Abstract
Background
There is a dearth of drug utilization studies for coronavirus disease 2019 (COVID-19) treatments in 2021 and beyond after the introduction of vaccines and updated guidelines; such studies are needed to contextualize ongoing COVID-19 treatment effectiveness studies during these time periods. This study describes utilization patterns for corticosteroids, interleukin-6 (IL-6) inhibitors, Janus kinase inhibitors, and remdesivir among hospitalized adults with COVID-19, over the entire hospitalization, and within hospitalization periods categorized by respiratory support requirements.
Methods
This descriptive cohort study included United States adults hospitalized with COVID-19 admitted from 1 January 2021 through 1 February 2022; data included HealthVerity claims and hospital chargemaster. The number and distribution of patients were reported for the first 3 drug regimen lines initiated.
Results
The cohort included 51 066 patients; the most common initial drug regimens were corticosteroids (23.4%), corticosteroids plus remdesivir (25.1%), and remdesivir (4.4%). IL-6 inhibitors and Janus kinase inhibitors were included in later drug regimens and were more commonly administered with both corticosteroids and remdesivir than with corticosteroids alone. IL-6 inhibitors were more commonly administered than Janus kinase inhibitors when patients received high-flow oxygen or ventilation.
Conclusions
These findings provide important context for comparative studies of COVID-19 treatments with study periods extending into 2021 and later. While prescribing generally aligned with National Institutes of Health COVID-19 treatment guidelines during this period, these findings suggest that prescribing preference, potential confounding by indication, and confounding by prior/concomitant use of other therapeutics should be considered in the design and interpretation of comparative studies.
Keywords: COVID-19, drug utilization, interleukin-6 inhibitors, Janus kinase inhibitors
This study provides an updated overview of inpatient COVID-19 drug utilization from January 2021 to February 2022, and suggests that prescribing preference and confounding by indication and prior treatments may influence the results of ongoing and future COVID-19 studies.
Graphical Abstract
Graphical abstract.
While the treatment landscape of coronavirus disease 2019 (COVID-19) has evolved rapidly since the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in late 2019, the burden of disease remains high in the United States (US), with novel variants of the virus continuing to contribute to hospitalizations and deaths [1]. Understanding longitudinal drug utilization patterns for patients hospitalized with COVID-19 is important for developing methods to evaluate the effectiveness of COVID-19 therapeutics using real-world data and to inform the ability of clinical practice to respond to the evolving availability of treatments and therapeutic guidelines. Several studies on inpatient COVID-19 drug utilization in the US have been published [2–15]. All but 2 of these studies described treatment patterns within the first year of the pandemic; Westhoff and colleagues [14] evaluated treatment patterns among pregnant women enrolled in a pregnancy registry through June 2021, and Diaczok and colleagues [15] included data through February 2021. Only 2 studies evaluated treatment patterns within levels of disease severity as defined by respiratory support requirement (RSR) [4, 12]. In the time following these study periods, the National Institutes of Health (NIH) COVID-19 treatment guidelines were revised to include interleukin-6 inhibitors (IL-6is) in April 2021 (US Food and Drug Administration [FDA] Emergency Use Authorization [EUA], November 2020) and Janus kinase inhibitors (JAKis) in July 2021 (FDA EUA, June 2021), as add-in therapy to dexamethasone or other systemic corticosteroids (CS) for patients with severe or critical COVID-19 disease (Supplementary Table 1.1) [16–22]. Since no currently published studies include the period during and after the addition of both JAKis and IL-6is, there is a need to better understand more recent drug utilization patterns. The objective of this study was to describe utilization patterns for CS, IL-6is, JAKis, and remdesivir among hospitalized adult patients with COVID-19 from January 2021 to February 2022 (1) during the entire patient's hospitalization; (2) during periods within the hospitalization, categorized by RSR; and (3) over calendar time.
METHODS
This observational descriptive cohort study characterizes drug utilization in adult patients hospitalized with COVID-19 in the US using HealthVerity Hospital chargemaster and Pharmacy and Medical Claims healthcare data, which comprises medical and pharmacy open and closed claims, and chargemaster administrative inpatient and outpatient hospital billing data. The data includes hospitals and other provider sites from all 50 states, and it includes all major payer types (commercial, Medicaid, and Medicare).
Patients eligible for inclusion in the study population were all those aged 18–84 years who had an inpatient hospital chargemaster event between 1 January 2021 and 1 February 2022, and a diagnosis of COVID-19 (International Classification of Diseases, Tenth Revision [ICD-10] code U07.1) at admission, or within the 7 days prior. Patients with missing age or sex, or without continuous medical claims enrollment during the 183 days (baseline period) before admission (60-day allowable gap) were excluded, to ensure observability in both the chargemaster and claims data.
