ABSTRACT
Ivermectin is an endectocide used widely to treat a variety of internal and external parasites. Field trials of ivermectin mass drug administration for malaria transmission control have demonstrated a reduction of Anopheles mosquito survival and human malaria incidence. Ivermectin will mostly be deployed together with artemisinin-based combination therapies (ACT), the first-line treatment of falciparum malaria. It has not been well established if ivermectin has activity against asexual stage Plasmodium falciparum or if it interacts with the parasiticidal activity of other antimalarial drugs. This study evaluated antimalarial activity of ivermectin and its metabolites in artemisinin-sensitive and artemisinin-resistant P. falciparum isolates and assessed in vitro drug-drug interaction with artemisinins and its partner drugs. The concentration of ivermectin causing half of the maximum inhibitory activity (IC50) on parasite survival was 0.81 μM with no significant difference between artemisinin-sensitive and artemisinin-resistant isolates (P = 0.574). The ivermectin metabolites were 2-fold to 4-fold less active than the ivermectin parent compound (P < 0.001). Potential pharmacodynamic drug-drug interactions of ivermectin with artemisinins, ACT-partner drugs, and atovaquone were studied in vitro using mixture assays providing isobolograms and derived fractional inhibitory concentrations. There were no synergistic or antagonistic pharmacodynamic interactions when combining ivermectin and antimalarial drugs. In conclusion, ivermectin does not have clinically relevant activity against the asexual blood stages of P. falciparum. It also does not affect the in vitro antimalarial activity of artemisinins or ACT-partner drugs against asexual blood stages of P. falciparum.
KEYWORDS: ivermectin, ivermectin metabolites, drug-drug interactions, antimalarial drugs, Plasmodium falciparum
INTRODUCTION
In 2021, there were an estimated 247 million malaria cases and 619,000 malaria deaths worldwide according to WHO estimates (1). Six countries in the Greater Mekong subregion (GMS)—Cambodia, China (specifically Yunnan Province and the Guangxi Zhuang Autonomous Region), Laos, Myanmar, Thailand, and Vietnam—have all pledged to aim for malaria elimination by 2030. Malaria prevention and treatment in this region relies heavily on safe and effective antimalarial drugs. Artemisinin resistance in Plasmodium falciparum has emerged and spread widely throughout the GMS (2, 3). It has also recently emerged in Rwanda (4, 5) and Uganda (6–8). The spread of antimalarial drug resistance is one of the most important obstacles to malaria elimination. New drugs are needed urgently for malaria treatment and elimination.
Ivermectin is a well-established antiparasitic drug with endectocidal properties. The primary target of ivermectin is the glutamate-gated chloride (GluCl) ion channel in the muscle and nerves of invertebrates (9–11). Ivermectin binds with high affinity and prevents the closure of the GluCl channel leading to an influx of chloride with subsequent hyperpolarization of the cell, leading to flaccid paralysis and potential death. Ivermectin has been used widely in mass drug administration (MDA) for the eradication of onchocerciasis and lymphatic filariasis. Recently, ivermectin has been proposed for use in MDA for malaria transmission control as it has potent mosquito-lethal properties against Anopheles mosquitoes (12–16). In Anopheles, ivermectin at sublethal concentrations delays time to refeeding, decreases locomotor activity, reduces fecundity, and inhibits Plasmodium development in the vector which could further impact transmission (17–21). Using ivermectin as a complementary approach for vector control can target mosquitoes that have changed their feeding behavior or survive from conventional vector control measures (i.e., indoor residual spraying and long-lasting insecticidal nets). Ivermectin MDA field trials have shown promising results in reducing wild Anopheles survival (14, 15, 22) and human malaria incidence (22, 23).
Ivermectin significantly reduces survival of Anopheles malaria vector (21, 24). In vitro mosquito-killing activity of ivermectin was used to predict mosquito-lethal effect in humans by pharmacokinetic-pharmacodynamic modeling. The simulated time above the lethal concentration that kills 50% of mosquitoes (LC50) after single dose administration of ivermectin (400 μg/kg) was 0.4 and 1.1 days in Anopheles dirus and Anopheles minimus mosquitoes, respectively (21). However, a clinical trial in healthy Thai adults showed much greater mosquito-killing activity after single dose ivermectin (400 μg/kg) treatment compared to in vitro ivermectin-spiked blood (24). This suggests that uncharacterized ivermectin metabolites may possess mosquito-lethal effects (24). When evaluated using in vitro systems, ivermectin is primarily metabolized by cytochrome P450 3A4. More than 10 ivermectin metabolites, mostly hydroxylated and demethylated, were identified using human liver microsomes (25, 26). These metabolites; 3”-O-demethyl ivermectin (M1), 4-hydroxymethyl ivermectin (M3), and 3”-O-demethyl, 4-hydroxymethyl ivermectin (M6) were found in human blood (26).
Ivermectin affects Plasmodium development in the Anopheles vector. Laboratory studies demonstrated that ivermectin at sublethal concentration on Anopheles vector inhibits P. falciparum and Plasmodium vivax sporogony by reducing oocyst prevalence and intensity (19, 21, 27–29). It has been reported that ivermectin inhibits the liver stages of Plasmodium berghei, similar to the pre-erythrocytic effects of primaquine (30), and it was also shown to inhibit the development of liver schizonts and hypnozoites of Plasmodium cynomolgi when evaluated in in vitro models (31). Ivermectin impaired both the in vitro sexual and asexual blood stage development in P. falciparum (32–34). However, the effect of ivermectin against the asexual blood stage showed very different levels of response in P. falciparum K1 strain when using different assays; IC50 of 8 μg/mL (equivalent to 9.1 μM) using the [3H] hypoxanthine incorporation assay (33) and 0.32 μg/mL (equivalent to 0.37 μM) using P. falciparum Histidine-Rich Protein 2 (HRP2) enzyme-linked immunosorbent assay (32). It was proposed that ivermectin blocked nucleo-cytoplasmic shuttling of P. falciparum signal recognition particle components (34, 35). Asexual stage P. vivax maturation was impaired when incubated with ivermectin-treated human plasma at 4 h after 200 μg/kg ivermectin single dose administration (28).
