Abstract
The 2018 International Federation of Gynecology and Obstetrics (FIGO) revision to the staging criteria for uterine cervical cancer adopted pathological staging for patients who underwent surgery. We investigated the correlation between clinicopathological factors and prognosis in patients with high-risk factors in accordance with the FIGO 2018 staging criteria by analyzing a real-world database of 6,192 patients who underwent radical hysterectomy at 116 institutions belonging to the Japan Gynecologic Oncology Group. A total of 1,392 patients were categorized into the high-risk group. Non-squamous cell carcinoma histology, regional lymph node metastasis, pT2 classification, and ovarian metastasis were identified as independent risk factors for mortality. Based on pathological findings, 313, 1003, and 76 patients were re-classified into FIGO 2018 stages IIB, IIIC1p, and IIIC2p, respectively. Patients with stage IIIC2p disease showed worse prognoses than those with stage IIB or IIIC1p disease. In patients with stage IIIC1p disease, overall survival was significantly better if their tumors were localized in the uterine cervix, except for single lymph node metastasis, with a 5-year overall survival rate of 91.8%. This study clarified the heterogeneity of the high-risk group and provided insights into the feasibility of upfront radical hysterectomy for a limited number of patients harboring high-risk factors.
Subject terms: Cervical cancer, Surgical oncology
Introduction
Uterine cervical cancer is a widely prevalent gynecological malignancy. Although most cervical cancers can be prevented through human papilloma virus (HPV) vaccination and appropriate cancer screening, not all individuals have access to these amenities1–4. According to a global database, in 2020, 604,127 patients were newly diagnosed with cervical cancer and 341,831 patients died from this cancer5. In Japan, 2,887 patients died of the disease in 20206. Thus, optimization of therapeutic strategies for advanced cervical cancer remains an important concern for both patients and clinicians.
In 2018, the International Federation of Gynecology and Obstetrics (FIGO) revised their staging system for uterine cervical cancer. The most important changes in the system are the transition from a clinical to a post-surgical pathological staging system for patients whose primary treatment involved surgery and the integration of lymph node metastasis in the staging criteria reflected in FIGO 2018 stage IIIC7.
Radical hysterectomy and definitive radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) are the fundamental therapeutic options for uterine cervical cancer without distant metastasis8–10. With the development of Okabayashi’s surgery, radical hysterectomy is more frequently performed in Japan than in other countries11–14. The National Comprehensive Cancer Network guidelines state that radical hysterectomy is a primary treatment option for patients categorized into FIGO 2018 clinical stages IB1, IB2, and IIA1, in which the tumor diameter is ≤ 40 mm. While radical hysterectomy is also listed as an option for patients in clinical stages IB3 and IIA2, CCRT is recommended with a stronger evidence level than surgery. Radical hysterectomy is not a recommended option for patients in stages IIB, III, or IVA9. In contrast, the latest version of the Japan Society of Gynecologic Oncology (JSGO) guidelines recommends either radical hysterectomy or RT/CCRT as the primary treatment for patients with FIGO 2018 clinical stage IB or IIA cervical cancer regardless of tumor diameter. Although CCRT is recommended, radical hysterectomy has also been proposed as an option under particular conditions for FIGO 2018 clinical stage IIB cases. Furthermore, upfront radical hysterectomy is proposed as a primary therapeutic option to CCRT for patients in FIGO 2018 stage IIIC if their T classification is T1 or T210.
Patients who undergo surgery as the primary treatment are classified as showing low, intermediate, or high risk for recurrence based on pathological findings. Adjuvant therapy is considered for patients with intermediate- or high-risk factors. Pathologically proven parametrial invasion and regional lymph node metastasis are considered high-risk factors, and adjuvant CCRT is recommended9,10,15,16. In contrast to low- and intermediate-risk patients, the clinicopathological features of postsurgical high-risk patients, who are positive for pathologically proven parametrial invasion and/or regional lymph node metastasis, have not been fully described as these patients are more likely to be treated with definitive RT/CCRT globally9,17. Another concern is that lymph node metastasis detected using clinical imaging modalities such as computed tomography (CT), magnetic resonance imaging, or positron emission tomography-CT had not been officially integrated in the cancer staging system for cervical cancer until the FIGO 2018 staging system was announced7,18; consequently, literature on therapeutic stratification based on lymph node metastasis in the treatment of cervical cancer is lacking. Considering the current popularity of the FIGO 2018 staging schema, we believe that it is especially important to clarify the clinicopathological features of FIGO 2018 stage IIIC population.
The Japanese Gynecologic Oncology Group (JGOG) has established a nationwide database with data from 6,192 patients who underwent radical hysterectomy. We had reported the clinicopathological features of the low- and intermediate-risk groups in the database19,20. This study aimed to elucidate the association between patient survival and clinicopathologic features and to investigate the potential of treatment optimization in the high-risk group by retrospectively analyzing the relevant JGOG data. Because the FIGO adopted postsurgical findings in the staging criteria for the first time in 20187, this study also aimed to assess the feasibility of the current staging system and clarify the clinicopathological characteristics of each FIGO 2018 stage in the high-risk group.