For eligible patients, drug utilization was described from admission through death, discharge, or 28 days of hospitalization. For each day in which a patient was hospitalized, use of CS, IL-6i, JAKi, and remdesivir was sourced from procedure codes (ie, Healthcare Common Procedure Coding System/Current Procedural Terminology, Fourth Edition [HCPCS/CPT-4], ICD-10, Procedure Coding System [PCS]), and free-text fields from the inpatient chargemaster data. Continuous episodes of each drug event were defined using a 10-day allowable gap (per recommended drug administration protocols in NIH guidelines) [18–22]. A 183-day washout was applied to ensure only incident drug use was captured; patients who continued a drug that they began prior to hospitalization during this washout period could not qualify for incident use capture in that corresponding drug regimen. Continuous overlapping episodes for each drug or drug class were considered combination regimens (Supplementary Figure 1.2). For combination regimens, patients were required to be new to the combination (even if they had prior use of the drugs individually). They were excluded if there was overlap of the drugs during the washout period (ie, to be considered incident CS + remdesivir, patients could not have prior overlap of CS and remdesivir on any day during the washout period). An extension was added to account for the expected half-lives of all IL-6i (+10 days) and CS (+2 days) episodes, given that the half-life for these drugs was greater than 1 day [23, 24]; thus, a continued course of CS use immediately following tocilizumab administration was defined as tocilizumab + CS combination therapy, rather than incident use of CS alone. Drug episodes that did not overlap with any other continuous drug episodes, after applying the extension, were considered monotherapies. The first drug regimen initiated is herein referred to as the first-line drug regimen. Any change to an incident drug regimen (eg, drug switching, augmentation, or discontinuation) was considered a subsequent drug regimen line (ie, second-line, third-line).
Sankey diagrams with descriptive statistics characterized the first 3 drug regimen lines over the time of hospitalization, and over the time categorized by RSR. Each hospital day was categorized using HCPCS/CPT and ICD-10-PCS codes and free-text chargemaster descriptions by increasing severity based on oxygen requirements: (1) no oxygen or ventilation, (2) any conventional supplemental oxygen (O2), (3) any noninvasive ventilation or high-flow oxygen (NIV/HFO), and (4) any invasive mechanical ventilation or extracorporeal membrane oxygenation (IMV/ECMO) [25]. Patients were assigned to their highest category on a given day. Sankey diagrams were used to illustrate the first 3 drug regimen lines occurring during hospitalization time coinciding with IMV/ECMO, NIV/HFO, or O2, respectively. Time on RSR began on the first day with evidence of an RSR (ie, for the O2 analyses, time began the first day a patient received O2), and ended at the earliest of hospital discharge, death, 28 days hospitalized, or an increase in RSR (ie, patients on O2 were censored on the day they began NIV/HFO or IMV/ECMO).
Counts and percentages of patients initiating drug regimen lines by calendar week of hospital admission were plotted as bar and line graphs. Patient characteristics at admission, at the time of treatment initiation, and during the baseline period prior to admission were described in the overall cohort, and by drug regimen line (Supplementary Figure 1.3). Descriptive statistics for the first 3 sequential drug regimens included the number and percentage of patients and the median time to treatment (calculated as days between the start of either admission [entire hospitalization analysis] or first day on RSR [for analyses by RSR] and each drug regimen line).
RESULTS
Among the 1 119 940 patients with both chargemaster and medical claims data, 51 066 patients were hospitalized with COVID-19 and eligible for inclusion in the final study cohort used for analysis (January 2021 to February 2022) (Supplementary Table 1.4). The median age was 55 years (interquartile range, 39–65 years) and 55% of patients were female (Table 1 and Supplementary Table 2.2). Most patients were from the South (47.7%) and the least from the Midwest (7.9%). More than half (51.3%) of patients were on Medicaid, 19.6% on Medicare, and 28.2% were commercially insured; region and insurance type varied across initiator subgroups. Urban areas and nonteaching hospitals were heavily represented (88.4% and 58.9%, respectively). Of all the treatments evaluated, patients initiating remdesivir had slightly higher comorbidity scores and the highest prevalence of tobacco/smoking history. The average follow-up time was 8.53 days (standard deviation, 7.12); 8.5% of patients were censored at death, 4.7% were censored at maximum follow-up, and the remaining 86.8% of patients were censored at discharge.
Table 1.
