Abstract
Aims
The primary objective of the Nurse‐led Intervention for Less Chronic Heart Failure (NIL‐CHF) Study is to develop a programme of care that cost‐effectively prevents the development of chronic heart failure (CHF).
Methods
NIL‐CHF is a randomized controlled trial of a hybrid, home‐ and clinic‐based, nurse‐led multidisciplinary intervention targeting hospitalized patients at risk of developing CHF. A target of 750 patients aged ≥45 years will be exposed to usual post‐discharge care or the NIL‐CHF intervention. The composite primary endpoint is all‐cause mortality or CHF‐related admission during 3–5 years of follow‐up. After 12 months recruitment, ∼300 eligible patients (40% of target) have been randomized. Overall, 73% are male and the mean age is 65 ± 10 years. The most common antecedents for CHF thus far are hypertension (70%, 95% CI, 64–75%), coronary artery disease (51%, 95% CI, 31–41%), and type 2 diabetes (26%, 95% CI, 21–31%), whereas 76% (95% CI, 69–82%) of patients have diastolic dysfunction, 29% (95% CI, 23–36%) left ventricular hypertrophy, 71% (95% CI, 64–78%) mitral valve dysfunction, and 7% (95% CI, 4–12%) have a left ventricular ejection fraction ≤45%.
Conclusion
As one of the largest randomized studies of its kind, NIL‐CHF will ultimately provide important insights into the potential to prevent CHF via prolonged and intensive disease management.
Keywords: Chronic heart failure, Prevention, Disease management, Hypertension
Introduction
Chronic heart failure (CHF) continues to be a deadly and disabling syndrome that has not only reached epidemic proportions within the ageing populations of high income countries,1 but is now appearing in low‐to‐middle income countries.2 The continued adverse impact of this complex syndrome defies the contemporary development and introduction of new pharmacological agents, devices, and management programmes.3 These have undoubtedly improved the prognosis of affected patients4 without directly addressing the root cause of CHF, which is inadequately controlled or managed antecedents (commonly atherosclerosis, hypertension, and/or type 2 diabetes) that leave affected individuals at risk of developing cardio‐renal dysfunction.1
It is within this context that we are currently in the process of refining our approach to optimizing the management of essentially ‘high‐risk’ patients to prevent cardiac dysfunction. This process essentially involves bridging the gap between predominantly short‐term secondary prevention programmes (i.e. cardiac rehabilitation)5 and specialist CHF programmes6 by focusing on longer term strategies. Despite the disappointment of a trial focusing on inherently low‐risk patients with CHF7 and the recently reported results of the COACH Study,8 the rationale for undertaking trials such as the STOP‐HF Study9 are well founded.10 The prospect of reducing the number of individuals who develop CHF within our ageing populations and therefore requiring a combination of costly and resource‐intensive pharmacological agents, devices, and management programmes is too attractive and desirable an outcome to be ignored.
Study design
Study rationale
Although there are effective treatment options and expert guidelines available for patients with chronic forms of cardiovascular disease (CVD) that precede the development of CHF, suboptimal outcomes persist due to a combination of several factors which may be important including: (i) significant gaps in the overall application of gold‐standard therapeutics to at‐risk individuals; (ii) the lack of flexible health‐care models to overcome individual factors such as treatment non‐adherence, poor knowledge, and suboptimal self‐care behaviours; and (iii) suboptimal application of adjunctive non‐pharmacological strategies (e.g. exercise programmes). Importantly, there are clear and additional theoretical benefits beyond simply applying cardio‐protective pharmacological agents.
Within the context of identifying high‐risk patients and applying optimal preventative strategies, there are two priority areas for the prevention of CHF within our ageing populations. First, screening for and optimally managing those most at risk of developing CHF in the primary care setting is a main concern, usually by detecting those with asymptomatic left ventricular systolic dysfunction who, for example, have an elevated brain natriuretic peptide level.11 This is the focus of the STOP‐HF Study.9 Secondly, targeting hospitalized patients with CVD at high risk of developing CHF is also important. This is the focus of NIL‐CHF making it a complementary study to STOP‐HF. Analogous with successful nurse‐led, multidisciplinary CHF management programmes (CHF‐MPs) that improve health outcomes in high‐risk patients with pre‐existing CHF,6 the approach of NIL‐CHF has great potential for a service development.