Ivermectin has been combined with dihydroartemisinin-piperaquine in MDA campaigns against malaria in The Gambia (22). This intervention was shown to be safe, well tolerate, and effective in reducing the prevalence of malaria in the region. Ivermectin has also been combined with seasonal malaria chemoprevention in a cluster-randomized trial evaluating sulfadoxine-pyrimethamine plus amodiaquine in Burkina Faso and reported no safety concerns (23). Furthermore, a healthy volunteer trial reported a small increase in ivermectin exposure of approximately 25% when combined with dihydroartemisinin-piperaquine but with no effect of primaquine co-administration (24). The lack of substantial pharmacokinetic drug-drug interactions and safety signals are promising, but there is still a paucity on the information of possible pharmacodynamic drug-drug interactions. The current study investigated the effects of ivermectin and its metabolites against asexual blood stages of artemisinin-sensitive and artemisinin-resistant P. falciparum isolates and the pharmacodynamic interactions of ivermectin when combined with commonly used antimalarial drugs.
RESULTS
Ivermectin parent compound, ivermectin aglycone, ivermectin monosaccharide, M1, M3, and M6 (Fig. S1) were selected to study antimalarial effects in this study. Antimalarial activity of ivermectin and its metabolites on asexual blood stage were investigated using a standard SYBR green I-based 72 h in vitro assay. Ivermectin parent compound showed antimalarial activity on asexual blood stage in a dose-dependent manner with mean (95% confidence interval) IC50 of 0.81 (0.67 to 0.95) μM and 0.81 (0.75 to 0.88) μM when tested against two artemisinin-sensitive and five artemisinin-resistant isolates, respectively (Table 1; Fig. S2). There was no significant difference in IC50 between artemisinin-sensitive and artemisinin-resistant isolates (P = 0.574). The IC50s of all ivermectin-related compounds, including its major metabolites, were 2-fold to 4-fold higher than ivermectin parent compound (Table 1). Ivermectin aglycone had the highest IC50 and all ivermectin metabolites showed less potency compared to ivermectin parent compound against all isolates (P < 0.001).
TABLE 1.
Antimalarial effect of ivermectin and its metabolites on asexual blood stages of P. falciparuma
| Parasite | Pfk13 mutation | Ivermectin | Ivermectin aglycone | Ivermectin monosaccharide | 3″-O-demethyl ivermectin (M1) | 4-hydroxymethyl ivermectin (M3) | 3″-O-demethyl, 4-hydroxymethyl ivermectin (M6) |
|---|---|---|---|---|---|---|---|
| Laboratory strain | |||||||
| NF54 | Wild type | 0.63 (0.46 to 0.80) |
2.55 (1.91 to 3.18) |
1.88 (1.39 to 2.38) |
1.99 (1.69 to 2.29) |
1.68 (1.22 to 2.14) |
2.57 (2.02 to 3.13) |
| Artemisinin-sensitive isolates | |||||||
| ARN3G | Wild type | 0.85 (0.55 to 1.10) |
2.72 (2.56 to 2.87) |
1.97 (1.56 to 2.39) |
1.91 (1.78 to 2.05) |
2.33 (1.78 to 2.87) |
2.80 (2.42 to 3.18) |
| ARK1G | Wild type | 0.76 (0.49 to 1.00) |
2.79 (2.14 to 3.43) |
2.14 (1.71 to 2.57) |
2.02 (1.47 to 2.57) |
1.82 (1.61 to 2.02) |
2.35 (2.28 to 2.43) |
| Mean of all artemisinin-sensitive isolates (n = 2) | 0.81 (0.67 to 0.95) |
2.75 (2.57 to 2.93) |
2.06 (1.87 to 2.24) |
1.97 (1.80 to 2.13) |
2.07 (1.74 to 2.41) |
2.58 (2.30 to 2.86) |
|
| Artemisinin-resistant isolates | |||||||
| APL4G | C580Y | 0.74 (0.66 to 0.81) |
3.02 (2.46 to 3.58) |
2.01 (1.57 to 2.45) |
2.03 (1.65 to 2.41) |
1.98 (1.75 to 2.20) |
2.66 (2.32 to 3.00) |
| APL5G | C580Y | 0.76 (0.47 to 1.10) |
4.23 (2.07 to 6.40) |
2.97 (1.89 to 4.06) |
1.91 (1.60 to 2.23) |
2.20 (1.46 to 2.94) |
2.85 (1.85 to 3.84) |
| APS2G | R539T | 1.06 (0.87 to 1.20) |
3.80 (3.53 to 4.06) |
3.13 (3.04 to 3.23) |
2.49 (2.04 to 2.94) |
2.84 (2.60 to 3.08) |
3.74 (3.24 to 4.24) |
| APS9G | C580Y | 0.79 (0.65 to 0.92) |
3.35 (2.60 to 4.10) |
2.56 (2.30 to 2.82) |
2.29 (1.89 to 2.69) |
2.21 (1.98 to 2.44) |
3.54 (3.05 to 4.03) |
| ARN2G | G449A | 0.78 (0.63 to 0.92) |
3.01 (2.25 to 3.78) |
2.46 (1.81 to 3.11) |
2.00 (1.89 to 2.10) |
2.40 (1.96 to 2.84) |
3.37 (2.81 to 3.93) |
| Mean of all artemisinin-resistant isolates (n = 5) | 0.81 (0.75 to 0.88) |
3.42 (3.10 to 3.74) |
2.58 (2.34 to 2.82) |
2.14 (2.00 to 2.28) |
2.30 (2.14 to 2.47) |
3.23 (2.97 to 3.49) |
|
All data are reported as mean IC50 (95% confidence interval), with the unit μM.
