Abstract
Background
The mammalian cell-based quadrivalent inactivated influenza vaccine (IIV4c) has advantages over egg-based quadrivalent inactivated influenza vaccine (IIV4e), as production using cell-derived candidate viruses eliminates the opportunity for egg adaptation. This study estimated the relative vaccine effectiveness (rVE) of IIV4c versus IIV4e in preventing cardiorespiratory hospitalizations during the 2019–2020 US influenza season.
Methods
We conducted a retrospective cohort study using electronic medical records linked to claims data of US individuals aged 18–64 years. We assessed rVE against cardiorespiratory hospitalizations and against subcategories of this outcome, including influenza, pneumonia, myocardial infarction and ischemic stroke, and respiratory hospitalizations. We used a doubly robust inverse probability of treatment weighting and logistic regression model to obtain odds ratios (ORs; odds of outcome among IIV4c recipients/odds of outcome among IIV4e recipients) adjusted for age, sex, race, ethnicity, geographic region, vaccination week, health status, frailty, and healthcare resource utilization. rVE was calculated as 100(1 − ORadjusted).
Results
In total, 1 491 097 individuals (25.2%) received IIV4c, and 4 414 758 (74.8%) received IIV4e. IIV4c was associated with lower odds of cardiorespiratory (rVE, 2.5% [95% confidence interval, 0.9%–4.1%]), respiratory (3.7% [1.5%–5.8%]), and influenza (9.3% [0.4%–17.3%]) hospitalizations among adults 18–64 years of age. No difference was observed for the other outcomes.
Conclusions
This real-world study conducted for the 2019–2020 season demonstrated that vaccination with IIV4c was associated with fewer cardiorespiratory, respiratory, and influenza hospitalizations compared with IIV4e.
Keywords: cardiovascular-respiratory illness, cell-based quadrivalent influenza vaccine, hospitalizations, real-world evidence, relative vaccine effectiveness
In a large US database linking electronic medical records to claims data during the 2019–2020 influenza season, odds of cardiorespiratory hospitalizations were lower with cell-based than with egg-based quadrivalent influenza vaccines among adults aged 18–64 years.
Disease and death due to influenza impose a significant burden. In the United States, influenza causes 140 000–710 000 hospitalizations annually and >50 000 deaths in high-severity seasons [1]. Influenza virus infection usually causes a self-limited upper respiratory tract infection, but its complications include secondary bacterial infections, exacerbations of chronic lung disease, and serious cardiovascular events, such as myocardial infarction and ischemic stroke [2–4].
To reduce the impact of influenza on individuals and society, the US Advisory Committee on Immunization Practices recommends annual influenza vaccination [5]. The traditional manufacturing process for influenza vaccines relies on fertilized chicken eggs, and vaccine seed viruses may adapt to avian receptors found within eggs, a process called egg adaptation. Egg adaptation can occur in the dominant antigenic region of the virus, leading to mutations in key viral antigens that may result in antigenic mismatch to circulating viruses and thereby reducing vaccine effectiveness. In contrast, propagating vaccine viruses in mammalian cell cultures can yield vaccine viruses more antigenically similar to seed strain viruses by eliminating egg adaptation [6–8].
In observational studies, the cell-based quadrivalent inactivated influenza vaccine (IIV4c; Flucelvax Quadrivalent; CSL Seqirus USA) has been shown to be more effective than traditional, egg-based quadrivalent inactivated influenza vaccines (IIV4e; Fluarix Quadrivalent and Flulaval Quadrivalent [GlaxoSmithKline Biologicals], Fluzone Quadrivalent [Sanofi Pasteur], and Afluria Quadrivalent [CSL Seqirus USA]) in preventing influenza-related medical encounters, with greater effect sizes observed during seasons with documented egg adaptation [9–18]. In the current study, we compared IIV4c and IIV4e for the prevention of cardiorespiratory hospitalizations and subsets of this outcome, including respiratory hospitalizations, influenza hospitalizations, pneumonia hospitalizations, and myocardial infarction and ischemic stroke hospitalizations, during the 2019–2020 influenza season among adults in the United States.
METHODS
Study Design
We conducted a retrospective cohort study during the 2019–2020 influenza season among US residents 18–64 years of age to evaluate the relative vaccine effectiveness (rVE) of IIV4c compared with IIV4e. Of note, vaccine effectiveness may be measured by comparing the frequency of health outcomes in vaccinated and unvaccinated individuals (ie, absolute vaccine effectiveness [aVE]) or comparing the frequency of health outcomes in individuals who received one type of vaccine with that in those who received a different vaccine (ie, rVE) [19]. The current study evaluates the rVE of IIV4c versus IIV4e. The study was designed, implemented, and reported in accordance with Good Pharmacoepidemiological Practice, applicable local regulations, and the ethical principles laid down in the Declaration of Helsinki. Study findings have been reported according to the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) recommendations [20, 21].
Study Objectives
The primary objective of this study was to estimate the adjusted rVE of IIV4c compared with IIV4e for the prevention of cardiorespiratory hospitalizations among individuals 18–64 years of age. The secondary objectives included estimation of the adjusted rVE in specific age subgroups comprising persons 18–49 or 50–64 years old. An exploratory objective was included for the subgroup of children and adolescents aged 4–17 years.
