Abstract
Objectives:
Preoperative deep venous thrombosis (DVT) can cause potentially life-threatening postoperative venous thromboembolism (VTE). Lower limb venous ultrasound (LLVU) is a modality that can detect DVT. However, the threshold for performing preoperative LLVU in the population undergoing colorectal resection is controversial. In this context, we evaluated whether a preoperative D-dimer value can identify patients who benefit from LLVU from the perspective of preventing postoperative symptomatic VTE.
Methods:
Patients undergoing colorectal resection in our institute from 2013 to 2020 were retrospectively enrolled (n=2071). We divided the patients into two groups: the clinical indication group (CG: including patients from 2013 to 2016, n=875) and the D-dimer-orientated group (DG: including patients from 2017 to 2020, n=1196). In the CG, LLVU was performed when DVT was clinically suspected; in the DG, preoperative LLVU was performed in patients with a preoperative D-dimer>1.0 μg/ml.
Results:
In the surveyed period, 277 LLVUs were performed, among which DVT was detected in 34 cases (12.3%). In the CG, DVT was detected in 0.7% of patients, whereas in the DG, it was detected in 2.3% of patients. Postoperative symptomatic VTE was significantly reduced in the DG at both 3 and 6 months after surgery (p=0.041 and 0.020, respectively). Moreover, Multivariate analysis showed that a past medical history of PE and treatment following the CG protocol were independent risk factors for postoperative symptomatic VTE within 6 months of surgery (p<0.0001 and =0.036, respectively).
Conclusions:
LLVU in patients with a preoperative D-dimer>1.0 μg/ml is a useful method to prevent postoperative symptomatic VTE.
Keywords: colorectal surgery, D-dimer, lower limb venous ultrasound, postoperative VTE, preoperative screening
Introduction
Venous thromboembolism (VTE) is a term covering deep vein thrombosis (DVT) to pulmonary embolism (PE). The pathogenesis of VTE is multifactorial, involving interactions between acquired or inherited predispositions and various risk factors[1]. Patients with cancer are six times more likely to develop VTE than patients without cancer, and VTE is reported to be the second leading cause of death in patients with cancer[2]. Thus, the importance of VTE screening in patients with cancer is widely recognized from the standpoint of preventing life-threatening complications. The prevalence of DVT in patients who undergo colorectal resection for colorectal cancer (CRC) is reported to be 7.8%[3]. Moreover, a previous report described that preoperative DVT is significantly associated with postoperative VTE[4]. In this context, it is important to preoperatively assess the potential risk of DVT in patients undergoing colorectal resection; however, no screening protocol has been established.
Lower limb venous ultrasound (LLVU) is one of the most reliable modalities to diagnose DVT. However, because it is unrealistic to perform preoperative LLVU in every patient, a screening method is required to identify patients at high risk for preoperative DVT. Stender et al. showed that abnormal preoperative D-dimer values were significantly associated with the occurrence of postoperative DVT in patients who underwent colorectal resection for CRC[5]. Moreover, Nakagawa et al. reported that a preoperative D-dimer>1.0 μg/ml was an independent risk factor for preoperative DVT[6]. Based on these reports, from the perspective of preventing postoperative VTE, an abnormal preoperative D-dimer value can be considered a pragmatic criterion for performing LLVU in patients who undergo colorectal resection.
The ultimate purpose of preoperative screening for DVT is to prevent postoperative VTE, which can be either symptomatic or asymptomatic. Asymptomatic postoperative VTE is sometimes employed as an endpoint to assess the efficacy of preoperative screening due to its relatively higher incidence than symptomatic VTE. However, because of the clinical significance of symptomatic postoperative VTE, the American guidelines recommend symptomatic VTE as the preferred endpoint over asymptomatic VTE[7]. Therefore, symptomatic postoperative VTE should be used as an endpoint to evaluate the clinical impact of preoperative screening procedures for DVT.
In our institute, preoperative LLVU was performed for patients undergoing colorectal resection until 2016 to evaluate the presence or absence of DVT only when DVT was clinically suspected; since 2017, we have routinely performed preoperative LLVU when patients exhibit a preoperative D-dimer>1.0 μg/ml. However, the clinical impact of routine LLVU on the occurrence of postoperative VTE is unclear. Given this background, the aim of the present study was to evaluate the clinical impact of the combination of preoperative D-dimer value and LLVU as a pragmatic screening method and its association with postoperative symptomatic VTE in patients undergoing colorectal resection.
