitory Cl− currents, which attenuates neuronal excitability. Abbreviations: GluAR – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ER – endoplasmic reticulum; GABAAR – γ-aminobutyric acid receptor; IL– interleukin; IP3 – inositol 1,4,5-trisphosphate; NLRP3 – NLR family pyrin domain containing 3; GluNR – N-methyl-d-aspartate receptor; TGF-β – transforming growth factor β; TNFα – tumor necrosis factor α; TNFR1 – tumor necrosis factor α receptor 1.
Since gliotransmitters modulate neuronal excitability, it is likely that disturbance of this astrocyte function is implicated in ictogenesis and epileptogenesis, as described in the following section.
Gliotransmission in the initiation and progression of epilepsy
Enhanced glutamate release and loss of astrocyte gap junction coupling
The most intensively studied gliotransmitter in the context of epilepsy is glutamate (Fig. 2). Extracellular glutamate levels are elevated in the hippocampus in human and experimental TLE, particularly before (interictally) and during seizures45,46. First hints for an involvement of astrocytic glutamate release in epileptogenesis came from studies showing that Ca2+ oscillations in astrocytes evoke SICs through extrasynaptic GluNRs in neurons and promotes synchronization of neuronal discharges36,37 (cf. above and Fig. 2). This led to the hypothesis that excessive release of the gliotransmitter underlies the hypersynchronous neuronal firing characterizing epileptic activity, and this was supported by the finding that astrocytic glutamate triggers neuronal paroxysmal depolarization shifts, which are the cellular correlates of interictal discharges47,48. However, other work suggested a modulatory rather than a causative role of the gliotransmitter49, e.g. by lowering the threshold for ictal activity50. This idea was recently supported by showing that in mice injected intracortically with kainate, astrocytic Ca2+ increases precede seizures by several seconds51. That Ca2+-dependent gliotransmission is crucially involved in epileptogenesis and neurodegeneration was also concluded from studies in rodents using pharmacological or genetic tools to inhibit astrocytic transmitter release52,53. In the sclerotic hippocampus of human and experimental TLE, astrocytes undergo dramatic morphological and functional alterations, which affects their sensitivity to glutamate and impairs their control of the extracellular glutamate concentration. Besides loss of gap junction coupling, glutamate transporters are replaced by ionotropic GluARs, particularly GluA1 flip leading to prolonged astroglial depolarization15,18,54. This activates a vicious circle, in which extracellular glutamate increasingly accumulates, depolarizes glia, and generates neuronal hyperactivity55.
ATP and purinergic receptors
Changes in extracellular ATP levels during seizures and epilepsy have been investigated in a number of studies with inconsistent results56. Among other reasons, this inconsistency is probably due to the difficulty of determining extracellular ATP levels because of its very short half-life and degradation to adenosine56. Nevertheless, using a luciferin-luciferase assay, Dossi and colleagues57 demonstrated that extracellular ATP levels increase significantly during ictal discharges in cortical tissue samples from patients with drug-resistant epilepsy. ATP and adenosine play opposite roles in epilepsy. While it is well established that extracellular adenosine exerts anticonvulsive and neuroprotective effects by acting on pre- or postsynaptic A1 receptors (A1Rs), activation of purinergic receptors by elevated extracellular ATP levels promote seizures and epileptogenesis43 (Fig. 2). In this context, the ionotropic P2X7R attracted much attention, as several studies indicate that its inhibition has anti-convulsive and neuroprotective effects43,58. As in human and rodent epileptic tissue P2X7Rs are mainly expressed by microglia and excitatory neurons (but not astrocytes) (Fig. 2), the protective effects of P2X7R inhibition are probably mediated by suppressing neuroinflammation and neuronal glutamate release43. However, the current data on the role of P2X7Rs in epilepsy are incomplete, partly contradictory and require further investigation. Among the metabotropic purinergic receptors, the Gq protein-coupled P2Y1 subtype (P2Y1R) has been implicated in epilepsy (Fig. 2). This receptor is activated by ADP and ATP, and is expressed by neurons, astrocytes and microglia59. Upregulation of P2Y1R expression was shown in the hippocampus of human and experimental TLE60. Inhibition of P2Y1R has anticonvulsant, antiepileptogenic and/or neuroprotective effects59–63, possibly due to inhibition of P2Y1R-mediated Ca2+-dependent glutamate release from astrocytes61–63. P2Y1Rs seem to be crucial in astrocyte-to-astrocyte propagation of Ca2+ waves and thus synchronisation of neuronal activity63, and in regulating synaptic activity by autocrine astrocytic ATP signalling61,62. However, others have failed to detect P2Y1Rs in astrocytes of epileptic tissue and suggested that the observed attenuation of inflammatory processes and antiepileptic effects were due to inhibition of microglial P2Y1Rs59,64. Thus, although there are still several inconsistencies and unanswered questions about the role of astrocytic ATP in epilepsy, evidence is accumulating that this gliotransmitter is actively involved in genesis and/or progression of the disease.
GABA and extrasynaptic GABAA receptors
Loss of GABAergic interneurons and synaptic inhibition have been shown in the hippocampus of human and experimental TLE65,66. Despite this loss, several studies reported preserved or increased tonic (extrasynaptic) GABAA receptor (GABAAR)-mediated currents in TLE, indicating high ambient GABA concentrations of non-neuronal origin65–67. Work from two groups suggested that in TLE mouse models, reactive astrocytes aberrantly overproduce GABA through de novo synthesis and/or decarboxylation of excess glutamate (Fig. 2). After its release into the extracellular space, possibly through Best1 channels, the gliotransmitter activates tonic GABAAR-mediated currents in excitatory neurons and reduces seizure susceptibility (Fig. 2), thus apparently compensating for the loss of interneurons66,67.
Summary
Overall, the above evidence underscores reactive astrocytes may release either excitatory or inhibitory gliotransmitters in epilepsy. A future challenge is to dissect out the involvement of each of these opposite functions, and which one might prevail, during epilepsy development. Additionally, although abnormal release of excitatory gliotransmitters seems to promote seizures and neurodegeneration, it might not itself be sufficient to trigger the pathology. It is conceivable, however, that in line with other astrocytic dysfunctions, it constitutes a main cause of some forms of epilepsy. For instance, loss of astrocytic gap junctional coupling and the consequential impairments in K+ and glutamate homeostasis are critically involved in the initiation and progression of TLE18. Accordingly, enhanced gliotransmission in concert with impairment of homeostatic functions of astrocytes might drive neuronal networks into epileptiform activity.
Therapeutic potential of targeting gliotransmission
The need of alternative strategies for efficient therapies in patients with pharmacoresistant epilepsy provided a rational to determine whether glial dysfunctions, such as aberrant gliotransmission, represent promising therapeutic targets43,53. Work by Tian et al47 showed that the antiseizure medications (ASMs) valproate, gabapentin, and phenytoin suppress astrocytic Ca2+ signaling during experimental seizures suggesting that these drugs exert part of their therapeutic effects by inhibiting gliotransmission. Several pharmacological agents targeting different sites of pathological gliotransmitter release affected epileptogenesis in experimental models43,53. In this context, the P2Y1R antagonists MRS2179 or MRS2500 were repeatedly shown to inhibit Ca2+-mediated gliotransmission and also to exert antiseizure, antiepileptogenic and neuroprotective effects in experimental models, at least when administered after status epilepticus (SE)59,61–63, a recurrent seizure event that triggers epilepsy. Dossi et al57 reported that high K+-induced ictal discharges in postoperative human epilepsy brain tissue cause ATP release through Panx1 channels, although it remained unclear in that study whether ATP was released by neurons or astrocytes. Inhibition of these channels with probenecid or mefloquine (approved drugs for the treatment of gout and malaria) efficiently blocked ictal discharges in human cortical brain tissue slices and displayed anticonvulsive effects in a mouse model of acquired epilepsy57.
Deregulation of astrocytic metabotropic glutamate receptors (mGluR) has been described in epilepsy68,69. In particular, mGlu1R and mGlu5R antagonists (LY456236, LY367385, MPEP) were reported to decrease neuronal excitability in an in vitro model of focal seizures and in ex vivo brain slices from kindled rats, probably by attenuating glutamate release from both astrocytes and pre-synaptic terminals50,61. P2X7R antagonists, such as A43807958 or JNJ-4796556770, reduced SE severity and the consequent neurodegeneration or chronic seizures, respectively, in a kainate model of epilepsy, presumably by inhibiting neuroinflammation (see also Ref71). Drugs increasing GABA expression or release from astrocytes may also be of therapeutic interest in epilepsy, but to our knowledge such studies have not yet been conducted. Thus, although there is experimental evidence supporting the therapeutic potential of targeting gliotransmission in epilepsy, no compounds affecting this process have yet been investigated in clinical trials. This is likely because the mechanisms of action of the compounds showing anticonvulsive/antiepileptogenic effects in experimental models are often not well understood, or drugs are non-specific or have poor brain penetration and side effects57. A deeper understanding of the mechanisms and impact of astrocytic gliotransmitter release in the normal and diseased brain is needed to allow the development of more specific drugs with clinical relevance for the treatment of epilepsy.
Cell metabolism
As early as the late 1970s it was already recognized that “the dynamic system that controls glucose and lipid metabolism, and thus electrolyte balance, is abnormal” in persons with epilepsy72. It is now well established that seizures cause profound changes in metabolism, and that maladaptive changes in biochemical pathways contribute to seizure generation and the development of epilepsy and its progression73,74. Astrocytes play a key role in the regulation of biochemical pathways related to adenosine, glucose, glutamate and GABA metabolism, and transmethylation (Fig. 3), which constitute a new frontier for therapy development with the prospect to not only treat seizures, but to treat epilepsy, its development, and its underlying comorbidities more comprehensively75.
Figure 3. Key mechanisms through which metabolic dysfunction in astrocytes contributes to increased neuronal excitability.

Changes in key metabolic enzymes or transporters lead to an imbalance in the key neurotransmitters and neuromodulators glutamate, glutamine, GABA, and adenosine. Glycolysis can lead to the formation of lactate, which is a key energy metabolite for neurons supplied by the astrocyte neuron lactate shuttle. Adenosine in the cell nucleus, regulated by ADK-L contributes to epigenetic reprogramming as driver of the epileptogenic process. Consequently, metabolic therapies, such as ketogenic diet, glucose restriction, adenosine, and ketones can reduce epileptic activity through multiple mechanisms. Abbreviations: ADO – adenosine; ADK-L – long isoform of adenosine kinase; ADK-S – short isoform of adenosine kinase; ANLS – astrocyte neuron lactate shuttle; A1R – adenosine A1 receptor; DNA-CH3 – DNA methylation; Gln – glutamine; GLT-1 – astroglial glutamate transporter 1; Glu – glutamate; GS – glutamine synthetase; KD – ketogenic diet; LDH – lactate dehydrogenase.