Data Sources
For the analysis, we used a US integrated data set of primary and specialty care data from Veradigm Health Insights electronic medical record (EMR) platforms (including components from Allscripts Tiers 1 and 2 and Practice Fusion) linked with pharmacy and medical claims data from Komodo Health. The Veradigm EMR platform comprised all primary care interactions for >120 million patients at the time the study was conducted. Komodo Health sources data both directly from payers (closed claims) as well as from broad-based healthcare sources, including clearinghouses, pharmacies, and software platforms and can capture a patient's activities, regardless of their insurance provider (open/closed claims). This study used all available claims data for the analysis. The data sources have been described in more detail elsewhere [22].
An algorithm developed by Datavant was used to deidentify patient-specific information to certify privacy and meet minimum protected health information standards in accordance with the Health Insurance Portability and Accountability Act (HIPAA) privacy rule. Patient-level deidentified tokens are generated deterministically in each data source, using fields such as name, date of birth, and sex. The final linked data set is created as a merge of the patient-level deidentified tokens in each individual data set and contains no protected health information. Research staff did not participate in the deidentification process and had no access to the data sets until all identifying information had been removed. Because this noninterventional, retrospective study used a certified HIPAA-compliant database, institutional review board approval was not necessary. Because this study used deidentified patient data from the EMR Veradigm data set, patient consent was not required.
Study Period
The Centers for Disease Control and Prevention (CDC) defines the influenza surveillance season as epidemiologic week 40 through week 20 of the subsequent year, corresponding to 30 September 2019 to 17 May 2020. In our study, we defined the influenza season as 30 September 2019 to 7 March 2020—that is, week 40 through week 10 of the subsequent year. We chose the end of the observation period as 7 March 2020, to avoid outcome misclassification owing to overlap with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. The influenza season also served as the outcome ascertainment period. The vaccination intake period was between 1 August 2019 and 31 January 2020. Sensitivity analyses with different observation periods (defined in Statistical Methods) were conducted to assess the impact of potential outcome misclassification due to low influenza activity and SARS-CoV-2 cocirculation.
Study Population
The primary analysis population included adults 18–64 years of age who resided in the United States and who received a single dose of IIV4c or IIV4e. The secondary analysis population included adults 18–49 or 50–64 years of age. Eligible persons also had ≥1 year of primary care medical history in the Veradigm EMR data set and continuous enrollment in the Komodo claims data 6 months before the vaccination date and after the end of the primary observation period (ie, 7 March 2020). Individuals were excluded if they had a record of influenza vaccination between 19 May and 31 July 2019, a record of an influenza-related medical encounter before becoming vaccinated or before 30 September 2019 (the start of the influenza season), or missing information on age, sex, or geographic region. An exploratory analysis included a pediatric population 4–17 years of age who met the same criteria. Children <9 years of age who received 2 influenza vaccine doses during the intake period were eligible for inclusion, but those ≥9 years of age who received 2 vaccine doses were excluded. Although the influenza vaccines studied here are licensed for individuals aged ≥4 years, we chose to evaluate only individuals aged 4–64 years, because high-dose or adjuvanted vaccines were more commonly used in, and are preferentially recommended for, individuals aged ≥65 years in the United States.
Influenza Vaccine Exposure
Codes for vaccines administered, Current Procedural Terminology codes, and national drug codes were used to identify patients with IIV4c or IIV4e from EMRs and/or claims data (Supplementary Table 1). To permit development of vaccine-specific antibodies, patients were considered fully vaccinated 14 days after vaccination. Children <9 years of age who received 2 vaccine doses were considered fully vaccinated 14 days after their second dose.
Outcomes
Cardiorespiratory hospitalizations were the outcome of interest in this study and included respiratory hospitalizations overall and by subcategory of respiratory hospitalization, that is, influenza hospitalizations and pneumonia hospitalizations. Hospitalizations for myocardial infarction and ischemic stroke were also evaluated. Outcomes were considered >14 days after influenza vaccination during the period from 30 September 2019 (week 40) to 7 March 2020 (week 10). Outcomes were identified by relevant diagnosis codes in any position on the claim or restricted to only the admitting position, also referred to as the primary position. Outcomes identified in the admitting diagnosis position are a subset of the “any” position; the “any” diagnosis includes the admitting diagnosis as well as the subsequent diagnosis, regardless of the admitting diagnosis. Results for each type of diagnosis are presented separately. Hospitalization for injury or trauma was evaluated as a negative control outcome in any diagnosis position as well as admitting diagnosis position only [23, 24]. Codes used to ascertain outcomes, from the International Classification of Diseases, Tenth Revision, Clinical Modification, are listed in Supplementary Table 2.
Covariates
Covariates included age, sex, race, ethnicity, US geographic region, week of vaccination (index week), individual comorbid conditions included in the Charlson Comorbidity Index (Supplementary Table 3) [25, 26], body mass index (<30 or ≥30 [calculated as weight in kilograms divided by height in meters squared]), smoking status, frailty index, number of outpatient visits, number of inpatient admissions, and baseline cardiovascular risk (determined by history of hypercholesterolemia, hypertension, type 2 diabetes, obesity, and hospitalizations for myocardial infarction, ischemic stroke, heart failure, or transient ischemic attack). Covariate baseline data were ascertained from EMRs and claims in the 12 months before vaccination.
