Abstract
In industrial fermentations, Penicillium chrysogenum uses sulfate as the source of sulfur for the biosynthesis of penicillin. By a PCR-based approach, two genes, sutA and sutB, whose encoded products belong to the SulP superfamily of sulfate permeases were isolated. Transformation of a sulfate uptake-negative sB3 mutant of Aspergillus nidulans with the sutB gene completely restored sulfate uptake activity. The sutA gene did not complement the A. nidulans sB3 mutation, even when expressed under control of the sutB promoter. Expression of both sutA and sutB in P. chrysogenum is induced by growth under sulfur starvation conditions. However, sutA is expressed to a much lower level than is sutB. Disruption of sutB resulted in a loss of sulfate uptake ability. Overall, the results show that SutB is the major sulfate permease involved in sulfate uptake by P. chrysogenum.
The filamentous fungus Penicillium chrysogenum is well known for its ability to produce penicillin (5, 39, 57). Penicillin biosynthesis starts with the condensation of the amino acids l-α-aminoadipic acid, l-Cys, and l-Val by the peptide synthetase δ(l-α-aminoadipyl)-l-cysteinyl-d-valine synthetase. The three precursor amino acids are synthesized in the cell as part of the primary metabolism of the fungus. To accommodate to the high demand for sulfur to be assimilated and incorporated into penicillin by high-producing strains (46, 54), inorganic sulfate is added to the medium as the source of sulfur for the formation of Cys (15, 39).
The uptake of sulfate, the first step in the pathway, has been studied by using mycelium and isolated plasma membrane vesicles from P. chrysogenum (4, 10, 17, 18, 46, 56, 60). These experiments indicated that sulfate is actively transported across the plasma membrane via a sulfate/proton symport mechanism.
Sulfate uptake is an important point of regulation of the sulfur metabolism in fungi. In Neurospora crassa, sulfate uptake is subject to a mechanism called sulfur (metabolite) repression or regulation, involving the action of positively and negatively acting regulatory proteins on the expression of sulfate permease-encoding genes (22, 27, 32). A similar situation holds for Aspergillus nidulans (30, 35, 36) and Saccharomyces cerevisiae (9, 55). In contrast, little is known about the mechanism and regulation of sulfate uptake in P. chrysogenum despite its possible significance in penicillin biosynthesis. Therefore, we set out to investigate sulfate permease-encoding genes from P. chrysogenum. The data shows that P. chrysogenum has two genes, designated sutA and sutB (sut for “sulfate transporter”), that encode putative sulfate transporters. Whereas the function of SutA remains to be elucidated, SutB was shown to be a functional sulfate transporter responsible for sulfate uptake in P. chrysogenum mycelium.
MATERIALS AND METHODS
Strains, plasmids, and libraries.
Escherichia coli LE392 [hdsR574 (rK− mK+) supE44 supF58 lacY1 galK2 galT22 metB1 trpR55] (34) and DH5α [φ80ΔlacZΔM15 recA1 endA1 gyrA96 thi-1 hdsR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169] (13) were used for phage handling and plasmid transformations, respectively. P. chrysogenum Wisconsin 54-1255 (Wis54-1255) has been described previously (8, 38). P. chrysogenum HP60 is a nicotinamide-requiring derivative of NRRL1951 (48). The construction of P. chrysogenum nr45 is described below. A. nidulans IG1 (sB3 pyrG89 pabaA1), carrying the sB3 mutation (1, 52), was derived from a cross between strains G191 (2) and 0198 obtained from the Glasgow Stock Collection (J. Clutterbuck, University of Glasgow). Plasmid pBluescript II KS (Stratagene) was used for cloning and sequencing in E. coli. Plasmid pGEM-T-easy (Promega) was used to clone PCR products. Plasmid pDJB2 is an A. nidulans transformation vector carrying the N. crassa pyr-4 gene as a selection marker (2). Plasmid pBSsutA contains a 5.5-kb PstI fragment (Fig. 1) cloned into the PstI site of pBluescript II KS. Plasmid pBSsutB contains a 4.3-kb BamHI fragment cloned into the BamHI site of pBluescript II KS. Plasmid pBSPsutB contains a 2.1-kb SalI fragment cloned into the SalI site of pBluescript II KS. Plasmid pBSsutB-XS contains an internal 1.0-kb XhoI-SalI fragment of sutB cloned into the MCS of pBluescript II KS. Plasmid pBSPsutBsutA was constructed as follows. Plasmid pBSsutB was digested with EcoRV (in the multiple-cloning site) and HincII (28 bp upstream of the sutB ATG start codon), and a 737-bp EcoRV-HincII fragment containing part of the sutB promoter region was isolated (Fig. 1). This fragment was cloned into plasmid pBSsutA, from which the sutA promoter region was removed by digestion with BstEII (49 bp upstream of the sutA ATG start codon), treatment with DNA polymerase (Klenow fragment), and digestion with EcoRV (in the multiple-cloning site). A genomic library of P. chrysogenum Q176 (38) DNA in phage λ-EMBL3a was a generous gift from H. Schwab, Technical University, Graz, Austria. DSM-Gist (Delft, The Netherlands) kindly provided the P. chrysogenum cDNA library.
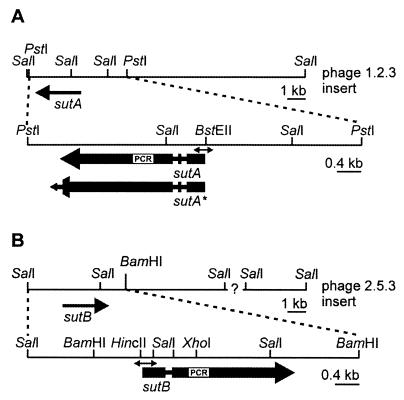
FIG. 1.
Physical map of the genomic DNA fragments containing the sutA (A) and sutB (B) genes. Genomic DNA fragments present in phages 1.2.3 and 2.5.3 carrying the sutA and sutB gene are depicted. The sutA and sutB open reading frames are indicated by the large, thick arrows, in which the narrow regions represent introns. For sutA, two versions are depicted, designated sutA and sutA*, the latter representing an extended open reading frame which would result if the last intron were spliced out. The sequences of the enlarged fragments (PstI-PstI for sutA and SalI-BamHI for sutB) are available in the GenBank database. The positions of the PCR fragments that were used to isolate the genes are indicated by the white boxes designated PCR. Regions that were used to probe expression in the Northern analysis are indicated by the double-headed arrows.
