Abstract
Nanomaterials feature exceptional, one-of-a-kind qualities that might be used in electronics, medicine, and other industries. Two-dimensional nanomaterials called borophene have a variety of intriguing characteristics, which helped them to leave an indelible impression in the fields of chemistry, material science, nanotechnology, and condensed matter physics. The concept of modelling the structure of a molecule or chemical network to a chemical graph and then quantitatively analysing them with the aid of topological descriptors was a major advance in the fields of mathematics and chemistry, with a wide range of applications. M-polynomial approach is a very versatile and quick method for computing the degree-based descriptors of chemical graphs or networks. The degree-based descriptors of the -Borophene nanosheet are established in this study utilising the M-polynomial technique. A program code that enables to generate the M-polynomial of any chemical structure was developed in Java platform and the same is displayed. At the conclusion, the numerical and graphical comparison based on the identified analytic expressions is also provided. Additionally, the QSPR analysis was also carried out and the outcoms are presented therein.
Subject terms: Chemistry, Mathematics and computing
Introduction
The discovery of the two dimensional nanomaterial, graphene bought a stupendous research zest in the field of two dimensional materials. It has led to a remarkable response in the material field and appreciably improved the applications of two dimensional materials in various fields. The unique and superlative properties, the outstanding performances and potential applications of these materials have grabbed major attention from the scientific experts. Two dimensional nanosheets are ultrathin compared to bulk materials and have majority of atoms exposed to the surface, so that it can have greater surface areas, higher chemical and physical activity and quantum confinement effects1. This nature enrich them with special photonic, electronic, catalytic, and magnetic properties. Also these nanomaterials have found great application in bio-like materials, drug carriers, biosensors, electronic devices, etc.
Among the two-dimensional allotropes of boron, borophene offers a variety of intriguing characteristics. Due to its minimal weight, borophene has exceptional mechanical stiffness and superconducting characteristics. It also possesses a number of exceptional therapeutic features, which expands the scope of its application in the medical and technological sectors. Owing to its remarkable structural endurance and high reliability, borophene has the potential to be used as a channel for drug distribution in immunotherapy and is predicted to have strong immunological function and catalytic activity1. These nanoparticles, with their exceptional physical and chemical attributes are ideal for a wide range of scientific and technological purposes, including higher energy fuel, elevated temperature devices, coatings, atomic engineering, and atmospheric emissions. In the areas of chemistry, bioengineering, nanoelectronics, and quantum physics, borophene has steadily gained significance. In contrast to graphene, borophene exhibits a triangular framework with hexagonal cavities (HH), and the distribution of these cavities changes over the entire structure. , , and honeycomb are the most researched borophene nanostructures. While the and variants of borophene have planar configurations devoid of vertical oscillations and various patterns of systematic boron voids, the phase of borophene has a corrugated structure, while the honeycomb borophene seems to have a structural resemblance to graphene2.
Graph theory, a sub-branch of discrete mathematics deals with the study of connection between things. This study was extended to chemical structures and networks paving way to the concept of chemical graph theory. Thus combining graph theory and chemistry, another sub-branch, chemical graph theory emerged, which utilised the concept of graph theory to characterize molecular structures3. Here the idea is to model the structure of a chemical compound or networks to chemical graph and is further analyzed quantitatively with the help of topological descriptors. Topological descriptors have found application in the field of chemical graph theory and are crucial in study of structural properties with many applications in structural chemistry. The IUPAC4 defines the topological index as “A topological index is a numerical value which is associated with the chemical constitution and is used for correlation of chemical structure with various physical properties, chemical reactivity, or biological activity”. Topological descriptors help in a very appealing way to determe the physical, chemical, biological or pharmacological properties of a chemical structure. It has application in vast areas of chemistry, informatics, biology, Quantitative Structure-Property Relationships (QSPR) and Quantitative Structure-Activity Relationships (QSAR), etc.3, 5–9. Wiener index is the first known structural descriptor, introduced by H. Wiener in 19475. Using the concept, Wiener defined the boiling points for alkanes. Subsequently, in 1972, Gutman and Trinajstić10 introduced the first and second Zagreb indices. Further, many modified forms and variants of Zagreb indices were introduced, such as modified Zagreb index11, Augmented Zagreb index12 etc. In the study of heat formation of heptanes and octanes, the augmented Zagreb index is useful. In 1975, Randić proposed the Randić index8, which has application in the field of drug design13. A generalized version of Randić index14 was also defined. Another variation of the Randić index, which is known as Harmonic index was first introduced and defined by Siemion Fajtlowicz15. In chemical graph theory, there are many more topological descriptors, especially many degree based descriptors. The application of these structural descriptors in various fields are remarkable16 and researchers working on this concept are coming up with new descriptors that correlates with the structural properties of a chemical structure more precisely17, 18.
