Abstract
Background
Olea europaea leaf extract (OELE) has potential health benefits and protects against cytotoxicity. This study investigated the possible ameliorative effect of OELE on cisplatin-induced nephrotoxicity in rats.
Methods
Rats were assigned into six groups; two groups received 150 mg/kg or 300 mg/kg of OELE, one group received a single dose of cisplatin (6 mg/kg) IP on the first day of the experiment, two groups received a single dose of cisplatin 150 mg/kg or 300 mg/kg of OELE on the first day then starting from the fifth day for 10 consecutive days, and one group acted as a control. Results and Conclusion. The findings showed that cisplatin-induced nephrotoxicity was evidenced by a significant increase in serum creatinine blood urea nitrogen (BUN) and a significant decrease in estimated creatinine clearance and potassium level, which corresponded with the alterations in the histopathology of the renal tissue. OELE significantly ameliorated the nephrotoxic effects of cisplatin as dose-dependent.
1. Introduction
Cisplatin is a platinum-derived chemotherapeutic agent frequently used as a standard antineoplastic drug for treating different types of malignancies in the lung, bladder, cervix, ovaries, and testes [1, 2]. Cisplatin is eliminated through the renal system and accumulates in the renal tubules [3], leading to increased cisplatin concentrations in the proximal tubule cells that are five times higher than the serum level [4]. Therefore, it is limited to patients with creatinine clearance greater than 60 ml/minute [5]. Although cisplatin induces various dose-limiting adverse effects, such as neurotoxicity, ototoxicity, gastrotoxicity, myelosuppression [2, 3], cardiotoxicity [6], testicular toxicity [7], and hepatorenal toxicity [8], nephrotoxicity is the most prominent adverse effect encountered in clinical practice. Nephrotoxicity induced by cisplatin is often seen after 10 days of cisplatin administration and can present with various types of symptoms, including acute kidney injury (AKI), renal tubular acidosis, hypomagnesemia, hyperuricemia, and hypocalcemia [9]. Clinical evidence has revealed that one-third of patients experience AKI after cisplatin administration, along with increases in blood urea nitrogen (BUN), serum creatinine, reduced glomerular filtration rate (GFR), and disturbed electrolytes [10, 11].
Olea europaea, also known as the olive tree, is an evergreen tree that is indigenous to the Mediterranean region, where it is widely utilized as traditional medicine for a variety of illnesses [12]. Among the different parts of the Olea europaea tree, the leaves have the highest antioxidant and scavenging capacity [13]. The extracted leaves of Olea europaea have drawn attention since they have phenolic compounds that are linked to various pharmacological activities [14]. Several in vitro and in vivo studies have demonstrated wide range of health benefits of the extracted Olea europaea leaves, such as cytotoxic activity against human breast cancer cells [15], an antiproliferative effect on leukemia cells [16, 17], an antiarrhythmic effect [18], a hypotensive effect [19], an anti-HIV effect [20], and an antimalaria effect [21]. The wide range of these pharmacological activities is mostly linked to oleuropein and its bioactive byproduct, hydroxytyrosol, which comprises the major active compound in olive leaves [22]. This endogenous compound in OELE possesses empower antioxidant activities [14] that provide sufficient protection against the reactive oxygen species (ROS) resulted from cell damage or burst [22]. Several studies on the antioxidants of hydroxytyrosol and oleuropein have been performed. A recent study showed that both oleuropein and hydroxytyrosol have hypolipidemic and hepatoprotective effects against high-fat diet-induced metabolic disorders [23]. The nephroprotective effect of Olea europaea leaf extract (OELE) has been reported on diclofenac-induced hepatotoxicity and kidney damage [24] and gentamicin-induced nephrotoxicity [25, 26]. In addition, the ameliorative effect of the extracted Olea europaea leaves has been reported on nephrotoxicity induced by cyclosporin [27] and cyclophosphamide in an animal model [28]. In human embryonic renal epithelial “GP-293” cells, oleuropein has exhibited a protective effect against cisplatin-induced cellular toxicity [29]. However, the nephroprotective effect of different doses of OELE against cisplatin-induced nephrotoxicity in a rat model has not been evaluated yet. Thus, the aim of this study is to evaluate the ameliorative effect of different doses of the Olea europaea leaf extract against cisplatin-induced nephrotoxicity in rat models. It also aimed to study the effect of the extracted leaves and/or cisplatin on the body's and organs' weight and potassium level as a major complication of acute kidney injury.
2. Materials and Methods
2.1. Animals and Treatment
Adult healthy Wistar male rats weighing 160 ± 20 g were brought from the Biology Department, Sana'a University. Animals were housed in a group of five in each metal cage under hygienic conditions at a temperature of 23 ± 2°C, relative humidity of 50–60%, and 12 h of light/dark cycles. All experiments were carried out in the light cycle according to the protocol of the Animal Care and Use Committee, National Research Council (Washington, 2011). Animals were identified by tail labeling and provided with standard rodent food with free access to water ad libitum. All procedures were reviewed and approved by the ethical committee at the University of Science and Technology (reference number: EAC/UST231). Before starting the experiments, the animals were kept for two weeks to ensure acclimatization.
2.2. Drugs and Natural Products
A cisplatin vial (1 mg/mL; Zuviplat®) in the clinical formulation was purchased from Zuvius Lifesciences Pvt. Ltd. (Mumbai, India).
The fresh leaves of Olea europaea were collected from the olive trees in the Sana'a region, Yemen, at the beginning of September 2016. Identification and authentication of the collected plant specimen were done by a botanist from the Faculty of Sciences, Department of Botany, Sana'a University. A voucher specimen was deposited in the Yemeni National Herbarium, Sana'a University, Yemen, with the collection number (Herbarium number: 718).
2.3. Induction of Acute Kidney Injury (AKI)
Acute kidney injury (AKI) was induced in rats by intraperitoneal administration of a single cisplatin dose (6 mg/kg; Zuviplat®) according to previous studies [30–32]. After the induction of AKI, the rat had free access to food and water.
2.4. Plant and Sample Preparation
The fresh leaves of Olea europaea were collected from the olive trees in the Sana'a region at the beginning of September 2016. The leaves were thoroughly washed using tap water to remove dust and then dried at room temperature in a dark place for 14 days. The dried leaves were ground using a blender to a fine powder and then packaged in a glass container and stored at 4°C for further analysis.
2.5. Preparation of Olea europaea Leaf Extraction
The air-dried leaves (170 g) were extracted with 95% ethanol (1 : 25 w⁄v) using a Soxhlet apparatus for 24 hours and concentrated using a rotary evaporator (Büchi®, Rotavapor R-200) at 40°C. The resulting extract, 120 g (a yield of 70.6%), was stored at 15–20°C in sealed desiccators, and the drying process was carried out in closed and controlled equipment using a Labconco freeze dryer to improve the quality of the final product [33]. The required amount of the extracted Olea europaea leaves was dissolved in normal saline and administered to the animals by oral gavage according to their body weight [33, 34].