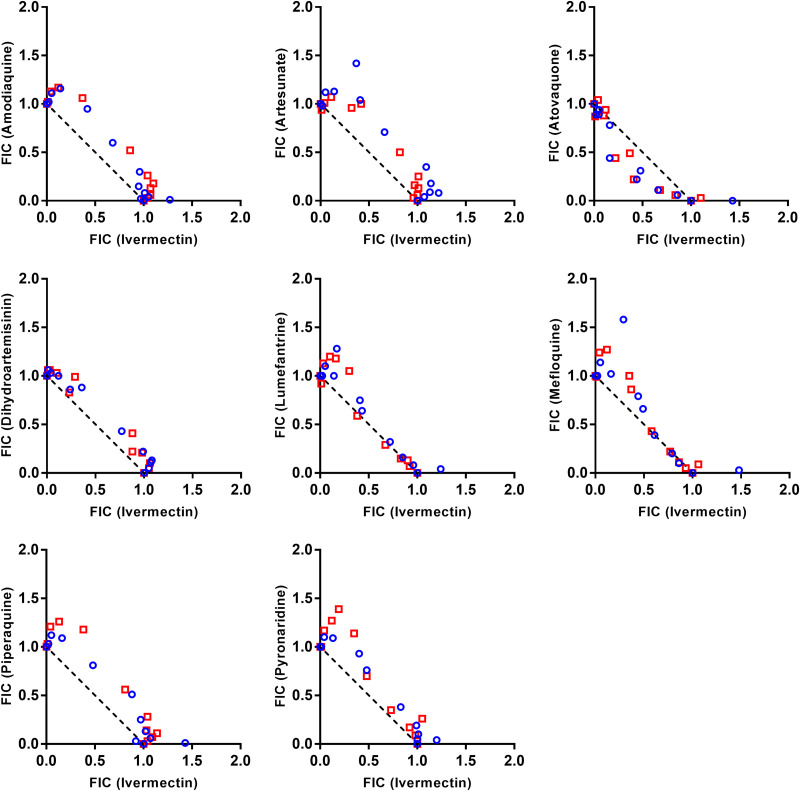
Ivermectin and antimalarial drug combination activity against P. falciparum was evaluated by a checkerboard analysis and presented as isobolograms and FIC indices. Eight combinations were tested with artemisinin-sensitive and artemisinin-resistant P. falciparum isolates. Isobologram analysis demonstrated no substantial interaction between ivermectin and amodiaquine, atovaquone, artesunate, dihydroartemisinin, lumefantrine, mefloquine, piperaquine, or pyronaridine (∑FIC > 0.5 and ≤4) (Fig. 1; Table 2).
FIG 1.
Pharmacodynamic interaction analysis. Isobologram analysis of ivermectin and antimalarial drugs against artemisinin-sensitive (blue circle symbol) and artemisinin-resistant (red square symbol) P. falciparum isolates, illustrated as fractional inhibitory concentration (FIC). The dashed line represents no interaction between the two drugs.
TABLE 2.
Antimalarial effects of ivermectin in combination with antimalarial drugs on asexual blood stages of P. falciparuma
| Drug combination(Drug A – Drug B) | Artemisinin-sensitive isolates (n = 2; NF54, ARN3G) |
Artemisinin-resistant isolates (n = 3; APL5G, APS2G, ARN2G) |
||||
|---|---|---|---|---|---|---|
| IC50 of ivermectin, (x103 nM) | IC50 of antimalarial, nM | ∑FIC | IC50 of ivermectin, (x103 nM) | IC50 of antimalarial, nM | ∑FIC | |
| Ivermectin – Amodiaquine | 0.94(0.72 to 1.16) | 11.42(7.94 to 14.90) | 1.18(1.09 to 1.27) | 1.18(0.82 to 1.53) | 12.31(10.83 to 13.79) | 1.21(1.13 to 1.30) |
| Ivermectin – Artesunate | 0.93(0.77 to 1.10) | 3.07(0.72 to 5.41) | 1.31(1.18 to 1.44) | 1.27(0.98 to 1.56) | 3.90(2.24 to 5.57) | 1.16(1.06 to 1.26) |
| Ivermectin – Atovaquone | 0.79(0.70 to 0.88) | 0.38(0.25 to 0.51) | 0.89(0.73 to 1.06) | 1.20(0.81 to 1.58) | 0.62(0.21 to 1.04) | 0.90(0.77 to 1.02) |
| Ivermectin – Dihydroartemisinin | 1.07(0.83 to 1.32) | 1.93(1.40 to 2.47) | 1.15(1.10 to 1.19) | 1.30(1.16 to 1.45) | 1.92(1.77 to 2.06) | 1.15(1.09 to 1.21) |
| Ivermectin – Lumefantrine | 0.95(0.70 to 1.19) | 7.40(2.11 to 12.70) | 1.13(1.03 to 1.22) | 1.41(0.99 to 1.83) | 6.14(3.96 to 8.32) | 1.09(0.98 to 1.21) |
| Ivermectin – Mefloquine | 0.78(0.62 to 0.93) | 10.96(4.05 to 17.87) | 1.15(1.03 to 1.27) | 1.13(0.92 to 1.27) | 23.08(10.2 to 35.97) | 1.14(1.02 to 1.25) |
| Ivermectin – Piperaquine | 0.80(0.66 to 0.95) | 12.94(10.09 to 15.78) | 1.21(1.10 to 1.31) | 1.06(0.84 to 1.27) | 12.16(9.46 to 14.85) | 1.26(1.15 to 1.37) |
| Ivermectin – Pyronaridine | 0.95(0.83 to 1.07) | 4.38(3.21 to 5.55) | 1.18(1.11 to 1.25) | 1.22(0.84 to 1.61) | 2.74(1.88 to 3.59) | 1.22(1.11 to 1.34) |
All data are reported as mean values (95% confidence interval).
The antimalarial activity of artesunate was further studied alone and in combination with a fixed dose of 50 ng/mL ivermectin by the trophozoite maturation assay. No difference was observed in IC50 values of artesunate alone or in combination with ivermectin in both artemisinin-sensitive (P = 0.385) and artemisinin-resistant (P = 0.546) isolates (Fig. S3).
DISCUSSION
The World Health Organization (WHO) has recommended a range of interventions to achieve the elimination of malaria (36). Ivermectin has a significant mosquito-lethal effect on many species of Anopheline mosquitoes. (17–19, 21, 28, 37). It has been proposed that ivermectin be used as a complementary malaria vector control tool (38). Although several studies have reported that ivermectin has a clear concentration-dependent mosquito-lethal effect, resulting in a reduced incidence of malaria, no study has evaluated the pharmacodynamic drug-drug interactions of ivermectin and the commonly used antimalarial drugs against the asexual blood stage of Plasmodium parasites. The in vitro activity of ivermectin was assessed against P. falciparum laboratory strains and isolates. Previous reports on the effect of ivermectin on the asexual blood stage of P. falciparum have reported IC50 values ranging from approximately 0.021 μM to 9 μM (Table S1) (32–34, 37, 39). Differences in IC50 values between studies may be due to variations in parasite strains, drug exposure times, and methods of assessment. In this study, the antimalarial activity of ivermectin was evaluated against artemisinin-sensitive and artemisinin-resistant P. falciparum isolates; however, there was no correlation between the artemisinin resistance status of the isolate and the ivermectin IC50 (~0.8 μM). A study from Gabon P. falciparum isolates reported relatively low IC50 of 0.14 μM in a chloroquine-sensitive isolate JH26, and 0.021 μM and 0.13 μM in chloroquine-resistant isolates JH1 and JH13, respectively. Also, there was no correlation between the effect of ivermectin and chloroquine resistance (32).
