Abstract
Chronic smoking is a primary risk factor for breast cancer due to the presence of various toxins and carcinogens within tobacco products. Nicotine is the primary addictive component of tobacco products and has been shown to promote breast cancer cell proliferation and metastases. Nicotine activates nicotinic acetylcholine receptors (nAChRs) that are expressed in cancer cell lines. Here, we examine the role of the α7 nAChR in coupling to heterotrimeric G proteins within breast cancer MCF-7 cells. Pharmacological activation of the α7 nAChR using choline or nicotine was found to increase proliferation, motility, and calcium signaling in MCF-7 cells. This effect of α7 nAChR on cell proliferation was abolished by application of Gαi/o and Gαq protein blockers. Specifically, application of the Gαi/o inhibitor pertussis toxin was found to abolish choline-mediated cell proliferation and intracellular calcium transient response. These findings were corroborated by expression of a G protein binding dominant negative nAChR subunit (α7345-348A), which resulted in significantly attenuating calcium signaling and cellular proliferation in response to choline. Our study shows a new role for G protein signaling in the mechanism of α7 nAChR-associated breast cancer growth.
Introduction
Epidemiological studies show that smoking of tobacco products can significantly increase a woman’s risk of developing breast cancer [1–4]. Thus, although cigarette smoke consists of a complex mixture of > 3000 chemicals, nicotine is a primary bioactive component of tobacco contributing to cancer risk [5]. A large body of published work relates nicotine exposure and the proliferation as well as metastatic potential of cancer cells within tissue such as lung and breast [6]. Molecular studies show that nicotine increases the proliferation of cancer cells through the activation of nicotinic acetylcholine receptors (nAChRs) expressed at the cell surface [7]. In some cases, nAChR expression can be altered during cancer cell growth and through hormones or toxin exposure [8].
The nAChR channel is a pentameric protein consisting of combinations of 16 isoforms of subunits including a subunits α1–α10, β1–β4, γ, δ, and/or ε [9, 10]. Homopentameric nAChRs including α7 and α9 exhibit high calcium permeability and thus can directly signal long-term cellular growth [11, 12]. Indeed, nicotine has been shown to activate α7 [13–17] and α9 [18–21] nAChRs in breast cancer cells. In cooperation with calcium influx, ligand activation of α7 nAChRs drives metabotropic signaling cascades important for growth and cytoskeletal motility [22–25]. In this study, we explored the role α7 nAChR interaction with G proteins in MCF-7 breast cancer cell proliferation, motility, and calcium signaling.
Materials and methods
Cell culture and transfection
Michigan Cancer Foundation-7 (MCF-7) cell line (ATCC, Manassas, VA, USA) cells was maintained as monolayers in advanced Dulbecco’s minimum essential medium (DMEM) containing phenol red and supplemented with 5% fetal bovine serum (FBS), 600 μg/ml L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 6 ml/500 ml 100 x non-essential amino acids (all from Invitrogen, CA, USA), and grown at 37°C in an incubator of 5% CO2 and 95% humidity. MCF-7 is a human breast cancer cell line with estrogen, progesterone and glucocorticoid receptors and is derived from the pleural effusion of a 69-year-old metastatic adenocarcinoma. MCF-7 cells were transfected with constructs encoding human α7345-348A or the control human α7 nAChR in pEYFP-C1 (Addgene) [24], GCaMP5G [26] using Lipofectamine 2000 (Thermo Fisher, Waltham, MA, USA). DNA was purified using a maxi prep kit (Xymo Research, Irvine, CA, USA).
Drug preparation
Choline, nicotine, cotinine, nornicotine, methyllycaconitine citrate (MLA) and α-bungarotoxin (BTX) were from Sigma Aldrich (St. Louis, MO, USA). Choline, MLA, and BTX were dissolved in distilled water. Nicotine, cotinine, and nornicotine were dissolved in ethanol. YM 254890 and pertussis toxin (PTX) were purchased from Tocris-Bio-Techne Corporation (Minneapolis, MN, USA) and dissolved in DMSO and distilled water, respectively. α-Conotoxin RgIA (CTX) was obtained from Alomone Labs (Jerusalem, Israel) and dissolved in distilled water.
Cellular fluorescence
Cell were permeabilized with 0.05% Triton X-100 then blocked with 10% goat serum (Life Technologies, Carlsbad, CA, USA) [27]. Surface α7 nAChRs were visualized in non-permeabilized cells using 100 nM Alexa Fluor (488 or 647) conjugated BTX as described [28, 29]. Imaging was performed on an inverted Zeiss LSM800 confocal microscope (Carl Zeiss Oberkochen, Germany). Analysis was carried out in ImageJ (NIH, Bethesda, MD, USA).
Cell proliferation (MTT assay)
Approximately 104 cells were seeded into triplicate wells of 12-well plates and allowed to attach overnight. Growth was assessed by an MTT assay after 3 days of drug incubation. Briefly, 1 ml of MTT [3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide] reagent (Promega) (0.5 mg/ml) was added to each well and incubated at 37°C for 30 min before the addition of 1 ml acidic isopropanol then vigorous re-suspension of the converted blue crystals. Cells were optically counted with a hemocytometer (Thermo Fisher) or the absorbance of the suspension was measured at 595 nm with background subtraction at 650 nm. Results are expressed as mean ± standard error of the mean (S.E.M.).