Statistical Methods
We assessed differences in baseline covariates between the exposure groups (IIV4c and IIV4e) using standardized mean differences, with an absolute value ≤0.1 indicating a negligible difference. Inverse probability of treatment weighting (IPTW) was used to balance exposure cohorts [27]. To create stabilized IPTW, we used propensity scores calculated for each exposure cohort using a multivariable logit model adjusted for all covariates listed above. Weights were truncated at the 99th percentile to attenuate any extreme variability from outliers. Using a doubly robust adjustment method, we estimated odds ratios (ORs; odds of outcome among IIV4c recipients/odds of outcome among IIV4e recipients) in the IPTW-weighted sample, using a multivariable logistic regression model that included all study covariates [28]. rVE was calculated as 100(1 − ORadjusted) and reported with 95% confidence intervals (CIs). Missing demographic variables were reported as “missing” or “not reported.” Patients with missing values for age, sex, or region were excluded from the study. Analyses were conducted using SQL and SAS software (version 9.4).
A secondary analysis was conducted among persons 18–49 or 50–64 years of age. In addition, an exploratory analysis was conducted for the subgroup of children and adolescents 4–17 years of age. Two sensitivity analyses were used to evaluate the robustness of study assumptions. First, the outcome observation period was restricted to the period of highest influenza activity as defined by the CDC (using the moving epidemic method, from 8 December 2019 [week 50], through 7 March 2020 [week 10]) [29]. Second, to avoid the potential impact of SARS-CoV-2 circulation even earlier than the primary analysis end date, a second sensitivity analysis evaluated the period between 30 September 2019 and 15 February 2020 (from week 40 through week 7).
RESULTS
Study Population
The overall study population included 7 347 376 patients, of whom 5 905 855 (80.4%) were adults aged 18–64 years included in the primary analysis and 1 441 521 (19.6%) were children and adolescents aged 4–17 years included in the exploratory analysis (Supplementary Tables 4 and 5). In the adult cohort, after the IPTW-weighted sample was truncated to remove extreme outliers, 1 432 038 persons received IIV4c and 4 414 758 received IIV4e. With the exception of Midwest residents, of whom a significantly larger proportion were IIV4e recipients, baseline demographic and clinical characteristics were well balanced among adults (Table 1 and Supplementary Table 3).
Table 1.
Patient Demographic Characteristics and Standardized Mean Differences Before and After Inverse Probability of Treatment Weighting in the 2019–2020 Influenza Season (30 September 2019—7 March 2020) in Adults Aged 18–64 Years
| Characteristic | Patients, No. (%) | Unweighted SMD | IPTW SMD | |
|---|---|---|---|---|
| IIV4c Recipients (n = 1 432 038) | IIV4e Recipients (n = 4 414 758) | |||
| Age, mean (SD), y | 47.4 (13.0) | 46.4 (13.3) | −0.08 | 0.05 |
| Age group, y | ||||
| 18–49 | 710 016 (47.6) | 2 240 092 (50.7) | 0.06 | −0.04 |
| 50–64 | 781 081 (52.4) | 2 174 666 (49.3) | −0.06 | 0.04 |
| Sex | ||||
| Female | 922 951 (61.9) | 2 726 031 (61.7) | 0.00 | 0.00 |
| Male | 568 146 (38.1) | 1 688 727 (38.3) | 0.00 | 0.00 |
| Race | ||||