Select Demographic and Clinical Characteristics of Hospitalized Cohort and Initiators of Selected Drug Regimens
| Characteristic | Hospitalized Cohort | Monotherapies | Combination Regimen | |||||
|---|---|---|---|---|---|---|---|---|
| CS | RDV | IL-6i + CS | JAKi + CS | CS + RDV | IL-6i + CS + RDV | JAKi + CS + RDV | ||
| N = 51 066 (100%) |
n = 17 012 (33.3%) |
n = 2313 (4.5%) |
n = 2722 (5.3%) |
n = 2295 (4.5%) |
n = 16 776 (32.8%) |
n = 1962 (3.8%) |
n = 2094 (4.1%) |
|
| Demographic and clinical characteristics | ||||||||
| Age, y | ||||||||
| Mean (SD) | 52.5 (16.6) | 55.2 (16.0) | 55.8 (15.6) | 56.0 (13.9) | 56.0 (13.4) | 55.4 (14.6) | 55.1 (13.7) | 55.4 (13.8) |
| Median (IQR) | 55 (39–65) | 58 (44–67) | 58 (45–67) | 57 (47–65) | 58 (47–65) | 57 (46–65) | 56 (46–64) | 57 (46–64) |
| Sex | ||||||||
| Female | 28 076 (55.0) | 8620 (50.7) | 1216 (52.6) | 1304 (47.9) | 1118 (48.7) | 8554 (51.0) | 924 (47.1) | 1007 (48.1) |
| US Census region | … | … | 1097 (47.4) | 1418 (52.1) | 1177 (51.3) | 8222 (49.0) | 1038 (52.9) | 1087 (51.9) |
| Northeast | 10 529 (20.6) | 3356 (19.7) | … | … | … | … | … | … |
| Midwest | 4016 (7.9) | 1538 (9.0) | 638 (27.6) | 726 (26.7) | 343 (14.9) | 3951 (23.6) | 607 (30.9) | 484 (23.1) |
| South | 24 364 (47.7) | 7907 (46.5) | 88 (3.8) | 201 (7.4) | 358 (15.6) | 857 (5.1) | 79 (4.0) | 161 (7.7) |
| West | 12 153 (23.8) | 4209 (24.7) | 1106 (47.8) | 1227 (45.1) | 994 (43.3) | 7764 (46.3) | 863 (44.0) | 908 (43.4) |
| Other/missing | 4 (<0.1) | 2 (<0.1) | 481 (20.8) | 568 (20.9) | 600 (26.1) | 4203 (25.1) | 413 (21.0) | 541 (25.8) |
| Insurance typea | ||||||||
| None recorded | 424 (0.8) | 138 (0.8) | 14 (0.6) | 21 (0.8) | 12 (0.5) | 118 (0.7) | 10 (0.5) | 6 (0.3) |
| Commercial only | 14 393 (28.2) | 4762 (28.0) | 591 (25.6) | 1000 (36.7) | 884 (38.5) | 5590 (33.3) | 764 (38.9) | 734 (35.1) |
| Medicare | 10 030 (19.6) | 3667 (21.6) | 611 (26.4) | 592 (21.7) | 424 (18.5) | 3401 (20.3) | 368 (18.8) | 379 (18.1) |
| Medicaid | 26 219 (51.3) | 8445 (49.6) | 1097 (47.4) | 1109 (40.7) | 975 (42.5) | 7667 (45.7) | 820 (41.8) | 975 (46.6) |
| Baseline clinical characteristics | ||||||||
| Comorbidity index (Gange) | ||||||||
| Mean (SD) | 3.1 (3.3) | 2.8 (3.0) | 3.3 (3.4) | 2.9 (2.9) | 2.5 (2.7) | 2.6 (2.9) | 2.7 (2.8) | 2.4 (2.7) |
| Median (IQR) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–4.0) | 2.0 (0.0–4.0) | 2.0 (1.0–4.0) | 2.0 (0.0–4.0) |
| Hospital characteristics | ||||||||
| Hospital setting | ||||||||
| Urban | 45 146 (88.4) | 15 007 (88.2) | 1831 (79.2) | 2400 (88.2) | 2084 (90.8) | 14 779 (88.1) | 1787 (91.1) | 1914 (91.4) |
| Rural | 4568 (8.9) | 1514 (8.9) | 428 (18.5) | 242 (8.9) | 155 (6.8) | 1474 (8.8) | 120 (6.1) | 126 (6.0) |
| Other/missing | 1352 (2.6) | 491 (2.9) | 54 (2.3) | 80 (2.9) | 56 (2.4) | 523 (3.1) | 55 (2.8) | 54 (2.6) |
| Hospital teaching status | ||||||||
| Teaching hospital | 20 500 (40.1) | 6614 (38.9) | 1096 (47.4) | 973 (35.7) | 726 (31.6) | 6185 (36.9) | 702 (35.8) | 754 (36.0) |
| Nonteaching | 30 097 (58.9) | 10 247 (60.2) | 1190 (51.4) | 1744 (64.1) | 1532 (66.8) | 10 445 (62.3) | 1256 (64.0) | 1305 (62.3) |
| Other/missing | 469 (0.9) | 151 (0.9) | 27 (1.2) | 5 (0.2) | 37 (1.6) | 146 (0.9) | 4 (0.2) | 35 (1.7) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CS, corticosteroids; IL-6i, interleukin-6 inhibitor; IQR, interquartile range; JAKi, Janus kinase inhibitor; RDV, remdesivir; SD, standard deviation.