Methods
Patients and study design
The current study was performed in accordance with the Declaration of Helsinki and was approved by research ethics committee of Osaka International Cancer Institute with approval number of 18033-3. Written consent has been obtained from all patients. Patients who underwent colorectal resection at the Osaka International Cancer Institute from 2013 to 2020 were retrospectively enrolled. Data collection was performed at the Department of Medical Informatics in our institute. A total of 2071 patients were identified and enrolled in this study. In our institute, until 2016, preoperative LLVU was performed when DVT was clinically suspected (e.g., swelling or redness of the lower limbs). There was no specific D-dimer value to perform LLVU in this period. Afterwards, since 2017, we have routinely performed LLVU in patients with a preoperative D-dimer values higher than 1.0 μg/ml as a screening procedure for DVT. In this study, we divided the patients into two groups: the clinical indication group (CG) included patients who underwent colorectal resection from 2013 to 2016 (n=875), and the D-dimer orientated group (DG) included patients who underwent colorectal resection from 2017 to 2020 (n=1196).
The patients' characteristics were reviewed for sex and age, preoperative body mass index (BMI), American Society of Anesthesiologists physical status (ASA-PS), epithelial or nonepithelial cancer, resected area (colon or rectum), surgical procedure (laparotomy/laparoscopy), clinical stage (TNM classification[8]), past medical history, preoperative tumor markers, preoperative D-dimer value and preoperative use of anticoagulants. The preoperative risk of every patient for VTE was assessed with the Khorana score[9]. Past medical history included known risk factors for DVT, such as smoking[10], hypertension[11], hyperlipidemia[11], diabetes mellitus[12], PE[13], myocardial infarction[14], heart failure[15], previous radiotherapy[11], and previous chemotherapy[16]. The preoperative tumor markers reviewed included CEA and CA 19-9.
Perioperative management
Preoperative D-dimer levels were measured in every patient enrolled in this study. Preoperative LLVU was performed according to the protocols described above. Patients were referred to cardiologists when DVT was detected by preoperative LLVU. Depending on the clinical indication, therapeutic intervention including anticoagulants or inferior vena cava filters was introduced. Patients wore compression stockings (CSs) during the operation in addition to intermittent pneumatic compression (IPC) devices. This management strategy was sometimes modified when the cardiologist provided specific instructions after considering gastrointestinal bleeding risk or other comorbidities. CSs and IPC devices were removed postoperatively when the patients were allowed to walk. Postoperative pharmacological thromboprophylaxis was introduced on the basis of the Japanese guidelines from the JCS Joint Working Group[17].
Assessment of postoperative VTE
Postoperative VTE generally includes a wide range of clinical conditions, from asymptomatic DVT to life-threatening PE. The American guidelines prefer postoperative symptomatic VTE as a primary endpoint to evaluate the efficacy of preoperative interventions to prevent postoperative thrombotic events[7]. In this context, in this study, our primary endpoint was the occurrence of postoperative symptomatic VTE, although the occurrence of postoperative asymptomatic VTE was also reviewed. An occurrence of VTE was evaluated within 3 months from surgery based on a previous randomized study[18], as well as within 6 months based on a previous report discussing persistent coagulopathy in CRC patients[19]. Symptomatic VTE is defined as VTE confirmed by radiological examinations with any kind of clinical symptoms caused by VTE, including skin redness, dyspnea, lower limb edema, or reduced saturated hemoglobin levels measured by pulse oximetry. Asymptomatic VTE is defined as VTE without any clinical symptoms and is accidentally detected in computed tomography (CT) or LLVU examinations. These clinical findings were collected retrospectively by referring to the patients' medical records. Postoperative screening for VTE was not routine in our clinical practice. Postoperative VTE was assessed by LLVU, contrast-enhanced CT or both.
Statistical analysis
Patient characteristics and pre- and postoperative factors were evaluated according to the preoperative LLVU policy using the chi-square test, Fisher's exact test, and the Mann-Whitney U test. The optimal cutoff levels of each clinical variable for predicting postoperative VTE were determined by constructing receiver operating characteristic curves (Youden index) for univariate and multivariate analyses. Variables with p<0.10 were incorporated into a logistic regression model to determine the independent risk factors for postoperative symptomatic VTE. The independent risk factors for postoperative VTE were evaluated in terms of odds ratios. Statistical significance was defined as p<0.05. All statistical analyses were performed with JMP software (JMP, version 13.2.1).
