Abstract
Modern genomic sequencing efforts are identifying new potential diagnostic and therapeutic targets more rapidly than existing methods can generate the peptide- and protein-based ligands required to study them. To address this problem, we have developed a Microfluidic Enrichment Device (MFED) enabling kinetic off-rate selection without the use of exogenous competitor. We tuned the conditions of the device (bed volume, flow rate, immobilized target) such that modest, readily achievable changes in flow rates favor formation or dissociation of target-ligand complexes based on affinity. Simple kinetic equations can be used to describe the behavior of ligand binding in the MFED and the kinetic rate constants observed agree with independent measurements. We demonstrate the utility of the MFED by showing a four-fold improvement in enrichment compared to standard selection. The MFED described here provides a route to simultaneously bias pools toward high-affinity ligands while reducing the demand for target-protein to less than a nanomole per selection.
Affinity reagent generation is at the core of developing diagnostics and therapeutics. Recent advancements in cancer genetics have helped to rapidly identify potential molecular targets on a genomic scale. Accelerated reagent generation is necessary to keep pace with the growing rate at which potential targets are identified. mRNA display is a selection technique that offers a rapid and cost-effective route towards generating reagents ranging in size from peptides1–3 to proteins4–10 and antibodies.11 In mRNA display, randomized libraries of polypeptides are covalently attached to their encoding mRNA, enabling wholly in vitro selection and directed evolution. To do this, a naïve initial library is subjected to a cyclic process involving selective enrichment and amplification. The result is that rare functional molecules from the initial library are enriched over several rounds of selection.
Most selection experiments aim to maximize the affinity (Kd) of the ligand for the target. Diffusion limits the on-rate of neutrally charged peptides to around 104-106 M−1s−1while off-rates tend to vary much more.12 Maximizing affinity therefore involves finding complexes with the slowest dissociation kinetics (koff) while maintaining near diffusion-limited on-rates. In prior work, Boder and Wittrup devised a strategy for improving the off-rates of pools using the target as a competitor in binding selections.13 This “off-rate selection” has resulted in very high affinity binders, including a femtomolar fluorescein antibody14 and single digit picomolar peptide binders to B-cell Lymphoma-extra Large (Bcl-xL, a pro-survival protein often overexpressed in cancer cells.)3 In this approach, a vast excess of free target is added (typically 100-fold) to create a condition where any ligand that dissociates from the target is lost, thereby biasing the pool toward molecules with lower dissociation rate constants. Competitor-driven screening thus limits optimal ligand development to systems where milligrams of target are readily accessible, significantly hindering genomic-scale ligand development.
Other techniques have been developed to perform off-rate selections without additional competitor. These include extensive washing and volume dilution techniques.15–18 Extensive washing removes ligands as they dissociate from beads with manual washes. The volume dilution technique relies on a large volume of buffer that is added to the beads after an initial binding step. This drastically decreases the concentration of target and ligand to impair the rebinding of lower affinity ligands as they dissociate from the target. In this paper, we present a new competitor-free off-rate selection technique that offers additional kinetic control compared to extensive washing and volume dilution techniques.
We have implemented a flow-based strategy enabling kinetic off-rate selections without exogenous competitor. To do this, we designed a Microfluidic Enrichment Device (MFED) consisting of 3D printed parts, a microfluidic channel, and a frit to enable experiments with non-magnetic beads. At low flow rates, a 5 μL bead bed volume with 100 pmol immobilized target rapidly captures mRNA displayed peptides similar to manual pull-down experiments because the residence time on the bed is sufficient to facilitate binding. Increasing the flow 20-fold decreases the residence time such that there is not enough time for the library to diffuse to the surface of the beads and bind. By varying the duration of bead washing after binding, we can enrich the library in binders with slower off-rates where the complex stays intact longer. Thus flow, rather than competitor, creates the conditions needed to perform an off-rate selection. Here, we demonstrate the utility of the device using previously identified Bcl-xL ligands by successfully enriching a high-affinity ligand over a lower-affinity one.3
The MFED is designed to load and wash beads in continuous flow.