Until recently, there was a paucity of data to suggest that it was cost‐effective to adopt a CHF‐MP approach. DeBusk et al.7 specifically studied patients with milder forms of CHF using an extended version of their successful telephone‐based MULTI‐FIT programme, with minimal success. However, meta‐analyses of CHF‐MPs (both in respect to the totality of evidence6 and more recently to ‘remote management’ techniques12) support the notion that in order to prevent (or delay progression to) CHF, a face‐to‐face, nurse‐led multidisciplinary management approach is most likely to provide the greatest benefits.13
Preliminary evidence to support NIL‐CHF
Our group recently published a report on the long‐term effects of a nurse‐led multidisciplinary home‐based intervention that showed, for the first time, that it is possible to prevent an incident admission for CHF with better disease management.14 During 7.5 years of follow‐up for 528 ‘high‐risk’ patients, those randomized to the study intervention (n = 260) were significantly less likely to be re‐admitted for an incident admission for CHF compared with usual post‐discharge care (n = 268) [adjusted relative risk 0.40, 95% CI, 0.18–0.88; P = 0.02]. All‐cause hospital costs were also significantly lower (14%) in the study intervention group ($AU 823 vs. $AU 960 per patient/year; P = 0.045).14 These ‘pilot’ data, when combined with the overall evidence and parallel efforts by other groups, underpin the intent and design of the prospectively planned NIL‐CHF Study.
Study hypothesis
The NIL‐CHF Study will test the hypothesis that relative to usual post‐discharge hospital and primary care, a nurse‐led, multidisciplinary management programme for hospitalized patients aged ≥45 years with pre‐existing CVD (but not CHF) and/or common antecedents of CHF, which implements gold‐standard pharmacological and non‐pharmacological therapy directed by advanced risk assessment and monitoring to achieve optimal cardiac, renal, and neurological protection, will reduce the incidence of admission for CHF or all‐cause mortality (composite end‐point) by a clinically significant amount (40% relative difference) during 3–5 years of follow‐up.
Study design
Figure 1 shows the overall design of the NIL‐CHF Study; a single‐centre, randomized controlled study of a sustained, post‐discharge, nurse‐led, multidisciplinary intervention compared with usual care in a target of 750 patients discharged from acute hospital care. The NIL‐CHF Study is registered with the Australian New Zealand Clinical Trials Registry (Number 12608000022369) and conforms to both the principles outlined in the Declaration of Helsinki15 and the CONSORT guidelines.16 The study has been approved by the Human Research Ethics Committee of the Alfred Hospital, Melbourne, Australia, and all subjects are required to provide written informed consent to participate.
Figure 1. Study schema.
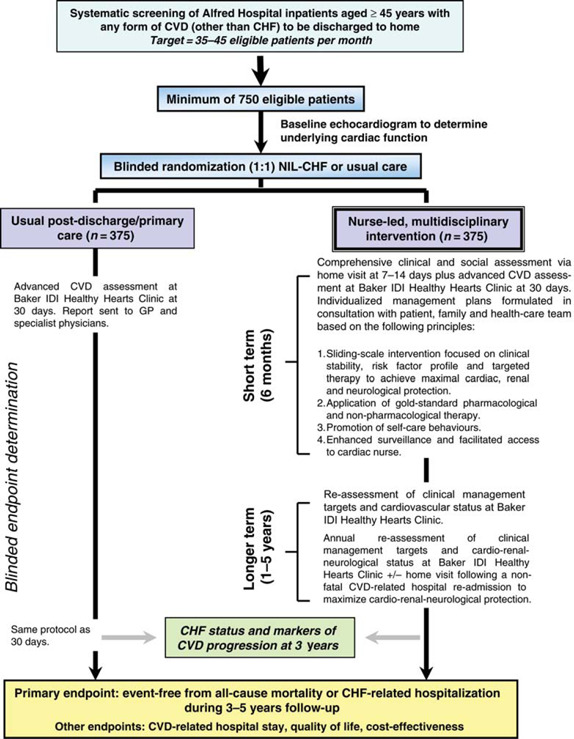
Study outcomes
The primary (composite) end‐point of the NIL‐CHF Study is event‐free survival from a CHF‐related hospitalization or all‐cause mortality during 3–5 years of follow‐up. Endpoints will be adjudicated by an independent and blinded Study End‐Point Committee. The applied definition of CHF will be derived from contemporary Australian expert guidelines:17 ‘CHF is a complex clinical syndrome with typical symptoms which may occur at rest or on effort, and that is frequently, but not exclusively, characterized by objective evidence of an underlying structural abnormality OR cardiac dysfunction that impairs the ability of the ventricle to fill with or eject blood (particularly during exercise)’.
