Abstract
Gut microbiota is a complex ecosystem, strictly linked to health and disease, as a balanced composition (referred as eubiosis) is necessary for several physiological functions, while an unbalanced composition (dysbiosis) is often associated to pathological conditions and/or diseases. An altered microbiota could be positively affected and partially restored through probiotic supplementation, among others. This review addresses the effects of probiotics in several conditions, used as case-studies (colorectal cancer, neuro-psychiatric diseases, intestinal diseases, obesity, diabetes, metabolic syndrome, immune system, and musculoskeletal system disorders) by pointing out the clinical outcomes, the mode of action, mainly related to the production of short chain fatty acids (SCFA), the impact of probiotic dose and mode of supplementation, as well as trying to highlight a hit of the most used genera.
Keywords: probiotics, disease, clinical trials, effects, genera
1. Introduction
Since 2001, Lederbergh and McCray highlighted the importance of microorganisms inhabiting the human body in health and disease; in fact, a close connection between the “state of health” of microbial communities and human health was recognized as a milestone (1, 2). Nowadays, “the assemblage of microorganisms (bacteria, archaea, eukaryotes, and viruses) present in a defined environment” is called Microbiota (3) and its composition changes according to the surrounding environment. In particular, the microbiota of the gastro-intestinal tract, generally known as gut microbiota, is a complex ecosystem composed of fungi, viruses, and bacteria, adapted to live on the mucus surface of the intestine or in its lumen, affected, among others, by the modality of childbirth (vaginal vs. cesarean), initial nutrition (breastfeeding vs. formula) and by the guest genotype (4).
The microbial ecosystem balance is called eubiosis and this status allows to perform several functions (nutritional, immunological, preventive actions, etc.); but, if this balance is lacking or altered, there is a condition of “dysbiosis.” Dysbiosis status is often associated to various diseases, such as asthma, chronic intestinal diseases, obesity, diabetes mellitus, psychiatric disorders, and many others (5). Several factors, such as antibiotics, smoking, alcohol, a sedentary life, diets low in fiber, poor chewing, psychophysical stress, chemotherapy, or abuse of drugs (laxatives, antidepressants, sleeping pills, analgesics) heavily affect microbiota balance and could lead to a dysbiotic status (6). An altered microbiota could be positively affected and partially restored through correct diet, and physical activity, although sometimes a supplementation of probiotics and/or prebiotics (e.g., fibers) could be necessary (7).
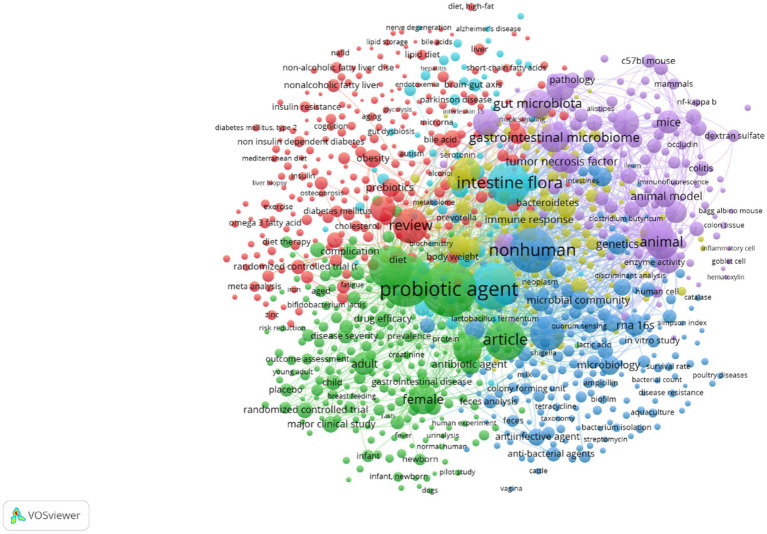
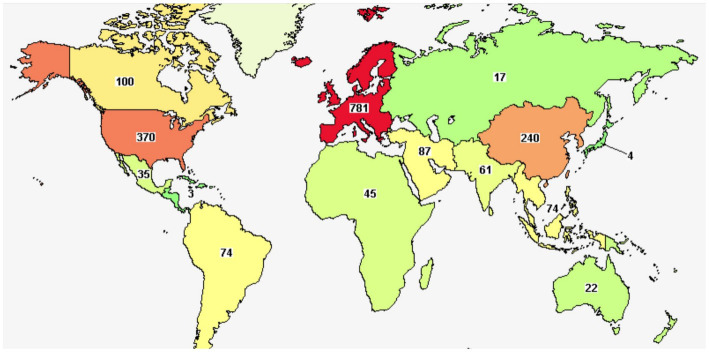
According to the definition of Food and Agriculture Organization/World Health Organization (8), slightly modified by Hill et al. (9), probiotics are “Live microorganisms which when administered in adequate amounts confer a health benefit on the host.” They represent a strategy to treat intestinal dysbiosis, as they could exert some important functions, that is (i) anti-inflammatory activity, essential for maintaining the immune response; (ii) to prevent the colonization by pathogenic microorganisms thanks to the physical barrier function; (iii) to produce antimicrobial substances (10). Thousands of authors studied probiotics and their effects on a wide variety of conditions; a search done on Scopus using two keywords (probiotics and disease) revealed for 2022–2023 more than 4,000 papers (research papers or reviews). The analysis of keywords and abstracts through VosViewer, a tool for networking and clustering of citations and reference details, pointed out a cluster linked to the effects of probiotics on many diseases (red clusters in Figure 1), including among others diabetes, liver diseases, cancer, neurological diseases, obesity etc., thus suggesting the interest toward this topic, also stressed by an overview on clinicaltrials.gov. When the search on this database was done (April 2023), there were more than 2000 items, addressing more than 900 conditions, mainly in Europe and United States (Figure 2).
Figure 1.
Clustering and most frequent keywords for the research papers and reviews published in 2022 and 2023 on the effects of probiotics on several disease. Elaboration through the software VosViewer.
Figure 2.
Studies on probiotics on clinicaltrials.gov.
The papers available on PubMed, and Scopus have some common keywords (intestinal flora, gut microbiota, microbiome) and generally postulate that the beneficial effect of probiotics relies upon the modulation of gut microbiota. In addition, another mode of action of probiotic into the gut is connected to the improvement of gut barrier mucosa; in fact, both an eubiotic gut microbiota and probiotics act at the level of signaling pathways, thus they cause an increase of the mucus, an enhanced production of defensins and proteins in the tight junctions (11). Finally, probiotics, could act on the immune systems, through its direct modulation or indirectly acting on gut microbiota.
It has been reported that 70% of immune cells are in the intestine, mainly in the small bowel, where they constitute the gut associated lymphoid tissue (GALT) (11), thus suggesting that gut is the main site of interaction between host immune systems and commensal microorganisms, either positive or pathogenic. Generally, the activation of the immune system is first based on the recognition of PRRs (pattern recognition receptors) by the microbial associated molecular patterns (MAMPs); MAMPs are components of microbial surface able to interact with the gut epithelium and stimulate the cells of the gut immune system at the lamina propria level (11). Therefore, T lymphocytes are activated, and helper T lymphocytes (Th) are differentiated, by favoring pro- or anti-inflammatory cytokines production (11).
Generally, an eubiotic gut microbiota and probiotics positively affect both host’s innate and adaptive immunity (12); concerning innate immunity, gut microbiota acts both locally and systemically, by influencing the development and function of antigen presenting cells (APCs), neutrophils and other innate cell types (12). Moreover, it has been reported the ability of gut microbiota and of some probiotics to affect innate immunity outside the gut milieu, for example by promoting the attenuation of inflammation processes at local levels (13, 14). There is also a role on adaptive immunity, due to the effect in the development of the most important subtypes of CD4+ T cells (or helper T cells, which are lymphocytes coordinating the response to diseases), that is Th1, Th2, Th17 and Treg (12, 15). In addition to T cells, an eubiotic gut microbiota could influence B cell maturation and immunoglobulin production (16).
The mechanisms by which gut microbiota and probiotics influence immune system include the production of various compounds; SCFA (short chain fatty acids; butyrate, acetate, formate), indole derivatives, and bile salts are, among others, the most important. An extensive description of the effects of indole derivatives on gut microbiota is in the review of Ye et al. (17); however, it is worth mentioning that indole derivatives, produced by gut microbes and some probiotic strains (e.g., Limosilactobacillus reuteri) through the metabolism of tryptophan are crucial, because they enhance intestinal epithelial cell function by regulating several genes involved in mechanical barrier formation. Moreover, they increase mucin and goblet cell secretion products, responsible of barrier of gut mucosa, and reduce the impact of possible pathogens (17).
