Abstract
Aims
The role of skeletal muscle estrogen and its ability to mitigate the negative impact of a high-fat diet (HFD) on obesity-associated metabolic impairments is unknown. To address this, we developed a novel mouse model to determine the role of endogenous 17β-estradiol (E2) production in males in skeletal muscle via inducible, skeletal muscle–specific aromatase overexpression (SkM-Arom↑).
Methods
Male SkM-Arom↑ mice and littermate controls were fed a HFD for 14 weeks prior to induction of SkM-Arom↑ for a period of 6.5 weeks. Glucose tolerance, insulin action, adipose tissue inflammation, and body composition were assessed. Indirect calorimetry and behavioral phenotyping experiments were performed using metabolic cages. Liquid chromatography mass spectrometry was used to determine circulating and tissue (skeletal muscle, hepatic, and adipose) E2 and testosterone concentrations.
Results
SkM-Arom↑ significantly increased E2 in skeletal muscle, circulation, the liver, and adipose tissue. SkM-Arom↑ ameliorated HFD-induced hyperglycemia, hyperinsulinemia, impaired glucose tolerance, adipose tissue inflammation, and reduced hepatic lipid accumulation while eliciting skeletal muscle hypertrophy.
Conclusion
Enhanced skeletal muscle aromatase activity in male mice induces weight loss, improves metabolic and inflammatory outcomes and mitigates the negative effects of a HFD. Additionally, our data demonstrate for the first time skeletal muscle E2 has anabolic effects on the musculoskeletal system.
Keywords: skeletal muscle, estrogen, obesity, metabolism, aromatase
Classically considered as a “female” hormone, 17β-estradiol (E2) regulates behavioral and metabolic processes via actions both centrally and peripherally in both male and females (1, 2). Centrally, E2 has been shown to regulate food intake and physical activity in both sexes (3, 4). Peripherally, E2 has been shown to elicit a multitude of metabolic effects including, but not limited to, mitochondrial dynamics, fatty acid oxidation, and glucose homeostasis/insulin sensitivity (1, 5, 6). With the advent of novel selective mouse models within the last 20 years, scientists have begun to understand and mechanistically explore the importance of E2 signaling on glucose metabolism and insulin sensitivity in both males and females (7-13). Here we extend our knowledge of E2 actions by focusing on E2's role in skeletal muscle and its ability to impact glucose metabolism and insulin sensitivity in male mice.
Skeletal muscle is a key component of regulating whole-body glucose homeostasis as it accounts for the majority of postprandial glucose disposal (14). Impairments in skeletal muscle insulin sensitivity is thought to be a primary culprit in the development of type II diabetes (14). To date, studies examining the impact of E2 signaling in skeletal muscle have focused on female mice and have produced discordant results (5, 6, 15, 16). For instance, skeletal muscle congenital deletion of the primary estrogen receptor, estrogen receptor-alpha (ERα), was found to be imperative for “metabolic homeostasis in females” (6), which was supported by Torres et al, who showed that E2 improves “bioenergetic function in the skeletal muscle” of females (5). However, a subsequent study found that inducible deletion of skeletal muscle ERα in females is “not required for regulation of muscle insulin sensitivity” (15). To begin to better understand the role of E2 in skeletal muscle, we have recently developed a novel mouse model that allows for inducible overexpression of aromatase (the enzyme necessary to convert androgens to estrogens) specifically in skeletal muscle (SkM-Arom↑) (16). We initially used this novel mouse model to better understand the role of E2 in skeletal muscle in females. Our initial data suggest that inducible augmentation of skeletal muscle estrogen production (at physiological levels) did not prevent or rescue obesity-associated metabolic dysfunction in gonadally intact or ovariectomized female mice, but did reduce adipose tissue inflammation and restored bone mineral density in female mice (16). These data suggest skeletal muscle E2 in females is not responsible for improvements in metabolic function.
What is not as well appreciated is that E2 is also critical in males. Prior to our work, no study in males has selectively explored skeletal muscle manipulation of E2 or its receptors. There are data studying whole-body E2 supplementation in males which found E2 therapy prevented high-fat-diet (HFD)–induced obesity and impairments in glucose metabolism (8), but data from this study do not indicate which tissue(s) are critical sites of action. Thus, we sought to utilize our SkM-Arom↑ model to determine the potential therapeutic impact of inducible skeletal muscle E2 production on obesity-associated metabolic impairments in males. We paired this with our ultrasensitive liquid chromatography mass spectrometry method of E2 quantification (16), state-of-the-art indirect calorimetry and behavioral phenotyping system, and metabolic assessments to allow us to begin to determine where E2 has its site of action (skeletal muscle, adipose tissue, and liver) as well as the content of E2 necessary to elicit any favorable outcomes. Our findings significantly advance our understanding of the metabolic benefits of endogenously produced E2 in males.
Materials and Methods
Animal Model
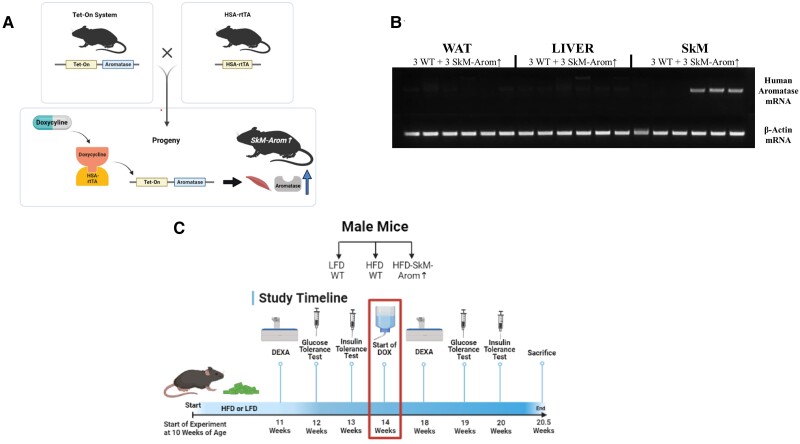
HSA-rtTA+/+ (17) and tet-human Aromatase+/− mice (18) on a C57BL/6 background were bred to generate a mouse model that allows for inducible overexpression of aromatase specifically in skeletal muscle upon doxycycline (DOX) treatment (HSA-rtTA+/−, tet-Aromatase+/−) (SkM-Arom↑) (Fig. 1A). “Wildtype (WT)” littermate controls included the following genotype: HSA-rtTA+/−, tet-Aromatase−/−. At 10 weeks of age, Male SkM-Arom↑ and littermate control mice were fed a HFD for 14 weeks prior to administration of DOX-supplemented water for an additional 6.5 weeks (20.5 weeks of dietary treatment in total). A cohort of 10-week-old littermate control WT mice were fed a low-fat diet (LFD) as well to serve as a gauge to determine the effectiveness of any benefit induced by increased skeletal muscle E2 production (Fig. 1C). We did not include a SkM-Arom↑ LFD group as the primary purpose of this investigation was to determine whether augmented skeletal muscle E2 production in the setting of obesity could serve as a potential therapy for obesity in males. All mice regardless of diet received DOX in their drinking water at a concentration of 0.1 mg/mL. The justification for exposing mice to a HFD for 14 weeks was to ensure the development of an obese phenotype characterized by impaired glucose metabolism and insulin resistance as verified by our pre-DOX treatment metabolic assessments. The sample size for all experimental groups in experiments was n = 16 to 18 mice/group. Mice were housed, 3 to 5/cage, maintained on a 12:12-hour light–dark cycle in a low stress environment (22 °C, 50% humidity, low noise), and given food and water ad libitum. All methods were in accordance with the American Association for Laboratory Animal Science, and the Institutional Animal Care and Usage Committee of the University of South Carolina reviewed and approved all experiments.