Traditionally, the structural descriptors were computed directly using the definitions, which are time consuming. Renowned researchers established several techniques to compute the structural descriptors19–21, inorder to save computational time. One among them was the polynomial representation of structural descriptors, which acquired broad attention and acceptance in the literature22. To compute wiener index, the Hosoya polynomial, which is also known as Wiener polynomial23, 24 was used. It was also useful in the computation of Hosoya index, Hyper Wiener index etc. The wiener index is obtained by evaluating the derivative of Hosoya polynomial by equating the variable to 1. Later, in 2015, the concept of M-polynomial was proposed by Deutsch and Klavžar25. The concept of M-polynomial is to provide a general polynomial with the help of which, one can determine various degree based structural descriptors.
In this work, various degree based descriptors of -Borophene nanosheet2 are computed using M-polynomial approach. The structural descriptors of borophene nanosheets and borophene nanotubes of other variations, namely, , and honeycomb were studied by researchers26–28. In this paper, the -Borophene nanosheet is denoted as , where r is the count of rows and c is the count of columns that hexagons with centre vertex are repeated. The molecular graph of with r taking value 3 and c taking value 4 is can be denoted as , and the same is presented in Fig. 1.
Figure 1.
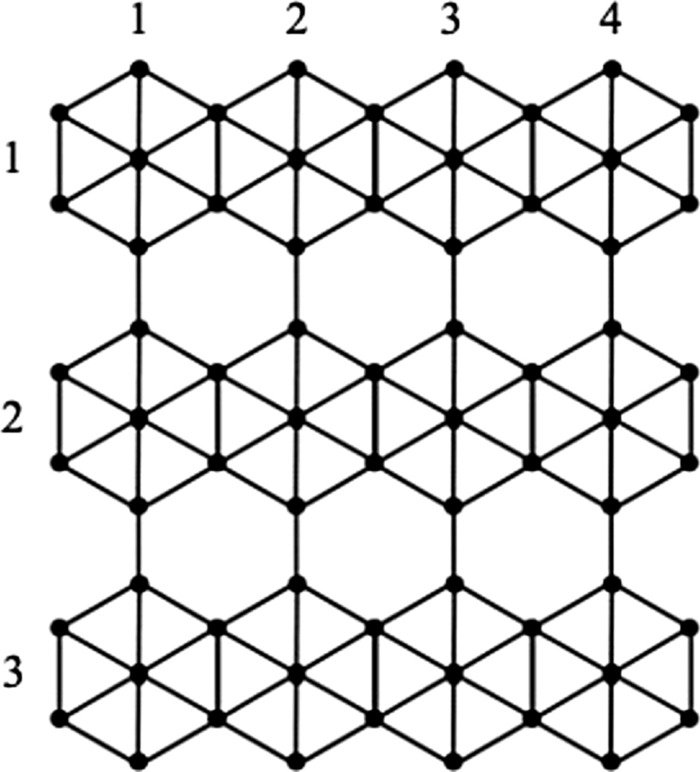
-Borophene nanosheet, , where and .
Mathematical concepts
This section comprises of the notations and the concepts that are employed for the study. Consider a simple graph , which is connected. The vertex set is denoted by and denotes the edge set of . The degree of a vertex is denoted as , which is the number of edges incident to the vertex.
The first Zagreb index and second Zagreb index10 are defined as,
These indices are recogonised to provide quantitative measures of molecular branching10 and even used in the study of complexity of chemical compounds16.
Researchers have developed modified versions Zagreb indices and among them two are the second modified Zagreb index11, and augmented Zagreb index12, .
In the line of Zagreb indices, the eminent scientist Randić proposed the Randić index8,
Later a generalized version of the Randić index was introduced, replacing by a fixed number , known as the general Randić index14 given by, .
The general reciprocal Randić index29 is defined as . When , the reciprocal Randć index is obtained.
Harmonic index15 is an another version of Randić index given by, .
The hyper Zagreb index30 and the forgotten index31 (also known as F-index) are defined as,
One of the major application of F-index is that it is found to be appropriate for testing the properties of drug related molecular structures31.
The sigma index32 is given by, .
To improve the Quantitative Structure-Activity-Property Relationships studies, Vukičević and Gašperov33 established a new class of structural descriptors, called as “discrete Adriatic indices” and it comprises of one hundred and forty-eight descriptors. The symmetric division index34 is one among them and is a significant predictor of physicochemical properties of chemical compounds. It is also noted that, among all the existing structural descriptors, the symmetric division index has the strongest correlation for predicting the total surface area of polychlorobiphenyls33. It is given as, .
The inverse sum index35, is defined as .
The atom bond connectivity index is utilized in theoretical chemistry for designing chemical compounds with specific physicochemical properties or given pharmacological and biological activities. The geometric arithmetic index and sum-connectivity index are also correlated with the physical and chemical properties of chemical compounds and used in QSPR and QSAR studies. The atom bond connectivity index36, , geometric arithmetic index37, and sum-connectivity index38, are defined as, , and .