Calcium imaging
Cells were transfected with GCaMP5G [26] and the signal was detected using a Zeiss LSM 800 at an acquisition rate of 1 frame per 256 ms for 75 sec at 2 x 2 binning as described previously [23]. Drugs were applied to the recording chamber via a gravity fed perfusion at a flow rate of 1 ml/sec. Regions of interest (ROIs) were normalized as ΔF/Fθ and analyzed using ImageJ (NIH) as described [23]. A total of 20–30 cells were imaged per experimental condition and experiments were performed in triplicate.
Cell motility assay
Cells were plated on 6-well plates at 80–90% confluence with complete DMEM containing vehicle or drug. The following day, a scratch was created in the cell monolayer using a sterile p1000 pipette tip. A photograph of the scratched area was taken immediately (0 h) and after a 24 h incubation in a 37°C, 5% CO2. Cell motility was determined by calculating the width of the scratch at 24 h as a percentage of 0 h.
Statistical analysis
Student’s two-tailed unpaired t-test, or one-way ANOVA test followed by Bonferroni post hoc test were used to compare means of individual groups with p < 0.05 as statistically significant.
Results
Nicotinic receptor ligands promote proliferation and motility in MCF-7 cells
Nicotine has been shown promote cancer cell proliferation within various cell lines and in animal systems [6, 7, 20]. We examined the effect of a 3-day treatment with nicotine (50 nM-1 μM) on MCF-7 cell proliferation (Fig 1A). Treatment with nicotine was found to produce a concentration dependent increase in cell proliferation (p<0.05; ANOVA). Similarly, treatment with the selective α7-nACh receptor agonist choline increased proliferation of MCF-7 cells (Fig 1B). Cotinine, is the primary metabolite of nicotine and can persist longer in the body that nicotine [30]. Nornicotine, like nicotine, is found in tobacco products and has carcinogenic properties [31]. Both cotinine and nornicotine activate mammalian nAChRs and contribute to addiction [32]. We tested the effect of a 3-day treatment with cotinine (0.1–10 μM) or nornicotine (0.1–10 μM) on cell proliferation. We found that both cotinine and nornicotine increased cell proliferation to levels comparable with choline and nicotine at the concentration range of 1–10 μM (Fig 1C and 1D).
Fig 1. Dose-response effect of nicotine, choline, and nicotine metabolites on breast cancer cell proliferation.
Approximately 104 MCF-7 cells were seeded into microwell plates and grown for 3 days in the presence of vehicle (control) or increasing concentrations of nicotine (A), choline (B), cotinine (C), and nornicotine (D). Cells were harvested and growth was determined by an MTT assay. Bars represent means ± SEM of at least 3 independent determinations. Asterisk denotes significant difference from control with p < 0.05.
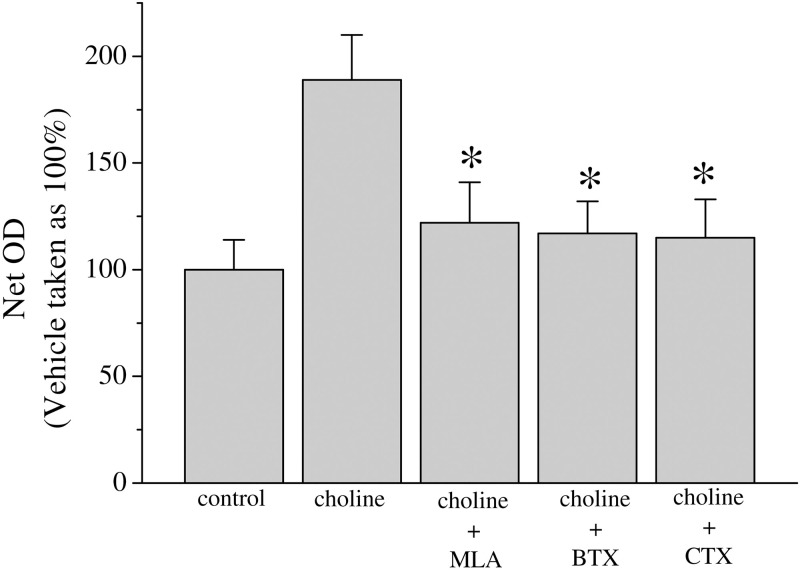
Nicotine can activate calcium signaling and lead to cell proliferation and the regulation of apoptosis within cancer cells [20]. We investigated the involvement of α7 and α9-nAChRs in breast cancer proliferation in response to 10 mM choline application, which activates both homopentameric receptor types [33]. Co-application of α7-nAChR antagonists: methyllycaconitine (10 μM, MLA) or α-bungarotoxin (100 nM, BTX), was found to significantly reduce cell proliferation in this experiment. Conotoxin RgIA (100 nM, CTX), an antagonist with higher potency for α9 and α10-nAChRs [34], also inhibited choline mediated proliferation (Fig 2).
Fig 2. Choline effect on proliferation in MCF-7 cells.