Media and growth conditions.
Manipulations with and growth of E. coli LE392 and DH5α were performed by standard methods (43). P. chrysogenum and A. nidulans growth media and conditions have been described previously (2, 16, 17). Where appropriate, sulfate salts were replaced by chloride salts, and methionine was added as indicated.
Gene cloning and sequencing.
Degenerate deoxyribonucleotide oligomers, designated sut-forw (5′-ACC TAC AAG GT[CT] [GA]T[CT] ATC [GA]A[CT] AC[TCA] CT[TGC] AA-3′) and sut-rev (5′-CC GAA [GT]GA CTT [TG]GA [GA]AT [TG]GC [TGA]AT [AG]TG [TC]TC-3′), were designed to correspond to two stretches of amino acid residues present in CYS-14p of N. crassa (TYKV[VI]I[NE]TLK and EHIAISKSFG) (23) and SUL1p and SUL2p of S. cerevisiae (TYKV[VI]I[NE]TLK and EHIAISKSFG) (9, 21, 49). PCR was performed on chromosomal P. chrysogenum DNA under standard conditions. PCR products of about 400 bp were isolated, treated with DNA polymerase (Klenow fragment), ligated into the SmaI site of pBluescript II KS, and sequenced. Of the 20 clones sequenced, two sets of 3 and 7 identical clones showed sequence similarity to known sulfate transporter-encoding genes but were different from each other. The PCR products were used to screen a genomic library of P. chrysogenum Q176 DNA in phage λ-EMBL3A by standard methods, resulting in the isolation of phages designated 1.2.3 and 2.5.3, which appeared to carry the sutA and sutB genes, respectively (Fig. 1). Further DNA manipulations to locate the genes on the phage-inserted DNA and to determine the nucleotide sequence were performed by standard methods (43).
cDNA analysis.
cDNAs comprising the coding regions of sutA and sutB were isolated by PCR with a P. chrysogenum cDNA library and the primers sutA-forw (5′-CCG CAG GTG ACC CTC CAG ACT ACG-3′) and sutA-rev (5′-GCT GGC CAA GAA CGG ATG CCC GCA-3′) for sutA and sutB-forw (5′-CAG TTC CCA ATA CAC TCC CCG TGG-3′) and sutB-rev (5′-CAG AGA GGT AGC AAG CAA TAG ATG-3′) for sutB. PCR was performed with the Expand High Fidelity PCR system (Boehringer Mannheim) as specified by the manufacturer. Specific PCR products were cloned into the pGEM-T-easy vector (Promega) and completely sequenced. For sutA, a fragment encompassing a tentative third intron was amplified with the primers sutA-in-forw (5′-CTC ATG GCG GAG GAG GCA CCC ACA-3′) and the aforementioned sutA-rev. To determine the sequences of the 5′- and 3′-untranslated regions of the sutA and sutB genes, primers which annealed in the coding regions but were directed outward of the genes were designed. For sutA, the primers rev-sutA (5′-GG GAT AAA GTC GAC TAT GAA GCC-3′) and forw-sutA (5′-CTC ATG GCG GAG GAG GCA CCC ACA-3′) were used, and for sutB, the primers rev-sutB (5′-GGG TGA TCT CGC GAA TCC AG-3′) and forw-sutB (5′-CCG TCA TGT CGA CTC TTA CCG-3′) were used. By using PCR (Expand Long Template PCR; Boehringer Mannheim), the cDNA library, and the primers mentioned above, the sutA and sutB flanking sequences as well as the vector backbone were amplified, treated with DNA polymerase (Klenow fragment), self-ligated, and sequenced.
Transformation of A. nidulans.
A. nidulans IG1 was transformed as described by Ballance and Turner (2), using 2 μg of pDJB2 mixed with 2 μg of pBSsutA, pBSsutB, or pBSPsutAsutB. Transformants were selected on minimal medium lacking uridine and uracil but supplemented with l-Met and p-aminobenzoic acid. After 5 days at 37°C, the transformants were tested for growth in the absence of methionine.
Construction of a P. chrysogenum sutB disruption mutant.
P. chrysogenum HP60 was transformed as described by Bull et al. (6), except that protoplasts were prepared from mycelium grown for 36 h.
A cotransformation was carried out with 5 μg of pBC1003 (which carries a phleomycin resistance marker [a gift from E. Friedlin, Biochemie GmbH]) mixed with 5 μg of pBSsutB-XS. pBSsutB-XS contains an internal 1.0-kb XhoI-SalI fragment of sutB cloned into the multiple-cloning site of pBluescript II KS (Fig. 1). Transformants were selected on medium containing 50 μg of phleomycin per ml in 25 ml of bottom agar overlaid with 20 ml of drug-free top agar into which transformed protoplasts had been mixed. Phleomycin-resistant transformants were tested for the ability to grow on sulfate as the sole sulfur source.
Sulfate uptake and expression studies.
A. nidulans strains were grown aerobically at 37°C for 16 h on glucose-containing minimal medium in which all sulfate salts were replaced by chloride salts. As required, l-Met (0.25 or 5 mM) and/or MgSO4 (0.1 or 2.0 mM) was added as the sulfur source(s). Where appropriate, uridine, uracil, and p-aminobenzoate were added to the medium at 10 mM, 20 mM, and 1 μg/ml, respectively. P. chrysogenum Wis54-1255 was grown aerobically at 25°C on a sulfur-sufficient (≥5 to 10 mM sulfate) main culture medium with lactose as the C source. After 24 h, the medium was exchanged either for fresh original (S-rich) medium or for sulfurless medium in which all sulfate salts were replaced by chloride salts; this was followed by 16 h of growth. P. chrysogenum HP60 and nr45 were precultured overnight (at 25°C) on starter culture medium supplemented with 10 mM l-Met. The starter cultures were used to inoculate (at a 1:10 ratio) main culture medium with glucose as the C source, supplemented with 20 mM Met. After growth for 40 h, the medium was exchanged either for a medium containing 10 mM Met and normal sulfate levels (S-rich medium) or for a medium lacking Met and with all sulfate salts replaced by chloride salts (S starvation medium), and growth was continued for 4 h.