Methods
The primary data included information on 6,192 patients diagnosed with uterine cervical cancer and treated with radical hysterectomy at 116 institutions under the JGOG between January 2004 and December 2008. Patient information was collected and analyzed after obtaining ethical approval from the institutional review board (IRB) at Tottori University (ethical approval number: 1946). The need for informed consent was waived due to the nature of the retrospective surveillance under the same approval by the IRB at Tottori University. All methods were performed in accordance with the relevant guidelines and regulations. The information consisted of age, FIGO 2008 clinical stage, clinical outcomes such as disease-free survival (DFS) and overall survival (OS), pathological tumor-node-metastasis (pTNM), histological diagnosis (squamous cell carcinoma [SCC] or non-SCC), tumor diameter, pelvic lymph node metastasis status, parametrial invasion, stromal invasion to the outer half, and lymphovascular space invasion. Peritoneal cytology results were reported, where applicable. If para-aortic lymphadenectomy was performed, the presence or absence of para-aortic lymph node metastasis was also recorded.
After excluding patients who received neoadjuvant chemotherapy or had distant metastasis, patients were categorized into the high-risk group if they showed positive results for lymph node metastasis and/or parametrial invasion. Patients without information about neoadjuvant chemotherapy, pTNM staging, or clinical outcomes were excluded. We also excluded cases with conflicting data for pTNM staging and parametrial invasion or lymph node metastasis.
Survival curves were determined using the Kaplan–Meier method and statistically compared using the log-rank test. Bonferroni correction was applied for post-hoc multiple comparisons. The chi-squared test was used to compare the distribution of adjuvant therapy. Hazard ratios for the clinical outcome and each parameter were determined using univariate and multivariate Cox regression models. A two-tailed p value that was less than 0.05 was considered significant. Statistical analyses were performed using JMP® Pro 16.0.0. (JMP Statistical Discovery, Cary, NC, USA).
Results
Patient characteristics
Based on patient selection criteria, 1,392 patients were included in the analysis. The patient selection scheme is shown in Supplementary Fig. S1. It must be noted that all patients underwent open laparotomy because minimally invasive approaches such as laparoscopic and robot-assisted surgery were not covered by public health insurance in Japan during the study period. Table 1 summarizes the patient characteristics. The most important point in the revised 2018 FIGO staging criteria for uterine cervical cancer was the adoption of a postsurgical staging system for patients who underwent surgery. Therefore, we reclassified all cases according to the FIGO 2018 staging criteria by referring to their pathological findings. Consequently, 313, 1006, and 76 patients were reclassified into FIGO 2018 stages IIB, IIIC1p, and IIIC2p, respectively (Table 1 and Supplementary Fig. S1).
Table 1.
Patient characteristics.
| Parameter | No. of patients or range (median) | % |
|---|---|---|
| No. of patients | 1392 | |
| Age (year) | 20–83 (49) | |
| Observation period (months) | 0–120 (59) | |
| Death from any cause | 295 | |
| Disease recurrence | 449 | |
| FIGO 2018 stage | ||
| IIB | 313 | 22.5 |
| IIIC1p | 1003 | 72.1 |
| IIIC2p | 76 | 5.5 |
| pT classification | ||
| pTIa | 1 | 0.1 |
| pTIb | 495 | 35.6 |
| pT2a | 159 | 11.4 |
| pT2b | 737 | 52.9 |
| Histology | ||
| SCC | 954 | 68.5 |
| nSCC | 438 | 31.5 |
| PLN metastasis | ||
| Negative | 315 | 22.6 |
| Positive | 1077 | 77.4 |
| PALN metastasis | ||
| Negative | 259 | 18.6 |
| Positive | 76 | 5.5 |
| Lymphadenectomy not performed | 1057 | 75.9 |
| Tumor diameter | ||
| ≤ 40 mm | 867 | 62.3 |
| > 40 mm | 513 | 36.9 |
| Unknown | 12 | 0.9 |
| LVSI | ||
| Negative | 194 | 13.9 |
| Positive | 1143 | 82.1 |
| Unknown | 55 | 4.0 |
| Stromal invasion | ||
| ≤ 1/2 | 238 | 17.1 |
| > 1/2 | 1020 | 73.3 |
| Unknown | 134 | 9.6 |
| Ovarian metastasis | ||
| Negative | 1317 | 94.6 |
| Positive | 32 | 2.3 |
| Unknown/preserved` | 43 | 3.1 |
| Peritoneal cytology | ||
| Negative | 411 | 29.5 |
| Positive | 51 | 3.7 |
| Unknown/not examined | 930 | 66.8 |
| Uterine corpus invasion | ||
| Negative | 1053 | 75.6 |
| Positive | 321 | 23.1 |
| Unknown | 18 | 1.3 |
| Adjuvant therapy | ||
| CCRT | 573 | 41.2 |
| CT | 350 | 25.1 |
| RT | 351 | 25.2 |
| None | 64 | 4.6 |
| Others/unknown | 54 | 3.9 |
FIGO The International Federation of Gynecology and Obstetrics, HR hazard ratio, SCC squamous cell carcinoma, nSCC non-squamous cell carcinoma, PLN pelvic lymph node, LVSI lymphovascular space invasion, CCRT concurrent chemoradiotherapy, CT chemotherapy, RT radiotherapy.
Most patients received adjuvant CCRT, RT, or chemotherapy. More specifically, 87.5%, 93.2%, and 92.1% of the patients who were classified into FIGO 2018 stage IIB, IIIC1p, and IIIC2p, respectively, received either adjuvant CCRT, RT, or chemotherapy. Because adjuvant chemotherapy was administered more frequently to patients with non-SCC histology for intermediate-risk factors20, we investigated whether histological differences were statistically associated with the type of adjuvant therapy in the high-risk group. As shown in Supplementary Table S1, chemotherapy was administered to 19.6% (175/891) and 45.7% (175/383) of the patients with SCC and non-SCC histology, respectively. The chi-square test indicated significant differences in the selection of adjuvant chemotherapy between the SCC and non-SCC histology groups (p < 0.001). We also assessed the association between types of adjuvant therapy and peritoneal cytology, as positive peritoneal cytology indicates the possibility of microscopic metastasis within peritoneal cavity, which may motivate clinicians to select adjuvant chemotherapy. As shown in Supplementary Table S2, no significant difference was observed in the results of the chi-square test (p = 0.468).