Previous work identified ivermectin metabolites generated by liver microsomes, primary human hepatocyte, and human blood after ivermectin administration (26). In this current study, we examined how three primary ivermectin metabolites (M1, M3, M6), as well as ivermectin monosaccharide and aglycone, affected both artemisinin-sensitive and resistant parasites. Ivermectin was more potent than ivermectin aglycone, ivermectin monosaccharide, and all ivermectin in vivo metabolites (M1, M3, and M6). The effect of ivermectin aglycone, which lacks the sugar moiety and has a hydroxy-group at C-13 position, presented >90% parasite growth at 1 μM concentrations in this study. The reduction of effect due to these molecular modifications has been shown previously (37, 39).
A single dose of 5 mg/kg ivermectin had no effect on the blood stages of Plasmodium berghei in rodents, resulting in the same level of parasitemia, gametocytemia, and exflagellation as vehicle control (29). The impact of ivermectin on different Plasmodium developmental stages has been evaluated, and found to inhibit liver-stage development of P. berghei (30, 37, 39) and Plasmodium cynomolgi in vitro (31). Three doses of 10 mg/kg ivermectin inhibited approximately 80% of P. berghei liver infections and enhanced host survival in 80% of the treated mice (30). However, no causal prophylactic effect of ivermectin was observed for P. cynomolgi infections in macaques (0.3 to 1.2 mg/kg) (31) or P. falciparum infections in a controlled human malaria infection model of ivermectin administration at 400 μg/kg (40). Ivermectin inhibited the sporogony of P. falciparum (27) and P. vivax (19, 21) at sublethal concentrations to Anopheles vectors by reducing oocyst prevalence and intensity. It remains unclear if the drug acts on mosquito midgut physiology or interferes with sporogony development. Ivermectin and avermectin derivatives showed no activity against gametes. However, they exhibited inhibitory effects against the late sporogony process of ookinete and oocyst formation in a mosquito-free in vitro assay to study the direct drug effect on sporogony in Plasmodium berghei (29).
The optimal dosage and regimen of ivermectin are key parameters to maintain plasma ivermectin concentration at effective levels (41). A standard dose of ivermectin for onchocerciasis and lymphatic filariasis were 150 μg/kg and 200 μg/kg. In comparison, the tested doses of ivermectin trials for malaria transmission control varied from 150 to 600 μg/kg (13–16, 22, 23). Peak concentrations of 56.8 ng/mL ivermectin was observed after a single dose of 400 μg/kg in healthy Thai adults (24). While peak concentrations of 64.1 ng/mL and 105.2 ng/mL were reported in malaria patients after receiving a 3-day treatment of dihydroartemisinin-piperaquine and ivermectin at 300 and 600 μg/kg/day, respectively (42). At this dosage, plasma ivermectin showed potent mosquitocidal effects against Anopheline mosquitoes (24, 43). In contrast, the asexual blood stage IC50 observed in this study was approximately 0.8 μM (equal to 712 ng/mL), which is 6-fold to 12-fold higher than clinically relevant peak plasma ivermectin concentration at commonly used doses and regimens. Thus, it is unlikely that ivermectin MDA for malaria transmission control will impact asexual blood stage malaria parasites. In addition, ivermectin-treated parasites showed increasing trends of sexual commitment in a transgenic P. falciparum NF54 strain that expressed endogenous mScarlet-tagged AP2-G, a specific marker for sexually committed ring stages (44).
During the malaria transmission season, ivermectin MDA alone (14, 15) and in combination with artemether-lumefantrine (13) or dihydroartemisinin-piperaquine (16, 22, 24) or albendazole (15, 23) significantly reduced mosquito survival and malaria cases. Although ivermectin MDA was designed to target mosquitoes that feed on humans, it is important to address the interaction between ivermectin and antimalarial drugs on asexual blood stages of P. falciparum. For instance, antagonistic interactions have been observed in vitro on P. falciparum when combining ivermectin and doxycycline (45). The results presented here demonstrate that the parasite killing effects of the commonly used antimalarial drugs is not altered when combined with ivermectin when evaluated in vitro. This suggests that there is no clinically important pharmacodynamic drug-drug interactions to consider for possible MDA administrations. However, limited data are available on pharmacokinetic drug-drug interactions between ivermectin and antimalarial drugs. A healthy volunteer trial in Thailand showed relatively minor increases in the exposure to both piperaquine and ivermectin when co-administered, but there was no drug-drug interaction reported for primaquine (24). The combination of ivermectin and ACTs in MDA campaigns need further monitoring of drug efficacy and pharmacokinetic drug interactions in endemic area.
In conclusion, ivermectin and its metabolites showed no antimalarial effects at clinically relevant concentrations, although ivermectin demonstrated stronger antimalarial activity than its metabolites. Furthermore, neither artemisinin-sensitive nor artemisinin-resistant P. falciparum isolates exhibited pharmacodynamic interactions between ivermectin and commonly used antimalarial drugs. These findings support that ivermectin is unlikely to interfere with the antimalarial activity of the commonly used antimalarial drugs.
MATERIALS AND METHODS
Parasite culture.