Dysregulation of adenosine metabolism
Adenosine is an evolutionary ancient master regulator of energy homeostasis76. Any energy crisis broadly leads to a drop in ATP and a resulting increase in adenosine, which acts as global inhibitor of metabolic and physiological activity. From an energetic standpoint, an epileptic seizure is a state of excessive energy consumption, which prompts the generation of adenosine acting as endogenous anticonvulsant and seizure terminator77. Adenosine-based inhibitory activity in the brain is largely mediated by activation of Gi protein coupled adenosine A1 receptors, which can be blocked by the non-selective adenosine receptor antagonists caffeine and theophylline which therefore can have proconvulsant properties78. Maladaptive overexpression of the largely astrocyte-based adenosine metabolizing enzyme ADK has emerged as a pathological hallmark of experimental and human TLE79. Specifically, in multiple epilepsy models, reactive astrogliosis is associated with an overexpression of ADK and the resulting adenosine deficiency is not only sufficient to trigger seizures80 but also contributes to the epileptogenic process81. Consequently, adenosine augmentation is an effective therapeutic strategy, not only for the suppression of epileptic seizures82, but also to interrupt epileptogenesis81. Therefore, a derangement in adenosine metabolism affects the pathophysiology of epilepsy broadly and offers new metabolism-based therapeutic options, which directly interfere with the epileptogenic process83.
Dysregulation of glutamate, glutamine, and GABA metabolism
An additional astrocyte based metabolic enzyme of importance for the pathophysiology of epilepsy is GS. This enzyme aminates glutamate into glutamine, which, after transport into inhibitory or excitatory neurons, can be converted into GABA or re-converted into glutamate, respectively. Therefore, GS plays an important role in the regulation of the glutamate and GABA balance, which is under metabolic control because GABAergic neurons are more sensitive to an hypoenergetic state11,84. In line with this mechanism, loss of GS activity in human TLE can cause seizures and supports the epileptogenic process by promoting (i) an increase in extracellular glutamate, regulated by astrocytes, and (ii) a decrease in GABA production in neurons because of a decrease in glutamine supply11. Specifically, neuroinflammation through oxidative stress inhibits GS activity suggesting that these processes may affect GS in epilepsy27. Dysregulation of extracellular glutamate is further exacerbated by impaired astrocytic glutamate clearance through the glutamate transporter-1 (GLT-1), which is downregulated in the epileptic brain18,54,85. Indeed, the lack of GLT-1 in mice causes epilepsy and increased brain injury86. Together, the consequences of this metabolic derangement contribute to both ictogenesis and epileptogenesis.
Role of the astrocyte-neuron lactate shuttle
Epileptic neuronal networks have a high demand for energy. Excessive synaptic activity stimulates astrocytic glycolysis causing a rapid drop of glucose and a corresponding rise in lactate, which becomes an essential energy source for neurons. Increased ictal glycogen and glucose utilization lead to the formation of lactate from pyruvate through lactate dehydrogenase (LDH). Through this mechanism, astrocytes can potentially provide a significant supply of energy via the astrocyte-neuron lactate shuttle to sustain the energy demands of hyperactive neuronal networks87. The hypothesis that seizure-induced lactate formation enables epileptic activity is supported by findings that LDH inhibitors such as stiripentol provide robust anti-seizure effects88. In this scenario, LDH inhibition likely interferes with glycolysis by limiting the availability of NAD+ and supporting the oxidative mitochondrial metabolism of pyruvate. However, a recent study demonstrated that both astrocytic glycolysis and the astrocyte-neuron lactate shuttle depend on the energy sensor astroglial AMP activated protein kinase (AMPK)89. The genetic deletion of AMPK in mice led to the depletion of lactate and a reduction in seizure threshold, whereas the deletion of AMPK in astrocytes, but not in neurons, triggered neuronal cell loss in mice and flies89. This apparent contradiction demonstrates the complexity of astrocyte metabolism in the regulation of lactate and brain energy homeostasis, which are of crucial importance for the balance of seizure thresholds.
Dysregulation of transmethylation reactions in epilepsy
The methylation hypothesis of epileptogenesis posits that maladaptive changes in DNA methylation drive and propagate the epileptogenic process90. Indeed, multiple studies show that epigenetic processes and in particular increased DNA methyltransferase activity and aberrant DNA methylation are intricately linked to the epileptogenic process81,91. In surgically resected hippocampal and neocortical specimens from patients with TLE and hippocampal sclerosis, progressive changes in DNA methylation were associated with prolonged epilepsy duration and induction of genes related to inflammatory processes, lending further support to the notion that aberrant DNA methylation is linked to the epileptogenic process92. Metabolically, all transmethylation reactions, including DNA methylation, are linked to adenosine metabolism through ADK81,93. The genetic disruption or pharmacological inhibition of ADK leads to an increase in adenosine, which shifts the thermodynamic equilibrium of the S-adenosylhomocysteine hydrolase reaction towards the formation of S-adenosylhomocysteine, a potent inhibitor of DNA methyltransferases. Conversely, pathological overexpression of ADK as part of the epileptogenic process, drives increased DNA methylation and thereby promotes epileptogenesis81.
Metabolic therapies for epilepsy and its prevention
Because astrocytes play a key role as metabolic master regulator of brain activity, and because maladaptive changes in metabolism and basic biochemistry are linked to seizure generation and the epileptogenic process, astrocytes are attractive targets for metabolic therapies75 (Fig. 3).
Ketones, fatty acids, and glucose restriction
The most widely used metabolic therapy is a high-fat, low-carbohydrate ketogenic diet, which forces the brain to use ketone bodies rather than glucose as the main energy source, and has been in clinical use for a century. Ketogenic diets enhance ketone body production in the liver, but astrocytes constitute a major site for fatty acid oxidation in the brain and they are the only cell types in the brain capable of producing ketone bodies. Through multiple mechanisms directly or indirectly linked to glucose restriction and elevated ketone bodies94, ketogenic diets and related metabolic therapies suppress seizures in a wide spectrum of pharmacoresistant epilepsies95. Intriguingly, both in clinical studies, as well as in preclinical models, it was shown that ketogenic diet-based therapeutic interventions had lasting disease modifying, and possibly antiepileptogenic properties83. Of note, ketogenic diet therapy affects gene expression differentially in neurons and astrocytes, with a marked reduction of astrocytic gene expression related to pathways controlling glutamatergic signalling, endoplasmic reticulum processing, and insulin signalling. Because glucose restriction is one of the mechanisms of ketogenic diets, the disruption of glycolysis with the glycolytic inhibitor 2-deoxy-D-glucose (2DG) has been considered as an alternative to a strict dietary regimen96. In rodent models 2DG exerts acute antiseizure effects97 with the involvement of a presynaptic mechanism98 and delayed kinding development in rats97. Similarly, in a controlled cortical impact model of neurotrauma in mice, 2DG attenuated the development of epileptiform activities in in vitro cortical slice preparations and after one week of in vivo treatment immediately after injury, supporting disease modifying properties of metabolic therapies99. Together those findings support the notion that glucose restriction rather than ketone production provides key therapeutic benefits of metabolic therapies. This is important, because astrocytes take up glucose from the blood capillaries via the glucose transporter GLUT-1 and thereby assume a role as gatekeeper for energy homeostasis in the brain.
Adenosine for epilepsy prevention
An additional, ketone-independent mechanism of ketogenic diet therapy is an increase in adenosine, a multifunctional metabolite controlled by astrocytic metabolic clearance, in conjunction with downregulation of ADK, which is a likely explanation for the disease modifying properties of metabolic therapies100,101. Because maladaptive increases of ADK expression in astrocytes drive the epileptogenic process through increased DNA methylation, therapeutic adenosine augmentation is a rational approach for epilepsy prevention. Thus, adenosine delivered via cell-based brain implants suppressed both kindling epileptogenesis and the development of chronic seizures in kainate induced epilepsy models80,102,103. Adenosine delivered locally and transiently to the hippocampus of rats after the onset of epilepsy via silk-based implants prevented epilepsy progression81, and a transient systemic dose of a small molecule ADK inhibitor attenuated the epileptogenic process in mice after an intrahippocampal injection of kainate104. Because one isoform of ADK is specifically expressed in the cell nucleus (ADK-L) the opportunity exists to directly target nuclear adenosine metabolism in an effort to capitalize on the epigenetic mechanisms of adenosine, while minimizing excessive rise of adenosine in the extracellular space. Importantly, a transient treatment with adenosine (for ten days after the onset of epilepsy in rats) or an adenosine elevating drug given transiently during the latent period of epileptogenesis (for five days beginning with a delay of three days after an epilepsy triggering SE) was found to provide lasting suppression of epilepsy progression and development, respectively81,104. Early intervention would also mitigate additional risk factors associated with progression of epilepsy such as sudden unexpected death in epilepsy (SUDEP) and the development of comorbidities and ASM resistance. The epilepsy preventing properties of adenosine are in line with clinical findings showing that a single nucleotide polymorphism in the Adk gene constitutes a biomarker for increased risk of posttraumatic epileptogenesis after a traumatic brain injury105. Together, those studies demonstrate that transient metabolic treatments and strategies, which decrease astrocytic adenosine metabolism and thereby increase adenosine availability have the unique potential to disrupt the epileptogenic process via an epigenetic mechanism75,83. The transient use of preventative treatments would mitigate adverse effects associated with chronic treatment regimens in established epilepsy.
Immune and inflammatory functions
Astrocytes have been shown to initiate, regulate, and amplify immune-mediated mechanisms in different CNS diseases5,26. These cells represent a source of immunologically relevant cytokines and chemokines in both human and experimental epilepsy5,26,27. Reciprocally, an inflammatory phenotype in astrocytes can be induced by soluble molecules (cytokines, chemokines, danger signals) either released by surrounding parenchymal cells, like reactive microglia106,107 or the pericytes and the microvasculature108,109, or imported by blood leukocytes110. Spatio-temporal dynamics of glia activation in rodents after SE showed rapid microglia reactivity (within 24 h) leading to subsequent activation of astrocytes that exhibit IP3 R2-mediated Ca2+ hyperactivity24, and express inflammatory molecules25. Notably, these reactive astrocytes are essential for promoting chronic enhanced seizure susceptibility after SE24. Increased neuronal activity is sufficient to elicit biosynthesis and release of pro-inflammatory molecules by astrocytes, and from other brain cells, a mechanism defined “neurogenic neuroinflammation”111. In support, activated neurons release danger signals such as HMGB1112,113, ATP114, prostaglandins115, and complement factors116,117 that activate their cognate receptors in astrocytes thereby inducing their inflammatory phenotype. Pattern-recognition receptors (PRRs) are innate immunity receptors activated by endogenous danger signals which are pivotal for the induction of the inflammatory cascade in glia and peripheral immune cells118.