Insurance status was defined via mutually exclusive categories, where patients with any Medicaid were assigned to the Medicaid group, patients with any Medicare and no Medicaid were assigned to the Medicare group, and patients with commercial insurance only were assigned to the commercial group.
More than half of the patients in the cohort (n = 30 938 [60.6%]) were hospitalized after both JAKis and IL-6is were added to the NIH guidelines after 8 July 2021 (Figure 1). The most common drug regimens initiated were CS monotherapy (33.3%) and CS + remdesivir (32.8%). Nearly all of the IL-6i use (>98%) was tocilizumab, and nearly all JAKi use (>99%) was baricitinib. Few patients initiated IL-6i + CS (5.3%), remdesivir (4.5%), JAKi + CS (4.5%), JAKi + CS + remdesivir (4.1%), and IL-6i + CS + remdesivir (3.8%). All other combinations of interest were each observed in <1% of the cohort (Supplementary Table 1.4).
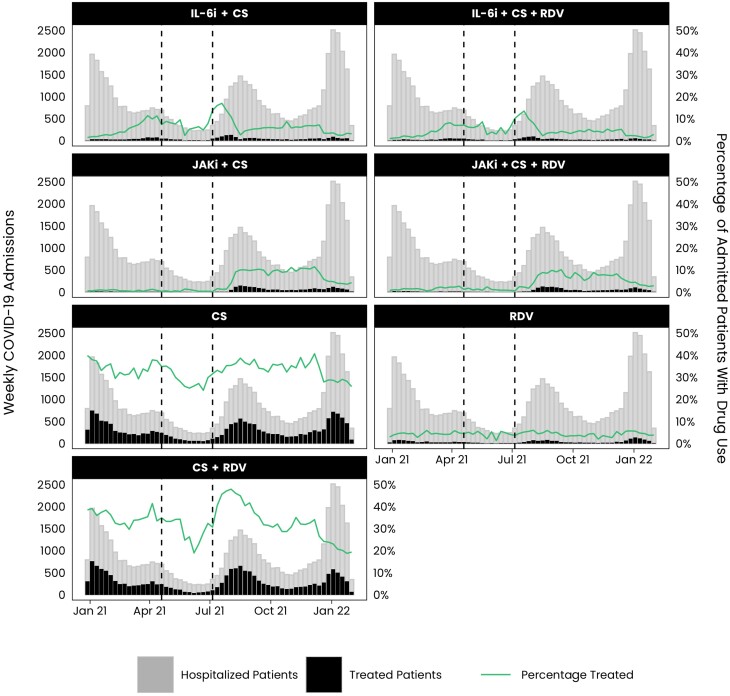
Figure 1.
Number (%) of weekly coronavirus disease 2019 (COVID-19) admissions treated with a drug regimen. Cohort entry date is from 1 January 2021 to 1 February 2022. Interleukin-6 inhibitor (IL-6i), Janus kinase inhibitor (JAKi), IL-6i + JAKi, IL-6i + remdesivir (RDV), JAKi + RDV, IL-6i + JAKi + corticosteroids (CS), IL-6i + JAKi + RDV, and IL-6i + JAKi + CS + RDV regimens were removed due to low sample size. Vertical lines mark changes in National Institutes of Health guidelines [18–22]. The first line occurs on 21 April 2021, marking when IL-6is were added to treatment guidelines, while the second line occurs on 8 July 2021, marking when JAKis were added to treatment guidelines.
Treatment Initiation Over Calendar Time
The number and percentage of patients initiating each regimen, stratified by the week of admission, are shown in Figure 1. The use of remdesivir monotherapy was relatively stable over time, whereas CS + remdesivir and CS monotherapy generally varied with hospitalization trends, with lower use during May–June 2021 and higher use in July–September 2021. Treatment regimens that included IL-6is increased gradually prior to their addition to the NIH treatment guidelines in April 2021, and use remained relatively higher through July 2021 before decreasing. Use of JAKis remained low until their addition to the NIH guidelines in July 2021. Prior to July 2021, IL-6i regimens were more common than JAKi regimens; however, the use of JAKis surpassed the levels of IL-6i shortly after July 2021. A decrease in the percentage of patients treated (all drug regimens) was seen in December 2021 through February 2022.