The MFED is designed to enable ligand capture and washing on beads (magnetic or non-magnetic) large enough to be trapped in the frit. The device tested here consists of a set of 3D printed parts and a 10 μm pore size frit (Figure 1).
Figure 1.

Design of the MFED. (A) Schematic illustration of bead washing on the MFED. (B) Picture of the assembled MFED. (C) Picture of the disassembled MFED, 1 cm scale bar indicated.
We have previously demonstrated the capacity of 3D printing to fabricate complex microfluidic device morphologies.19 This device is fabricated using a benchtop digital light processing stereolithography printer from a clear methacrylate-based resin. The device connects two segments of microfluidic tubing (1/16” inner diameter) to either side of the frit. Flow is driven by a syringe pump over a wide range of rates (from < 1 μL/min to > 2 mL/min).
Flow rate and residence time determines ligand binding and dissociation.
The MFED is designed to function in two modes—to load ligand (here, mRNA display libraries) onto target-modified beads and to wash nonspecific and weak binders off the bead bed. For loading, the flow rate must be adjusted such that the second-order binding reaction can reach equilibrium during the time the ligand transits the bed. To test this process, we measured the binding of a high affinity radiolabeled peptide ligand previously described (E1 peptide, Kd = 40 pM) to immobilized Bcl-xL.3 In order to mimic the enrichment that occurs in a selection, the E1 peptide was constructed in mRNA display format—as an mRNA-peptide fusion where the C-terminus of the peptide is covalently attached to its mRNA via a puromycin bearing linker.
We then explored the formation of the E1 ligand-Bcl-xL complex under different flow conditions (Figure 2). The E1 peptide has an extremely slow dissociation rate constant (koff = 7.4 × 10−6 s−1), such that >95% of complexes formed should remain stable during the course of any experiment. At low flow rates (<12.5 μL/min) E1-Bcl-xL complex formation is essentially quantitative. However, as the flow is increased, we observed a steady decrease in fraction of E1 peptide binding the immobilized target, with very little binding at flow rates of 500 μL/min and higher.