Immunohistochemical and large-scale transcriptomic studies in human brain tissue from TLE patients, and epileptogenic developmental pathologies, together with studies in animal models, showed induction of various inflammatory pathways in reactive astrocytes5,26,27, some of which contribute to hyperexcitability and seizures, as discussed later.
Below we describe the astrocyte innate immunity properties relevant for seizure generation, cell loss and neurological comorbidities in epilepsy.
Astrocyte dysfunction linked to their inflammatory state
Here we focus on the inflammatory phenotype of astrocytes, considering both cell autocrine and paracrine responses to cytokines and danger signals released following epileptogenic lesions or during recurrent seizures. Astrocytes are key players to sustain the neuroinflammatory response in epilepsy as shown by their transcriptomic profiles and by histological co-localization of inflammatory markers with astrocyte-specific markers27. The key role of the crosstalk between astrocytes and neuronal and non-neuronal cells, in particular microglia and the microvasculature, in hyperexcitability and seizure generation has been confirmed in genetic and acquired epilepsies, and most recently in glioneuronal tumor-related epileptogenesis5,26,119.
The following sections describe the neuromodulatory effects of inflammatory mediators expressed by astrocytes, and how inflammatory astrocytes lose their homeostatic functions5,26,120, thus contributing to an extracellular milieu permissive to hyperexcitability and cell death.
Neuronal hyperexcitability driven by cytokines and chemokines
Cytokines and chemokines possess neuromodulatory properties evoked by their neuronal receptors 118,121. In normal brain physiology, these immunity-related molecules play a role in synaptic transmission and plasticity121, however, upon overproduction they can induce network hyperexcitability and reduce seizure threshold by modifying the expression and the function of voltage-gated and receptor-coupled ion channels27,121. Cytokines released after brain injury or hyperthermia may induce astrocyte dysfunctions, including block of gap junction coupling, which results in impaired ion and transmitter homeostasis and the generation of spontaneous recurrent seizures18,122,123.
BBB dysfunction
Astrocytic endfeet ensheath blood vessels providing, together with endothelial cells and pericytes, structural barriers that promote vascular integrity and function124. Astrocytes contribute to the maintenance of tight-junctions between endothelial cells and regulate movements of water, soluble molecules, and immune cells across brain parenchyma. BBB permeability properties are altered in acquired epilepsies and this phenomenon occurs early on, and persists, after the epileptogenic injury in animal models124, and is often associated with newly generated leaky vessels125. Perivascular astrocytes are pivotally involved in structural and functional changes of the microvasculature in epilepsy by releasing cytokines, such as vascular endothelial growth factor (VEGF), IL-1β, HMGB1 and TNF, chemokines and downstream effectors, which activate their receptors in pericytes and endothelial cells of microvessels108,126. As a consequence, BBB permeability is affected at multiple levels, including disruption of tight-junctions, increased transendothelial vesicular transport, and leukocyte trafficking. A converging consequence of BBB dysfunction in epilepsy is brain extravasation of serum albumin and the subsequent activation of transforming growth factor receptor 2 (TGFβRII)-SMAD signaling in perivascular astrocytes. This leads to transcriptional activation of inflammatory genes and downregulation of Kir4.1, GLT-1, and AQP4 density as well as subcellular reorganization of Cx43 in astrocytic endfeet124,127. This chain of events reduces seizure thresholds and promotes epilepsy development in rodents. In accord, pharmacological blockade of TGFβRII-SMAD signaling for 3 weeks post-SE rescued some of the molecular changes in astrocytes and reduced epilepsy incidence and chronic seizure frequency124 (but see Ref128). Mimicking BBB dysfunction in rodents is sufficient to induce epileptogenesis124. Albumin induces TGFβ1 and inhibits astrocyte gap junction coupling129, thus reinforcing signaling activation, and elicits reactive astrogliosis through mitogen-activated protein kinase (MAPK) pathways130.
The close contact of astrocytes with BBB constituents allows the sensing of immune molecules released by circulating leukocytes and facilitates astrocyte and peripheral immune cell interactions, which may shape the astrocyte activation state and function.
Extracellular matrix and synaptic remodeling
Inflammatory astrocytes in human TLE and related animal models express high levels of matrix metallopeptidase family proteins under the control of TGFβR131 and IL-1β signalings132,133, thereby inducing extracellular matrix remodeling and pathological synaptic plasticity. In particular, astrocytes mediate excitatory synaptogenesis in vitro, and in vivo before epilepsy develops, by activation of TGFβR signaling124 and they are possibly involved in axonal sprouting134, and are implicated in the degradation of perineuronal nets around fast-spiking inhibitory interneurons, as shown in brain tissue from TLE patients and rodent models of acquired epilepsy124,135,136. These modifications are associated with hyperexcitability and spontaneous seizure generation, and they are prevented by TGFβR signaling blockers which also inhibited epilepsy development in animals124,136–138 (but see Ref128). Notably, endothelial cells activated in vitro by inflammatory stimuli can induce an extracellular matrix remodeling profile in astrocytic primary cultures109.
Treatment with an MMP2/9 inhibitor reduced seizure severity in the rapid-kindling model and decreased the number of chronic spontaneous seizures and cognitive deficits in the kainate model during seven days treatment post-SE and up to seven weeks after drug withdrawal133.
Oxidative stress
Neuroinflammation and oxidative stress (OS) are linked phenomena, and the “redox proteome” is intimately related to immune responses. There is evidence for the redox-based control of hyperexcitability and seizures in epilepsy139, which mainly involves astrocytes. Astrocytes are major producers of glutathione (GSH) from glutamate, cysteine and glycine, and the astrocyte-neuronal GSH shuttle replenishes the neuronal GSH pool. This provides a major antioxidant defence mechanism in the brain by neutralising reactive oxygen species (ROS) and hydrogen peroxide140. Biomarkers of OS [e.g., inducible nitric oxide, cysteine transporter (xCT), transcriptional nuclear factor Nrf2] are increased in astrocytes of rodent brain during epileptogenesis and human and rodent chronic epileptic foci141,142. Astrocytic mitochondrial function seems to be maintained during seizures, when neuronal mitochondria depolarize and fail143 suggesting that astrocytes provide sustained antioxidant defence. Moreover, increased neuronal firing activates astrocytic Nrf2, thus promoting antioxidant, anti-inflammatory and cytoprotective effects144. These homeostatic functions of astrocytes co-exist with their inflammatory features, in agreement with the complex transcriptomic spectrum of astrocytes described during epileptogenesis in a SE mouse model25, and in other CNS diseases7,107. To add to this complexity, a given molecular alteration may result in opposing functional outcomes. For example, elevated extracellular glutamate is a key contributor to cortical hyperexcitability, epileptiform activity, and excitotoxicity145. In addition to GLUT-1, extracellular glutamate is also controlled by the xCT antiporter system. Increased expression and activity of xCT in astrocytes not only promotes GSH biosynthesis but may also result in enhanced extracellular glutamate levels, which correlates with glioma-associated epilepsy146. Accordingly, sulfasalazine, the FDA-approved drug blocking xCT, reduces established seizures in vivo147.
Preclinical studies demonstrate that the altered redox status in epilepsy is sensitive to therapeutic targeting by activation of the Nrf2 pathway with brain-penetrant small molecules or replenishing the GSH pool with N-acetylcysteine. These interventions decrease established chronic seizures, but also arrest disease progression when administered after SE and before epilepsy develops, also affording neuroprotection and improvement of cognitive deficits27,141,142. This evidence supports that antioxidant defense mechanisms are inefficient in epilepsy, therefore their implementation may offer new therapeutic opportunities.
Dysregulation of iron metabolism
Recent evidence from genetic epilepsies due to mTOR pathway dysregulation, indicates that OS and iron metabolism are tightly linked and may act synergistically to exacerbate cell dysfunction in epilepsy148. Iron is a key element for CNS development and proper function, and astrocytes are crucial players for maintaining iron homeostasis149. Accordingly, high concentrations of unbound Fe2+ enhance the generation of ROS, thereby promoting cell dysfunction or death150. Intracerebral hemorrhages after stroke or neurotrauma, and the consequent epilepsy, are characterized by brain iron deposition, and intracortical iron injection evokes seizures in experimental models150. Moreover, BBB dysfunction may contribute to brain iron overload by leakage of iron-rich blood components. A recent study addressed iron metabolism-based alterations in surgically resected brain specimens from patients with TLE or in autoptic tissue from patients who died after SE, and in a rat model of TLE151. The study showed that iron overload is likely due to BBB leakage and it accumulates in neurons with pycnotic morphology, in microglia and astrocytes, the latter cells showing also elevated ferritin levels. Human astrocyte cultures challenged with iron and ROS increased their antioxidant and iron-binding capacity, but simultaneously developed pro-inflammatory features upon chronic exposure151. Thus, reducing iron uptake into astrocytes should reduce their inflammatory state with potential therapeutic effects in epilepsy149,150.
Mechanisms of drug-resistance
Release of glutamate and inflammatory mediators by perivascular astrocytes may contribute to up-regulation of multidrug transport proteins on BBB endothelial cells. These proteins, in particular p-glycoprotein (Pgp), are overexpressed at the luminal side of endothelial cells and astrocytic endfeet in TLE patients and in developmental glioneuronal lesions152. Pgp overexpression may limit access of several ASMs to their brain targets thus reducing their therapeutic effects and contributing to drug-resistance152. A strict association was found between astrocytic COX-2, prostaglandin E2 and Pgp expression in microvessels in brain specimens from TLE patients and animal models,153,154. Notably, COX-2 inhibitors prevented an increase in Pgp expression and function in rats with chronic seizures, and reversed ASM-resistance in these rats154.