| White | 744 013 (49.9) | 2 234 669 (50.6) | 0.01 | −0.03 |
| Black | 82 287 (5.5) | 233 554 (5.3) | −0.01 | −0.02 |
| Asian | 46 772 (3.1) | 128 557 (2.9) | −0.01 | 0.01 |
| Other | 43 659 (2.9) | 126 272 (2.9) | 0.00 | 0.01 |
| Unknown/not reported | 574 366 (38.5) | 1 691 706 (38.3) | 0.00 | 0.04 |
| Ethnicity | ||||
| Hispanic | 100 607 (6.7) | 286 375 (6.5) | −0.01 | 0.02 |
| Non-Hispanic | 1 198 058 (80.3) | 3 498 589 (79.2) | −0.03 | 0.01 |
| Unknown/not reported | 192 432 (12.9) | 629 794 (14.3) | 0.04 | −0.03 |
| Geographic region, n (%) | ||||
| Northeast | 292 493 (19.6) | 875 573 (19.8) | 0.01 | 0.04 |
| Midwest | 182 681 (12.3) | 1 097 101 (24.9) | 0.33 | −0.17a |
| South | 812 350 (54.5) | 1 511 138 (34.2) | −0.42 | 0.07 |
| West | 203 573 (13.7) | 930 946 (21.1) | 0.20 | 0.04 |
| No. of outpatient visits, mean (SD) | 5.6 (7.7) | 5.9 (7.6) | 0.04 | −0.02 |
| No. of inpatient admissions, mean (SD) | 0.2 (1.1) | 0.2 (1.2) | 0.02 | 0.00 |
| CCI score, mean (SD) | 0.7 (1.3) | 0.7 (1.3) | 0.02 | −0.02 |
| No. of CCI comorbidities | ||||
| 0 | 1 007 682 (67.6) | 2 904 151 (65.8) | −0.04 | 0.03 |
| 1 | 251 147 (16.8) | 797 053 (18.1) | 0.03 | −0.02 |
| 2 | 110 801 (7.4) | 348 345 (7.9) | 0.02 | −0.01 |
| 3 | 55 802 (3.7) | 173 126 (3.9) | 0.01 | −0.01 |
| ≥4 | 65 665 (4.4) | 192 083 (4.4) | 0.00 | 0.00 |
| Cardiovascular risk factors | ||||
| Hospitalizations | ||||
| Myocardial infarction | 2794 (0.2) | 9405 (0.2) | 0.01 | −0.01 |
| Ischemic stroke | 2447 (0.2) | 8086 (0.2) | 0.00 | −0.01 |
| Heart failure | 4091 (0.3) | 12 534 (0.3) | 0.00 | 0.00 |
| Transient ischemic attack | 919 (0.1) | 2740 (0.1) | 0.00 | 0.00 |
| Hypercholesterolemia | 103 042 (6.9) | 301 855 (6.8) | 0.00 | 0.00 |
| Hypertension | 439 194 (29.5) | 1 334 305 (30.2) | 0.02 | −0.02 |
| Type 2 diabetes | 206 856 (13.9) | 637 072 (14.4) | 0.02 | −0.01 |
| Tobacco use | ||||
| Current | 23 412 (1.6) | 76 981 (1.7) | 0.01 | −0.02 |
| Former | 14 894 (1.0) | 44 092 (1.0) | 0.00 | 0.00 |
| Never | 26 141 (1.8) | 87 097 (2.0) | 0.02 | −0.01 |
| Unknown/not reported | 1 426 650 (95.7) | 4 206 588 (95.3) | −0.02 | 0.02 |
| BMI, mean (SD)b | 30.0 (7.1) | 30.3 (7.2) | … | … |
| BMI categoryb | ||||
| <18.5 | 5362 (0.4) | 16 903 (0.4) | 0.00 | 0.00 |
| 18.5–24.9 | 91 662 (6.1) | 271 772 (6.2) | 0.00 | 0.00 |
| 25–29.9 | 117 866 (7.9) | 347 840 (7.9) | 0.00 | 0.00 |
| ≥30 | 175 690 (11.8) | 553 635 (12.5) | 0.02 | −0.03 |
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; IIV4c, cell-based quadrivalent inactivated influenza vaccine; IIV4e, egg-based quadrivalent inactivated influenza vaccine; IPTW, inverse probability of treatment weighting; SD, standard deviation; SMD, standardized mean difference.
SMD value ≥0.1.
BMI is calculated as weight in kilograms divided by height in meters squared. Percentages for BMI do not sum to 100% because the data to calculate BMI were not available for all patients.
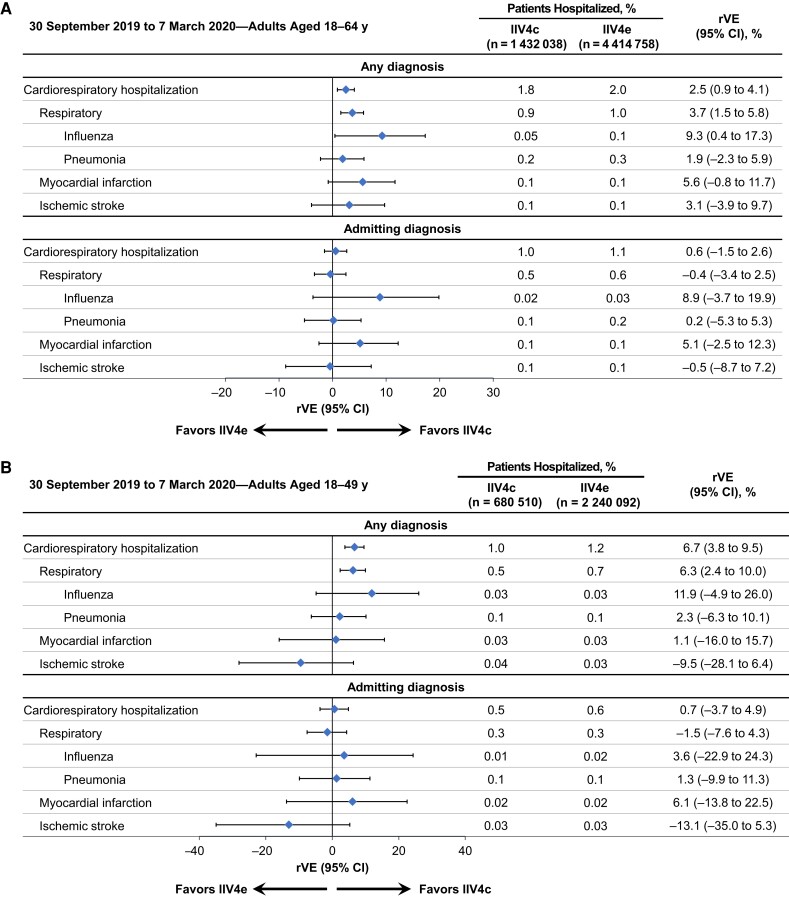
Cardiorespiratory Hospitalization—Any Diagnosis Position
For the full observation period, 1.8% of IIV4c and 2.0% of IIV4e recipients were hospitalized for a cardiorespiratory disease in any diagnosis position (Figure 1 [adjusted analyses] and Supplementary Figure 1 [unadjusted analyses]). Approximately half of the hospitalizations were respiratory in both vaccine cohorts.