Mycelium for sulfate uptake studies was harvested by suction filtration, washed with ice-cold 0.9% NaCl, and resuspended in 50 mM potassium phosphate (pH 6.0) at approximately 10 ml/g (wet weight). After the mycelium had been cooled for at least 30 min on ice, it was aerated for 15 min at that temperature prior to the uptake experiments. After preincubation of the mycelium at 25°C for 3 min, sulfate ([35S]Na2SO4; specific activity, 10 to 20 mCi/mmol [ICN Pharmaceuticals]) was added to a final concentration of 10 mM. Samples were drawn and processed as described previously (17). To check the energy dependency of sulfate uptake, deenergization of the mycelium was performed with the protonophore carbonyl cyanide-m-chlorophenylhydrazone (10 mM final concentration), which was added at the start of the 3-min preincubation period. Dry weights of lyophilized samples were determined.
Mycelium for total-RNA isolation was harvested by suction filtration and immediately frozen in liquid nitrogen. The mycelium was ground in liquid nitrogen with a mortar and pestle, and RNA was isolated from the powdered mycelium with Trizol (Gibco BRL) as specified by the manufacturer. Electrophoresis and Northern blotting were carried out essentially as described previously (45). To ensure that hybridization was specific for sutA or sutB, probes for sutA and sutB were made from gene regions which have the lowest sequence identity (less than 40%) (Fig. 1) and hybridization was performed under stringent conditions. A sutA-specific fragment was amplified with primers sutA-N-forw (5′-CCG CAG GTG ACC CTC CAG ACT ACG-3′) and sutA-N-rev (5′-GCC CCA GAT GTA ACG GCG AAC-3′), and a sutB-specific fragment was obtained with sutB-N-forw (5′-CAG TTC CCA ATA CAC TCC CCG TGG-3′) and sutB-N-rev (5′-ATT GAG CAA GTA TCT GCC CAG-3′). For the constitutively expressed actA gene, an actA cDNA clone was isolated from a cDNA library of P. chrysogenum by PCR with primers ACT-1 (5′-CAG TCG AAG CGT GGT ATC CTC-3′) and ACT-2 (5′-ACG TGG ATA CCG CCA GAC TCG-3′) under standard conditions as specified by the manufacturer (Pharmacia Biotech). A DNA fragment to probe pcbC expression was kindly provided by DSM-Gist. Labeling was performed with an oligolabeling kit (Pharmacia Biotech), as specified by the manufacturer, and [α-32P]dCTP.
GenBank accession numbers.
The sutA and sutB sequences can be found in the GenBank database with accession no. AF163975 and AF163974, respectively.
RESULTS
Cloning of the sutA and sutB genes.
Two putative sulfate transporter-encoding genes, sutA and sutB, were cloned from P. chrysogenum genomic DNA by a PCR-based approach as described in Materials and Methods. The nucleotide sequences of a 5.5-kb region encompassing the sutA gene and of a 5.8-kb region encompassing the sutB gene were determined. The sutA and sutB genes encode single polypeptides of 746 and 842 amino acid residues, respectively, with predicted molecular masses of 81.5 kDa (SutA) and 91.9 kDa (SutB). cDNA analysis showed that the coding regions are interrupted by two introns (63 and 60 nucleotides (nt) [nt 260 to 322 and 469 to 528]) for sutA and by one intron (59 nt [nt 457 to 515]) for sutB. These regions fit the intron consensus sequence 5′-GTNNGT......CT[GA]AC...YAG-3′ (numbering is relative to the ATG start codon). The intron in sutB is at exactly the same position as the second intron of sutA with respect to the amino acid sequence. The sutA gene was suspected to contain an additional intron in the 3′ region (nt 2279 to 2332 [5′-GTCAGAN28CTGAAN12TAG-3′]). Splicing out of this putative intron would extend the amino acid sequence identity between SutA and SutB (see below). Therefore, three independently isolated cDNAs were analyzed, with special attention paid to this region. From none of these cDNAs was the suspected intron spliced out. Furthermore, a fragment encompassing the putative intron was amplified by PCR with the cDNA library. One major band was detected, with the suspected intron not spliced out. A very faint band was detected (<5% abundance) from which the intron was putatively spliced out. cDNA analysis showed that the 5′ untranslated regions are ≥60 nt (sutA) and ≥171 nt (sutB). Sequences directly upstream of the transcribed but untranslated regions of sutA and sutB are particularly CT rich and contain TATA- and CCAAT-like sequences that may be involved in transcription. cDNA analysis showed that the 3′ untranslated regions are 342 nt (sutA) and 423 nt (sutB), not including the poly(A) tails.
Genetic complementation of the A. nidulans sB3 mutant.
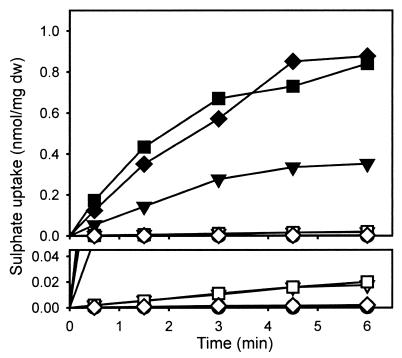
To investigate the function of the proteins encoded by the sutA and sutB genes, their ability to complement the sB3 (sulfate permease) mutation of A. nidulans was tested by cotransformation with pDJB2 and plasmids pBSsutA or pBSsutB, using the pyr-4 gene of pDJB2 as a selectable marker. Plasmids pBSsutA and pBSsutB contain about 2.5 and 0.8 kb of the respective promoter regions (Fig. 1). Of 50 Pyr+ transformants, 14 showed complementation of the sB3 mutation by the sutB gene from their ability to grow on a medium with sulfate as the sole sulfur source. Of these 14 clones, 2, named M27 and M63, were used for sulfate uptake studies. Strains M27 and M63, as well as the parental sB3 mutant strain IG1 and strain R21 (wild type for sB), were grown for 16 h on an S-poor medium containing 0.25 mM Met as the sole S source. After being harvested, mycelium was resuspended in phosphate buffer and sulfate uptake was studied. Strains M27 and M63 showed uptake levels comparable to that of R21, whereas sulfate uptake by IG1 was undetectably low (Fig. 2). When the strains were grown on S-rich medium containing 5 mM Met and 2 mM MgSO4, sulfate uptake by R21 was repressed more than 500-fold, while sulfate uptake of the sutB+ strains M27 and M63 was repressed approximately 50-fold (Fig. 2).