Figure 2.
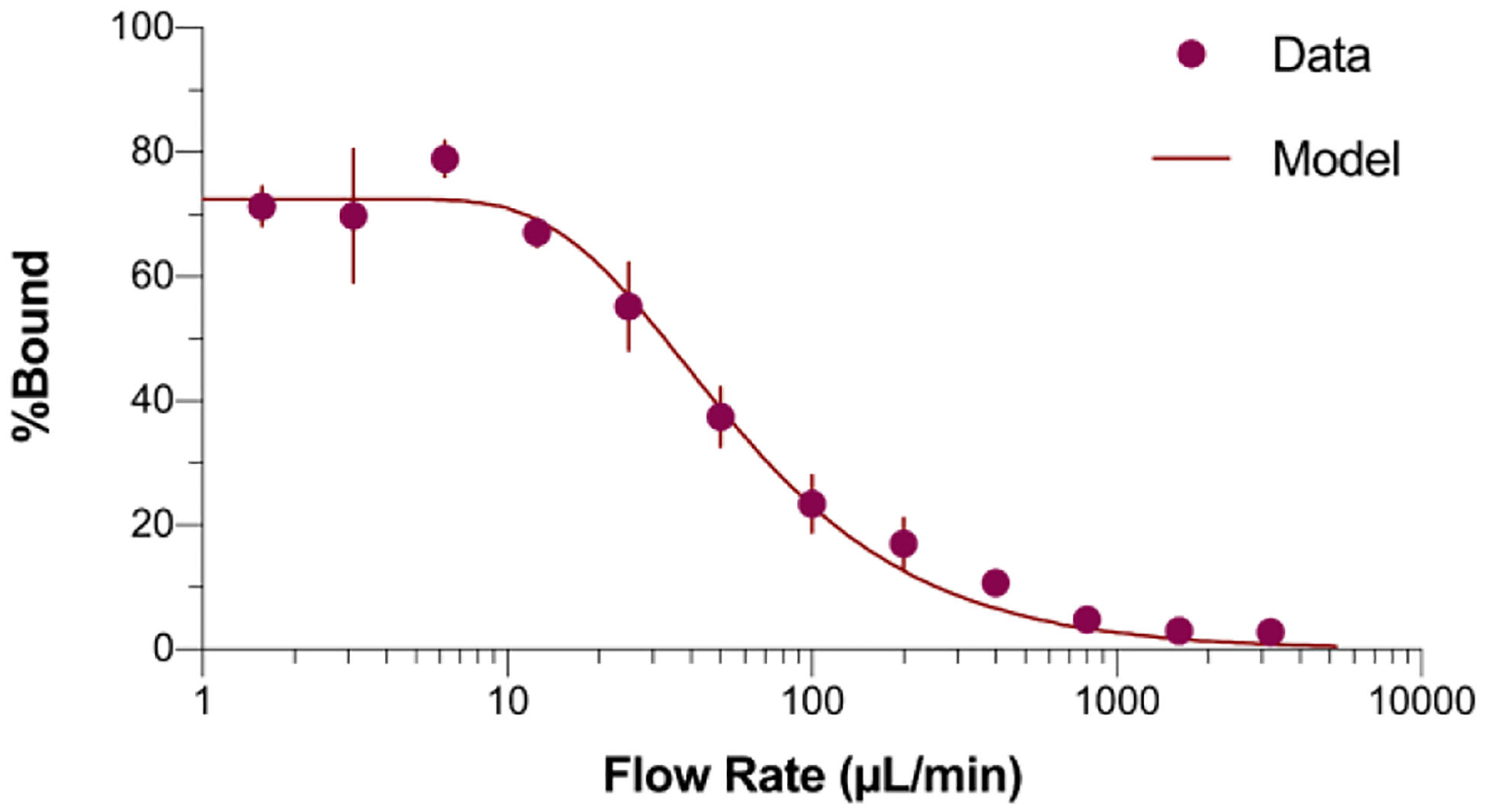
Characterizing and modeling E1 peptide-mRNA fusion binding at various flow rates. The percent bound increases as the flow rate decreases and plateaus at 73%. Error bars represent the standard deviation of percent bound over three trials. The best-fit model gives kon = (2.5 ± 4) × 104 M−1s−1.
The binding vs. flow curve is essentially the mirror image of the E1-Bcl-xL formation reaction, with low flow rates giving a residence time on the bead bed long enough for the reaction to go to completion and high flow providing a short enough residence time for almost no complex formation. The curve can be fit to a pseudo-first-order on-rate model, under the condition of excess target where ligand flow is directed towards a bead bed. The residence time (τ) of ligand flowing through the bead bed is a function of volume of the bead bed (V), the packing efficiency of the beads (η), and the flow rate (Q):
| [1] |
The bead packing efficiency (η), was estimated as 74%, the close packing density of regular spheres, giving τ values of 6 seconds for 12.5 μL/min and 150 msec for 500 μL/min, respectively.
Since the dissociation of E1 is much slower than the time scale of these experiments, we can model the percentage of ligand bound (B) as a function of maximum ligand binding (Bmax), association constant (kon), initial target concentration ([T]0), and residence time (τ):
| [2] |
This analysis gives kon = (2.5 ± 0.4) x 104 M−1s−1, which is in agreement with prior work on the diffusion limit of ligands.19 The Bmax of ~75% is typical for peptides constructed from oligos as mRNA display fusions monitored by radioactive labeling. The fact that Bmax is not 100% is likely due to chemical synthesis errors in template construction, with 75% efficiency corresponding to an error rate of ~ 0.5% per position ((0.995)63= 73%, insertions, deletions, and mutations).20
High flow rates mimic the conditions needed for “optimal” selection of ligands.