Comparison of survival according to FIGO 2018 stage and patterns of positive risk factors
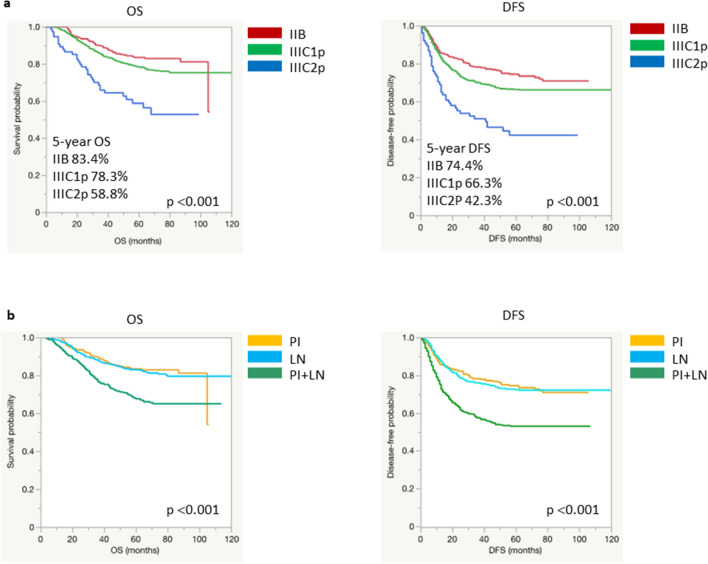
To assess the feasibility of the FIGO 2018 staging criteria, we determined survival curves according to FIGO 2018 stage (Fig. 1a). While patients in stages IIB and IIIC1p showed similar survival trends, those in stage IIIC2p showed poor OS and DFS. Post-hoc analysis revealed a significant difference in both OS and DFS between stages IIIC2p and IIB or IIIC1 (Supplementary Fig. S2). In terms of risk factors, 654 patients were diagnosed with only pelvic and/or para-aortic lymph node metastasis, 313 patients were diagnosed with only parametrial invasion, and 425 patients were diagnosed with both high-risk factors. To understand the association between risk factors and prognosis, we compared survival curves on the basis of the pattern of positive risk factors. Although patients showing either high-risk factor showed similar OS and progression-free survival (PFS) trends, the OS and PFS of those exhibiting both risk factors were significantly worse, as shown in Fig. 1b.
Figure 1.
Comparison of survival by FIGO 2018 stage and by the pattern of positive risk factors. (a) Kaplan–Meier curves for each FIGO 2018 stage. (b) Kaplan–Meier curves according to the pattern of positive risk factors. FIGO The International Federation of Gynecology and Obstetrics, OS overall survival, DFS disease-free survival, PI parametrial invasion, LN lymph node metastasis.
Association between overall survival and pathological factors
To further assess the influence of pathological factors on patient mortality, we performed univariate and multivariate Cox regression analyses. The results of univariate analysis indicated that all the pathological factors were associated with OS (Table 2). Since peritoneal cytological evaluation was not performed in over half of the 1,392 patients included in this study, peritoneal cytology data were excluded from subsequent multivariate analyses. Non-SCC, pT classification, pelvic or para-aortic lymph node metastasis, and ovarian metastasis were identified as independent risk factors for OS (Table 2). Importantly, tumor diameter, depth of stromal invasion, and lymphovascular space invasion (LVSI) were not significantly associated with the OS of high-risk patients. LVSI was identified as an independent risk factor for disease recurrence, in addition to non-SCC, pT classification, pelvic or para-aortic lymph node metastasis, and ovarian metastasis. Patients who did not receive adjuvant therapy were also at risk of recurrence (Supplementary Table S3).
Table 2.
