Abstract
Objectives:
Daikenchuto (DKT) enhances the contraction of the internal anal sphincter (IAS) in patients with constipation and fecal incontinence; however, the mechanism of its action is unknown. We investigated the effects of the active ingredients of DKT (hydroxy-α-sanshool (HAS) and hydroxy-β-sanshool (HBS)) on the contractile activity of the canine rectum and IAS.
Methods:
Three male beagle dogs were prepared for each of the HAS, HBS, and control groups. Force transducers were attached to the rectal and IAS surfaces of the dogs, and the contractile responses were measured by telemetry under conscious conditions. HAS (10 mg/body) and HBS (2.5 mg/body) were administered intrarectally at doses previously identified from an effective dose of DKT extract (1.5 g/body), and contractile responses were recorded up to 6 h after administration. Contractile activity of the rectum and IAS was evaluated by observing the area under the curve (AUC) of the recorded contraction waveform. Plasma concentrations of HAS and HBS were measured before and after administration to confirm IAS exposure to both ingredients.
Results:
The mean AUC values of the IAS for the control, HAS, and HBS groups at 10 min after administration were 115, 87, and 220 (g-min), respectively, indicating a higher contraction in the HBS group, which was maintained for approximately 3 h. As for the rectum, no contractile response was observed in either the HAS or HBS groups. Plasma concentrations of both ingredients peaked at 20 min after administration.
Conclusions:
HBS could be involved in the contractile action of DKT on the IAS.
Keywords: Daikenchuto, internal anal sphincter, fecal incontinence, telemetry system, hydroxy-α-sanshool, hydroxy-β-sanshool
Introduction
Fecal incontinence (FI) is a condition defined as involuntary or uncontrollable loss of feces[1]. FI is problematic owing to its high prevalence (affecting approximately 2% of the adult population)[2] and the marked disruption it imposes on daily life, even in less severely affected patients. Etiologies vary, and dysfunction of the anal sphincter, in particular, represents a crucial background factor in the development of FI. The internal anal sphincter (IAS) is an involuntary smooth muscle that plays a major role in maintaining resting anal pressure. Further, its sustained contraction prevents unintentional defecation. Resting anal pressure is measured using anal manometry and is used in clinical practice to indicate IAS function. IAS dysfunction due to age-related decline or sphincter defects is one of the leading causes of FI, and strengthening IAS contractility is key to FI treatment[2,3].
Although surgical[4] and pharmacotherapeutic strategies[5-7] are available to treat FI, a treatment for strengthening of IAS contraction remains to be established. Daikenchuto (DKT) is a traditional Japanese herbal medicine (Kampo) made from ginseng, processed ginger, and Japanese pepper. Retrospective and prospective clinical studies have been recently conducted on the effects of DKT in patients with FI[8,9]. These studies have shown that the Cleveland Clinic Florida Fecal Incontinence Score, a severity index for patients with FI, decreased with a tendency toward improved symptoms and increased resting anal pressure after DKT administration.
In a previous study, based on the hypothesis that the above-mentioned increase in resting anal pressure after DKT administration is due to IAS contraction, we examined intrarectal administration of DKT to conscious dogs (1.5 g/5.0 mL/body) and found that IAS contraction was induced[10]. We reported that the ingredients of DKT, hydroxy-α-sanshool (HAS), and hydroxy-β-sanshool (HBS), were detected in plasma and peaked at 2 h after DKT administration[10]. Further, these sanshools have shown high plasma concentrations in human pharmacokinetic studies of DKT[11]. Furthermore, HAS and HBS induced contractile effects on the isolated intestine of rats and guinea pigs[12] and enhanced intestinal transit[13], indicating their direct role in contractile effects on intestinal smooth muscle. However, the active ingredients of DKT that affect IAS contraction remain unknown.
Therefore, we aimed to determine whether HAS or HBS had a greater impact on the contraction of IAS. We synthesized and selectively administered HAS and HBS to the canine rectum to compare the differences in the contractile activity of IAS.