2.6. Phytochemical Screening
Screening for the presence or absence of flavonoids, alkaloids, anthraquinones, saponins, tannins, glycosides, and terpenoids was qualitatively carried out as described in [35, 36].
2.7. Acute Oral Toxicity Study
An acute oral toxicity study of the extracted leaves was carried out according to the OECD 425 guidelines and methods of Jaykaran et al. [37]. After a two-week acclimatization period, twelve rats weighing 180 ± 20 g were randomly assigned into three groups, with four rats in each group, as follows: Group I served as a control group, which has free access to rodent food and water; Group II was given 2 g/kg of the extract of Olea europaea leaves; and group III was given 5 g/kg of the same extract for 72 hours. The extract was previously dissolved in water and given to the animals by oral gavage (once a day) for 72 hours. Rats from the two treatment groups were observed individually for any sign of toxicity after the treatment, as described in [37, 38].
2.8. Study Design
Thirty male Wistar rats with age 2-3 months, weighing 160 ± 20 g, were randomly assigned into six groups, with five rats per group, as follows: the control group, which had free access to water for 15 days; the cisplatin (CIS) group was given a single intraperitoneal dose (6 mg/kg) of cisplatin and utilized as a positive control; two groups (treated groups: CIS/OELE-150/kg and CIS/OELE-300/kg) were given a single dose of intraperitoneal cisplatin (6 mg/kg) on the first day, and then 150 mg/kg and 300 mg/kg of the extracted leaves of Olea europaea, starting from the 5th day to the 15th day; two groups were given 150 mg/kg and 300 mg/kg (OELE-150/kg, and OEL-300/kg) of OELE for 15 days according to the method [28] and utilized as a negative control. On the sixth day of the experiment, blood samples were withdrawn for nephrotoxicity evaluation, and at the end of the experiment (15th day), blood samples were collected using a heart puncture under halothane anesthesia, and both kidneys were dissected for histopathological study [28].
2.9. Biochemical Parameters
Kidney function tests, including serum creatinine (Chema Diagnostica, Monsano (AN)-Italy), blood urea nitrogen (Chema Diagnostica, Monsano (AN)-Italy), estimated creatinine clearance, and potassium level (Spinreact, Ctra.Santa Coloma, Girona, Spain), were evaluated using the COBAS 6000 analyzer.
2.10. Creatinine Clearance Estimation
Creatinine clearance estimation was carried out using a recently published model. This model depends on plasma creatinine and the weight of rats for the estimation of creatinine clearance with high accuracy, regardless of other factors such as the age of the rats, comorbidities, and treatment. The model was validated and found to have congruent results with the measured creatinine clearance. This model was recently released as a web-based calculator (https://idal.uv.es/aclara) to facilitate its use [39].
2.11. Histopathology of the Kidney
The animals were sacrificed by decapitation under light anesthesia using halothane in desiccator specialized for anesthesia on the same day of blood collection. Both kidneys were dissected immediately. The organs were washed using normal saline water and weighed. Both kidneys were preserved in 10% phosphate buffer and formalin for histopathologic investigations [40]. Kidneys were embedded in paraffin wax and chopped into small serial pieces, cut at 4 μm using a rotary microtome. They were stained with hematoxylin and eosin and then observed under a light microscope (Leica Microsystems, Germany) by a histopathologist in a blind manner.
Different histopathological alterations, including tubular necrosis, proteinaceous casts, and medullary congestion, were examined. The severity of these alterations was categorized as follows: normal renal tissue indicating no damage, mild damage characterized by the presence of unicellular or patchy isolated damage, moderate damage representing renal damage of less than 25% but greater than the mild, severe damage representing renal damage between 25% and 50%, and very severe damage indicating damage exceeding 50% of the renal tissue [40].
2.12. Statistical Analyses
All data were expressed as means ± standard error of the means (SEM). Statistical analysis was carried out using GraphPad Prism version 7.0 (GraphPad Software, Inc., San Diego, USA). Comparisons of biomarkers among the different study groups were evaluated by one-way ANOVA followed by Tukey's posttest. Two-way ANOVA followed by the Bonferroni posttest was also used to compare groups at different time points (the 6th day and the last day of the experiment as repeated-measures factors). The results of this study are considered significant at a P value less than 0.05.
3. Results
3.1. Phytochemical Screening
Phytochemical screening tests of OELE showed the presence of flavonoids, anthraquinones, tannins, saponins, alkaloids, and terpenoids.
3.2. Findings of the Acute Oral Toxicity Study
Administration of 2 g/kg and 5 g/kg of the extracted leaves to different groups of rats for 72 hours did not show signs of poisoning such as lacrimation, hair erection, convulsion, coma, or death, a significant difference in the body weights, compared to the control group. The findings showed no significant difference in the hemoglobin level (Hb) or white blood cell (WBC) and other biochemical studies compared with the control group. These findings indicated that this plant has LD50 greater than 5 g/kg.
3.3. Results of the Main Experiment
3.3.1. Effect of OELE and/or Cisplatin on Serum Creatinine Levels in Rats
A single intraperitoneal dose of cisplatin (6 mg/kg) administered on the first day of the experiment resulted in persistent AKI on the sixth and fifteenth days of the experiment, as evidenced by a significant increase in serum creatinine levels (P < 0.0001; Figure 1) compared to the control group. However, the administration of 300 mg/kg of ethanol-extracted Olea europaea leaves on day 5 of the experiment showed a significant improvement (P < 0.0001) in serum creatinine when compared with the cisplatin-treated group. Similar findings were observed when a second blood sample was collected on the 15th day of the experiment. The finding revealed persistent kidney damage on the 15th day of cisplatin administration, as evidenced by a persistent increase in the serum creatinine levels (P < 0.0001), compared with the control group, as shown in Figure 2. However, continuous administration of 300 mg/kg of ethanol-extracted Olea europaea leaves showed a significant (P < 0.0001) improvement in serum creatinine levels compared with the cisplatin-treated group. On the other hand, the lower dose (150 mg/kg) of the extracted Olea europaea leaves did not show significant improvement in the levels of serum creatinine at the two time points (the 6th and the 15th days) of the experiment when compared with the cisplatin-treated group. Normal rats receiving either 150 mg/kg or 300 mg/kg of the extracted leaves alone did not show any alteration in serum creatinine levels compared to the control group.
Figure 1.

Effect of OELE on day 6 of cisplatin administration. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05; CIS vs. (CIS/OELE-150 and CIS/OELE-300): ++++P < 0.0001, +++P < 0.001, and ++P < 0.01, +P < 0.05. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
Figure 2.