Univariate and multivariate analysis of overall survival for the 1,392 patients.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (continuous) | 0.992 | 0.983–1.002 | 0.988 | 0.977–1.000 | ||
| Histology | ||||||
| SCC | 1 (reference) | 1 (reference) | ||||
| nSCC | 2.060 | 1.638–2.591 | < 0.001 | 2.381 | 1.814–3.127 | < 0.001 |
| pT classification | ||||||
| pT1a | NA | NA | NA | NA | NA | NA |
| pT1b | 1 (reference) | 1 (reference) | ||||
| pT2a | 2.085 | 1.421–3.061 | < 0.001 | 2.181 | 1.401–3.396 | < 0.001 |
| pT2b | 1.881 | 1.426–2.480 | < 0.001 | 2.332 | 1.621–3.356 | < 0.001 |
| pT2a | 1 (reference) | 1 (reference) | ||||
| pT2b | 0.902 | 0.645–1.261 | 0.546 | 1.069 | 0.712–1.606 | 0.746 |
| PLN metastasis | ||||||
| Negative | 1 (reference) | 1 (reference) | ||||
| Positive | 1.483 | 1.097–2.005 | 0.011 | 1.997 | 1.366–2.920 | < 0.001 |
| PALN metastasis | ||||||
| Negative/not performed | 1 (reference) | 1 (reference) | ||||
| Positive | 2.474 | 1.705–3.590 | < 0.001 | 1.668 | 1.017–2.737 | 0.043 |
| Tumor diameter | ||||||
| ≤ 40 mm | 1 (reference) | 1 (reference) | ||||
| > 40 mm | 1.629 | 1.293–2.052 | < 0.001 | 1.283 | 0.978–1.683 | 0.072 |
| LVSI | ||||||
| Negative | 1 (reference) | 1 (reference) | ||||
| Positive | 1.977 | 1.290–3.029 | 0.002 | 1.336 | 0.827–2.157 | 0.237 |
| Stromal invasion | ||||||
| ≤ 1/2 | 1 (reference) | 1 (reference) | ||||
| > 1/2 | 2.122 | 1.425–3.161 | < 0.001 | 1.367 | 0.856–2.183 | 0.190 |
| Ovarian metastasis | ||||||
| Negative/preserved | 1 (reference) | 1 (reference) | ||||
| Positive | 3.682 | 2.253–6.019 | < 0.001 | 2.254 | 1.232–4.122 | < 0.001 |
| Corpus invasion | ||||||
| Negative | 1 (reference) | 1 (reference) | ||||
| Positive | 1.709 | 1.337–2.185 | < 0.001 | 1.266 | 0.935–1.715 | 0.127 |
| Adjuvant therapy | ||||||
| CCRT | 1 (reference) | 1 (reference) | ||||
| CT | 1.071 | 0.804–1.427 | 0.637 | 0.837 | 0.596–1.176 | 0.306 |
| RT | 1.024 | 0.766–1.368 | 0.875 | 1.119 | 0.806–1.555 | 0.501 |
| None | 0.884 | 0.427–1.685 | 0.707 | 1.120 | 0.504–2.487 | 0.781 |
| CT | 1 (reference) | 1 (reference) | ||||
| RT | 0.955 | 0.694–1.315 | 0.779 | 1.337 | 0.917–1.948 | 0.131 |
| None | 0.825 | 0.427–1.594 | 0.566 | 1.337 | 0.591–3.025 | 0.485 |
| RT | 1 (reference) | 1 (reference) | ||||
| None | 0.863 | 0.446–1.671 | 0.863 | 1.000 | 0.443–2.260 | 0.999 |
| Peritoneal cytology | ||||||
| Negative | 1 (reference) | |||||
| Positive | 2.126 | 1.327–3.405 | 0.002 | |||
HR Hazard ratio, CI confidence interval, SCC squamous cell carcinoma, nSCC non-squamous cell carcinoma, PLN pelvic lymph node, PALN para-aortic lymph node, LVSI lymphovascular space invasion, CCRT concurrent chemoradiotherapy, CT chemotherapy, RT radiotherapy.
Assessments of clinicopathological findings and patient survival by FIGO 2018 stage
Based on the FIGO 2018 staging criteria, the most high-risk patients are classified into stage IIB, IIIC1p, or IIIC2p7. To further elucidate the characteristics of each stage, we evaluated the association of clinicopathological factors and OS in stages IIB, IIIC1p, and IIIC2p independently.
The results for each substage are summarized in Tables 3, 4 and 5. In FIGO 2018 stage IIB, non-SCC histology and tumor diameter > 40 mm were identified as independent risk factors for survival from the results of the multivariate analysis (Table 3). For stage IIIC1p, we first focused on the number of lymph node metastases. Although the number of lymph node metastases and OS did not show a clear correlation (Supplementary Fig. S3), patients with multiple pelvic lymph node metastases showed significantly worse OS than those with a single lymph node metastasis (Supplementary Fig. S3). Multivariate analysis further validated the independent influence of multiple lymph node metastases on patient survival, in addition to non-SCC histology, pT2 classification, corpus invasion, and ovarian metastasis (Table 4). Because most of the risk factors identified via multivariate analysis were related to extra-cervical tumor extension, we further compared the survival between patients without extra-cervical lesions except for single lymph node involvement and the rest of the patients in stage IIIC1p. Patients whose tumors were limited to the uterine cervix and a single lymph node showed significantly better OS, with a 5-year overall survival rate of 91.8% (Supplementary Fig. S3).
Table 3.
Univariate and multivariate analysis of overall survival in the patients classified into FIGO 2018 stage IIB.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| (No. of patients) | HR | 95% CI | p value | HR | 95% CI | p value |
| Age (continuous) | 0.990 | 0.967–1.013 | 0.992 | 0.967–1.017 | ||
| Histology | ||||||
| SCC (211) | 1 (reference) | 1 (reference) | ||||
| nSCC (102) | 2.399 | 1.385–4.156 | 0.002 | 3.402 | 1.743–6.637 | < 0.001 |
| Tumor diameter | ||||||
| ≤ 40 mm (194) | 1 (reference) | 1 (reference) | ||||
| > 40 mm (116) | 2.548 | 1.452–4.471 | 0.001 | 2.988 | 1.599–5.584 | < 0.001 |
| LVSI | ||||||
| Negative (60) | 1 (reference) | 1 (reference) | ||||
| Positive (241) | 1.028 | 0.497–2.127 | 0.940 | 1.017 | 0.453–2.283 | 0.967 |
| Ovarian metastasis | ||||||
| Negative/preserved (307) | 1 (reference) | 1 (reference) | ||||
| Positive (3) | 6.425 | 1.556–26.522 | 0.010 | 2.935 | 0.621–13.868 | 0.174 |
| Corpus invasion | ||||||
| Negative (211) | 1 (reference) | 1 (reference) | ||||
| Positive (102) | 1.051 | 0.585–1.887 | 0.867 | 0.701 | 0.360–1.367 | 0.297 |
| Adjuvant therapy | ||||||
| CCRT (118) | 1 (reference) | 1 (reference) | ||||
| CT (57) | 0.906 | 0.394–2.084 | 0.816 | 0.680 | 0.285–1.623 | 0.385 |
| RT (99) | 1.437 | 0.765–2.697 | 0.259 | 1.490 | 0.727–3.055 | 0.276 |
| None (31) | 0.431 | 0.100–1.858 | 0.259 | 0.382 | 0.084–1.730 | 0.212 |
| CT | 1 (reference) | 1 (reference) | ||||
| RT | 1.585 | 0.702–3.581 | 0.267 | 2.191 | 0.889–5.399 | 0.088 |
| None | 0.476 | 0.101–2.240 | 0.347 | 0.561 | 0.116–2.702 | 0.471 |
| RT | 1 (reference) | 1 (reference) | ||||
| None | 0.300 | 0.070–1.280 | 0.104 | 0.256 | 0.056–1.181 | 0.081 |
FIGO The International Federation of Gynecology and Obstetrics, HR hazard ratio, CI confidence interval, SCC squamous cell carcinoma, nSCC non-squamous cell carcinoma, LVSI lymphovascular space invasion, CCRT concurrent chemoradiotherapy, CT chemotherapy, RT radiotherapy.