Results
Patient characteristics
The details of the patient characteristics are summarized in Table 1. The preoperative risk for VTE was comparable between the two groups based on the Khorana score (p=0.28). Laparotomy was performed more often in the CG than in the DG (54.3% vs. 14.3%, p<0.0001). A past medical history of heart failure was significantly more frequent in the CG than in the DG (4.7% vs. 1.9%, p=0.0004). The other parameters showed no significant difference between the two groups.
Table 1.
Patient Characteristics.
| Total (n=2071) | CG (n=875) | DG (n=1196) | p value | |
|---|---|---|---|---|
| Sex (male/female) | 0.14 | |||
| male | 1120 (54.1%) | 490 (56.0%) | 630 (52.7%) | |
| female | 951 (45.9%) | 385 (44.0%) | 566 (47.3%) | |
| Age (years old) (mean, range) | 66 (25 - 94) | 66 (25 - 91) | 66 (28 - 94) | 0.43 |
| BMI† (kg/m2) (mean. range) | 22.35 (12.8 - 39.6) | 22.3 (13.3 - 39.6) | 22.4 (12.8 - 39.6) | 0.27 |
| ASA-PS‡ | 0.31 | |||
| 1-2 | 1760 (85.0%) | 704 (80.5%) | 1056 (88.3%) | |
| 3-6 | 194 (9.4%) | 70 (8.0%) | 124 (10.4%) | |
| Non-epithelial tumor | 12 (0.58%) | 4 (0.46%) | 8 (0.67%) | 0.77 |
| Resected area | 0.067 | |||
| colon | 1113 (53.7%) | 450 (51.4%) | 663 (55.4%) | |
| rectum | 958 (46.3%) | 425 (48.6%) | 533 (44.6%) | |
| Surgical Procedure | <0.0001 | |||
| laparotomy | 646 (31.2%) | 475 (54.3%) | 171 (14.3%) | |
| laparoscopy | 1425 (68.8%) | 400 (45.7%) | 1025 (85.7%) | |
| clinical Stage | 0.29 | |||
| I | 761 (36.7%) | 330 (37.7%) | 431 (36.0%) | |
| II-IV | 1020 (49.3%) | 416 (47.5%) | 604 (50.5%) | |
| Past history | ||||
| smoking | 1182 (57.1%) | 493 (56.3%) | 689 (57.6%) | 0.57 |
| hypertension | 695 (33.6%) | 299 (34.2%) | 396 (33.1%) | 0.64 |
| hyperlipidemia | 153 (7.4%) | 60 (6.9%) | 93 (7.8%) | 0.45 |
| diabetes mellitus | 251 (12.1%) | 115 (13.1%) | 136 (11.4%) | 0.25 |
| pulmonary embolism | 90 (4.3%) | 47 (5.4%) | 43 (3.6%) | 0.063 |
| myocardial infarction | 15 (0.7%) | 5 (0.6%) | 10 (0.8%) | 0.60 |
| heart failure | 64 (3.1%) | 41 (4.7%) | 23 (1.9%) | 0.0004 |
| radiotherapy | 119 (5.7%) | 41 (4.7%) | 78 (6.5%) | 0.085 |
| chemotherapy | 171 (8.3%) | 73 (8.3%) | 98 (8.2%) | 0.94 |
| Preoperative tumor marker (median, range) | ||||
| CEA (ng/ml) | 3.1 (0.5 - 16696.4) | 2.95 (0.5 - 16696.4) | 3.2 (0.5 - 5377.6) | 0.12 |
| CA19-9 (U/ml) | 7 (2 - 100000) | 8 (2-91365) | 6 (2-100000) | 0.75 |
| Preoperative D-dimer (μg/ml) (median, range) | 0.7 (0.1 - 93.2) | 0.7 (0.3 - 43.5) | 0.7 (0.4 - 26.9) | 0.66 |
| Preoperative risk for VTE§ based on Khorana score | 0.28 | |||
| low | 1681 (81.2%) | 720 (82.3%) | 961 (80.4%) | |
| intermediate or high | 390 (18.8%) | 155 (17.7%) | 235 (19.7%) | |
| Preoperative pharmacological thromboprophylaxis | 223 (10.8%) | 86 (9.8%) | 137 (11.5%) | 0.25 |
†BMI, body mass index; ‡ASA-PS, American Society of Anesthesiologists physical status; §VTE, venous thromboembolism
Preoperative LLVU and DVT
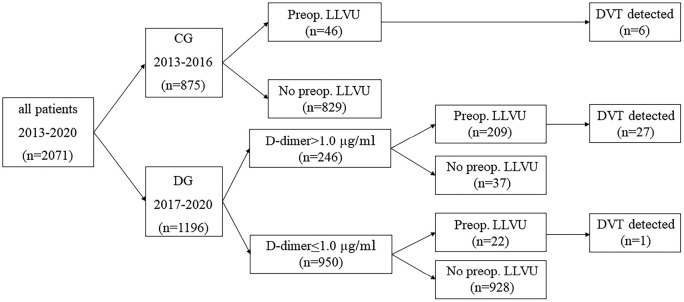