Figure 1.
Model description and experimental design. (A) Mouse model description, (B) Northern blot showing specificity of the HSA-rtTA model to induce human aromatase expression specifically in skeletal muscle (6.5 weeks after doxycycline [DOX] treatment), and (C) experimental design. HFD, high-fat diet; LFD, low-fat diet, and dual x-ray absorptiometry.
Diets
The LFD utilized in this experiment was the open-source, purified AIN-76A diet (3.79 kcal/g) (69%, 12%, 19% of total calories from carbohydrate, fat, and protein, respectively). The HFD (4.57 kcal/g) was a purified diet comprising 47%, 40%, and 13% of total calories from carbohydrate, fat, and protein, respectively, with saturated fat making up 12% of total calories in order to mimic the standard American diet (BioServ, Frenchtown, NJ). Details and previous use of this diet are provided elsewhere (19-28).
Body Weight and Body Composition
Body weight was monitored on a weekly basis throughout the study. Body composition was assessed after 11 weeks of diet and again after 4 weeks of DOX treatment to use lean mass as the basis for the dose of glucose and insulin administration for glucose and insulin tolerance tests, respectively. For this procedure, mice were briefly anesthetized via isoflurane inhalation, and lean mass, fat mass, percent body fat, and bone mineral density (BMD) were assessed by dual-energy X-ray absorptiometry (Lunar PIXImus).
Metabolic Assessment
Fasting blood glucose and insulin levels were assessed after 12 weeks of dietary treatment and then again after 5 weeks of DOX treatment (19 weeks of diet consumption) (Fig. 1). After a 5-hour fast (29), blood samples were collected from the tip of the tail. A glucometer (Bayer Contour, Mishawaka, IN) was used to determine blood glucose concentrations in whole blood. Collected blood was centrifuged at 4000 rpm for 10 minutes at 4 °C. Plasma insulin concentrations were analyzed according to the manufacturer's instructions using a mouse insulin enzyme-linked immunosorbent assay kit (Mercodia, Winston Salem, NC) (antibody ID: RRID:AB_2783837). Glucose and insulin tolerance tests (GTTs and ITTs, respectively) were performed after 12 and 13 weeks of dietary treatment and then again after 19 and 20 weeks of dietary treatment (5 and 6 weeks of DOX treatment), respectively. For these procedures, mice were fasted for 5 hours, and glucose or insulin was administered intraperitoneally at 2 g/kg or 0.75 U/kg lean mass, respectively. A glucometer (Bayer Contour) was used to measure blood glucose concentrations (tail sampling) intermittently over a 2-hour period (0, 15, 30, 60, 90, and 120 minutes) for GTTs and intermittently over a 1-hour period (0, 15, 30, 45, and 60 minutes) for ITTs. Baseline glucose levels were subtracted accordingly, and the area of the curve was generated as previously described (30). The area under the curve of the area of the curve was calculated using the trapezoidal rule. Blood was collected from the tip of the tail during the GTT at 0 minutes for each group to determine fasting insulin levels. Fasting serum was collected (using nonheparinized capillary tubes) for free fatty acid analysis at the 0- and 30-minute time points of the ITT to assess insulin's ability to inhibit lipolysis. Free fatty acids were analyzed using a commercially available kit according to the manufacturer's instructions (Wako Diagnostics, Richmond, VA).
Tissue Collection
At the termination of the experiment (20.5 weeks of dietary treatment and 6.5 weeks of DOX administration), mice were euthanized via isoflurane inhalation for tissue collection. Blood was collected from the inferior vena cava using heparinized syringes and subsequently centrifuged to isolate plasma. Gonadal, mesenteric, and perirenal fat pads (visceral fat), as well as liver and skeletal muscle (gastrocnemius), were removed and immediately snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
Quantitative Real-Time Polymerase Chain Reaction and Northern Blot
An EZNA Total RNA Kit (Omega Bio-Tek, Norcross, GA) including DNASE treatment was used to isolate RNA from gonadal adipose tissue, liver, and skeletal muscle. Bio-Rad reverse transcription reagents, SsoAdvanced Universal Probes Supermix, and probe assays (Bio-Rad, Hercules, CA) were used to reverse transcribe and analyze the expression of the following genes in adipose tissue: CD68 (qMmuCEP0027967), CD11c (qMmuCEP0055567), CD206 (qMmuCEP0054272), MCP-1 (qMmuCEP0056726), TNF-α (qMmuCEP0028054), and TLR2 (qMmuCIP0037139). Potential reference genes (GAPDH [qMmuCEP0039581], β-Actin [qMmuCEP0039589], HMBS [qMmuCIP0035290], TBP [qMmuCIP0042759], H2AFV [qMmuCEP0057536], UBC [qMmuCIP0034811], PPIA [qMmuCEP0043519], and IPO8 [qMmuCEP0042052]) were analyzed for stability using Qbase+ software (Biogazelle, Ghent, Belgium) for each tissue analyzed (31). The optimal number of reference genes was determined by Qbase+, and the geometric mean of these genes (IPO8, PPIA, and TBP) was used as the normalization factor. Gene expression was quantified using the ΔΔCT method and Qbase+ software. In order to ensure that induction of human aromatase was specific to skeletal muscle in the SkM-Arom↑ model, a Northern blot was run on isolated mRNA from adipose tissue, liver, and skeletal muscle from a cohort of SkM-Arom↑ and littermate control mice (n = 3/group) for Arom: (5′-CCTTGCACCCAGATGAGACT-3′ [forward]; 5′-GACAGCACAACAACCAGCAC-3′ [reverse]) and β-Actin (5′-GGCTGTATTCCCCTCCATCG-3′ [forward]; 5′-CCAGTTGGTAACAATGCCATGT-3′ [reverse]) using GoTaq Green Master Mix (Promega, Madison, WI) (Fig. 1B).