Methods
Materials
The chemical structures of HAS and HBS used in this study are shown in Figure 1. Both ingredients were synthesized for experimental use by TSUMURA & CO. (Tokyo, Japan). The doses for HAS and HBS were set at 10 mg/5.0 mL/body and 2.5 mg/5.0 mL/body, respectively. These doses were determined based on the amounts of ingredients present in 1.5 g of DKT, which has previously been reported to have contractile effects on IAS[10]. Each ingredient was suspended in a solution of 1.0% sodium carboxymethyl cellulose (CMC-Na) and administered intrarectally. The control group was administered 5.0 mL of 1.0% CMC-Na solution alone. Three dogs in each group of HAS, HBS, and control were tested for contractile response and plasma levels.
Figure 1.

Chemical structures of (A) hydroxy-α-sanshool and (B) hydroxy-β-sanshool.
Experimental animals
Male TOYO beagle dogs (Kitayama Labes Co Ltd, Iwakuni, Japan) aged 8-11 months were used in the experiments. To select the beagle dogs, all dogs were administered phenylephrine hydrochloride intrarectally, and the resulting IAS contractions were confirmed. Breeding environments were similar to those used in a previous study[10]. The dogs were provided with standard laboratory food (300 g/d, DS-A; Oriental Yeast, Tokyo, Japan) and water (1,800 g/d) from their arrival at the facility until the end of the study. These dogs were housed in an animal room with a temperature maintained between 21.6 and 25.4°C, a relative humidity of 43.3% and 100.0%, and the lighting was switched on and off every 12 h. All experimental procedures were performed at the Kumamoto laboratory of LSIM Safety Institute Corporation in accordance with the “Guidelines for the Care and Use of Laboratory Animals” approved by the Laboratory Animal Committee of LSIM Safety Institute Corporation (approval no. 2021-0002, protocol no. P200477).
Preparation of animals
We followed a previously reported animal preparation procedure[10]. After inducing anesthesia, we performed laparotomy on beagle dogs and force transducers (F-12IS; Star Medical, Tokyo, Japan) were implanted in both rectum and IAS to measure their contractile response. Beagle dogs were fasted for at least 18 h prior to administration of dosing solution, and a glycerin enema was administered at least 1 h prior to administration to wash the intestine.
Measurement of contractile activity of the rectum and IAS
We followed the same procedure to record the contractile activity of the rectum and IAS as described in a previous study[10]. The contractile activities were measured and analyzed using a telemetry system consisting of an 8-channel transmitter/receiver and amplifier (GTS-850; Star Medical, Tokyo, Japan). One hour after intestinal wash with the glycerin enema, the transducer electrode fixed on the canine back was connected to the transmitter to initiate measurements.
Dogs were placed in a hammock, and a 5.0-mL dosing solution was injected into their rectum by inserting a dosing catheter through the anus. After 1 h, the dogs were removed from the hammock, allowing free movement. Rectal and IAS contractile responses were recorded continuously via an amplifier (DAT-85A; Star Medical, Tokyo, Japan) connected to the transducer and were stored on a computer system for subsequent analysis. Waveforms for rectal and IAS contraction were recorded up to 6 h after administration.
Evaluation of contractile response
We evaluated the contractile responses of the rectum and IAS using the areas under the curve (AUCs) of the recorded contraction waveforms. We calculated AUCs using a specially designed program (Analyze II with DAT-85A; Star Medical, Tokyo, Japan). The sampling rate for the contractile response was 100 ms, and the AUC was calculated from the output values at each sampling rate as an integral value based on the contractile response (g) and time (min), expressed in g-min. AUCs were calculated over a 6-h period, with AUCs being examined at a 10-min interval for the first hour and at a 1-h interval for the next 5 h.
Measurement of plasma concentrations of HAS and HBS
A blood sample (2 mL) was collected from the cephalic vein of each dog using a heparinized syringe before and 20 min, 2 h, and 4 h after administration of the test ingredients (HAS 10 mg/body or HBS 2.5 mg/body). Plasma was separated via centrifugation (1,830 × g, 10 min, 4°C) and stored at -80°C. Plasma concentrations of HAS and HBS were determined using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (LC: Shimadzu Nexera X2 System; Shimadzu, Kyoto, Japan; MS/MS: QTRAP 5500; AB SCIEX, Tokyo, Japan).