Antiinflammatory therapies
Interventional studies in rodent models of epilepsy helped to establish that the activation of specific inflammatory pathways in brain cells lowers neuronal excitability threshold thereby promoting seizures, and reciprocally the ensuing seizures perpetuate neuroinflammation27. In particular, specific antiinflammatory treatments, also including anti-oxidant drugs, were shown to decrease both acute and chronic seizures in animal models27. Additionally, such drugs, transiently administered after SE either before the clinical onset of epilepsy or right afterwards, could interfere with epileptogenesis thus mediating strong reduction in ensuing seizures, neuroprotection and in some instances also improving cognitive deficits27,155. Since the molecular targets of these treatments are the ictogenic inflammatory molecules induced in reactive astrocytes, this cell population is likely to be significantly involved in the observed therapeutic effects.
Some drugs tested in animals are already in medical use for autoinflammatory or autoimmune diseases and are well-tolerated, therefore, they may be considered for treating patients with drug-resistant epilepsy, or individuals at high risk of developing seizures after acute brain injuries27,28. One recent example of the therapeutic response is represented by the compassionate use of the antiinflammatory drug anakinra, the human recombinant IL-1 receptor antagonist, in patients with unremitting seizures due to new onset refractory SE27,156, or the traditional use of immunosuppressive steroids in pediatric epileptic encephalopathies27.
In vivo monitoring of astrocyte reactivity
The availability of minimally invasive tools to monitor astrocyte activation in vivo may both help to better understand the astrocyte involvement in the pathophysiology of epilepsy and to discover biomarkers for epilepsy outcomes. To meet these goals, two complementary approaches have been developed in animals and humans, namely in vivo imaging of reactive astrocytes and measurement of astrocyte-associated molecules in biofluids (Fig. 4). These two approaches, and related tools and applied methodologies, permit in principle to monitor astrocytic activation during epileptogenesis in the same animal, and possibly in humans, and to investigate whether therapeutic interventions have an impact on astrocyte reactivity. Additionally, they may provide epilepsy biomarkers as diagnostic tools to identify an epileptic focus, to predict the effect of new treatments, or to prospectively identify the risk of developing epilepsy and pharmacoresistance. However, the evidence for the use of astrocyte-based biomarkers of epilepsy is currently still weak. In particular, the majority of relevant studies did not include a receiver operating characteristic (ROC) analysis of the results, the sample size of the analyzed cohorts is generally small, and validation cohorts for candidate biomarkers have been used rarely.
Figure 4. Imaging modalities and biofluids fingerprint of astrocyte reactivity.
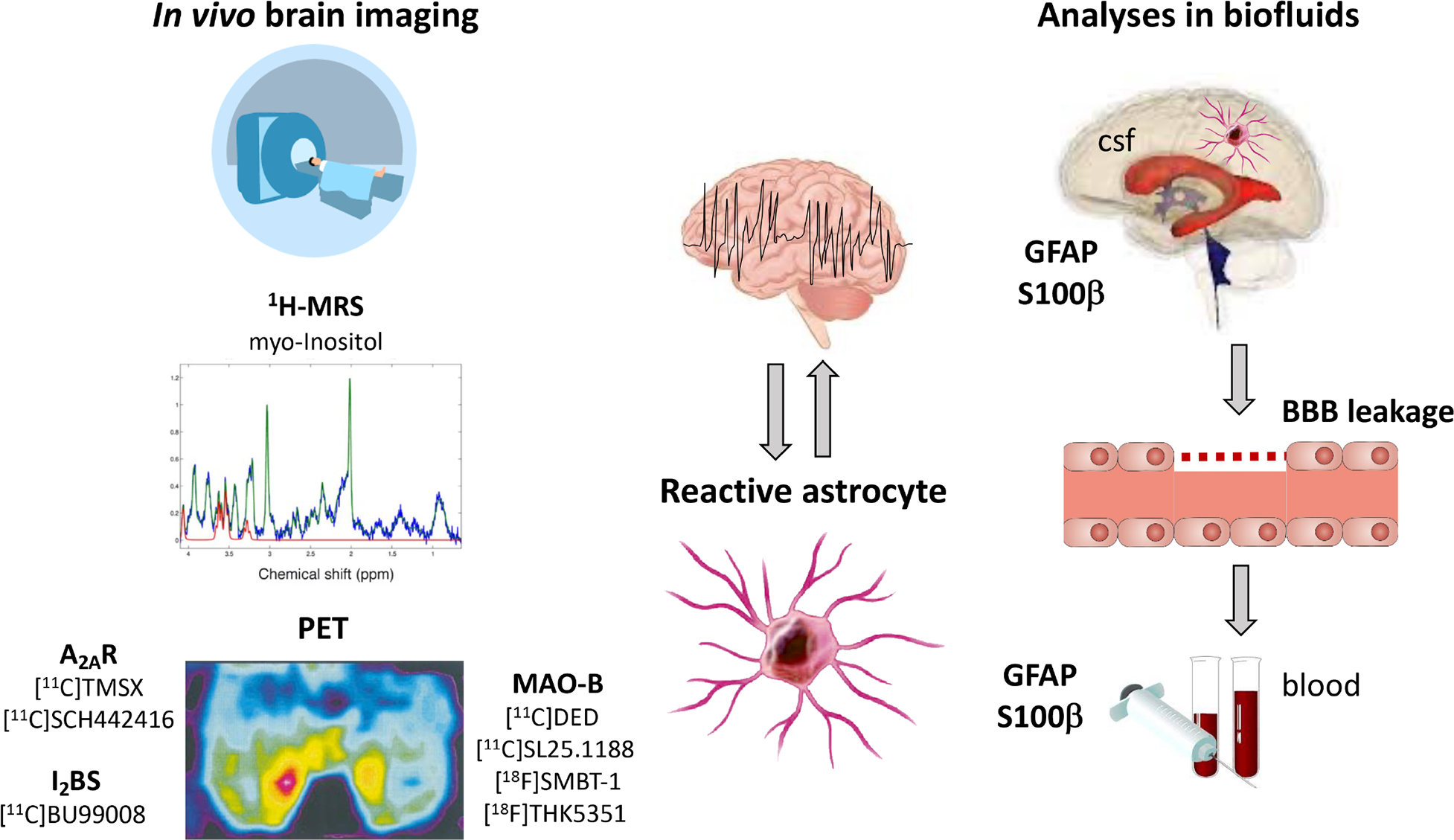
Brain imaging using proton magnetic resonance spectroscopy (1H-MRS) and positron emission tomography (PET) are minimally invasive tools to monitor astrocyte activation in vivo by targeting molecular markers enriched in reactive astrocytes, namely myo-Inositol, adenosine 2A receptors (A2AR), imidazoline2 binding sites (I2BS), monoamine-oxidase B (MAO-B). Additionally, analysis of circulating markers of astrocytes such as GFAP and S100β in blood or CSF may provide an index of astrocyte reactivity or injury. These astrocyte-related biomarkers may help to identify epileptic foci, to monitor epileptogenesis or the effect of therapies.
Moreover, the specificity of potential biomarkers for epilepsy compared with other CNS diseases has not been validated. Whether these candidate markers are useful for monitoring epileptogenesis in humans has not been tested as yet. Despite these limitations, neuroimaging and blood proteins have great potential as biomarkers due to minimal invasiveness, accessibility, and feasibility of quantification.
Imaging of reactive astrocytes
Human epilepsy.
Proton magnetic resonance spectroscopy (1H-MSR) and positron emission tomography (PET) are imaging techniques used in epilepsy patients to help identify the epileptogenic zone. 1H-MSR identifies various cell metabolites including myo-Inositol (mIns) which is considered an astrocyte marker157. 1H-MSR coupled with GFAP immunohistochemistry showed that increased mIns levels are associated with reactive astrogliosis157, supporting the view that mIns level mirrors alterations in astrocyte activation. mIns was increased in the epileptogenic zone of patients with structural epilepsies, namely TSC, Rasmussen’s encephalitis and TLE158–160. However, hippocampal mIns variably changed in TLE patients compared to non-neurological controls161 which may be due to the plethora of ASMs, the different 1H-MSR protocols used in the studies, or differences in seizures between the cohorts analysed. This variability may hinder the usefulness of mIns measurement to help detecting the epileptic focus.
An alternative molecular target of reactive astrocytes that has been tested in epilepsy is monoamine-oxidase B (MAO-B), an enzyme induced in astrocytes in pathological conditions. The autoradiographic binding of [3H]-L-deprenyl, an irreversible MAO-B inhibitor, was increased in surgical hippocampal tissue from TLE patients versus autopsy controls, and it correlated with histologically assessed reactive astrogliosis162. This study prompted PET development using [11C]Deuterium-deprenyl as a diagnostic tool for localizing the epilepsy focus. [11C]Deuterium-deprenyl signal was increased in the sclerotic hippocampus of TLE patients compared to non-neurological controls163,164, and the identification of the epileptic focus was equivalent to PET with [18F]FDG, the gold standard approach as determined in a subsequent validation study163. The diagnostic value of [11C]Deuterium-deprenyl was confirmed by single-photon emission computed tomography using the MAO-B inhibitor [123I]Ro43–0463165. The histopathological examination of the epileptogenic zone confirmed the presence of reactive astrogliosis. However, differently from seizures originating in the temporal lobe, the [11C]Deuterium-deprenyl signal was unchanged in patients with neocortical epilepsy thus highlighting potential mechanistic differences in glial functions or in the type of reactive gliosis between the two clinical conditions163. Optimized MAO-B-based PET tracers were recently developed in patients with psychiatric or neurodegenerative conditions166 fostering further application in epilepsy.
Additional astrocyte-based PET molecular targets were developed in chronic neurodegenerative diseases which may inform about their potential use in epilepsy. Examples are the imidazoline2 binding sites (I2BS) localized in the outer membrane of mitochondria in astrocytes167 and which regulate GFAP expression168, and adenosine A2A receptors expressed by reactive astrocytes in the hippocampus of TLE patients169. Notably, I2BS and A2A PET tracers were used to map disease progression in multiple sclerosis, Alzheimer’s disease and Parkinson disease166,170–172.
Experimental models.
1H-MSR was applied to experimental models of acquired epilepsy to measure mIns levels during epileptogenesis as an index of astrocytic reactivity to be correlated with pathological outcomes. In rat models of SE-induced epileptogenesis, hippocampal mIns increased within days after the acute injury173 and before the onset of spontaneous seizures174. The latter study established a negative correlation between mIns level and the extent of neuronal cell loss in epileptic rats 174. Morever, hippocampal mIns level predicted the onset of epilepsy in a SE rat model with 65% epilepsy incidence, and ROC analysis provided a measure of good predictive accuracy (AUC=0.83)175. This set of evidence suggests mIns is a potential prognostic biomarker of epileptogenesis which could be validated in humans exposed to epileptogenic injuries.