Hepatic Lipid Content
Lipids were isolated from the liver from each mouse utilizing a modified Folch extraction method and quantified gravimetrically, as previously described (23, 27). In brief, a portion of the liver (≈200 mg) was weighed, and homogenized in 2 mL of chloroform:methanol (2:1 v/v). After rotating on a mixer for 20 minutes, 500 μL of 0.6% NaCl was added to the solution, the tubes were vortexed, and subsequently centrifuged for 5 minutes at 1500g. The resulting bottom layer containing the lipid fraction was removed and placed in a preweighed glass vial. The vial was placed in an oven overnight at 37 °C to allow organic solvent evaporation. After evaporation the vial containing the lipids was weighed. Hepatic lipid content was determined as the following: (postvial weight – previal weight)/(tissue weight of liver used).
Extraction of Steroids
Extraction of steroids was performed as previously described (16). Prior to processing samples, internal standards (Sigma Aldrich, St. Louis, MO) for testosterone (-23,4-13C3), and E2 (D5) were added to each tissue and plasma sample. Calibration curves utilizing testosterone (Sigma Aldrich, Catalog# T037), and E2 (Sigma Aldrich, Catalog# E-060) were used to determine the quantity of each steroid. Steroids were extracted from ≈150 to 200 μL of plasma via vortex using 500 μL of methyl tert-butyl ether (MTBE) (×2). The resulting upper phase was removed and placed into a glass vial where it was dried down using N2. The dried down steroids were derivatized as previously described utilizing 1-methylimidazole-2-sulfonyl chloride (32). For tissue samples, skeletal muscle (1 gastrocnemius), and ≈100 mg of adipose tissue and liver were cut into sections with a razor blade, weighed, and homogenized in 1 mL of acetonitrile for 3 minutes using a bead beater (Biospec Products, Bartlesville, OK). The samples were centrifuged 5 minutes × 12 000g and the resulting supernatant was removed and placed in glass vials. Subsequently, the tissue samples were resuspended in 1 mL of acetonitrile, homogenized, and centrifuged, and the supernatant collected for a second time. The supernatants were dried down using N2 and were resuspended in 200 mM sodium acetate. MTBE was added to the sample for liquid–liquid extraction of the steroids (×2). The MTBE layer was removed and dried under nitrogen gas prior to derivatization as previously described (32). After derivatization, the samples were placed in 0.2 PVDF microspin filters (Fisher Scientific, Waltham, MA) and centrifuged to remove any insoluble material prior to mass spectrometry analysis. Analyte recovery for plasma and tissue was >80%.
Chromatography and Mass Spectrometry
Analysis was carried out on a Q Exactive HF-X hybrid quadrupole-orbitrap mass spectrometer with a Vanquish HPLC on the front end (Thermo Electron, Waltham, MA) as previously described (16).
Indirect Calorimetry and Behavioral Phenotyping
Male SkM-Arom↑ and WT littermate controls (n = 4/group) underwent body composition analysis (dual x-ray absorptiometry), and were put on a HFD before being placed into a Promethion multichannel continuous measurement indirect calorimetry system (Sable System International, Las Vegas, NV) on a 12-hour light and 12-hour dark cycle for an 11-day period. After a 1-day acclimation period mice were given DOX-supplemented water. Subsequently, food intake, water consumption, body mass, total activity, total energy expenditure, resting energy expenditure, oxygen consumption, carbon dioxide production, respiratory quotient, and animal locomotion (all meters) were determined and analyzed over a 10-day period. All data (besides respiratory exchange ratio [RER] and all meters = 2-tailed Student's t-test) were analyzed using analysis of covariance with lean mass as a covariate utilizing the MMPC Statistical Analysis Page. CalR was used for the creation of the RER, food intake, energy balance, locomotor activity, and all-meters figures over time.
Skeletal Muscle Hypertrophy Assessment
Skeletal muscle water content was assessed by weighing half of a gastrocnemius muscle (cut vertically) before and after drying overnight (90 °C). Skeletal muscle myofibrillar and sarcoplasmic fractions were extracted and quantified from the remaining half of the gastrocnemius muscle according to a previously established methodology (33).
Statistical Analysis
All data were analyzed using commercially available statistical software: Prism 9 (GraphPad Software, La Jolla, CA). A 1-way analysis of variance was used for all group comparisons followed by a Newman–Keuls post hoc test. Any statistical test that did not pass the equal-variance test (Bartlett's test) was log-transformed and then reanalyzed. Data are presented as means ± SE, and the level of statistical significance was set at P < .05.
Results
SkM-Arom↑ Leads to Increased Skeletal Muscle E2 Production Yielding Elevated Circulating and Tissue E2 Contents
Six and a half weeks of SkM-Arom↑ significantly increased skeletal muscle E2 content compared with WT LFD and HFD mice (P < .05) (Table 1). Consistent with our previous publication (16), we also found an elevated E2 concentration in plasma, adipose tissue, and liver (P < .05), and although higher than the normal range for both male and female mice, the plasma values of E2 in the SkM-Arom↑ mice were within the normal range for pregnant mice (34, 35). The large variations in the testosterone concentrations are not surprising as it has previously been shown that there is a “striking” individual variation (>30 fold differences) in testosterone levels in male rodents as well as large daily fluctuations (36).
Table 1.