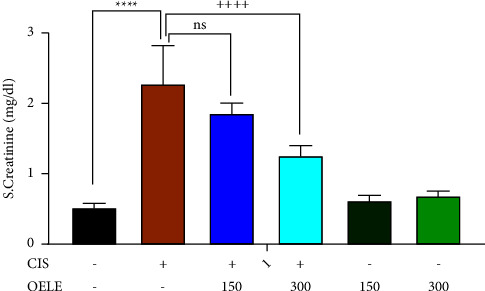
Effect of OELE on day 15 of cisplatin administration. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05; CIS vs. (CIS/OELE-150 and CIS/OELE-300): ++++P < 0.0001, +++P < 0.001, ++P < 0.01, and +P < 0.05. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
The effect of the time factor and continuous administration of the extracted leaves on serum creatinine is shown in Table 1. On the 15th day, all groups treated with cisplatin (cisplatin, CIS/OELE-150/kg, and CIS/OELE-300/kg) exhibited a significant decrease (P < 0.0001) in the mean serum creatinine levels compared to the levels observed on the 6th day of the experiment.
Table 1.
Differences in serum creatinine level at different time points.
| Test details (day 6–day 15) | Mean 1 (day 6) | Mean 2 (day 15) | SEM | P value |
|---|---|---|---|---|
| Control | 0.458 | 0.528 | 0.07138 | >0.9999 |
| CIS | 3.925 | 2.3 | 0.07981 | <0.0001 |
| CIS/OELE-150 | 3.7 | 1.88 | 0.07981 | <0.0001 |
| CIS/OELE-300 | 2.803 | 1.27 | 0.07981 | <0.0001 |
| OELE-150 | 0.4375 | 0.625 | 0.07981 | 0.1751 |
| OELE-300 | 0.508 | 0.694 | 0.07138 | 0.1015 |
CIS: cisplatin; OELE: Olea europaea leaf extract; SEM: standard error of the mean. Bold values indicates significant differences.
3.3.2. Effect of OELE and/or Cisplatin on Urea Levels in Rats
The ameliorative effect of different doses of OELE against cisplatin-induced nephrotoxicity was also evaluated by measuring blood urea levels on the 6th and 15th days of the experiment. Administration of cisplatin on day one resulted in AKI, as evidenced by the significant (P < 0.0001) increase in urea levels on both 6th and 15th days of the experiment when compared with the control group. However, continuous administration of 300 mg/kg of the extracted leaves by oral gavage on the 6th day resulted in a significant improvement in kidney function on the 6th (++P < 0.01) and 15th (+P < 0.05) days of the experiment when compared with the cisplatin-treated group. However, the lower dose of the extract (150 mg/kg) did not show a significant improvement in the urea levels on the 6th (P > 0.05) and 15th (P > 0.05) days of the experiment when compared with the cisplatin-treated group, as shown in Figures 3 and 4.
Figure 3.

Changes in urea levels on day 6 of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.0001; CIS vs. (CIS/OELE-150 and CIS/OELE-300): ++P < 0.01. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
Figure 4.
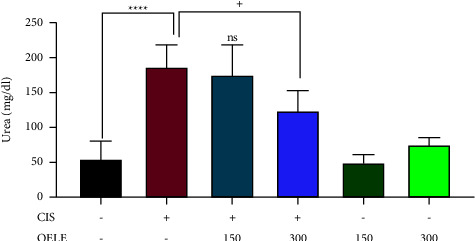
Changes in urea levels on day 15 of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.0001; CIS vs. (CIS/OELE-150 and CIS/OELE-300): +P < 0.05. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
Table 2 presents the impact of cisplatin and/or extracted leaves on urea levels at two different time points. On the 15th day, all the groups treated with cisplatin (cisplatin, CIS/OELE-150, and CIS/OELE-300) exhibited a significant decrease (P < 0.0001) in the mean blood urea levels compared to the levels observed on the 6th day of the experiment.
Table 2.
Differences in serum urea of the rats at different time points.
| Test details (day 6–day 15) | Mean 1 (day 6) | Mean 2 (day 15) | SEM | Adjusted P value |
|---|---|---|---|---|
| Control | 38.74 | 54.98 | 17.18 | 0.9287 |
| CIS- | 441.8 | 186.8 | 19.21 | <0.0001 |
| CIS/OELE-150 | 404.5 | 175.5 | 19.21 | <0.0001 |
| CIS/OELE-300 | 311.5 | 124 | 19.21 | <0.0001 |
| OELE-150 | 38.45 | 49.65 | 19.21 | 0.9934 |
| OELE-300 | 30.69 | 75.46 | 17.18 | 0.0973 |
CIS: cisplatin; OELE: Olea europaea leaf extract; SEM: standard error of the mean. Bold values indicates significant differences.
3.3.3. Effect of Cisplatin with/without the Extracted Leaves of Olea europaea on Creatinine Clearance
As shown in Table 3 and Figure 5, cisplatin-treated groups showed a significant reduction in the estimated creatinine clearance compared to the control group (P value <0.0001). Administration of high doses of the extracted Olea europaea leaves showed nonsignificant improvement in the estimated creatinine clearance. However, the low dose of the plant did not show any improvement in the estimated creatinine clearance.
Table 3.
Effect of OELE and/or cisplatin on the creatinine clearance.
| ANOVA table | F (5, 18) = 63.82 | P < 0.0001 | |||
|---|---|---|---|---|---|
| Test details | Mean 1 | Mean 2 | Mean diff. | SE of diff. | Adjusted Pvalue |
| Control group vs. CIS | 0.4273 | 0.11 | 0.3174 | 0.02423 | <0.0001 |
| Control group vs. OELE-150 | 0.4273 | 0.3987 | 0.02862 | 0.02423 | 0.8400 |
| Control group vs. OELE-300 | 0.4273 | 0.3568 | 0.07052 | 0.02285 | 0.0598 |
| CIS vs. CIS/OELE-150 | 0.11 | 0.1206 | −0.01068 | 0.02759 | 0.9987 |
| CIS vs. CIS/OELE-300 | 0.11 | 0.1785 | −0.06858 | 0.02759 | 0.1801 |
CIS: cisplatin; OELE: Olea europaea leaf extract; SEM: standard error of the mean. Bold values indicates significant differences.
Figure 5.
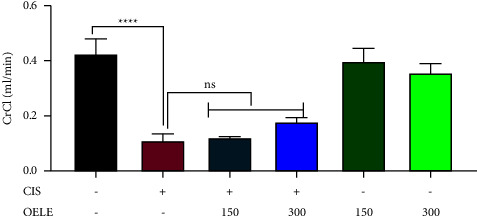
Estimated creatinine clearance on the last day of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.0001; CIS vs. (CIS/OELE-150 and CIS/OELE-300): ++P < 0.01. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
3.3.4. Effect of OELE and/or Cisplatin on Electrolytes in Rats
The data illustrated a significant reduction in the serum potassium levels in the cisplatin-treated group on the 6th day (P < 0.05; Figure 6). Also, a further reduction was detected on the 15th day (P < 0.001; Figure 7) of the experiment compared with the control group. However, the administration of 150 mg/kg or 300 mg/kg of OELE for the cisplatin-treated group resulted in a further reduction in potassium levels on the 6th and 15th days of the experiment, and the lower dose of OELE resulted in a significant reduction in potassium levels on the 15th day compared with the cisplatin group. No significant differences in the potassium levels were observed when the rats received different doses of the extracted leaves of Olea europaea alone compared with the control group on both 6th and 15th days of the experiment.
