Abstract
The cell membrane is a complex mixture of lipids, proteins, and other components. By forming dynamic lipid domains, different membrane molecules can selectively interact with each other to control cell signaling. Herein, we report several new types of lipid-DNA conjugates, termed as “DNA Zippers”, which can be used to measure cell membrane dynamic interactions and the formation of lipid domains. Dependent on the choice of lipid moieties, cholesterol- and sphingomyelin-conjugated DNA Zippers specifically locate in and detect membrane liquid ordered domains, while in contrast, tocopherol-DNA Zipper can be applied for the selective imaging of liquid disordered phases. These versatile and programmable probes can be further engineered into membrane competition assays to simultaneously detect multiple types of membrane dynamic interactions. These DNA Zipper probes can be broadly used to study the correlation between lipid domain and various cellular processes, such as the epithelial–mesenchymal transition.
Keywords: Cell membrane analysis, DNA probes, fluorescence imaging, lipid-DNA conjugates, lipid domains, membrane order
Graphical Abstract
Different membrane lipids and proteins can dynamically diffuse, conjugate, and separate from each other on cell membranes.1 The cell plasma membrane adopts a heterogenous structural pattern: certain lipids and proteins preferentially interact with others to form lipid domains.2-5 These membrane compartments can facilitate the formation of stabilized or transient signaling complexes, and play critical roles in modulating cell signaling processes, such as the immune cell activation, pathogen entry into host cells, and endocytosis.6-8 Dysregulation of membrane domains is correlated with various diseased conditions including cancers, autoimmune disorders, and cardiovascular diseases.9-13
Nowadays, fluorescent probes and advanced fluorescence microscopy techniques are mostly widely used to study lipid domains.2,14,15 These fluorescent probes can be normally divided into two categories: fluorescent molecules with preferential partition in membrane domains (e.g., cyanine dyes, lipid-binding agents, and fluorescent lipid analogues) and solvatochromic dyes whose signals are sensitive to the lipid packing in the membranes (e.g., Laurdan and Di-4-ANNEPDHQ).16-18 However, because of the diverse compositions and variable packing states of the plasma membranes, currently there is no single probe that can provide indisputable domain imaging in living cell membranes.2,16,19
We have recently developed alternative DNA-based fluorescent probes to image membrane orders in living cells.20,21 In our so-called “DNA Zipper” system, membrane cholesterol–cholesterol interaction was used as an indicator of membrane liquid ordered (Lo) phases. Cholesterols are known to be enriched in the Lo domains,22-24 their high local concentration induces more effective and frequent (large kon) membrane cholesterol–cholesterol encounters within Lo domains. To visualize these transient cholesterol–cholesterol (large koff) interactions, in our DNA Zipper design, cholesterol is conjugated with either a Förster resonance energy transfer (FRET) donor dye or a corresponding acceptor fluorophore via a short piece of DNA strand (Fig. 1a). The donor and acceptor DNA strands can hybridize with each other to extend the duration (reduced koff) of membrane cholesterol–cholesterol interactions, as well as the corresponding fluorescence signals. DNA probes are chosen because their hybridization can be precisely regulated by fine-tuning the strand sequence and length. As a result, optimal probes can be identified that will stabilize membrane cholesterol–cholesterol interactions just enough to visualize lipid domain-associated events, but not random membrane encounters. This optimal DNA Zipper probe has been determined to contain a 6–8-base-pair (bp)-long-duplex region.21
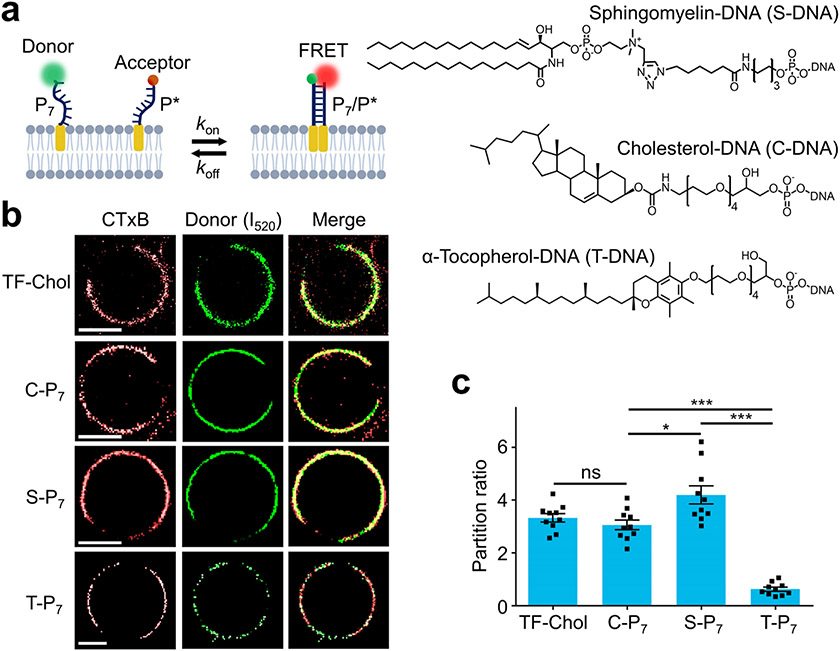
Figure 1.
Design and membrane partitioning of DNA Zippers. (a) Schematic of the DNA Zipper system. Chemical structures of the lipid moieties were also shown. (b) Giant plasma membrane vesicles (GPMV) were extracted from the MCF-7 cells and stained with 2.5 ng/μL CTxB and 20 nM lipid-DNA or BODIPY-cholesterol (TF-Chol) for 30 min at 4°C. Scale bar, 5 μm. (c) By comparing the membrane distribution of the CTxB fluorescence with that of the lipid-DNA signal, the partition ratio of TF-Chol and each lipid-DNA probe was determined. Shown are the mean and standard error of the mean (SEM) values obtained from the membranes of at least 10 GPMVs in each case. *p< 0.05, ***p< 0.001 in two-tailed Student’s t-test; ns, not significant. C-P7, S-P7, and T-P7 represent cholesterol-, sphingomyelin-, and tocopherol-modified DNA donor probes.