Tissue and plasma steroid concentrations
| WT LFD | WT HFD | SkM-Arom↑ | |
|---|---|---|---|
| Skeletal muscle | |||
| E2 (pg/g tissue) | 4.1 ± 1.5a | 5.0 ± 2.0a | 94.2 ± 17.3b |
| T (pg/g tissue) | 166.5 ± 34.3 | 2087 ± 1783 | 104.4 ± 11.2 |
| Plasma | |||
| E2 (pg/mL) | 10.1 ± 0.73a | 11.5 ± 1.2a | 64 ± 15.8b |
| T (pg/mL) | 1028 ± 443.5 | 14227 ± 7632 | 587 ± 85.9 |
| Adipose tissue | |||
| E2 (pg/g tissue) | 8.0 ± 1.7a | 4.7 ± 1.6a | 102.7 ± 20.1b |
| T (pg/g tissue) | 4093 ± 2024 | 7416 ± 2615 | 1323.7 ± 258 |
| Liver | |||
| E2 (pg/g tissue) | 10.6 ± 2.7a | 6.9 ± 1.6a | 46.3 ± 6.9b |
| T (pg/g tissue) | 48.3 ± 6.3 | 63.2 ± 14.2 | 37.1 ± 2.8 |
SkM-Arom↑ increases tissue and plasma E2 concentrations. Tissue and circulating E2 and testosterone were analyzed after 6.5 weeks of doxycycline treatment (n = 6–10/group). Data are presented as mean ± SE. Letters that do not match are statistically different from one another (P < .05).
Abbreviations: E2, 17β-estradiol; HFD, high-fat diet; LFD, low fat diet; SkM-Arom↑, skeletal muscle–specific aromatase overexpression; T, testosterone; WT, wild type.
Increased Circulating and Tissue E2 Content Reduces Body Weight, Adiposity, and Adipose Tissue Inflammation
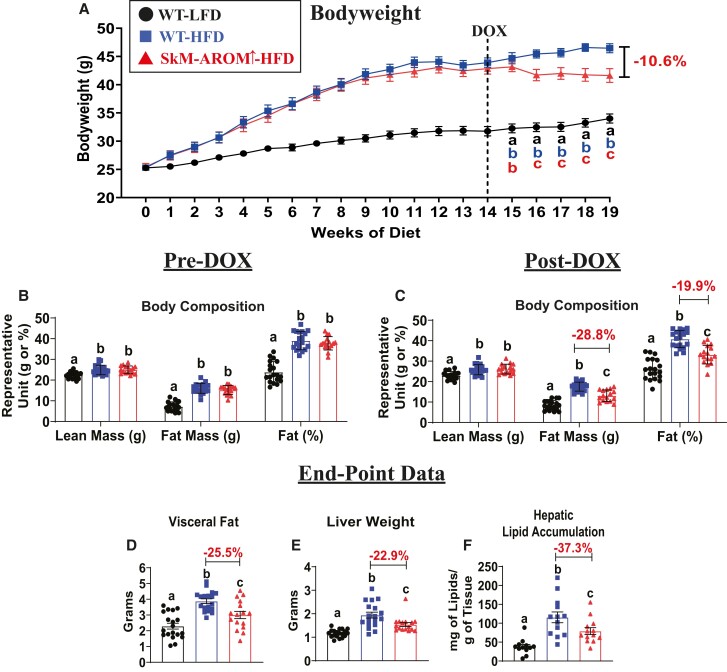
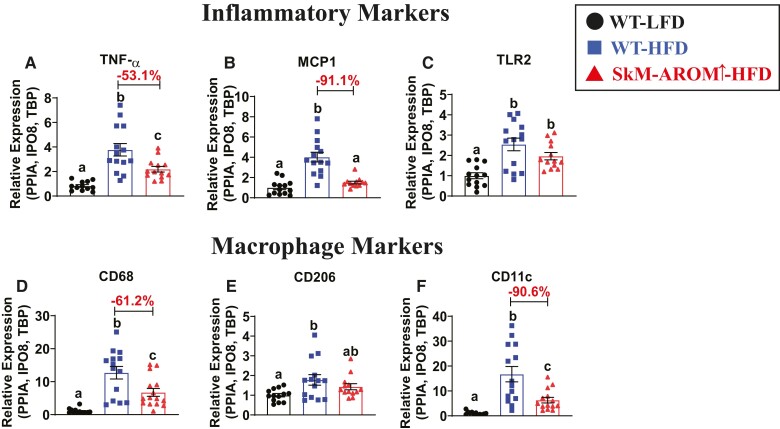
Prior to induction of SkM-Arom↑, we confirmed that male mice placed on a HFD displayed an increase in body weight, lean mass, fat mass, and body fat% relative to WT-LFD mice (Fig. 2A and 2B) (P < .05). Selective upregulation of muscle E2 using our SkM-Arom↑ model resulted in increased levels of E2 which facilitated weight loss (−11%) (Fig. 2A), particularly in the form of fat mass (−29% total fat and −26% visceral fat) (Fig. 2C and 2D) and reduced liver weight and hepatic lipid accumulation (−37%) (Fig. 2E and 2F) (P < .05). Gene expression of proinflammatory markers, TNF-α and MCP-1, as well as the pan macrophage marker, CD68, and the proinflammatory macrophage marker, CD11c, were found to be significantly reduced (51-91%) in the adipose tissue of SkM-Arom↑ HFD mice compared with WT-HFD littermate controls (Fig. 3) (P < .05). Furthermore, SkM-Arom↑ reduced adipose tissue CD206 expression (Fig. 3E), but had no appreciable effect on TLR2 expression (Fig. 3C).
Figure 2.
Body weight, body composition, and hepatic lipid accumulation are beneficially affected by increased tissue and circulating E2. (A) Body weight and (B, C) body composition were assessed pre- and post-doxycycline (DOX) treatment and (D) visceral fat (sum of gonadal, mesentery, and perirenal fat pads), (E) liver weight, and (F) hepatic lipid accumulation were assessed post-DOX in male wildtype (WT) LFD- and HFD-fed mice and SkM-Arom↑ HFD-fed mice (n = 16–18). Data are presented as mean ± SE. Bar graphs not sharing a common letter are significantly different from one another (P < .05).
Figure 3.
Adipose tissue inflammation is reduced in SkM-Arom↑ mice. After 20.5 weeks of LFD or HFD consumption, including 6.5 weeks of SkM-Arom↑ via DOX treatment, gene expression of gonadal adipose tissue inflammatory markers (A) TNF-α, (B) MCP-1, (C) TLR2, and macrophage markers (D) CD68 (pan macrophage marker), (E) CD206 (M2 macrophage marker), and (F) CD11c (M1 macrophage marker) were assessed (n = 16-18). Data are presented as mean ± SE. Bar graphs not sharing a common letter are significantly different from one another (P < .05).