Artemisinin-sensitive (n = 2) and artemisinin-resistant (n = 5) P. falciparum isolates were obtained from clinical studies conducted on the Thailand-Cambodia border (2, 46). All parasite isolates were mycoplasma free. Parasites were cultured at 5% parasitemia and 5% hematocrit in culture medium. Culture medium consisted of Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma catalog no. R6504) containing 50 mg/L hypoxanthine (Sigma catalog no. H9377), 3 mg/L thiamine (Sigma catalog no. T1270), 6 mg/L L-ascorbic acid (Sigma catalog no. A5960), 30 mg/L CaCl2 (Sigma catalog no. C4901), 26 mg/L KH2PO4 (Merck catalog no. A681173), 16 mg/L MgSO4 (Sigma catalog no. M8150), 1 g/L d-glucose (Sigma catalog no. G7021), 5.96 g/L HEPES (Sigma catalog no. H3375), 2 g/L NaHCO3 (Sigma catalog no. S5761), and 10% human serum. Culture medium was replaced daily and incubated at 37°C in a 5% CO2 incubator.
Ivermectin, ivermectin metabolites, and antimalarial drugs.
Ivermectin parent compound (Sigma, catalog no. I8898), ivermectin aglycone (Cayman Chemical, catalog no. 19442), ivermectin monosaccharide (Clearsynth, catalog no. CS-CM-00113) were purchased commercially. The ivermectin metabolite, 4-hydroxymethyl ivermectin (M3), was synthesized from parent compound (WuXi AppTec). The 3″-O-demethyl ivermectin (M1) structure could not be produced by chemical synthesis and therefore bacterial strains were used to generate the 3″-O-demethyl ivermectin (M1) from parent compound, and the 3″-O-demethyl, 4-hydroxymethyl ivermectin (M6) from M3 (Hypha Discovery). A previously developed LC-MS/MS method was used to confirm that the synthesized compounds contained the stated metabolites (26). Ivermectin compounds were prepared as stock solutions at 2 mg/mL in DMSO.
Artesunate (Artesunate for Injection; registration no. 1C 3/35 [N], Guilin No.2 Pharmaceutical Factory) was dissolved in 5% NaHCO3 at 60 mg/mL. Amodiaquine was dissolved in 70% ethanol at 1 mg/mL. Atovaquone and dihydroartemisinin were dissolved in DMSO at 1 mg/mL. Lumefantrine was dissolved in absolute ethanol at 1 mg/mL. Mefloquine and piperaquine were dissolved in 0.1 M H3PO4 at 1 mg/mL. Pyronaridine was dissolved in RPMI 1640 medium at 1 mg/mL. All antimalarial drugs were kindly provided by Worldwide Antimalarial Resistance Network (WWARN). Stock solutions were kept at −80°C and diluted with culture medium before the assay was set up.
Drug sensitivity on asexual blood stage.
Ivermectin and ivermectin metabolites were prepared by a 2-fold serial dilution (0.02 to 10 μM) in RPMI 1640 medium supplemented with 0.5% AlbuMAX II (Thermo Fisher Scientific catalog no.11021045) in flat bottom 96-well plates at 50 μL/well. Asexual blood stage parasites, predominantly at the ring stage, were prepared at 1% parasitemia and 2% hematocrit. In each well, 50 μL of parasite suspension was added and gently mixed with the compounds. After 72 h of incubation, SYBR green I staining was used to detect parasite growth (47, 48). Each well was filled with 100 μL of 2×SYBR-green I lysis buffer (0.1% wt/vol saponin, Sigma catalog no.47036; 1%vol/vol Triton X-100, Bio-Rad catalog no. 161-0407; 5 mM EDTA, Sigma catalog no. E7889; and 20 mM Tris-HCl, Sigma catalog no. T5941). Plates were incubated in the dark for 30 min before the fluorescence signal was measured on a microplate reader (Synergy H1, BioTek) using a 485-nm excitation filter and a 520-nm emission filter. Assays were performed for at least three independent biological replicates with technical duplicates in each isolate. The percentage of parasite growth and IC50 were calculated using GraphPad Prism version 8. Statistical significance was determined by Student's t test and nonparametric Mann-Whitney U tests.
Pharmacodynamic drug-drug interactions with antimalarial drugs.
The effects of ivermectin parent compound in combination with antimalarial drugs against the asexual blood stage of P. falciparum were evaluated using the checkerboard technique (49, 50). Briefly, two-dimensional checkerboard titration was prepared in flat-bottom 96-well plates in a volume of 50 μL. The assay plate was prepared with combinations of ivermectin (0.05 to 10 μM) and individual antimalarial drugs (0.20 to 100 nM amodiaquine, 0.10 to 50 nM artesunate, 0.02 to 10 nM atovaquone, 0.1 to 50 nM dihydroartemisinin, 0.39 to 200 nM lumefantrine, 0.78 to 400 nM mefloquine, 0.39 to 200 nM piperaquine, and 0.20 to 100 nM pyronaridine). Asexual blood stage parasites, predominantly at the ring stage, were prepared at 1% parasitemia and 2% hematocrit. In each well, 50 μL of parasite suspension was added to a final volume of 100 μL and gently mixed with the compounds. After 72 h of incubation, parasite growth was assessed by DNA content using a SYBR green I-based fluorescence staining (47, 48). Each well was filled with 100 μL of 2×SYBR-green I lysis buffer. Plates were incubated in the dark for 30 min before the fluorescence signal was measured on a microplate reader (Synergy H1, BioTek) using a 485-nm excitation filter and a 520-nm emission filter. The interactions between two compounds were evaluated using isobolograms and derived fractional inhibitory concentrations (FIC). The sum of FICs (ΣFIC) were calculated by a fraction of the IC50s in each drug combination and the IC50s of the single drug according to equation 1.
| (1) |
where IC50A+B is the IC50 of drug A in combination with drug B, IC50B+A is the IC50 of drug B in combination with drug A, IC50 A and IC50 B are the IC50 of drug A and drug B alone, respectively. ΣFIC of ≤0.5, 0.5 < FIC ≤ 4 and FIC > 4 indicated synergistic, indifferent, and antagonistic effects, respectively (51, 52). The isobolograms and derived mean FIC values were calculated from at least three independent biological replicates with technical duplicates in each assay.