However, cholesterols are not exclusively partitioned in lipid domains or naturally interact with each other. Instead, cholesterols pack tightly in between the saturated acyl chains of sphingolipids (especially sphingomyelins), fill the void, and hold saturated lipids together to form gel-like Lo phases.25-27 As a result, compare to cholesterol–cholesterol interactions, the membrane cholesterol–sphingomyelin conjugation may serve as a more reliable indicator of membrane Lo domains. Herein, our first goal is to develop DNA Zipper to image these transient membrane cholesterol-sphingomyelin interactions and test their efficiency in distinguishing Lo phases in living cell membranes.
Meanwhile, unsaturated membrane lipids also play critical roles in regulating membrane order and lipid domains.28-31 These unsaturated lipids generally partition into liquid disordered (Ld) phases, which are shown to modulate immune cell inflammation, exhibit dietary benefits, and associate with cardiovascular protection.32-34 Our second goal is to engineer novel DNA Zippers that can assess membrane disordered phases. Membrane tocopherol-mediated interactions are chosen because they are known to predominantly partition into Ld phases.35
Our results indeed indicate that cholesterol–sphingomyelin and sphingomyelin–sphingomyelin interaction-based DNA Zipper probes can be better suited to image membrane Lo domains than cholesterol–cholesterol probes. These “second-generation” Lo-DNA Zipper exhibit enhanced Lo partitioning, greater sensitivity to changes in the membrane order, and improved membrane persistence with reduced internalization. With less interference from intracellular background signals, these probes can be used for more reliable imaging of Lo domains. Additionally, membrane tocopherol–tocopherol interactions can be used to visualize Ld regions in cell membranes. We also established a novel competition assay to reveal the relative preference of in situ membrane interactions among different lipid pairs. Lastly, DNA Zipper was used to investigate the potential correlation between membrane domain and epithelial–mesenchymal transition process.
We first synthesized three types of oligonucleotides that were respectively conjugated with cholesterol, sphingomyelin, and tocopherol (Fig. 1a). For each lipid-DNA conjugate, two DNA strands were prepared containing a 7-bp hybridization region (Table S1), denoted as the donor (P7) and acceptor (P*) probe, as they were conjugated with either a FRET donor (Atto488) or acceptor dye (Cy5) (Fig. 1a).
To test if these lipid-DNA conjugates can partition into different membrane domains, giant plasma membrane vesicles (GPMVs) were used since GPMVs lack actin cytoskeleton and can easily generate phase separation.36 Cholera toxin B subunit (CTxB) was used as a fluorescent marker for Lo domains. After incubating GPMVs with CTxB and 20 nM of cholesterol-, sphingomyelin-, or tocopherol-modified donor DNA strand (i.e., C-P7, S-P7, or T-P7), the membrane colocalization of Atto488 and CTxB signals was used to determine the domain partition ratio (Lo/Ld) of each lipid-DNA. As expected, both C-P7 (Lo/Ld= 3.0±0.3) and S-P7 (Lo/Ld= 4.2±0.4) are highly enriched in the Lo phases, while T-P7 (Lo/Ld=0.6±0.1) generally partition in Ld domains (Fig. 1b and 1c). Using BODIPY-cholesterol (TF-Chol) as reference37,38, DNA conjugation (C-P7) will not influence the membrane partitioning of lipids.
We wondered if all these lipid-DNA conjugates can be modified onto live-cell membranes. To test this, 100 nM cholesterol-, sphingomyelin-, or tocopherol-modified donor (C-P7, S-P7, and T-P7) and acceptor probes (C-P*, S-P*, and T-P*) were separately incubated with MCF-7 breast cancer cells. Indeed, significant cell membrane fluorescence was observed within 5 min upon incubation, with maximum signals shown in ~20 min (Fig. S1). Sphingomyelin-and tocopherol-modified DNA strands exhibited a slightly higher MCF-7 membrane modification than cholesterol-DNA. By correlating membrane probe densities with fluorescence levels (Fig. S2), after a 20-min incubation, 100 nM C-P7 or C-P* can provide similar membrane probe density on MCF-7 cells as that with 90 nM S-P7, S-P*, T-P7, or T-P* (Fig. S3). After removing free probes, membrane-modified C-P7 and T-P7 was stable for another ~30 min, and then cellular internalization and membrane dissociation was clearly observed (Fig. S4). In contrast, S-P7 persistently stayed on cell membranes for >60 min with minimal internalization.
We also characterized the membrane diffusion coefficient of C-P*, S-P*, and T-P* using a fluorescence recovery after photobleaching (FRAP) assay. The greatest diffusivity was observed for the T-P* probe (0.37±0.05 μm2/s), while C-P* (0.18±0.03 μm2/s) and S-P* (0.15±0.02 μm2/s) showed similar diffusion coefficients in MCF-7 cell membranes (Fig. S5). This variation in the membrane diffusivity between tocopherol- and cholesterol/sphingomyelin-based probes is likely correlated with their difference in the membrane domain partitioning.