Figure 1.
Adjusted relative vaccine effectiveness (rVE) of cell-based quadrivalent inactivated influenza vaccine (IIV4c) versus egg-based quadrivalent inactivated influenza vaccine (IIV4e) between 30 September 2019 and 7 March 2020 in adults aged 18–64 years (A), 18–49 years (B), or 50–64 years (C). Abbreviation: CI, confidence interval.
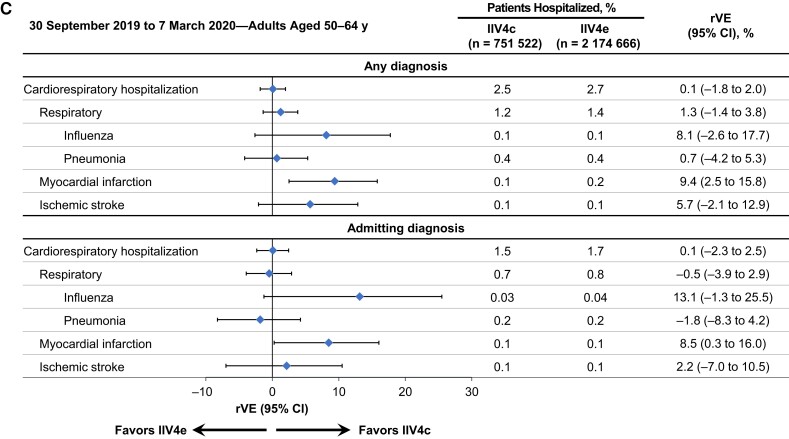
Among adults 18–64 years of age, IIV4c was more effective than IIV4e in preventing cardiorespiratory hospitalizations, with higher effect sizes for respiratory and especially influenza hospitalizations (Figure 1A). The adjusted rVE for IIV4c versus IIV4e a was 2.5% (95% CI, 0.9%–4.1%) for cardiorespiratory, 3.7% (1.5%–5.8%) for respiratory, and 9.3% (0.4%–17.3%) for influenza hospitalizations. Among adults aged 18–49 years, rVE point estimates were higher than in the overall population for respiratory and influenza hospitalizations, although the 95% CI for influenza hospitalizations crossed the null (Figure 1B). In the older age subgroup (50–64 years), IIV4c was associated with fewer myocardial infarctions than IIV4e (Figure 1C). The rVE against stroke hospitalizations did not favor either vaccine in the overall population or in the age subgroups. In the exploratory analysis of children aged 4–17 years, 0.01% and 0.03% of pediatric recipients of IIV4c and IIV4e, respectively, had an influenza hospitalization in the “any diagnosis” position. The associated rVE was high but with a wide 95% CI owing to small numbers of hospitalized patients (50.9% [95% CI, −20.1% to 79.9%]; Supplementary Figure 2).
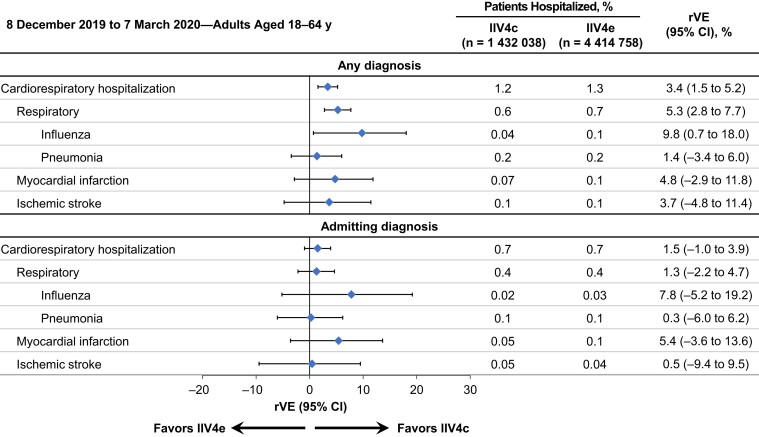
The rVE patterns for the overall population were similar during the peak influenza season between 8 December 2019 and 7 March 2020, when influenza activity was highest, with an rVE of 9.8% (95% CI, 0.7%–18.0%) for influenza hospitalizations (moving epidemic method sensitivity analysis; Figure 2) compared with the overall rVE of 9.3% (0.4%–17.3%) for the full study period. Patterns were similar when the study period was shortened to avoid confounding factors related to SARS-CoV-2 (30 September 2019 through 15 February 2020; Supplementary Figure 3).
Figure 2.