Secondary endpoints will also examine the potential of the intervention to make a positive impact, both from an individual (e.g. cardiac function, functional status,18 health‐related quality of life,19,20 cognitive function,21 and mental health22,23) and health‐care system perspective (e.g. all‐cause hospital stay).
Health economic analysis
An economic analysis (e.g. cost per quality‐adjusted life year averted) using gold‐standard methods24 will also be undertaken. Health‐care costs will be independently monitored during study follow‐up. These include: (i) the cost of applying the study intervention; (ii) community‐based health‐care costs; (iii) prescribed pharmacotherapy; and (iv) inpatient/outpatient hospital activity. All costs will be standardized (adjusting for inflation) to the last full financial year of the study.
Patient cohort and selection criteria
All patients admitted to a 450‐bed tertiary referral hospital servicing the wider metropolitan area of Melbourne, Australia (population 3.5 million), are screened for study eligibility. Patients without CHF are eligible if they are aged ≥45 years and discharged to home following an index admission characterized (clinical documentation plus prescription of long‐term treatment) by active treatment for: (i) a chronic form of CVD and/or; (ii) type 2 diabetes and/or; (iii) hypertension. Patients are excluded if they are diagnosed with a congenital condition, surgically repairable or significant valvular disease, terminal malignancy, live beyond a 40 km radius from the hospital, or have experienced an acute coronary event (e.g. acute myocardial infarction) associated with cardiac dysfunction within 30 days.
Study power
We have used outcome data from our earlier work in 198 patients with CVD (followed‐up for >7 years who contributed to >95% of new CHF admissions14) in order to conservatively estimate that the composite primary endpoint will occur in at least 20% of usual care patients (15% incident CHF admission and 5% death without prior CHF admission) over a median of 4.5 years. With a minimum of 350 patients in each group, NIL‐CHF has >80% power to detect a 40% variation in the primary endpoint between the two groups (absolute difference of 12 vs. 20%) and 85% power to detect a 45% variation of the same (11 vs. 20%). Allowing for patient drop‐out (estimated 10% based on previous studies), intention‐to‐treat analyses, and power to compare secondary endpoints, a total of 750 patients will be recruited.
Study randomization
All eligible patients who consent to participate will be randomized on a 1:1 basis to the two arms of the study.
Baseline and 3‐year cardiovascular assessments
All patients will undergo an advanced cardiovascular assessment at a dedicated outpatient clinic at 30 days post‐discharge (i.e. when most are clinically stable). This includes a baseline evaluation of cardiac function via echocardiography, atherosclerotic burden via measurement of carotid intima‐medial thickness, ankle brachial pressure index and, in a subset of patients, arterial compliance/endothelial function via gold‐standard pulse wave velocity.25
Study patients will be characterized by the echocardiographic assessment into five groups: (i) no evidence of a cardiac abnormality; (ii) asymptomatic systolic dysfunction as defined by a left ventricular ejection fraction of ≤45%; (iii) asymptomatic diastolic dysfunction as defined by the presence of peak diastolic tissue velocity of ≤8 cm/s or at least mild diastolic dysfunction;26 (iv) combination of systolic and diastolic dysfunction (ii and iii) and; (v) other cardiac abnormality [other than (ii) or (iii)].
The same protocol will be applied at 3 years to determine potentially important changes in cardiovascular health status over the medium term. All results are reviewed (in a blinded manner) by a qualified cardiologist and a report of results is sent to the patient's general practitioner (GP).
The NIL‐CHF intervention
In line with the development and application of home‐based, multidisciplinary CHF‐MPs,27 the intervention is co‐ordinated and applied by a qualified cardiac nurse with advanced training in the management of CVD and diabetes. In broad terms, the intervention includes at least one home visit and an 18‐month visit to a dedicated NIL‐CHF clinic to initiate short‐ and long‐term strategies to prevent the development of CHF.