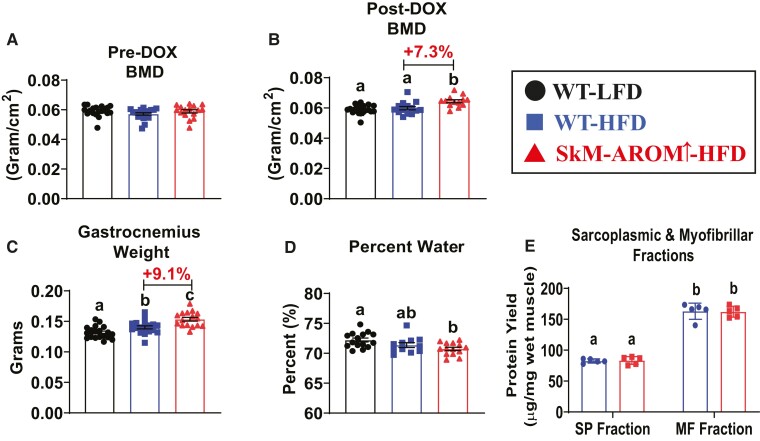
Elevated Skeletal Muscle E2 Production Elicits Anabolic Effects on the Musculoskeletal System
There were no differences in BMD across the groups prior to SkM-Arom↑ (Fig. 4A). However, post-DOX treatment, SkM-Arom↑ mice presented with a significant increase in BMD (Fig. 4B) (P < .05). We found the weight of the gastrocnemius to be increased by 9% in the SkM-Arom↑ mice relative to the WT-HFD mice (Fig. 4C) (P < .05). To determine if this increase in skeletal muscle mass was due to increased water weight, we dried out the skeletal muscle and determined that the increase in skeletal muscle mass displayed by the SkM-Arom↑ mice was not a result of increased water retention (Fig. 4D) (P < .05). We found that there was no difference in the protein content of the sarcoplasmic or myofibrillar fractions when normalized to wet weight between the HFD groups (Fig. 4E), providing evidence that the increase in skeletal muscle mass was a result of skeletal muscle hypertrophy. These results highlight the regulatory effects of E2 on the musculoskeletal system in male mice.
Figure 4.
SkM-Arom↑ promotes anabolic effects on the musculoskeletal system. Bone mineral density (BMD) was assessed (A) pre- and (B) post-DOX treatment and (C) the gastrocnemius weight, (D) percent water weight of the gastrocnemius, (E) as well as the protein contents of the sarcoplasmic and myofibrillar fractions of the gastrocnemius were assessed (n = 5–18) at the termination of the study. Data are presented as mean ± SE. Bar graphs not sharing a common letter are significantly different from one another (P < .05).
HFD-Induced Impairments in Glucose Tolerance, Hyperinsulinemia, and Adipose Tissue Insulin Action, but not Exogenous Insulin-stimulated Glucose Metabolism, are Ameliorated by Increased E2 Content
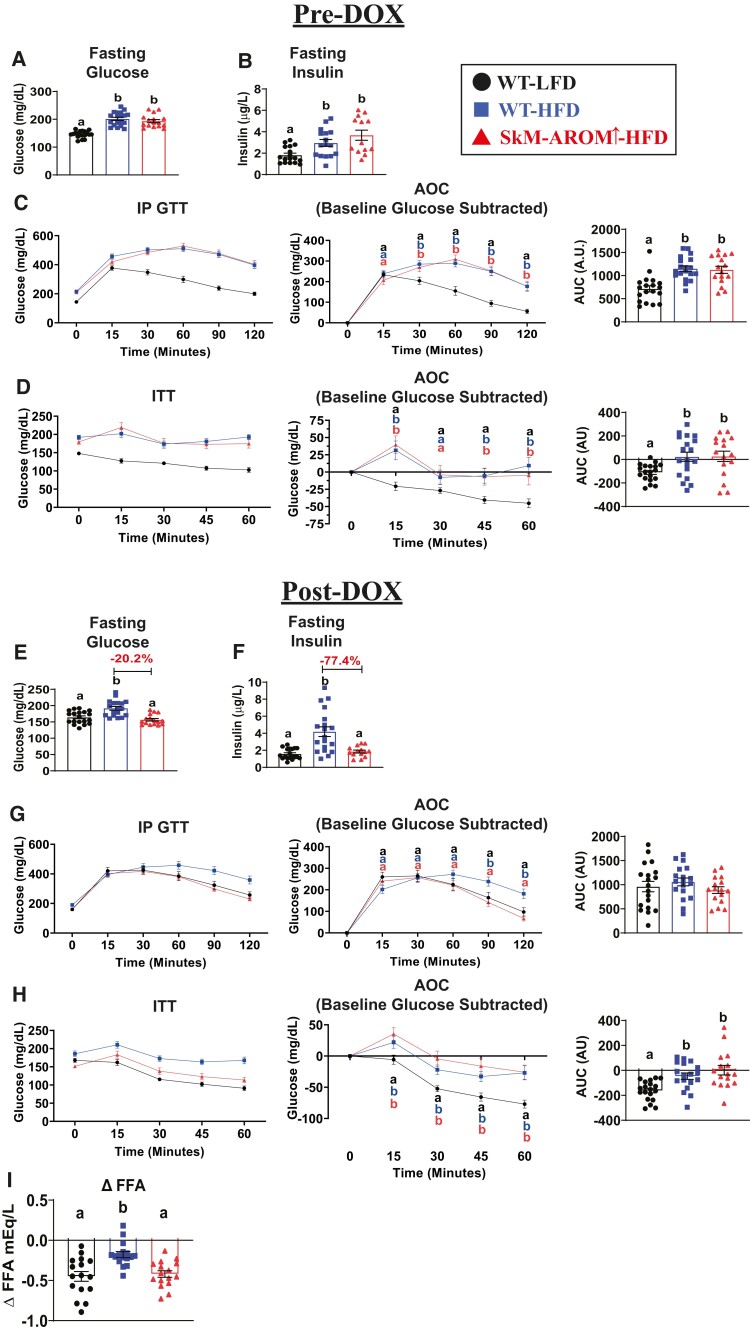
Before the initiation of SkM-Arom↑, both HFD groups exhibited a similar degree of hyperglycemia and hyperinsulinemia, as well as impaired glucose tolerance and insulin action relative to WT-LFD mice (Fig. 5A-5D) (P < .05). Post-DOX treatment, SkM-Arom↑ completely resolved their hyperglycemia, hyperinsulinemia, and impaired glucose tolerance induced by HFD feeding (Fig. 5E-5G) (P < .05). The ability of exogenous insulin to inhibit adipose tissue lipolysis was also enhanced in SkM-Arom↑ mice relative to WT HFD littermate controls (Fig. 5I) (P < .05). However, impairments in exogenous insulin-stimulated glucose metabolism were not mitigated by SkM-Arom↑ (Fig. 5H) (P < .05).