Boder and Wittrup used off-rate selections to maximize the binding free energy in directed evolution experiments.13, 14 The key to their optimization was establishing conditions where ligands (library members) that dissociate from the target cannot rebind. Their work achieved this condition by adding a large molar excess (100X) of free target as competitor. Here, the ligand binding vs. flow experiments (Figure 2) demonstrate that the no-rebinding condition can also be met at high flow rates, without using exogenous competitor. This is an important observation because it can drastically reduce the amount of target protein needed to perform an in vitro selection by ~100 fold—from milligram scale, to 10–100 micrograms. This fact alone greatly expands the number of proteins that can be targeted by mRNA display, as target production is a major bottleneck in affinity reagent generation.21
MFED loading and washing with high affinity and moderate affinity ligands.
The flow experiments with the high affinity mRNA-display peptide ligand (E1) established conditions for loading and washing target-bound beads with the MFED. Using this information, we tested eluting a moderate affinity ligand (Pep2 Kd ~ 65 nM) vs. the high affinity E1 peptide, both presented in mRNA display format (Figure 3). The MFED was first used to load and wash radiolabeled [35S]-labeled E1 and Pep2 mRNA fusions separately. Samples were loaded into the MFED at 25 μL/min and washed at 500 μL/min. The loading step represents a compromise—lower flow rates (1 – 10 μL/min, Figure 2) give more quantitative binding, but slow the overall function and selection cycle time of the device due to the relatively large volumes in the loading step (500–1000 μL). Loading at 25 μL/min allows both fairly rapid library/bead binding while retaining most of the steady-state binding for E1 and Pep2 (52% and 12%, respectively). Also, since the fraction loaded depends on the formation rate constant (kon) these slightly accelerated loading conditions may provide a small positive bias for complexes with faster on-rates. This approach thus effectively retains functional library members on the bead bed. Radiolabeled peptide fusion elution and binding were determined by scintillation counting the flow-through during loading, the buffer used to wash the bead bed, and, at the end of the experiment, the beads themselves. Figure 3 shows the fraction of each peptide fusion initially introduced that remains bound to the beads as a function of wash time. It also shows the ratio of E1:Pep2 remaining on the beads.
Figure 3.

MFED loading and washing E1 (●) and Pep2 (●) mRNA-peptide fusions. Error bars indicate the standard deviation of three trials. Initial binding for E1 (52%) shows little decay during washing, whereas Pep2 binding (12 ± 1%) washes out with a first order rate constant koff = 9 ± 2 × 10−3 s−1. The ratio of E1:Pep2 bound (∎) plateaus after ~1,000 seconds when the Pep2 binding reaches background binding (1.4 ± 0.3%).
Washing has a dramatically different effect on peptide ligands with different affinities. For the Pep2 mRNA-peptide fusion, dissociation is well fit by a first-order model (eqn. 3; Figure 3) with percent bound (B), wash time (τw), dissociation constant (koff), minimum percent bound (Bmin), and initial percent bound (B0):
| [3] |
This fit yields a koff = 9 × 10−3 s−1, which is in excellent agreement with manual results (koff = 1.1 × 10−2 s−1) Pep2 (Figure S1). This off rate is also consistent with there being no rebinding to the beads once dissociation occurs. However, we noted that the ligand retention did not achieve 0% binding. We suspect that this is due to hindered diffusion from the pores of agarose beads.
Peptide fusions with Kd ~ 100 nM thus fall to background levels after <15 minutes. On the other hand, ~90% of the E1 peptide fusions are retained during the 30 minute washing step. To achieve an optimal enrichment of E1 over Pep2, conditions need to be found where the ratio of the stronger and weaker binder is maximized. During MFED washing, the ratio of E1:Pep2 bound rises rapidly and plateaus after ~1,000 seconds.