For be a graph and be the number of edges such that , the M-polynomial is depicted as25,
The following operators are required while computing the degree based descriptors using the M-polynomial concept25.
The formulas for deriving the degree based structural descriptors from M-polynomials are given in Table 122, 25.
Table 1.
The formulas for deriving the degree based descriptors from M-polynomials.
| Degree based descriptor | t(g, h) | Derivation from |
|---|---|---|
| First Zagreb | ||
| Second Zagreb | gh | |
| Second modified Zagreb | ||
| Hyper Zagreb | ||
| Harmonic | ||
| General Randić | ||
| General reciprocal Randić | ||
| Forgotten | ||
| Symmetric division | ||
| Inverse sum | ||
| Sigma | ||
| Augmented Zagreb |
M-Polynomial
This section comprise of the direct computation method of determining the M-polynomial for the proposed structure and a progam code that generates the M-polynomial of any chemical graph.
M-Polynomial of -borophene nanosheet
Theorem 1
Let be the chemical graph of the - borophene nanosheet as seen in Fig. 1, then it’s M-polynomial is
Proof
Let . has vertices and edges. The edge set can be partitioned into sets , based on the vertex degree and by observation, clearly it can be said that there are nine types of edge partitions, say , , , , , , , and for G. The edge partitions for are shown in Fig. 2.
Figure 2.
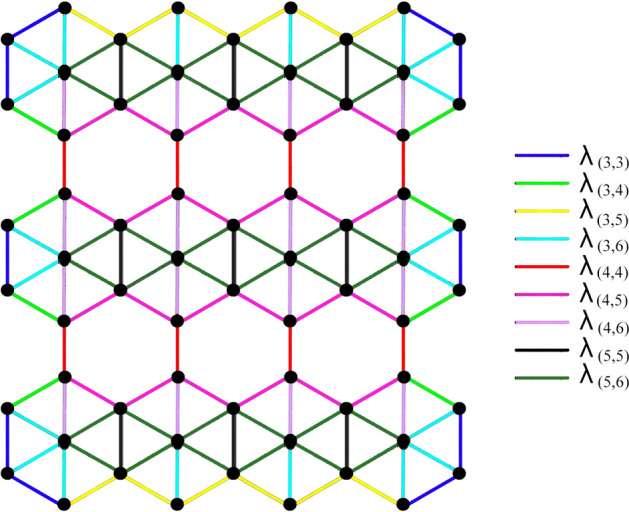
Edge partitions of -Borophene Nanosheet, .
It can be also observed that
; ; ;
; ; ;
; ; .
By definition of M-polynomial,
Therefore,
Hence the proof.
M-Polynomial of a chemical graph
In this section, a program code using Java programming language was developed, that enables us to generate the M-polynomial of any chemical graph. The program code is as follows.
Program Code:
var totalinput = "";
var inputx = "";
var inputy = "";
var inputequ = "";
var inputequ = document.getElementById("inputequ");
var inputx = document.getElementById("inputx");
var inputy = document.getElementById("inputy"); var inputbutton = document.getElementById("addquation");
var resultcontainer = document.getElementById("result");
window.addEventListener("DOMContentLoaded", (event) => {
inputbutton.addEventListener("click", myFunction);
});
function myFunction() {
var xval = inputx.value;
var yval = inputy.value;
var eqval = inputequ.value;
//console.log(resultcontainer.innerHTML); if (resultcontainer.innerHTML != ""){
resultcontainer.innerHTML += " + ";
}
resultcontainer.innerHTML += "(" + eqval + ") * g<sup>"+xval+"</sup> * h<sup>"+yval+"</sup>";
}
Output Window:
Once the program is runned, the output window as given in Fig. 3 is obtained. In this window, the edge partition data of the chemical structure is needed to be inputed inorder to obtain the M-polynomial. Suppose there are x number of edges with end vertex degree , then in ‘Input X’ column, we have to input the value of , in ‘Input Y’ column, we have to input the value of and in ‘Input Equation’ column, we have to input the value of x. Next, the inputed data is added by clicking the ‘Add’ button. Likewise, each data is added and as a final outcome, the required M-polynomial equation is obtained.
Figure 3.
The output window for the given program code for generating M-polynomial.
Degree based descriptors of : borophene nanosheet
Results based on M-polynomial approach
This section comprises of theorems based on the derivation of degree based descriptors of - borophene nanosheet, using M-polynomial.
Theorem 2
For
Proof
The M-polynomial of is,
Let . Then,
By using Table 1,
Theorem 3
For
Proof
The proof is similar to Theorem 2. Let , then
Theorem 4
For
Proof
Let , then by using the Table 1,
Theorem 5
For
Proof
Let . Then by using Table 1,
Results based on edge partition technique
This section comprises of theorems based on the computation of degree based descriptors of —borophene nanosheet, . In this section, the degree counting method and edge partition technique is applied and the expressions are derived from the definition of the corresponding degree based descriptors.