One study used [18F]-deprenyl autoradiography in ex vivo brain slices from SE-exposed rats for investigating astrocytic activation during epileptogenesis176. It was found that the signal increased in the epileptic circuitry during disease progression supporting further PET-based approaches.
Whether in vivo astrocyte imaging may help to develop pharmacodynamic biomarkers of drug effect is still unexplored. In support, neural stem cell transplantation, which provides anti-inflammatory effects in a mouse model of Alzheimer’s disease, was associated with both decreased astrocyte proliferation and mIns levels as assessed by 1H-MRS177.
Additional tools could be developed for monitoring astrocyte reactivity by exploiting existing imaging modalities that include astrocyte’s contribution to the generation of MRI or PET signal (Box 2).
BOX 2. Additional tools to monitor in vivo astrocyte reactivity.
[18F]FDG PET signal in human epilepsy foci is considered an index of neuronal glucose consumption during intense neuronal activity. Recent evidence re-evaluated the cell-specific contribution to [18F]FDG PET signal by considering that astrocytes are critical for glucose uptake and for coupling neuronal activity to glucose utilization201 (see above astrocyte-neuron lactate shuttle). This provides a new perspective for interpretation of [18F]FDG PET signal changes in epilepsy.
Diffusion-weighted MRI (DW-MRI) allows to detect changes in water molecules trajectories in brain parenchyma consequent to alterations in cell volume and/or water circulation in the extracellular space. Since reactive astrocytes are often characterized by hypertrophic cell body, in principle changes in DW-MRI-associated measures may reflect astrogliosis. In this context, an advanced DW-MRI sequence was recently developed to image astrocytes and microglia reactivity to inflammatory stimuli in the gray matter of rats, by building a multicompartment tissue model based on glial cell morphology202.
Functional MRI (fMRI) is based on the blood oxygenation level-dependent (BOLD) signal, which is considered a surrogate marker of neuronal activity. However, optogenetic astrocyte activation in the cortex of awake transgenic mice, whose cortical neurons or astrocytes express channel rhodopsin-2, evoked an fMRI BOLD response in the absence of changes in local neuronal activation203. This response was associated with oxidative glucose metabolism at the site of astrocyte, but not neuron, evoked signal, as assessed by mass spectrometry in brain sections. This evidence implies that astrocytes contribute to the generation of the BOLD signal, although the extent of this contribution in pathophysiological conditions awaits further investigation for a correct interpretation of data.
Measurements of astrocyte-associated molecules in biofluids
The majority of studies on circulating markers of astrocytes measured GFAP and S100β in blood or CSF with the intent of identifying potential biomarkers for epilepsy. The cerebral origin of these proteins was supported by studies showing increased CSF levels of GFAP in children with epilepsy of various aetiologies178 or S100β in adult TLE patients179 compared to respective controls. Since S100β is a releasable protein while GFAP is retained intracellularly in healthy astrocytes, their respective increase in biofluids is likely to reflect astrocyte reactivity or injury.
GFAP
Serum GFAP was higher in epilepsy patients with seizures of focal or generalized onset, although no correlation was found with seizure frequency or disease duration180,181. Notably, GFAP serum level was higher in patients with epileptic seizures compared with psychogenic seizures and controls180 offering a potential diagnostic tool to differentiate between the two types of seizures (ROC analysis provided an AUC=0.68), thus reducing misdiagnosis and the need of video-EEG monitoring. Moreover, children with epilepsy and well controlled seizures also displayed increased GFAP serum level vs controls, suggesting this protein may be a marker of the underlying pathology, rather than purely reflecting cell damage due to recurrent seizures181.
Recent studies reported anti-GFAP antibodies in blood -in the absence of neuronal antibodies- in ~5% of children and adult epilepsy patients182. This autoimmune response may occur in predisposed individuals as consequence of GFAP elevation in peripheral blood. Whether this is a bystander effect of seizures or contributes to epileptogenesis needs further investigation.
S100β
A systematic review and meta-analysis showed that blood level of S100β was significantly higher in epilepsy patients compared to healthy controls183. In particular, serum S100β levels were more elevated in TLE patients with hippocampal sclerosis compared to those without hippocampal sclerosis184, possibly reflecting the extent of reactive astrogliosis and the structural damage in the epileptic focus. Interpretation of these data, however, has to consider that S100β is also expressed by other cell types in the brain, including neurons and NG2 glia185.
Conclusions
Rapidly growing evidence from both human epilepsy and animal models has linked astrocyte dysfunction to neuronal network perturbations leading to seizures and cell loss. Since astrocytes are broadly involved in brain homeostasis, and oversee large number of synapses, their functional alterations may lead to widespread and progressive changes in neuronal network activation, thus contributing to the development and aggravation of epilepsy. Most of our knowledge on the role of astrocytes in epilepsy is derived from animal models, which have the unique advantage to offer the possibility of monitoring the epileptogenic process longitudinally, and which allow for the identification of commonalities in the pathogenic and/or homeostatic processes across models. However, it is important to consider that any preclinical work may have implicit bias based on the experimental rigor of each individual study as it pertains to factors such as reproducibility, blinding, statistical power, age, genetic background, and sex186. Importantly, the pathological role of astrocytes was intensively studied in human drug-resistant focal epilepsies, thanks to the availability of resected brain tissue, which allowed the validation of animal model findings. In general, findings from animal studies are in agreement with validation studies from human resected epileptogenic tissue. However, information on the role of astrocytes in genetic forms of epilepsy, although plausible, remains still elusive (Box 3). Supportive evidence includes the effect of gliotransmission on thalamo-cortical circuitry that is involved in genetic generalized epilepsy44, the evidence of reactive and inflammatory astrocytes in the somatosensory cortex of GAERS rats, an animal model of absence seizures187, or dysfunctional and inflammatory astrocytes in mouse models of genetic epileptic encephalopathy188, or Lafora progressive myoclonus epilepsy189.
BOX 3. Outstanding questions.
Heterogeneity of astrocyte properties and dysfunctions across brain regions is insufficiently understood
Which mechanisms astrocytes use for gliotransmitter release, whether multiple gliotransmitters are co-released by individual astrocytes and under which conditions, is still largely unknown
Pathophysiological effects of ATP release vs adenosine degradation, and their cognate receptors activation, need further elucidation: autocrine vs. paracrine effects, target cells, pre- vs post-synaptic effects
Information on the role of astrocytes in genetic forms of epilepsy remains elusive
Reactivity of astrocytes in epilepsy reflects complex combinations of compensatory and detrimental phenotypes, dynamics and consequences need better understanding
Selective drugs specifically targeting pathological mechanisms activated in astrocytes, with good BBB penetration, and limited side-effects are still needed
Although mechanistic investigations in epilepsy models highlight that several signaling pathways are modified in reactive astrocytes, there is still inconsistency and open questions (Box 3). This includes the role of ATP and purinergic receptors, or how the complexity of energy metabolism affects seizure threshold (cf. related sections), pointing out the need for further investigations. Moreover, the reactivity of astrocytes in epilepsy is accompanied by dynamic transcriptomic and molecular changes, reflecting complex combinations of compensatory and detrimental phenotypes during epileptogenesis. This is exemplified by inflammatory and OS mediators that may coexist with anti-inflammatory and detoxification molecules in astrocytes from the same brain area, and in some instances are co-expressed by the same cells27,190. Another point of consideration refers to the context-specific role of some signaling pathways activated in astrocytes, such as the dual role of P2Y1 receptors or COX-2 and prostaglandins in exacerbating or reducing seizures depending on timing of activation in the epilepsy model59,191. Single cell transcriptomics and accompanying functional approaches may help to clarify these aspects that are crucial for better understanding the consequences of astrocyte reactivity in epilepsy, and to guide focused therapeutic interventions. In order to elucidate the dynamic role of astrocytes in the pathogenesis of epilepsy, innovative cell-specific approaches may be used, which include gene therapy to express light-sensitive opsins that allow to modulate cell activation192, or gene editing with CRISPR-CAS technologies193, or chemogenetic compounds that selectively target a genetically-modified G protein-coupled receptor (DREADD) or a chimeric ion channel (PSAM)194. These new approaches have been mostly used for modulating neuronal activity but they can be extended to glial cells, and possibly envisaged as therapeutic interventions, although their clinical application is still remote.
Notably, before drugs targeting astrocyte dysfunctions are developed and validated in the clinic, it is crucial to elucidate the proper time of treatment initiation and duration during epilepsy development and to select the most suitable patient population. Animal models can help determining the dynamics of astrocytic changes and their consequences for pathological outcomes, but there are limitations for translating these animal-based dynamics to the clinical context. The identification of imaging biomarkers of astrocyte activation/reactivity states, or biofluid signatures reflecting astrocyte dysfunctional state (as discussed above), may help for designing effective treatment schedules and for patient stratification in clinical studies. However, validation of such biomarkers is required in prospective studies in larger cohorts of well-phenotyped patients with differing epilepsy etiology.
In the near future, astrocyte related research may open new opportunities to develop a largely unexplored therapeutic niche with more selective drugs specifically targeting pathological mechanisms, with good BBB penetration, and limited side-effects (Box 2). These may include modulators of gliotransmission and gap junction coupling, metabolic therapies, anti-inflammatory/anti-oxidant treatments, and drugs mitigating BBB dysfunction. A resolution of those processes that chiefly involve astrocytes during pathogenesis may offer new therapeutic opportunities for patients with pharmacoresistant epilepsies, and more broadly in CNS diseases associated with astrocytes dysfunctions195.
BOX 4. Literature search strategy.
We searched PubMed for peer-reviewed publications published between January 1990 and April 2022 with the term “astrocytes”. We then refined our search terms to be “astrocytes” AND (as individual combinatory terms) “epilepsy”, “epileptogenesis”, “seizure(s)”, “(neuro)inflammation”, “imaging”, “biomarker(s)”, “neurobiology”, “metabolism”, “gliotransmission”, “treatment(s)”. Selection criteria from full-text outputs were novelty of study findings and their relevance to neurologists, with inclusion decided collectively by all authors. One relevant historical reference outside the search timeframe was also included.
Key points.
In healthy conditions, astrocytes play key functions in the CNS which are compromised in disease states such as epilepsy, thus contributing to pathologic changes in synaptic transmission and to hyperexcitability.
The metabolic and biochemical underpinnings of epilepsy are tightly linked to astrocyte pathophysiology.