Figure 1 summarizes the protocols of the preoperative examinations (i.e., D-dimer value and LLVU) and their results. In the CG, 5.3% (46/875) of the patients underwent preoperative LLVU based on clinical indications, which included swelling, redness, or warmth of the affected limb, the presence of Homans sign, and a markedly elevated D-dimer value (> 30 μg/ml). Of these 46 patients, DVT was detected in 6 patients (6/46: 13.0%). In the DG, 37 of the patients did not undergo LLVU despite having abnormal preoperative D-dimer values (37/246: 15.0%), among whom 9 patients underwent emergency surgery. The reason for the lack of LLVU in the remaining 28 patients was unknown. In contrast, 22 patients in the DG underwent LLVU despite having a normal D-dimer value (22/950: 2.3%) due to clinical symptoms suggestive of DVT, such as lower limb swelling or redness; among these patients, DVT was detected in 1 patient. In the DG, among 246 patients with a D-dimer value higher than 1.0 μg/ml, preoperative LLVU was performed in 209 patients (209/246: 85.0%), among whom DVT was detected in 27 patients (27/209: 12.9%). Overall, 277 LLVUs were performed, among which DVT was detected in 34 cases (12.3%). In the CG, DVT was detected in 0.7% (6/875) of patients, whereas in the DG, it was detected in 2.3% (28/1196) of patients. The patients undergoing LLVU in the CG exhibited a significantly higher level of preoperative D-dimer (range: 0.5-43.5 μg/ml) than those undergoing LLVU in the DG (range: 0.5-26.9 μg/ml) (p=0.0006).
Figure 1.
Preoperative LLVU treatment profile in the CG and DG.
LLVU: lower limb venous ultrasound, DVT: deep venous thrombosis
Preoperative management of DVT
The management of preoperatively detected DVT was dependent on the risk of developing embolism. Low-risk DVT indicated a chronic, organized DVT which was unlikely to develop embolism; whereas high-risk DVT indicated an acute, friable DVT which was likely to cause embolism. Low-risk DVT was observed in 5 patients from the CG (5/6: 83.3%) and in 24 patients from the DG (24/28: 85.7%) without a significant difference between the two groups (p>0.99). These patients required no specific preoperative and postoperative prophylaxis for VTE. On the other hand, high-risk DVT was observed in 1 patient from CG, who underwent preoperative treatment with oral rivaroxaban. A decrease in size of DVT was confirmed by a follow-up LLVU. Subcutaneous administration of enoxaparin was performed as postoperative prophylaxis. In DG, high-risk DVT was observed in 4 patients. All of them underwent preoperative prophylaxis including anticoagulant agents (oral rivaroxaban or intravenous heparin) or IVC filter placement, the effectiveness of which was confirmed by LLVU or contrast-enhanced computed tomography. Intravenous heparin was administered as postoperative prophylaxis for VTE.
Postoperative VTE
Postoperative prophylactic anticoagulants were administered in 43 patients in the CG (4.9%) versus 79 patients in the DG (6.6%), which was not a significant difference (p=0.11). Table 2 shows the results regarding postoperative VTE in this study. The occurrence of postoperative symptomatic VTE was evaluated at 3 and 6 months after surgery. The appearance of asymptomatic VTE was also evaluated in both groups. The incidence of symptomatic VTE was significantly lower in the DG at both 3 and 6 months after surgery than in the CG (0.2% vs. 0.8%, p=0.041; 0.3% vs. 1.1%, p=0.020, respectively). In contrast, the incidence of postoperative asymptomatic VTE after 6 months was significantly higher in the DG than in the CG (1.4% vs. 0.3%, p=0.012).
Table 2.