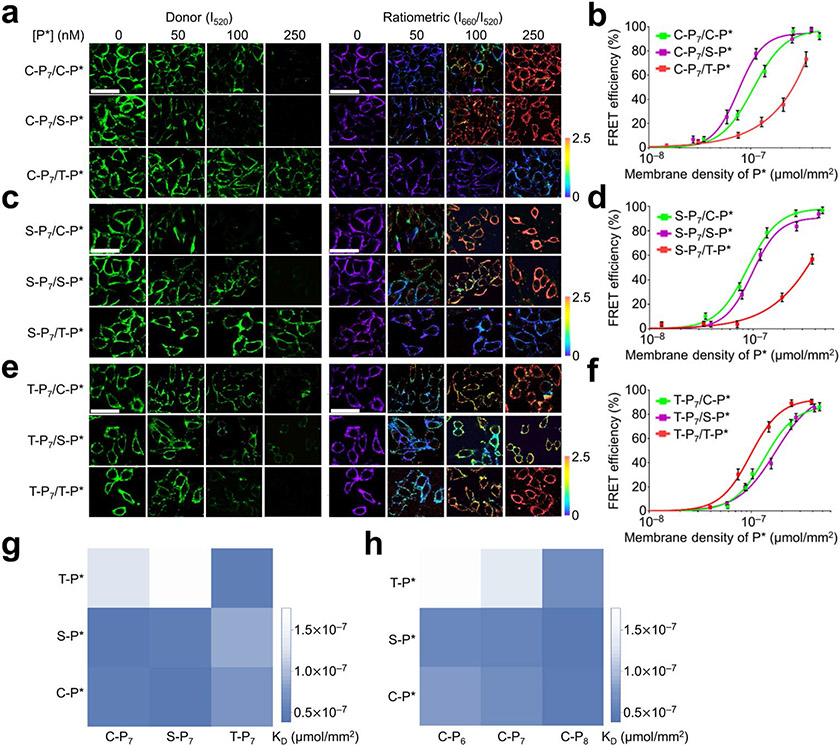
We next studied how the membrane interaction pattern of DNA Zippers is regulated by the lipid moiety. After incubating MCF-7 cells with 100 nM C-P7 or 90 nM S-P7 or T-P7 and removing excess probes, 0–500 nM C-P*, S-P*, or T-P* was added, respectively. To compare the membrane association efficiency of each DNA Zipper pair, membrane FRET efficiency (E) was calculated based on changes in donor fluorescence (I520, λex/λem= 488/520 nm) before (IPn) and after (IPn,p*) adding the acceptor DNA, i.e., E= 1−(IPn,P*/IPn). Based on the correlation between the FRET efficiency and membrane DNA density (Fig. 2a-f), apparent membrane dissociation constant, KD, for each lipid-DNA pair was determined.
Figure 2.
Characterization of DNA Zippers on MCF-7 cell membranes. (a) 100 nM of C-P7 probe, (c) 90 nMof S-P7 probe, and (e) 90 nMof T-P7 probe was incubated with 0–250 nM of C-P*, C-P*, or C-P* probe on the MCF-7 cells for 20 min at 25°C. Samples were then imaged at the same temperature via excitation via a 488 nm laser and the fluorescence emission of Atto488 (I520) and Cy5 (I660) was collected at ~520 and ~660 nm, respectively. Pseudo-color I660/I520 ratiometric images were also shown. Scale bar, 20 μm. (b, d, f) The FRET efficiency was calculated based on the changes in the donor fluorescence intensity. Shown are the mean and SEM values from at least 20 individual cells in each P* concentration, (g) Apparent membrane dissociation constant, KD, for each pair of lipid-DNA conjugates was shown. (h) The effect of DNA duplex length on the membrane DNA Zipper binding efficiency. C-P7, S-P7, and T-P7 represent cholesterol-, sphingomyelin-, and tocopherol-modified DNA donor probes. C-P6, C-P7, and C-P8 represent cholesterol-DNA donor probe with 6, 7, or 8-nucleotide-long hybridization region with the acceptor probe modified with cholesterol (C-P*), sphingomyelin (S-P*), or tocopherol (T-P*).
As shown in Fig. 2g and Table S2, the strongest DNA Zipper interactions were observed with C-P7/S-P* and S-P7/C-P* (KD= 3.8×10−8 and 4.2×10−8 μmol/mm2), which can be explained by the preferential membrane cholesterol–sphingomyelin interactions.23-27 T-P7/T-P* also exhibited strong membrane interactions (KD= 5.0×10−8 μmol/mm2). In contrast, tocopherol-DNA only weakly bound with C-P* or S-P*, with KD values in the range of 1.3×10−7–1.8×10−7 μmol/mm2. These results suggest that tocopherol partitions in different membrane domains as cholesterol/sphingomyelin, and thus, cholesterol–tocopherol and sphingomyelin–tocopherol interactions have less chance to occur. It is worth mentioning that C-P7/T-P* interaction appeared to be stronger than S-P7/T-P* (Table S2), which may indicate less Lo domain restriction of cholesterol as compared to sphingomyelin.
We also studied the effect of DNA duplex length on membrane FRET efficiency by synthesizing C-P6 and C-P8 that can hybridize with P* via 6- or 8-base pairs. After incubating C-P6 and C-P8 with different concentrations of C-P*, S-P* or T-P*, on MCF-7 cells, DNA-duplex-length-dependent FRET signals were clearly observed (Fig. S6). As the base-pair number increased from P6/P* to P8/P*, a gradual decrease in the apparent membrane dissociation constant was shown in all these DNA Zippers (Fig. 2h and Table S2). Cholesterol–sphingomyelin membrane interaction was the strongest, followed by cholesterol–cholesterol interactions. Cholesterol–tocopherol and sphingomyelin–tocopherol interactions were always the least favored ones on MCF-7 cell membranes.