Approximately 104 MCF-7 cells were seeded into microwell plates and grown for 3 days in the presence of vehicle (control), choline (10 mM), choline + methyllycaconitine (10 μM, MLA), choline + α-bungarotoxin (100 nM, BTX), and choline + α-Conotoxin RgIA (100 nM, CTX). Cells were harvested and growth was determined by an MTT assay. Bars represent means ± SEM of at least 3 independent determinations. Asterisk denotes significant difference from choline treated group with p < 0.05.
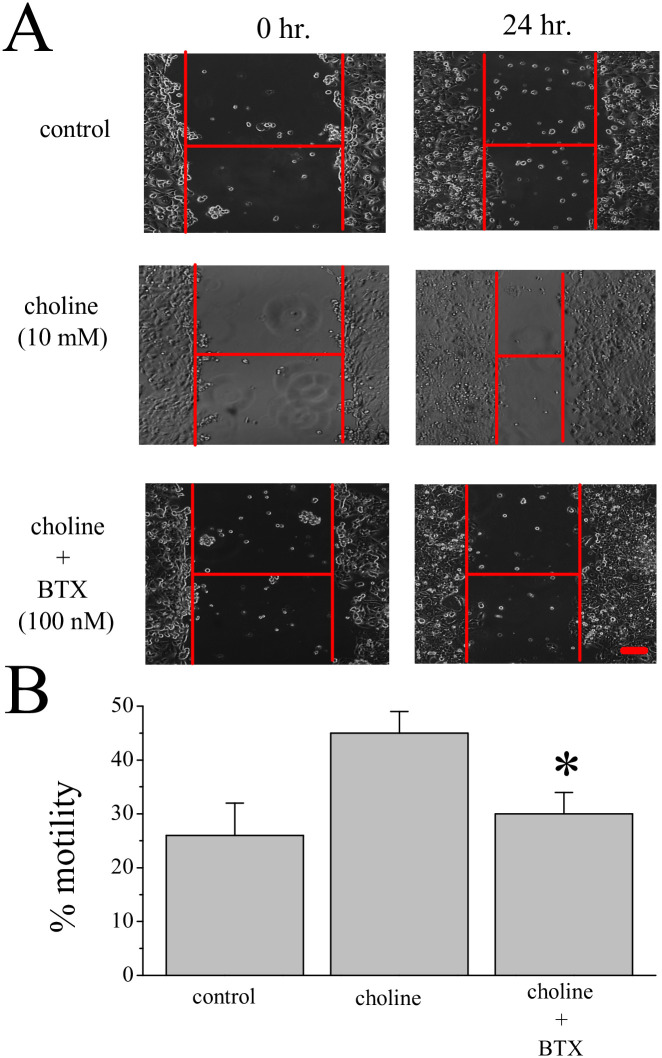
The wound healing (or scratch) assay can measure cancer cell migration in vitro [35]. Based on earlier studies that show that α7 nAChR activation can modify the cytoskeleton and cell motility as well as structural change [23, 36], we tested the effect of choline on the motility of MCF-7 cells. A 24-hour treatment with 10mM choline was found increase cell motility by 2-fold when compared to the control. This effect of choline on motility was virtually eliminated by co-application of 100 nM BTX (Fig 3).
Fig 3. Choline increases cell motility.
(A) Images of MCF-7 cells at the start (0 hr) and end (24 hr) of the motility assay. Experimental groups: vehicle (control), choline (10 mM), and choline with α-bungarotoxin (100 nM, BTX). (B) Average motility measures at 24 hr from 3 independent experiments. Asterisk denotes significance p < 0.05. Scale = 100μm.
G protein coupling to α7 nAChR drives intracellular calcium signaling and cell proliferation
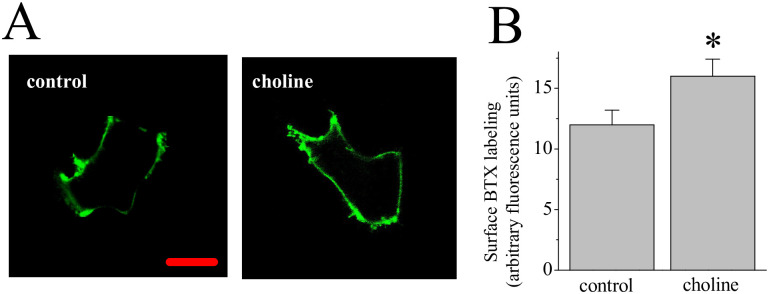
Ligand stimulation of α7 nAChRs impacts receptor synthesis and trafficking to the cell surface in cancer cells [37]. We examined α7 nAChR expression at the cell surface after a 3-day treatment with 10 mM choline. In this experiment, we used a BTX-Alexa Fluor 488 conjugate to label non-permeabilized MCF-7 cells (S1 Fig), comparing choline treated to control cells. As shown in Fig 4, treatment with choline was associated with an increase in the BTX-Alexa Fluor 488 signal at the cell surface in comparison to the control condition.
Fig 4. An effect of choline on surface labeling for the nAChR.
(A) MCF-7 cells were incubated with 100 nM α-bungarotoxin (BTX) Alexa Fluor 488. Images of representative cells showing labeling at 72 hr in control and choline (10 mM) treated cells. Scale = 5μm (B) Average BTX fluorescence values. Asterisk denotes significance p < 0.05 (n = 24–27).