Table 4.
Univariate and multivariate analysis of overall survival in the patients classified into FIGO 2018 stage IIIC1p.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| (No. of patients) | HR | 95% CI | p value | HR | 95% CI | p value |
| Age (continuous) | 0.995 | 0.983–1.006 | 0.989 | 0.976–1.003 | ||
| Histology | ||||||
| SCC (697) | 1 (reference) | 1 (reference) | ||||
| nSCC (306) | 2.019 | 1.540–2.645 | < 0.001 | 2.157 | 1.560–2.983 | < 0.001 |
| pT classificaion | ||||||
| T1a (1) | NA | NA | NA | NA | NA | NA |
| T1b (474) | 1 (reference) | 1 (reference) | ||||
| T2a (149) | 2.165 | 1.452–3.228 | < 0.001 | 2.158 | 1.355–3.436 | 0.001 |
| T2b (379) | 2.359 | 1.731–3.214 | < 0.001 | 2.367 | 1.594–3.515 | < 0.001 |
| T2a | 1 (reference) | 1 (reference) | ||||
| T2b | 1.089 | 0.756–1.569 | 0.645 | 1.097 | 0.715–1.684 | 0.672 |
| Tumor diameter | ||||||
| ≤ 40 mm (629) | 1 (reference) | 1 (reference) | ||||
| > 40 mm (366) | 1.384 | 1.053–1.819 | 0.020 | 1.034 | 0.750–1.425 | 0.840 |
| LVSI | ||||||
| Negative (130) | 1 (reference) | 1 (reference) | ||||
| Positive (834) | 2.222 | 1.291–3.824 | 0.004 | 1.411 | 0.767–2.596 | 0.268 |
| Stromal invasion | ||||||
| ≤ 1/2 (200) | 1 (reference) | 1 (reference) | ||||
| > 1/2 (701) | 2.065 | 1.346–3.169 | < 0.001 | 1.134 | 0.687–1.874 | 0.622 |
| No. of positive PLN metastasis | ||||||
| Single (424) | 1 (reference) | 1 (reference) | ||||
| Multiple (574) | 2.626 | 1.912–3.607 | < 0.001 | 2.089 | 1.458–2.994 | < 0.001 |
| Ovarian metastasis | ||||||
| Negative/preserved (945) | 1 (reference) | 1 (reference) | ||||
| Positive (18) | 3.663 | 1.937–6.928 | < 0.001 | 2.377 | 1.193–4.735 | 0.014 |
| Corpus invasion | ||||||
| Negative (793) | 1 (reference) | 1 (reference) | ||||
| Positive (192) | 2.022 | 1.506–2.715 | < 0.001 | 1.440 | 1.002–2.069 | 0.049 |
| Adjuvant therapy | ||||||
| CCRT (432) | 1 (reference) | 1 (reference) | ||||
| CT (261) | 1.075 | 0.777–1.487 | 0.664 | 0.940 | 0.643–1.375 | 0.751 |
| RT (242) | 0.831 | 0.580–1.189 | 0.311 | 0.969 | 0.651–1.443 | 0.879 |
| None (31) | 1.407 | 0.652–3.033 | 0.384 | 1.855 | 0.661–5.296 | 0.241 |
| CT | 1 (reference) | 1 (reference) | ||||
| RT | 0.773 | 0.524–1.141 | 0.195 | 1.031 | 0.659–1.612 | 0.894 |
| None | 1.309 | 0.598–2.864 | 0.500 | 1.973 | 0.692–5.619 | 0.203 |
| RT | 1 (reference) | 1 (reference) | ||||
| None | 1.693 | 0.762–3.762 | 0.196 | 1.913 | 0.663–5.518 | 0.230 |
| Peritoneal cytology | ||||||
| Negative (277) | 1 (reference) | |||||
| Positive (41) | 1.914 | 1.092–3.355 | 0.023 | |||
FIGO The International Federation of Gynecology and Obstetrics, HR hazard ratio, CI confidence interval, SCC squamous cell carcinoma, nSCC non-squamous cell carcinoma, PLN pelvic lymph node, LVSI lymphovascular space invasion, CCRT concurrent chemoradiotherapy, CT chemotherapy, RT radiotherapy.
Table 5.