SCFA are produced through the fermentation of non-digestible carbohydrates and amino acids in the colon and play a major role in maintaining the barrier function of gut (18). They are absorbed by the colonocytes and used as fuel for the colonic mucosal epithelial cells (19), but at the same time they directly act on gut mucosa; for example, butyrate contributes to reduce oxidative stress, thus stabilizing gut mucosa and reducing the translocation of LPS (Lipopolysaccharide) (12). Also, bile salts are essential for immunity in a bidirectional crosstalk between host and microbiota. Primary bile salts, or host-derived bile salts, shape and modify the composition of microbiota, generally reducing the levels of Gram-negative bacteria; while those synthesized by microbiota contribute to a further modulation of microbiota itself and act on both innate and adaptive immunity, for example by reducing the levels of pro-inflammatory cytokines, or enhancing Treg cells differentiation (20).
SCFA and derivatives from tryptophan could also play a significant role in reducing inflammatory status. SCFA bind to specific receptors on intestinal epithelial cells, thus they inhibit NF-κB pathway, Treg cell suppression, and pro-inflammatory cytokine production by neutrophils and macrophages (21). For example, butyrate could control gut inflammation through the induction of Treg cell differentiation (22). In addition, tryptophan (deriving from diet) and indolic acid derivatives (for example IPA, indole-3-propionic acid) bind to receptors expressed on immune cells, promote IL-10 production with anti-inflammatory activity and decrease TNF-α release (21).
It is worth mentioning that the ability of potential probiotics to modulate the immune system and ameliorate inflammatory status depend on the strains and a comprehensive overview of the effects at species level is missing (23). Other topics missing in the literature are the technological aspects of the problems (production and dose of probiotics). Therefore, the main goal of this paper is an overview of the effects of probiotics on some representative conditions, addressing some key-points, like the clinical effects, and the mode of action of probiotics, if available; the elucidation of aspects common to all strains of a species, and finally a focus on the importance of a correct dose.
There are many pathological conditions; however, by authors’ choice only research papers and some representative conditions were chosen, as best models for future studies, that is colorectal cancer, neuro-psychiatric diseases, intestinal diseases, obesity, diabetes, metabolic syndrome, which are probably the most addressed topics in the literature, along with two minor issues (immune system, and musculoskeletal system disorders), which are promising ways but with a few evidence.
For each pathological conditions, the effects of probiotics are described, and the list of studies and outcomes is in reported, along with the kind of probiotic, or the probiotics mix, the target of the study (humans or animal model), and the achievable and measurable outcomes (Supplementary Table S1).
2. Colorectal cancer
Colorectal cancer (CRC) is the most frequent neoplastic form of the gastrointestinal tract; its incidence is experiencing a progressive increase, due to a gradual aging of the population, the adoption of sedentary lifestyle, and unbalanced diets (24), as also suggested by the higher incidence rates in Australia and New Zealand, North America, and Europe (25). Although it is a multi-etiological condition, it should be considered the genetic susceptibility of each individual, as well as some environmental factors connected to carcinogenesis, like caloric intake, obesity, alcohol or smoking (26–37). Focusing on gut microbiota, CRC patients often develop a dysbiosis due to the use of antibiotics, radiation therapy, and chemotherapy, and their gut microbiota is characterized by an increased pathogenic bacteria abundance, decreased SCFA-producing bacteria and SCFA levels (38, 39) and butyrate seems the most affected compound, as it could be successfully used as a potential biomarker of CRC risk or as an early warning signal of the disease onset (40). Conversely, high levels of SCFA have antineoplastic properties, due to a combination of several mechanisms, like the downregulation of the canonical Wnt signaling pathway linked to colonic carcinogenesis, the limitation of proliferation and migration of neoplastic cells, the suppression of tumor angiogenesis, the induction of apoptosis and the promotion of neoplastic colonocytes differentiation (40).
Although the production of SCFA probably exerts a major role in the anti-carcinogenic activity of probiotics, there are also some other direct and indirect effects, briefly summarized in Figure 3, including the ability to catch and adsorb carcinogenic compounds, as well as by stimulating host’s antitumor activity through the stabilization of the tight junctions or the production of defensins. Other effects include the antagonistic activity toward putrefactive microbiota and the creation of a microenvironment into the colon unfavorable for the carcinogenesis.
Figure 3.
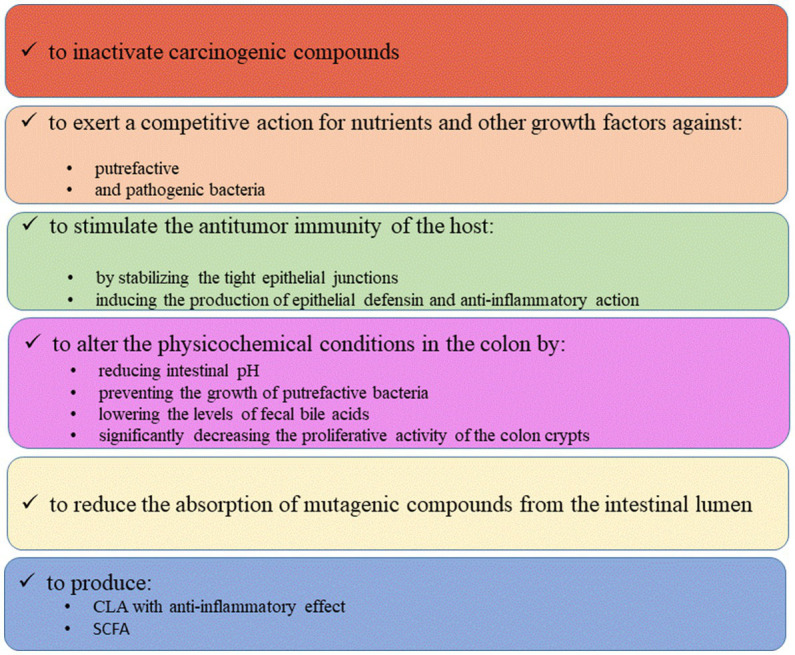
Probiotic effects on CRC.
Many research papers and clinical trials have addressed the role of probiotics in the CRC onset and/or mitigation and a comprehensive overview of the most important trials is in the paper of Hou et al. (40); Supplementary Table S1 shows some relevant studies. In particular, Bacteroides fragilis exerts anti-inflammatory and anticancer effect, as it can alter the composition of the microbiota, inactivating carcinogenic compounds, competing with pathogens or CRC-promoting bacteria and stimulating the immune response (41); similar effects could be observed for Lactobacillus acidophilus MTCC 5401 (42) and Faecalibacterium prausnitzii (43), while Lactococcus lactis subsp. cremoris C60 and Lacticaseibacillus casei ATCC334 probably exerted a preventive and an inhibitory effect on the cells responsible for CRC (44, 45). In addition, an emergent butyrate-producing probiotic, Butyricicoccus pullicaecorum exerted antitumor effect and showed good acid and bile tolerance; it was also able to reduce pathogen population and to prevent necrotic enteritis (41).
Shang et al. (46) demonstrated the effectiveness of a probiotic mix composed of Bifidobacterium longum, Bifidobacterium bifidum, L. acidophilus and Lactiplantibacillus plantarum in mice, able to reduce the tendency of CRC cells to migrate in different body tissues. Furthermore, the tumor size in mice feed with probiotic mixture was significantly smaller than the control group. In another study, Dong et al. (47) investigated Ligilactobacillus salivarius effect on CRC cells, via oral administration in male mice. The authors reported that probiotic induced the suppression of dimethylhydrazine (DMH) production, both in the early and post-early stages of carcinogenesis. DMH is a potent carcinogen used to induce colon cancers in animals, particularly mice. These results therefore suggest that daily oral administration of L. salivarius could effectively prevent CRC carcinogenesis by inhibiting cell proliferation and inducing apoptosis in DMH-induced tumor models.
Probiotics could also counteract dysbiosis occurring in most patients after CRC resection and improve the biodiversity of bacterial biota. In this context, Park et al. (48) observed improvements in postoperative intestinal dysbiosis with the use of probiotics in CRC surgical resection patients. Sixty patients, aged between 18 and 75, with sigmoid colon adenocarcinoma and anterior resection of the same, were divided into two groups: 29 and 31 patients feed with a probiotic mixture (Bifidobacterium animalis subsp. lactis HY8002, L. casei HY2782 and L. plantarum HY7712) and the placebo respectively, for 4 weeks. Probiotics led to an increased production of SCFA by colon bacteria, decreased microbes associated with the development of CRC (mainly Alloprevotella and Porphyromonas) and improved postoperative recovery of patients. Particularly interesting were the data obtained from the measurement of faecal zonulin, a protein that acts on the tight junctions of the intestine, regulating its permeability; high levels are associated with a deterioration of the intestinal mucosa, which does not adequately perform its protective function. The authors found that zonulin significantly decreased in the group fed with probiotic mixture compared to the placebo group.
The efficiency of L. plantarum was also observed by Yoon et al. (49); the authors evaluated the effect of L. plantarum CJLP243 (isolated from kimchi, a traditional fermented product of Korea) on intestinal function and quality of life toward 36 patients aged 20–75, who have undergone rectal resection and were admitted undergoing the reversal of the ileostomy. Unfortunately, a significant number of patients reported symptoms including diarrhea, fecal incontinence, and other complications. The patients were divided into two groups: 19 and 17 patients who took placebo and probiotic respectively, once a day for the duration of 3 weeks. The results showed that there were no significant differences between the two groups regarding the improvement of symptoms; however, by comparing the post-operative results between the first and third weeks, the administration of the probiotic showed a tendency to improve intestinal function and quality of life.