We further tested the cell membrane persistence of DNA Zippers. S-P7/S-P* and C-P7/S-P* displayed significantly greater membrane stability than C-P7/C-P* and T-P7/T-P* (Fig. S7). For example, after 2-hour incubation, >94% of both donor (I520) and acceptor (I660) fluorescence of S-P7/S-P* are still visible on cell membranes. C-P7/S-P* also exhibited good persistence: ~80% C-P7 and ~92% S-P* cell membrane signals still exist. In contrast, only ~74% C-P7/C-P* and ~52% T-P7/T-P* fluorescence remained on cell membranes under the same condition. Obvious internalized signals were shown with these cholesterol- or tocopherol-conjugated DNAs (Fig. S7). This fact may be related with the natural outer leaflet localization and slow membrane flip-flop of sphingomyelin.39-41
To further study if these membrane FRET signals are correlated with membrane order, we extracted GPMVs from MCF-7 and determined the colocalization of DNA Zipper and CTxB signals (Fig. S8). To normalize cell-to-cell variations in membrane probe densities, I660/I520 ratiometric signal was used. The membrane I660/I520 signals of C-P7/C-P*, C-P7/S-P* and S-P7/S-P* are largely located in the Lo phases, with a Pearson correlation coefficient’s r= 0.77, 0.84, and 0.86, respectively. The slightly lower Lo preference of C-P7/C-P* further suggested that sphingomyelin-based probes can be better used than cholesterol for more accurate reporting of membrane order. As expected, T-P7/T-P* interactions (r= 0.19) were significantly enriched in the Ld phases. All these results indicated that DNA Zippers can indeed be used to measure membrane order and lipid domains.
We next asked if these DNA Zippers could be used to image changes in the membrane order. We first studied the effect of methyl-β-cyclodextrin (MβCD) on DNA Zipper signals. MβCD is widely used to sequester membrane cholesterols and disrupt membrane order.42 T-P7/T-P* signal was increased after the MβCD treatment, which could be explained by the enlarged membrane Ld phases after the disruption of Lo. In contrast, after pre-treating MCF-7 cells with 10 mM MβCD, significant loss of membrane I660/I520 signals was observed from C-P7/C-P*, C-P7/S-P*, and S-P7/S-P* (Fig. 3a and 3b). C-P6/C-P*, C-P8/C-P*, C-P6/S-P*, and C-P8/S-P* also exhibited obvious decrease in the membrane ratiometric signal, though to a lesser extent (Fig. S9). Consistent with our previous report,21 7-base-pair DNA Zipper is the optimal sensor for imaging membrane order.
Figure 3.
Imaging membrane order with DNA Zippers. (a) MCF-7 cells were first treated with 10 mM of MβCD for 30 min, and then each pair of P7 and P* probes was added for 20 min at 25°C before imaging. Cells without adding MβCD was used as the control. Pseudo-color I660/I520 ratiometric images were shown via excitation through a 488 nm laser. Scale bar, 20 μm. (b) Ratiometric signals on individual MCF-7 cell membranes as measured in the presence or absence of the MβCD pre-treatment. (c) MCF-7 cells were pre-treated with a mixture of 1 mM cholesterol and 10 mM of MβCD for 30 min (cholesterol supplementation) or a mixture of 1.5 mM sphingomyelin and 10 mM of MαCD for 5 min (sphingomyelin supplementation), and then each pair of P7 and P* probes was added for 20 min at 25°C before imaging. Cells without the pre-treatment was used as the control. Pseudo-color I660/I520 ratiometric images were shown via excitation through a 488 nm laser. Scale bar, 20 μm. (d) Ratiometric signals on individual MCF-7 cell membranes as measured in the presence or absence of the cholesterol or sphingomyelin supplementation. (e) Ratiometric signals on individual MCF-7 cell membranes either without the pre-treatment (0 mM) or pre-treated with a mixture of 10 mM of MβCD and 0.1-, 0.25-, or 0.75-mM cholesterol for 30 min at 25°C. Shown are the mean and SEM values obtained from at least 20 cell membranes in each case. *p< 0.05, ***p< 0.001 in two-tailed Student’s t-test; ns, not significant. C-P7, S-P7, and T-P7 represent cholesterol-, sphingomyelin-, and tocopherol-modified DNA donor probes. C-P*, S-P*, and T-P* represent the corresponding cholesterol-, sphingomyelin-, and tocopherol-modified acceptor strands.
We further studied the effect of sphingomyelin and cholesterol supplementation on DNA Zipper signals, which supplementing is known to promote the formation of Lo domains.43,44 Indeed, C-P7/C-P*, C-P7/S-P*, and S-P7/S-P* exhibited enhanced ratiometric signals upon pre-treatment with 1 mM cholesterol or 1.5 mM sphingomyelin (Fig. 3c and 3d). Interestingly, S-P7/S-P* was more sensitive to the membrane addition of cholesterol (104% enhancement) than sphingomyelin (42% increasement). In comparison, C-P7/C-P* exhibited a slightly larger signal increase upon adding sphingomyelin (83%) than cholesterol (71%). C-P7/S-P* provided the most significant fluorescence enhancement towards both sphingomyelin (105%) and cholesterol (134%) treatment (Fig. 3d).
To test if DNA Zippers can be used to detect small changes in membrane order, we supplemented MCF-7 cells with 0–0.75 mM of cholesterol and added C-P7/C-P*, C-P7/S-P*, and S-P7/S-P* for ratiometric imaging. Consistently, C-P7/S-P* exhibited the greatest sensitivity (Fig. 3e and S10). Even with the addition of 0.25 mM cholesterol, a significant increase in C-P7/S-P* signals could be still detected. The use of cholesterol-sphingomyelin pairs can potentially enhance the sensitivity of DNA Zippers for studying membrane order. We have further demonstrated the successful application of C-P7/S-P* for detecting membrane order on MDCK and Jurkat cells (Figure S11).