The main objectives of the intervention are: (i) to establish a clear health‐care plan using expert guidelines to manage the patients underlying CVD and; (ii) using the above clinical plan as a point of reference, to establish a personal management regime to optimize the treatment of CVD (and any other concurrent illnesses) and reduce the probability of further morbidity and a premature death. In collaboration with the patient, their family/carers and health‐care team (including the patient's GP and community pharmacist), the study intervention focuses on the individual's circumstances and needs with a strong emphasis on self‐care behaviours (changes in which are evaluated over time using a modified tool for patients with CHF28).
As shown in Figure 1, the study intervention will comprise two key phases of support:
Short–medium term. Study patients will receive a detailed home visit at 7–14 days post‐hospital discharge, which will focus on gathering information to determine the clinical stability and optimal management of the patient.29 Using key information from this visit and that of the clinic‐based assessment at 30 days post‐hospital discharge, an individualized, sliding‐scale programme of intervention that includes a component of telephone ‘coaching’30 (see below for more detail) will be implemented over 6 months.
Longer term. Study patients will be able to contact the cardiac nurse for continued advice and support. Advanced cardiovascular assessments will be repeated at 18 months and 3 years in order to adjust treatment plans under the guidance of the patient's GP and/or cardiologist.
If patients are re‐admitted to hospital for any cardiovascular reason, a repeat home visit will be implemented at 7–14 days post‐discharge and the long‐term health‐care plan for the patient will be revised accordingly.
Rather than offering every patient the same level of support, based on the home visit assessment and results of advanced clinical phenotyping via the NIL‐CHF clinic visit, patient follow‐up will be adjusted according to the following three key criteria:
Clinical stability. Consistent with other studies that undertake home visits in recently discharged patients,31 the potential for discovering patients who have signs or symptoms indicative of an impending crisis or at high risk due to inappropriate drug use is still relatively high. Immediate intervention to correct this will be applied.
Appropriate management. The appropriateness of prescribed pharmacological and non‐pharmacological treatment will be compared with expert guidelines specific to the patient's clinical profile (e.g. secondary prevention).32
Risk profile. The study nurse will also focus on other important factors that may positively or negatively impact on the patient's future health status. This includes the pattern of disease awareness, self‐care abilities, cognitive function, mental health status, social support, and of course, the pattern of treatment adherence. These factors will be specifically measured using a range of validated tools.18–23,28
Table Table 1 shows how the above criteria are used to generate a traffic light system of potential risk for developing CHF; this will be used to guide the frequency and intensity of subsequent management for optimal cardiac, renal, and neurological protection.
Table Table 1.
Adjustment of the intensity of the NIL‐CHF intervention according to the patient profile
| Priority | Clinical status | Management | Risk profile | Intervention | |
|---|---|---|---|---|---|

|
High/intense | Clinically unstable | Key deficits | Multiple risk factors | Urgent intervention to promote clinical stability. Ongoing surveillance to optimize management and minimize risk factors for CHF |

|
Medium | Stable | Areas of deficit | One or more risk factors | Sustained follow‐up to optimize management and address identified factor(s) contributing to increased risk of CHF |

|
Low | Stable | Gold‐standard | No risk factors | No further intervention other than routine contact and surveillance |
Key therapeutic targets and their evaluation
Table 2 summarizes the key therapeutic targets, how they will be addressed by the NIL‐CHF intervention, and how the impact of the intervention will be ultimately evaluated (in respect to preventing progression to CHF or a fatal event).
Table 2.