FIG. 2.
Sulfate uptake by four different strains under high-sulfate (5 mM Met and 2 mM MgSO4) or low-sulfate (0.25 mM Met) conditions. Shown are the wild-type strain A. nidulans R21 (high, ◊; low, ⧫), the sulfate uptake-deficient strain A. nidulans IG1 (high, ○; low, ●), carrying the mutant sB3 allele of the sulfate permease gene sB, and strains M27 (high, ▿; low, ▾) and M63 (high, □; low, ■), both of which are A. nidulans IG1 stains complemented with the P. chrysogenum sutB gene. The lower panel is a partial magnification of the upper panel to indicate that some residual sulfate uptake can be detected under high-sulfate conditions. dw, dry weight.
In contrast to the results for sutB, of 50 tested Pyr+ transformants cotransformed with pBSsutA and pDJB2, none showed complementation of the sB3 phenotype. To circumvent the possibility that sutA did not complement the A. nidulans IG1 sB3 mutation because of low expression of the sutA gene (see below), the 2.5-kb promoter region of sutA present in pBSsutA was replaced by the 0.8-kb promoter region upstream of sutB on pBSsutB, yielding plasmid pBSPsutBsutA. When A. nidulans IG1 was cotransformed with pDJB2 and pBSPsutBsutA, none of the tested Pyr+ clones showed complementation of the sB3 mutation as judged by their ability to grow on a medium with sulfate as the sole sulfur source. Northern analysis showed that in some of these transformants the sutA gene was expressed under the control of the sutB promoter during growth on S-poor medium. One of these clones (designated BA2) was used for sulfate uptake studies. After growth for 16 h on an S-poor medium containing 0.25 mM Met as the sole S source, sulfate uptake was measured, but not detected (not shown). These data demonstrate that SutB is a sulfate transporter. The function of SutA remains to be elucidated.
Expression of sutA and sutB, and disruption of sutB in P. chrysogenum.
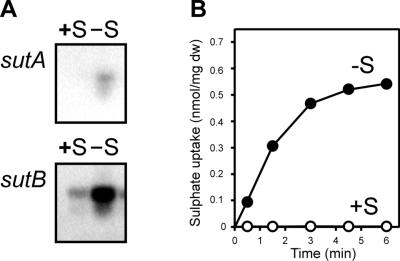
Northern analysis with P. chrysogenum Wis54-1255 showed that transcription of sutA and sutB was almost completely repressed when the strain was grown (for 40 h) under S-sufficient conditions (i.e., normal levels of sulfate) on main culture medium with lactose as the C source. When, the mycelium was starved for sulfur for 16 h, after 24 h of growth on main culture medium, expression of both sutA and sutB was induced. However, expression of sutB was an order of magnitude stronger than that of sutA (Fig. 3A). The level of sutA and sutB expression corresponded to sulfate uptake by mycelium grown under S-rich and S starvation conditions (Fig. 3B).
FIG. 3.
Northern blots showing the expression of sutA and sutB in P. chrysogenum Wis54-1255 (equal amounts of RNA were loaded [not shown]) (A) and corresponding sulfate uptake levels (B). Wis54-1255 was grown for 24 h in a sulfur-sufficient medium with lactose as the C source, after which the medium was exchanged either for fresh medium of the same composition (+S) or for fresh medium in which all sulfate salts were replaced by chloride salts (−S), and growth was continued for 16 h. dw, dry weight.
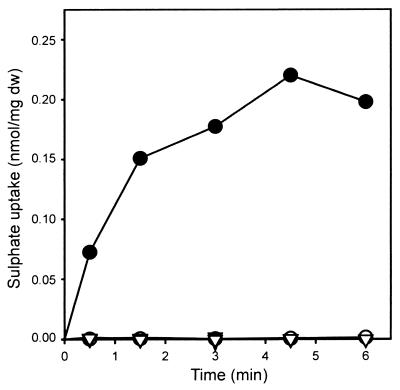
To study the function of sutB and sutA in P. chrysogenum, the sutB gene of P. chrysogenum HP60 was disrupted by homologous integration of an internal fragment of sutB following cotransformation with a phleomycin resistance vector. Of 100 phleomycin-resistant transformants tested, only one, nr45, failed to grow normally on sulfate as the sole sulfur source. This strain, which grew normally on medium supplemented with methionine, also showed resistance to selenate, indicative of a lesion in an early step in sulfate assimilation (1). DNA extracted from this strain and probed with pBSsutB-XS confirmed the disruption of sutB by homologous integration of pBSsutB-XS (data not shown). Sulfate uptake by strain HP60 grown for 44 h on an S-rich main culture medium (containing normal amounts of sulfate salts and 10 to 20 mM Met) was completely repressed compared to that by strain HP60 grown for 40 h under S-sufficient conditions and then starved for 4 h on S-less main culture medium (no sulfate salts, no l-Met) (Fig. 4). No sulfate uptake was detected for the sutB disruptant strain nr45, grown either under S-rich or under S starvation conditions (Fig. 4). These data demonstrate that SutB is the major sulfate permease involved in sulfate uptake by P. chrysogenum.
FIG. 4.
Sulfate uptake by P. chrysogenum HP60 (○, ●) (wild type) and nr45 (▿, ▾) (sutB disruptant). The strains were grown for 40 h in a sulfur-sufficient medium with glucose as the C source supplemented with 20 mM Met. Subsequently, growth was allowed for 4 h on fresh medium of the same composition supplemented with 10 mM Met (open symbols) or on fresh medium without Met in which all sulfate salts were replaced by chloride salts (solid symbols). dw, dry weight.