Treatment Patterns Over All Hospitalization Time
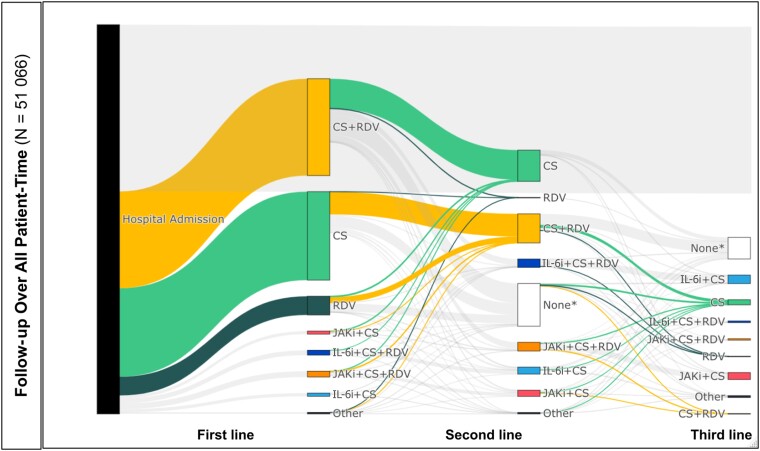
The Sankey diagrams in Figure 2 highlight treatment patterns for initiating CS, CS + remdesivir, and remdesivir, as those regimens were the most common over time (additional details can be found in Table 2 and Supplementary Table 2.3). More than half of patients (57.7%) received at least 1 incident drug regimen of interest during their hospitalization. The most common first-line regimens were CS + remdesivir (25.1%) and CS alone (23.4%). These drug regimens comprised a similar proportion of second-line therapies but were less commonly observed as third-line. There was substantial crossover between CS alone and CS + remdesivir as patients moved from first-line to second-line treatment. Of patients with first-line CS alone, 25.6% augmented to CS + remdesivir combination therapy for their second-line treatment; of those with first-line CS + remdesivir, 30.6% of patients discontinued or completed their remdesivir regimen to have CS alone as their second-line regimen. Remdesivir was the next most prevalent regimen in the first-line treatment (4.4%), though use of remdesivir monotherapy was rare in the second and third lines of treatment.
Figure 2.
Treatment pathways. *None: Did not receive incident treatment of interest during hospitalization. Abbreviations: CS, corticosteroids; IL-6i, interleukin-6 inhibitor; JAKi, Janus kinase inhibitor; RDV, remdesivir.
Table 2.
Descriptive Characteristics of Drug Regimen Pathways by Drug Regimen Line
| Characteristic | Drug Group | |||||
|---|---|---|---|---|---|---|
| No. (%) of Patients in Drug Group (Relative to Remaining Patients) |
Median Time to Treatment, Days (Follow-up Start to Treatment) |
|||||
| First-Line | Second-Line | Third-Line | First-Line | Second-Line | Third-Line | |
| Total patients continuing to line (cumulative % continuing to line) | ||||||
| Total patients remaining | 51 066 (100) | 17 707 (34.7) | 6259 (12.3) | … | … | … |
| No. of patients with each drug regimen (% with each drug regimen of those remaining) | ||||||
| CS | 11 947 (23.4) | 4159 (23.6) | 683 (11.0) | 0 | 5 | 5 |
| RDV | 2271 (4.4) | 17 (0.1) | 18 (0.3) | 0 | 3 | 10 |
| CS + RDV | 12 832 (25.1) | 3894 (22.1) | 46 (0.7) | 0 | 1 | 3 |
| IL-6i + CS | 422 (0.8) | 922 (5.2) | 1120 (18.1) | 1 | 5 | 5 |
| IL-6i + CS + RDV | 632 (1.2) | 1098 (6.2) | 218 (3.5) | 0 | 1 | 3 |
| Any IL-6i combination therapy (IL-6i + CS and IL-6i + CS + RDV) |
1054 (2.1) | 2020 (11.4) | 1338 (21.4) | … | … | … |
| JAKi + CS | 403 (0.8) | 818 (4.6) | 911 (14.7) | 1 | 5 | 5 |
| JAKi + CS + RDV | 761 (1.5) | 1146 (6.5) | 187 (3.0) | 0 | 1 | 3 |
| Any JAKi combination therapy (JAKi + CS and JAKi + CS + RDV) |
1164 (2.3) | 1964 (11.1) | 1098 (17.5) | … | … | … |
| No treatmenta | 21 603 (42.3) | 5408 (30.7) | 2814 (45.4) | 0 | 5 | 7 |
Abbreviations: CS, corticosteroids; IL-6i, interleukin-6 inhibitor; JAKi, Janus kinase inhibitor; RDV, remdesivir.
No treatment in the first-line column indicates patients who never received treatment during their hospital time and therefore were not assigned a first-line treatment.
Sankey diagrams highlighting regimens that include IL-6is and JAKis can be found in Supplementary Figure 2.1B and 2.1C, respectively. Regimens that included IL-6is and JAKis became more common in later drug regimen lines after other drugs (largely CS and CS + remdesivir) were first administered. A similar number of patients initiated IL-6i combination regimens and JAKi regimens across all regimen lines (0.8% initiated first-line IL-6i and first-line JAKi combinations, 5.2% and 4.6% initiated second-line IL-6i and JAKi combinations, and 18.1% and 14.7% initiated third-line IL-6i and JAKi combinations, respectively). The median time from admission to first-line drug regimen was 0 days for CS and for all combinations including remdesivir, and 1 day for JAKi + CS and IL-6i + CS. Median time from admission to second- and third-line drug regimen lines ranged from 1 to 5 days and 3 to 7 days, respectively.