Theorem 6
For
Proof
Let . has vertices and edges. The edge set partitions based on the vertex degree are , , , , , , , and for G, where, . Also,
; ; ; ; ;
; ; ; .
By definition, Atom bond connectivity index,
Geometric arithmetic index,
Sum connectivity index,
Discussion
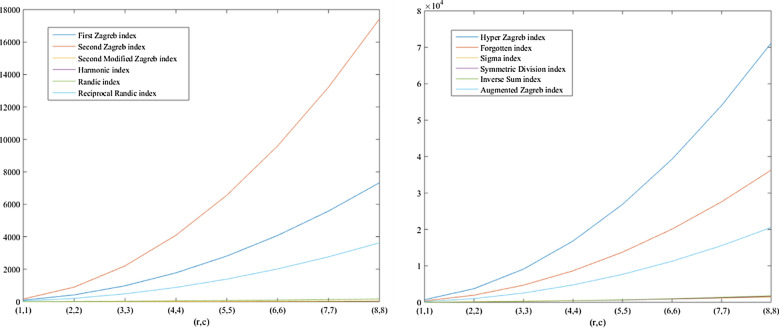
In the previous section, the degree based descriptors of -Borophene nanosheet were derived from the M-polynomial of the graph. Using those analytic expressions, the numerical values of the descriptors for some fixed value of the parameters r and c of were computed and are presented in Tables 2 and 3. The graph plotted using the M-polynomial function of the nanosheet is depicted in Fig. 4. The numerical comparison of degree based descriptors computed in Theorem 6 between some fixed values of the parameters r and c are shown in Table 4. The numerical computations was done with the help of MATLAB sofware. The computed numerical values were plotted inorder to obtain a graphical comparison between the descritors and parameters r and c, and the same are exhibited in Figs. 5 and 6.
Table 2.
Computed numerical values of degree based descriptors for .
| (r, c) | (1,1) | (2,2) | (3,3) | (4,4) | (5,5) | (6,6) |
|---|---|---|---|---|---|---|
| First Zagreb | 90 | 416 | 978 | 1776 | 2810 | 4080 |
| Second Zagreb | 162 | 894 | 2204 | 4092 | 6558 | 9602 |
| Second modified Zagreb | 1 | 2.9939 | 6.0261 | 10.0967 | 15.2056 | 21.3528 |
| Harmonic | 3.3333 | 11.5196 | 24.6382 | 42.6892 | 65.6724 | 93.5880 |
| Randić | 3.4142 | 11.7541 | 25.0599 | 43.3317 | 66.5694 | 94.7731 |
| Reciprocal Randić | 43.4558 | 203.5623 | 480.8595 | 875.3476 | 1387.0265 | 2015.8962 |
Table 3.
Computed numerical values of degree based descriptors for .
| (r, c) | (1,1) | (2,2) | (3,3) | (4,4) | (5,5) | (6,6) |
|---|---|---|---|---|---|---|
| Hyper Zagreb | 702 | 3732 | 9106 | 16,824 | 26,886 | 39,292 |
| Forgotten | 378 | 1944 | 4698 | 8640 | 13,770 | 20,088 |
| Sigma | 54 | 156 | 290 | 456 | 654 | 884 |
| Symmetric division | 27 | 104.5 | 231.4 | 407.6 | 633.1 | 908 |
| Inverse sum | 21 | 99.6642 | 236.5244 | 431.5805 | 684.8326 | 996.2807 |
| Augmented Zagreb | 170.3612 | 1009.5078 | 2538.5007 | 4757.3399 | 7666.0253 | 11,264.5571 |
Figure 4.
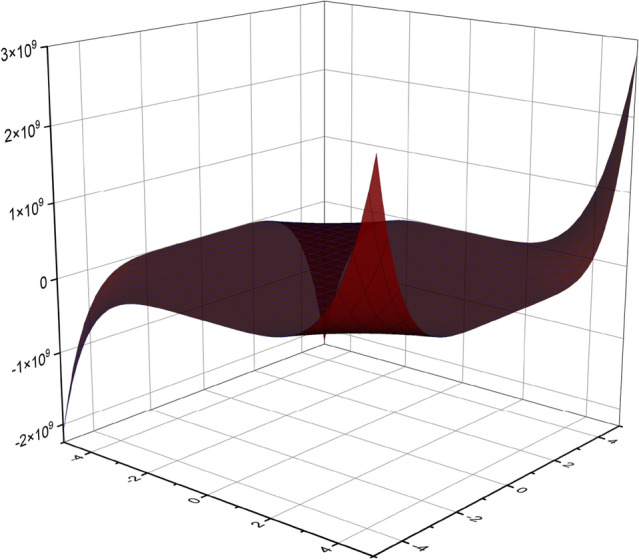
Graphical representation of M-polynomial for the nanosheet, .