DISCUSSION
We have cloned two P. chrysogenum genes, sutA and sutB, one of which, sutB, encodes a functional sulfate permease. sutB complements the sB3 mutation of A. nidulans, and disruption of sutB in P. chrysogenum abolishes sulfate uptake. SutB appears to be the major sulfate permease present during mycelial growth and should therefore be located at the plasma membrane. SutB probably represents the high-affinity high-capacity 2H+/SO42− symport system, which has been kinetically characterized by Hillenga et al. (17). In line with this, both the Km for SutB (20 to 30 mM for SO42−) and the inhibition profile for SutB (S2O32− > S2O52− ∼ SO32−) after expression of the sutB gene in the A. nidulans sB3 strain (57a), resemble the characteristics of the P. chrysogenum system in mycelium (4, 17, 60). The physiological function of SutA remains unclear. Expression of SutA in mycelium is low and is not enhanced in the early growth stages (57b), unlike the situation indicated for N. crassa cys-13 (31). It is unlikely that SutA represents a low-affinity system, since P. chrysogenum nr45 (sutB disruptant) does not grow on a medium with high sulfate concentrations without methionine. sutA may encode a thiosulfate, tetrathionate, or sulfite transporter (56) or may function as a sulfate transporter in the vacuolar membrane (18).
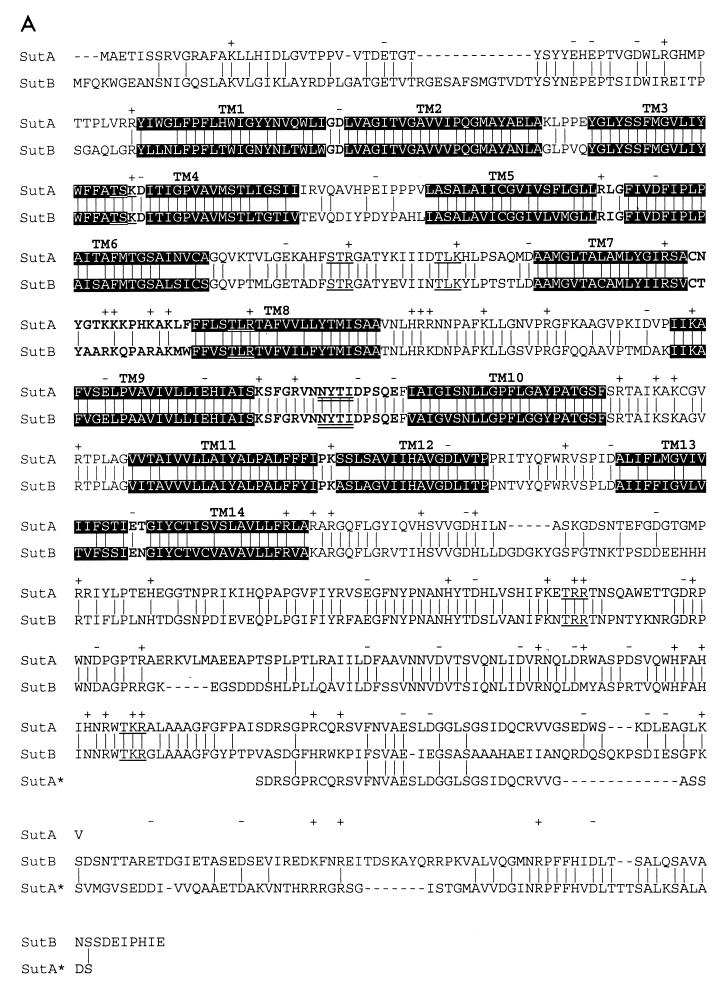
SutA seems to be truncated at its C terminus in comparison to SutB. Although the sutA genomic sequence suggests that an intron (nt 2279 to 2332 with respect to the ATG start codon) runs over the stop codon, cDNA analysis showed that it is not removed from the mRNA. If this intron were spliced out, SutA would be extended by 54 amino acid residues, which is very similar to the C terminus of SutB (Fig. 5) and to the C termini of SUL1p and SUL2p from S. cerevisiae (9, 49). It will be interesting to see whether (physiological) conditions exist that facilitate the removal of the putative intron from the primary transcript, resulting in SutA proteins with greater similarity to SutB and other sulfate transporters.
FIG. 5.
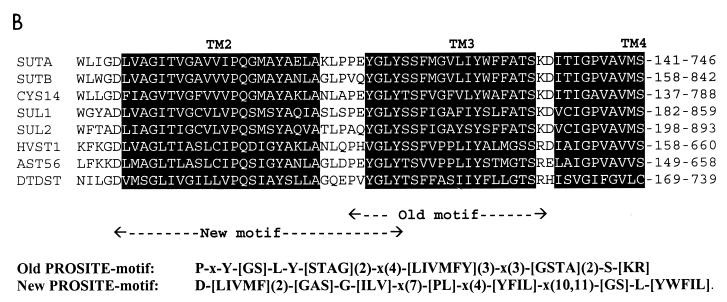
(A) Alignment of SutA and SutB amino acid sequences. Indicated are identical residues (|); charges (+, −), predicted protein kinase C phosphorylation sites ([ST]X[RK]) (59), and predicted N glycosylation sites (NX[ST]X, with X1P) (12, 33) which are present in both SutA and SutB; predicted transmembrane helices (black background); and predicted extracellular loops (bold type). Also shown is the alignment of an extended version of SutA, named SutA* (see Fig. 1 and Discussion). Alignments were made with ClustalX (20), and hydropathy profile analysis was done with MemGen version 4.08 (28, 29). (B) Alignment of SutA and SutB amino acid sequences with sequences of some examples of the SulP family of sulfate permeases whose the function has been proven experimentally (except SutA). Shown are the regions around the old sulfate permease motif (44) and the region around the proposed new sulfate permease motif. Alignments were made with the ClustalX program (20). SUTA, P. chrysogenum SutA (accession no. AF163975) (this work); SUTB, P. chrysogenum SutB (AF163974) (this work); CYS14, N. crassa Cys14 (BP23622) (19, 31); SUL1, S. cerevisiae Sul1 (P38359) (9, 49); SUL2, S. cerevisiae Sul2 (S64926 and Z73264) (YLR092w) (9); HVST1, Hordeum vulgare HVST1 (U52867) (51); AST56, Arabidopsis thaliana Ast56 (AB012047 and S74246) (53); DTDST, Homo sapiens DTDST (P50443) (14, 41). The old motif fails to recognize SutB, Sul2, and HVST1.