3. Neuro-psychiatric diseases
Many human and animal studies support the idea that gut microbiota plays an important role for cognitive functions, in the regulation of mood and emotions, and in the interpersonal interactions and communications (50). Gut microbiota can modulate brain activity and behavior; therefore, its manipulation can be applied in the treatment of neuropsychiatric disorders such as autism spectrum disorders, depression, etc. (51, 52). The idea that probiotic could positively affect the clinical outcomes of depression was first postulated in 1910 when Hubert J. Norman and Georges Porter Philipps found an improvement in the symptoms after taking lactobacilli (53). Later then, this idea has been confirmed by several studies and clinical trials, although the mode of action of probiotic on behavior and neuro-psychiatric diseases is still unclear, as in some cases symptoms improvement and amelioration are not related to a modification in gut microbiota (53).
Supplementary Table S1 reports 33 scientific articles concerning the effect of probiotics in subjects with neuro-psychiatric diseases. Twelve articles refer to autism (ASD), a neurobiological developmental disorder, characterized by severe and generalized impairment of both communication skills and social interaction. Subjects affected by ASD, especially in children aged 2 to 11 years, show a stereotypical use of movements, language or objects, excessive adherence to routine situations, routines, rituals, and fixation for particular or restricted interests abnormally in duration or intensity (54). The benefits of probiotics depend on the microorganisms. For example, an anti-inflammatory effect was found following the administration of Bifidobacterium spp. (55), while improvement of gastrointestinal disorders and neuro-behavioral symptoms was achieved by microbial mixtures composed of several strains of Bifidobacterium, Lactobacillus, Streptococcus genera as well as L. plantarum PS128, Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG (56–62). In particular, the effectiveness of L. plantarum PS128 relied upon the age of the children, as the best results were obtained on infants (60).
For anxiety and depression, the outputs showed an improvement in the gut microbiota with a reduction in depressive and anxious behavior (63–65). In particular, Abildgaard et al. (64) proposed a mixture of probiotics (B. bifidum W23, Bifidobacterium lactis W52, L. acidophilus W37, Levilactobacillus brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19, L. lactis W58) as potential treatment strategy in major depressive disorders (MDD) to reduce depressive behavior. Some studies reported improvement in behavioral abnormalities and reduction in the main symptoms of depression in humans, after the administration of strains belonging to the genera Lactobacillus and Bifidobacterium (66–71). Another possible use of probiotics refers to dementia and cognitive deterioration. The intake of Enterococcus faecium together with inulin (72) and Bifidobacterium breve A1 (73) improved learning and memory skills, language, attention and orientation in the elderly people. In addition, some studies on animals showed an improvement in the intestinal barrier and spatial learning through the administration of L. casei LC122, of B. longum BL986 and of Clostridium butyricum (74, 75).
For Parkinson’s disease (PD) Tamtajii et al. (76) and Magistrelli et al. (77) observed that L. acidophilus, B. bifidum, L. reuteri, Limosilactobacillus fermentum and L. salivarius allowed an improvement in MDS-UPDRS (Movement Disorder Society-Unified Parkinson’s Disease Rating Scale) scores and a significant reduction in pro-inflammatory cytokine levels and reactive oxygen species (ROS), with a possible weight of the stage of the disease and sex. In animal models, Barichella et al. (78) showed that the genera Lactobacillus and Bifidobacterium could improve intestinal integrity and reduce anxiety, depression and stress.
Anorexia nervosa (AN) consists of an altered perception of one’s own body, in particular weight. In fact, people who are in this condition try to keep their body weight as low as possible through a strong dietary restriction, inducing vomiting and practicing intense physical activity. AN most frequently affects young women, although recently it has also targeted men; it can often be associated with psychological problems such as depression, anxiety, low self-esteem, alcohol abuse, and self-harm (79, 80). L. plantarum P8 determined a reduction in anxiety and stress (81) while B. fragilis reduced gastro-intestinal pains and caused as a secondary effect an increase serotonin production (82); it is not clear if these effects have a connection or are independent outcomes (Supplementary Table S1). In animals, Lactobacillus spp. promoted weight gain (83) and improved the behavioral abnormalities in stressed mice involving the microbiota-brain gut axis (84). Moreover, Akkermansia muciniphila, considered a potential candidate for improving metabolic disorders associated with anorexia, obesity, diabetes, liver disease, favored the restoration of a compromised intestinal barrier (85).
Probiotics were also studied in relation to the benefits they bring for other diseases affecting the brain systems. For example, the administration of L. acidophilus, B. bifidum and B. longum, improved the cognitive function of Alzheimer’s patients (humans and in animals) (86, 87), while strains of L. rhamnosus GG and B. animalis subsp. lactis Bb12 led to an improvement of the symptoms related to schizophrenia (such as delirium, hallucinations, language, and disorganized behavior, etc.) (88). Furthermore, in women aged 20–40 affected by multiple sclerosis, a mixture of probiotics (Lacticaseibacillus paracasei, L. plantarum, L. acidophilus, L. delbrueckii, B. longum, Bifidobacterium infantis, B. breve, Streptococcus thermophilus) improved the symptoms by modulating the anti-inflammatory immune response (89). Referring to multiple sclerosis, Altieri et al. (90) in a recent review described how microbiota change in MS patients and proposed probiotics as useful tools to improve the symptoms of MS patients.
4. Intestinal diseases
Generally, probiotics could positively impact on gastrointestinal disorders (GI) (abdominal pain or discomfort, swelling and flatulence) through metabolic effects resulting from enzymatic activity and the crosstalk with the central nervous system, by improving gut function (91). In addition, there are several evidence on positive effects on Inflammatory Bowel Disease (IBD) and Irritable Bowel Syndrome (IBS).
Concerning IBD, Ferreira-Halder et al. (43) and Lopetuso et al. (92) highlighted the anti-inflammatory effect performed by F. prausnitzii and A. muciniphila. F. prausnitzii contributes substantially to the health of the intestine and is considered a biomarker not only for human health but also for diagnosis and subsequent treatment (43). On the other hand, A. muciniphila has been shown to be effective in immune and metabolic regulation; it ensures increased function of the intestinal barrier showing a direct and beneficial effect on the host’s response. In addition, its use is considered safe if aimed at human studies (93).
In patients with ulcerative colitis, probiotics act as a barrier against harmful microorganisms. A consortium of 8 probiotic strains (VSL3, composed of L. casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, B. longum subsp. longum, B. breve and B. longum subsp. infantis, Streptococcus salivarius subsp. thermophilus) was effective in maintaining a state of remission (94), while Azad et al. (95) reported that Lb. acidophilus restored the balance of inflammatory cytokines and Th17/Treg cells in mice induced colitis, and showed beneficial effects in the prevention of cancer and intestinal inflammation (95).
In addition, several analyses have shown the effectiveness of the administration of probiotics in premature infants, with a reduction of both the development of enterocolitis and the risk of sepsis in old age. In particular, Dermyshi et al. (96) supported the benefits of L. acidophilus-B. infantis blend.
IBS causes swelling, vomiting, diarrhea, abdominal pain, frequency of stools, and probiotics could improve these symptoms. Two formulations containing different probiotic strains (F1 = L. acidophilus, L. reuteri; F2 = L. plantarum, L. rhamnosus, B. animalis subsp. lactis) were administered to humans, thus gaining a relief in bloating, abdominal pain, constipation, abdominal cramps, and flatulence (97). Similar effects were observed through the administration of Bacillus coagulans MTCC 5856 (98), and L. plantarum DSM 9843 (99). Other studies reported the improvement of IBS symptoms due to several lactobacilli (100, 101).
5. Obesity
Gut microbiota is involved in the control of body weight, energy homeostasis and inflammation states; therefore, it plays an important role in the pathophysiology of obesity. Firmicutes and Bacteroidetes are the two phyla involved in microbial dysbiosis and in the development of obesity. The ratio between these phyla is very important; in fact, Bervoets et al. (102) studied the gut microbiota of 26 overweight and obese children and 27 skinny children and found that obese children have a higher ratio of Firmicutes to Bacteroidetes.
Supplementary Table S1 focuses on some application of probiotics toward overweight and obese subjects. Kadooka et al. (103) administered fermented milk containing Lactobacillus gasseri SBT2055 (200 g/day) to 87 overweight adults for 12 weeks. Reductions in visceral and subcutaneous fat, body weight and BMI (Body Mass Index) compared to the control group, were observed. Furthermore, the consumption of yogurts supplemented with capsules, containing 109 CFU of Lactobacillus amylovorus and L. fermentum by 28 overweight participants, led to a reduction in total body fat mass (104). Regarding gut microbiota, the researchers observed a significant reduction of Clostridium cluster IV (for L. amylovorus consumption), together with an increase of Lactobacillus in both treatments and concluded that when the gut microbial composition is modulated through probiotic consumption, this can positively alter energy metabolism and body composition (104). An additional study on 70 overweight and obese children revealed that a combination of probiotics, prebiotics and vitamins A, E and C for 8 weeks, significantly reduced BMI, waist circumference, waist/hip ratio, LDL cholesterol and triglycerides (105).