Statistical analysis
Data on AUCs and plasma concentrations of the ingredients for the three dogs in each group are expressed as the mean ± standard error of the mean (SEM). The AUCs of the HAS and HBS groups were compared with those of the control group using Dunnett's multiple comparison method. We used Excel 365 (Microsoft Corporation) to calculate the mean and SEM. Further, R version 4.0.5 (The R Foundation for Statistical Computing) was used for multiple comparisons.
Results
Effects on the contractile response of IAS
Example waveforms of contractile responses of the IAS for the control, HAS, and HBS are shown in Figure 2. Figure 3 shows the AUCs measured every 10 min up to 1 h after administration (Figure 3A) and every hour up to 6 h after administration (Figure 3B). The mean AUCs were compared between the control and HAS- or HBS-treated groups (Table 1). The HBS-treated group showed a high tendency to contract 10 min after administration and maintained it for approximately 3 h (Figure 3A). The contractile effect in the HBS group was also observed in the AUC for all 6 h (Figure 4). On the contrary, the AUC of the HAS group did not differ from that of the control group, indicating no enhancement in contraction (Figure 3, 4).
Figure 2.

Examples of contractile response wave of the internal anal sphincter in each group. Test substances were administered at the open arrow and contractile waves were continuously recorded up to 6 h after administration. A: control; B: hydroxy-α-sanshool (HAS) 10 mg/5.0 mL/dog; C: hydroxy-β-sanshool (HBS) 2.5 mg/5.0 mL/dog.
Figure 3.
Effects of intrarectal administration of the control, hydroxy-α-sanshool (HAS), and hydroxy-β-sanshool (HBS) on the area under the curve (AUC) of the contractile response wave of the internal anal sphincter. (A) Time course of the mean AUC in each treatment group up to 60 min after administration. (B) Time course of the mean AUC in each treatment group up to 6 h after administration.
Closed circle, control; open triangle, HAS; open circle, HBS. Data are expressed as mean ± standard error of the mean. n = 3 in each group.
Table 1.
Area under the Curve (AUC) of the Contractile Response Wave of the Internal Anal Sphincter.
| Time after
Administration |
Control | Hydroxy-α-sanshool
(10 mg/body) |
Hydroxy-β-sanshool
(2.5 mg/body) |
|||
|---|---|---|---|---|---|---|
| AUC
(g-min) |
AUC
(g-min) |
p-value | AUC
(g-min) |
p-value | ||
| 10-min segment of the first 1 h | Pre | 17±6 | 10±5 | 0.669 | 14±9 | 0.913 |
| 10 min | 115±32 | 87±15 | 0.702 | 220±33 | 0.064 | |
| 20 min | 91±58 | 63±12 | 0.838 | 185±32 | 0.230 | |
| 30 min | 100±29 | 72±28 | 0.786 | 104±42 | 0.995 | |
| 40 min | 47±6 | 50±7 | 0.993 | 85±37 | 0.418 | |
| 50 min | 55±13 | 34±8 | 0.723 | 67±34 | 0.904 | |
| 60 min | 84±19 | 30±15 | 0.255 | 76±33 | 0.959 | |
| 1 h segment | Pre | 144±17 | 97±33 | 0.552 | 157±45 | 0.950 |
| 1 h | 493±123 | 336±32 | 0.615 | 736±179 | 0.356 | |
| 2 h | 462±147 | 295±4 | 0.550 | 577±149 | 0.738 | |
| 3 h | 249±52 | 240±31 | 0.997 | 509±185 | 0.251 | |
| 4 h | 210±14 | 233±4 | 0.484 | 220±20 | 0.862 | |
| 5 h | 193±69 | 198±34 | 0.998 | 334±74 | 0.258 | |
| 6 h | 188±62 | 175±61 | 0.987 | 250±78 | 0.752 | |
Data are expressed as the mean ± standard error of the mean. n = 3 in each group.
P-values of each treatment group (hydroxy-α-sanshool or hydroxy-β-sanshool) vs control were calculated from Dunnett’s multiple comparison test.
Figure 4.