The individual experiments with E1 and Pep2 peptide fusions demonstrate conditions for efficient loading and washing for ligands typical in mRNA display selections (Kd = 100 nM) and those optimized for antibody-like affinity (Kd ≤ 1 nM). More importantly, during washing, the ability to achieve high flow rates (500 μL/min) with small bed volumes and short residence times prohibits ligand/target rebinding, enabling competitor-free off-rate selections.
Testing MFED enrichment of a high affinity ligand (E1) vs. a lower affinity ligand (Pep2).
In a typical manual mRNA display selection, peptide ligands with their attached encoding cDNA bind immobilized target. Unbound ligands are then washed using a filter, and the remaining ligands along with their encoding cDNA are collected and amplified by polymerase chain reaction (PCR). Our optimization of the washing conditions in the MFED suggested that the MFED would be more effective than manual selections in enriching high affinity ligands. The kinetic binding data in Figure 3 shows a 4.3-fold enrichment of E1 over Pep2 initially. After washing, this ratio can be improved to >40-fold by facilitating an off-rate based enrichment. To quantify the actual increase of enrichment, we measured the relative abundance of a mixed ligand population before and after selection using the MFED and manual selection.
Prior to selection, we checked whether PCR would introduce bias during the amplification of E1 and Pep2 cDNA. PCR bias is present when some templates amplify more efficiently than others, which can lead to a change in relative abundance that could potentially be misinterpreted as enrichment. Since E1 and Pep2 are different lengths (126 and 96 bp, respectively), they can be distinguished on an agarose gel. To ensure that there is no PCR bias between E1 and Pep2, we mixed the two cDNAs such that these species gave similar band intensities on an agarose gel (Figure S2). This mixture was then serially diluted such that each sample required a different number of PCR cycles to be amplified to achieve visible band intensities. The ratio of band intensities remains unchanged with samples PCR amplified for different numbers of cycles, thereby confirming that there is no PCR bias between E1 and Pep2 (Figure S2).
To demonstrate that selection with the MFED is capable of outperforming a typical manual mRNA display selection, we used mixtures of E1 and Pep2 as our starting materials. The different lengths of their coding DNA enabled us to easily quantify enrichment by determining the relative intensities of each species on an agarose gel.
To test this, we prepared mixtures of E1 and Pep2 at different ratios. E1 and Pep2 were co-translated at various ratios (E1:Pep2 at 1:2, 1:38, 1:67, 1:135, and 1:292), reverse transcribed, and a sample at each ratio was then used in either an MFED or traditional manual mRNA display selection. Mixing the two templates immediately prior to the translation step (rather than an earlier step in the mRNA display cycle, e.g., PCR, transcription, or ligation) reduces the potential biases for one clone over the other prior to selection. After reverse transcription, half of each mixture was used for the MFED selection and the other half was used for manual selection. Splitting each mixture in half directly before selection also reduces the variation/bias in the samples due to post-translation steps such as dT purification, reverse transcription, or sample handling.
In manual selections, each sample was incubated with bead-immobilized Bcl-xL in a rotating tube for an hour and washed over a filter three times, with no off-rate selective pressure added. In MFED selections, the samples were loaded onto bead-immobilized Bcl-xL at a flow rate of 25 μL/min and then washed at 500 μL/min for 15 min, which our data above determined to be conditions that would be expected to yield an off-rate selective pressure. In each case, after the selections were completed, the beads were transferred into PCR solution and each sample was PCR amplified until the bands were visible on an agarose gel (Figures 4 and S3). Image analysis was performed using Image Studio Lite®, and molar ratios were extracted from a standard curve (Figure S3).
Figure 4.