Probiotics can reduce cholesterol levels through bile salt hydrolase (an enzyme that hydrolyzes bile salts into amino acid residues and free bile salts). 200 g/day of yogurt containing S. thermophilus, L. delbrueckii subsp. bulgaricus, L. acidophilus LA-5, and B. animalis BB12 for 9 weeks to 70 women in the third trimester of pregnancy resulted in significant reductions in total cholesterol, LDL cholesterol, and high-density lipoprotein (HDL) cholesterol, as well as serum triglyceride concentrations (106). Probiotic supplementation also reduced blood lipid concentrations (107).
A. muciniphila administered to animals led to a reduction in fat mass and body weight; moreover, it favored the restoration of the intestinal barrier function and, if administered to humans, improved inflammation, insulin resistance and blood sugar level (108).
Many authors reported that the action of probiotic toward obesity is mediated by SCFA, which probably could be involved in body weight regulation, and maintenance, as well as in energy intake and expenditure (109–111). Although there are several hypotheses, the most probable mechanism involves the ability of propionate and butyrate to bind to G-protein-coupled receptors in the colon leading to the production of the gut hormones peptide YY and glucagon-like peptide 1, thus influencing satiety and glucose homeostasis (109). In addition, SCFA activate intestinal gluconeogenesis, and the released glucose mediates a signal to brain through portal nerves for satiety and insulin sensitivity, or they can also affect peripheral metabolism in the liver (enhanced lipid oxidation, lower lipid storage), skeletal muscles (increase of glycogen synthesis and reduction of glycolysis), pancreas (increase of insulin and reduction of glucagon synthesis and release) or adipose tissue (reduction of insulin mediated adiposity) (109, 110). The evidence available in the literature suggest that that increasing SCFA production could be a preventive measure to counteract gastro-intestinal dysfunction, obesity, and type 2 diabetes mellitus (109, 110), although longer term trials and data are required, also to elucidate the exact role of the initial imprinting of gut microbiota and how it can respond to probiotic intervention.
6. Diabetes
Generally systemic inflammation involve microbiota as it modulates inflammation, interacts with nutrients, influences intestinal permeability, glucose and lipid metabolism, insulin sensitivity and the body’s energy balance. The microbiota of diabetic patients is poorly populated by useful microorganisms (Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia) which have anti-inflammatory activity, are butyrate-producing and are promoters of low intestinal permeability and may have inhibitory activity against carbohydrates-degrading enzymes, reducing postprandial hyperglycemia. On the contrary, there are many microorganisms favoring the production of inflammatory molecules and the alteration of intestinal permeability such as Ruminococcus, Fusobacterium, and Blautia (112, 113). In any case, considering that diabetes is closely linked to food choices and habits, it is certainly essential to make adequate decisions in this regard; for example, an active lifestyle could improve insulin resistance, while taking foods rich in fibers, largely represented by prebiotics, is certainly a positive choice for wise prevention.
Positive effects such as increased insulin sensitivity and improvement of microbial diversity were found following administration of L. reuteri DSM 17938 to patients with type 2 diabetes (114).
Toejing et al. (115) administered L. paracasei HII01 (50 × 109 CFU/day) to 50 T2DM (type 2 diabetes mellitus) patients to evaluate the effect on glycemia and observed that after 12 weeks fasting blood glucose (FBG) level significantly decreased. Furthermore, probiotics reduced the plasma levels of lipopolysaccharide (LPS), inflammatory markers (TNF-α, IL-6) and C-reactive protein (hsCRP). A reduction in pathogenic microorganisms together with improvement in beneficial bacteria were also observed; therefore, the authors concluded that L. paracasei HII01 could play a potential role as an adjuvant treatment in type 2 diabetes.
A potential antidiabetic effect was also observed by using another Lactobacillus strain: Wu et al. (116) investigated the performances of L. rhamnosus LRa05 on glucose metabolism and gut microbiota in T2DM mice. The treatment with 109 CFU/day of L. rhamnosus resulted in a reduction in the fasting blood glucose (FBG) levels (by 53.5%), lowered insulin resistance, alleviated metabolic lipopolysaccharide-related inflammation and relieved hepatic oxidative stress. Further positive effects were found on the gut microbiota composition; in fact, SCFA producing microorganisms, such as Alloprevotella and Bacteroides, increased with a reduction of proinflammatory microorganisms such as Odoribacter and Mucispirillum (116).
Manaer et al. (117) reported the benefits of Lactobacillus and yeasts on T2DM mice. Probiotics (Lactobacillus kefiranofaciens, L. plantarum, Lactobacillus helveticus, L. lactis, Issatchenkia orientalis), isolated from traditional fermented cheese whey (TFCW), were used to prepare a mix from camel milk (CPCM) to feed db/db mice. The authors studied how these strains affect gut microbiota, glucose and lipid metabolism, liver and renal functions. CPCM reduced fasting blood glucose (FBG), oral glucose tolerance test and glycosylated hemoglobin HbAlc, increased C-Protein, modulated lipid metabolism and improved liver. Finally, CPCM increased LAB and Bifidobacterium population in intestinal tract and decreased Escherichia.
Razmpoosh et al. (118) evaluated the effect of 7 probiotics (L. acidophilus, L. casei, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus), and 100 mg of fructo-oligosaccharide (FOS) with lactose as carriers, on lipid profile and glycemic control in 60 patients. They were equally divided into 2 groups (group 1 took probiotics and group 2 took a placebo, for 6 weeks). A significant decrease in the fasting plasma glucose (FPG) and increase of high density of lipoprotein cholesterol (HDL-C), was observed. No significant differences in the levels of insulin, triglycerides, total cholesterol, insulin resistance and anthropometric measurements (weight, waist circumference and body mass index).
7. Metabolic syndrome
Metabolic syndrome (MetS) is a pathology characterized by an excess in abdominal fat, arterial hypertension, impaired fasting plasma glucose (FPG) or insulin resistance, whose diagnoses and treatments are often similar to those of obesity (119). Supplementary Table S1 lists 6 papers concerning the study of the effect of some probiotics in subjects with MetS.
Corb Aron et al. (108) and Ottman et al. (93) used A. muciniphila to evaluate its effect on volunteers with MetS. They observed that the probiotic degrades mucin by stimulating the production of new mucous layer (108) and contributes to immune and metabolic regulation by increasing the intestinal barrier (93). At the same time, the metabolic activity of A. muciniphila led to the production of SCFA with beneficial effect to the host and members of the microbiota (93).
Instead L. plantarum (120), L. acidophilus and some Bifidobacterium species (B. bifidum, B. lactis, and B. longum) (121) mainly led to a reduction in blood sugar and cholesterol. In particular, reduction in LDL cholesterol, blood glucose, and homocysteine levels when postmenopausal women were treated with L. plantarum for 90 days (120).
8. Musculoskeletal system
The role of probiotics in the control of musculoskeletal diseases is a topic of great interest; osteoporosis (characterized by a decrease in bone strength, a low mineral density of the bone tissue, with consequent fragility and aging) (122), osteoarthritis (a non-inflammatory arthropathy involving cartilage and bone remodeling) or bone fragility, and microbiota changes are closely related (123).
It has been demonstrated that the synergistic action of L. casei with type II collagen (CII) and glucosamine (GS) (potential prebiotic), administrated to arthritic rats, led to an effective reduction of pain and cartilage destruction. Moreover, a reduced expression of numerous proinflammatory cytokines, resulted (124).
Supplementary Table S1 reports some cases concerning the use of different Lactobacillus strains to relieve bone, joint and muscle disorders. The ability of probiotics to reduce pain and cartilage destruction has been highlighted in experiments conducted on animals (125) together with numerous effects, such as antimicrobial, antioxidant, anti-inflammatory (126), the ability to determine an increase in calcium (127) and recovery of joint strength (128) in humans.
Steves et al. (125) and Paul et al. (126) demonstrated that L. casei and L. acidophilus improved intestinal dysbiosis and the symptoms of rheumatoid arthritis after long-term repeated use thanks to their anti-inflammatory, antimicrobial and antioxidant properties. These microorganisms act symbiotically in the intestine to establish their colonization and consequently increase the integrity of the cell layers of the gastro-intestinal tract, maintain the nutritional support of the host and reduce the severity of inflammatory conditions.
