Abstract
Mammographic breast cancer screening is effective in reducing breast cancer mortality. Nevertheless, several limitations are known. Therefore, developing an alternative or complementary non-invasive tool capable of increasing the accuracy of the screening process is highly desirable. The objective of this study was to identify circulating microRNA (miRs) ratios associated with BC in women attending mammography screening. A nested case–control study was conducted within the ANDROMEDA cohort (women of age 46–67 attending BC screening). Pre-diagnostic plasma samples, information on life-styles and common BC risk factors were collected. Small-RNA sequencing was carried out on plasma samples from 65 cases and 66 controls. miR ratios associated with BC were selected by two-sample Wilcoxon test and lasso logistic regression. Subsequent assessment by RT-qPCR of the miRs contained in the selected miR ratios was carried out as a platform validation. To identify the most promising biomarkers, penalised logistic regression was further applied to candidate miR ratios alone, or in combination with non-molecular factors. Small-RNA sequencing yielded 20 candidate miR ratios associated with BC, which were further assessed by RT-qPCR. In the resulting model, penalised logistic regression selected seven miR ratios (miR-199a-3p_let-7a-5p, miR-26b-5p_miR-142-5p, let-7b-5p_miR-19b-3p, miR-101-3p_miR-19b-3p, miR-93-5p_miR-19b-3p, let-7a-5p_miR-22-3p and miR-21-5p_miR-23a-3p), together with body mass index (BMI), menopausal status (MS), the interaction term BMI * MS, life-style score and breast density. The ROC AUC of the model was 0.79 with a sensitivity and specificity of 71.9% and 76.6%, respectively. We identified biomarkers potentially useful for BC screening measured through a widespread and low-cost technique. This is the first study reporting circulating miRs for BC detection in a screening setting. Validation in a wider sample is warranted.
Trial registration: The Andromeda prospective cohort study protocol was retrospectively registered on 27-11-2015 (NCT02618538).
Subject terms: Computational biology and bioinformatics, Molecular biology, Biomarkers, Risk factors, Breast cancer, Cancer prevention, Cancer screening, Tumour biomarkers, Cancer, Cancer
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in females (2,261,419 new cases, 11.7% of all cancer sites) and the leading cause of women cancer death worldwide (684,996 deaths, 6.9% of all sites) as reported in the Global Cancer Statistics report for 20201.
Currently, the primary screening tool for early detection is mammography. Although it is demonstrated to be effective in reducing cancer mortality (around 15% in women younger than 50 years, and between 14 and 23% in older women), it does have certain limitations including interval cancers, false positive rate, overdiagnosis, radiation exposure and inflexible scheduling2,3. Notably, the updated European Breast Screening Guidelines of the European Commission Initiative for Screening and Diagnosis strongly recommended biennial mammography screening in the context of an organised program for women 50–69 years. Organised mammography screening is suggested, but with a conditional recommendation, also for younger (45–49 years) and older women (70–74 years), while screening interval (biennial or triennial) in these age ranges is still under debate4.
Lately, age extensions, diverse imaging technologies and combinations of different risk factors have been considered for optimizing BC screening protocols. BC risk is a composite measure, including the contribution of reproductive history (i.e. menarche, menopause, age at first pregnancy), family history, previous breast biopsies, prior chest irradiation, and breast density5,6. The state-of-the-art BC risk algorithms also include (epi)genetic biomarkers, such as single nucleotide polymorphisms (SNPs) or microRNAs (miRs)6–8. miRs are a class of small non-coding RNA molecules which function as negative regulators of gene expression by directing specific mRNA cleavage or translational inhibition9. Dysregulated tissue and circulating miR profiles have been associated with diagnosis, prognosis and sometimes survival in BC10,11. Moreover, many dysregulated miRs are reproducibly found in body fluids such as plasma and serum. They are believed to be protected from degradation by association with secreted membrane vesicles or RNA-binding proteins12. miRs may represent valuable markers for BC early diagnosis, prognosis as well as conceivable treatment targets13. Hence, circulating miRs have the potential to be suitable as minimally invasive biomarkers for early cancer detection. Numerous reports on miRs for BC detection have been published10; however, to our knowledge, none were analysed in a BC screening context.