Key therapeutic targets and their evaluation
| Therapeutic target | Key interventions | Primary evaluation |
|---|---|---|
| Optimize individual factors to promote safe and effective self‐management | Home assessment of individual awareness and social circumstances at 7–14 days | Health‐related quality of life |
| Referral to social worker | Mental health status | |
| Individual targets negotiated | Self‐care status (knowledge and adherence) | |
| Optimize cardiovascular risk profile | Comprehensive risk profiling at 30 day clinic | Absolute cardiovascular risk assessment (primary and secondary) |
| Comprehensive reports to GP and specialists | Change in body fatness, blood pressure, lipid profile, and smoking status | |
| Coaching to individualized targets | ||
| Develop chronic disease management plan | ||
| Annual NIL‐CHF clinic | ||
| Optimize clinical management of pre‐existing diabetes and CVD | Treatment review relative to clinical diagnoses and supplementary clinical profiling (NIL‐CHF clinic) | Adherence to gold‐standard guidelines for pharmacological and non‐pharmacological management |
| Referral to home pharmacy reviews and management | Cardiac function (e.g. presence/absence of left ventricular hypertrophy and systolic and diastolic function) | |
| Referral to specialist services (e.g. diabetes clinic, social workers, and dieticians) | ||
| Annual NIL‐CHF clinic | ||
| Optimize clinical management of potentially related co‐morbidity | Assessment of renal function, carotid intima‐medial thickness, ankle‐brachial index | Adherence to gold‐standard guidelines for pharmacological and non‐pharmacological management |
| Comprehensive reports to GP and specialists | Renal function | |
| Referral to specialist services (e.g. renal and vascular clinics) | Carotid intima‐medial thickness | |
| Ankle brachial index | ||
| Cognitive status | ||
| Prevent progression to CHF or a fatal event | Combination of all of the above during study follow‐up (3–5 years) | Primary composite endpoint |
| Multivariate analyses of independent correlates of event‐free survival | ||
| Cardiovascular‐specific and all‐cause hospital events and stay |
Usual post‐discharge care
No restrictions on discharge planning and post‐discharge follow‐up will be imposed on those patients randomized to the usual care group. All patients randomized to usual care will be scheduled for routine follow‐up via the outpatient department of the Alfred Hospital and will undergo advanced cardiovascular assessment, as necessary.
Current status
A key challenge to any large clinical study is recruitment, which may reflect the clinical relevance and/or patient or clinician acceptance of the study. After 12 months of full‐time recruitment (commencing June 2008), the NIL‐CHF Study had recruited ∼300 eligible patients (≈40% of target patients).
Of these 300 patients, 73% are male and the mean age is 65 ± 10 years. The most common antecedents for CHF thus far are hypertension (70%, 95% CI, 64–75%), coronary artery disease (51%, 95% CI, 31–41%), and type 2 diabetes (26%, 95% CI, 21–31%). Other conditions commonly linked to CHF include chronic atrial fibrillation (8%, 95% CI, 5–12%) and other forms of atherosclerotic disease including peripheral and cerebrovascular disease (19%, 95% CI, 15–24%). Baseline profiling shows that 76% (95% CI, 69–82%) of patients have some form of diastolic dysfunction, 29% (95% CI, 23–36%) left ventricular hypertrophy, 71% (95% CI, 64–78%) mitral valve dysfunction, and 7% (95% CI, 4–12%) have a left ventricular ejection fraction ≤45% indicative of asymptomatic left ventricular systolic dysfunction. A further 11% (95% CI, 7–16%) of patients have been found to have clinically significant carotid intima‐medial thickness.
Of those patients subject to home visits by the study nurse, 8% were clinically unstable, 17% required adjustments to their clinical management, and 52% needed educational support and longer term surveillance. Our sliding‐scale approach to further management in the immediate to longer term highlighted that 20% of recruited patients were designated as high risk (red) (95% CI, 14–28%), 72% (95% CI, 63–79%) moderate risk (amber), and 8% low risk (green) (95% CI, 4–14%). On the basis of these criteria, patients were managed accordingly in the areas of clinical status, management, and overall risk profile, as specified in Table Table 1.