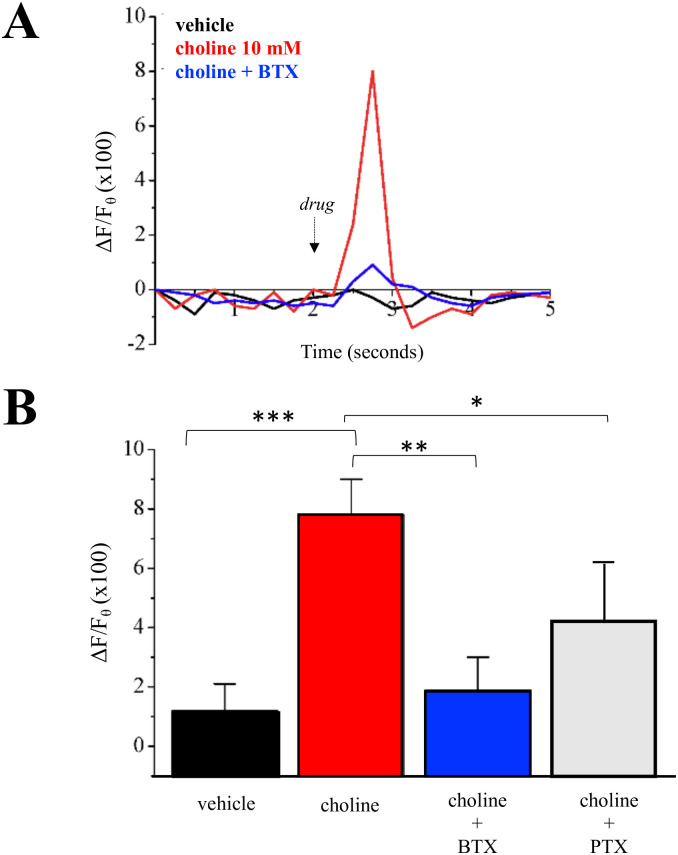
Ligand stimulation of the α7 nAChR rapidly increases intracellular calcium levels by extracellular calcium influx through open nAChRs, the activation of voltage-gated calcium channels, and the activation of calcium induced calcium release (CICR) as well as inositol induced calcium release (IICR) from the ER [22]. We examined the effect of 10 mM choline on intracellular calcium within MCF-7 cells. As shown in Fig 5A, application of choline resulted in a calcium transient that appeared within 0.5 sec of drug application and lasted for ~1 second. This calcium transient was not seen when BTX was present in the application solution. Statistical analysis of calcium transient peaks between drug treatment groups confirms that BTX abolishes choline-associated calcium responses within MCF-7 cells (Fig 5B).
Fig 5. Choline mediated change in intracellular calcium in MCF-7 cells.
(A) Calcium transients (ΔF/Fθ) measured using GCaMP5G in response to drug application. Vehicle (control), choline (10 mM), and choline with α-bungarotoxin (100 nM, BTX). (B) Average calcium peaks across experimental groups. Choline with pertussis toxin (5 μg/ml, PTX) (n = 21–25 cells). Statistical significance: *p<0.05; **p<0.01, ***p<0.001.
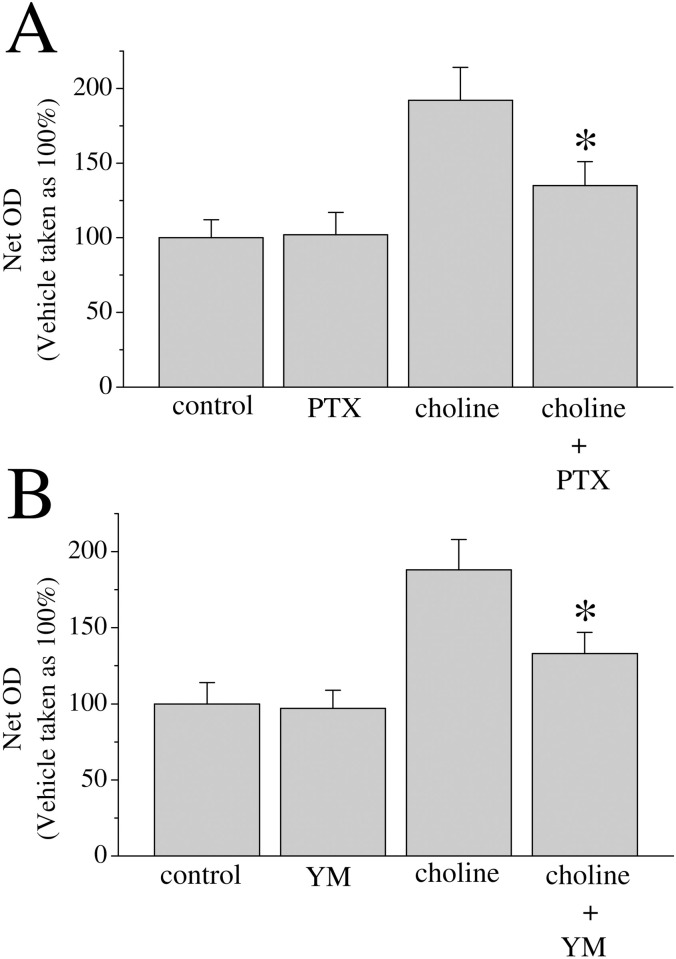
In previous studies we have shown that G protein interaction is involved in α7 nAChR-mediated IICR [22]. We tested the effect of the Gαi/o inhibitor PTX on choline-mediated calcium transients. Co-application of PTX was found to reduce the choline calcium transient by over 30% an effect that was found to be statistically significant (Fig 5B). We further explored G protein activity in nAChR-mediated cell growth. The 3-day proliferation assay was repeated in MCF-7 cells treated with 10 mM choline alone, choline with PTX (5 μg/ml), or choline with the Gαq inhibitor YM 254890 (1 μM). Analysis indicates that application of PTX or YM 254890 indicates a significant reduction in choline-mediated proliferation when G protein blockers are present (Fig 6).