The antimalarial effect of artesunate when combined with a fixed dose of ivermectin was further investigated by the trophozoite maturation assay (53). Two-fold serial dilutions of artesunate alone (0.001 to 1 μM) and artesunate combined with a fixed dose ivermectin at 50 ng/mL, the observed clinical peak concentration of ivermectin after administration of ivermectin (150 μg/kg) (54), were prepared in flat-bottom 96-well plates. Parasites, predominantly at the ring stage, were prepared at 1% parasitemia and 2% hematocrit, and then incubated with the drug for 24 h. Thick and thin blood films were harvested and stained with Field’s stain. The staging of parasite development was investigated using a light microscope, and the numbers of trophozoites were counted per 100 parasitized red blood cells. Trophozoites were identified by morphology, size, nuclear/cytoplasm ratios, and visible pigment. IC50 was evaluated from the inhibition of the parasite development from ring to trophozoites compared to parasites in drug-free control wells using GraphPad Prism version 8. Statistical significance was determined by Student's t test and nonparametric Mann-Whitney U tests.
ACKNOWLEDGMENTS
We thank the Department of Clinical Pharmacology, Mahidol Oxford Tropical Medicine Research Unit (MORU), and Worldwide Antimalarial Resistance Network (WWARN) for providing compounds and antimalarial drugs. We thank Thanat Chookajorn, Markus Winterberg, and all staff from the Malaria Laboratory, Mahidol Oxford Tropical Medicine Research Unit (MORU), and Cell and Tissue Culture Research Unit (CTCRU), Faculty of Tropical Medicine, Mahidol University for advice and technical assistance. This work was supported by the Royal Golden Jubilee PhD Program (PHD/0142/2559), DEAN-MORU scholarship, Faculty of Tropical Medicine, Mahidol University, The Wellcome Trust of Great Britain, and the Bill & Melinda Gates Foundation (INV-008941).
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the U.S. Department of the Army or the U.S. Department of Defense.
Footnotes
[This article was published on 20 June 2023 with incorrect affiliation information for Nicholas J. White and some superfluous wording associated with equation 1. The affiliation information was updated and the superfluous wording was eliminated in the current version, posted on 29 June 2023.]
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2022. World malaria report 2022. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han K-T, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R. Tracking Resistance to Artemisinin Collaboration (TRAC), et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, Smithuis FM, Hlaing TM, Tun KM, van der Pluijm RW, Tripura R, Miotto O, Menard D, Dhorda M, Day NPJ, White NJ, Dondorp AM. 2017. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 17:491–497. doi: 10.1016/S1473-3099(17)30048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straimer J, Gandhi P, Renner KC, Schmitt EK. 2022. High prevalence of Plasmodium falciparum K13 mutations in Rwanda is associated with slow parasite clearance after treatment with artemether-lumefantrine. J Infect Dis 225:1411–1414. doi: 10.1093/infdis/jiab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, Habimana RM, Rucogoza A, Moriarty LF, Sandford R, Piercefield E, Goldman I, Ezema B, Talundzic E, Pacheco MA, Escalante AA, Ngamije D, Mangala J-LN, Kabera M, Munguti K, Murindahabi M, Brieger W, Musanabaganwa C, Mutesa L, Udhayakumar V, Mbituyumuremyi A, Halsey ES, Lucchi NW. 2021. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 21:1120–1128. doi: 10.1016/S1473-3099(21)00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asua V, Conrad MD, Aydemir O, Duvalsaint M, Legac J, Duarte E, Tumwebaze P, Chin DM, Cooper RA, Yeka A, Kamya MR, Dorsey G, Nsobya SL, Bailey J, Rosenthal PJ. 2021. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis 223:985–994. doi: 10.1093/infdis/jiaa687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asua V, Vinden J, Conrad MD, Legac J, Kigozi SP, Kamya MR, Dorsey G, Nsobya SL, Rosenthal PJ. 2019. Changing molecular markers of antimalarial drug sensitivity across Uganda. Antimicrob Agents Chemother 63:e01818-18. doi: 10.1128/AAC.01818-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S-I, Yamauchi M, Opio W, Emoto S, Anywar DA, Kimura E, Palacpac NMQ, Odongo-Aginya EI, Ogwang M, Horii T, Mita T. 2021. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385:1163–1171. doi: 10.1056/NEJMoa2101746. [DOI] [PubMed] [Google Scholar]
- 9.Lynagh T, Lynch JW. 2012. Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol Sci 33:432–441. doi: 10.1016/j.tips.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Lynagh T, Lynch JW. 2012. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front Mol Neurosci 5:60. doi: 10.3389/fnmol.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers JI, Gray M, Kuklinski W, Johnson LB, Snow CD, Black WC, Partin KM, Foy BD. 2015. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J Exp Biol 218:1478–1486. doi: 10.1242/jeb.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaccour C, Lines J, Whitty CJM. 2010. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis 202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 13.Ouédraogo AL, Bastiaens GJH, Tiono AB, Guelbéogo WM, Kobylinski KC, Ouédraogo A, Barry A, Bougouma EC, Nebie I, Ouattara MS, Lanke KHW, Fleckenstein L, Sauerwein RW, Slater HC, Churcher TS, Sirima SB, Drakeley C, Bousema T. 2015. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin Infect Dis 60:357–365. doi: 10.1093/cid/ciu797. [DOI] [PubMed] [Google Scholar]
- 14.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, Foy BD. 2010. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J 9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alout H, Krajacich BJ, Meyers JI, Grubaugh ND, Brackney DE, Kobylinski KC, Diclaro JW, Bolay FK, Fakoli LS, Diabaté A, Dabiré RK, Bougma RW, Foy BD. 2014. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J 13:417. doi: 10.1186/1475-2875-13-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smit MR, Ochomo EO, Aljayyoussi G, Kwambai TK, Abong'o BO, Chen T, Bousema T, Slater HC, Waterhouse D, Bayoh NM, Gimnig JE, Samuels AM, Desai MR, Phillips-Howard PA, Kariuki SK, Wang D, Ward SA, Ter Kuile FO. 2018. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 18:615–626. doi: 10.1016/S1473-3099(18)30163-4. [DOI] [PubMed] [Google Scholar]