Figure 6.

(a) Changes in serum potassium level on day 6 of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗P < 0.05; CIS vs. (CIS/OELE-150 and CIS/OELE-300). CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group. (b) Changes in serum potassium level on day 15 of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control: ∗∗∗P < 0.001; CIS vs. (CIS/OELE-150 and CIS/OELE-300): +++P < 0.001. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
Figure 7.
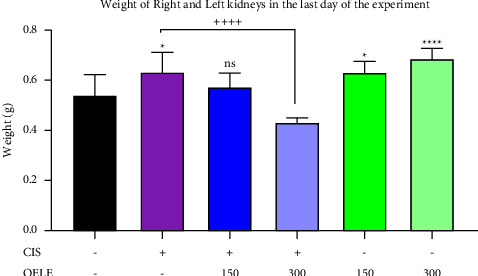
Weight of the right and left kidneys of the rats at the end of the experiment. Notes: each bar is expressed as mean ± SEM. Cisplatin vs. control group: ∗∗∗∗P < 0.001 and ∗P < 0.05; CIS vs. (CIS/OELE-150 and CIS/OELE-300): ++++P < 0.001. CIS: cisplatin; OELE: Olea europaea leaf extract; ns: nonsignificant; n = 5 rats/group.
The effect of the time factor on the potassium levels was studied in the presence of other variables. As shown in Table 4, the findings showed no significant (P > 0.05) differences in the potassium levels between the different groups on the 6th and 15th days of the experiment.
Table 4.
Differences in serum potassium of the rats at different time points.
| Test details (day 6–day 15) | Mean 1 (day 6) | Mean 2 (day 15) | SEM | Adjusted P value |
|---|---|---|---|---|
| Control | 6.1 | 6.56 | 0.1966 | ns |
| CIS | 5.45 | 5.6 | 0.2198 | ns |
| CIS/OELE-150 | 5.225 | 4.85 | 0.2198 | ns |
| CIS/OELE-300 | 5.25 | 5.25 | 0.2198 | ns |
| OELE-150 | 6.35 | 6.375 | 0.2198 | ns |
| OELE-300 | 6.3 | 6.22 | 0.1966 | ns |
CIS: cisplatin; OELE: Olea europaea leaf extract; SEM: standard error of the mean.
3.3.5. Effect of OELE and/or Cisplatin on Rats' Weight
As shown in Table 5, regular increases in the body weight of rats were observed in the control group. There was a significant (P < 0.01) increase in the weight of rats between day 1 and day 15 of the experiment. However, all the cisplatin-treated groups (cisplatin, CIS/OELE-150, and CIS/OELE-300) showed a reduction in weight on day 15 compared with the weight on day 1. This effect was significant (P < 0.01 and P < 0.0001) when cisplatin was concurrently administered with either 300 mg/kg or 150 mg/kg of the extract, respectively.
Table 5.
Change in body weight between 1st and 15th days of the experiment.
| Body weight (day 1–day 15) | Mean 1 (day 6) | Mean 2 (day 15) | % Change | P value |
|---|---|---|---|---|
| Control | 130 | 143.6 | +10.5 | 0.0054 |
| CIS | 156.3 | 150 | +4 | 0.5363 |
| CIS/OELE-150 | 150.3 | 121.3 | −19.3 | <0.0001 |
| CIS/OELE-300 | 154 | 137 | −11 | 0.0071 |
| OELE-150 | 159 | 170.5 | +7.2 | 0.0451 |
| OELE-300 | 169.4 | 169.4 | 0 | >0.9999 |
CIS: cisplatin; OELE: Olea europaea leaf extract; SEM: standard error of the mean. Bold values indicates significant differences.
Oral administration of 300 mg/kg of OELE alone prevented weight gain, while a lower dose (150 mg/kg) of the same extract did not prevent weight gain as appeared on day 15 when compared with the weight of the same rats on day 1.
3.3.6. Effect of Cisplatin and/or OELE on the Weight of Rats' Kidneys on the Last Day of the Experiment
At the end of the treatment period, a significant increase (P < 0.05) in the weight of both kidneys was detected in the cisplatin-treated group when compared with the control group. In addition, normal rats that received different doses of the extract for 15 days showed a significant increase in the weight of the kidneys at a higher dose (300 mg/kg; P < 0.0001) and a lower dose (150 mg/kg; P < 0.05) of the extract compared with the control group. However, administration of a higher dose (300 mg/kg) of the extracted leaves to the cisplatin-treated group resulted in a significant (P < 0.01) reduction in the weight of the kidneys on the last day of the treatment period compared with the cisplatin-treated group. No significant (P > 0.05) difference in the weight of the kidneys was detected when the cisplatin-treated rats received a lower dose (150 mg/kg) of the same extract, as shown in Figure 7.
3.4. The Results of the Histopathologic Examination
3.4.1. The Effect of Cisplatin and/or OELE on the Histology of the Kidney
Histopathological study showed several structural changes and alterations in the hematoxylin and eosin-stained kidney section of cisplatin-treated rats. These alterations were shown as moderate interstitial inflammatory cells mainly lymphocytes with multiple foci of tubulitis along with multiple foci of coagulative necrosis and calcification, as shown in Figures 8(b)–8(f). However, administration of 300 mg/kg of the extracted Olea europaea leaves ameliorated cisplatin-induced interstitial inflammation of the renal tissue to the mild stage, as shown in Figure 8(h). Moreover, the lower dose (150 mg/kg) of the extracted leaves in cisplatin-treated rats revealed amelioration of the interstitial inflammation but induced hemorrhage, as shown in Figure 8(g).
Figure 8.