Fig 6. G-protein inhibitors block choline-mediated MCF-7 cell proliferation.
Approximately 104 MCF-7 cells were seeded into microwell plates for 3 days then assessed via an MTT assay: (A) vehicle (control), pertussis toxin (5 μg/ml, PTX), choline (10 mM), and choline with PTX, (B) vehicle (control), YM 254890 (1 μM), choline (10 mM), and choline with YM 254890. Bars represent means ± SEM of 3 independent experiments. Asterisk denotes significant difference from choline treated group (p < 0.05).
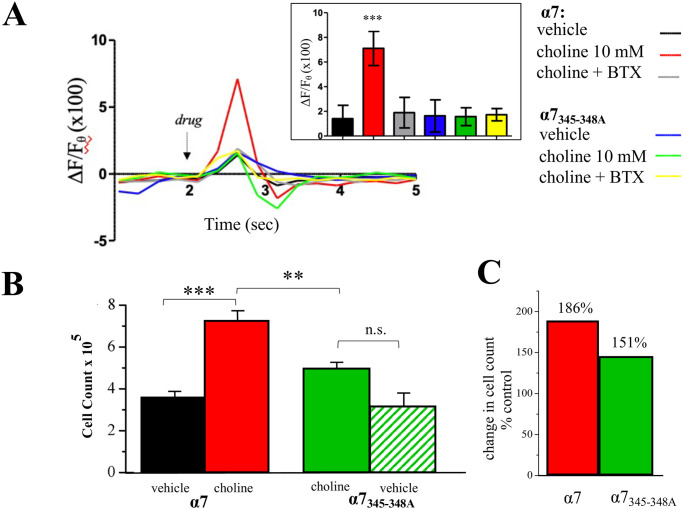
We have shown that α7 nAChRs directly bind G proteins through a binding sequence that is located within the intracellular loop region of the receptor [24]. Site directed mutagenesis of 4 amino acids at this site creates a dominant negative α7 subunit (α7345–348A) that is unable to bind G proteins [24]. In previous work, the expression of α7345–348A is sufficient to impair wild-type α7 nAChR calcium signaling and cell growth [36]. We transfected MCF-7 cells with α7345–348A and as shown in Fig 7, the expression of this mutant was seen sufficient in abolishing calcium transient responses to choline application. We also examined the effect of α7345–348A on proliferation in MCF-7 cells. An analysis of α7345–348A expression indicates that this mutant subunit does not significantly alter MCF-7 growth in the absence of a cholinergic ligand (Fig 7B). However, in cells transfected with α7345–348A, a 3-day treatment with choline did not increase proliferation as evidenced by a comparison of choline effect on cell number between wild-type α7 and α7345–348A expressing cells (Fig 7B and 7C).
Fig 7. G protein regulation of α7 nAChR calcium signaling.
(A) Representative calcium transients following application of vehicle (control), choline (10 mM), and choline with α-bungarotoxin (100 nM, BTX) in cells transfected with wild-type α7 or the mutant α7345-348A subunit. Inset: average of calcium transient peak values (ΔF/Fθ) across experimental groups (n = 21–25). (B) Approximately 104 MCF-7 cells were seeded into microwell plates for 3 days then assessed via the MTT assay: vehicle (control) or choline (10 mM) in cells transfected with wild-type α7 or the mutant α7345-348A subunit. (C) A comparison of choline mediated proliferation in cells transfected with wild-type α7 or the mutant α7345-348A subunit relative to the vehicle control. Statistical significance **p<0.01, ***p<0.001.
Discussion
Evidence indicates a link between tobacco product use and increased risk to oral, lung, and breast cancer [38]. While there maybe diverse mechanisms by which nicotine can promote cancer cell progression, they all involve the activation of nAChRs on target cells [7, 20, 39]. Indeed, various nAChR couple to cancer processes that regulate cell division, morphology, and can increase angiogenesis and modify inflammatory responses in microenvironment of the cancer cells [7, 25, 40]. Recently, nAChRs have been shown to promote MCF-7 breast cancer cell proliferation via the activation of ERK1/2 phosphorylation [14] and drive epithelial to mesenchymal transition (EMT) [17].