Effect of intrarectal administration of the control, hydroxy-α-sanshool (HAS), and hydroxy-β-sanshool (HBS) on the area under the curve (AUC) of the contractile response wave of the internal anal sphincter for all 6 h. Open column, control; hatched column, HAS; closed column, HBS. Data in the bar graph are expressed as mean ± standard error of the mean. Open circles represent data from each animal. n=3 in each group.
Effects on the contractile response of the rectum
Changes observed in AUC for rectal contraction response after administration in control, HAS, and HBS groups are shown in Figure 5. A significant difference between the control and HAS-treated groups was evident 30 min after administration. However, the contraction amplitude was small, and no notable contraction was observed in either the HAS group or HBS group.
Figure 5.
Effect of intrarectal administration of control, hydroxy-α-sanshool (HAS), and hydroxy-β-sanshool (HBS) on the area under the curve (AUC) of the contractile response wave of the rectum. (A) Time course of the mean AUC in each treatment group up to 60 min after administration. (B) Time course of the mean AUC in each treatment group up to 6 h after administration. Closed circle, control; open triangle, HAS; open circle, HBS. Data are expressed as mean ± standard error of the mean. n = 3 in each group.
* indicates p < 0.01 i.e., a significant difference compared to the control group (Dunnett’s multiple comparison test)
Plasma concentrations of HAS and HBS
Plasma concentrations of HAS and HBS before and after administration of each ingredient showed that neither HAS nor HBS was detectable in plasma before administration (Figure 6), and each peaked at 20 min after administration. Further, HAS and HBS were detected in the HAS and HBS groups, respectively (Figure 6A, 6B, HAS: 39.6 ng/mL in the HAS group; HBS: 38.1 ng/mL in the HBS group). Both values decreased after 2 h and almost disappeared from plasma after 4 h.
Figure 6.
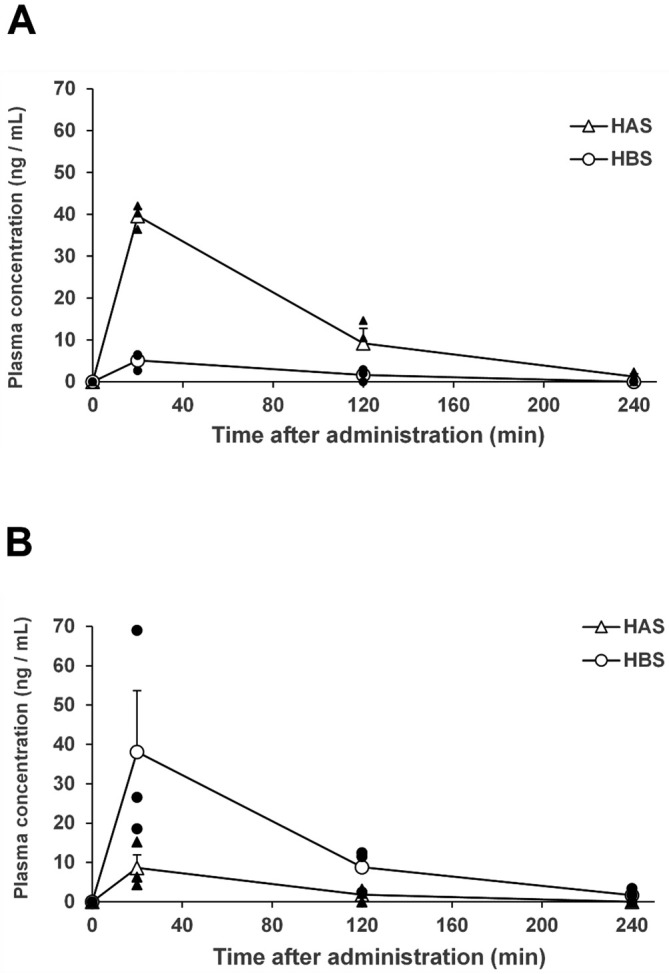
Plasma concentrations of HAS and HBS after intrarectal administration of (A) hydroxy-α-sanshool (HAS) or (B) hydroxy-β-sanshool (HBS). Open triangle, the mean concentration of HAS; open circle, the mean concentration of HBS; closed triangle, the individual value of HAS; closed circle, the individual value of HBS. Data are expressed as mean ± standard error of the mean.
n = 3 in each group.