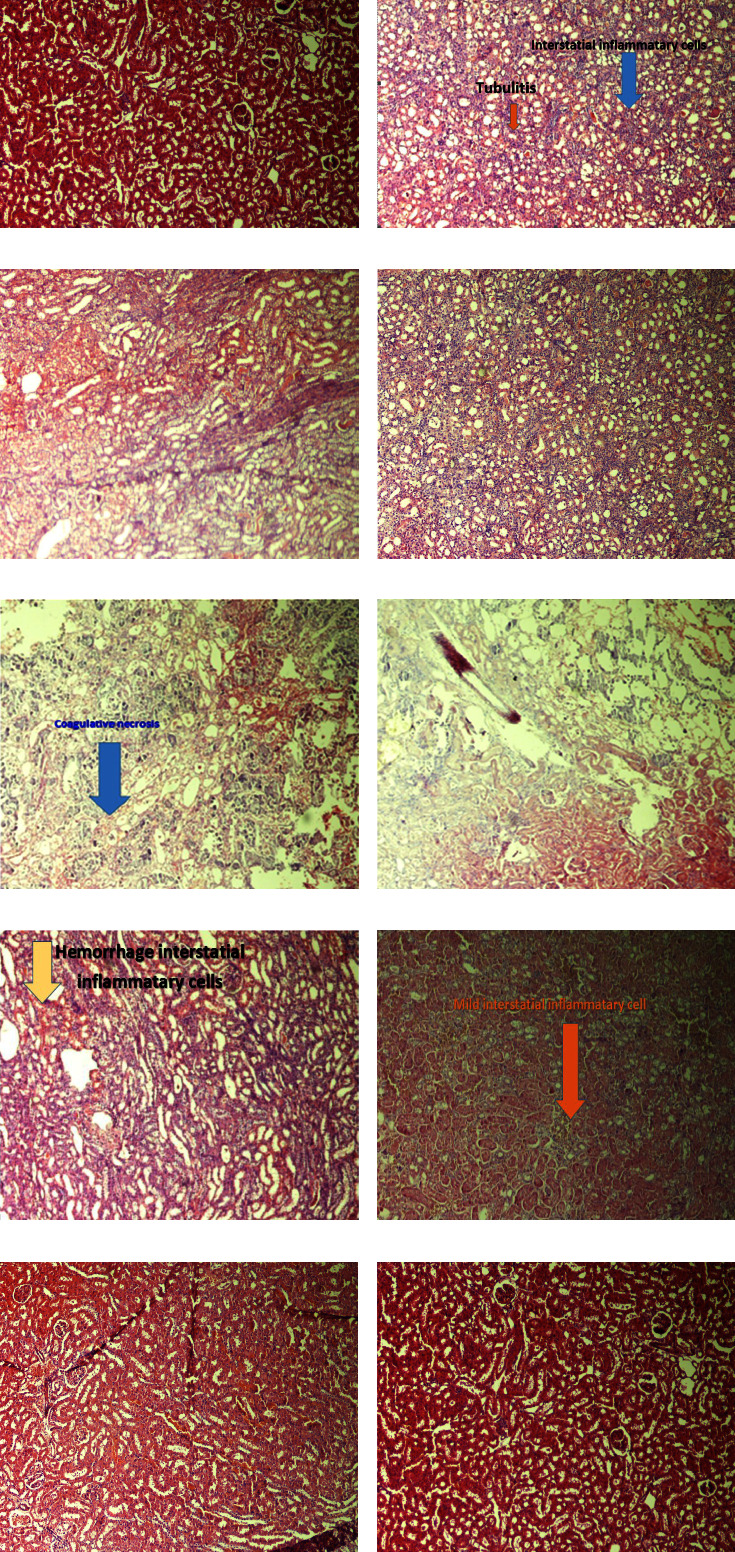
Effect of cisplatin and/or OELE on kidney histology of experimental groups: (a) section from the normal control group show normal renal tissues, (b, c) tissue sections from cisplatin-treated rats show renal tissues with moderate interstitial inflammatory cells mainly lymphocytes with multiple foci of tubulitis, (d–f) tissue sections from cisplatin-treated rats show renal tissues with multiple foci of coagulative necrosis and calcification, (g) tissue section from cisplatin-treated rats which received a dose of 150 mg/kg of the extracted Olea europaea leaves shows renal tissues with mild and hemorrhage interstitial inflammatory cells, (h) tissue section from cisplatin-treated rats which received a dose of 300 mg/kg of the extracted Olea europaea leaves shows renal tissues with mild interstitial inflammatory cells, (i) tissue section from normal rats received a Doe of 300 mg/kg of the extracted Olea europaea leaves alone shows normal renal tissues, and (j) tissue section from normal rats received a dose of 150 mg/kg of the extracted Olea europaea leaves shows normal renal tissues.
Control rats and normal rats received either 300 mg/kg or 150 mg/kg of OELE and showed normal morphology and histological structure of glomeruli and renal tubule, as shown in Figures 8(a), 8(i), and 8(j).
4. Discussion
The renal system plays a crucial role in the regulation of blood volume and the excretion of toxic materials from the body. Kidneys have a high capacity level for drug uptake, making them vulnerable to drug toxicity and injury. An efficient therapeutic agent to attenuate cisplatin-induced nephrotoxicity is not yet available. Therefore, manipulation with an endogenous antioxidant by chemoprotective agents, such as natural products rich in antioxidants or supplementation with traditional antioxidants, has become an alternative modality. A single dose (6 mg/kg) of cisplatin was intraperitoneally injected into rats on the first day of the experiment. Two doses (150 mg/kg and 300 mg/kg) of extracted Olea europaea leaves (OEL) were administered to different groups of rats by oral gavage, starting on the 5th day of cisplatin administration until the end of the experiment. Serum biomarkers about kidney function, as well as electrolyte changes, were measured on days 6 and 15 of cisplatin administration. The outcomes of this work showed a significant increase in the serum creatinine, and blood urea nitrogen, and a significant decrease in the potassium level in cisplatin-treated rats was detected. However, administration of a higher dose of the extracted OEL ameliorated cisplatin-induced AKI as evidenced by a significant reduction in the serum creatinine and blood urea nitrogen. In other words, the reduction of toxic parameters means improvement in kidney function. Moreover, the weights of rats were studied in this experiment. It revealed that cisplatin and a higher dose of the extracted OEL prevented weight gain when administered separately and resulted in further reduction in body weight when administered concurrently. Furthermore, cisplatin and OELE caused a significant increase in rats' kidneys when administered separately compared to the control group. However, the weight of the kidney was significantly reduced when cisplatin and a higher dose of OELE were administered concurrently.
The findings of histopathologic examination also confirmed the presence of renal tissue damage in the cisplatin-treated rats. These damages were observed as interstitial inflammation, lymphocyte infiltration, multiple foci of tubulitis, coagulase necrosis, and calcification of the renal tissue. These consequences of cisplatin administration were ameliorated by the administration of different doses of OELE.
The results of the acute toxicity study showed that the crude extract of OEL did not cause physical and behavioral changes as well as mortality during 72 hours of the follow-up toxicity period after administration of 2 g/kg and 5 g/kg of the extracted leaves to different groups. The higher dose during the toxicity study represents more than 15 times the higher tested dose (300 mg/kg) of the extracted leaves. It has been revealed that a good candidate substance for further studies is one with a three times lethal dose (LD50) higher than the minimum effective dose 38, making the doses of the crude extract in this study suitable for further studies.
In the present study, a significant reduction in the body weight of all cisplatin-treated rats was found on the last day of the experiment. The findings of this study are in agreement with other studies that have shown a total body weight reduction after cisplatin administration [41–44]. It seems that weight reduction that occurs after cisplatin administration is attributed to cisplatin-induced anorexia and gastrointestinal disturbances [45, 46]. Connected with weight reduction in cisplatin-treated rats, this study revealed a significant increase in kidneys' weight in this group compared with the control group. This study is in agreement with another finding which shows a correlation between cisplatin treatment and an increase in kidney weight [43, 47, 48].