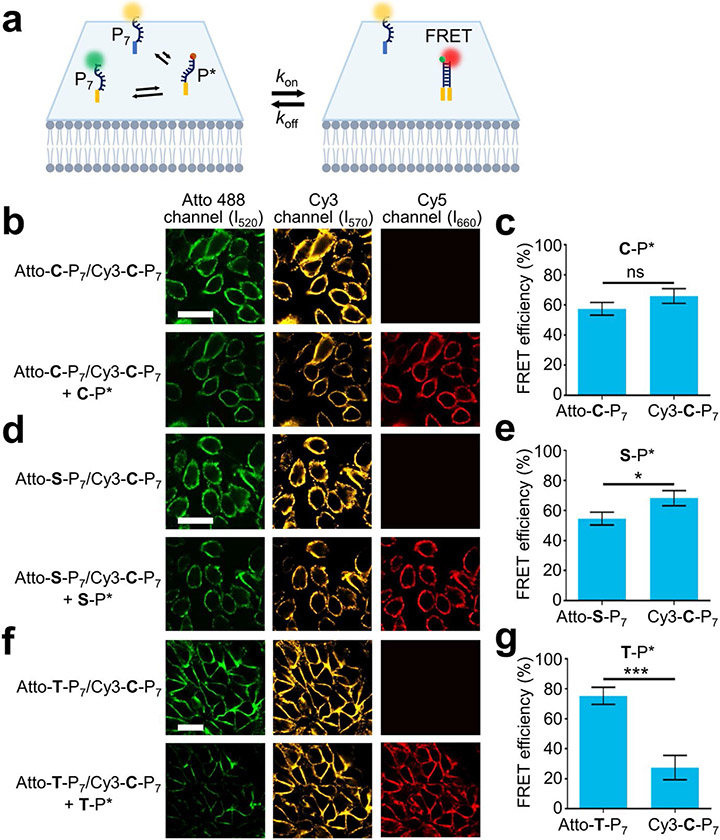
To test if multiple lipid interactions can be detected on the same cell membrane simultaneously, we further developed a competition assay by conjugating P7 probes with two different donor dyes, Atto488 and Cy3 (Fig. 4a). Both dyes can form FRET pairs with Cy5. After demonstrating Atto488 and Cy3 can be imaged together with minimal spectral overlap and crosstalk from Cy5 (Fig. S12a), we verified that after mixing Cy3-modified cholesterol-P7 (i.e., Cy3-C-P7) and Cy5-modified cholesterol-P* probes (i.e., C-P*), highly effective MCF-7 cell membrane FRET signals could be detected (Fig. S12b). To test if Atto488/Cy5 and Cy3/Cy5 FRET signals can be simultaneously detected, we incubated equal amount of Atto488- and Cy3-labeled cholesterol-P7 probes, Atto-C-P7 and Cy3-C-P7, with MCF-7 cells, and added 100 nM C-P*. As the same cholesterol-P7 probe was used, we expect to observe a similar level of quenching in both Atto488 and Cy3 signals. Indeed, membrane FRET efficiency (E) of Atto-C-P7/C-P* and Cy3-C-P7/C-P* was determined as 57% and 66%, respectively (Fig. 4b, 4c and S13).
Figure 4.
Development of a cell membrane competition assay. (a) Schematic of the membrane competition assay where two donor fluorophore-labeled lipid-P7 probes compete for an acceptor dye-labeled lipid-P* probe. (b, d, f) Membrane competition assays were performed by adding Cy3-C-P7 and an Atto488-modified competitive probe with the MCF-7 cells for 20 min at 25°C. After washing away excess probes, 100 nM of C-P* or 90 nM of S-P* or T-P* was added. Fluorescence images were taken after excitation with a 488 nm, 561 nm, and 640 nm laser, respectively for the Atto488, Cy3, and Cy5 channels. Scale bar, 25 μm. (c, e, g) The FRET efficiency was calculated based on the changes in the donor fluorescence intensity in either Atto488 or Cy3 channel. Shown are the mean and standard deviation values from at least 20 individual cells in each case. *p< 0.05, ***p< 0.001 in two-tailed Student’s t-test; ns, not significant. Atto-C-P7, Atto-S-P7, and Atto-T-P7 represent Atto488-modified cholesterol-, sphingomyelin-, and tocopherol-DNA donor probes. Cy3-C-P7 represent Cy3-modified competitor cholesterol-DNA donor probe. C-P*, S-P*, and T-P* represent Cy5-modified cholesterol-, sphingomyelin-, and tocopherol-DNA acceptor strands.
To further examine if this competition assay can distinguish the relative preference of membrane interactions, we applied Atto488-modified C-P6 and C-P8 probes to compete with Cy3-C-P7 for the membrane binding of C-P*. As expected, Cy3-C-P7/C-P* interaction (E= 83%) was much preferred than Atto-C-P6/C-P* (E= 25%) (Fig. S13). Meanwhile, much more frequent membrane Atto-C-P8/C-P* interactions (E= 78%) was shown as compared to Cy3-C-P7/C-P* (E= 25%).
We next applied this competition assay to reveal variations in the membrane interaction patterns among different lipids. To test if sphingomyelin has stronger membrane interaction with cholesterol or another sphingomyelin, we incubated Atto-S-P7 and Cy3-C-P7 with S-P* on MCF-7 cells. Only slightly greater quenching of Cy3-C-P7 signals was observed than Atto-S-P7 (Fig. 4d and 4e), suggesting a similar strength of membrane cholesterol–sphingomyelin and sphingomyelin–sphingomyelin interactions. We also studied if tocopherol–tocopherol interaction is preferable to cholesterol–tocopherol. After mixing Atto-T-P7, Cy3-C-P7, and T-P* with MCF-7 cells, a dominated Atto-T-P7/T-P* interaction signal (E= 76%) was shown as compared to Cy3-C-P7/T-P* (E= 27%) (Fig. 4f and 4g), which is consistent with different membrane domain partitioning of tocopherol and cholesterol.