- 17.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, Sylla M, Foy BD. 2010. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop 116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampaio VS, Beltrán TP, Kobylinski KC, Melo GC, Lima JBP, Silva SGM, Rodriguez ÍC, Silveira H, Guerra MGVB, Bassat Q, Pimenta PFP, Lacerda MVG, Monteiro WM. 2016. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J 15:491. doi: 10.1186/s12936-016-1540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobylinski KC, Escobedo-Vargas KS, López-Sifuentes VM, Durand S, Smith ES, Baldeviano GC, Gerbasi RV, Ballard S-B, Stoops CA, Vásquez GM. 2017. Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi. Malar J 16:474. doi: 10.1186/s12936-017-2125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampaio VdS, Rivas GBdS, Kobylinski K, Pinilla YT, Pimenta PFP, Lima JBP, Bruno RV, Lacerda MVG, Monteiro WM. 2017. What does not kill it makes it weaker: effects of sub-lethal concentrations of ivermectin on the locomotor activity of Anopheles aquasalis. Parasit Vectors 10:623. doi: 10.1186/s13071-017-2563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobylinski KC, Ubalee R, Ponlawat A, Nitatsukprasert C, Phasomkulsolsil S, Wattanakul T, Tarning J, Na-Bangchang K, McCardle PW, Davidson SA, Richardson JH. 2017. Ivermectin susceptibility and sporontocidal effect in Greater Mekong Subregion Anopheles. Malar J 16:280. doi: 10.1186/s12936-017-1923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabira ED, Soumare HM, Conteh B, Ceesay F, Ndiath MO, Bradley J, Mohammed N, Kandeh B, Smit MR, Slater H, Peeters Grietens K, Broekhuizen H, Bousema T, Drakeley C, Lindsay SW, Achan J, D'Alessandro U. 2022. Mass drug administration of ivermectin and dihydroartemisinin–piperaquine against malaria in settings with high coverage of standard control interventions: a cluster-randomised controlled trial in The Gambia. Lancet Infect Dis 22:519–528. doi: 10.1016/S1473-3099(21)00557-0. [DOI] [PubMed] [Google Scholar]
- 23.Foy BD, Alout H, Seaman JA, Rao S, Magalhaes T, Wade M, Parikh S, Soma DD, Sagna AB, Fournet F, Slater HC, Bougma R, Drabo F, Diabaté A, Coulidiaty AGV, Rouamba N, Dabiré RK. 2019. Efficacy and risk of harms of repeat ivermectin mass drug administrations for control of malaria (RIMDAMAL): a cluster-randomised trial. Lancet 393:1517–1526. doi: 10.1016/S0140-6736(18)32321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobylinski KC, Jittamala P, Hanboonkunupakarn B, Pukrittayakamee S, Pantuwatana K, Phasomkusolsil S, Davidson SA, Winterberg M, Hoglund RM, Mukaka M, van der Pluijm RW, Dondorp A, Day NPJ, White NJ, Tarning J. 2020. Safety, pharmacokinetics, and mosquito-lethal effects of ivermectin in combination with dihydroartemisinin-piperaquine and primaquine in healthy adult Thai subjects. Clin Pharmacol Ther 107:1221–1230. doi: 10.1002/cpt.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Z, Andrew NW, Arison BH, Luffer-Atlas D, Wang RW. 1998. Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica 28:313–321. doi: 10.1080/004982598239597. [DOI] [PubMed] [Google Scholar]
- 26.Tipthara P, Kobylinski KC, Godejohann M, Hanboonkunupakarn B, Roth A, Adams JH, White NJ, Jittamala P, Day NPJ, Tarning J. 2021. Identification of the metabolites of ivermectin in humans. Pharmacol Res Perspect 9:e00712. doi: 10.1002/prp2.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobylinski KC, Foy BD, Richardson JH. 2012. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malar J 11:381. doi: 10.1186/1475-2875-11-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinilla YT, C P Lopes S, S Sampaio V, Andrade FS, Melo GC, Orfanó AS, Secundino NFC, Guerra MGVB, Lacerda MVG, Kobylinski KC, Escobedo-Vargas KS, López-Sifuentes VM, Stoops CA, Baldeviano GC, Tarning J, Vasquez GM, Pimenta PFP, Monteiro WM. 2018. Promising approach to reducing Malaria transmission by ivermectin: sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Negl Trop Dis 12:e0006221. doi: 10.1371/journal.pntd.0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azevedo R, Mendes AM, Prudêncio M. 2019. Inhibition of Plasmodium sporogonic stages by ivermectin and other avermectins. Parasit Vectors 12:549. doi: 10.1186/s13071-019-3805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendes AM, Albuquerque IS, Machado M, Pissarra J, Meireles P, Prudêncio M. 2017. Inhibition of plasmodium liver infection by ivermectin. Antimicrob Agents Chemother 61:e02005-16. doi: 10.1128/AAC.02005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanachayangkul P, Im-Erbsin R, Tungtaeng A, Kodchakorn C, Roth A, Adams J, Chaisatit C, Saingam P, Sciotti RJ, Reichard GA, Nolan CK, Pybus BS, Black CC, Lugo-Roman LA, Wegner MD, Smith PL, Wojnarski M, Vesely BA, Kobylinski KC. 2020. Safety, Pharmacokinetics, and activity of high-dose ivermectin and chloroquine against the liver stage of plasmodium cynomolgi Infection in Rhesus Macaques. Antimicrobial Agents and Chemotherapy 64:e00741-20. doi: 10.1128/AAC.00741-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho LPd, Sandri TL, Melo EJTd, Fendel R, Kremsner PG, Mordmüller B, Held J. 2019. Ivermectin impairs the development of sexual and asexual stages of plasmodium falciparum in vitro. Antimicrobial Agents and Chemotherapy 63. doi: 10.1128/AAC.00085-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasveld P, Russell B, Kotecka B, Rieckmann K. 2003. Lack of in vitro effect of ivermectin on Plasmodium falciparum. Southeast Asian J Trop Med Public Health 34:552–553. [PubMed] [Google Scholar]