The main aims of the present study were: to identify miR ratios associated with BC through sequencing of small RNAs in a nested case–control study within a large cohort of women attending the BC screening program; to investigate the consistency of the results using Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR), a widespread and low-cost technology; to identify an RT-qPCR based miR ratio signature to be validated in further cohorts of women attending BC screening programs.
Methods
This case–control study followed the strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for reporting observational studies14.
Study population
ANDROMEDA was a multicentre prospective cohort study on women attending BC screening in two centres in Italy15. The eligible population of the study consisted of women of age 46–67 invited to breast screening in the cities of Turin and Biella (two Northern Italian cities in Piedmont), where BC screening is a long-standing practice well known by the people living in the area16. Enrolment started in July 2015 for Turin and in May 2016 for Biella, and by the end of the recruitment phase (March 2018), 26,640 women had been included in the study. The cohort has been followed to date through the screening archives to observe the onset of new BC cases. At the time of BC screening appointment, all eligible women were offered to participate. After a detailed explanation of the study protocols, written informed consent was obtained from each participant. Women who agreed to participate in ANDROMEDA were asked, immediately at the enrolment desk, to fill in a short risk questionnaire to collect information on general BC risk factors (reproductive and BC family history, previous breast biopsies, basic physical activity level, body mass index (BMI) and alcohol consumption). In addition, they were asked to fill in a detailed risk questionnaire on diet, physical activity, smoking habits, general state of health and psychological distresses. Life-style information was gathered and employed to build a comprehensive life-style score, as proposed by Romaguera and colleagues17 on the EPIC cohort, based on adherence to the World Cancer Research Fund (WCRF) recommendations18. The score, ranging from 0 to 7, includes BMI, physical activity, high energy–density foods, plant foods, animal foods, alcoholic drinks, and breastfeeding. The five-year absolute risk was obtained on all samples as estimated by Petracci and colleagues19.
Women were also invited to undergo anthropometric measurements (height, weight, body composition, and waist circumference) and to provide a blood sample for serum, plasma and buffy coat storing. Blood specimens were aliquoted, processed and stored at −80 °C.
Incident BC cases were identified through record linkage with screening archives, cancer registries and hospital discharge cards. Intrinsic subtypes of BC were defined using the clinicopathologic surrogate definition reported at the 13th St Gallen International BC Conference20. Ethical approval was obtained from the Ethics Committee of each participating center (Ethical and deontological institutional review board of the A.O.U Città della Salute e della Scienza of Turin with the protocol number 78326 on 11.07.2013—and Ethical Committee of Novara with the protocol number 248/CE and study number CE 27/15). The research was performed in accordance with the Declaration of Helsinki guidelines. Informed consent was obtained from all participants. The study was registered in ClinicalTrials.gov with the number NCT02618538, on November 27th, 2015.
Breast density evaluation
Standard digital mammographies (DM) were performed and read by two expert radiologists. Breast density was calculated during breast examination through two different algorithms: Breast Imaging Reporting and Data System (BI-RADS)21 and Tabar22. The BI-RADS classified the breast density into category 1—almost fatty (< 25% glandular component); category 2—scattered fibroglandular densities (25–50% glandular); category 3—heterogeneously dense (51–75% glandular); and category 4—extremely dense (> 75% glandular)21. Similarly, Tabar classification was adopted as follows: I (balanced proportion of all components of breast tissue with a slight predominance of fibrous tissue), II (predominance of fat tissue), III (predominance of fat tissue with retroareolar residual fibrous tissue), IV (predominantly nodular densities), V (predominantly fibrous tissue)22. For subsequent analyses, considering sample distribution and risk classification, the patterns of higher density for both classifications were grouped in a unique category (i.e. BI-RADS 4–5, and Tabar IV–V).