Figure 5.
Hyperglycemia, hyperinsulinemia, and impaired glucose tolerance is resolved in SkM-Arom↑ mice. (A, E) Fasting blood glucose, (B, F) fasting blood insulin, (C, G) glucose tolerance, (D, H) and insulin action were assessed pre- and post-DOX treatment. Additionally, (I) the Δ plasma free fatty acid (FFA) concentration in response to insulin administration was determined post-DOX treatment (n = 16-18). Data are presented as mean ± SE. Bar graphs not sharing a common letter are significantly different from one another (P < .05).
Action of E2 in the Brain Leads to Decreased Food Intake Leading to a Negative Energy Balance
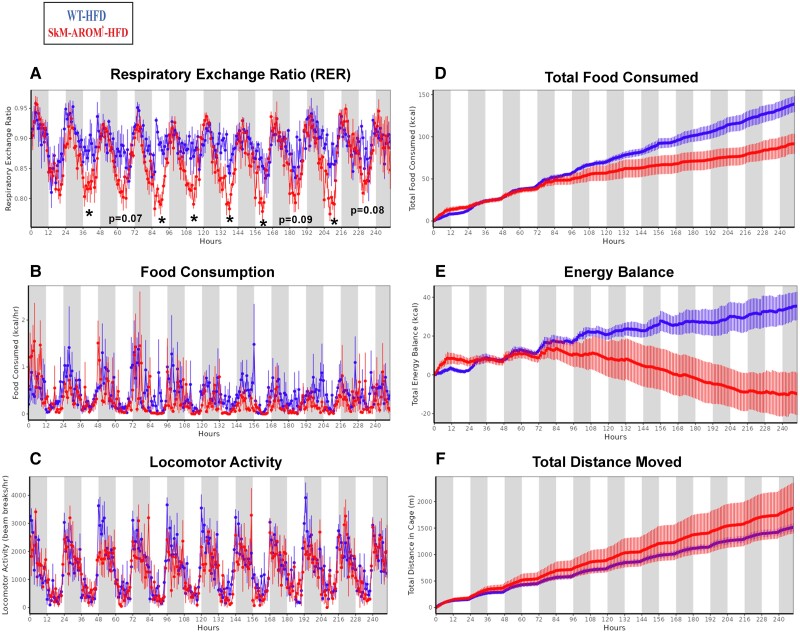
Mice were placed in metabolic cages in conjunction with DOX treatment to monitor the effect of SkM-Arom↑ on metabolic and behavioral outcomes in real time (Fig. 6). The RER was found to be significantly decreased in SkM-Arom↑ mice within 48 hours of DOX treatment (Fig. 6A) (P < .05). After 5 days of DOX treatment, SkM-Arom↑ mice started to exhibit a decrease in food intake resulting in a negative energy balance providing evidence for the central effects of E2 action (Fig. 6B, 6D, and 6E) (P ≤ .05). No significant differences were found with respect to physical activity (Fig. 6C and 6F) or any other measured parameter upon analysis of covariance adjustment for lean mass (Table 2).
Figure 6.
The SkM-Arom↑ phenotype is characterized by a reduced RER and a negative energy balance induced by a decrease in energy intake. Male SkM-Arom↑ and WT littermate controls (n = 4/group) were fed a HFD and placed into a Promethion multichannel continuous measurement indirect calorimetry system (Sable System International, Las Vegas, NV) on a 12-hour light and 12-hour dark cycle for an 11-day period. After a 1-day acclimation period, mice were given DOX-supplemented water (initiation of DOX-treated water is considered hour “0”). Data are presented as mean ± SE. *P < .05.
Table 2.
Indirect calorimetry data
| Unadjusted | ANCOVA adjusted | |||||
|---|---|---|---|---|---|---|
| WT HFD | SkM-Arom↑-HFD | P value | WT HFD | SkM-Arom↑-HFD | P value | |
| Total EE (kcal/day) | 9.89 (±0.21) | 9.71 (±0.206) | .55 | 9.84 (±0.218) | 9.76 (±0.218) | .82 |
| Energy intake (kcal/day) | 13.11 (±1.29) | 7.84 (±1.291) | .03 | 13.07 (±1.368) | 7.88 (±1.368) | ≤.05 |
| Resting EE (kcal/hour) | 0.33 (±0.007) | 0.32 (±0.007) | .21 | 0.33 (±0.007) | 0.32 (±0.007) | .68 |
| Nonresting EE (kcal/day) | 1.92 (±0.13) | 2.05 (±0.125) | .48 | 1.97 (±0.132) | 2 (±0.132) | .87 |
| O2 consumption (light cycle avg) | 1.34 (±0.028) | 1.28 (±0.028) | .23 | 1.32 (±0.029) | 1.3 (±0.029) | .62 |
| CO2 production (light cycle avg) | 1.17 (±0.025) | 1.07 (±0.025) | .038 | 1.16 (±0.027) | 1.09 (±0.027) | .14 |
| Avg RER (light cycle avg) | 0.86 (±0.007) | 0.79 (±0.007) | .001 | |||
| All meters (day) | 118.13 (±19.84) | 173.66 (±39.13) | .33 | |||
Average temperature 23.21 (±0.154) °C.
Male SkM-Arom↑ and WT littermate controls (n = 4/group) were fed a HFD and placed into a Promethion multichannel continuous measurement indirect calorimetry system (Sable System International, Las Vegas, NV, USA) on a 12-h light and 12-h dark cycle for a 11-day period. After a 1-day acclimation period, mice were given DOX-supplemented water. Data is presented as mean ± SE.
Abbreviations: ANCOVA, analysis of covariance; E2, 17β-estradiol; HFD, high-fat diet; LFD, low fat diet; SkM-Arom↑, skeletal muscle–specific aromatase overexpression; T, testosterone; WT, wild type; EE, energy expenditure; RER, respiratory exchange ratio.
Discussion
E2 is often considered a “female” sex hormone due to its greater abundance in females relative to males. However, what is often neglected is the fundamental role that E2 has in regulating male physiology including metabolic and anabolic outcomes (37). For instance, male whole-body aromatase knockout mice display increased adiposity, and impaired hepatic fatty acid β-oxidation, which can be reversed with exogenous E2 treatment (38-40). Additionally, male global, hepatic, and adipose tissue ERα knockout mice display an impaired metabolic phenotype highlighting the importance of estrogen signaling to regulate metabolism in males (7, 11-13). Utilizing our novel mouse model, which indelibly increases skeletal muscle–specific E2 production, paired with an ultrasensitive liquid chromatography mass spectrometry assay we determined for the first time the importance of skeletal muscle–derived E2 and its ability to mitigate the negative consequences of a HFD in males. This is of interest as it has been established that aromatase expression exists in human skeletal muscle (41, 42).