Treatment Patterns Over Time Categorized by Respiratory Support Requirements
Sankey diagrams describing treatment patterns over time, stratified by increasing severity of RSR, can be found in Supplementary Figure 2.1D–L, Table 2, and Supplementary Table 2.3; 11 382 (22.3%) patients contributed at least 1 day to O2, 9999 (19.6%) to NIV/HFO, and 6277 (12.3%) to IMV/ECMO. Among patients with at least 1 day on O2, 48.4% had at least 1 incident drug regimen on the same day or after initiating O2 and prior to censoring or worsening of RSR to NIV or IMV; patients who either did not initiate any drugs or initiated drugs prior to beginning O2 were considered as never having an incident drug regimen while on O2. Similarly, 56.2% of patients with at least 1 day on NIV/HFO and 41.8% of patients with at least 1 day on IMV/ECMO had at least 1 incident drug regimen on the same day or after initiating NIV/HFO or IMV/ECMO, respectively.
Across all RSRs, CS and CS + remdesivir were the most common first-line regimens. Second-line CS and CS + remdesivir were more common among patients receiving O2 than those receiving NIV/HFO or IMV/ECMO; third-line CS was most common among patients receiving O2 and NIV/HFO relative to IMV/ECMO. Remdesivir monotherapy was primarily a first-line drug regimen and was more common among O2- and NIV/HFO-treated patients, compared to patients receiving IMV/ECMO (Supplementary Figure 2.1D, G, and J). Combinations that included JAKis were more common than combinations that included IL-6is across nearly all treatment lines during time on O2 (Supplementary Figure 2.1E and 2.1F). Conversely, IL-6i combinations were more common than JAKi combinations across all drug regimen lines during time on NIV/HFO and IMV/ECMO (Supplementary Figure 2.1H–I and K–L). Notably, JAKi combinations were observed in IMV/ECMO groups (first-line: 4.0%, second-line: 7.0%, third-line: 5.7%) (Supplementary Figure 2.1L).
DISCUSSION
This study of patients hospitalized with COVID-19 is the first to describe real-world drug utilization patterns for CS, IL-6is, JAKis, and remdesivir throughout 2021 and early 2022 in a large US data source. As anticipated based on NIH treatment guidelines for therapeutic management of hospitalized patients with COVID-19 and distributions of patients by severity level, the most common therapies over the study period were CS and CS + remdesivir. Temporal trends in the use of these drugs varied. While the percentage of patients using remdesivir monotherapy was relatively stable over calendar time, the percentage of patients using either CS + remdesivir or CS use alone followed hospitalization trends, with lower use during May–June 2021 coinciding with relatively fewer hospitalizations, and higher use in July–September 2021 coinciding with an increase in hospitalizations. The reasons for this relative increase is unclear. IL-6i (primarily tocilizumab) and JAKi (primarily baricitinib) uptake increased corresponding with changes in NIH treatment guidelines to include tocilizumab (in April 2021) and baricitinib (in July 2021). JAKi use, which had been lower than that of IL-6i, surpassed IL-6i use after its addition to the NIH treatment guidelines in July 2021. Despite high numbers of hospitalizations, a decrease in the percentage of patients treated (all drug regimens) was seen in December 2021–February 2022. Potential reasons for the decrease during this time might include changes in treatment patterns or an increased proportion of incidental diagnoses associated with the highly contagious SARS-CoV-2 variant Omicron. These findings illustrate that there was substantial variation in prescribing practice by treatment over the study period; thus, adjusting for or stratifying by calendar time in comparative observational studies is crucial to ensure comparability between exposure groups.
CS, which was recommended for use among patients requiring any level of respiratory support, and CS + remdesivir, which was recommended for use in patients requiring supplemental O2 or NIV/HFO, were the most common first- and second-line treatments, overall and by RSR. Remdesivir was usually administered as an initial drug treatment and was rare for patients with IMV/ECMO. These findings suggest that prescribing largely followed NIH treatment guidelines in place during the study period, with patients often receiving CS, remdesivir, or a combination as their first-line drug regimen before augmenting with an IL-6i or JAKi.