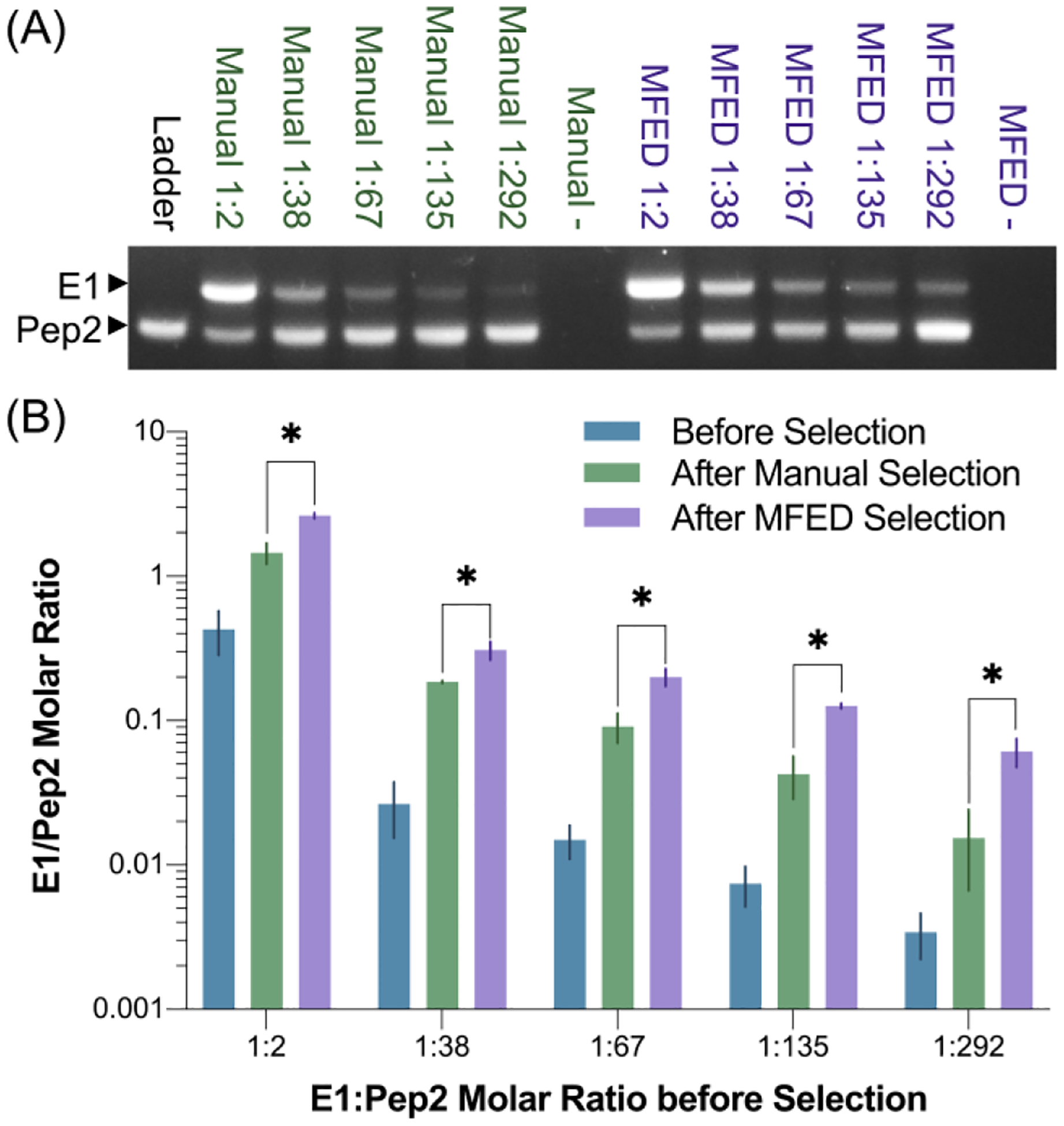
Manual versus MFED enrichment of E1 over Pep2. Selection was performed on various starting ratios of E1:Pep2 (1:2, 1:38, 1:67, 1:135, and 1:292). Resulting PCR products were run on agarose gels (A and S3). Gel images were analyzed to extract molar ratios before and after selection (B and S3). MFED selection consistently outperformed manual selection, averaging 13-fold enrichment compared to 5-fold enrichment observed with manual selection (* indicates p<0.05).