Adjusted relative vaccine effectiveness (rVE) of cell-based quadrivalent inactivated influenza vaccine (IIV4c) versus egg-based quadrivalent inactivated influenza vaccine (IIV4e) between 8 December 2019 and 7 March 2020, in adults 18–64 years of age. Abbreviation: CI, confidence interval.
Cardiorespiratory Hospitalization—Admitting Diagnosis Position
When hospitalization data were restricted to admitting diagnoses, the rates of cardiorespiratory hospitalizations decreased from 1.8% to 1.0% in the IIV4c group and from 2.0% to 1.1% in the IIV4e group (Figure 1 [adjusted analyses] and Supplementary Figure 1 [unadjusted analyses]). In the overall population, the rVE point estimate for influenza as an admitting diagnosis was similar to the rVE for any influenza hospitalization (8.9% [95% CI, −3.7% to 19.9%]), but the 95% CI was wider and included the null (Figure 1A). Among adults 50–64 years of age, IIV4c was associated with fewer myocardial infarctions than IIV4e, similar to the results for myocardial infarction hospitalizations recorded in any diagnosis position (Figure 1C).
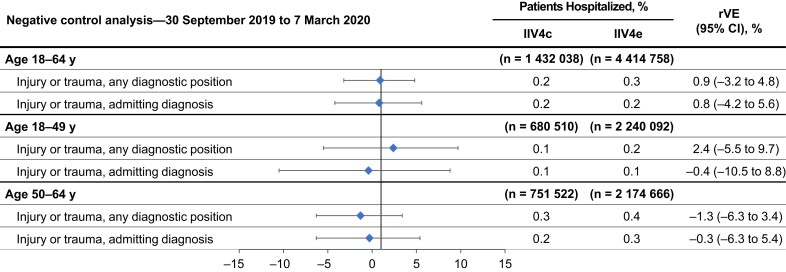
Negative Control Outcome
Similar proportions of patients were hospitalized with an injury or trauma, and, after weighting and adjustment, rVEs indicated that vaccine exposure was not associated with the negative control outcome (Figure 3).
Figure 3.
Negative control analysis comparing the effect of vaccination with cell-based quadrivalent inactivated influenza vaccine (IIV4c) versus egg-based quadrivalent inactivated influenza vaccine (IIV4e) on the incidence of hospitalizations for injury or trauma in each cohort between 30 September 2019 and 7 March 2020, after adjustment. Abbreviations: CI, confidence interval; rVE, relative vaccine effectiveness.
DISCUSSION
In our study conducted during the 2019–2020 influenza season in the United States, IIV4c, compared with IIV4e, was associated with fewer cardiorespiratory, respiratory, and influenza hospitalizations with a diagnosis in any position among vaccinated adults 18–64 years of age, with the greatest differences observed for influenza hospitalizations. No difference in the effectiveness of vaccines was observed in the overall population (18–64 years of age) for myocardial infarction and stroke.
During the 2019–2020 season, the predominant circulating strain in adults was A(H1N1)pdm09, along with B/Victoria cocirculation [30]. The CDC estimated overall aVE for all influenza vaccines to be 39% (95% CI, 32%–44%) in the 2019–2020 season, and aVE in adults ranged between 34% and 40% [31]. Adaptive viral mutations can occur during propagation of influenza vaccine viruses in embryonated chicken eggs, which may affect antigenicity [32–34]. In contrast, virus propagation in mammalian cells eliminates the potential for egg adaptation [35]. For A(H1N1)pdm09 viruses, 6B.1A subclades 5A, 5B, and 7 predominated globally, while the vaccine virus was clade 6B.1A1, indicating genetic drift [36]. Whereas the CDC found that circulating and vaccine A(H1N1) viruses were antigenically similar based on antigenic characterization with ferret antiserum, the World Health Organization stated that, based on human serology studies, circulating A(H1N1) viruses had decreased antigenic similarity to cell-propagated reference virus and even more pronounced differences when compared with an egg-propagated reference virus, indicating potential egg adaptation [36–38].
Among B/Victoria viruses, clade V1A.3 viruses predominated (97%), but the vaccine virus belonged to the V1A.1 clade [37]. Fewer circulating B/Victoria viruses were antigenically similar to the egg-propagated vaccine reference virus compared with the cell-propagated vaccine reference virus (60% vs 8%) [39]. However, the B/Victoria vaccine virus provided good cross-protection, as indicated by the CDC's estimate of a strain-specific aVE (45%) for B/Victoria, which is consistent with the aVE during seasons where B/Victoria vaccine virus was well matched to circulating viruses [31]. Our findings suggest that cell-based vaccines provided better protection than egg-based vaccines during this influenza season, with limited circulation of A(H3N2), the strain known to be particularly subject to egg-adaptive changes [6, 40, 41].
No difference in effectiveness was found for the cardiovascular end points among the adult population, except in the 50–64-year age subgroup for myocardial infarction hospitalizations, which favored IIV4c. We may consider that these end points may be more relevant in elderly adults, as individuals ≥65 years of age are more likely to have cardiovascular disease. Alternatively, if effect sizes against this outcome are small, our study may not have had sufficient sample size to detect a difference.