Table 4.
Computed numerical values of degree based descriptors for .
| (r, c) | (1,1) | (2,2) | (3,3) | (4,4) | (5,5) | (6,6) |
|---|---|---|---|---|---|---|
| Atom bond connectivity | 7.7416 | 29.3422 | 64.7228 | 113.8837 | 176.8247 | 253.5459 |
| Geometric arithmetic | 11.6568 | 47.0069 | 106.1935 | 189.2167 | 296.0764 | 426.7725 |
| Sum connectivity | 4.4495 | 16.5419 | 36.3176 | 63.7766 | 98.9187 | 141.7441 |
Figure 5.
Comparison graph based on the descriptors of -borophene nanosheet.
Figure 6.
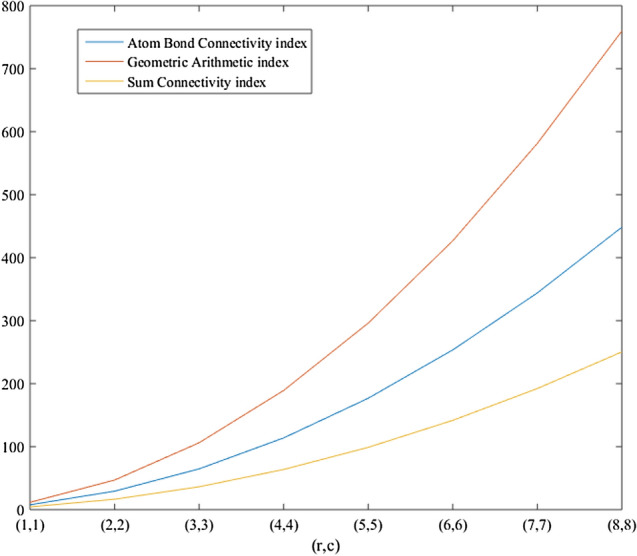
Comparison graph based on the descriptors of -borophene nanosheet.
Quantitative structure-activity-property relationship model
Theoretical topological descriptors are numerical representations of chemical compounds that encode the topological and chemical information of the structure. A variety of descriptors, such as physicochemical, constitutional and geometrical, electrostatic, topological and quantum chemical indices, have been developed and are widely used in quantitative structure-activity-property research to predict biological activities and chemical properties. The process begins with a suitable molecular topological descriptor and ends with some inference, hypothesis, and prediction about the molecule’s behaviour, properties and characteristics. The availability of high-quality experimental data, as well as its correctness, are vital in this investigation. The selection of the optimal topological descriptor for modelling in analysis is a crucial phase in the QSPR/QSAR process. Because there is no consensus on the best molecular description, all feasible descriptors are determined. A major application of QSPR/QSAR models is that the properties, activities, behavior, etc. of a newly designed or untested chemical compound can be inferred from the molecular structure of similar compounds whose properties, activities, characteristics, etc. have already been assessed. Recently researchers are coming up with relevant results in this field of research.
A flow chart diagram is drawn based on the QSPR/QSAR modelling with respect to structural descriptors of chemical structures, especially with respect to the borophene nanosheet. The same is presented in Figure 7.
Figure 7.
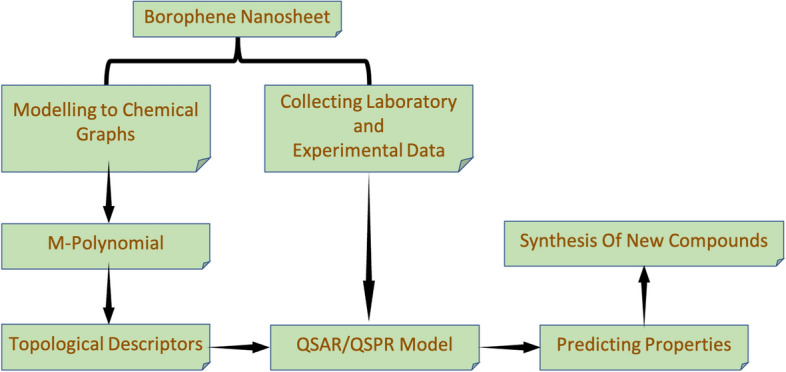
QSAR/QSPR flow chart model.
Linear regression model
As mentioned, , , are the most researched borophene nanostructures. In this section, the linear regression models for few properties of these borophene nanostructures with their degree based descriptors are obtained using least square procedure.
The following regression model is considered for the analysis.
where P and SD stands for property and structural descriptor respectively.
For the study, the properties such as shear modulus (G), Young’s modulus (Y) and Poisson’s ratio are considered. The numerical values of various topological descriptors and the properties39 for corresponding borophene nanostuctures are given in Tables 5 and 6 respectively.
Table 5.