Univariate and multivariate analysis of overall survival in the patients classified into FIGO 2018 stage IIIC2p.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| (No. of patients) | HR | 95% CI | p value | HR | 95% CI | p value |
| Age (continuous) | 0.986 | 0.957–1.017 | 0.959 | 0.909–1.009 | ||
| Histology | ||||||
| SCC (46) | 1 (reference) | 1 (reference) | ||||
| nSCC (30) | 1.017 | 0.790–3.269 | 0.191 | 1.303 | 0.412–4.125 | 0.652 |
| pT classificaion | ||||||
| T1b (21) | 1 (reference) | 1 (reference) | ||||
| T2a (10) | 1.261 | 0.315–5.048 | 0.743 | 0.872 | 0.135–5.615 | 0.885 |
| T2b (45) | 2.344 | 0.946–5.806 | 0.066 | 1.497 | 0.345–6.498 | 0.590 |
| T2a | 1 (reference) | 1 (reference) | ||||
| T2b | 1.859 | 0.556–6.216 | 0.314 | 1.717 | 0.342–8.611 | 0.511 |
| Tumor diameter | ||||||
| ≤ 40 mm (44) | 1 (reference) | 1 (reference) | ||||
| > 40 mm (31) | 2.178 | 1.057–4.488 | 0.035 | 1.881 | 0.541–6.535 | 0.320 |
| Stromal invasion | ||||||
| ≤ 1/2 (7) | 1 (reference) | 1 (reference) | ||||
| > 1/2 (57) | 2.699 | 0.364–19.996 | 0.331 | 0.841 | 0.095–7.479 | 0.877 |
| Ovarian metastasis | ||||||
| Negative/preserved (65) | 1 (reference) | 1 (reference) | ||||
| Positive (11) | 1.447 | 0.554–3.777 | 0.451 | 0.611 | 0.066–5.678 | 0.665 |
| Corpus invasion | ||||||
| Negative (49) | 1 (reference) | 1 (reference) | ||||
| Positive (27) | 1.631 | 0.803–3.314 | 0.176 | 1.394 | 0.441–4.400 | 0.572 |
| Adjuvant therapy | ||||||
| CCRT (26) | 1 (reference) | 1 (reference) | ||||
| CT (34) | 0.875 | 0.337–2.270 | 0.783 | 0.908 | 0.220–3.738 | 0.893 |
| RT (10) | 5.707 | 2.112–15.423 | < 0.001 | 5.694 | 1.346–24.084 | 0.018 |
| None (2) | 10.324 | 1.179–90.391 | 0.035 | 31.191 | 1.509–644.748 | 0.026 |
| CT | 1 (reference) | 1 (reference) | ||||
| RT | 6.526 | 2.506–16.999 | < 0.001 | 6.274 | 1.751–22.485 | 0.018 |
| None | 11.805 | 1.373–101.479 | 0.025 | 34.369 | 2.073–569.752 | 0.014 |
| RT | 1 (reference) | 1 (reference) | ||||
| None | 1.809 | 0.218–14.981 | 0.583 | 5.478 | 0.299–100.284 | 0.252 |
| Peritoneal cytology | ||||||
| Negative (30) | 1 (reference) | |||||
| Positive (9) | 1.452 | 0.555–3.794 | 0.447 | |||
| LVSI | ||||||
| Negative (4) | 1 (reference) | |||||
| Positive (68) | NA | NA | NA | |||
| PLN metastasis | ||||||
| Negative (2) | 1 (reference) | |||||
| Positive (74) | NA | NA | NA | |||
FIGO The International Federation of Gynecology and Obstetrics, HR hazard ratio, CI confidence interval, SCC squamous cell carcinoma, nSCC non-squamous cell carcinoma, PLN pelvic lymph node, LVSI lymphovascular space invasion, CCRT concurrent chemoradiotherapy, CT chemotherapy, RT radiotherapy.
Among the 76 FIGO 2018 IIIC2p cases, neither pathological factor was significantly associated with patient survival in a multivariate analysis. However, patients treated with adjuvant RT showed significantly worse OS than those treated with adjuvant CCRT or chemotherapy, even though the number of patients who received each adjuvant therapy was relatively small (Table 5).
Discussion
In this study, we investigated the association between patient prognosis and clinicopathological features in surgically treated patients with uterine cervical cancer harboring high-risk factors. Overall, the results indicated that patient prognosis was heterogeneous, even in the high-risk group.
As shown in Table 2, non-SCC histology, pT2a/pT2b classification, pelvic or para-aortic lymph node metastasis, and ovarian metastasis were identified as independent risk factors for OS in the high-risk group. In contrast, LVSI, deep stromal invasion, and tumor diameter, which have been categorized as intermediate-risk factors21,22, did not influence OS. These results validate the significance of parametrial invasion and lymph node metastasis in patient prognosis. Simultaneously, non-SCC histology and ovarian metastasis were identified as potential risk factors among the high-risk groups. Because pT2a represents vaginal invasion, pathological vaginal invasion may be another risk factor. Importantly, these factors were also identified as risk factors for mortality in the low- and intermediate-risk groups19,20. Our findings suggest that, in addition to conventional risk factors, other pathological parameters that are not currently deemed indisputable risk factors should be considered when planning therapeutic strategies in patients with surgically treated cervical cancer.