9. Immune system disorders
It is known that probiotics can also bring benefits through the modulation of the immune system. Supplementary Table S1 shows 3 articles focused on the effect of probiotics on the modulation of the immune system. Among the most significant results, there are the bactericidal and antitumor effect with production of proinflammatory and anti-inflammatory cytokines in humans, by E. faecium (95) and the development of regulatory cells in the gastrointestinal epithelium in animals, by strains of L. reuteri (99). Finally, Han et al. (129) treated mice with L. rhamnosus HDB1258 and observed that it enhanced the immune response by activating innate immunity. In addition, L. rhamnosus suppressed systemic inflammation by increasing the expression ratio of anti-inflammatory cytokines and modulated the microbiota composition.
10. Probiotic species, dose, delivery, and production
This review shows that there are significant effects of probiotics on a wide variety of conditions; moreover, a focus at genus/species level on research papers with a robust design beyond and with proven effects (ca. 160) suggests the efficacy of lactobacilli (L. plantarum, L. casei, L. acidophilus, L. reuteri, among others) and bifidobacteria (B. longum, B. infantis, B. animalis, B. bifidum or B. breve), with promising evidence for a new generation of probiotics (mainly A. muciniphila, B. fragilis, and F. prausnitzii; Figure 4). Apart from species, the identification of the dose required to gain a measurable output is controversial. Many probiotic supplements contain 1 to 10 billion CFU per dose, up to 50 billion CFU or more; however, higher CFU counts do not necessarily improve health effects. In fact, depending on the disorder, it may happen that even a lower dose can be effective or even better than a higher dose (130). Supplementary Table S1 shows the doses, when available, for the different trials; generally, the concentrations for the most important commercial preparations of Lactobacillus spp. and Lactobacillus related genera are from 109 to 1010 CFU, while for Bifidobacterium spp. at 108–1010 CFU, for Pediococcus acidilactici 109 CFU, for Streptococcus thermophilus 108 CFU, for yeast strains such as Saccharomyces boulardii 109 CFU, Bacillus subtilis 109 CFU and A. muciniphila 108 CFU (131). It is worth mentioning that the dose is also a function of storage conditions, as some preparations should be stored at room temperature, while others require refrigeration; therefore, a thermal abuse could heavily affect probiotic survival. The International Scientific Association for Probiotics and Prebiotics advises manufacturers to list expected probiotic concentration on the “expiration” or “use by” date on the product label when stored at proper conditions and suggests consumers to avoid preparations listing the dose of probiotic at the time of production (132).
Figure 4.
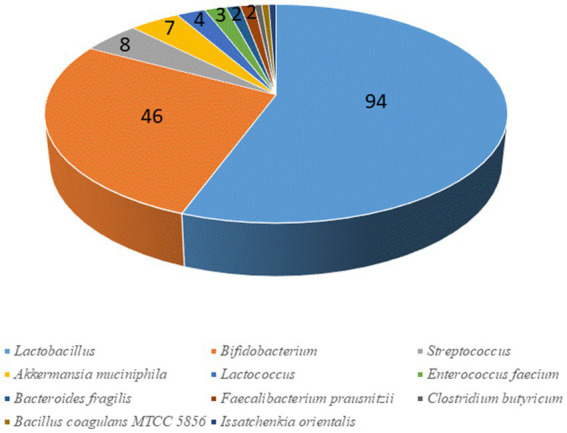
Probiotic genera mostly used in clinical trials.
Strictly linked to the dose, the second critical point is the duration of supplementation, but for this aspect there is not a consensus in the literature; generally, it is believed that probiotics should be assumed for several weeks (at least from 2 to 4 weeks) to gain achievable outputs (133). However, the supplementation could be either short-term or long-term, with short-term interventions suggested only for acute gastro-intestinal conditions (5–7 days for acute diarrhea in infants and children, from 1 to 4 weeks for antibiotic-associated diarrhea, a few weeks for constipation) (133, 134), while other conditions require long-term supplementation, up to 2–3 months for IBD, 3–6 months for Chron disease, atopic dermatitis, or psychiatric diseases (133–136).
Another critical point is the delivery. Probiotics are marketed in different forms such as capsules, tablets, films, or hydrogels, and for oral delivery the microencapsulation in hydroxypropyl methylcellulose phthalate (HPMCP), hydroxypropylmethyl cellulose acetated succinate, and cellulose acetate phthalate (CAP) is used to minimize the exposure of probiotics to gastric acids, reducing their viability loss in the stomach (137). It is a matter of debate if oral delivery mediated by foods could result in a higher impact of probiotics (138), while other ways of delivery, less used at least for the studies reported in this review, are nasal, transdermal, rectal, and vaginal (137).
Also, production could affect viability and thus health effects of probiotics; fermentation is the most common method of producing commercial probiotics: in a large fermentation vessel, single-strain probiotics are inoculated into a liquid broth that is stirred to prevent bacterial settlement and with pH kept under control. When the production concerns anaerobic species, gasses such as nitrogen, hydrogen, and carbon dioxide, are controlled. Microbial growth is controlled by cell density measurements and light/fluorescence microscopes are used to check for unwanted contaminations. Once batch fermentation is complete, a filtered and concentrated cells suspension is either spray-dried or freeze-dried but previously, cryoprotectants or lyoprotectants are added to prevent loss of microbial viability (131).
To increase the production rate the batch fermentation is integrated with crossflow membrane ultra/microfiltration; when the desired cell density is reached, toxic metabolites and/or acids are removed through a membrane. Fresh medium is continuously pumped into the fermenter by varying flow rates to ensure a constant total volume. The cell suspension can then be extracted in batches or continuously (131).
Another effective method to enhance the production of probiotics is the immobilization in natural biopolymers such as protein-based biopolymers, polysaccharides, lipids, and synthetic polymers or coating for the protection of probiotics against moistures or gasses (oxygen/carbon dioxide) (131). Cells are immobilized in polysaccharide hydrogels, then placed in a fermenter, where the medium is regularly supplemented, and the cells periodically removed to ensure proper dilution. This strategy is used to improve overall growth rate and cell viability. The benefits of this approach are the continuous and controlled delivery of probiotics to the gut, a higher viability, and lower costs, while the some limits are the restricted biocompatibility of some immobilization agents, and the complexity of production processes (131).
11. Conclusions and perspectives
The use of probiotics could be a promising strategy to counteract side or secondary effects in several pathological conditions; the evidence and data hereby reported suggest a benefit in CRC both as a preventive measure to avoid carcinogenesis or during medical treatments to favor recovery, or in improving cognitive functions, in ameliorating the symptoms of some intestinal diseases (e.g., IBD), or to counteract obesity, diabetes and other metabolic syndromes. The effect is generally mediated through the modulation of gut microbiota, as well as on the production of significant amounts of SCFA, which exert in turn several physiological functions, and the final output could be symptoms amelioration or disease remission, although the use of different clinical outcomes is a challenge, as it makes difficult a comparison of different trials and research papers.
At species level, most data are available on Lactobacillaceae and on Bifidobacterium spp., even if evidence is available for A. muciniphila, B. fragilis, and F. prausnitzii. However, there are some issues that should be addressed, related to the duration of the supplementation (short-term or long-term), dose, as each study suggests a different dose (ranging from 108 to 1010 CFU). Concerning the way of supplementation, oral delivery is preferred, but there is still a debate on the usefulness of a supplementation through food.
Moreover, most papers focus on the medical point of view, while there is a dark side not addressed, that is the technological story connected to probiotic productions, the way of supplementation (with food or as supplements), the shelf life, and the dose at the time of consumptions, among others. Further efforts are required to address both medical issues and technological/microbiological challenges for an effective use of probiotics as concurrent strategies for many pathological conditions; there are promising evidence and data, but we are still at a preliminary level, as an effective and efficient use of probiotics should be based on the clear definition of a “before” (dose, storage, way of supplementation, duration etc.) and an “after” (outputs clearly evidenced and defined).