Discussion
In this study, HAS and HBS were synthesized and administered to the canine rectum to elucidate the mechanism of action of DKT-induced IAS contraction. The results showed that the IAS in the HBS group tended to contract more strongly than in the control group, with the peak contraction occurring at 10 min after the administration. However, no contractions were observed with HAS. Neither HAS nor HBS showed notable rectal contractions.
Both HAS and HBS are derived from Japanese pepper, one of the constituent botanical raw materials of DKT, and they are known to contract the intestinal smooth muscle in animals. These ingredients have been reported to contract small intestinal smooth muscle cells isolated from mice[14] and enhance the motility of the colon isolated from rats[13], suggesting that inhibition of two-pore domain potassium channels (KCNK) is involved in contraction[13]. Furthermore, HBS induces the contraction of the rat-isolated ileum, and this contraction involves the release of acetylcholine from intrinsic cholinergic nerves and tachykinin from sensory nerves[12]. The intestinal tract periodically contracts and relaxes to move its contents, whereas IAS constantly contracts to prevent FI. The anal tone generated by this sustained IAS contraction is mainly controlled by the sympathetic nervous system[15]. Based on this sympathetic involvement, clinical studies of α-adrenergic agonists for the treatment of FI were investigated[16]. However, if HAS or HBS causes contraction of the IAS remains to be confirmed, and the present study is the first attempt in exploring and examining the effects of HAS and HBS on IAS. The findings of IAS contractility and the increasing effects of HBS in the present study suggest that HBS increases anal resting pressure observed in DKT.
While the anal tone is mainly controlled by the sympathetic nervous system, the effects of DKT on the sympathetic nervous system have not been clarified. Ca2+-activated Cl- channels (ANO1) expressed in the interstitial cells of Cajal (ICC) in the IAS are reportedly involved in maintaining the tone. The slow waves generated in ICCs propagate to neighboring IAS cells, triggering contractions. The sum of these muscle contractions is thought to be involved in tone maintenance[17]. The relationship between the contractile responses exhibited by DKT and HBS and functional proteins, such as these receptors and channels, should be investigated in the future.
In this study, IAS contraction was not observed with HAS administration; however, both HAS and HBS are known to exert contractile effects on intestinal smooth muscles[13,14], although their mechanisms of action are not entirely consistent. Bautista et al. reported KCNK3/9/18 inhibition by HAS in mouse neurons[18]. Kubota et al. reported that HAS increases colonic transit by inhibiting KCKN3/9, whereas HBS increases colonic transit by specifically blocking KCNK3, based on experiments for transit in the isolated colon[13]. Therefore, differences in IAS contraction induced by the administration of HAS and HBS is possibly attributed to the diversity of the distributions and subtypes of KCNK in IAS tissue.
In beagle dogs, no rectal contractions were observed with intrarectal administration of DKT extract[10]. Similarly, we did not observe any notable rectal contractions in beagle dogs after HAS or HBS administration. This finding can be attributed to the physiological differences between the rectum and IAS; a high density of α-adrenergic receptors in the IAS maintains sympathetic contraction, whereas a lower density of α-adrenergic receptors has been reported in the rectal region than in the IAS[15]. In addition, the distribution of ICCs, which are essential for intestinal contraction and maintenance of anorectal tone, differs between the rectum and IAS[19], and these differences in innervation and anatomic region may contribute to regional differences in HBS action.
High plasma concentrations of each ingredient were detected in the HAS and HBS groups after intrarectal administration, and the time of peak HBS concentration in the HBS group was 20 min. The peak IAS contractile response was observed 10 min after HBS administration (Figure 3), suggesting that the contraction was initially caused by the direct effect of HBS administered to the rectum, followed by the contractile effect of the ingredient absorbed from the vessels. However, during the contractile response, HBS had almost disappeared from the plasma within 2-3 h after administration, suggesting that HBS may have sustained effects, and one possibility is that the ingredients administered through the rectal route remain in the luminal tissue, thereby exerting sustained effects.