The Results Regarding Postoperative LLVU† and VTE‡.
| Total (n=2071) | CG (n=875) | DG (n=1196) | p value | |
|---|---|---|---|---|
| Number of postoperative LLVUs | 266 (12.8%) | 115 (13.1%) | 151 (12.6%) | 0.74 |
| Symptomatic VTE | ||||
| within 3 months from surgery | 9 (0.4%) | 7 (0.8%) | 2 (0.2%) | 0.041 |
| PE§ | 3 (0.1%) | 2 (0.2%) | 1 (0.1%) | |
| proximal DVT|| | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | |
| distal DVT | 4 (0.2%) | 4 (0.5%) | 0 (0%) | |
| within 6 months from surgery | 13 (0.6%) | 10 (1.1%) | 3 (0.3%) | 0.020 |
| PE | 3 (0.1%) | 2 (0.2%) | 1 (0.1%) | |
| proximal DVT | 5 (0.2%) | 3 (0.3%) | 2 (0.2%) | |
| distal DVT | 5 (0.2%) | 5 (0.6%) | 0 (0%) | |
| Asymptomatic VTE | ||||
| within 6 months from surgery | 20 (1.0%) | 3 (0.3%) | 17 (1.4%) | 0.012 |
| PE | 1 (0.05%) | 0 (0%) | 1 (0.1%) | |
| proximal DVT | 7 (0.3%) | 0 (0%) | 7 (0.6%) | |
| distal DVT | 12 (0.6%) | 3 (0.3%) | 9 (0.8%) |
†LLVU, lower limb venous ultrasound; ‡VTE, venous thromboembolism; §PE, pulmonary embolism; ||DVT, deep venous thromboembolism
Univariate and multivariate analysis to identify risk factors for postoperative symptomatic VTE
The clinical variables listed in Table 3 were assessed for an association with the occurrence of postoperative symptomatic VTE within 6 months of surgery using univariate and multivariate logistic regression models. Past medical history of smoking and PE, use of postoperative anticoagulants, as well as treatment following the CG protocol showed an association with the occurrence of postoperative VTE (p=0.088, <0.0001, 0.002, 0.020, respectively) in univariable analysis and were incorporated into multivariable analysis. After multivariate analysis, a past medical history of PE and treatment following the CG protocol were independent risk factors for postoperative symptomatic VTE within 6 months of surgery (p<0.0001 and =0.036, respectively).
Table 3.
Univariate and Multivariate Analysis for Factors of Postoperative Symptomatic VTE† at 6 Months from Surgery.
| Univariate
Number of patients |
p value | Multivariate
OR‡ (95 % CI§) |
p value | |
|---|---|---|---|---|
| Sex | 0.28 | |||
| male | 5 | |||
| female | 8 | |||
| Age (years old) | 0.58 | |||
| ≥68 | 7 | |||
| <68 | 6 | |||
| BMI¶ (kg/m2) | 0.63 | |||
| ≥27 | 0 | |||
| <27 | 13 | |||
| Resected area | 0.28 | |||
| colon | 5 | |||
| rectum | 8 | |||
| Surgical procedure | 0.13 | |||
| laparotomy | 7 | |||
| laparoscopy | 6 | |||
| clinical Stage | >0.99 | |||
| I, II, III | 8 | |||
| IV | 1 | |||
| Past history | ||||
| smoking | 4 | 0.088 | 0.44 (0.13 - 1.55) | 0.20 |
| hypertension | 3 | 0.56 | ||
| hyperlipidemia | 1 | >0.99 | ||
| diabetes mellitus | 1 | >0.99 | ||
| pulmonary embolism | 9 | <0.0001 | 27.30 (7.27 - 102.43) | <0.0001 |
| myocardial infarction | 0 | >0.99 | ||
| heart failure | 1 | 0.34 | ||
| radiotherapy | 0 | >0.99 | ||
| chemotherapy | 1 | >0.99 | ||
| Preoperative CEA (ng/ml) | 0.55 | |||
| ≥5 | 5 | |||
| <5 | 8 | |||
| Preoperative CA19-9 (U/ml) | 0.66 | |||
| ≥38 | 2 | |||
| <38 | 11 | |||
| Preoperative D-dimer (μg/ml) | 0.72 | |||
| >1.0 | 4 | |||
| ≤1.0 | 9 | |||
| Postoperative anticoagulants | 0.002 | |||
| use | 10 | 3.29 (0.78 - 13.91) | 0.11 | |
| nonuse | 3 | |||
| LLVU†† policy | 0.020 | |||
| CG | 10 | 4.20 (1.10 - 16.08) | 0.036 | |
| DG | 3 |
†VTE, venous thromboembolism; ‡OR, odds ratio; §CI, confidential interval; ¶BMI, body mass index; ††LLVU, lower limb venous ultrasound
Discussion
Our results showed that in patients undergoing colorectal resection, the incidence of postoperative symptomatic VTE was significantly lower when preoperative LLVU was performed in patients with a D-dimer value>1.0 μg/ml than when LLVU was performed based on clinical indications. Wada et al. performed the same preoperative screening protocol for DVT as in our study in preoperative patients with gastric cancer[20]. Although they reported that neoadjuvant chemotherapy was significantly associated with the preoperative detection of DVT, they did not discuss the clinical impact of the preoperative screening protocol on the occurrence of postoperative VTE. Our study demonstrated that patients with a preoperative D-dimer>1.0 μg/ml may benefit from LLVU from the perspective of preventing postoperative symptomatic VTE.