Since our study is based on clinical diagnosis of the outcomes of interest, influenza-related hospitalizations were not laboratory confirmed. The peak influenza season, or moving epidemic method analysis, aimed to improve outcome specificity in the absence of laboratory confirmation of influenza. Findings were similar in terms of both magnitude and direction between the full outcome assessment period (30 September 2019 to 7 March 2020) and the peak influenza season (8 December 2019 to 7 March 2020). Patterns were also similar when the study period was shortened further to 15 February 2020, to avoid outcome misclassification due to SARS-CoV-2 cocirculation. Of note, cardiorespiratory hospitalizations may occur as a result of complications of influenza, even after a patient has recovered and may no longer test positive for influenza, making laboratory confirmation for these outcomes irrelevant.
Several limitations apply to our research related to the noninterventional design of the study. We used routinely collected EMR and claims data to assess real-world outcomes in cardiorespiratory hospitalizations. As a result, this study is subject to limitations due to variations in patient and provider behavior. For example, patient use of healthcare resources may be intermittent or opportunistic. This is unlikely to affect hospitalization outcomes, but variations in the amount and quality of available data may affect the balancing of patients by baseline characteristics. This limitation was minimized by restricting the analysis to active patients in the data set. Next, clinicians may prioritize different codes when documenting the primary reason for a healthcare encounter based on factors such as the resource intensiveness of the encounter or disease manifestations that occasioned the visit. For this reason, we examined the rVEs of hospitalization outcomes both in the admitting position and in any position. The admitting diagnosis is the initial working diagnosis established at the time of admission and could be related to manifestations of cardiorespiratory illness that prompted the individual to seek care, such as shortness of breath, for example. Similar rVE magnitude was observed for influenza hospitalizations in the admitting diagnosis compared with any diagnosis; however, the lower bound of the CI crossed the null, likely owing to the substantially reduced number of cases available for the analysis of the admitting diagnosis.
Our study also has several important strengths. The integrated data set combines the clinical details of EMR data with the comprehensive care details of claims data. Exposure, outcome, and covariate information were ascertained similarly across all exposure cohorts, limiting the possibility of differential misclassification. The data set was drawn from a broad geographic sample, minimizing regional differences, and the population demographics are representative of the overall US population, which supports the generalizability of the results [22]. Any demographic and clinical heterogeneity between vaccination groups was mitigated by the IPTW approach, which balanced demographic confounders across vaccine types. Furthermore, we used doubly robust methods to aim to account for any residual confounding in the weighted sample. A negative control outcome of injury/trauma hospitalizations was included in the analyses to detect residual bias in the weighted and adjusted analyses and showed no association with the vaccines of interest. Our study results corroborate findings from real-world studies of the 2019–2020 season as well as those conducted during previous 2017–2018 and 2018–2019 influenza seasons and support the trend favoring IIV4c relative to IIV4e [9, 11, 12, 15, 17, 18].
In conclusion, the purpose of this retrospective cohort study was to evaluate the vaccine effectiveness of IIV4c relative to IIV4e among adults, using data from the 2019–2020 influenza season in the United States. The study findings demonstrated that, during the 2019–2020 influenza season, IIV4c was associated with fewer cardiorespiratory, respiratory, and influenza hospitalizations than IIV4e among individuals 18–64 years of age.
Supplementary Material
Acknowledgments
Medical consultants C. Gordon Beck and Amanda M. Justice provided editorial and medical writing support, which was funded by CSL Seqirus.
Author contributions. M. I. and M. H. were involved in study conception, design, and conceptual frameworks. L. L. G., A. D., and M. B. were involved in the analysis. J. P. B. and J. R. O. provided regular feedback on each of these steps. All authors were involved in the interpretation of data. M. I. drafted the manuscript, and A. D., J. P. B., J. R. O., L. L. G., M. B., and M. H. revised the manuscript critically. All authors made substantive intellectual contributions to the development of this manuscript and approved the final version.
Financial support. This study was supported by CSL Seqirus.
Contributor Information
Mahrukh Imran, CSL Seqirus, Kirkland, Quebec, Canada.
Juan Puig-Barbera, FISABIO, Valencia, Spain.
Justin R Ortiz, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Lorena Lopez-Gonzalez, Veradigm, Chicago, Illinois, USA.
Alex Dean, Veradigm, Chicago, Illinois, USA.
Machaon Bonafede, Veradigm, Chicago, Illinois, USA.
Mendel Haag, CSL Seqirus Netherlands B.V., Amsterdam, the Netherlands.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention. Disease burden of flu. 2022. Available at: https://www.cdc.gov/flu/about/burden/index.html. Accessed 1 March 2022.
- 2. Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol 2016; 1:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sellers SA, Hagan RS, Hayden FG, Fischer WA II. The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses 2017; 11:372–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrales-Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis 2010; 10:83–92. [DOI] [PubMed] [Google Scholar]
- 5. Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 influenza season. MMWR Recomm Rep 2020; 69:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajaram S, Van Boxmeer J, Leav B, Suphaphiphat P, Iheanacho I, Kistler K. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared to cell-based isolation and the possible implications for vaccine effectiveness. Presented at: IDWeek 2018; 3–7 October 2018, San Francisco, CA; abstract 2556.