The current study has a few limitations. First, since this was an exploratory study, the number of animals in each group was limited, and no significant difference was observed in IAS contraction in the HBS group. The present findings contradict the significant contraction observed in a prior report following DKT administration, suggesting that HBS alone may not exert a sufficient contractile effect. Second, we selected HAS and HBS as test substances owing to the high plasma concentrations of these ingredients after administration of DKT in human pharmacokinetic studies[11] and the results of pharmacological effects on the intestinal tract. However, in addition to these two ingredients derived from Japanese pepper, DKT contains ingredients derived from processed ginger and ginseng. In particular, [6]-gingerol, an ingredient of processed ginger, inhibits the contraction of rat-excised small intestine[20]. Since the contractile response of HBS did not reach the level seen with DKT, the contractile effects of DKT may be a result of the interrelated effects of several ingredients. However, the individual effects of other DKT-derived ingredients and their interactions on IAS contraction and various other related issues remain unresolved. Third, intra-anal pressure has not been evaluated in this study; therefore, whether IAS contractions cause an increase in intra-anal pressure remains unclear. One of the objectives of this study was to examine the effects of the DKT ingredients in conscious dogs, and measuring intra-anal pressure in conscious dogs is difficult owing to the lack of manometry with a telemetry system. In addition, since the IAS of the dogs was fitted with a transducer, using the same animal could have affected the internal pressure measurement. Thus, the effect of DKT on intra-anal pressure in conscious dogs should be further explored. Finally, the route of administration used in this study was different from that in the clinical practice of DKT. In a previous report, DKT was administered intrarectally to determine its direct effect on the rectoanal area; therefore, HAS and HBS were also administered intrarectally to determine the direct effect similarly in this study. The effects of oral administration of DKT remain to be investigated in the future.
In conclusion, the results suggest that HBS contributes more to the DKT-induced IAS contraction than HAS. However, rectal contractility was not enhanced by either HAS or HBS. Thus, we believe that DKT may prevent FI by increasing anal pressure without increasing rectal pressure and hence, its effects warrant further exploration.
Conflicts of Interest
This study was done in collaboration with TSUMURA & CO. Kotaro Maeda received research funding and honoraria for consultation from TSUMURA & CO.; Toshinobu Sasaki is an employee of TSUMURA & CO.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kotaro Maeda and Toshinobu Sasaki. The first draft of the manuscript was written by Kotaro Maeda and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Approval by Institutional Review Board (IRB)
All experimental procedures were performed at the Kumamoto laboratory of LSIM Safety Institute Corporation in accordance with the “Guidelines for the Care and Use of Laboratory Animals” approved by the Laboratory Animal Committee of LSIM Safety Institute Corporation (approval no. 2021-0002).
Acknowledgements
This work was supported by a grant from TSUMURA & CO.
We would like to thank Mr. Kazuaki Sasaki and other members of LSIM Safety Institute Corporation for their technical support for this work.
We also would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Maeda K, Yamana T, Takao Y, et al. Japanese Practice Guidelines for Fecal Incontinence Part 1 -Definition, Epidemiology, Etiology, Pathophysiology and Causes, Risk Factors, Clinical Evaluations, and Symptomatic Scores and QoL Questionnaire for Clinical Evaluations- English Version. J Anus Rectum Colon. 2021 Jan; 5(1): 52-66. doi: 10.23922/jarc.2020-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamm MA. Faecal incontinence. BMJ. 1998 Feb; 316(7130): 528-32. doi: 10.1136/bmj.316.7130.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul T, Ahlberg M, Biondo S, et al. International validation of the low anterior resection syndrome score. Ann Surg. 2014 Apr; 259(4): 728-34. doi: 10.1097/SLA.0b013e31828fac0b. [DOI] [PubMed] [Google Scholar]