Expression of both sutA and sutB is induced when P. chrysogenum is grown under S starvation conditions (Fig. 3 and 4). Also, in A. nidulans, SutB-mediated sulfate uptake is subject to sulfur regulation (Fig. 2). S regulation is a well-documented phenomenon in N. crassa, A. nidulans, and S. cerevisiae (32, 55). For sutB, the 800 nt upstream of the start codon that are present on pBSsutB are almost completely sufficient for S regulation in A. nidulans (Fig. 2 and 4). In N. crassa, S regulation is positively mediated by the DNA-binding protein CYS-3p (22, 23, 26, 27, 32). Recently, a positively acting CYS-3p homologue has been found in A. nidulans (37). No CYS-3p homologue has been reported for P. chrysogenum. Sequences that weakly resemble the CYS-3p binding-site consensus ATGRYRYCAT (26, 27) are present upstream of sutA and sutB at positions −2481 (ATTGTACAAT), −1871 (ATTACGTGTT), −1513 (GTCGCGTGAC), −813 (GTCACGTACC), and −312 (CTGACGTTCG) (sutA) and −1516 (ATGACGTGAT), −983 (ATTATGTAAT), −394 (ACAACGTGGA), and −231 (ATTGCGCCAT) (sutB) with respect to the ATG start codon. Other sequences in the sutA and sutB promoter regions resemble the consensus binding site TCACGTG, which is recognized in S. cerevisiae by the Cbf1p-Met4p-Met28p complex (24, 25, 55) (sutA, positions −2209, −2105, −1870, −1512, −904, and −812; sutB, positions −1909, and −1515 [note that some of these sites are part of putative CYS3p homologue-binding sites]), or the consensus binding site AAANTGTG of the positive regulators Met31p and Met32p (3) (sutA, positions −2129, −1600, and −1000; sutB, positions −1483, −1475, and −876). A possible function for these cis-acting elements and their proposed trans-acting binding factors remains to be investigated.
Hydropathy analysis (28, 29, 47, 58) of SutA and SutB shows a pattern typical for a polytopic membrane protein, with 14 putative hydrophobic transmembrane (TM) helices in the N-terminal part of the protein followed by a long C-terminal extension (Fig. 5A). The overall sequence identity of the SutA and SutB proteins is 66% (Fig. 5A). Both proteins show significant homology to eukaryotic sulfate permeases from fungi, plants, and animals (data not shown). These proteins are clustered, together with a number of prokaryotic proteins, in the so-called SulP superfamily of sulfate permeases, which belongs to the class of secondary transporters (40, 42). These proteins all contain a motif which has become known as the sulfate permease signature. Originally this motif was defined as P-x- Y-[GS]-L-Y-[STAG](2)-x(4)-[LIVMF](2)-Y-x(3)-[GSTA](2)-S-[KR](44, 50), and it runs over the TM helix 3 (depicted in Fig. 5B). This motif is present in both SutA and SutB. However, a database search with this motif fails to recognize many putative and experimentally proven sulfate permeases, including SutB (Fig. 5B). Therefore, a new motif is proposed with the sequence D-[LIVFM](2)-[GAS]-G-[ILV]-x(7)-[PL]-x(15, 16)-[GS]-L-[YWFIL], which starts at TM helix 2 and runs into TM helix 3 (depicted in Fig. 5B). This motif is both complete and specific in the recognition of (putative) sulfate permeases in sequence databases.
According to the topology model of the whole SulP family, based on hydropathy profile analysis (data not shown), the N termini of both SutA and SutB are located in the cytosol. The C-terminal domain of both systems is predicted to be located in the cytosol as well, in line with topology data for the human DRA-encoded sulfate transporter (7). Previously published models were based on alignments of a small number of eukaryotic sulfate permeases (see e.g., references 9, 11, 50, and 51) and predicted 12 or fewer TM helices. However, a hydropathy profile based on the 50 presently available sequences (not shown) predicts 14 TM helices for most eukaryotic sulfate permeases and 13 TM helices for the prokaryotic sulfate permeases. The predicted TM helix 1 appears to be present in a subset of eukaryotic sulfate permeases, including SutA and SutB (P. chrysogenum), SUL1p, SUL2p, and SULXp (S. cerevisiae), CYS-14p (N. crassa), and some plant, nematode, and mammalian sulfate permeases, but it is lacking in other eukaryotic sulfate permeases and in all prokaryotic permeases. The current model predicts the presence of TM helices 13 and 14, whereas in most previous models a single TM helix was predicted. However, the previously proposed topology models disobey the so-called positive-inside rule (14, 47, 50, 58), while the prediction of TM helix 14 yields a topology with a charge distribution which is in better agreement with the positive-inside rule, as seen in Fig. 5.
Summarizing, P. chrysogenum contains two genes, designated sutA and sutB, that encode putative sulfate transporters. SutB is the system responsible for sulfate uptake in mycelium of P. chrysogenum, whereas the role of SutA remains to be determined. Future studies will address the regulation and expression of these systems in relation to the high demand for sulfur during penicillin biosynthesis.
ACKNOWLEDGMENTS
This project was supported by the EC, Eurofung Cell Factory-RTD4CY96-0535.
We thank DSM-Gist. (Delft, the Netherlands) for determining the nucleotide sequences and providing the P. chrysogenum cDNA library and the pcbC probe, and we thank J. S. Lolkema and D.-J. Slotboom for discussions with respect to the SutA and SutB topology model.