Astrocyte-mediated inflammation can promote epileptogenesis and seizure recurrence, especially when the endogenous resolution mechanisms fail.
Imaging of astrocyte activation states and related blood fingerprint of soluble molecules may represent prognostic and diagnostic biomarkers of epilepsy useful for patient stratification in clinical studies.
A resolution of those processes that chiefly involve astrocytes during pathogenesis constitute a new frontier for therapy development.
Acknowledgements
The authors gratefully acknowledge their sources of support Era-Net Neuron Ebio2, and American Epilepsy Society Seed Grant and NORSE Institute (AV), the Associazione Italiana Contro l’Epilessia (AICE-FIRE) (TR). DB is funded through the National Institutes of Health (NIH grants NS103740, NS065957, NS127846, the Office of the Assistant Secretary of Defense for Health Affairs through the Epilepsy Research Program under Award No. W81XWH2210638, and a Catalyst Award from CURE Epilepsy. CS acknowledges support by grants from the EU (H2020-MSCA-ITN project No 722053 EU-GliaPhD) and BMBF (16GW0182 CONNEXIN, 01DN20001 CONNEX). AE acknowledges support by grants from the EU (H2020-MSCA-ITN project No 722053 EU-GliaPhD; H2020-Twinning project EpiEpiNet (No 952455) and the Dutch Organization for Medical Sciences (ZonMw).
Footnotes
Competing interests
The authors declare no competing interests
References
- 1. Khakh BS & Deneen B The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42, 187–207 (2019). *This review emphasizes that astrocytes represent a diverse population of cells and that they display brain area- and disease-specific properties and functions. Knowledge gaps are identified that need to be addressed in order to elucidate astrocyte diversity and its physiological relevance in the CNS.
- 2.Archie SR, Al Shoyaib A & Cucullo L Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 13, 1779 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacVicar BA & Newman EA Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 7, a020388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araque A, Parpura V, Sanzgiri RP & Haydon PG Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22, 208–215 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC & Rogawski MA Glia and epilepsy: excitability and inflammation. Trends Neurosci 36, 174–84 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Verhoog QP, Holtman L, Aronica E & van Vliet EA Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front Neurol 11, 591690 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Escartin C et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24, 312–325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middeldorp J & Hol EM GFAP in health and disease. Prog Neurobiol 93, 421–443 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Griffin WS et al. Overexpression of the neurotrophic cytokine S100 beta in human temporal lobe epilepsy. J Neurochem 65, 228–33 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronica E et al. Expression and regulation of voltage-gated sodium channel beta1 subunit protein in human gliosis-associated pathologies. Acta Neuropathol 105, 515–523 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Eid T, Tu N, Lee T-SW & Lai JCK Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem Int 63, 670–681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griemsmann S et al. Characterization of Panglial Gap Junction Networks in the Thalamus, Neocortex, and Hippocampus Reveals a Unique Population of Glial Cells. Cereb Cortex 25, 3420–3433 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ammothumkandy A et al. Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nat Neurosci 25, 493–503 (2022). *This study reports that a longer duration of epilepsy is associated with a sharp decline in neuronal production and persistent numbers in astrogenesis. Further, immature neurons in MTLE are mostly inactive; however, immature astroglia are present in every MTLE case and their location and activity in the hippocampus are dependent on epileptiform-like activity.
- 14.Yang L et al. Astrocyte activation and memory impairment in the repetitive febrile seizures model. Epilepsy Res 86, 209–220 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Bedner P, Jabs R & Steinhäuser C Properties of human astrocytes and NG2 glia. Glia 68, 756–767 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Li J et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat Commun 12, 3958 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devinsky O et al. Epilepsy. Nat Rev Dis Primers 4, 18024 (2018). [DOI] [PubMed] [Google Scholar]
- 18. Bedner P et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138, 1208–1222 (2015). *This study provides the first direct evidence that loss of astrocytic gap junction coupling plays a key role in the initiation and progression of epilepsy.
- 19.Messing A, Brenner M, Feany MB, Nedergaard M & Goldman JE Alexander disease. J Neurosci 32, 5017–5023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feige L, Zaeck LM, Sehl-Ewert J, Finke S & Bourhy H Innate Immune Signaling and Role of Glial Cells in Herpes Simplex Virus- and Rabies Virus-Induced Encephalitis. Viruses 13, 2364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robel S et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci 35, 3330–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortinski PI et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13, 584–91 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao L et al. Silencing of circIgf1r plays a protective role in neuronal injury via regulating astrocyte polarization during epilepsy. FASEB J 35, e21330 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Sano F et al. Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus. JCI Insight 6, 135391 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maupu C et al. Diisopropylfluorophosphate-induced status epilepticus drives complex glial cell phenotypes in adult male mice. Neurobiol Dis 152, 105276 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Aronica E, Ravizza T, Zurolo E & Vezzani A Astrocyte immune response in epilepsy. Glia 60, 1258–68 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Vezzani A, Balosso S & Ravizza T Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15, 459–472 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Klein P et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 59, 37–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bushong EA, Martone ME, Jones YZ & Ellisman MH Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22, 183–192 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savtchouk I & Volterra A Gliotransmission: Beyond Black-and-White. J Neurosci 38, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiacco TA & McCarthy KD Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neurosci 38, 3–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parpura V et al. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Nedergaard M Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771 (1994). [DOI] [PubMed] [Google Scholar]
- 34. Bezzi P et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7, 613 (2004). *This study provides the first proof for the presence of transmitter vesicles in identified astrocytes in situ.
- 35.Bohmbach K, Schwarz MK, Schoch S & Henneberger C The structural and functional evidence for vesicular release from astrocytes in situ. Brain Res Bull 136, 65–75 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Fellin T et al. Neuronal Synchrony Mediated by Astrocytic Glutamate through Activation of Extrasynaptic NMDA Receptors. Neuron 43, 729–743 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Angulo MC, Kozlov AS, Charpak S & Audinat E Glutamate Released from Glial Cells Synchronizes Neuronal Activity in the Hippocampus. J Neurosci 24, 6920–6927 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jourdain P et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10, 331–339 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Araque A, Castillo PE, Manzoni OJ & Tonini R Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 124, 13–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henneberger C, Papouin T, Oliet SHR & Rusakov DA Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papouin T, Dunphy J, Tolman M, Foley JC & Haydon PG Astrocytic control of synaptic function. Philos Tran R Soc lond B Biol Sci 372, 20160154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araque A, Martín ED, Perea G, Arellano JI & Buño W Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22, 2443–2450 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beamer E, Kuchukulla M, Boison D & Engel T ATP and adenosine—Two players in the control of seizures and epilepsy development. Prog Neurobiol 204, 102105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon B-E & Lee CJ GABA as a rising gliotransmitter. Front Neural Circuits 8, 141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavus I et al. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57, 226–235 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Soukupova M et al. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscience 301, 246–253 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Tian G-F et al. An astrocytic basis of epilepsy. Nat Med 11, 973–981 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang N, Xu J, Xu Q, Nedergaard M & Kang J Astrocytic Glutamate Release-Induced Transient Depolarization and Epileptiform Discharges in Hippocampal CA1 Pyramidal Neurons. J Neurophysiol 94, 4121–4130 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G & Haydon PG Astrocytic Glutamate Is Not Necessary for the Generation of Epileptiform Neuronal Activity in Hippocampal Slices. J Neurosci 26, 9312–9322 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gómez-Gonzalo M et al. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol 8, e1000352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heuser K et al. Ca2+ Signals in Astrocytes Facilitate Spread of Epileptiform Activity. Cereb Cortex 28, 4036–4048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clasadonte J, Dong J, Hines DJ & Haydon PG Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proc Natl Acad Sci USA 110, 17540–17545 (2013). *This study shows that genetic inhibition of the astrocytic transmitter release machinery attenuates epileptiform activity and neurodegeneration through a pathway involving NMDARs.
- 53.Riquelme J, Wellmann M, Sotomayor-Zárate R & Bonansco C Gliotransmission: A Novel Target for the Development of Antiseizure Drugs. Neuroscientist 26, 293–309 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Seifert G, Hüttmann K, Schramm J & Steinhäuser C Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon’s horn sclerosis. J Neurosci 24, 1996–2003 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seifert G, Schilling K & Steinhauser C Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7, 194–206 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Beamer E, Conte G & Engel T ATP release during seizures - A critical evaluation of the evidence. Brain Res Bull 151, 65–73 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Dossi E et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci Transl Med 10, eaar3796 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Jimenez-Pacheco A et al. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 54, 1551–1561 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Alves M et al. Context-Specific Switch from Anti- to Pro-epileptogenic Function of the P2Y1 Receptor in Experimental Epilepsy. J Neurosci 39, 5377–5392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alves M et al. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia 58, 1603–1614 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Álvarez-Ferradas C et al. Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 63, 1507–1521 (2015). [DOI] [PubMed] [Google Scholar]
- 62. Nikolic L et al. Blocking TNFα-driven astrocyte purinergic signaling restores normal synaptic activity during epileptogenesis. Glia 66, 2673–2683 (2018). *This study revealed that pathologically elevated TNFα contributes to epileptogenesis, by boosting hyperexcitation through an autocrine mechanism involving purinergic signaling, astrocyte glutamate release, and modulation of neuronal transmitter release.
- 63.Wellmann M, Álvarez-Ferradas C, Maturana CJ, Sáez JC & Bonansco C Astroglial Ca2+-Dependent Hyperexcitability Requires P2Y1 Purinergic Receptors and Pannexin-1 Channel Activation in a Chronic Model of Epilepsy. Front Cell Neurosci 12, 446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alves M, Smith J & Engel T Differential Expression of the Metabotropic P2Y Receptor Family in the Cortex Following Status Epilepticus and Neuroprotection via P2Y1 Antagonism in Mice. Front Pharmacol 10, 558 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pavlov I & Walker MC Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology 69, 55–61 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Müller J et al. Astrocytic GABA Accumulation in Experimental Temporal Lobe Epilepsy. Front Neurol 11, 614923 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pandit S et al. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 68, 1065–1080 (2020). *This study shows that reactive astrocytes in experimental TLE aberrantly overproduce and release GABA, which in turn inhibits neuronal excitability and network activity through activation of tonic GABAAR-mediated currents in excitatory neurons.