The percentage of patients on JAKi and IL-6i combinations varied by drug regimen line and RSR. Combinations including CS and remdesivir (JAKi + CS + remdesivir and IL-6i + CS + remdesivir) were mostly observed as second-line treatments, while JAKi + CS and IL-6i + CS were mostly third-line treatments. However, these patterns may be attributed in part to overlaps between initial treatments recommended for those on lower RSR (eg, CS alone or CS + remdesivir), and augmentation with IL-6is or JAKis with worsening severity. This study also found that JAKi and IL-6i combination regimens had similar utilization over the entire hospitalization; however, JAKi combinations were more commonly used during hospitalization time characterized by receipt of O2, whereas IL-6i combinations were more commonly used during receipt of NIV/HFO and IMV/ECMO, aligning with the guidelines. Notably, JAKi combinations were observed during receipt of IMV/ECMO, even though this was not recommended during the study period; this trend preceded the update of the guidelines on 8 August 2022 to include JAKi combination for patients on IMV/ECMO. Differences between JAKis and IL-6is with regard to use during receipt of O2 and NIV/HFO were unanticipated, given that both drug regimens were recommended for patients with these RSRs during the study period. These findings suggest that prescribing practice varied by severity of disease; adjustment or stratification for COVID-19 severity in future observational comparative analyses may help mitigate risk of confounding by disease severity in these studies.
This study has several strengths. HealthVerity-linked chargemaster and claims data offer a unique, large source of inpatient treatment data across all US regions. The period covered by this study (which includes hospitalizations as recent as February 2022) spans the inclusion of both JAKis and IL-6is in the NIH COVID-19 treatment guidelines, filling an important gap in the existing published literature. Finally, this study describes drug utilization over the entire hospitalization and by RSR. However, there are limitations that may impact these findings. First, while our dataset was large with all states represented, some regions (ie, the South and Northeast) were overrepresented and others (ie, Midwest) were underrepresented relative to US census data; therefore our findings may not be generalizable to all US hospitalized COVID-19 patients during the study period. Second, the inpatient medication data are largely from nonadjudicated chargemaster data, which are collected for administrative use, rather than research purposes, and include nonstandardized free-text descriptions, and, therefore, may be subject to some misclassification. Comprehensive text search strings were used to minimize treatment misclassification due to the free-text nature of the data. In addition, though records of medication billed are proxies for patients receiving and consuming the medication, medication nonadherence is anticipated to be relatively low in the inpatient medically supervised setting. Additionally, although patients may have received a COVID-19 diagnosis prior to or upon admission for an unrelated hospitalization, a 7-day window prior to admission to confirm a COVID-19 diagnosis was chosen to minimize including hospital-acquired or incidental diagnoses of COVID-19 cases. Next, our analyses categorizing hospital-time by RSR censors patients when there is an increase in RSRs, but patients are not censored if there is a decrease in RSRs (eg, a patient on IMV/ECMO improving, and switching to O2). Thus, interpretation of these findings should consider that each RSR category may include both patients who remain at that RSR level until censoring and patients who improve in RSR prior to censoring. Supplemental oxygen may have been underrecorded, with those patients misclassified as having received no respiratory support. Finally, we are unable to measure factors such as barriers to access, which may influence treatment patterns.
CONCLUSIONS
The study findings suggest that inpatient prescribing in HealthVerity was generally in line with NIH COVID-19 treatment guidelines. They also highlight that studies on drugs typically initiated later in hospitalization (ie, JAKis or IL-6is) should consider prior or concomitant use of other drugs as potential confounding factors. While this study provides context for drug use in 2021 through February 2022, treatment guidelines continue to evolve. Prescribing preference, access to drug regimens, and potential confounding by indication even within RSR levels should be considered in the design and interpretation of future comparative studies. In the meantime, descriptive drug utilization studies should continue to be prioritized to support future comparative studies extending through 2022 and beyond.
Supplementary Material
Contributor Information
Sarah E Vititoe, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Priya Govil, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Aidan Baglivo, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Elisha Beebe, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Elizabeth M Garry, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Nicolle M Gatto, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Tamar Lasky, Office of the Commissioner, US Food and Drug Administration, Silver Spring, Maryland, USA.
Aloka Chakravarty, Office of the Commissioner, US Food and Drug Administration, Silver Spring, Maryland, USA.
Marie C Bradley, Division of Epidemiology, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, USA.
Silvia Perez-Vilar, Division of Epidemiology, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, USA.
Donna R Rivera, Oncology Center of Excellence, US Food and Drug Administration, Silver Spring, Maryland, USA.
Kenneth Quinto, Office of Medical Policy, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, USA.
Andrew Clerman, Division of Pulmonology, Allergy, and Critical Care, Office of Immunology and Inflammation, Office of New Drugs, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, USA.
Anil Rajpal, Division of Rheumatology and Transplant Medicine, Office of Immunology and Inflammation, Office of New Drugs, Center for Drug Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, USA.
Vera Frajzyngier, Aetion, Inc, Scientific Research and Strategy, New York, New York, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . We wish to thank Melanie Wang, MPH (Aetion, Inc) and Sachin Shah, MBA (US Food and Drug Administration [FDA]) for management and coordination of this collaborative research effort, and Andrew R. Weckstein (Aetion, Inc) for review and scientific contributions.