REFERENCES
- 1.Arst H N., Jr Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature. 1968;219:268–270. doi: 10.1038/219268a0. [DOI] [PubMed] [Google Scholar]
- 2.Ballance D J, Turner G. Development of a high frequency transforming vector for Aspergillus nidulans. Gene. 1985;36:321–331. doi: 10.1016/0378-1119(85)90187-8. [DOI] [PubMed] [Google Scholar]
- 3.Blaiseau P L, Isnard I D, Surdin-Kerjan Y, Thomas D. Met31p and Met32p, two related zinc-finger proteins, are involved in transcriptional regulation of the yeast sulfur amino acid metabolism. Mol Cell Biol. 1997;17:3640–3648. doi: 10.1128/mcb.17.7.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradfield G, Somerfield P, Meyn T, Holby M, Babcock D, Bradley D, Segel I H. Regulation of sulfate transport in filamentous fungi. Plant Physiol. 1970;46:720–727. doi: 10.1104/pp.46.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brakhage A A. Molecular regulation of β-lactam biosynthesis in filamentous fungi. Microbiol Mol Biol Rev. 1998;62:547–585. doi: 10.1128/mmbr.62.3.547-585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull J H, Smith D J, Turner G. Transformation of Penicillium chrysogenum with a dominant selectable marker. Curr Genet. 1988;13:377–382. doi: 10.1007/BF00365658. [DOI] [PubMed] [Google Scholar]
- 7.Byeon M K, Frankel A, Papas T S, Henderson K W, Schweinfest C W. Human DRA functions as a sulfate transporter in Sf9 insect cells. Protein Expression Purif. 1998;12:67–74. doi: 10.1006/prep.1997.0809. [DOI] [PubMed] [Google Scholar]
- 8.Cantoral J M, Gutiérrez S, Fierro F, Gil-Espinosa S, van Liempt H, Martín J F. Biochemical characterization and molecular genetics of nine mutants of Penicillium chrysogenum impaired in penicillin biosynthesis. J Biol Chem. 1993;268:737–744. [PubMed] [Google Scholar]
- 9.Cherest H, Davidian J-C, Thomas D, Benes V, Ansorge W, Surdin-Kerjan Y. Molecular characterization of two high affinity sulfate transporters in Saccharomyces cerevisiae. Genetics. 1997;145:627–635. doi: 10.1093/genetics/145.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuppoletti J, Segel I H. Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry. 1975;14:4712–4718. doi: 10.1021/bi00692a023. [DOI] [PubMed] [Google Scholar]
- 11.Everett L A, Glaser B, Beck J C, Idol J R, Buchhs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis A D, Sheffield V C, Green E D. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 12.Gavel Y, von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3:433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 14.Hästbacka J, de la Chapelle A, Mahtani M M, Clines G, Reeve-Daly M P, Daly M, Hamilton B A, Kusumi K, Trivedi B, Weaver A, Coloma A, Lovett M, Buckler A, Kaitila I, Lander E S. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 15.Herschbach G J M, van der Beek C P, van Dijck P W M. The penicillins: properties, biosynthesis, and fermentation. In: van Damme E, editor. Biotechnology of industrial antibiotics. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 45–140. [Google Scholar]
- 16.Hillenga D J, Versantvoort H J M, Driessen A J M, Konings W N. Structural and functional properties of plasma membranes from the filamentous fungus Penicillium chrysogenum. Eur J Biochem. 1994;224:581–587. doi: 10.1111/j.1432-1033.1994.t01-1-00581.x. [DOI] [PubMed] [Google Scholar]
- 17.Hillenga D J, Versantvoort H J M, Driessen A J M, Konings W N. Sulfate transport in Penicillium chrysogenum plasma membranes. J Bacteriol. 1996;178:3953–3956. doi: 10.1128/jb.178.13.3953-3956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter D R, Segel I H. Evidence for two distinct pools of inorganic sulfate in Penicillium notatum. J Bacteriol. 1985;162:881–887. doi: 10.1128/jb.162.3.881-887.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarai G, Marzluf G A. Sulfate transport in Neurospora crassa: regulation, turnover, and cellular localization of the CYS-14 protein. Biochemistry. 1991;30:4768–4773. doi: 10.1021/bi00233a019. [DOI] [PubMed] [Google Scholar]
- 20.Jeanmougin F, Thompson J D, Gouy M, Higgins D G, Gibson T. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y H, Jang Y K, Kim M J, Rad M R, Kirchrath L, Seong R H, Hong S H, Hollenberg C P, Park S D. Characterization of sfp2, a putative sulfate permease gene of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;214:709–715. doi: 10.1006/bbrc.1995.2343. [DOI] [PubMed] [Google Scholar]
- 22.Ketter J S, Marzluf G A. Molecular cloning and analysis of the regulation of cys-14+, a structural gene of the sulfur regulatory circuit of Neurospora crassa. Mol Cell Biol. 1988;8:1504–1508. doi: 10.1128/mcb.8.4.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ketter J S, Jarai G, Fu Y-H, Marzluf G A. Nucleotide sequence, messenger RNA stability, and DNA recognition elements of cys-14, the structural gene for sulfate permease II in Neurospora crassa. Biochemistry. 1991;30:1780–1787. doi: 10.1021/bi00221a008. [DOI] [PubMed] [Google Scholar]
- 24.Kuras L, Cherest H, Surdin-Kerjan Y, Thomas D. A heteromeric complex containing the centromere binding factor 1 and two basic leucine zipper factors, Met4 and Met28, mediates the transcription activation of yeast sulfur metabolism. EMBO J. 1996;15:2519–2529. [PMC free article] [PubMed] [Google Scholar]
- 25.Kuras L, Barbey R, Thomas D. Assembly of a bZIP/bLHL transcription activation complex: formation of the yeast Cbf1/Met4/Met28 complex is regulated through Met28 stimulation of Cbf1 DNA binding. EMBO J. 1997;16:2441–2451. doi: 10.1093/emboj/16.9.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Marzluf G A. Determination of the Neurospora crassa CYS3 sulfur regulatory protein consensus DNA-binding site: amino-acid substitutions in the CYS3 bZIP domain that alter DNA-binding specificity. Curr Genet. 1996;30:298–304. doi: 10.1007/s002940050136. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Zhou L, Marzluf G A. Functional in vivo studies of the Neurospora crassa cys-14 gene upstream region: importance of CYS3-binding sites for regulated expression. Mol Microbiol. 1996;22:109–117. doi: 10.1111/j.1365-2958.1996.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 28.Lolkema J S, Slotboom D-J. Estimation of structural similarity of membrane proteins by hydropathy profile alignment. Mol Membr Biol. 1998;15:33–42. doi: 10.3109/09687689809027516. [DOI] [PubMed] [Google Scholar]