Discussion
The NIL‐CHF Study responds to the modern‐day challenge of limiting the ‘epidemic’ of CHF within our ageing population in a pragmatic and cost–effective manner. As such, there is much to be achieved by applying evidence‐based therapeutics (e.g. angiotensin‐converting enzyme inhibitors and beta‐blockers) and supportive strategies (e.g. lifestyle modification and enhanced self‐care) to prevent CHF in high‐risk patients.3 However, there is a paucity of evidence to translate research into practice, particularly to achieve long‐term benefits. NIL‐CHF targets recently hospitalized patients with a range of CVD states and uses comprehensive cardiovascular assessment and risk profiling to implement gold‐standard therapeutics via a nurse‐led, multidisciplinary home‐ and clinic‐based intervention. In Australia alone (population 22 million), the potential impact of this type of intervention is substantial as there are close to 100 000 hospitalizations relating to CHF each year33 with the related economic impact per annum most probably costing in excess of $AU 1 billion per annum in line with other Western nations.34 Importantly, if the NIL‐CHF intervention is proven successful, it is likely to have a significant impact on other costly CVD‐related events (e.g. acute myocardial infarction, stroke, and advanced renal disease). At the very minimum, this intervention has the potential to prevent six CHF‐related admissions costing ∼$AU 42 000 plus two fatal events per 100 ‘treated’ patients.
In conclusion, therefore, we aim to apply a range of well‐established principles, including home visits35 and a host of secondary prevention strategies,36 and to prevent the development of CHF in high‐risk patients with established CVD or its antecedents, over the medium to longer term. If positive, NIL‐CHF will provide an important bridge between effective CHF management (based on a well‐developed body of evidence) and prevention.
Acknowledgements
The authors gratefully acknowledge the cardiac research nurses (Mr Wayne Dawson, Ms Lia Bailey, Ms Jessica Wood, and Ms Geraldine Lee) for patient recruitment, home visits, and data collection, Ms Trischa Edwards for the echocardiographic assessments and Dr Chiew Wong for the echocardiographic reporting. The data management team and Project Co‐ordinator, Ms Alicia Calderone, are sincerely thanked together with the Investigators: Principal Investigator, Professor Simon Stewart; and Chief Investigators, Professor Garry Jennings, Dr Peter Bergin, Dr Barbora de Courten, Ms Geraldine Lee, Dr Melinda Carrington, and Dr Chiew Wong.
Funding
This work was supported by a National Health and Medical Research Council Project (grant application 472662). S.S. and M.J.C. are supported by the National Health and Medical Research Council of Australia.
Conflict of interest: none declared.
References
- 1. Krum H Abraham WT Heart failure. Lancet. 2009;373:941–955. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 2. Stewart S Wilkinson D Hansen C Vaghela V Mvungi R McMurray J Sliwa K Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. 2008;118:2360–2367. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- 3. Dickstein K Cohen‐Solal A Filippatos G McMurray JJ Ponikowski P Poole‐Wilson PA Stromberg A van Veldhuisen DJ Atar D Hoes AW Keren A Mebazaa A Nieminen M Priori SG Swedberg K ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4. Jhund PS Macintyre K Simpson CR Lewsey JD Stewart S Redpath A Chalmers JW Capewell S McMurray JJ Long‐term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 5. Clark AM Hartling L Vandermeer B McAlister FA Meta‐analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143:659–672. doi: 10.7326/0003-4819-143-9-200511010-00010. [DOI] [PubMed] [Google Scholar]
- 6. McAlister FA Stewart S Ferrua S McMurray JJ Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 7. DeBusk RF Miller NH Parker KM Bandura A Kraemer HC Cher DJ West JA Fowler MB Greenwald G Care management for low‐risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004;141:606–613. doi: 10.7326/0003-4819-141-8-200410190-00008. [DOI] [PubMed] [Google Scholar]
- 8. Jaarsma T van der Wal MH Lesman‐Leegte I Luttik ML Hogenhuis J Veeger NJ Sanderman R Hoes AW van Gilst WH Lok DJ Dunselman PH Tijssen JG Hillege HL van Veldhuisen DJ Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 9. McDonald K Conlon C Ledwidge M Disease management programs for heart failure: not just for the ‘sick’ heart failure population. Eur J Heart Fail. 2007;9:113–117. doi: 10.1016/j.ejheart.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10. Stewart S Stopping heart failure in its tracks. Eur J Heart Fail. 2007;9:118–119. doi: 10.1016/j.ejheart.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11. de Lemos JA McGuire DK Khera A Das SR Murphy SA Omland T Drazner MH Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study Am Heart J 2009. 157 746 753e742 [DOI] [PubMed] [Google Scholar]