Patient consent. This is a secondary analysis of de-identified data without access to personal identifying information and without direct enrollment of patients (eg, in contrast to interventional studies or other primary data collection studies requiring patient participation). All patient-level and provider-level data within the database contain synthetic identifiers to protect the privacy of individuals and data contributors. Therefore this study does not include factors necessitating patient consent.
Disclaimer. This article reflects the views of the authors and should not be construed to represent the views or policies of the FDA.
Financial support . This article is part of a US FDA Broad Agency Announcement contract to use the Aetion Evidence Platform to develop a system of studies and a systematic process for the rapid assessment of COVID-19 medical countermeasures. This includes identifying and analyzing fit-for-purpose data sources to characterize inpatient COVID-19 patient populations and risk factors for COVID-19–related complications and to explore methods for scientific evaluation of potential interventions for inpatient treatment of COVID-19.
References
- 1. Centers for Disease Control and Prevention . COVID data tracker weekly review.2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed 27 January 2022.
- 2. Vaduganathan M, van Meijgaard J, Mehra MR, Joseph J, O’Donnell CJ, Warraich HJ. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA 2020; 323:2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivera DR, Peters S, Panagiotou OA, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) cohort study. Cancer Discov 2020; 10:1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin KJ, Schneeweiss S, Tesfaye H, et al. Pharmacotherapy for hospitalized patients with COVID-19: treatment patterns by disease severity. Drugs 2020; 80:1961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dabestani A, DeAngelo D, Chhay SR, Larson BJ, Ganio MC. Medication utilization in patients in New York hospitals during the COVID-19 pandemic. Am J Health Syst Pharm 2020; 77:1885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 2020; 3:e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan L, Liu H, Li N, et al. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered. Medicine (Baltimore) 2021; 100:e23923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Best JH, Kong AM, Kaplan-Lewis E, et al. Treatment patterns in US patients hospitalized with COVID-19 and pulmonary involvement. J Med Virol 2021; 93:5367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta HB, An H, Andersen KM, et al. Use of hydroxychloroquine, remdesivir, and dexamethasone among adults hospitalized with COVID-19 in the United States: a retrospective cohort study. Ann Intern Med 2021; 174:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watanabe JH, Kwon J, Nan B, Abeles SR, Jia S, Mehta SR. Medication use patterns in hospitalized patients with COVID-19 in California during the pandemic. JAMA Netw Open 2021; 4:e2110775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayodele O, Ren K, Zhao J, et al. Real-world treatment patterns and clinical outcomes for inpatients with COVID-19 in the US from September 2020 to February 2021. PLoS One 2021; 16:e0261707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis 2022; 75:e450–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scott A, Chambers R, Reimbaeva M, et al. Real-world retrospective analysis of patient characteristics, healthcare resource utilization, costs, and treatment patterns among unvaccinated adults with COVID-19 diagnosed in outpatient settings in the United States. J Med Econ 2022; 25:287–98. [DOI] [PubMed] [Google Scholar]
- 14. Westhoff WJ, Smith LH, Wyszynski DF, Hernandez-Diaz S. COVID-19 pharmacotherapy utilization patterns during pregnancy: International Registry of Coronavirus Exposure in Pregnancy. Pharmacoepidemiol Drug Saf 2022; 31:804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaczok B, Nair G, Lin CH, et al. Evolution of prescribing practices and outcomes in the COVID-19 pandemic in metropolitan areas. Infez Med 2022; 30:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes drug for treatment of COVID-19.2021. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-treatment-covid-19. Accessed 12 September 2022.
- 17. US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes drug combination for treatment of COVID-19.2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19. Accessed 12 September 2022.
- 18. National Institutes of Health . COVID-19 treatment guidelines.2022. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf. Accessed 6 September 2022.
- 19. National Institutes of Health . COVID-19 treatment guidelines (Archive, 17 December 2020).2020. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-12-17-2020.pdf. Accessed 6 September 2022.
- 20. National Institutes of Health . COVID-19 treatment guidelines (Archive, 21 April 2021).2021. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-04-21-2021.pdf. Accessed 6 September 2022.
- 21. National Institutes of Health . COVID-19 treatment guidelines (Archive, 8 July 2021).2021. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-07-08-2021.pdf. Accessed 6 September 2022.
- 22. National Institutes of Health . COVID-19 treatment guidelines (Archive, 8 August 2021).2021. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-08-08-2022.pdf. Accessed 6 September 2022.
- 23. US Food and Drug Administration . ACTEMRA (tocilizumab) injection [package insert].2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125276s114lbl.pdf. Accessed 6 September 2022.
- 24. US Food and Drug Administration . Dexamethasone sodium injection [package insert].2014. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/084916s066lbl.pdf. Accessed 6 September 2022.
- 25. Garry EM, Weckstein AR, Quinto K, et al. Categorization of COVID-19 severity to determine mortality risk. Pharmacoepidemiol Drug Saf. 2022. doi: 10.1002/pds.5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.