Six and a half weeks of SkM-Arom↑ successfully increased skeletal muscle E2. Similar to our previous findings in females (16), E2 produced in skeletal muscle also increased E2 in circulation and elicited systemic effects including a beneficial effect on BMD. Because males have higher endogenous testosterone levels that provided the precursor for conversion into E2, male SkM-Arom↑ had higher concentrations of muscle and circulating E2 than female SkM-Arom↑ mice (16). Elevated E2 elicited central (brain) and peripheral effects that combined to reverse the obese phenotype. Our metabolic cage data further demonstrated beneficial effects of E2 to induce hypophagia, which is likely primarily responsible for the decrease in body weight, as resting energy expenditure and physical activity were unchanged. Importantly, our data demonstrate a metabolic benefit of E2 in males to induce weight loss without having catabolic effects on the musculoskeletal system.
Utilizing our metabolic cage phenotyping system, we were able to determine the RER of the SkM-Arom↑ was significantly reduced compared with WT littermates and this change in RER was independent of any changes to body weight, food intake, or physical activity, suggesting that increased E2 levels impacted fuel partitioning, ultimately promoting fat oxidation. With respect to the liver, it has previously been established that E2 signaling regulates hepatic lipid accumulation and E2 deficiency impairs hepatic fat oxidation (7, 39). The SkM-Arom↑ model resulted in a significant increase in hepatic E2 content and decreased hepatic lipid accumulation. An elevated E2 concentration in adipose tissue was associated with a robust decrease in adipose tissue inflammation as exemplified by a 51% to 91% decrease in inflammatory marker gene expression, indicating the potent anti-inflammatory effects of E2.
With respect to metabolic outcomes, the elevated circulating and tissue E2 content induced by SkM-Arom↑ completely resolved hyperglycemia and hyperinsulinemia induced by HFD feeding, restored glucose handling, and improved the antilipolytic effect of insulin action in adipose tissue. With respect to E2 action in the liver, previous research has shown that hepatic E2 signaling prevents hepatic insulin resistance in HFD-fed male mice (7). The ITT results suggest skeletal muscle insulin action was not benefited by the elevated level of skeletal muscle E2 induced by SkM-Arom↑. However, it should be noted that a limitation of our study is that we did not directly assess hepatic and skeletal muscle insulin action. This would be of interest for future studies.
Previous research in male and female diabetic patients has shown weight loss induced by a hypocaloric diet can reverse hepatic insulin resistance, hyperglycemia, and hyperinsulinemia independent of any changes to insulin-stimulated peripheral glucose metabolism (43). Importantly, this is consistent with the phenotype we were able to generate in SkM-Arom↑ male mice. An interesting and unexpected outcome we observed was the anabolic effects on the musculoskeletal system exhibited by the SkM-Arom↑ mice despite the hypophagia and weight loss that typically promotes catabolic effects on skeletal muscle (44). In our previously published experiment in female mice, we did not see any significant change in gastrocnemius size (16). Our data suggest the combination of anabolic hormones (eg, testosterone and dihydrotestosterone) present at higher levels in males than in females may be responsible for eliciting the hypertrophy. This is supported by the fact that both E2 and dihydrotestosterone have previously been shown to elicit skeletal muscle hypertrophy in male mice (45). Of further interest was the fact that skeletal muscle hypertrophy was elicited without any concurrent impact on insulin action (as assessed by the ITT). This suggests that E2 likely regulates its effects on skeletal muscle hypertrophy and insulin action via two independent signaling mechanisms.
In summary, utilizing a novel mouse model, we show that E2 originating from skeletal muscle mitigates the negative consequences of HFD-induced obesity in males. One of the strengths of this investigation is that the tissue quantity of E2 was assessed, providing insight into the tissue content of E2 that may be required to elicit any tissue-specific effect. A limitation of our investigation is that we were not able to delineate whether the central or peripheral effects of E2 action were most responsible for the anti-obesity effects induced by skeletal E2 production. However, our data demonstrate the benefit targeted therapies may have to reduce the metabolic consequences of Western diet consumption in males. More importantly, our novel mouse model enabled us to demonstrate endogenous production of skeletal muscle E2 has a profound antiobesity effect in males without causing skeletal muscle catabolism. The fact that our findings differ in males when compared with females further suggests the importance of studying sex differences. Moreover, our data further emphasize the importance of estrogens in males and its impact on metabolic function.
Acknowledgments
We would like to thank Dr. John McCarthy (University of Kentucky) and the Center for Muscle Biology at the University of Kentucky for kindly providing the HSA-rtTA animals as well as Dr. Priscilla Furth (Georgetown University) for kindly providing the tet-Aromatase animals.
Abbreviations
- BMD
bone mineral density
- DOX
doxycycline
- E2
17β-estradiol
- EE
energy expenditure
- ERα
estrogen receptor-alpha
- GTT
glucose tolerance test
- HFD
high-fat diet
- ITT
insulin tolerance test
- LFD
low-fat diet
- MTBE
methyl tert-butyl ether
- RER
respiratory exchange ratio
- SkM-Arom↑
skeletal muscle–specific aromatase overexpression
- WT
wild type
Contributor Information
Christian A Unger, Department of Pathology, Microbiology, and Immunology, University of South Carolina-School of Medicine, Columbia, SC 29209, USA.
Ahmed K Aladhami, Department of Pathology, Microbiology, and Immunology, University of South Carolina-School of Medicine, Columbia, SC 29209, USA; University of Baghdad, Nursing College, Baghdad, Iraq.
Marion C Hope, III, Department of Pathology, Microbiology, and Immunology, University of South Carolina-School of Medicine, Columbia, SC 29209, USA.
William E Cotham, Department of Chemistry and Biochemistry, College of Arts and Science, University of South Carolina, Columbia, SC 29209, USA.
Kendall W Nettles, Department of Integrative Structural and Computational Biology, Wertheim UF Scripps Institute for Biomedical Innovation and Technology, Jupiter, FL 33458, USA.
Deborah J Clegg, Department of Internal Medicine, Texas Tech Health Sciences Center, El Paso, TX 79905, USA.
Kandy T Velázquez, Department of Pathology, Microbiology, and Immunology, University of South Carolina-School of Medicine, Columbia, SC 29209, USA.