- 68.Aronica E et al. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci 12, 2333–44 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Aronica E et al. Ionotropic and metabotropic glutamate receptor protein expression in glioneuronal tumours from patients with intractable epilepsy. Neuropathol Appl Neurobiol 27, 223–237 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Jimenez-Pacheco A et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J Neurosci 36, 5920–32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer W et al. Critical Evaluation of P2X7 Receptor Antagonists in Selected Seizure Models. PLOS ONE 11, e0156468 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Natelson S, Miletich DJ, Seals CF, Visintine DJ & Albrecht RF Clinical biochemistry of epilepsy. I. Nature of the disease and a review of the chemical findings in epilepsy. Clin Chem 25, 889–897 (1979). [PubMed] [Google Scholar]
- 73.Boison D The Biochemistry and Epigenetics of Epilepsy: Focus on Adenosine and Glycine. Front Mol Neurosci 9, 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel M A Metabolic Paradigm for Epilepsy. Epilepsy Curr 18, 318–322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boison D & Steinhäuser C Epilepsy and astrocyte energy metabolism. Glia 66, 1235–1243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boison D & Yegutkin GG Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell 36, 582–596 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.During MJ & Spencer DD Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 32, 618–624 (1992). [DOI] [PubMed] [Google Scholar]
- 78.Boison D Methylxanthines, seizures, and excitotoxicity. Handb Exp Pharmacol 200, 251–266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aronica E, Sandau US, Iyer A & Boison D Glial adenosine kinase--a neuropathological marker of the epileptic brain. Neurochem Int 63, 688–695 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li T et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest 118, 571–582 (2008). *This work unravelled a novel astrocyte-based mechanism of epileptogenesis, which forms the basis for the adenosine kinase hypothesis of epileptogenesis.
- 81. Williams-Karnesky RL et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Invest 123, 3552–3563 (2013). *This work describes the discovery of a novel function of an isoform of adenosine kinase, expressed in the cell nucleus, as regulator of DNA methylation. Therapeutic adenosine augmentation prevents maladaptive DNA methylation and thereby prevents epileptogenesis.
- 82.Gouder N, Fritschy J-M & Boison D Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia 44, 877–885 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Boison D & Rho JM Epigenetics and epilepsy prevention: The therapeutic potential of adenosine and metabolic therapies. Neuropharmacology 167, 107741 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sandhu MRS et al. Astroglial Glutamine Synthetase and the Pathogenesis of Mesial Temporal Lobe Epilepsy. Front Neurol 12, 665334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peterson AR & Binder DK Astrocyte Glutamate Uptake and Signaling as Novel Targets for Antiepileptogenic Therapy. Front Neurol 11, 1006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tanaka K et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702 (1997). *This seminal paper provides the first direct evidence that dysregulation of astrocytic glutamate homeostasis plays a key role in epilepsy and its development.
- 87.Pellerin L & Magistretti PJ Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91, 10625–10629 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sada N, Lee S, Katsu T, Otsuki T & Inoue T Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347, 1362–1367 (2015). *This landmark paper discovered lactate dehydrogenase as major metabolic component and therapeutic target for the treatment of epilepsy. Importantly, clinically used drugs are already available to target this metabolic mechanism.
- 89.Muraleedharan R et al. AMPK-Regulated Astrocytic Lactate Shuttle Plays a Non-Cell-Autonomous Role in Neuronal Survival. Cell Rep 32, 108092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobow K & Blumcke I The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia 52 Suppl 4, 15–9 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Miller-Delaney SFC et al. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 138, 616–631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martins-Ferreira R et al. Epilepsy progression is associated with cumulative DNA methylation changes in inflammatory genes. Prog Neurobiol 209, 102207 (2022). [DOI] [PubMed] [Google Scholar]
- 93.Boison D et al. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA 99, 6985–6990 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simeone TA, Simeone KA, Stafstrom CE & Rho JM Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 133, 233–241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.deCampo DM & Kossoff EH Ketogenic dietary therapies for epilepsy and beyond. Curr Opin Clin Nutr Metab Care 22, 264–268 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Ockuly JC et al. Behavioral, cognitive, and safety profile of 2-deoxy-2-glucose (2DG) in adult rats. Epilepsy Res 101, 246–252 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Stafstrom CE et al. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol 65, 435–447 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan Y-Z, Sutula TP & Rutecki PA 2-Deoxy-d-glucose reduces epileptiform activity by presynaptic mechanisms. J Neurophysiol 121, 1092–1101 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Koenig JB et al. Glycolytic inhibitor 2-deoxyglucose prevents cortical hyperexcitability after traumatic brain injury. JCI Insight 5, 126506 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lusardi TA et al. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology 99, 500–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Masino SA et al. A ketogenic diet suppresses seizures in mice through adenosine A₁ receptors. J Clin Invest 121, 2679–2683 (2011). *This paper describes the discovery of a novel mechanism through which a high-fat low-carbohydrate metabolic treatment (ketogenic diet) prevents seizures in epilepsy.
- 102.Li T et al. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain 130, 1276–1288 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Li T, Ren G, Kaplan DL & Boison D Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res 84, 238–241 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandau US et al. Transient use of a systemic adenosine kinase inhibitor attenuates epilepsy development in mice. Epilepsia 60, 615–625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Diamond ML et al. Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia 56, 1198–1206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eyo UB, Murugan M & Wu L-J Microglia-Neuron Communication in Epilepsy. Glia 65, 5–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). *This paper shows that reactive microglia by secreting inflammatory cytokines induces dysfunctions in astrocytes that lose their homeostatic and cell survival promoting functions while inducing the death of neurons and oligodendrocytes.
- 108.Giannoni P et al. The pericyte-glia interface at the blood-brain barrier. Clin Sci 132, 361–374 (2018). [DOI] [PubMed] [Google Scholar]
- 109. Taylor X et al. Activated endothelial cells induce a distinct type of astrocytic reactivity. Commun Biol 5, 282 (2022). *This paper shows that a subtype of neurotoxic astrocytes is induced by activated endothelial cells that is distinct from astrocytes activated by microglia. These astrocytes exhibit a distinctive extracellular matrix remodeling profile.
- 110.Varvel NH et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci USA 113, E5665–74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xanthos DN & Sandkuhler J Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15, 43–53 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Maroso M et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16, 413–9 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Zurolo E et al. Activation of TLR, RAGE and HMGB1 signaling in malformations of cortical development. Brain 134, 1015–32 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Fields RD Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol 22, 214–219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rojas A, Chen D, Ganesh T, Varvel NH & Dingledine R The COX-2/prostanoid signaling cascades in seizure disorders. Expert Opin Ther Targets 23, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gruber V-E et al. Increased expression of complement components in tuberous sclerosis complex and focal cortical dysplasia type 2B brain lesions. Epilepsia 63, 364–374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benson MJ et al. A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models. Neurobiol Dis 76, 87–97 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Vezzani A, Maroso M, Balosso S, Sanchez MA & Bartfai T IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25, 1281–9 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Zimmer TS et al. Tuberous Sclerosis Complex as Disease Model for Investigating mTOR-Related Gliopathy During Epileptogenesis. Front Neurol 11, 1028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Binder DK & Steinhauser C Functional changes in astroglial cells in epilepsy. Glia 54, 358–68 (2006). [DOI] [PubMed] [Google Scholar]
- 121.Vezzani A & Viviani B Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Khan D et al. Experimental febrile seizures impair interastrocytic gap junction coupling in juvenile mice. J Neurosci Res 94, 804–813 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Deshpande T et al. Constitutive deletion of astrocytic connexins aggravates kainate-induced epilepsy. Glia 68, 2136–2147 (2020). [DOI] [PubMed] [Google Scholar]
- 124.Löscher W & Friedman A Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int J Mol Sci 21, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Lanen RH et al. Microvascular changes associated with epilepsy: A narrative review. J Cereb Blood Flow Metab 41, 2492–2509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamanaka G et al. The Neuroinflammatory Role of Pericytes in Epilepsy. Biomedicines 9, 759 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deshpande T et al. Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 65, 1809–1820 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Henning L, Steinhäuser C & Bedner P Initiation of Experimental Temporal Lobe Epilepsy by Early Astrocyte Uncoupling Is Independent of TGFβR1/ALK5 Signaling. Front Neurol 12, 660591 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Braganza O et al. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia 53, 1898–906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ralay Ranaivo H & Wainwright MS Albumin activates astrocytes and microglia through mitogen-activated protein kinase pathways. Brain Res 1313, 222–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ralay Ranaivo H, Hodge JN, Choi N & Wainwright MS Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J Neuroinflammation 9, 68 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Korotkov A et al. Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of microRNA-155 in cultured human astrocytes. J Neuroinflammation 15, 211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Broekaart DW et al. The matrix metalloproteinase inhibitor IPR-179 has antiseizure and antiepileptogenic effects. J Clin Invest 131, 138332 (2021). *This study shows that increased MMPs expression in astrocytes is a prominent hallmark of the human epileptogenic brain and that MMP inhibition mediates antiseizure and antiepileptogenic effects in rodent epilepsy models and attenuates seizure-induced cognitive decline.
- 134.Twible C, Abdo R & Zhang Q Astrocyte Role in Temporal Lobe Epilepsy and Development of Mossy Fiber Sprouting. Front Cell Neurosci 15, 725693 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rankin-Gee EK et al. Perineuronal net degradation in epilepsy. Epilepsia 56, 1124–1133 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Kim SY et al. TGFβ signaling is associated with changes in inflammatory gene expression and perineuronal net degradation around inhibitory neurons following various neurological insults. Sci Rep 7, 7711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pollock E, Everest M, Brown A & Poulter MO Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis 70, 21–31 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Chaunsali L, Tewari BP & Sontheimer H Perineuronal Net Dynamics in the Pathophysiology of Epilepsy. Epilepsy Curr 21, 273–281 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parsons ALM et al. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants (Basel) 11, 157 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dringen R Metabolism and functions of glutathione in brain. Prog Neurobiol 62, 649–71 (2000). [DOI] [PubMed] [Google Scholar]
- 141.Pearson-Smith JN & Patel M Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int J Mol Sci 18, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Terrone G, Balosso S, Pauletti A, Ravizza T & Vezzani A Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 167, 107742 (2020) *This review paper reports evidence for disease-modifications mediated by combinations of anti-inflammatory and anti-oxidant treatments in animal models of acquired epilepsy.