In various neuronal cells high calcium signaling through the homopentameric α7 nAChR directs actin associated cytoskeletal dynamics leading to observable changes synaptic growth and plasticity [25, 41]. An analogous mechanism for α7 nAChR-calcium signaling to the cytoskeleton exists in non-neuronal cells including immune and epithelial cells [42, 43]. In this case, nAChR activation may drive changes in cell proliferation and/or metastatic transition involving the regulation of the cytoskeleton in cancer cells [44]. Our observations suggest that cholinergic ligands (e.g., nicotine) can alter α7 nAChR expression at the cell membrane of breast cancer cells. This may explain aspects of chronic nicotine exposure on cancer risk in smokers and suggests that trafficking of nAChRs may contribute to cancer progression.
The metabotropic activity of the α7 nAChR is driven by protein interactions of the receptor’s intracellular (M3-M4) loop [45]. We have shown the existence of a G protein binding region within the α7 nAChR M3-M4 loop [24]. Coupling between the nAChR and various G proteins, including Gαi and Gαq, was found to participate in important aspects of nAChR signaling [25]. At present it is not clear if this G protein binding site within the α7 nAChR favors association with specific Gα subunits. Based on in vivo analysis of G protein interactions with the α7 nAChR in the adult rodent brain, various G protein subunits appear able to bind the nAChR [24].
In this study, functional interaction between the α7 nAChR and Gαi appears to contribute to MCF-7 cell proliferation and motility based on experiments that show that blocking Gαi/o activity with PTX can abolish choline-associated cell proliferation. In calcium imaging experiments however PTX was found to significantly reduce the choline calcium transient response but not completely abolish it thus suggesting additional (non-Gαi) contributions to the α7 nAChR calcium signal. This is consistent with experiments using the Gαq inhibitor YM 254890 showing an effect of the G protein subunit on calcium mediated MCF-7 growth. Our results are supportive of earlier finding that show that Gαq can bind the α7 nAChR and promote calcium store release [23] and Gαi can activate pathways important for breast cancer growth [46]
Mammalian α7, α9, and heteromeric α9α10 combinations are the nAChR types with the greatest Ca2+ permeability [11, 47, 48]. It is important to note that many cancer cells express homopentameric nAChRs that are found to stimulate cancer cell proliferation, metastasis, and inhibit cancer cell apoptosis [7, 20]. The α9 nAChR contributes to breast cancer growth and EMT through activation of PI3K or MAPK signaling pathways [49]. In this study we also find a significant effect of α-Conotoxin RgIA, that is known to have a higher potency for α9 than α7 nAChRs, on MCF-7 cell growth. Thus, in future studies it will be important to explore the involvement of G proteins in α9 nAChR signaling in breast cancer cells.
Supporting information
MCF-7 cells were labeled with 100 nM BTX. Cell membranes were not permeabilized in these labeling experiments. Bottom panel shows a representative image of a labeled cell. Scale bar = 5μm.
(TIFF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
Funding provided by the Kuwait Foundation for the Advancement of Sciences (PN19-13PT-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Slattery ML, Curtin K, Giuliano AR, Sweeney C, Baumgartner R, Edwards S, et al. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat. 2008;109(1):101–11. Epub 2007/06/28. doi: 10.1007/s10549-007-9629-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015;154(2):213–24. Epub 2015/11/08. doi: 10.1007/s10549-015-3628-4 . [DOI] [PubMed] [Google Scholar]
- 3.Reynolds P. Smoking and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):15–23. Epub 2012/11/28. doi: 10.1007/s10911-012-9269-x . [DOI] [PubMed] [Google Scholar]
- 4.Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG, et al. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob Control. 2011;20(1):e2. Epub 2010/12/15. doi: 10.1136/tc.2010.035931 . [DOI] [PubMed] [Google Scholar]
- 5.Subhan M, Saji Parel N, Krishna PV, Gupta A, Uthayaseelan K, Uthayaseelan K, et al. Smoking and Pancreatic Cancer: Smoking Patterns, Tobacco Type, and Dose-Response Relationship. Cureus. 2022;14(6):e26009. Epub 2022/07/22. doi: 10.7759/cureus.26009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuller HM. Cell type specific, receptor-mediated modulation of growth kinetics in human lung cancer cell lines by nicotine and tobacco-related nitrosamines. Biochem Pharmacol. 1989;38(20):3439–42. Epub 1989/10/15. doi: 10.1016/0006-2952(89)90112-3 . [DOI] [PubMed] [Google Scholar]
- 7.Khodabandeh Z, Valilo M, Velaei K, Pirpour Tazehkand A. The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy. Breast Cancer. 2022;29(5):778–89. Epub 2022/05/19. doi: 10.1007/s12282-022-01369-7 . [DOI] [PubMed] [Google Scholar]