- 29.Lolkema J S, Slotboom D-J. Hydropathy profile alignment: a tool to search for structural homologues of membrane proteins. FEMS Microbiol Rev. 1998;22:305–322. doi: 10.1111/j.1574-6976.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 30.Lukaszkiewicz Z, Paszewski A. Hyper-repressible operator-type mutant in sulphate permease gene of Aspergillus nidulans. Nature. 1976;159:337–338. doi: 10.1038/259337a0. [DOI] [PubMed] [Google Scholar]
- 31.Marzluf G A. Genetic and biochemical studies of distinct sulfate permease species in different developmental stages of Neurospora crassa. Arch Biochem Biophys. 1970;138:254–263. doi: 10.1016/0003-9861(70)90306-1. [DOI] [PubMed] [Google Scholar]
- 32.Marzluf G A. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annu Rev Microbiol. 1997;51:73–96. doi: 10.1146/annurev.micro.51.1.73. [DOI] [PubMed] [Google Scholar]
- 33.Miletich J P, Broze G J., Jr Beta protein C is not glycosylated at asparagine 329: the rate of translation may influence the frequency of usage at asparagine X-cysteine sites. J Biol Chem. 1990;265:11397–11404. [PubMed] [Google Scholar]
- 34.Murray N E, Brammar W E, Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977;150:53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- 35.Natorff R, Balinska M, Paszewski A. At least four regulatory genes control sulphur metabolite repression in Aspergillus nidulans. Mol Gen Genet. 1993;238:185–192. doi: 10.1007/BF00279546. [DOI] [PubMed] [Google Scholar]
- 36.Natorff R, Piotrowska M, Paszewski A. The Aspergillus nidulans sulphur regulatory gene sconB encodes a protein with WD40 repeats and an F-box. Mol Gen Genet. 1998;257:255–263. doi: 10.1007/s004380050646. [DOI] [PubMed] [Google Scholar]
- 37.Natorff R, Sieñko M, Brzywczy J, Paszewski A. Abstracts of the 20th Fungal Genetics Conference. 1999. The Aspergillus nidulans METR sulphur regulator belongs to bZIP transcriptional factors, abstr. 78; p. 59. [Google Scholar]
- 38.Newbert R W, Barton B, Greaves P, Harper J, Turner G. Analysis of a commercially improved Penicillium chrysogenum strain series: involvement of recombigenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J Ind Microbiol Biotechnol. 1997;19:18–27. doi: 10.1038/sj.jim.2900411. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen J. Physiological engineering aspects of Penicillium chrysogenum. Lyngby, Denmark: Technical University of Denmark; 1995. [Google Scholar]
- 40.Paulsen I T, Sliwinski M K, Nelissen B, Goffeau A, Saier M H., Jr Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 1998;430:116–125. doi: 10.1016/s0014-5793(98)00629-2. [DOI] [PubMed] [Google Scholar]
- 41.Rossi A, Bonaventura J, Delezoide A-L, Cetta G, Superti-Furga A. Undersulfation of proteoglycans synthesized by chondrocytes from a patient with achondrogenesis type 1B homozygous for and L483P substitution in the diastrophic dysplasia sulfate transporter. J Biol Chem. 1996;271:18456–18464. doi: 10.1074/jbc.271.31.18456. [DOI] [PubMed] [Google Scholar]
- 42.Saier M H., Jr Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archea and eukarya. Adv Microb Physiol. 1998;40:81–136. doi: 10.1016/s0065-2911(08)60130-7. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Sandal N N, Marcker K A. Similarities between a soybean nodulin, Neurospora crassa sulphate permease II and a putative tumor suppressor. Trends Biochem Sci. 1994;19:19. doi: 10.1016/0968-0004(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 45.Schuurs T A, Schaeffer E A M, Wessels J G H. Homology-dependent silencing of the SC3 gene in Schizophyllum commune. Genetics. 1997;147:589–596. doi: 10.1093/genetics/147.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segel I H, Johnson M J. Accumulation of intracellular inorganic sulfate by Penicillium chrysogenum. J Bacteriol. 1961;81:91–98. doi: 10.1128/jb.81.1.91-98.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sipos L, von G. Heijne Predicting the topology of eukaryotic membrane proteins. Eur J Biochem. 1993;213:1333–1340. doi: 10.1111/j.1432-1033.1993.tb17885.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith D J, Bull J H, Edwards J, Turner G. Amplification of the isopenicillin N synthetase gene in a strain of Penicillium chrysogenum producing high levels of penicillin. Mol Gen Genet. 1989;216:492–497. doi: 10.1007/BF00334395. [DOI] [PubMed] [Google Scholar]
- 49.Smith F W, Hawkesford M J, Prosser I M, Clarkson D T. Isolation of a cDNA from Saccharomyces cerevisiae that encodes a high affinity sulphate transporter at the plasma membrane. Mol Gen Genet. 1995;247:709–715. doi: 10.1007/BF00290402. [DOI] [PubMed] [Google Scholar]
- 50.Smith F W, Ealing P M, Hawkesford M J, Clarkson D T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci USA. 1995;92:9373–9377. doi: 10.1073/pnas.92.20.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith F W, Hawkesford M J, Ealing P M, Clarkson D T, Van den Berg P J, Belcher A R, Warrilow A G S. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- 52.Spencer B, Hussey E C, Orsi B A, Scott J. Mechanism of choline-O-sulphate utilization in fungi. Biochem J. 1968;106:461–469. doi: 10.1042/bj1060461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, de Almeida Engler J, Engler G, van Montagu M, Saito K. Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:11102–11107. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tardrew P L, Johnson M J. Sulfate utilization by penicillin-producing mutants of Penicillium chrysogenum. J Bacteriol. 1958;76:400–405. doi: 10.1128/jb.76.4.400-405.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas D, Surdin-Kerjan Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1997;61:503–532. doi: 10.1128/mmbr.61.4.503-532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tweedie J W, Segel I H. Specificity of transport processes for sulfur, selenium, and molybdenum anions by filamentous fungi. Biochim Biophys Acta. 1970;196:95–106. doi: 10.1016/0005-2736(70)90170-7. [DOI] [PubMed] [Google Scholar]
- 57.Van De Kamp M, Driessen A J M, Konings W N. Compartmentalization and transport in β-lactam antibiotic biosynthesis by filamentous fungi. Antonie Leeuwenhoek. 1999;75:41–78. doi: 10.1023/a:1001775932202. [DOI] [PubMed] [Google Scholar]
- 57a.van de Kamp, M. Unpublished results.
- 57b.van de Kamp, M., et al. Unpublished results.
- 58.von Heijne G. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 59.Woodget J R, Gould K L, Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986;161:177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto L A, Segel I H. The inorganic sulfate transport system of Penicillium chrysogenum. Arch Biochem Biophys. 1966;114:523–538. doi: 10.1016/0003-9861(66)90376-6. [DOI] [PubMed] [Google Scholar]