Several preoperative factors are reported to be associated with postoperative DVT in patients with colorectal resection, among which elevated D-dimer was reported by Stender et al. to be associated with a 6.5-fold higher risk of postoperative DVT[5]. Although this study suggests that preoperative D-dimer can identify patients at risk for postoperative DVT, it does not refer to the impact of preoperative D-dimer on the occurrence of postoperative symptomatic VTE, which is the preferred outcome over asymptomatic VTE[21]. The current study demonstrated that D-dimer alone did not have a significant association with postoperative symptomatic VTE (p=0.72; Table 3); in contrast, treatment following the DG protocol, in which patients with a preoperative D-dimer>1.0 μg/ml underwent LLVU, significantly prevented the occurrence of postoperative symptomatic VTE (p=0.036). This result suggests that both identifying high-risk patients by using the D-dimer value as well as detecting preoperative DVT by LLVU and providing proper management may have played important roles in the prevention of postoperative symptomatic VTE. Although our results also suggest that preoperative LLVU can possibly be omitted in patients with a D-dimer≤1.0 μg/ml, the confirmation of this hypothesis requires further studies with prospective designs.
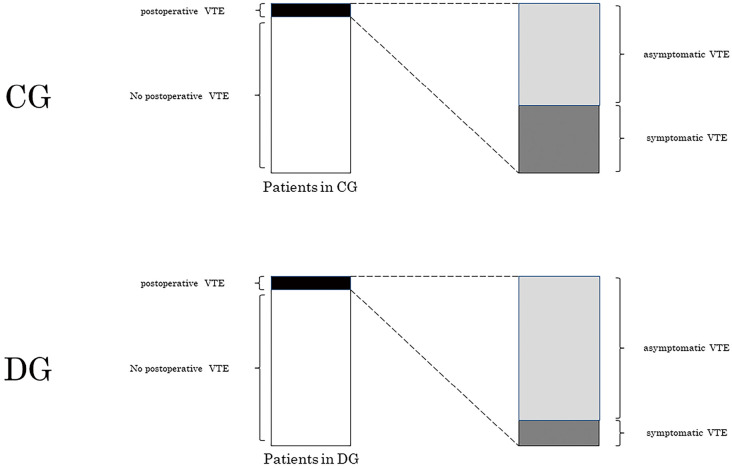
It is difficult to establish clinical evidence of good quality regarding the prevention of postoperative symptomatic VTE because a large number of patients must be enrolled given the low incidence of the disease. To overcome this problem, some studies set postoperative asymptomatic VTE, which is a more common finding than postoperative symptomatic VTE, as a surrogate endpoint to assess the efficacy of preoperative intervention[22]. However, because the American guidelines recommend symptomatic VTE as a more favorable endpoint than asymptomatic VTE[7], we set postoperative symptomatic VTE as our primary endpoint. In our study, postoperative asymptomatic DVT was significantly more frequent in the DG than in the CG. This seemingly contradicting observation is probably attributable to the larger number of patients in the DG with preoperative DVT confirmed by LLVU. In the current study, an overall rate of postoperative VTE (i.e., asymptomatic and symptomatic VTE) was comparable between the CG (13/875: 1.5%) and the DG (20/1196: 1.7%) (p=0.86). Although our screening method did not reduce the rate of “overall” occurrence of postoperative VTE, it significantly reduced the rate of postoperative symptomatic VTE in the DG compared with that in the CG, probably resulting in a relative increase in the rate of asymptomatic VTE in the DG (Figure 2). Moreover, this result indicates that postoperative asymptomatic VTE cannot be a surrogate endpoint for postoperative symptomatic VTE in cancer patients.