- 143.Kovac S, Domijan A-M, Walker MC & Abramov AY Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death and Disease 5, e1442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Habas A, Hahn J, Wang X & Margeta M Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci USA 110, 18291–18296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Patel DC, Tewari BP, Chaunsali L & Sontheimer H Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci 20, 282–297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sørensen MF et al. High expression of cystine-glutamate antiporter xCT (SLC7A11) is an independent biomarker for epileptic seizures at diagnosis in glioma. J Neurooncol 138, 49–53 (2018). [DOI] [PubMed] [Google Scholar]
- 147.Alcoreza O, Jagarlamudi S, Savoia A, Campbell SL & Sontheimer H Sulfasalazine decreases astrogliosis-mediated seizure burden. Epilepsia 63, 844–854. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zimmer TS et al. Chronic activation of anti-oxidant pathways and iron accumulation in epileptogenic malformations. Neuropathol Appl Neurobiol 46, 546–563 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh N et al. Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid Redox Signal 20, 1324–1363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen S et al. Iron Metabolism and Ferroptosis in Epilepsy. Front Neurosci 14, 601193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zimmer TS et al. Seizure-mediated iron accumulation and dysregulated iron metabolism after status epilepticus and in temporal lobe epilepsy. Acta Neuropathol 142, 729–759 (2021). *This study suggests that seizure-mediated, chronic neuronal iron uptake plays a role in neuronal dysfunction/loss in TLE-HS. On the other hand, astrocytes sequester iron, specifically in chronic epilepsy. This function might transform astrocytes into a highly resistant, pro-inflammatory phenotype potentially contributing to pro-epileptogenic inflammatory processes.
- 152.Löscher W, Potschka H, Sisodiya SM & Vezzani A Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol Rev 72, 606–638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weidner LD et al. The expression of inflammatory markers and their potential influence on efflux transporters in drug-resistant mesial temporal lobe epilepsy tissue. Epilepsia 59, 1507–1517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potschka H Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia 51, 1333–1347 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Terrone G, Frigerio F, Balosso S, Ravizza T & Vezzani A Inflammation and reactive oxygen species in status epilepticus: Biomarkers and implications for therapy. Epilepsy Behav 101(Pt B), 106275 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Lai Y-C et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol 7, 2467–2474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harris JL, Choi I-Y & Brooks WM Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci 7, 202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matsuo N et al. The correlation between 1H-MR spectroscopy and clinical manifestation with tuberous sclerosis complex. Neuropediatrics 38, 126–129 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Hammen T et al. Non-invasive detection of hippocampal sclerosis: correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed 21, 545–52 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Turkdogan-Sozuer D, Ozek MM, Sav A, Dincer A & Pamir MN Serial MRI and MRS studies with unusual findings in Rasmussen’s encephalitis. Eur Radiol 10, 962–6 (2000). [DOI] [PubMed] [Google Scholar]
- 161.Obenaus A Neuroimaging biomarkers for epilepsy: advances and relevance to glial cells. Neurochem Int 63, 712–718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumlien E, Hilton-Brown P, Spannare B & Gillberg PG In vitro quantitative autoradiography of [3H]-L-deprenyl and [3H]-PK11195 binding sites in human epileptic hippocampus. Epilepsia 33, 610–7 (1992). [DOI] [PubMed] [Google Scholar]
- 163.Kumlien E, Nilsson A, Hagberg G, Langstrom B & Bergstrom B PET with 11C-deuterium-deprenyl and 18F-FDG in focal epilepsy. Acta Neurol Scand 103, 360–366 (2001). [DOI] [PubMed] [Google Scholar]
- 164. Kumlien E et al. Positron emission tomography with [11C]deuterium-deprenyl in temporal lobe epilepsy. Epilepsia 36, 712–21 (1995). *This study provides the first clinical evidence of imaging astrocytes in epilepsy patients using PET
- 165.Buck A et al. Monoamine oxidase B signle-photon emission tomography with [123]Ro 43–0463: imaging in volunteers and patients with temporal lobe epilepsy. Eur J Nucl Med 25, 464–470 (1998). [DOI] [PubMed] [Google Scholar]
- 166. Harada R et al. Imaging of Reactive Astrogliosis by Positron Emission Tomography. Front Neurosci 16, 807435 (2022). *This article presents an extensive overview of the tools available to image reactive astrogliosis using PET tracers in the clinical setting.
- 167.Regunathan S, Feinstein DL & Reis DJ Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J Neurosci Res 34, 681–688 (1993). [DOI] [PubMed] [Google Scholar]
- 168.Olmos G, Alemany R, Escriba PV & García-Sevilla JA The effects of chronic imidazoline drug treatment on glial fibrillary acidic protein concentrations in rat brain. Br J Pharmacol 111, 997–1002 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Patodia S et al. Adenosine kinase and adenosine receptors A1 R and A2A R in temporal lobe epilepsy and hippocampal sclerosis and association with risk factors for SUDEP. Epilepsia 61, 787–797 (2020). [DOI] [PubMed] [Google Scholar]
- 170.Matthews PM & Datta G Positron-emission tomography molecular imaging of glia and myelin in drug discovery for multiple sclerosis. Expert Opin Drug Discov 10, 557–570 (2015). [DOI] [PubMed] [Google Scholar]
- 171.Wilson H et al. Imidazoline 2 binding sites reflecting astroglia pathology in Parkinson’s disease: an in vivo 11C-BU99008 PET study. Brain 142, 3116–3128 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Sun M-J, Liu F, Zhao Y-F & Wu X-A In Vivo Positron Emission Tomography Imaging of Adenosine A2A Receptors. Front Pharmacol 11, 599857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu Y et al. Metabolic changes in early poststatus epilepticus measured by MR spectroscopy in rats. J Cereb Blood Flow Metab 35, 1862–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Filibian M et al. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 53, 1907–16 (2012). [DOI] [PubMed] [Google Scholar]
- 175. Pascente R et al. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol Dis 93, 146–155 (2016). *This article identifies hippocampal mIns levels measured using 1H-MRS as prognostic biomarker of epileptogenesis in experimental models.
- 176.Bascuñana P et al. Ex vivo characterization of neuroinflammatory and neuroreceptor changes during epileptogenesis using candidate positron emission tomography biomarkers. Epilepsia 60, 2325–2333 (2019). [DOI] [PubMed] [Google Scholar]
- 177.Chen S-Q et al. (1)H-MRS evaluation of therapeutic effect of neural stem cell transplantation on Alzheimer’s disease in AβPP/PS1 double transgenic mice. J Alzheimers Dis 28, 71–80 (2012). [DOI] [PubMed] [Google Scholar]
- 178.Gurnett CA, Landt M & Wong M Analysis of cerebrospinal fluid glial fibrillary acidic protein after seizures in children. Epilepsia 44, 1455–1458 (2003). [DOI] [PubMed] [Google Scholar]
- 179.Steinhoff BJ et al. Cisternal S100 protein and neuron-specific enolase are elevated and site-specific markers in intractable temporal lobe epilepsy. Epilepsy Res 36, 75–82 (1999). [DOI] [PubMed] [Google Scholar]
- 180. Simani L, Elmi M & Asadollahi M Serum GFAP level: A novel adjunctive diagnostic test in differentiate epileptic seizures from psychogenic attacks. Seizure 61, 41–44 (2018). *This article reports clinical evidence that GFAP serum level distinguish epileptic vs psychogenic seizures.
- 181.Elhady M et al. Circulating glial fibrillary acidic protein and ubiquitin carboxy-terminal hydrolase-L1 as markers of neuronal damage in children with epileptic seizures. Childs Nerv Syst 37, 879–884 (2021). [DOI] [PubMed] [Google Scholar]
- 182.Savaş M et al. Glial fibrillary acidic protein (GFAP)-antibody in children with focal seizures of undetermined cause. Acta Neurol Belg 121, 1275–1280 (2021). [DOI] [PubMed] [Google Scholar]
- 183. Liang K-G et al. Increased Serum S100B Levels in Patients With Epilepsy: A Systematic Review and Meta-Analysis Study. Front Neurosci 13, 456 (2019). *This paper reports a meta-analysis demonstrating that blood level of S100β are increased in epilepsy patients compared to healthy controls.
- 184.Lu C et al. Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: a case-control study. Neurosci Lett 484, 139–142 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Moshrefi-Ravasdjani B et al. Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct Funct 222, 831–847 (2017). [DOI] [PubMed] [Google Scholar]
- 186.Galanopoulou AS, French JA, O’Brien T & Simonato M Harmonization in preclinical epilepsy research: A joint AES/ILAE translational initiative. Epilepsia 58 (Suppl 4), 7–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Akin D et al. IL-1beta is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence. Neurobiol Dis 44, 259–69 (2011). [DOI] [PubMed] [Google Scholar]
- 188.Thompson JA et al. Astrocyte reactivity in a mouse model of SCN8A epileptic encephalopathy. Epilepsia Open 7, 280–292 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lahuerta M et al. Reactive Glia-Derived Neuroinflammation: a Novel Hallmark in Lafora Progressive Myoclonus Epilepsy That Progresses with Age. Mol Neurobiol 57, 1607–1621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arena A et al. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: molecular insights into their interdependence. Brain Pathol 29, 351–365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang J & Dingledine R Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci 34, 413–423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Borodinova AA, Balaban PM, Bezprozvanny IB, Salmina AB & Vlasova OL Genetic Constructs for the Control of Astrocytes’ Activity. Cells 10, 1600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Duarte F & Déglon N Genome Editing for CNS Disorders. Front Neurosci 14, 579062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bradley SJ, Tobin AB & Prihandoko R The use of chemogenetic approaches to study the physiological roles of muscarinic acetylcholine receptors in the central nervous system. Neuropharmacology 136, 421–426 (2018). [DOI] [PubMed] [Google Scholar]
- 195.Lee H-G, Wheeler MA & Quintana FJ Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov 21, 339–358 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McTague A, Howell KB, Cross JH, Kurian MA & Scheffer IE The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol 15, 304–316 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Northrup H et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr Neurol 123, 50–66 (2021). [DOI] [PubMed] [Google Scholar]
- 198.Kondo T et al. Modeling Alexander disease with patient iPSCs reveals cellular and molecular pathology of astrocytes. Acta Neuropathol Commun 4, 69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sase S, Takanohashi A, Vanderver A & Almad A Astrocytes, an active player in Aicardi-Goutières syndrome. Brain Pathol 28, 399–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.van Heteren JT et al. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutières syndrome. Glia 56, 568–578 (2008). [DOI] [PubMed] [Google Scholar]
- 201.Magistretti PJ & Pellerin L The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry 1, 445–452 (1996). [PubMed] [Google Scholar]
- 202.Garcia-Hernandez R et al. Mapping microglia and astrocytes in vivo using diffusion MRI. Sci Adv 8, eabq2923 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Takata N et al. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia 66, 2013–2023 (2018). [DOI] [PubMed] [Google Scholar]