- 34.Panchal M, Rawat K, Kumar G, Kibria KM, Singh S, Kalamuddin M, Mohmmed A, Malhotra P, Tuteja R. 2014. Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell Death Dis 5:e994. doi: 10.1038/cddis.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walunj SB, Wang C, Wagstaff KM, Patankar S, Jans DA. 2022. Conservation of importin α; function in apicomplexans: ivermectin and GW5074 target Plasmodium falciparum importin α; and inhibit parasite growth in culture. Int J Molecular Sciences [Internet] 23. doi: 10.3390/ijms232213899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 2021. Global technical strategy for malaria 2016–2030, 2021 update. WHO, Geneva, Switzerland. [Google Scholar]
- 37.Singh L, Fontinha D, Francisco D, Mendes AM, Prudêncio M, Singh K. 2020. Molecular design and synthesis of ivermectin hybrids targeting hepatic and erythrocytic stages of plasmodium parasites. J Med Chem 63:1750–1762. doi: 10.1021/acs.jmedchem.0c00033. [DOI] [PubMed] [Google Scholar]
- 38.Billingsley P, Binka F, Chaccour C, Foy B, Gold S, Gonzalez-Silva M, Jacobson J, Jagoe G, Jones C, Kachur P, Kobylinski K, Last A, Lavery JV, Mabey D, Mboera D, Mbogo C, Mendez-Lopez A, Rabinovich NR, Rees S, Richards F, Rist C, Rockwood J, Ruiz-Castillo P, Sattabongkot J, Saute F, Slater H, Steer A, Xia K, Zullinger R. 2020. A roadmap for the development of ivermectin as a complementary malaria vector control tool. Am J Trop Med Hyg 102:3–24. doi: 10.4269/ajtmh.19-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh L, Fontinha D, Francisco D, Prudêncio M, Singh K. 2022. Synthesis and antiplasmodial activity of regioisomers and epimers of second-generation dual acting ivermectin hybrids. Sci Rep 12:564. doi: 10.1038/s41598-021-04532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzger WG, Theurer A, Pfleiderer A, Molnar Z, Maihöfer-Braatting D, Bissinger AL, Sulyok Z, Köhler C, Egger-Adam D, Lalremruata A, Esen M, Lee Sim K, Hoffman S, Rabinovich R, Chaccour C, Alonso P, Mordmüller BG, Kremsner PG. 2020. Ivermectin for causal malaria prophylaxis: a randomised controlled human infection trial. Trop Med Int Health 25:380–386. doi: 10.1111/tmi.13357. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. 2022. Endectocide and ectocide products for malaria transmission control. WHO, Geneva, Switzerland. [Google Scholar]
- 42.Smit MR, Ochomo EO, Waterhouse D, Kwambai TK, Abong'o BO, Bousema T, Bayoh NM, Gimnig JE, Samuels AM, Desai MR, Phillips-Howard PA, Kariuki SK, Wang D, Ter Kuile FO, Ward SA, Aljayyoussi G. 2019. Pharmacokinetics-pharmacodynamics of high-dose ivermectin with dihydroartemisinin-piperaquine on mosquitocidal activity and QT-prolongation (IVERMAL). Clin Pharmacol Ther 105:388–401. doi: 10.1002/cpt.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smit MR, Ochomo EO, Aljayyoussi G, Kwambai TK, Abong'o BO, Bousema T, Waterhouse D, Bayoh NM, Gimnig JE, Samuels AM, Desai MR, Phillips-Howard PA, Kariuki SK, Wang D, Ward SA, Ter Kuile FO. 2019. Human direct skin feeding versus membrane feeding to assess the mosquitocidal efficacy of high-dose ivermectin (IVERMAL Trial). Clin Infect Dis 69:1112–1119. doi: 10.1093/cid/ciy1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thommen BT, Passecker A, Buser T, Hitz E, Voss TS, Brancucci NMB. 2022. Revisiting the effect of pharmaceuticals on transmission stage formation in the malaria parasite Plasmodium falciparum. Front Cell Infect Microbiol 12. doi: 10.3389/fcimb.2022.802341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nodari R, Corbett Y, Varotto-Boccazzi I, Porretta D, Taramelli D, Epis S, Bandi C. 2020. Effects of combined drug treatments on Plasmodium falciparum: in vitro assays with doxycycline, ivermectin and efflux pump inhibitors. PLoS One 15:e0232171. doi: 10.1371/journal.pone.0232171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Pluijm RW, Tripura R, Hoglund RM, Pyae Phyo A, Lek D, Ul Islam A, Anvikar AR, Satpathi P, Satpathi S, Behera PK, Tripura A, Baidya S, Onyamboko M, Chau NH, Sovann Y, Suon S, Sreng S, Mao S, Oun S, Yen S, Amaratunga C, Chutasmit K, Saelow C, Runcharern R, Kaewmok W, Hoa NT, Thanh NV, Hanboonkunupakarn B, Callery JJ, Mohanty AK, Heaton J, Thant M, Gantait K, Ghosh T, Amato R, Pearson RD, Jacob CG, Gonçalves S, Mukaka M, Waithira N, Woodrow CJ, Grobusch MP, van Vugt M, Fairhurst RM, Cheah PY, Peto TJ, von Seidlein L, Dhorda M, Maude RJ, Winterberg M. Tracking Resistance to Artemisinin Collaboration, et al. 2020. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet 395:1345–1360. doi: 10.1016/S0140-6736(20)30552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. 2007. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother 51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dery V, Duah NO, Ayanful-Torgby R, Matrevi SA, Anto F, Quashie NB. 2015. An improved SYBR Green-1-based fluorescence method for the routine monitoring of Plasmodium falciparum resistance to anti-malarial drugs. Malar J 14:481. doi: 10.1186/s12936-015-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Irujo JJ, Villahermosa ML, Alberdi E, Santiago E. 1996. A checkerboard method to evaluate interactions between drugs. Biochem Pharmacol 51:635–644. doi: 10.1016/s0006-2952(95)02230-9. [DOI] [PubMed] [Google Scholar]
- 50.Bellio P, Fagnani L, Nazzicone L, Celenza G. 2021. New and simplified method for drug combination studies by checkerboard assay. MethodsX 8:101543. doi: 10.1016/j.mex.2021.101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob Agents Chemother 48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 53.Chotivanich K, Tripura R, Das D, Yi P, Day NPJ, Pukrittayakamee S, Chuor CM, Socheat D, Dondorp AM, White NJ. 2014. Laboratory detection of artemisinin-resistant Plasmodium falciparum. Antimicrob Agents Chemother 58:3157–3161. doi: 10.1128/AAC.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baraka OZ, Mahmoud BM, Marschke CK, Geary TG, Homeida MMA, Williams JF. 1996. Ivermectin distribution in the plasma and tissues of patients infected with Onchocerca volvulus. Eur J Clin Pharmacol 50:407–410. doi: 10.1007/s002280050131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01730-22-s0001.docx, DOCX file, 0.2 MB (248.3KB, docx)