- 7. Katz JM, Naeve CW, Webster RG. Host cell-mediated variation in H3N2 influenza viruses. Virology 1987; 156:386–95. [DOI] [PubMed] [Google Scholar]
- 8. Rocha EP, Xu X, Hall HE, Allen JR, Regnery HL, Cox NJ. Comparison of 10 influenza A (H1N1 and H3N2) haemagglutinin sequences obtained directly from clinical specimens to those of MDCK cell- and egg-grown viruses. J Gen Virol 1993; 74(pt 11):2513–8. [DOI] [PubMed] [Google Scholar]
- 9. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis 2019; 220:1255–64. [DOI] [PubMed] [Google Scholar]
- 10. Barr IG, Donis RO, Katz JM, et al. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018; 3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boikos C, Sylvester GC, Sampalis JS, Mansi JA. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin Infect Dis 2020; 71:e665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boikos C, Fischer L, O'Brien D, Vasey J, Sylvester GC, Mansi JA. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018–2019 influenza season in the United States. Clin Infect Dis 2021; 73:e692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imran M, Ortiz JR, McLean HQ, et al. Relative effectiveness of cell-based versus egg-based quadrivalent influenza vaccines in children and adolescents in the United States during the 2019–2020 influenza season. Pediatr Infect Dis J 2022; 41:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boikos C, Imran M, Nguyen VH, Ducruet T, Sylvester GC, Mansi JA. Effectiveness of the cell-derived inactivated quadrivalent influenza vaccine in individuals at high risk of influenza complications in the 2018–2019 United States influenza season. Open Forum Infect Dis 2021; 8:ofab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imran M, Ortiz JR, McLean HQ, et al. Relative effectiveness of cell-based versus egg-based quadrivalent influenza vaccines in adults during the 2019–2020 influenza season in the United States. Open Forum Infect Dis 2022; 9:ofac532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Divino V, Ruthwik Anupindi V, DeKoven M, et al. A real-world clinical and economic analysis of cell-derived quadrivalent influenza vaccine compared to standard egg-derived quadrivalent influenza vaccines during the 2019–2020 influenza season in the United States. Open Forum Infect Dis 2022; 9:ofab604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Divino V, Krishnarajah G, Pelton SI, et al. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine 2020; 38:6334–43. [DOI] [PubMed] [Google Scholar]
- 18. Krishnarajah G, Divino V, Postma MJ, et al. Clinical and economic outcomes associated with cell-based quadrivalent influenza vaccine vs. standard-dose egg-based quadrivalent influenza vaccines during the 2018–19 influenza season in the United States. Vaccines (Basel) 2021; 9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis NM, Chung JR, Uyeki TM, Grohskopf L, Ferdinands JM, Patel MM. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin Infect Dis 2022; 75:170–5. [DOI] [PubMed] [Google Scholar]
- 20. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–4. [DOI] [PubMed] [Google Scholar]
- 22. Boikos C, Imran M, De Lusignan S, Ortiz JR, Patriarca PA, Mansi JA. Integrating electronic medical records and claims data for influenza vaccine research. Vaccines (Basel) 2022; 10:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi X, Miao W, Tchetgen ET. A selective review of negative control methods in epidemiology. Curr Epidemiol Rep 2020; 7:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006; 35:337–44. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 26. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57:1288–94. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vega T, Lozano JE, Meerhoff T, et al. Influenza surveillance in Europe: establishing epidemic thresholds by the moving epidemic method. Influenza Other Respir Viruses 2013; 7:546–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. FluView summary ending on September 26, 2020. 2020. Available at: https://www.cdc.gov/flu/weekly/weeklyarchives2019-2020/Week39.htm. Accessed 10 May 2023.
- 31. Centers for Disease Control and Prevention. US flu VE data for 2019–2020. 2020. Available at: https://www.cdc.gov/flu/vaccines-work/2019-2020.html. Accessed 10 May 2023.
- 32. Flannery B, Fry AM. Comparing influenza vaccine types: the path toward improved influenza vaccine strategies. J Infect Dis 2019; 220:1237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monto AS, Petrie JG. Improving influenza vaccine effectiveness: ways to begin solving the problem. Clin Infect Dis 2019; 69:1824–6. [DOI] [PubMed] [Google Scholar]
- 34. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajaram S, Boikos C, Gelone DK, Gandhi A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother 2020; 8:2515135520908121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. Recommended composition of influenza virus vaccines for use in the 2020–2021 northern hemisphere influenza season. 2020. Available at: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2020-2021-northern-hemisphere-influenza-season. Accessed 10 May 2023.
- 37. Dawood FS, Chung JR, Kim SS, et al. Interim estimates of 2019–20 seasonal influenza vaccine effectiveness—United States, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Epperson S, Davis CT, Brammer L, et al. Update: influenza activity—United States and worldwide, May 19-September 28, 2019, and composition of the 2020 southern hemisphere influenza vaccine. MMWR Morb Mortal Wkly Rep 2019; 68:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Food and Drug Administration, Center for Biologics Evaluation and Research. 159th Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting; 4 March 2020; Silver Spring, MD.
- 40. Levine MZ, Martin ET, Petrie JG, et al. Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 influenza season. J Infect Dis 2019; 219:1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.