Figure 2.
Our hypothesis of an increased number of asymptomatic VTE in DG.
An overall incidence of postoperative VTE (i.e., asymptomatic and symptomatic VTE) did not significantly differ between the two groups (black bar on the left). However, the rate of symptomatic VTE was significantly higher in the CG than in the DG, which resulted in a relative increase in the rate of asymptomatic VTE in the DG (gray bar on the right). CG, clinical indication group; DG, D-dimer orientated group; VTE, venous thromboembolism
Since preoperative DVT can result in postoperative VTE[4], preoperatively detecting DVT is of clinical importance. The prevalence of preoperative DVT in patients with colorectal resection varies from study to study. Although Stender et al. reported that the prevalence is 7.8%[3], we must be aware that the study predominantly included Caucasian patients, and 26% of them had an ASA-PS of III-IV. In contrast, Alcalay et al. reported that the prevalence of DVT in Asian patients with CRC is approximately 2%[23]. Considering that preoperative LLVU revealed 28 patients in the DG had DVT in our study (28/1196: 2.3%), the use of a combination of the D-dimer value and LLVU may be a potent strategy to identify almost every patient with DVT. The preoperative recruitment of most DVT patients based on the combination of D-dimer value and LLVU, followed by proper management, probably resulted in a significantly decreased occurrence rate of postoperative symptomatic VTE.
There are some limitations in this study that need to be addressed. First, due to the retrospective nature of this study, we need to be careful in interpreting the results of the study regarding the clinical efficacy of the preoperative D-dimer value and LLVU in association with postoperative symptomatic VTE. Specifically, a threshold of D-dimer value to perform preoperative LLVU was not prepared in the CG. Second, postoperative pharmacological thromboprophylaxis was not a routine intervention in our patients, although it is recommended in the guidelines from the American Society of Clinical Oncology[24]. Due to the unique epidemiology of VTE in our country, the Japanese guidelines recommend either IPC devices or postoperative unfractionated heparin for cancer patients aged over 40 undergoing major surgeries[17]. This difference in the indications for postoperative thromboprophylaxis between Japan and America implies that caution is needed in the interpretation of the observed results. Third, due to the high false positivity of D-dimer discussed above, some hospitals may face financial problems in performing LLVU for all patients with abnormal preoperative D-dimer values. The application of our LLVU strategy requires considerably high costs in terms of human resources and equipment.
In conclusion, the preoperative D-dimer value was a useful marker because the rate of postoperative symptomatic VTE was significantly reduced by performing preoperative LLVU in patients with a D-dimer value higher than 1.0 μg/ml. This simple screening procedure for D-dimer and LLVU is beneficial in because it can potentially reduce the incidence of fatal postoperative complications following colorectal surgery.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
HI and MY have made substantial contributions to the conception and design of the work, and the acquisition, analysis, and interpretation of data, and have drafted the work and revised it. NN, NH, JN, CM have made substantial contributions to the conception and design of the work, and the analysis, and interpretation of data. HM, MO and MS have made substantial contributions to the conception and design of the work, and have drafted the work and revised it. All authors read and approved the final manuscript.
Approval by Institutional Review Board (IRB)
The current study has been approved by the research ethics committee of Osaka International Cancer Institute with approval number 18033-3.
Acknowledgements
We express our appreciation to Dr. Nariaki Matsuura, the president of Osaka International Cancer Institute, and to cooperative staff in the Clinical Research Administration Center for providing permission to plan and conduct the present study. We are also grateful to our colleagues in the Department of Onco-Cardiology and Department of Rehabilitation for their caring support of the patients.