Author contributions
MC, DC, and AB: conceptualization. MS, BS, and AB: methodology. AR, DC, and AB: investigation and data. AB and DC: writing–original draft preparation. DC, AB, BS, AR, MS, and MC: writing–review and editing. MC: supervision. MS and MC: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1209238/full#supplementary-material
References
- 1.Lederberg J. McCray AT ‘Ome sweet’ omics. A genealogical treasury of words. Scientist. (2001) 15:8. [Google Scholar]
- 2.Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, Chang MW. Microbiome and human health: Current understanding, engineering, and enabling technologies. Chem Rev. (2023) 123:31–72. doi: 10.1021/acs.chemrev.2c00431, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirscfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut. (2016) 65:330–9. doi: 10.1136/gutjnl-2015-309990, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capurso L. Il Microbiota Intestinale. Recenti Prog Med. (2016) 107:257–66. doi: 10.1701/2296.24680, PMID: [DOI] [PubMed] [Google Scholar]
- 5.De Siena M, Laterza L, Matteo MV, Mignini I, Schepis T, Rizzatti G, et al. Gut and Reproductive Tract Microbiota Adaptation during Pregnancy: New Insights for Pregnancy-Related Complications and Therapy. Microorganisms. (2021) 9:1–15. doi: 10.3390/microorganisms9030473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marangoni F, Poli A. Microbiota intestinale; salute umana e prebiotici. Milano: Pacini ED; (2017). [Google Scholar]
- 7.Campaniello D, Corbo MR, Sinigaglia M, Speranza B, Racioppo A, Altieri C, et al. How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients. (2022) 14:2456. doi: 10.3390/nu14122456, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FAO/WHO . Food and Agriculture Organization of the United Nations/World Health Organization. Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Geneva: World Health Organization; (2001). [Google Scholar]
- 9.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Gasbarrini A, Laterza L. La microbiota revolution: nuove conoscenze sul ruolo del microbiota intestinale e possibili scenari nell’uso dei probiotici In: Marangoni F, Restani P, Morelli L, Gasbarrini A, Laterza L, Careddu D, editors. Review sull’integrazione alimentare: evidenza dalla ricerca scientifica e nuove frontiere di sviluppo. 2nd ed. Milano: Edra; (2019). 44–8. [Google Scholar]
- 11.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. (2014) 44:1–8. doi: 10.1016/j.medmal.2013.10.002, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Choden T, Cohen NA. The gut microbiome and the immune system. Expl Med. (2022) 3:219–33. doi: 10.37349/emed.2022.00087 [DOI] [Google Scholar]
- 13.Wu X, Tian J, Wang S. Insight into non-pathogenic Th17 cells in autoimmune diseases. Front Immunol. (2018) 9:1112. doi: 10.3389/fimmu.2018.01112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohkubo T, Tsuda M, Suzuki S, El Borai N, Yamamura M. Peripheral blood neutrophils of germ-free rats modified by in vivo granulocyte-colony-stimulating factor and exposure to natural environment. Scand J Immunol. (1999) 49:73–7. doi: 10.1046/j.1365-3083.1999.00456.x, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Qian LJ, Kang SM, Xie JL, Huang L, Wen Q, Fan YY, et al. Early-life gut microbial colonization shapes Th1/Th2 balance in asthma model in BALB/c mice. BMC Microbiol. (2017) 17:135. doi: 10.1186/s12866-017-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. Lancet. (2022) 82:104163. doi: 10.1016/j.ebiom.2022.104163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, et al. Dual role of indoles derived from intestinal microbiota on human health. Front Immunol. (2022) 13:903526. doi: 10.3389/fimmu.2022.903526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. doi: 10.1080/19490976.2015.1134082, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvi PS, Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. (2021) 10:1775. doi: 10.3390/cells10071775, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godlewska U, Burlanda E, Wypych TP. Bile acids in immunity: bidirectional mediators between the host and the microbiota. Front Immunol. (2022) 13:949033. doi: 10.3389/fimmu.2022.949033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of Interleukin-10 receptor. Am J Pathol. (2018) 188:1183–94. doi: 10.1016/j.ajpath.2018.01.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrlich AM, Pacheco AR, Henrick BM, Taft D, Xu G, Huda MN, et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. (2020) 20:357. doi: 10.1186/s12866-020-02023-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ménard O, Butel MJ, Gaboriau-Routhiau V, Waligora-Dupriet AJ. Gnotobiotic mouse immune response induced by Bifidobacterium sp. strains isolated from infants. Appl Environ Microbiol. (2008) 74:660–6. doi: 10.1128/AEM.01261-07, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponz de Leon M, Rossi G, di Gregorio C, De Gaetani C, Rossi F, Ponti G, et al. Epidemiology of colorectal cancer: the 21-year of expirience of a specialised registry. Intern Emerg Med. (2007) 2:269–79. doi: 10.1007/s11739-007-0077-z, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Dionigi R. Chirurgia - Basi teoriche e Chirurgia generale - Chirurgia specialistica. Milano: Masson; (2019). [Google Scholar]
- 26.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol. (2019) 14:89–103. doi: 10.5114/pg.2018.81072, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grancher A, Michel P, Di Fiore F, Sefrioui D. Aspirin and colorectal cancer. Bull Cancer. (2018) 105:171–80. doi: 10.1016/j.bulcan.2017.09.013, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Alcoholado SC, Ordóñez R, Otero A, Plaza-Andrade I, Laborda-Illanes A, Medina JA, et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal cancer. Int J Mol. (2020) 21:6782. doi: 10.3390/ijms21186782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan SY, DesMeules M. Energy intake, physical activity, energy balance, and cancer: epidemiologic evidence. Methods Mol Biol. (2009) 472:191–215. doi: 10.1007/978-1-60327-492-0_8 [DOI] [PubMed] [Google Scholar]
- 30.Balhareth A, Aldossary MY, McNamara D. Impact of physical activity and diet on colorectal cancer survivors’ quality of life: a systematic review. World J Surg Oncol. (2019) 17:17–153. doi: 10.1186/s12957-019-1697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. (2006) 55:285–91. doi: 10.1136/gut.2005.073163, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minkyeong K, Kyong P. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. (2018) 10:1963. doi: 10.3390/nu10121963, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenders M, Siersema PD, Overvad K, Tjønneland A, Olsen A, Boutron-Ruault MC, et al. Subtypes of fruit and vegetables, variety in consumption and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2015) 137:2705–14. doi: 10.1002/ijc.29640, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Zhang SM, Moore SC, Lin J, Cook NR, Manson J, Lee I-M, et al. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. (2006) 15:108–15. doi: 10.1093/aje/kwj016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S-Y, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. (2019) 1:67–76. doi: 10.1093/aje/kwy208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botteri E, Borroni E, Sloan EK, Bagnardi V, Bosetti C, Peveri G, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: A meta-analysis. Am J Gastroenterol. (2020) 12:1940–9. doi: 10.14309/ajg.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 37.Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers. (2013) 5:676–713. doi: 10.3390/cancers5020676, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. (2012) 6:320–9. doi: 10.1038/ismej.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germfree and conventional mice. Gastroenterol. (2017) 153:1621–33. doi: 10.1053/j.gastro.2017.08.022, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Hou H, Chen D, Zhang K, Zhang W, Liu T, Wang S, et al. Gut microbiota derived short-fatty acids and colorectal cancer: ready for clinical transition. Cancer Lett. (2022) 526:225–35. doi: 10.1016/j.canlet.2021.11.027, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Torres-Maravilla E, Boucard AS, Mohseni AH, Taghinezhad SS, Cortes-Perez NG, Bermudez-Humaran LG. Role of gut microbiota and probiotics in Colorectal Cancer: Onset and progression. Microorganisms. (2021) 9:1021. doi: 10.3390/microorganisms9051021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deol PK, Khare P, Bishnoi M, Kondepudi KK, Kaur IP. Coadministration of ginger extract–Lactobacillus acidophilus (cobiotic) reduces gut inflammation and oxidative stress via downregulation of COX-2, i-NOS, and c-Myc. Phytother Res. (2018) 32:1950–6. doi: 10.1002/ptr.6121, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Ferreira-Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Prat Res Cl Ga. (2017) 31:643–8. doi: 10.1016/j.bpg.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Kakizaki N, Okuno A, Maekawa T, Tsuji NM. Lactococcus lactis subsp. cremoris C60 restores T cell population in small intestinal lamina propria in aged Interleukin-18 deficient mice. Nutrients. (2020) 12:3287. doi: 10.3390/nu12113287, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwama T, Fujiva M, Konishi H, Tanaka H, Murakamu Y, Kuonogi T, et al. Bacteria-derived ferrichrome inhibits tumor progression in sporadic colorectal neoplasms and colitis-associated cancer. Cancer Cell Int. (2021) 21:21. doi: 10.1186/s12935-020-01723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang F, Jiang X, Wang H, Chen S, Wang X, Liu Y, et al. The inhibitory effects of probiotics on colon cancer cells: in vitro and in vivo studies. J Gastrointest Oncol. (2020) 11:1224–32. doi: 10.21037/jgo-20-573, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y, Zhu J, Zhang M, Ge S, Zhao L. Probiotic Lactobacillus salivarius Ren prevent dimethylhydrazine-induced colorectal cancer through protein kinase B inhibition. Appl Microbiol Biotechnol. (2020) 104:7377–89. doi: 10.1007/s00253-020-10775-w, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Park IJ, Lee JH, Kye BH, Oh HK, Cho YB, Kim YT, et al. Effects of PrObiotics on the symptoms and Surgical ouTComes after Anterior REsection of colon cancer (POSTCARE): A randomized, double-blind, placebo-controlled trial. J Clin Med. (2020) 9:2181. doi: 10.3390/jcm9072181, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon BJ, Oh HK, Lee J, Cho JR, Kim MJ, Kim DW, et al. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: a randomized controlled trial. Color Dis. (2021) 23:901–10. doi: 10.1111/codi.15463, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Sarkar A, Harty S, Lehto SM, Moeller AH, Dinan TG, Dunbar RIM, et al. The microbiome in psychology and cognitive neuroscience. Trends Cogn Sci. (2018) 22:611–36. doi: 10.1016/j.tics.2018.04.006, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Kelly JR, Borre Y, Obrien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Accettulli A, Corbo MR, Sinigaglia M, Speranza B, Campaniello D, Racioppo A, et al. Psycho-Microbiology, a new frontier for probiotics: An exploratory overview. Microorganisms. (2022) 10:2141. doi: 10.3390/microorganisms10112141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieërs G, Belkhir L, Enaud R, Leclercq S, De Foy JMP, Dequenne I, et al. How probiotics affect the microbiota. Front Cell Infect Microbiol. (2020) 9:454. doi: 10.3389/fcimb.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimaldi R, Gibson G, Vulevic J, Giallourou N, Castro-Mejía J, Hansen L, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. (2018) 6:1–13. doi: 10.1186/s40168-018-0523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fattorusso A, Di Genova L, Dell’Isola GB, Mencaroni E, Esposito S. Autism spectrum disorders and the gut microbiota. Nutrients. (2019) 11:521. doi: 10.3390/nu11030521, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grossi E, Melli S, Dunca D, Terruzzi V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med Case Rep. (2016) 4:1–5. doi: 10.1177/2050313X16666231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, et al. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr Neurosci. (2018) 21:676–81. doi: 10.1080/1028415X.2017.1347746 [DOI] [PubMed] [Google Scholar]
- 58.Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. (2019) 14:e0210064. doi: 10.1371/journal.pone.0210064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duque ALRF, Demarqui FM, Santoni MM, Zanelli CF, Adorno MAT, Dragan M, et al. Effect of probiotic, prebiotic, and synbiotic on the gut microbiota of autistic children using an in vitro gut microbiome model. Food Res Int. (2021) 149:110657. doi: 10.1016/j.foodres.2021.110657, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Liu YW, Liong MT, Chung YE, Huang HY, Peng WS, Cheng YF, et al. Effects of Lactobacillus plantarum PS128 on children with autism spectrum disorder in Taiwan: a randomized, double-blind, placebo-controlled trial. Nutrients. (2019) 11:820. doi: 10.3390/nu11040820, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong XJ, Liu J, Li J, Kwong K, Koh M, Sukijthamapan P, et al. Probiotics and oxytocin nasal spray as neuro-social-behavioral interventions for patients with autism spectrum disorders: a pilot randomized controlled trial protocol. Pilot Feasibility Stud. (2020) 6:20. doi: 10.1186/s40814-020-0557-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr Res. (2015) 77:823–8. doi: 10.1038/pr.2015.51, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Cheng R, Xu W, Wang J, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem Biophys Res Commun. (2021) 566:170–6. doi: 10.1016/j.bbrc.2021.06.018, PMID: [DOI] [PubMed] [Google Scholar]
- 64.Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. (2017) 79:40–8. doi: 10.1016/j.psyneuen.2017.02.014, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Reis DJ, Ilardi SS, Punt SEW. The anxiolytic effect of probiotics: a systematic review and meta-analysis of the clinical and preclinical literature. PLoS One. (2018) 13:e0199041. doi: 10.1371/journal.pone.0199041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann General Psychiatry. (2017) 16:14. doi: 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghodarz Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. (2016) 32:315–20. doi: 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 68.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. (2019) 102:13–23. doi: 10.1016/j.neubiorev.2019.03.023, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr. (2019) 38:522–8. doi: 10.1016/j.clnu.2018.04.010, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. (2021) 76:32–40. doi: 10.1093/gerona/glaa090, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma T, Jin H, Kwol LY, Sun Z, Liong MT, Zhang H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol Stress. (2021) 14:100294. doi: 10.1016/j.ynstr.2021.100294, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romo-Araiza A, Gutierrez-Salmean G, Galvan EJ, Hernandez-Frausto M, Herrera- Lopez G, Romo-Parra H, et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci. (2018) 10:416. doi: 10.3389/fnagi.2018.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve a1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. (2019) 10:511–20. doi: 10.3920/BM2018.0170, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, et al. Lactobacillus and Bifidobacterium Improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res. (2019) 63:e1900603. doi: 10.1002/mnfr.201900603, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. (2015) 2015:412946. doi: 10.1155/2015/412946, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. (2019) 38:1031–5. doi: 10.1016/j.clnu.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 77.Magistrelli L, Amoruso A, Mogna L, Graziano T, Cantello R, Pane M, et al. Probiotics may have beneficial effects in Parkinson’s Disease: In vitro evidence. Front Immunol. (2019) 10:969. doi: 10.3389/fimmu.2019.00969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology. (2016) 87:1274–80. doi: 10.1212/WNL.0000000000003127, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Mendez-Figueroa V, Biscaia JM, Mohedano RB, Blanco-Fernandez A, Bailen M, Bressa C, et al. Can gut microbiota and lifestyle help us in the handling of anorexia nervosa patients? Microorganisms. (2019) 7:58. doi: 10.3390/microorganisms7020058, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Achamrah N, Déchelotte P, Coëffier M. New therapeutic approaches to target gut-brain axis dysfunction during anorexia nervosa. Clin Nutr Exp. (2019) 28:33–41. doi: 10.1016/j.yclnex.2019.01.006 [DOI] [Google Scholar]
- 81.Lew LC, Hor YY, Yusoff NAA, Choi SB, Yusoff MSB, Roslan NS, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr. (2018) 38:2053–64. doi: 10.1016/j.clnu.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 82.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cells. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb Pathog. (2017) 106:162–70. doi: 10.1016/j.micpath.2016.11.002, PMID: [DOI] [PubMed] [Google Scholar]
- 84.Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. (2017) 7:43859. doi: 10.1038/srep43859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. (2017) 8:1765. doi: 10.3389/fmicb.2017.01765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rezaeiasl Z, Salami M, Sepehri G. The effects of probiotic Lactobacillus and Bifidobacterium strains on memory and learning behavior, long-term potentiation (LTP), and some biochemical parameters in β-amyloid-induced rat’s model of Alzheimer’s Disease. Prev Nutr Food Sci. (2019) 24:265–73. doi: 10.3746/pnf.2019.24.3.265, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. (2019) 38:2569–75. doi: 10.1016/j.clnu.2018.11.034, PMID: [DOI] [PubMed] [Google Scholar]
- 88.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CLG, Schweinfurth LAB, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. (2014) 16:13m01579. doi: 10.4088/PCC.13m01579, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. (2018) 83:1147–61. doi: 10.1002/ana.25244, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altieri C, Speranza B, Corbo MR, Sinigaglia M, Bevilacqua A. Gut-Microbiota, and multiple sclerosis: Background, evidence, and perspectives. Nutrients. (2023) 15:942. doi: 10.3390/nu15040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eales J, Gibson P, Whorwell P, Kellow J, Yellowlees A, Perry RHJ, et al. Systematic review and meta-analysis: the effects of fermented milk with Bifidobacterium lactis CNCM I-2494 and lactic acid bacteria on gastrointestinal discomfort in the general adult population. Therapeutic Adv Gastroenterol. (2017) 10:74–88. doi: 10.1177/1756283X16670075, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopetuso LR, Quagliarello A, Schiavoni M, Petito V, Russo A, Reddel S, et al. Towards a disease-associated common trait of gut microbiota dysbiosis: the pivotal role of Akkermansia muciniphila. Dig Liver Dis. (2020) 52:1002–10. doi: 10.1016/j.dld.2020.05.020, PMID: [DOI] [PubMed] [Google Scholar]
- 93.Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol. (2017) 31:637–42. doi: 10.1016/j.bpg.2017.10.001, PMID: [DOI] [PubMed] [Google Scholar]
- 94.Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. World J Gastroenterol. (2016) 22:7186–202. doi: 10.3748/wjg.v22.i32.7186, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azad MAK, Sarker M, Li T, Yin J. Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int. (2018) 2018:9478630. doi: 10.1155/2018/9478630, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dermyshi E, Wang Y, Yan C, Hong W, Qiu G, Gong X, et al. The “Golden Age” of probiotics: A systematic review and Meta-Analysis of randomized and observational studies in preterm infants. Neonatology. (2017) 112:9–23. doi: 10.1159/000454668, PMID: [DOI] [PubMed] [Google Scholar]