In this study, the administration of cisplatin significantly caused clinical and histopathological manifestations of kidney dysfunction, including weight loss, infiltration of leukocytes, and necrosis. Moreover, cisplatin administration induced damage in the renal vasculature, leading to declining creatinine clearance. In this study, AKI was manifested as an increase in the serum creatinine level, blood urea nitrogen, and a decrease in the potassium level. This effect is in agreement with several experimental and clinical studies that showed that a single shot of cisplatin generated an overproduction of free radicals, which caused oxidative stress damage and polyunsaturated fatty acid peroxidation in the kidney tissue [43, 49, 50]. Different mechanisms of cisplatin-induced nephrosis have been illustrated in the literature.
The first underline mechanism of cisplatin-induced nephrosis is mostly attributed to the disturbances in the oxidative stress balance, which is manifested by the increase in the reactive oxygen species (ROS) production, especially superoxide anions and hydroxyl radicals and depleted in the enzymatic and nonenzymatic renal antioxidant defense system [51]. Excessive ROS results in destruction of cellular proteins and lipids as well as DNA [52]. However, administration of a higher dose of OELE caused improvement in the kidney function test which might be attributed to restore antioxidant defense capacity in the kidney. The strong antioxidant activity of OEL has been linked to the presence of polyphenolic contents, including oleuropein, hydroxytyrosol, luteolin, and orthodiphenols; as a result, these compounds could prevent the generation of reactive oxygen species [53–55].
The second underline mechanism involved in cisplatin-induced nephrotoxicity is the inflammation of the renal tissue [56]. It has been revealed that cisplatin causes the promotion of a series of inflammatory cytokines, such as IL-1β and TNF [57]. However, treatment using OEL prevented the production of inflammatory cytokines, including TNF-α and IL-1β in many organs, including testicular tissue and gastric tissue [38, 58, 59]. Similarly, Al-Quraishy et al. reported that the gastroprotective effect of OELE was mediated by maintaining the antioxidant activity and restraining inflammation of gastric mucosa by preventing the mobilization of the activated leukocytes and inhibiting nuclear factor-KB (NF-kB) [38]. In line with previous studies, the findings of this study showed that OELE decreased cisplatin-induced tubulointerstitial lesions, lymphocyte infiltration, and coagulative necrosis of the renal tissue. This anti-inflammation effect of OELE has a dose-dependent effect.
The third underline mechanism of cisplatin-induced acute renal damage is renal epithelial apoptosis [60]. The potential molecular mechanisms of cisplatin-induced apoptosis are related to increase the expressions of Bax and p53 genes and suppressing the expression of Bcl-2. However, Olea europaea compounds have a beneficial effect against cisplatin-induced nephrotoxicity in vitro. For example, in the “GP-293” cell line, incubation of cisplatin-treated cells with oleuropein at a dose of 20 μg/ml could prevent cellular apoptosis by preventing the activation of caspase-3 and reducing Bax: Bcl2 elevation ratio [29]. Another study has shown that treatment with the extracted OEL prevents apoptosis by downregulating Bax and upregulating Bcl-2 in the testicular tissue of cisplatin-treated rats [58].
In this study, serum electrolyte imbalances were detected following cisplatin administration. For example, serum potassium was significantly decreased in cisplatin-treated groups as compared with the control group, and it showed that cisplatin had a cumulative effect on potassium reduction, meaning that the hypokalemic effect of cisplatin on the last day is more prominent compared with the potassium level on the 6th day of the experiment. The higher dose of OELE (300 mg/kg) preserved potassium at a higher level compared with the lower dose of the extracted leaves. This might be related to the restoration effect of the kidney function by the higher dose of OELE, and thus maintained potassium level better than that of the lower dose. The findings of this study are in agreement with other findings that showed a reduction in the potassium concentration in the cisplatin-treated group [42, 47]. On the other hand, a study has shown that hyperkalemia is detected in rats following a single intraperitoneal cisplatin treatment [61].
In this study, a significant improvement in kidney function was observed in the second time frame when compared to the first-time frame. This improvement might result from the ameliorative effect of this extract. Another explanation for this improvement was the washout that happened to the cisplatin concentration from the body after a period of 15 days of administration.
5. Conclusion
In conclusion, this study reveals that a single intraperitoneal dose of cisplatin to Wistar rats induces histopathological alterations of the renal tissue, appearing as moderate interstitial inflammation and coagulative necrosis and calcification along with AKI and electrolyte disturbances as evidenced by elevated serum creatinine and blood urea nitrogen and decrease in the serum potassium level. However, the administration of the extracted Olea europaea leaves markedly ameliorates cisplatin-induced acute renal injury as explored by improving the renal tissue and reducing creatinine and blood urea nitrogen levels in cisplatin-treated rats, and this effect is obvious with a higher dose of the extract and at the second time point, which proved that the beneficial effect of this extract on renal function is both dose-dependent and time-dependent.
5.1. Limitation
This study had several limitations. One limitation was that this study did not assess renal oxidative stress biomarkers, inflammatory markers, and genetic testing. This limitation had consequences on the interpretation of the underline mechanism of the ameliorative effect of the crude extract. In addition, this study evaluated the effect of the crude extract, making the identification of the potential active compound that has the ameliorative effect another limitation. Therefore, further studies are recommended to address this limitation to understand the exact underline mechanism of this effect and to identify the potential active compound that exhibits the ameliorated effect of extracting Olea europaea leaves.
Acknowledgments
The authors thank the University of Science and Technology Hospital and Department of Botany at Sana'a University for aiding in laboratory tests and the experimental plant authentication.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The study was carried out after getting permission from the Ethics Committee (reference no.: EAC/UST231), Faculty of Medicine and Health Science, University of Science and Technology, Sana'a, Yemen. The Committee approved all study protocols according to the International Guide of Laboratory Animal Care and Use.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors made different parts of this paper either in the design of the work, acquisition of data, analysis of the data, or reviewing the article.