Numerical values of degree based descriptors for the borophene structures.
| Descriptor | |||
|---|---|---|---|
| First Zagreb | 90 | 416 | 978 |
| Second Zagreb | 162 | 894 | 2204 |
| Second modified Zagreb | 1 | 2.9939 | 6.0261 |
| Harmonic | 3.333 | 11.5196 | 24.6382 |
| Hyper Zagreb | 702 | 3732 | 9106 |
| Forgotten | 378 | 1944 | 4698 |
| Sigma | 54 | 156 | 290 |
| Symmetric division | 27 | 104.5333 | 231.4 |
| Inverse sum | 21 | 99.6642 | 236.5244 |
| Augmented Zagreb | 170.361 | 1009.5078 | 2538.5007 |
| Atom bond connectivity | 7.7416 | 29.342 | 64.7228 |
| Geometric arithmetic | 11.6568 | 47.0069 | 106.1935 |
| Sum connectivity | 11.6568 | 47.0069 | 106.1935 |
Table 6.
Values of the properties for the borophene structures.
| Descriptor | |||
|---|---|---|---|
| Shear modulus | 94 | 68.5 | 60.5 |
| Young’s modulus | 398 | 179 | 198.5 |
| Poisson’s ratio | − 0.04 | 0.176 | 0.116 |
Using the Microsoft Excel analytical tools, the regression analysis was carried out and the correlation coefficients () generated are given in Tables 7 and 8.
Table 7.
Correlation coefficients () between the properties and degree based descriptors of the borophene structures.
| Descriptor | Shear modulus | Young’s modulus | Poisson’s ratio |
|---|---|---|---|
| First Zagreb | 0.76 | 0.93 | 0.98 |
| Second Zagreb | 0.95 | 0.9999 | 0.97 |
| Second modified Zagreb | 0.89 | 0.71 | 0.56 |
| Harmonic | 0.79 | 0.56 | 0.39 |
| Hyper Zagreb | 0.96 | 0.9994 | 0.97 |
| Forgotten | 0.97 | 0.9983 | 0.96 |
| Sigma | 0.05 | 0.35 | 0.52 |
| Symmetric division | 0.36 | 0.06 | 0.13 |
| Inverse sum | 0.75 | 0.91 | 0.97 |
| Augmented Zagreb | 0.95 | 0.9999 | 0.97 |
Table 8.
Correlation coefficients () between the properties and degree based descriptors of the borophene structures.
| Descriptor | Shear modulus | Young’s modulus | Poisson’s ratio |
|---|---|---|---|
| Atom bond connectivity | 0.60 | 0.33 | 0.14 |
| Geometric arithmetic | 0.29 | 0.01 | 0.2 |
| Sum connectivity | 0.65 | 0.39 | 0.2 |
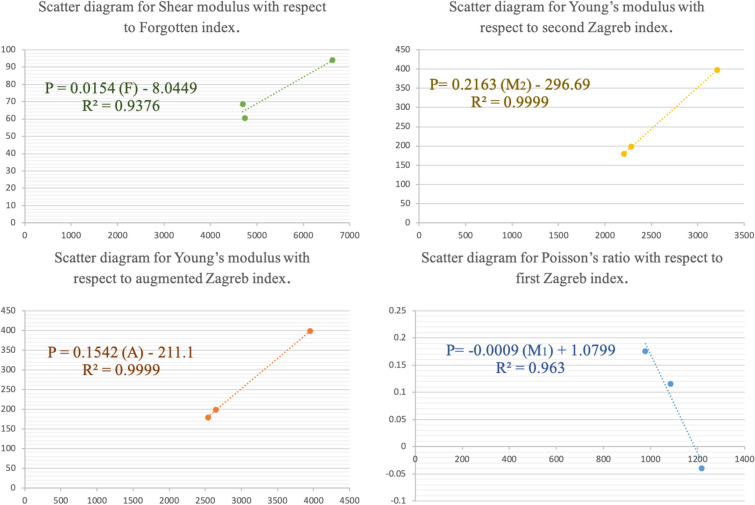
Based on the correlation coefficient (), the regression model for Forgotten index, (F) is found to be best fitted for the shear modulus, and the regression equation can be given as
For Young’s modulus, the regression model for both second Zagreb, and augmented Zagreb index, (A) is found to have same correlation coefficent.
The regression equation with respect to first Zagreb index can be given as
The regression equation with respect to augmented Zagreb index can be given as
For Poisson’s ratio, the regression model for first Zagreb index, is found to be best fitted and the regression equation can be given as
The scatter diagrams that show relation between properties and their best fitted descriptors are given in Fig. 8.
Figure 8.
Scatter diagram showing relation between the property and the descriptor.
Thus it can be concluded that, the above mentioned properties of different variations of borophene nanostructures can be predicted with the help of regression equations using the computed topological descriptors.