In each trial, the MFED outperformed manual selection, averaging a three-fold improvement in enrichment of E1 over Pep2. This suggests that a selection performed with the MFED and off-rate selective pressures would likely require fewer cycles of enrichment versus a traditional mRNA display selection, and therefore could reduce the time it takes to perform a selection from a naïve library. This result shows that the MFED is superior to traditional mRNA display selection in distinguishing ligands based on their off-rates and confirms that the device is capable of performing competitor-free, off-rate based selection.
Conclusions
In our effort to develop automated mRNA display, we designed the MFED as a more economical microfluidic alternative to the conventional competitor based off-rate selection technique. The MFED described here is an innovative device that facilitates a competitor-free, off-rate selection of mRNA display ligands. We demonstrated that the MFED selection is superior to the manual, non-off-rate-based technique owing to its continual free-ligand removal mechanism. The device utilizes continuous flow to facilitate ligand binding and washing with predictable kinetics. With only 100 pmol of target on 5 μL of beads, we demonstrated that ligands can be efficiently loaded at 25 μL/min and effectively washed at 500 μL/min. Both flow rates are easily attainable with a syringe pump, and the change in flow rate is only 20-fold. Fabrication of MFED devices is achieved with no specialized lithography equipment and utilizes low cost materials. The reduction in the amount of protein target that must be expressed and purified to perform off-rate selections enables access to targets that are difficult or expensive to produce in large quantities. Lastly, the combination of the lower sample requirements with the higher enrichment achieved with the MFED device argues that the MFED is an attractive selection improvement to accelerate the discovery of peptide and protein ligands against novel cancer targets for diagnostic and therapeutic uses.
Supplementary Material
ACKNOWLEDGMENTS
The work was funded by the National Cancer Institute (NCI) of the National Institutes of Health (NIH), award number CA204708.
Footnotes
- Supporting_info.pdf
- MFED_designfiles.zip
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Jalali-Yazdi F; Corbin JM; Takahashi TT; Roberts RW, Robust, Quantitative Analysis of Proteins using Peptide Immunoreagents, in Vitro Translation, and an Ultrasensitive Acoustic Resonant Sensor. Analytical chemistry 2014, 86 (10), 4715–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalali-Yazdi F; Takahashi TT; Roberts RW, General, Label-Free Method for Determining K d and Ligand Concentration Simultaneously. Analytical chemistry 2015, 87 (23), 11755–11762. [DOI] [PubMed] [Google Scholar]
- 3.Jalali-Yazdi F; Huong Lai L; Takahashi TT; Roberts RW, High-Throughput Measurement of Binding Kinetics by mRNA Display and Next-Generation Sequencing. Angewandte Chemie International Edition 2016, 55 (12), 4007–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross GG; Junge JA; Mora RJ; Kwon H-B; Olson CA; Takahashi TT; Liman ER; Ellis-Davies GCR; McGee AW; Sabatini BL; Roberts RW; Arnold DB, Recombinant Probes for Visualizing Endogenous Synaptic Proteins in Living Neurons. Neuron 2013, 78 (6), 971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L; Hung K-C; Takahashi TT; Joo K-I; Lim M; Roberts RW; Wang P, Antibody-Mimetic Ligand Selected by mRNA Display Targets DC-SIGN for Dendritic Cell-Directed Antigen Delivery. Acs Chemical Biology 2013, 8 (5), 967–977. [DOI] [PubMed] [Google Scholar]