- 8.Shehwana H, Keskus AG, Ozdemir SE, Acikgöz AA, Biyik-Sit R, Cagnan I, et al. CHRNA5 belongs to the secondary estrogen signaling network exhibiting prognostic significance in breast cancer. Cell Oncol (Dordr). 2021;44(2):453–72. Epub 2021/01/21. doi: 10.1007/s13402-020-00581-x . [DOI] [PubMed] [Google Scholar]
- 9.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36(2):96–108. Epub 2015/02/03. doi: 10.1016/j.tips.2014.12.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96(Pt B):302–11. Epub 2014/12/03. doi: 10.1016/j.neuropharm.2014.11.003 . [DOI] [PubMed] [Google Scholar]
- 11.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35(1):1–8. Epub 2003/12/13. doi: 10.1016/j.ceca.2003.08.006 . [DOI] [PubMed] [Google Scholar]
- 12.Corradi J, Bouzat C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol Pharmacol. 2016;90(3):288–99. Epub 2016/05/18. doi: 10.1124/mol.116.104240 . [DOI] [PubMed] [Google Scholar]
- 13.Pepper C, Tu H, Morrill P, Garcia-Rates S, Fegan C, Greenfield S. Tumor cell migration is inhibited by a novel therapeutic strategy antagonizing the alpha-7 receptor. Oncotarget. 2017;8(7):11414–24. Epub 2017/01/13. doi: 10.18632/oncotarget.14545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalantari-Dehaghi M, Parnell EA, Armand T, Bernard HU, Grando SA. The nicotinic acetylcholine receptor-mediated reciprocal effects of the tobacco nitrosamine NNK and SLURP-1 on human mammary epithelial cells. Int Immunopharmacol. 2015;29(1):99–104. Epub 2015/05/20. doi: 10.1016/j.intimp.2015.04.041 . [DOI] [PubMed] [Google Scholar]
- 15.Nishioka T, Kim HS, Luo LY, Huang Y, Guo J, Chen CY. Sensitization of epithelial growth factor receptors by nicotine exposure to promote breast cancer cell growth. Breast Cancer Res. 2011;13(6):R113. Epub 2011/11/17. doi: 10.1186/bcr3055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata N, Sekino Y, Kanda Y. Nicotine increases cancer stem cell population in MCF-7 cells. Biochem Biophys Res Commun. 2010;403(1):138–43. Epub 2010/11/09. doi: 10.1016/j.bbrc.2010.10.134 . [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta P, Rizwani W, Pillai S, Kinkade R, Kovacs M, Rastogi S, et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124(1):36–45. Epub 2008/10/11. doi: 10.1002/ijc.23894 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fararjeh AS, Tu SH, Chen LC, Cheng TC, Liu YR, Chang HL, et al. Long-term exposure to extremely low-dose of nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induce non-malignant breast epithelial cell transformation through activation of the a9-nicotinic acetylcholine receptor-mediated signaling pathway. Environ Toxicol. 2019;34(1):73–82. Epub 2018/09/28. doi: 10.1002/tox.22659 . [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Zhangsun M, Dong S, Liu Y, Qian J, Zhangsun D, et al. Differential Expression of Nicotine Acetylcholine Receptors Associates with Human Breast Cancer and Mediates Antitumor Activity of αO-Conotoxin GeXIVA. Mar Drugs. 2020;18(1). Epub 2020/01/23. doi: 10.3390/md18010061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pucci S, Zoli M, Clementi F, Gotti C. α9-Containing Nicotinic Receptors in Cancer. Front Cell Neurosci. 2021;15:805123. Epub 2022/02/08. doi: 10.3389/fncel.2021.805123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang LC, Lin CL, Qiu JZ, Lin CY, Hsu KW, Tam KW, et al. Nicotinic Acetylcholine Receptor Subtype Alpha-9 Mediates Triple-Negative Breast Cancers Based on a Spontaneous Pulmonary Metastasis Mouse Model. Front Cell Neurosci. 2017;11:336. Epub 2017/11/23. doi: 10.3389/fncel.2017.00336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King JR, Ullah A, Bak E, Jafri MS, Kabbani N. Ionotropic and Metabotropic Mechanisms of Allosteric Modulation of α7 Nicotinic Receptor Intracellular Calcium. Mol Pharmacol. 2018;93(6):601–11. Epub 2018/03/29. doi: 10.1124/mol.117.111401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordman JC, Kabbani N. Microtubule dynamics at the growth cone are mediated by α7 nicotinic receptor activation of a Gαq and IP3 receptor pathway. Faseb j. 2014;28(7):2995–3006. Epub 2014/04/02. doi: 10.1096/fj.14-251439 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King JR, Nordman JC, Bridges SP, Lin MK, Kabbani N. Identification and Characterization of a G Protein-binding Cluster in α7 Nicotinic Acetylcholine Receptors. J Biol Chem. 2015;290(33):20060–70. Epub 2015/06/20. doi: 10.1074/jbc.M115.647040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabbani N, Nichols RA. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol Sci. 2018;39(4):354–66. Epub 2018/02/13. doi: 10.1016/j.tips.2018.01.002 . [DOI] [PubMed] [Google Scholar]
- 26.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–40. Epub 2012/10/05. doi: 10.1523/JNEUROSCI.2601-12.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168(5):697–703. Epub 2005/02/25. doi: 10.1083/jcb.200407191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J, Quik M. A role for the nicotinic alpha-bungarotoxin receptor in neurite outgrowth in PC12 cells. Neuroscience. 1993;56(2):441–51. Epub 1993/09/01. doi: 10.1016/0306-4522(93)90344-f . [DOI] [PubMed] [Google Scholar]