Lastly, we applied DNA Zippers to analyze membrane order changes during epithelial–mesenchymal transition (EMT) of cancer cells. During EMT, the traits of cells shift from epithelial to more migratory and invasive mesenchymal states.45,46 The epithelial and mesenchymal states exhibit distinct lipid profiles.47-49 It is hypothesized that enhanced mesenchymal cell membrane motility is correlated with the destabilization of lipid domains.32,50,51 Controversially, the formation of lipid domains was also reported as important for activating EMT.52-54 We thus wanted to investigate what is the correlation between membrane order and EMT.
EMT of MCF-7 cells was triggered by adding TGF-β1. The successful EMT induction was confirmed by the downregulation of E-cadherin and upregulation of N-cadherin after 24–72-h induction (Fig. S14). After adding C-P7/S-P* different days following TGF-β1 induction, interestingly, no significant changes in DNA Zipper signals were observed, as compared to non-treated control cells (Fig. S15). It could potentially suggest that initial EMT induction may not be significantly linked to changes in membrane order. However, a complete investigation on this matter is beyond the scope of this study.
In this study, we developed “second-generation” DNA Zipper that can be used to image both liquid ordered and liquid disordered domains in living cell membranes. Compared to cholesterol-based DNA Zipper, sphingomyelin/cholesterol complex-based DNA probes exhibit improved cell membrane persistence, better Lo partitioning selectivity, and higher sensitivity for detecting membrane order changes. As a result, these sphingomyelin/cholesterol-DNA Zippers are more reliable and advanced tools for detecting lipid domains. Meanwhile, we reported novel tocopherol-based DNA Zipper for imaging liquid disordered phases. The modular and versatile design of these lipid-DNA probes can be potentially used for detecting a broad range of membrane dynamic interactions and compartments in living cells.
The DNA Zipper probes exhibit several unique features: (1) The hybridization strength of DNA duplex can be precisely and predictably tuned to accommodate membrane interactions of different binding affinities. (2) The highly selective and programmable nature of DNA probes allow multiple pairs of membrane interactions to be simultaneously detected as shown in our competition assay. (3) DNA Zipper can function simply by imaging after a brief incubation, which can be easily used by a broad scientific community. (4) DNA probes can be readily synthesized and modified with a wide range of fluorophores and lipid moieties to enable sensitive cellular imaging via standard fluorescence microscopes.
Synthetic lipid-DNA conjugates have gained much attention recently for biophysical studies on living cell membranes.55 Other powerful DNA Zipper probes may emerge lately for the real-time detection of cell membrane lipid–lipid, lipid–protein, and protein–protein interaction patterns. These emerging probes will dramatically improve our ability to understand the mysterious cell membrane organization, heterogeneity, and functions.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge NIH R35GM133507, Alfred P. Sloan Research Fellowship, Camille Dreyfus Teacher-Scholar Award, and start-up grant from UMass Amherst to M. You, and Paul Hathaway Terry Scholarship to Y. Bagheri. We are grateful to Dr. James Chambers for the assistance in fluorescence imaging. We also thank every other member of the You Lab for useful discussion.
Footnotes
Supporting Information
The supporting information is available free of charge online.
Experimental methods, DNA sequences, membrane interaction KD values, probe membrane insertion and persistence, FRAP results, measurement of lipid domain changes, membrane competition assay, and immunoassay for EMT.
The authors declare no competing financial interest.
References
- (1).Groves JT; Kuriyan J Molecular Mechanisms in Signal Transduction at the Membrane. Nat. Struct. Mol. Biol 2010, 17 (6), 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Sezgin E; Levental I; Mayor S; Eggeling C The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol 2017, 18 (6), 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Simons K; Toomre D Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol 2000, 1, 31–39. [DOI] [PubMed] [Google Scholar]
- (4).Cheng X; Smith JC Biological Membrane Organization and Cellular Signaling. Chem. Rev 2019, 119 (9), 5849–5880. [DOI] [PubMed] [Google Scholar]
- (5).Bagheri Y; Ali AA; You M Current Methods for Detecting Cell Membrane Transient Interactions. Front. Chem 2020, 8, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Varshney P; Yadav V; Saini N Lipid Rafts in Immune Signalling: Current Progress and Future Perspective. Immunology 2016, 149 (1), 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ripa I; Andreu S; López-Guerrero JA; Bello-Morales R Membrane Rafts: Portals for Viral Entry. Front. Microbiol 2021, 12, 631274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lajoie P; Nabi IR Lipid Rafts, Caveolae, and Their Endocytosis. Int. Rev. Cell Mol. Biol 2010, 282 (C), 135–163. [DOI] [PubMed] [Google Scholar]