Conclusion
In this work, the degree based structural descriptors of -borophene nanosheet were computed using the M-polynomial approach. That degree based structural descriptors can be routinely computed from the M-polynomial. This helps in reducing the problem of determining the expression in each particular case to a single problem of determining the M-polynomial, thus making the process general, fast and efficient. Our work is reduced to determining the edge partitions due to the developed program code’s relative ease of use. The descriptors is having application in disparate fields and thus the study is relevant in present scenario. The analytic expressions derived and the numerical and graphical comparison together with the Quantitative Structure-Activity-Property Relationship model may help researchers and scientists in future advancements in the field of nanomaterials and to study the nature and behaviour of the -borophene nanosheet and other borophene nanostructures without any laboratory experimentations.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number (R.G.P.2/163/44). Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R192), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author contributions
Conceptualization, D.A. and R.I.; methodology, M.U.G.; software, H.K.; validation, A.B. and H.K.; formal analysis, E.S.V.; investigation, M.U.G.; resources, A.T.N.; data curation, H.K.; writing—original draft preparation, R.I.; writing—review and editing, R.I.; visualization, Rashad Ismail; supervision, D.A. and M.U.G.; project administration, A.B.; All authors have read and agreed to the published version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The Acknowledgements section in the original version of this Article was incomplete. It now reads: The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number (R.G.P.2/163/44). Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R192), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/10/2023
A Correction to this paper has been published: 10.1038/s41598-023-40229-y
References
- 1.Ou M, Wang X, Yu L, Liu C, Tao W, Ji X, Mei L. The emergence and evolution of borophene. Adv. Sci. 2021;8(12):2001801. doi: 10.1002/advs.202001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chand H, Kumar A, Krishnan V. Borophene and boron-based nanosheets: Recent advances in synthesis strategies and applications in the field of environment and energy. Adv. Mater. Interfaces. 2021;8(15):2100045. doi: 10.1002/admi.202100045. [DOI] [Google Scholar]
- 3.Bonchev D. Chemical Graph Theory: Introduction and Fundamentals. CRC Press; 1991. [Google Scholar]
- 4.Van de Waterbeemd H, Carter RE, Grassy G, Kubinyi H, Martin YC, Tute MS, Willett P. Glossary of terms used in computational drug design (IUPAC Recommendations 1997) Pure Appl. Chem. 1997;69(5):1137–1152. doi: 10.1351/pac199769051137. [DOI] [Google Scholar]
- 5.Wiener H. Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1947;69(1):17–20. doi: 10.1021/ja01193a005. [DOI] [PubMed] [Google Scholar]
- 6.Ghani, M. U. et al. A Paradigmatic Approach to Find the Valency-Based K-Banhatti and Redefined Zagreb Entropy for Niobium Oxide and a Metal–Organic Framework. Molecules27(20), 6975 (2022). [DOI] [PMC free article] [PubMed]
- 7.Randic M. Novel molecular descriptor for structure-property studies. Chem. Phys. Lett. 1993;211(4–5):478–483. doi: 10.1016/0009-2614(93)87094-J. [DOI] [Google Scholar]
- 8.Randic M. Characterization of molecular branching. J. Am. Chem. Soc. 1975;97(23):6609–6615. doi: 10.1021/ja00856a001. [DOI] [Google Scholar]
- 9.Shirakol S, Kalyanshetti M, Hosamani SM. QSPR analysis of certain distance based topological indices. Appl. Math. Nonlinear Sci. 2019;4(2):371–386. doi: 10.2478/AMNS.2019.2.00032. [DOI] [Google Scholar]
- 10.Gutman I, Trinajstic N. Graph theory and molecular orbitals. Total ϕ-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972;17(4):535–538. doi: 10.1016/0009-2614(72)85099-1. [DOI] [Google Scholar]
- 11.Hao J. Theorems about Zagreb indices and modified Zagreb indices. MATCH Commun. Math. Comput. Chem. 2011;65:659–670. [Google Scholar]
- 12.Furtula B, Graovac A, Vukicevic D. Augmented Zagreb index. J. Math. Chem. 2010;48(2):370–380. doi: 10.1007/s10910-010-9677-3. [DOI] [Google Scholar]
- 13.Bollobás B, Erdös P. Graphs of extremal weights. Ars Combinat. 1998;50:225. [Google Scholar]
- 14.Li X, Shi Y. A survey on the Randic index. MATCH Commun. Math. Comput. Chem. 2008;59(1):127–156. [Google Scholar]
- 15.Fajtlowicz S. On conjectures of Grafitti II. Congr. Numb. 1987;60:189–197. [Google Scholar]
- 16.Eliasi M, Vukicevic D. Comparing the multiplicative Zagreb indices. MATCH Commun. Math. Comput. Chem. 2013;69:765–773. [Google Scholar]
- 17.Ali A, Furtula B, Redžepovic I, Gutman I. Atom-bond sum-connectivity index. J. Math. Chem. 2022;60(10):2081–2093. doi: 10.1007/s10910-022-01403-1. [DOI] [Google Scholar]
- 18.Amin S, Rehman MA, Naseem A, Khan I, Andualem M. Treatment of COVID-19 patients using some new topological indices. J. Chem. 2022;2022:7309788. doi: 10.1155/2022/7309788. [DOI] [Google Scholar]
- 19.Ghani MU, et al. Computation of Zagreb polynomial and indices for silicate network and silicate chain network. J. Math. 2023;2023:9722878. doi: 10.1155/2023/9722878. [DOI] [Google Scholar]
- 20.Khan AR, et al. Characterization of temperature indices of silicates. Silicon. 2023;1:1–7. [Google Scholar]
- 21.Ghani MU, et al. Hex-derived molecular descriptors via generalised valency-based entropies. IEEE Access. 2023;11:42052. doi: 10.1109/ACCESS.2023.3248507. [DOI] [Google Scholar]
- 22.Rajpoot, A. & Selvaganesh, L. Extension of M-Polynomial and Degree Based Topological Indices for Nanotube (2021).