- 6.Mora RJ; Roberts RW; Arnold DB, Recombinant probes reveal dynamic localization of CaMKIIα within somata of cortical neurons. Journal of Neuroscience 2013, 33 (36), 14579–14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CA; Nie J; Diep J; Al-Shyoukh I; Takahashi TT; Al-Mawsawi LQ; Bolin JM; Elwell AL; Swanson S; Stewart R; Thomson JA; Soh HT; Roberts RW; Sun R, Single-Round, Multiplexed Antibody Mimetic Design through mRNA Display. Angewandte Chemie-International Edition 2012, 51 (50), 12449–12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson CA; Adams JD; Takahashi TT; Qi H; Howell SM; Wu TT; Roberts RW; Sun R; Soh HT, Rapid mRNA-Display Selection of an IL-6 Inhibitor Using Continuous-Flow Magnetic Separation. Angewandte Chemie International Edition 2011, 50 (36), 8295–8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao H-I; Olson CA; Hwang S; Deng H; Wong E; Baric RS; Roberts RW; Sun R, mRNA Display Design of Fibronectin-based Intrabodies That Detect and Inhibit Severe Acute Respiratory Syndrome Coronavirus Nucleocapsid Protein. Journal of Biological Chemistry 2009, 284 (26), 17512–17520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cetin M; Evenson WE; Gross GG; Jalali-Yazdi F; Krieger D; Arnold D; Takahashi TT; Roberts RW, RasIns: genetically encoded intrabodies of activated Ras proteins. Journal of molecular biology 2017, 429 (4), 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi R; Chen BR; Vibat CRT; Huang N; Lee C-W; Chang G, In vitro nanobody discovery for integral membrane protein targets. Scientific reports 2014, 4, 6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlosshauer M; Baker D, Realistic protein–protein association rates from a simple diffusional model neglecting long-range interactions, free energy barriers, and landscape ruggedness. Protein Science 2004, 13 (6), 1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boder ET; Wittrup KD, Optimal screening of surface-displayed polypeptide libraries. Biotechnology progress 1998, 14 (1), 55–62. [DOI] [PubMed] [Google Scholar]
- 14.Boder ET; Midelfort KS; Wittrup KD, Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proceedings of the National Academy of Sciences 2000, 97 (20), 10701–10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y; Adams JD; Turner K; Cochran FV; Gambhir SS; Soh HT, Controlling the selection stringency of phage display using a microfluidic device. Lab on a chip 2009, 9 (8), 1033–1036. [DOI] [PubMed] [Google Scholar]
- 16.Lou X; Qian J; Xiao Y; Viel L; Gerdon AE; Lagally ET; Atzberger P; Tarasow TM; Heeger AJ; Soh HT, Micromagnetic selection of aptamers in microfluidic channels. Proceedings of the National Academy of Sciences 2009, 106 (9), 2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian J; Lou X; Zhang Y; Xiao Y; Soh HT, Generation of highly specific aptamers via micromagnetic selection. Analytical chemistry 2009, 81 (13), 5490–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh SS; Ahmad KM; Cho M; Kim S; Xiao Y; Soh HT, Improving aptamer selection efficiency through volume dilution, magnetic concentration, and continuous washing in microfluidic channels. Analytical chemistry 2011, 83 (17), 6883–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhargava KC; Ermagan R; Thompson B; Friedman A; Malmstadt N, Modular, discrete micromixer elements fabricated by 3D printing. Micromachines 2017, 8 (5), 137. [Google Scholar]
- 20.Liu R; Barrick JE; Szostak JW; Roberts RW, [19] Optimized synthesis of RNA-protein fusions for in vitro protein selection. 2000. [DOI] [PubMed] [Google Scholar]
- 21.Venkataraman A; Yang K; Irizarry J; Mackiewicz M; Mita P; Kuang Z; Xue L; Ghosh D; Liu S; Ramos P, A toolbox of immunoprecipitation-grade monoclonal antibodies to human transcription factors. Nature methods 2018, 15 (5), 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