Adherence to the guidelines regarding adjuvant therapy for patients primarily treated with radical hysterectomy is an unresolved issue in Japan. Although the current Japanese guidelines recommend adjuvant CCRT for treating high-risk patients10, adjuvant chemotherapy was adopted in approximately a quarter of the patients, especially in the non-SCC population. One reason for this may be to avoid radiation-related adverse events23,24. Indeed, Ikeda et al. reported that an increasing proportion of patients were treated with adjuvant chemotherapy in Japan25. Moreover, radiation has been considered to be less effective for adenocarcinoma in several studies, which may lead physicians to prescribe adjuvant chemotherapy for non-SCC cervical cancer12,26–28. Nevertheless, evidence for the feasibility of adjuvant chemotherapy in high-risk groups is currently inadequate. Unfortunately, we estimate that our database had substantial physician and institutional biases, making it difficult to appropriately assess the influence of adjuvant therapy. In the light of the current situation, the JGOG is conducting a prospective study to compare adjuvant CCRT and chemotherapy29. The results of this study will provide a certain indication regarding this concern.
Another concern is related to surgical radicality and the site of recurrence. A study by Matsuo et al. reported a lower risk of distant metastasis and a higher risk of local recurrence among patients with node-positive FIGO2008 stage IB-IIB cervical cancer who received adjuvant chemotherapy, which indicates that a satisfactory local control rate should be achieved through radical hysterectomy if adjuvant chemotherapy is selected30.
In classifications of cervical cancer based on the FIGO 2018 staging criteria, stages IIB, IIIC1p, and IIIC2p corresponded to the most high-risk cases. We believe that the inclusion of cases in which para-aortic lymph nodes were pathologically examined is an advantage of the current investigation of the feasibility of the FIGO 2018 staging criteria. Our analysis supported the feasibility of FIGO 2018 IIB, IIIC1p, and IIIC2p classifications from the perspective of prognosis. Patients categorized into stage IIIC2p showed significantly worse prognosis than those classified into stages IIB and IIIC1p. Interestingly, adjuvant RT was indicated as a risk factor for mortality compared with adjuvant CCRT and CT. Despite the previously mentioned limitations in the assessment of adjuvant therapy, our findings highlight the necessity of intensive adjuvant therapy for surgically treated patients with FIGO 2018 stage IIIC2p disease. Moreover, the prognosis observed in this study raised a concern regarding the feasibility of surgery-based therapeutic strategies in patients presenting with clear para-aortic lymph node metastasis as indicated by clinical imaging.
Patients with FIGO2018 IIIC1p cervical cancer showed heterogeneous prognoses based on the number of lymph node metastases and status of tumor extension. While definitive CCRT is generally considered if lymph node metastasis is suspected on preoperative clinical imaging, our results indicated that a patient was likely to have a favorable prognosis with the upfront surgery approach if the tumor was estimated to be limited to the uterine cervix except for single lymph node involvement. This finding is concordant with previous retrospective studies that reported the number of lymph node metastases as a risk factor for patients with stage IIIC1p cervical cancer31,32. On the other hand, the treatment strategies for patients with suspected multiple lymph node metastasis or tumor extension beyond uterine cervix should be optimized for each patient based on performance status, preexisting comorbidities, expected adverse effects, cost-effectiveness, and so on, along with careful preoperative physical evaluation and multiple clinical imaging.
One limitation of this study is that the current database consisted of information collected only from surgically treated patients. Therefore, direct comparison of treatment outcomes between primary radical hysterectomy with subsequent adjuvant therapy and definitive CCRT was not possible. Not limited to patients treated with upfront surgery, the prognosis of patients with FIGO2018 IIIC1 disease was reported to be heterogeneous depending on local tumor factors in a retrospective cohort study33. On the other hand, a nationwide clinicopathological database containing information on patients with uterine cervical cancer harboring high-risk factors is of significant value because these patients are more likely to be treated with RT/CCRT globally. Our findings and the existing literature highlight the need for larger-scale and more inclusive surveillance to optimize the management of FIGO 2018 stage IIIC1 cases.
In conclusion, this study clarified the heterogeneous outcomes associated with the clinicopathological features of patients presenting high-risk factors who underwent radical hysterectomy. The results reinforce the need for optimizing therapeutic strategies for this population, which should be further investigated in future studies.
Supplementary Information
Acknowledgements
The authors would like to thank all participants in this project. The authors would also like to thank Editage for the English proofreading.
Author contributions
This study was conceptualized by M.S., K.M., and M.M. and designed by S.S., M.S., D.A., and M.M. Data acquisition was performed by M.S., Y.T., T.N., T.S., D.A., and M.M. Data was curated and statistically analyzed by S.S., M.S., K.T., and Z.W. The initial manuscript was written by S.S., M.S., and Z.W. The tables, figure, and supplementary information were prepared by S.S. and M.S. The manuscript was edited by S.S., M.S., and K.M. All the authors reviewed the results and the manuscript.
Funding
This study was financially supported by the JSPS KAKENHI with the following Grant number: JP17K11265 (to M.S.).
Data availability
The dataset analyzed in this study is available from the corresponding author on reasonable request.
Competing interests
D.A. received honoraria from Merck Sharp and Dohme and Chugai Pharmaceutical. All other authors declare no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-39014-8.
References
- 1.Prudden HJ, et al. Understanding the public health value and defining preferred product characteristics for therapeutic human papillomavirus (HPV) vaccines: World health organization consultations, October 2021–March 2022. Vaccine. 2022;40(41):5843–5855. doi: 10.1016/j.vaccine.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L, et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health. 2022;10(8):e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni L, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 4.Machalek D, et al. Impact of one and two human papillomavirus (HPV) vaccine doses on community-level HPV prevalence in South African adolescent girls: Study protocol and rationale for a pragmatic before-after design. BMJ Open. 2022;12(2):e059968. doi: 10.1136/bmjopen-2021-059968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Information Service, National Cancer Center, Japan. Cancer Statistics (Vital Statistics of Japan, Ministry of Health, Labour and Welfare).