Reilly T Enos, Department of Pathology, Microbiology, and Immunology, University of South Carolina-School of Medicine, Columbia, SC 29209, USA.
Funding
This work was supported by NIH grants to RTE (K01-AT010348) and KTV (R00-AT009206).
Disclosures
The authors have no conflicts of interest.
Data Availability
Original data generated and analyzed during this study are included in this published article.
References
- 1. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morselli E, Santos RS, Gao S, et al. Impact of estrogens and estrogen receptor-alpha in brain lipid metabolism. Am J Physiol Endocrinol Metab. 2018;315(1):E7‐E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122(1-3):65‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krause WC, Rodriguez R, Gegenhuber B, et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature. 2021;599(7883):131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torres MJ, Kew KA, Ryan TE, et al. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018;27(1):167‐179.e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ribas V, Drew BG, Zhou Z, et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8(334):334ra354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu L, Martinez MN, Emfinger CH, Palmisano BT, Stafford JM. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am J Physiol Endocrinol Metab. 2014;306(10):E1188‐E1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dakin RS, Walker BR, Seckl JR, Hadoke PW, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int J Obes (Lond). 2015;39(10):1539‐1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rochira V, Madeo B, Zirilli L, Caffagni G, Maffei L, Carani C. Oestradiol replacement treatment and glucose homeostasis in two men with congenital aromatase deficiency: evidence for a role of oestradiol and sex steroids imbalance on insulin sensitivity in men. Diabet Med. 2007;24(12):1491‐1495. [DOI] [PubMed] [Google Scholar]
- 10. Fox CS, Yang Q, Cupples LA, et al. Sex-specific association between estrogen receptor-alpha gene variation and measures of adiposity: the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(11):6257‐6262. [DOI] [PubMed] [Google Scholar]
- 11. Davis KE, Neinast MD, Sun K, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab. 2013;2(3):227‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):E304‐E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Handgraaf S, Riant E, Fabre A, et al. Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes. 2013;62(12):4098‐4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10(3):785‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iñigo MR, Amorese AJ, Tarpey MD, et al. Estrogen receptor-α in female skeletal muscle is not required for regulation of muscle insulin sensitivity and mitochondrial regulation. Mol Metab. 2020;34:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aladhami AK, Unger CA, Hope MC, Cotham WE, Velázquez KT, Enos RT. Augmenting skeletal muscle estrogen does not prevent or rescue obesity-linked metabolic impairments in female mice. Endocrinology. 2022;163(11):bqac146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwata M, Englund DA, Wen Y, et al. A novel tetracycline-responsive transgenic mouse strain for skeletal muscle-specific gene expression. Skelet Muscle. 2018;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Díaz-Cruz ES, Sugimoto Y, Gallicano GI, Brueggemeier RW, Furth PA. Comparison of increased aromatase versus ERα in the generation of mammary hyperplasia and cancer. Cancer Res. 2011;71(16):5477‐5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enos RT, Velázquez KT, McClellan JL, Cranford TL, Walla MD, Murphy EA. Lowering the dietary omega-6: omega-3 does not hinder nonalcoholic fatty-liver disease development in a murine model. Nutr Res. 2015;35(5):449‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Enos RT, Velázquez KT, Murphy EA. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J Nutr Biochem. 2014;25(6):600‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enos RT, Velázquez KT, McClellan JL, et al. High-fat diets rich in saturated fat protect against azoxymethane/dextran sulfate sodium-induced colon cancer. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G906‐G919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velázquez KT, Enos RT, Carson MS, et al. Mir155 deficiency aggravates high-fat diet-induced adipose tissue fibrosis in male mice. Physiol Rep. 2017;5(18):e13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bader JE, Enos RT, Velázquez KT, et al. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am J Physiol Endocrinol Metab. 2019;316(3):E358‐E372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cranford TL, Enos RT, Velázquez KT, et al. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int J Obes (Lond). 2016;40(5):844‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enos RT, Davis JM, Velázquez KT, et al. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54(1):152‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Enos RT, Velázquez KT, Carson MS, et al. A low dose of dietary quercetin fails to protect against the development of an obese phenotype in mice. PLoS One. 2016;11(12):e0167979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aladhami AK, Unger CA, Ennis SL, et al. Macrophage tumor necrosis factor-alpha deletion does not protect against obesity-associated metabolic dysfunction. FASEB J. 2021;35(7):e21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Unger CA, Aladhami AK, Hope MC 3rd, et al. Congenital adiponectin deficiency mitigates high-fat-diet-induced obesity in gonadally intact male and female, but not in ovariectomized mice. Sci Rep. 2022;12(1):16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9-10):525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Virtue S, Vidal-Puig A. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab. 2021;3(7):883‐886. [DOI] [PubMed] [Google Scholar]
- 31. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids. 2015;99(Pt A):84‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roberts MD, Young KC, Fox CD, et al. An optimized procedure for isolation of rodent and human skeletal muscle sarcoplasmic and myofibrillar proteins. J Biol Methods. 2020;7(1):e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Majewski AR, Chuong LM, Neill HM, Roberts AL, Jerry DJ, Dunphy KA. Sterilization of silastic capsules containing 17β-estradiol for effective hormone delivery in Mus musculus. J Am Assoc Lab Anim Sci. 2018;57(6):679‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCormack JT, Greenwald GS. Progesterone and oestradiol-17β concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol. 1974;62(1):101‐107. [DOI] [PubMed] [Google Scholar]
- 36. Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92(4):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 37. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97(23):12735‐12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nemoto Y, Toda K, Ono M, et al. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105(12):1819‐1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145(4):1842‐1848. [DOI] [PubMed] [Google Scholar]
- 41. Larionov AA, Vasyliev DA, Mason JI, Howie AF, Berstein LM, Miller WR. Aromatase in skeletal muscle. J Steroid Biochem Mol Biol. 2003;84(4):485‐492. [DOI] [PubMed] [Google Scholar]
- 42. Matsumine H, Hirato K, Yanaihara T, Tamada T, Yoshida M. Aromatization by skeletal muscle. J Clin Endocrinol Metab. 1986;63(3):717‐720. [DOI] [PubMed] [Google Scholar]
- 43. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCarthy D, Berg A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients. 2021;13(7):2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Svensson J, Movérare-Skrtic S, Windahl S, Swanson C, Sjögren K. Stimulation of both estrogen and androgen receptors maintains skeletal muscle mass in gonadectomized male mice but mainly via different pathways. J Mol Endocrinol. 2010;45(1):45‐57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article.