References
- 1.Heit JA. Epidemiology of venous thromboembolism. Nature Reviews Cardiology. 2015 Aug; 12(8): 464-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of Thrombosis and Haemostasis. 2007 Mar; 5(3): 632-4. [DOI] [PubMed] [Google Scholar]
- 3.Stender MT, Nielsen TSH, Frøkjær JB, et al. High preoperative prevalence of deep venous thrombosis in patients with colorectal cancer. British Journal of Surgery. 2007 Sep; 94(9): 1100-3. [DOI] [PubMed] [Google Scholar]
- 4.Pandey A, Thakur B, Hogg F, et al. The role of preoperative deep vein thrombosis screening in neurooncology. Journal of Neurosurgery. 2018 Mar; 130(1): 38-43. [DOI] [PubMed] [Google Scholar]
- 5.Stender MT, Frøkjaer JB, Larsen TB, et al. Preoperative plasma D-dimer is a predictor of postoperative deep venous thrombosis in colorectal cancer patients: a clinical, prospective cohort study with one-year follow-up. Dis Colon Rectum. 2009 Mar; 52(3): 446-51. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa K, Watanabe J, Suwa Y, et al. Clinical analysis of preoperative deep vein thrombosis risk factors in patients with colorectal cancer: Retrospective observational study. Annals of Gastroenterological Surgery. 2019 Apr; 3(4): 451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb; 141(2 Suppl): e185S-e94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James DB, Mary KG, Christian W. International Union Against Cancer (UICC): TNM Classification of Malignant Tumours. 8th ed. Oxford: Wiley-Blackwell; 2017. 272 p. [Google Scholar]
- 9.Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008 May; 111(10): 4902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y-J, Liu Z-H, Yao F-J, et al. Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis. PLoS Medicine. 2013 Sep; 10(9): e1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Xue Y, Peng Y, et al. Analysis of risk factors for deep venous thrombosis in patients with gynecological malignant tumor: A clinical study. Pakistan Journal of Medical Sciences. 2019 Jan-Feb; 35(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ageno W, Becattini C, Brighton T, et al. Cardiovascular Risk Factors and Venous Thromboembolism: a meta-analysis. Circulation. 2008 Jan; 117(1): 93-102. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Indresano AT, O'Ryan F. Venous Thromboembolism in Oral and Maxillofacial Surgery: A Review of the Literature. Journal of Oral and Maxillofacial Surgery. 2011 Mar; 69(3): 840-4. [DOI] [PubMed] [Google Scholar]
- 14.Rinde LB, Lind C, Småbrekke B, et al. Impact of incident myocardial infarction on the risk of venous thromboembolism: the Tromsø Study. Journal of Thrombosis and Haemostasis. 2016 Jun; 14(6): 1183-91. [DOI] [PubMed] [Google Scholar]
- 15.Smilowitz NR, Zhao Q, Wang L, et al. Risk of Venous Thromboembolism after New Onset Heart Failure. Scientific Reports. 2019 Nov; 9(1): 17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandalà M, Barni S, Prins M, et al. Acquired and inherited risk factors for developing venous thromboembolism in cancer patients receiving adjuvant chemotherapy: a prospective trial. Annals of Oncology. 2010 Apr; 21(4): 871-6. [DOI] [PubMed] [Google Scholar]
- 17.Jcs Joint Working G. Guidelines for the Diagnosis, Treatment and Prevention of Pulmonary Thromboembolism and Deep Vein Thrombosis (JCS 2009) - Digest Version. Circulation Journal. 2011; 75(5): 1258-81. [DOI] [PubMed] [Google Scholar]
- 18.Vedovati MC, Becattini C, Rondelli F, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Annals of Surgery. 2014 Apr; 259(4): 665-9. [DOI] [PubMed] [Google Scholar]
- 19.Walker AJ, West J, Card TR, et al. Variation in the risk of venous thromboembolism in people with colorectal cancer: a population-based cohort study from England. Journal of Thrombosis and Haemostasis. 2014 May; 12(5): 641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada T, Fujiwara H, Morita S, et al. Incidence of and risk factors for preoperative deep venous thrombosis in patients undergoing gastric cancer surgery. Gastric Cancer. 2017 Sep; 20(5): 872-7. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb; 141(2 Suppl): 7s-47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segers AEM, Prins MH, Lensing AWA, et al. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? Journal of Thrombosis and Haemostasis. 2005 May; 3(5): 1099-102. [DOI] [PubMed] [Google Scholar]
- 23.Alcalay A, Wun T, Khatri V, et al. Venous Thromboembolism in Patients With Colorectal Cancer: Incidence and Effect on Survival. Journal of Clinical Oncology. 2006 Mar; 24(7): 1112-8. [DOI] [PubMed] [Google Scholar]
- 24.Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. Journal of Clinical Oncology. 2020 Feb; 38(5): 496-520. [DOI] [PubMed] [Google Scholar]