- 97.Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, et al. A randomized, double-blind, placebo-controlled trial: The efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. Biomed Res Int. (2016) 2016:4740907. doi: 10.1155/2016/4740907, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Majeed M, Nagabhushanam K, Natarajan S, Sivakumar A, Ali F, Pande A, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: A double blind randomized placebo-controlled pilot clinical study. Nutr J. (2016) 15:21. doi: 10.1186/s12937-016-0140-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lyra A, Hillila M, Huttunen T, Mannikko S, Taalikka M, Tennila J, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. (2016) 22:10631–42. doi: 10.3748/wjg.v22.i48.10631, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome–A 12 week double-blind study. Aliment Pharmacol Ther. (2014) 40:51–62. doi: 10.1111/apt.12787, PMID: [DOI] [PubMed] [Google Scholar]
- 102.Bervoets L, Van Hoorenbeeck K, Kortleven I, Van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. (2013) 5:10. doi: 10.1186/1757-4749-5-10, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, et al. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr. (2013) 110:1696–703. doi: 10.1017/S0007114513001037, PMID: [DOI] [PubMed] [Google Scholar]
- 104.Omar JM, Chan YM, Jones ML, Prakash S, Jones PJH. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in healthy persons. J Funct Foods. (2013) 5:116–23. doi: 10.1016/j.jff.2012.09.001 [DOI] [Google Scholar]
- 105.Safavi M, Farajian S, Kelishadi R, Mirlohi M, Hashemipour M. The effects of synbiotic supplementation on some cardio-metabolic risk factors in overweight and obese children: a randomized triple-masked controlled trial. Int J Food Sci Nutr. (2013) 64:687–93. doi: 10.3109/09637486.2013.775224, PMID: [DOI] [PubMed] [Google Scholar]
- 106.Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J Matern Fetal Neonatal Med. (2012) 25:1552–6. doi: 10.3109/14767058.2011.640372, PMID: [DOI] [PubMed] [Google Scholar]
- 107.Jiang J, Wu C, Zhang C, Zhao J, Yu L, Zhang H, et al. Effects of probiotic supplementation on cardiovascular risk factors in hypercholesterolemia: A systematic review and meta-analysis of randomized clinical trial. J Funct Foods. (2020) 74:104–77. doi: 10.1016/j.jff.2020.104177 [DOI] [Google Scholar]
- 108.Corb Aron RAC, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, et al. Recognizing the benefits of pre−/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms. (2021) 9:618. doi: 10.3390/microorganisms9030618, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mitheus G, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. (2020) 11:411–55. doi: 10.3920/BM2020.0057, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. (2022) 23:1105. doi: 10.3390/ijms23031105, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alsharairi NA. The role of short-chain fatty acids in mediating very low-calorie ketogenic diet-infant gut microbiota relationships and its therapeutic potential in obesity. Nutrients. (2021) 13:3702. doi: 10.3390/nu13113702, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. (2020):51102590. doi: 10.1016/j.ebiom.2019.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang S, Xiao Y, Tian F, Zhao J, Zhang H, Chen W. Rational use of prebiotics for gut microbiota alterations: specific bacterial phylotypes and related mechanisms. J Funct Foods. (2020) 66:1–13. doi: 10.1016/j.jff.2020.103838 [DOI] [Google Scholar]
- 114.Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. (2017) 19:579–89. doi: 10.1111/dom.12861, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Toejing P, Khampithum N, Sirilun S, Chaiyasut C, Lailerd N. Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: A randomized clinical trial. Foods. (2021) 10:1455. doi: 10.3390/foods10071455, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu T, Zhang Y, Li W, Zhao Y, Long H, Muhindo EM, et al. Lactobacillus rhamnosus LRa05 Ameliorate hyperglycemia trough a regulating glucagon-mediated signaling pathway and gut microbiota in type 2 diabetic mice. J Agric Food Chem. (2021) 69:8797–806. doi: 10.1021/acs.jafc.1c02925, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Manaer T, Yu L, Nabi XH, Dilidaxi D, Liu L, Sailike J. The beneficial effects of the composite of probiotics from camel milk on glucose and lipid metabolism, liver and renal function and gut microbiota in db/db mice. BMC Complement Med Ther. (2021) 21:127. doi: 10.1186/s12906-021-03303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr. (2019) 13:175–82. doi: 10.1016/j.dsx.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 119.Zheng J, Cheng G, Li Q, Jiao S, Feng C, Zhao X, et al. Chitin oligosaccharide modulates gut microbiota and attenuates high-fat-diet-induced metabolic syndrome in mice. Mar Drugs. (2018) 16:66. doi: 10.3390/md16020066, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barreto FM, Colado Simão AN, Morimoto HK, Lozovoy MAB, Dichi MDI, da Silva H, et al. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition. (2014) 30:939–42. doi: 10.1016/j.nut.2013.12.004, PMID: [DOI] [PubMed] [Google Scholar]
- 121.Kassaian N, Feizi A, Aminorroaya A, Amini M. Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: a randomized controlled trial. Diabetes Metab Syndr. (2019) 13:2991–6. doi: 10.1016/j.dsx.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 122.Porwal K, Pal S, Kulkarni C, Singh P, Sharma S, Singh P. A prebiotic, short-chain fructo-oligosaccharides promotes peak bone mass and maintains bone mass in ovariectomized rats by an osteogenic mechanism. Biomed Pharmacother. (2020) 129:1–9. doi: 10.1016/j.biopha.2020.110448 [DOI] [PubMed] [Google Scholar]
- 123.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res. (2016) 31:1638–46. doi: 10.1002/jbmr.2887, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.So JS, Song MK, Kwon HK, Lee CG, Chae CS, Sahoo A, et al. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. (2011) 88:358–66. doi: 10.1016/j.lfs.2010.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- 125.Steves CJ, Bird S, Williams FM, Spector TD. The microbiome and musculoskeletal conditions of aging: a review of evidence for impact and potential therapeutics. J Bone Miner Res. (2016) 31:261–9. doi: 10.1002/jbmr.2765, PMID: [DOI] [PubMed] [Google Scholar]
- 126.Paul AK, Paul A, Jahan R, Jannat K, Bondhon TA, Hasan A, et al. Probiotics and amelioration of rheumatoid arthritis: significant roles of Lactobacillus casei and Lactobacillus acidophilus. Microorganisms. (2021) 9:1070. doi: 10.3390/microorganisms9051070, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gohel MK, Prajapati JB, Mudgal SV, Pandya HV, Singh US, Trivedi SS, et al. Effect of probiotic dietary intervention on calcium and haematological parameters in geriatrics. J Clin Diagn Res. (2016) 10:5–9. doi: 10.7860/JCDR/2016/18877.7627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lei M, Hua LM, Wang DW. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benef Microbes. (2016) 7:631–7. doi: 10.3920/BM2016.0067 [DOI] [PubMed] [Google Scholar]
- 129.Han SK, Shin YJ, Lee DY, Kim KM, Yang SJ, Kim DS, et al. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. (2021) 21:146. doi: 10.1186/s12866-021-02192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.National Institute of Health . Office of Dietary Supplements Probiotics. Fact sheets for health professionals. (2022). Available at: https://ods.od.nih.gov/factsheets/Probiotics-HealthProfessional/ (Accessed June 14, 2023).
- 131.Hathi Z, Mettu S, Priya A, Athukoralalage S, Lam TN, Choudhury NR, et al. Methodological advances and challenges in probiotic bacteria production: Ongoing strategies and future perspectives. Biochem Eng J. (2021) 176:108199. doi: 10.1016/j.bej.2021.108199 [DOI] [Google Scholar]
- 132.International Association for Porbiotics and Prebiotics . Deciphering a probiotic label. (2022). Available at: http://isappscience.org/wp-content/uploads/2019/04/EU_Probiotic_labeling_rev1029.pdf (Accessed June 14, 2023).
- 133.Islam SUI. Clinical uses of probiotics. Medicine. (2016) 95:5. doi: 10.1097/MD.0000000000002658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller LE, Lehtoranta L, Lehtinen MJ. Short-term probiotic supplementation enhances cellular immune function in healthy elderly: systematic review and meta-analysis of controlled studies. Nutr Res. (2016) 64:1–8. doi: 10.1016/j.nutres.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 135.Forth E, Buehner B, Storer A, Sgarbossa C, Milev R, Meyyappan AC. Systematic review of probiotics as adjuvant treatment for psychiatric disorders. Front Behav Neurosci. (2023) 17:1111349. doi: 10.3389/fnbeh.2023.1111349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Amirami E, Milajerdi A, Mirzaeih JH, Mansournia MA, Hallajzadeh J, et al. The effects of probiotic supplementation on mental health, biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2023) 49:102361. doi: 10.1016/j.ctim.2020.102361 [DOI] [PubMed] [Google Scholar]
- 137.Baral KC, Bajracharya R, Lee SH, Han H-K. Advancements in the pharmaceutical applications of probiotics: dosage forms and formulation technology. Int J Nanomedicine. (2021) 16:7535–56. doi: 10.2147/IJN.S337427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cunningham M, Vinderola G, Charalampopoulos D, Leeber S, Sanders ME, Grimaldi R. Applying probiotics and prebiotics in new delivery formats – is the clinical evidence transferable? Trends Food Sci Technol. (2021) 11:495–506. doi: 10.1016/j.tifs.2021.04.009 [DOI] [Google Scholar]
- 139.Santocchi E, Guiducci L, Prosperi M, Calderoni S, Gaggini M, Apicella F, et al. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: a randomized controlled trial. Front Psych. (2020) 11:550593. doi: 10.3389/fpsyt.2020.550593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. (2015) 138:179–87. doi: 10.1016/j.physbeh.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 141.Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. (2019) 12:576–88. doi: 10.1002/aur.2079 [DOI] [PubMed] [Google Scholar]
- 142.El-Ansary A, Bacha AB, Bjørklund G, Al-Orf N, Bhat RS, Moubayed N, et al. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis. (2018) 331155–64. doi: 10.1007/s11011-018-0212-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.