- (9).Greenlee JD; Subramanian T; Liu K; King MR Rafting down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2021, 81 (1), 815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).McDonald G; Deepak S; Miguel L; Hall CJ; Isenberg DA; Magee AI; Butters T; Jury EC Normalizing Glycosphingolipids Restores Function in CD4+ T Cells from Lupus Patients. J. Clin. Invest 2014, 124 (2), 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Krishnan S; Nambiar MP; Warke VG; Fisher CU; Mitchell J; Delaney N; Tsokos GC Alterations in Lipid Raft Composition and Dynamics Contribute to Abnormal T Cell Responses in Systemic Lupus Erythematosus. J. Immunol 2004, 172 (12), 7821–7831. [DOI] [PubMed] [Google Scholar]
- (12).Michel V; Bakovic M Lipid Rafts in Health and Disease. Biol. Cell 2007, 99 (3), 129–140. [DOI] [PubMed] [Google Scholar]
- (13).Benarroch EE Lipid Rafts, Protein Scaffolds, and Neurologic Disease. Neurology 2007, 69 (16), 1635–1639. [DOI] [PubMed] [Google Scholar]
- (14).Simons K; Gerl MJ Revitalizing Membrane Rafts: New Tools and Insights. Nat. Rev. Mol. Cell Biol 2010, 11 (10), 688–699. [DOI] [PubMed] [Google Scholar]
- (15).Yu J; Fischman DA; Steck TL Selective Solubilization of Proteins and Phospholipids from Red Blood Cell Membranes by Nonionic Detergents. J. Supramol. Cell. Biochem 1973, 1 (3), 233–248. [DOI] [PubMed] [Google Scholar]
- (16).Klymchenko AS Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res 2017, 50 (2), 366–375. [DOI] [PubMed] [Google Scholar]
- (17).Klymchenko AS; Kreder R Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol 2014, 21 (1), 97–133. [DOI] [PubMed] [Google Scholar]
- (18).Amaro M; Reina F; Hof M; Eggeling C; Sezgin E Laurdan and Di-4-ANEPPDHQ Probe Different Properties of the Membrane. J. Phys. D. Appl. Phys 2017, 50 (13), 134004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sezgin E; Sadowski T; Simons K Measuring Lipid Packing of Model and Cellular Membranes with Environment Sensitive Probes. Langmuir 2014, 30 (27), 8160–8166. [DOI] [PubMed] [Google Scholar]
- (20).You M; Lyu Y; Han D; Qiu L; Liu Q; Chen T; Sam Wu C; Peng L; Zhang L; Bao G; Tan W DNA Probes for Monitoring Dynamic and Transient Molecular Encounters on Live Cell Membranes. Nat. Nanotechnol 2017, 12(5), 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bagheri Y; AN AA; Keshri P; Chambers J; Gershenson A; You M Imaging Membrane Order and Dynamic Interactions in Living Cells with a DNA Zipper Probe. Angew. Chemie Int. Ed 2022, 61 (6), e202112033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hjort Ipsen J; Karlström G; Mourtisen OG; Wennerström H; Zuckermann MJ Phase Equilibria in the Phosphatidylcholine-Cholesterol System. BBA - Biomembr. 1987, 905 (1), 162–172. [DOI] [PubMed] [Google Scholar]
- (23).Hanada K; Nishijima M; Akamatsu Y; Pagano RE Both Sphingolipids and Cholesterol Participate in the Detergent Insolubility of Alkaline Phosphatase, a Glycosylphosphatidylinositol-Anchored Protein, in Mammalian Membranes. J. Biol. Chem 1995, 270 (11), 6254–6260. [DOI] [PubMed] [Google Scholar]
- (24).Ahmed SN; Brown DA; London E On the Origin of Sphingolipid/Cholesterol-Rich Detergent-Insoluble Cell Membranes: Physiological Concentrations of Cholesterol and Sphingolipid Induce Formation of a Detergent-Insoluble, Liquid-Ordered Lipid Phase in Model Membranes. Biochemistry 1997, 36(36), 10944–10953. [DOI] [PubMed] [Google Scholar]
- (25).Brown DA; London E Structure and Function of Sphingolipid- and Cholesterol-Rich Membrane Rafts. J. Biol. Chem 2000, 275(23), 17221–17224. [DOI] [PubMed] [Google Scholar]
- (26).Radhakrishnan A; Li XM; Brown RE; McConnell HM Stoichiometry of Cholesterol-Sphingomyelin Condensed Complexes in Monolayers. Biochim. Biophys. Acta - Biomembr 2001, 1511 (1), 1–6. [DOI] [PubMed] [Google Scholar]
- (27).Lozano MM; Hovis JS; Moss FR; Boxer SG Dynamic Reorganization and Correlation among Lipid Raft Components. J. Am. Chem. Soc 2016, 138 (31), 9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Simons K; Ikonen E Functional Rafts in Cell Membranes. Nature 1997, 387 (6633), 569–572. [DOI] [PubMed] [Google Scholar]
- (29).Levental KR; Lorent JH; Lin X; Skinkle AD; Surma MA; Stockenbojer EA; Gorfe AA; Levental I Polyunsaturated Lipids Regulate Membrane Domain Stability by Tuning Membrane Order. Biophys. J 2016, 110 (8), 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sych T; Gurdap CO; Wedemann L; Sezgin E How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes (Basel). 2021, 11 (5), 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wassail SR; Brzustowicz MR; Shaikh SR; Cherezov V; Caffrey M; Stillwell W Order from Disorder, Corralling Cholesterol with Chaotic Lipids: The Role of Polyunsaturated Lipids in Membrane Raft Formation. Chem. Phys. Lipids 2004, 132 (1), 79–88. [DOI] [PubMed] [Google Scholar]
- (32).Tisza MJ; Zhao W; Fuentes JSR; Prijic S; Chen X; Levental I; Chang JT Motility and Stem Cell Properties Induced by the Epithelialmesenchymal Transition Require Destabilization of Lipid Rafts. Oncotarget 2016, 7(32), 51553–51568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gutiérrez S; Svahn SL; Johansson ME Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci 2019, 20 (20), 5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Mason RP; Libby P; Bhatt DL Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol 2020, 40, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Atkinson J; Harroun T; Wassail SR; Stillwell W; Katsaras J The Location and Behavior of α-Tocopherol in Membranes. Mol. Nutr. Food Res 2010, 54 (5), 641–651. [DOI] [PubMed] [Google Scholar]