References
- 1.Morgan K. P., Buie L. W., Savage S. W. The role of mannitol as a nephroprotectant in patients receiving cisplatin therapy. The Annals of Pharmacotherapy . 2012;46(2):276–281. doi: 10.1345/aph.1q333. [DOI] [PubMed] [Google Scholar]
- 2.Cepeda V., Fuertes M. A., Castilla J., Alonso C., Quevedo C., Pérez J. M. Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents in Medicinal Chemistry . 2007;7(1):3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 3.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney International . 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann J. T., Kollmannsberger C., Kanz L., Bokemeyer C. Platinum organ toxicity and possible prevention in patients with testicular cancer. International Journal of Cancer . 1999;83(6):866–869. doi: 10.1002/(sici)1097-0215(19991210)83:6<866::aid-ijc34>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Hoek J., Bloemendal K. M., Van der Velden L. A., et al. Nephrotoxicity as a dose-limiting factor in a high-dose cisplatin-based chemoradiotherapy regimen for head and neck carcinomas. Cancers . 2016;8(2):p. 21. doi: 10.3390/cancers8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsayed A., Elkomy A., Alkafafy M., et al. Ameliorating effect of lycopene and N-acetylcysteine against cisplatin-induced cardiac injury in rats. Pakistan Veterinary Journal . 2022;42(1):107–111. [Google Scholar]
- 7.Sallam A. O., Rizk H. A., Emam M. A., et al. The ameliorative effects of L-carnitine against cisplatin-induced gonadal toxicity in rats. Pakistan Veterinary Journal . 2021;41(01):147–151. doi: 10.29261/pakvetj/2020.082. [DOI] [Google Scholar]
- 8.Elsayed A., Elkomy A., Elkammar R., et al. Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-93196-7.13979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller R., Tadagavadi R., Ramesh G., Reeves W. Mechanisms of cisplatin nephrotoxicity. Toxins . 2010;2(11):2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer L. Nephrotoxicity as a cause of acute kidney injury in children. Pediatric Nephrology . 2008;23(12):2159–2173. doi: 10.1007/s00467-007-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh G.-S., Kim H.-J., Shen A., et al. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolytes & Blood Pressure . 2014;12(2):55–65. doi: 10.5049/ebp.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özcan M. M., Matthäus B. A review: benefit and bioactive properties of olive (Olea europaea L.) leaves. European Food Research and Technology . 2017;243(1):89–99. doi: 10.1007/s00217-016-2726-9. [DOI] [Google Scholar]
- 13.Japón-Luján R., Luque-Rodríguez J., Luque de Castro M. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. Journal of Chromatography A . 2006;1108(1):76–82. doi: 10.1016/j.chroma.2005.12.106. [DOI] [PubMed] [Google Scholar]
- 14.Goulas V., Exarchou V., Troganis A. N., et al. Phytochemicals in olive‐leaf extracts and their antiproliferative activity against cancer and endothelial cells. Molecular Nutrition & Food Research . 2009;53(5):600–608. doi: 10.1002/mnfr.200800204. [DOI] [PubMed] [Google Scholar]
- 15.Taamalli A., Arráez-Román D., Ruiz-Torres V., et al. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: phenolic composition and cytotoxicity against human breast cancer cells. Food and Chemical Toxicology . 2012;50(6):1817–1825. doi: 10.1016/j.fct.2012.02.090. [DOI] [PubMed] [Google Scholar]
- 16.Fares R., Bazzi S., Baydoun S. E., Abdel-Massih R. M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant Foods for Human Nutrition . 2011;66(1):58–63. doi: 10.1007/s11130-011-0213-9. [DOI] [PubMed] [Google Scholar]
- 17.Samet I., Han J., Jlaiel L., Sayadi S., Isoda H. Olive (Olea europaea) leaf extract induces apoptosis and monocyte/macrophage differentiation in human chronic myelogenous leukemia K562 cells: insight into the underlying mechanism. Oxidative medicine and cellular longevity . 2014;2014:16. doi: 10.1155/2014/927619.927619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somova L., Shode F., Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine . 2004;11(2-3):121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- 19.Scheffler A., Rauwald H., Kampa B., Mann U., Mohr F., Dhein S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. Journal of Ethnopharmacology . 2008;120(2):233–240. doi: 10.1016/j.jep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Lee-Huang S., Zhang L., Lin Huang P., Chang Y.-T., Huang P. L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochemical and Biophysical Research Communications . 2003;307(4):1029–1037. doi: 10.1016/s0006-291x(03)01292-0. [DOI] [PubMed] [Google Scholar]
- 21.Misganaw D., Engidawork E., Nedi T. Evaluation of the anti-malarial activity of crude extract and solvent fractions of the leaves of Olea europaea (Oleaceae) in mice. BMC Complementary and Alternative Medicine . 2019;19(1):p. 171. doi: 10.1186/s12906-019-2567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rietjens S. J., Bast A., Haenen G. R. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. Journal of Agricultural and Food Chemistry . 2007;55(18):7609–7614. doi: 10.1021/jf0706934. [DOI] [PubMed] [Google Scholar]
- 23.Fki I., Sayadi S., Mahmoudi A., Daoued I., Marrekchi R., Ghorbel H. Comparative study on beneficial effects of hydroxytyrosol-and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. BioMed Research International . 2020;2020:15. doi: 10.1155/2020/1315202.1315202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soussi R., Hfaiedh N., Sakly M., Ben Rhouma K. The aqueous extract of Olea europaea leaves protects from haematotoxicity and kidney damage induced by diclofenac in Swiss albino mice. RSC Advances . 2019;9(40):23361. doi: 10.1039/c9ra01670h.23352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavafi M., Ahmadvand H., Toolabi P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran J Kidney Dis . 2012;6(1):25–32. [PubMed] [Google Scholar]
- 26.Abbas M. T., Ali A. J., Abbas I. S., et al. Effect of Olea Europea (Olive oil) on gentamicin induced hepatorenal toxicity in male rats. Karbala Journal of Pharmaceutical Sciences . 2015;(10) [Google Scholar]
- 27.Mostafa-Hedeab G., Sati L. M., Elnaggar H. M., et al. Ameliorating effect of olive leaf extract on cyclosporineinduced nephrotoxicity in rats. Iranian journal of kidney diseases . 2015;9(5):361–368. [PubMed] [Google Scholar]
- 28.Alhaithloul H. A., Alotaibi M. F., Bin-Jumah M., Elgebaly H., Mahmoud A. M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomedicine & Pharmacotherapy . 2019;111:676–685. doi: 10.1016/j.biopha.2018.12.112. [DOI] [PubMed] [Google Scholar]
- 29.Kaeidi A., Rezaei M., Rashidipour M. Oleuropein reduces cisplatin-induced nephrotoxicity in human embryonic renal epithelial “GP-293” cells. Herbal Medicines Journal . 2016;1(1):18–23. [Google Scholar]
- 30.Mohan I. K., Khan M., Shobha J. C., et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemotherapy and Pharmacology . 2006;58(6):802–808. doi: 10.1007/s00280-006-0231-8. [DOI] [PubMed] [Google Scholar]