- 12. Clark RA Inglis SC McAlister FA Cleland JG Stewart S Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta‐analysis. BMJ. 2007;334:942. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sochalski J Jaarsma T Krumholz HM Laramee A McMurray JJ Naylor MD Rich MW Riegel B Stewart S What works in chronic care management: the case of heart failure. Health Aff (Millwood) 2009;28:179–189. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 14. Pearson S Inglis SC McLennan SN Brennan L Russell M Wilkinson D Thompson DR Stewart S Prolonged effects of a home‐based intervention in patients with chronic illness. Arch Intern Med. 2006;166:645–650. doi: 10.1001/archinte.166.6.645. [DOI] [PubMed] [Google Scholar]
- 15. Rickham PP Human experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D Schulz KF Altman D The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 17. Krum H Jelinek MV Stewart S Sindone A Atherton JJ Hawkes AL Guidelines for the prevention, detection and management of people with chronic heart failure in Australia 2006. Med J Aust. 2006;185:549–557. doi: 10.5694/j.1326-5377.2006.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 18. American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, authors. ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19. Brazier JE Roberts J The estimation of a preference‐based measure of health from the SF‐12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 20. Devlin N Hansen P Herbison P Macran S A ‘new and improved’ EQ‐5D valuation questionnaire? Results from a pilot study. Eur J Health Econ. 2005;6:73–82. doi: 10.1007/s10198-004-0263-0. [DOI] [PubMed] [Google Scholar]
- 21. Nasreddine ZS Phillips NA Bedirian V Charbonneau S Whitehead V Collin I Cummings JL Chertkow H The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22. Arroll B Khin N Kerse N Screening for depression in primary care with two verbally asked questions: cross‐sectional study. BMJ. 2003;327:1144–1146. doi: 10.1136/bmj.327.7424.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radloff LS The CES‐D Scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 24.Cost‐effectiveness in Health and Medicine. Oxford: Oxford University Press; 1996. [Google Scholar]
- 25. Taylor AJ Merz CN Udelson JE 34th Bethesda Conference: Executive summary–can atherosclerosis imaging techniques improve the detection of patients at risk for ischemic heart disease? J Am Coll Cardiol. 2003;41:1860–1862. doi: 10.1016/s0735-1097(03)00363-2. [DOI] [PubMed] [Google Scholar]
- 26. European Study Group on Diastolic Heart Failure, authors. How to diagnose diastolic heart failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 27.Improving Outcomes in Chronic Heart Failure: Specialist Nurse Intervention from Research to Practice. 2nd ed. London, UK: BMJ Books; 2004. [Google Scholar]
- 28. Riegel B Carlson B Moser DK Sebern M Hicks FD Roland V Psychometric testing of the self‐care of heart failure index. J Card Fail. 2004;10:350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 29. Stewart S Marley JE Horowitz JD Effects of a multidisciplinary, home‐based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354:1077–1083. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 30. Vale MJ Jelinek MV Best JD How many patients with coronary heart disease are not achieving their risk‐factor targets? Experience in Victoria 1996–1998 versus 1999–2000. Med J Aust. 2002;176:211–215. doi: 10.5694/j.1326-5377.2002.tb04375.x. [DOI] [PubMed] [Google Scholar]
- 31. Stewart S Horowitz JD Detecting early clinical deterioration in chronic heart failure patients post‐acute hospitalisation‐a critical component of multidisciplinary, home‐based intervention? Eur J Heart Fail. 2002;4:345–351. doi: 10.1016/s1388-9842(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 32.National Vascular Disease Prevention Alliance, authors. Guidelines for the Assessment of Absolute Cardiovascular Disease Risk. National Heart Foundation of Australia; 2009. [Google Scholar]
- 33. Najafi F Dobson AJ Jamrozik K Recent changes in heart failure hospitalisations in Australia. Eur J Heart Fail. 2007;9:228–233. doi: 10.1016/j.ejheart.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 34. Stewart S Jenkins A Buchan S McGuire A Capewell S McMurray JJ The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 2002;4:361–371. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 35. Elkan R Kendrick D Dewey M Hewitt M Robinson J Blair M Williams D Brummell K Effectiveness of home based support for older people: systematic review and meta‐analysis. BMJ. 2001;323:719–725. doi: 10.1136/bmj.323.7315.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wood D De Backer G Faergeman O Graham I Mancia G Pyorala K Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Atherosclerosis. 1998;140:199–270. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]