- 7.Bhatla N, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019;145(1):129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 8.Landoni F, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Cervical Cancer (Version 1.2022).https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (2022).
- 10.Japan Society of Gynecologic Oncology. Guidelines for treatment of uterine cervical cancer; Japan Society of Gynecologic Oncology (JSGO) 2022 edition. (In Japanese)
- 11.Shimada M, et al. Rethinking the significance of surgery for uterine cervical cancer. J. Obstet. Gynaecol. Res. 2022;48(3):576–586. doi: 10.1111/jog.15112. [DOI] [PubMed] [Google Scholar]
- 12.Mikami M, et al. Surgical principles for managing stage IB2, IIA2, and IIB uterine cervical cancer (Bulky Tumors) in Japan: A survey of the Japanese gynecologic oncology group. Int. J. Gynecol. Cancer. 2014;24(7):1333–1340. doi: 10.1097/IGC.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 13.Shigeta S, et al. Epidemiological guideline influence on the therapeutic trend and patient outcome of uterine cervical cancer in Japan: Japan society of gynecologic oncology guideline evaluation committee project. Gynecol. Oncol. 2020;159(1):248–255. doi: 10.1016/j.ygyno.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Sakuragi N, et al. Oncological outcomes after Okabayashi-Kobayashi radical hysterectomy for early and locally advanced cervical cancer. JAMA Netw. Open. 2020;3(5):e204307. doi: 10.1001/jamanetworkopen.2020.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters WA, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J. Clin. Oncol. 2000;18(8):1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 16.Falcetta FS, et al. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst. Rev. 2016;11(11):CD005342. doi: 10.1002/14651858.CD005342.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marth C, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl_4):iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 18.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya Y, et al. Prognostic factors of 2018 FIGO stage IB-IIA cervical cancer with absence of high/ intermediate surgical-pathological risk factors. Jpn. J. Clin. Oncol. 2022;52(11):1289–1296. doi: 10.1093/jjco/hyac125. [DOI] [PubMed] [Google Scholar]
- 20.Shigeta S, et al. Risk assessment in the patients with uterine cervical cancer harboring intermediate risk factors after radical hysterectomy: A multicenter, retrospective analysis by the Japanese gynecologic oncology group. Int. J. Clin. Oncol. 2022;27(9):1507–1515. doi: 10.1007/s10147-022-02198-6. [DOI] [PubMed] [Google Scholar]
- 21.Sedlis A, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A gynecologic oncology group study. Gynecol. Oncol. 1999;73(2):177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 22.Van de Putte G, Lie AK, Vach W, Baekelandt M, Kristensen GB. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol. Oncol. 2005;99(1):106–112. doi: 10.1016/j.ygyno.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Hosaka M, et al. Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J. Obstet. Gynaecol. Res. 2008;34(4):552–556. doi: 10.1111/j.1447-0756.2008.00739.x. [DOI] [PubMed] [Google Scholar]
- 24.Deura I, et al. Incidence and risk factors for lower limb lymphedema after gynecologic cancer surgery with initiation of periodic complex decongestive physiotherapy. Int. J. Clin. Oncol. 2015;20(3):556–560. doi: 10.1007/s10147-014-0724-0. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda M, et al. The trend and outcome of postsurgical therapy for high-risk early-stage cervical cancer with lymph node metastasis in Japan: A report from the Japan society of gynecologic oncology (JSGO) guidelines evaluation committee. J. Gynecol. Oncol. 2021;32(3):e44. doi: 10.3802/jgo.2021.32.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyahara S, et al. The impact of histological subtype on survival outcome of patients with stage IIB-IVA cervical cancer who received definitive radiotherapy. Tohoku J. Exp. Med. 2021;255(4):303–313. doi: 10.1620/tjem.255.303. [DOI] [PubMed] [Google Scholar]
- 27.Shimada M, et al. Comparison of the outcome between cervical adenocarcinoma and squamous cell carcinoma patients with adjuvant radiotherapy following radical surgery: SGSG/TGCU intergroup surveillance. Mol. Clin. Oncol. 2013;1(4):780–784. doi: 10.3892/mco.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishio S, et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix: An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol. Oncol. 2019;153(1):13–19. doi: 10.1016/j.ygyno.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Furusawa A, et al. A randomized phase III trial of adjuvant chemotherapy versus concurrent chemoradiotherapy for postoperative cervical cancer: Japanese gynecologic oncology group study (JGOG1082) Int. J. Gynecol. Cancer. 2021;31(4):623–626. doi: 10.1136/ijgc-2020-002344. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo K, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: Systemic chemotherapy versus pelvic irradiation. Int. J. Cancer. 2017;141(5):1042–1051. doi: 10.1002/ijc.30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anchora LP, et al. Should the number of metastatic pelvic lymph nodes be integrated into the 2018 FIGO staging classification of early stage cervical cancer? Cancers (Basel). 2020;12(6):1552. doi: 10.3390/cancers12061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan DD, Tang Q, Tu YQ, Chen JH, Lv XJ. A comprehensive analysis of the factors of positive pelvic lymph nodes on survival of cervical cancer patients with 2018 FIGO stage IIIC1p. Cancer Manag. Res. 2019;11:4223–4230. doi: 10.2147/CMAR.S204154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol. Oncol. 2019;152(1):87–93. doi: 10.1016/j.ygyno.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed in this study is available from the corresponding author on reasonable request.