- 29.Nordman JC, Kabbani N. An interaction between α7 nicotinic receptors and a G-protein pathway complex regulates neurite growth in neural cells. J Cell Sci. 2012;125(Pt 22):5502–13. Epub 2012/09/08. doi: 10.1242/jcs.110379 . [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P 3rd. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56(5):483–93. Epub 1994/11/01. doi: 10.1038/clpt.1994.169 . [DOI] [PubMed] [Google Scholar]
- 31.Pinto MI, Thissen J, Hermes N, Cunningham A, Digard H, Murphy J. Chemical characterisation of the vapour emitted by an e-cigarette using a ceramic wick-based technology. Sci Rep. 2022;12(1):16497. Epub 2022/10/04. doi: 10.1038/s41598-022-19761-w employed by BAT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54(7):743–53. Epub 1997/11/14. doi: 10.1016/s0006-2952(97)00117-2 [DOI] [PubMed] [Google Scholar]
- 33.Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39(13):2515–24. Epub 2000/10/25. doi: 10.1016/s0028-3908(00)00124-6 . [DOI] [PubMed] [Google Scholar]
- 34.Ellison M, Feng ZP, Park AJ, Zhang X, Olivera BM, McIntosh JM, et al. Alpha-RgIA, a novel conotoxin that blocks the alpha9alpha10 nAChR: structure and identification of key receptor-binding residues. J Mol Biol. 2008;377(4):1216–27. Epub 2008/02/26. doi: 10.1016/j.jmb.2008.01.082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrak NH, Khajah MA, Luqmani YA. Hypoxic environment may enhance migration/penetration of endocrine resistant MCF7- derived breast cancer cells through monolayers of other non-invasive cancer cells in vitro. Sci Rep. 2020;10(1):1127. Epub 2020/01/26. doi: 10.1038/s41598-020-58055-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King JR, Kabbani N. Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J Neurochem. 2016;138(4):532–45. Epub 2016/05/12. doi: 10.1111/jnc.13660 . [DOI] [PubMed] [Google Scholar]
- 37.Aali N, Motalleb G. The effect of nicotine on the expressions of the α7 nicotinic receptor gene and Bax and Bcl-2 proteins in the mammary gland epithelial-7 breast cancer cell line and its relationship to drug resistance. Cell Mol Biol Lett. 2015;20(5):948–64. Epub 2016/01/27. doi: 10.1515/cmble-2015-0056 . [DOI] [PubMed] [Google Scholar]
- 38.Frazer K, Bhardwaj N, Fox P, Stokes D, Niranjan V, Quinn S, et al. Systematic Review of Smoking Cessation Inventions for Smokers Diagnosed with Cancer. Int J Environ Res Public Health. 2022;19(24). Epub 2022/12/24. doi: 10.3390/ijerph192417010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mucchietto V, Crespi A, Fasoli F, Clementi F, Gotti C. Neuronal Acetylcholine Nicotinic Receptors as New Targets for Lung Cancer Treatment. Curr Pharm Des. 2016;22(14):2160–9. Epub 2016/02/05. doi: 10.2174/1381612822666160203144114 . [DOI] [PubMed] [Google Scholar]
- 40.Piovesana R, Salazar Intriago MS, Dini L, Tata AM. Cholinergic Modulation of Neuroinflammation: Focus on α7 Nicotinic Receptor. Int J Mol Sci. 2021;22(9). Epub 2021/06/03. doi: 10.3390/ijms22094912 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chrestia JF, Esandi MDC, Bouzat C. Cannabidiol as a modulator of α7 nicotinic receptors. Cell Mol Life Sci. 2022;79(11):564. Epub 2022/10/26. doi: 10.1007/s00018-022-04600-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordman JC, Muldoon P, Clark S, Damaj MI, Kabbani N. The α4 nicotinic receptor promotes CD4+ T-cell proliferation and a helper T-cell immune response. Mol Pharmacol. 2014;85(1):50–61. Epub 2013/10/11. doi: 10.1124/mol.113.088484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003;72(18–19):2055–61. Epub 2003/03/12. doi: 10.1016/s0024-3205(03)00083-3 . [DOI] [PubMed] [Google Scholar]
- 44.Proietti S, Catizone A, Masiello MG, Dinicola S, Fabrizi G, Minini M, et al. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J Pineal Res. 2018;64(4):e12467. Epub 2018/01/18. doi: 10.1111/jpi.12467 . [DOI] [PubMed] [Google Scholar]
- 45.Kabbani N, Woll MP, Levenson R, Lindstrom JM, Changeux JP. Intracellular complexes of the beta2 subunit of the nicotinic acetylcholine receptor in brain identified by proteomics. Proc Natl Acad Sci U S A. 2007;104(51):20570–5. Epub 2007/12/14. doi: 10.1073/pnas.0710314104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Citi S, Guerrera D, Spadaro D, Shah J. Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases. 2014;5(4):1–15. Epub 2014/12/09. doi: 10.4161/21541248.2014.973760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fucile S, Renzi M, Lax P, Eusebi F. Fractional Ca(2+) current through human neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2003;34(2):205–9. Epub 2003/06/18. doi: 10.1016/s0143-4160(03)00071-x . [DOI] [PubMed] [Google Scholar]
- 48.Fucile S, Sucapane A, Eusebi F. Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium. 2006;39(4):349–55. Epub 2006/02/03. doi: 10.1016/j.ceca.2005.12.002 . [DOI] [PubMed] [Google Scholar]
- 49.Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst. 2010;102(17):1322–35. Epub 2010/08/25. doi: 10.1093/jnci/djq300 . [DOI] [PubMed] [Google Scholar]