- (36).Sezgin E; Kaiser HJ; Baumgart T; Schwille P; Simons K; Levental I Elucidating Membrane Structure and Protein Behavior Using Giant Plasma Membrane Vesicles. Nat. Protoc 2012, 7 (6), 1042–1051. [DOI] [PubMed] [Google Scholar]
- (37).Pinkwart K; Schneider F; Lukoseviciute M; Sauka-Spengler T; Lyman E; Eggeling C; Sezgin E Nanoscale Dynamics of Cholesterol in the Cell Membrane. J. Biol. Chem 2019, 294 (34), 12599–12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gaibelet G; Tercé F; Allart S; Lebrun C; Collet X; Jamin N; Orlowski S; Gaibelet G; Tercé F; Allart S; Lebrun C; Collet X; Jamin N; Orlowski S Fluorescent Probes for Detecting Cholesterol-Rich Ordered Membrane Microdomains: Entangled Relationships between Structural Analogies in the Membrane and Functional Homologies in the Cell. AIMS Biophys. 2017, 4 (1), 121–151. [Google Scholar]
- (39).Fujimoto T; Parmryd I Interleaflet Coupling, Pinning, and Leaflet Asymmetry-Major Players in Plasma Membrane Nanodomain Formation. Front. Cell Dev. Biol 2017, 4, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Haest CWM; Kamp D; Deuticke B Transbilayer Reorientation of Phospholipid Probes in the Human Erythrocyte Membrane. Lessons from Studies on Electroporated and Resealed Cells. Biochim. Biophys. Acta - Biomembr 1997, 1325 (1), 17–33. [DOI] [PubMed] [Google Scholar]
- (41).Smeets EF; Comfurius P; Bevers EM; Zwaal RFA Calcium-Induced Transbilayer Scrambling of Fluorescent Phospholipid Analogs in Platelets and Erythrocytes. BBA - Biomembr. 1994, 1195 (2), 281–286. [DOI] [PubMed] [Google Scholar]
- (42).Zidovetzki R; Levitan I Use of Cyclodextrins to Manipulate Plasma Membrane Cholesterol Content: Evidence, Misconceptions and Control Strategies. Biochim. Biophys. Acta - Biomembr 2007, 1768 (6), 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Xiao M; Lai W; Wang X; Qu X; Li L; Pei H DNA Mediated Self-Assembly of Multicellular Microtissues. Microphysiological Syst. 2018, 2, 1. [Google Scholar]
- (44).Cheng HT; Megha; London E Preparation and Properties of Asymmetric Vesicles That Mimic Cell Membranes. Effect upon Lipid Raft Formation and Transmembrane Helix Orientation. J. Biol. Chem 2009, 284 (10), 6079–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Ribatti D; Tamma R; Annese T Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol 2020, 13(6), 100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Loh CY; Chai JY; Tang TF; Wong WF; Sethi G; Shanmugam MK; Chong PP; Looi CY The E-Cadherin and n-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8(10), 1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Sampaio JL; Gerl MJ; Klose C; Ejsing CS; Beug H; Simons K; Shevchenko A Membrane Lipidome of an Epithelial Cell Line. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (5), 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nieva C; Marro M; Santana-Codina N; Rao S; Petrov D; Sierra A The Lipid Phenotype of Breast Cancer Cells Characterized by Raman Microspectroscopy: Towards a Stratification of Malignancy. PLoS One 2012, 7 (10), e46456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Sabtu SN; Sani SFA; Looi LM; Chiew SF; Pathmanathan D; Bradley DA; Osman Z Indication of High Lipid Content in Epithelial-Mesenchymal Transitions of Breast Tissues. Sci. Rep 2021, 11, 3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Edmond V; Dufour F; Poiroux G; Shoji K; Malleter M; Fouque A; Tauzin S; Rimokh R; Sergent O; Penna A; Dupuy A; Levade T; Theret N; Micheau O; Segui B; Legembre P Downregulation of Ceramide Synthase-6 during Epithelial-to-Mesenchymal Transition Reduces Plasma Membrane Fluidity and Cancer Cell Motility. Oncogene 2015, 34 (8), 996–1005. [DOI] [PubMed] [Google Scholar]
- (51).Zhao W; Prijic S; Urban BC; Tisza MJ; Zuo Y; Li L; Tan Z; Chen X; Mani SA; Chang JT Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res. 2016, 76 (7), 2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Causeret M; Taulet N; Comunale F; Favard C; Gauthier-Rouvière C N-Cadherin Association with Lipid Rafts Regulates Its Dynamic Assembly at Cell-Cell Junctions in C2C12 Myoblasts. Mol. Biol. Cell 2005, 16 (5), 2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Fernéndez-Muñoz B; Yurrita MM; Martín-Villar E; Carrasco-Ramírez P; Megías D; Renart J; Quintanilla M The Transmembrane Domain of Podoplanin Is Required for Its Association with Lipid Rafts and the Induction of Epithelial-Mesenchymal Transition. Int. J. Biochem. Cell Biol 2011, 43 (6), 886–896. [DOI] [PubMed] [Google Scholar]
- (54).Wu Y; Zhao Y; He X; He Z; Wang T; Wan L; Chen L; Yan N Hydroxypropyl-β-cyclodextrin Attenuates the Epithelial-to-mesenchymal Transition via Endoplasmic Reticulum Stress in MDA-MB-231 Breast Cancer Cells. Mol. Med. Rep 2020, 21 (1), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Ali AA; Bagheri Y; You M Digging into the Biophysical Features of Cell Membranes with Lipid-DNA Conjugates. Q. Rev. Biophys 2022, 55, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.