- 31.Kim J. S., Son J. Y., Kim K. S., et al. Protective effects of Dendropanax morbifera against cisplatin-induced nephrotoxicity without altering chemotherapeutic efficacy. Antioxidants . 2019;8(8):p. 256. doi: 10.3390/antiox8080256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali B. H., Abdelrahman A., Al Suleimani Y., et al. Effect of concomitant treatment of curcumin and melatonin on cisplatin-induced nephrotoxicity in rats. Biomedicine & Pharmacotherapy . 2020;131 doi: 10.1016/j.biopha.2020.110761.110761 [DOI] [PubMed] [Google Scholar]
- 33.Ahmad-Qasem M. H., Cánovas J., Barrajón-Catalán E., Micol V., Cárcel J. A., García-Pérez J. V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innovative Food Science & Emerging Technologies . 2013;17:120–129. doi: 10.1016/j.ifset.2012.11.008. [DOI] [Google Scholar]
- 34.Bahloul N., Boudhrioua N., Kouhila M., Kechaou N. Effect of convective solar drying on colour, total phenols and radical scavenging activity of olive leaves (Olea europaea L.) International Journal of Food Science and Technology . 2009;44(12):2561–2567. doi: 10.1111/j.1365-2621.2009.02084.x. [DOI] [Google Scholar]
- 35.Sofowora A. Medicinal Plants and Traditional Medicine in Africa . Somerset, NJ, USA: Wiley; 1993. [DOI] [PubMed] [Google Scholar]
- 36.Herborne J. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis . Berlin, Germany: Springer; 1973. [Google Scholar]
- 37.Jaykaran B. P., Kantharia N., Yadav P., Panwar A. Acute toxicity study of an aqueous extract of Ficus racemosa Linn. bark in albino mice. The Internet Journal of Toxicology . 2008;6(1) [Google Scholar]
- 38.Al-Quraishy S., Othman M. S., Dkhil M. A., Abdel Moneim A. E. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomedicine & Pharmacotherapy . 2017;91:338–349. doi: 10.1016/j.biopha.2017.04.069. [DOI] [PubMed] [Google Scholar]
- 39.Pellicer-Valero Ó J., Massaro G. A., Casanova A. G., et al. Neural network-based calculator for rat glomerular filtration rate. Biomedicines . 2022;10(3):p. 610. doi: 10.3390/biomedicines10030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobuchi S., Shintani T., Sugiura T., et al. Renoprotective effects of γ-aminobutyric acid on ischemia/reperfusion-induced renal injury in rats. European Journal of Pharmacology . 2009;623(1-3):113–118. doi: 10.1016/j.ejphar.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Krettli A. U., Adebayo J. O., Krettli L. G. Testing of natural products and synthetic molecules aiming at new antimalarials. Current Drug Targets . 2009;10(3):261–270. doi: 10.2174/138945009787581203. [DOI] [PubMed] [Google Scholar]
- 42.Lin M.-T., Ko J.-L., Liu T.-C., Chao P.-T., Ou C.-C. Protective effect of D-methionine on body weight loss, anorexia, and nephrotoxicity in cisplatin-induced chronic toxicity in rats. Integrative Cancer Therapies . 2018;17(3):813–824. doi: 10.1177/1534735417753543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel-Daim M. M., Abushouk A. I., Donia T., et al. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environmental Science and Pollution Research . 2019;26(13):13509. doi: 10.1007/s11356-019-04780-4.13502 [DOI] [PubMed] [Google Scholar]
- 44.Mi X., Hou J., Wang Z. Ye han, shen ren, jun-nan hu, chen chen &wei Li. The protective effects of maltol on cisplatin-induced nephrotoxicity through the AMPK-mediated PI3K/akt and p53 signaling pathways. Scientific Reports . 2018;8(1):1–12. doi: 10.1038/s41598-018-34156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palipoch S., Punsawad C., Chinnapun D., Suwannalert P. Histopathology of small intestine induced by cisplatin in male wistar rats. Walailak Journal of Science and Technology . 2013;10(6):657–663. [Google Scholar]
- 46.Alhadeff A. L., Holland R. A., Zheng H., Rinaman L., Grill H. J., De Jonghe B. C. Excitatory hindbrain–forebrain communication is required for cisplatin-induced anorexia and weight loss. Journal of Neuroscience . 2017;37(2):362–370. doi: 10.1523/jneurosci.2714-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R., Hassan W., Ahmad F. U. D., Jabeen Q., Ahmed H., Iqbal O. Citrus aurantium ameliorates cisplatin-induced nephrotoxicity. BioMed Research International . 2019;2019:9. doi: 10.1155/2019/3960908.3960908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nematbakhsh M., Ashrafi F., Nasri H., et al. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences . 2013;18(5):370–373. [PMC free article] [PubMed] [Google Scholar]
- 49.Shino Y., Itoh Y., Kubota T., Yano T., Sendo T., Oishi R. Role of poly (ADP-ribose) polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radical Biology and Medicine . 2003;35(8):966–977. doi: 10.1016/s0891-5849(03)00470-2. [DOI] [PubMed] [Google Scholar]
- 50.Park H.-M., Cho J.-M., Lee H.-R., Shim G., Kwak M.-K. Renal protection by 3H-1, 2-dithiole-3-thione against cisplatin through the Nrf2-antioxidant pathway. Biochemical Pharmacology . 2008;76(5):597–607. doi: 10.1016/j.bcp.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Fang C., Lou D., Zhou L., et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacologica Sinica . 2021;42(12):1951–1969. doi: 10.1038/s41401-021-00620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian Q., Chen W., Cao Y., et al. Targeting Reactive Oxygen Species in Cancer via Chinese Herbal Medicine. Oxidative Medicine and Cellular Longevity . 2019;2019:23. doi: 10.1155/2019/9240426.9240426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salah M. B., Abdelmelek H., Abderraba M. Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Medicinal Chemistry . 2012;2(5):107–111. [Google Scholar]
- 54.Servili M., Sordini B., Esposto S., et al. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants . 2013;3(1):1–23. doi: 10.3390/antiox3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visioli F., Poli A., Gall C. Antioxidant and other biological activities of phenols from olives and olive oil. Medicinal Research Reviews . 2002;22(1):65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 56.Geyikoglu F., Emir M., Colak S., et al. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. Journal of Food and Drug Analysis . 2017;25(2):447–459. doi: 10.1016/j.jfda.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eid A. H., Abdelkader N. F., Abd El-Raouf O. M., Fawzy H. M., El-Denshary E. E. D. S. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Archives of Pharmacal Research . 2016;39(12):1693–1702. doi: 10.1007/s12272-016-0833-6. [DOI] [PubMed] [Google Scholar]
- 58.Almeer R. S., Abdel Moneim A. E. Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxidative Medicine and Cellular Longevity . 2018;2018:11. doi: 10.1155/2018/8487248.8487248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahmed K. M. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. The Saudi dental journal . 2013;25(4):141–147. doi: 10.1016/j.sdentj.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang L., Yang X., Li X., et al. Butein sensitizes HeLa cells to cisplatin through the AKT and ERK/p38 MAPK pathways by targeting FoxO3a. International Journal of Molecular Medicine . 2015;36(4):957–966. doi: 10.3892/ijmm.2015.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alsuhaibani A. M. Effect of Nigella sativa against cisplatin induced nephrotoxicity in rats. Italian journal of food safety . 2018;7(2):p. 7242. doi: 10.4081/ijfs.2018.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