- 4.Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Ann Surg. 2010 Mar; 251(3): 441-9. doi: 10.1097/SLA.0b013e3181cf8ed0. [DOI] [PubMed] [Google Scholar]
- 5.Markland AD, Burgio KL, Whitehead WE, et al. Loperamide versus psyllium fiber for treatment of fecal incontinence: the fecal incontinence prescription (Rx) management (FIRM) randomized clinical trial. Dis Colon Rectum. 2015 Oct; 58(10): 983-93. doi: 10.1097/DCR.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 6.Maeda K, Mimura T, Yoshioka K, et al. Japanese practice guidelines for fecal incontinence part 2-examination and conservative treatment for fecal incontinence-English version. J Anus Rectum Colon. 2021 Jan; 5(1): 67-83. doi: 10.23922/jarc.2020-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda K, Katsuno H, Tsunoda A, et al. Japanese practice guidelines for fecal incontinence part 3-surgical treatment for fecal incontinence, fecal incontinence in a special conditions- English version. J Anus Rectum Colon. 2021 Jan; 5(1): 84-99. doi: 10.23922/jarc.2020-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe T, Kunimoto M, Hachiro Y, et al. Clinical efficacy of Japanese herbal medicine daikenchuto in the management of fecal incontinence: a single-center, observational study. J Anus Rectum Colon. 2019 Oct; 3(4): 160-6. doi: 10.23922/jarc.2019-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimazutsu K, Watadani Y, Ohge H. Efficacy and safety of the Japanese herbal medicine daikenchuto (DKT) in elderly fecal incontinence patients: a prospective study. J Anus Rectum Colon. 2022 Jan; 6(1): 32-9. doi: 10.23922/jarc.2021-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda K, Katsuno H, Kono T. The Japanese extracted herbal medicine daikenchuto increases the contractile activity of the internal anal sphincter muscle in conscious dogs. J Anus Rectum Colon. 2020 Oct; 4(4): 193-200. doi: 10.23922/jarc.2020-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munekage M, Ichikawa K, Kitagawa H, et al. Population pharmacokinetic analysis of daikenchuto, a traditional Japanese medicine (Kampo) in Japanese and US health volunteers. Drug Metab Dispos. 2013 Jun; 41(6): 1256-63. doi: 10.1124/dmd.112.050112. [DOI] [PubMed] [Google Scholar]
- 12.Satoh K, Hashimoto K, Hayakawa T, et al. Mechanism of atropine-resistant contraction induced by dai-kenchu-to in guinea pig ileum. Jpn J Pharmacol. 2001 May; 86(1): 32-7. doi: 10.1254/jjp.86.32. [DOI] [PubMed] [Google Scholar]
- 13.Kubota K, Ohtake N, Ohbuchi K, et al. Hydroxy-α sanshool induces colonic motor activity in rat proximal colon: a possible involvement of KCNK9. Am J Physiol Gastrointest Liver Physiol. 2015 Apr; 308(7): G579-90. doi: 10.1152/ajpgi.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokita Y, Akiho H, Nakamura K, et al. Contraction of gut smooth muscle cells assessed by fluorescence imaging. J Pharmacol Sci. 2015 Mar; 127(3): 344-51. doi: 10.1016/j.jphs.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Tichenor SD, Buxton IL, Johnson P, et al. Excitatory motor innervation in the canine rectoanal region: role of changing receptor populations. Br J Pharmacol. 2002 Dec; 137(8): 1321-9. doi: 10.1038/sj.bjp.0704987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheetham MJ, Kamm MA, Phillips RK. Topical phenylephrine increases anal canal resting pressure in patients with faecal incontinence. Gut. 2001 Mar; 48(3): 356-9. doi: 10.1136/gut.48.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobine C, Hannah E, Zhu MH, et al. ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J Physiol. 2017 Mar; 595(6): 2021-41. doi: 10.1113/JP273618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautista DM, Sigal YM, Milstein AD, et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008 Jul; 11(7): 772-9. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horiguchi K, Keef KD, Ward SM. Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol. 2003 May; 284(5): G756-67. doi: 10.1152/ajpgi.00294.2002. [DOI] [PubMed] [Google Scholar]
- 20.Chatturong U, Kajsongkram T, Tunsophon S, et al. Ginger extract and [6]-gingerol inhibit contraction of rat entire small intestine. J Evid Based Integr Med. 2018 Jan-Dec; 23: 2515690X18774273. doi: 10.1177/2515690X18774273. [DOI] [PMC free article] [PubMed] [Google Scholar]