- 23.Hosoya H. On some counting polynomials in chemistry. Discret. Appl. Math. 1988;19(1–3):239–257. doi: 10.1016/0166-218X(88)90017-0. [DOI] [Google Scholar]
- 24.Gutman I. Some properties of the Wiener polynomial. Graph Theory Notes N. Y. 1993;125:13–18. [Google Scholar]
- 25.Deutsch, E. & Klavžar, S. M-polynomial and degree-based topological indices. Preprint at http://arXiv.org/1407.1592 (2014).
- 26.Afzal D, Afzal F, Hussain S, Chaudhry F, Thapa DK. Investigation on boron alpha nanotube by studying their M-polynomial and topological indices. J. Math. 2022;2022:1–7. [Google Scholar]
- 27.Hussain S, Afzal F, Afzal D, Cancan M, Ediz S, Farahani MR. Analyzing the boron triangular nanotube through topological indices via M-polynomial. J. Discret. Math. Sci. Cryptogr. 2021;24(2):415–426. doi: 10.1080/09720529.2021.1882158. [DOI] [Google Scholar]
- 28.Liu JB, Shaker H, Nadeem I, Hussain M. Topological aspects of boron nanotubes. Adv. Mater. Sci. Eng. 2018;2018:5729291. doi: 10.1155/2018/5729291. [DOI] [Google Scholar]
- 29.Favaron O, Mahéo M, Saclé JF. Some eigenvalue properties in graphs (conjectures of Graffiti-II) Discret. Math. 1993;111(1–3):197–220. doi: 10.1016/0012-365X(93)90156-N. [DOI] [Google Scholar]
- 30.Shirdel, G. H., Rezapour, H. & Sayadi, A. M. The Hyper-Zagreb Index of Graph Operations (2013).
- 31.Furtula B, Gutman I. A forgotten topological index. J. Math. Chem. 2015;53(4):1184–1190. doi: 10.1007/s10910-015-0480-z. [DOI] [Google Scholar]
- 32.Gutman I, Togan M, Yurttas A, Cevik AS, Cangul IN. Inverse problem for sigma index. MATCH Commun. Math. Comput. Chem. 2018;79(2):491–508. [Google Scholar]
- 33.Vukicevic D, GaŠperov M. Bond additive modeling 1. Adriatic indices. Croat. Chem. Acta. 2010;83(3):243–260. [Google Scholar]
- 34.Gupta CK, Lokesha V, Shwetha SB, Ranjini PS. On the Symmetric division deg index of graph. Southeast Asian Bull. Math. 2016;40:1. [Google Scholar]
- 35.Sedlar J, Stevanovic D, Vasilyev A. On the inverse sum indeg index. Discret. Appl. Math. 2015;184:202–212. doi: 10.1016/j.dam.2014.11.013. [DOI] [Google Scholar]
- 36.Estrada, E., Torres, L., Rodriguez, L. & Gutman, I. An Atom-Bond Connectivity Index: Modelling the Enthalpy of Formation of Alkanes (1998).
- 37.Vukicevic D, Furtula B. Topological index based on the ratios of geometrical and arithmetical means of end-vertex degrees of edges. J. Math. Chem. 2009;46(4):1369–1376. doi: 10.1007/s10910-009-9520-x. [DOI] [Google Scholar]
- 38.Zhou B, Trinajstic N. On general sum-connectivity index. J. Math. Chem. 2010;47(1):210–218. doi: 10.1007/s10910-009-9542-4. [DOI] [Google Scholar]
- 39.Wang ZQ, Lü TY, Wang HQ, Feng YP, Zheng JC. Review of borophene and its potential applications. Front. Phys. 2019;14:1–20. doi: 10.1007/s11467-019-0884-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.