Selection of cases and controls
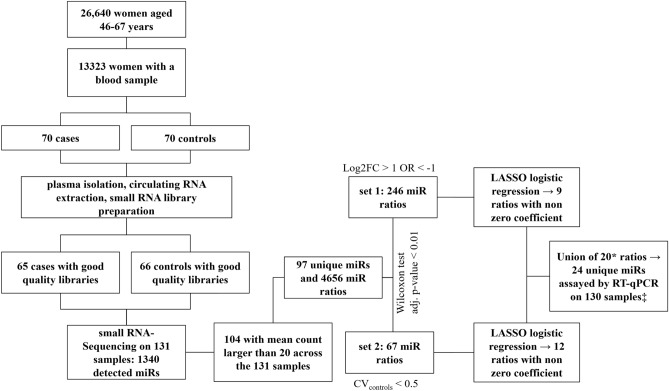
For the present study, a case–control study nested within the cohort was conducted. For the nested case–control study, both cases and controls were selected among the participants in the ANDROMEDA cohort study who accepted to provide blood samples at recruitment (n = 14,323, 53.8% of the total). Women with a personal history of BC, with a severe disease or who were unable to give informed consent were excluded from the study. Cases were restricted to women with incident BCs diagnosed within June, 2018 for whom blood was collected before any treatment (n = 70). Moreover, due to the relatively short period of time between blood storage and cases/controls extraction, random sampling, without variable matching, of 70 controls from women who did not experience any BC event before June 2018 was performed. No interval cancers were observed among the controls. The study flow-chart is reported in Fig. 1.
Figure 1.
Flow-chart of the study. * The let-7f-5p-2_miR-103a-3p-2 ratio was removed as miR-103a-3p-2 and miR-103a-3p-1, found in the let-7f-5p-1_miR-103a-3p-1 ratio, had almost identical counts and their ratio partners had identical mature miR sequences. ‡ One sample had to be excluded in the RT-qPCR step due to insufficient plasma volume.
SNP genotyping and polygenic risk score calculation
For SNP genotyping, genomic DNA was isolated from 200 µl of buffy coat by means of MagMAX DNA Multi-sample Ultra 2.0 kit (Thermo Fisher Scientific, Waltham, MA, USA). DNA concentration and purity were checked by Nanodrop Spectrophotometer (Thermo Fisher). Libraries were prepared starting from 15 ng of DNA and according to the Ion AmpliSeq Library Kit 2.0 protocol for sequencing on the Ion PGM system. The custom panel (Ion AmpliSeq Custom Panel) that selectively covered 80 SNPs target sequences was designed through AmpliSeq Designer (www.ampliseq.com). Ion Xpress Barcodes kit (1–16, 17–32 and 33–48), Ion Ampliseq custom Primer Pool and Ion AmpliSeq Library Kit 2.0-384LV were used in conjunction to obtain libraries. Ion Library Equalizer kit was used to normalise for DNA concentration. Equalised barcoded libraries were pooled and sequenced using Ion PGM Hi-Q OT2 kit and Ion PGM Hi-Q Sequencing Kit on Ion PGM 318 chip V2 on an Ion Torrent PGM (Thermo Fisher Scientific).
Variant calling was performed utilising the Variant Caller plugin within the Torrent Suite Software version 5.10 (Thermo Fisher). The polygenic risk score (PRS) was calculated by adding the multiplications of log odds ratio of each of the 77 SNPs23 by the genotype at respective loci (0 for wildtype, 1 for heterozygous variant and 2 for homozygous variant).
Plasma sample collection and small-RNA sequencing analysis
Plasma isolation from EDTA-tube blood samples, hemolysis check and circulating RNA extraction were carried out as previously described24. For library preparation, the Ion Total RNA-Seq kit v2 protocol (Thermo Fisher) with the recommendations for low input RNA quantity was followed as described in25. Barcoded primers from Ion Xpress™ RNA-Seq Barcode 01–16 Kit, Thermo Fisher, or synthesised by Eurofins Genomics as custom oligonucleotides (barcodes 17–24) were used. Differentially barcoded small-RNA libraries were pooled and checked by Bioanalyzer System and DNA 1000 Kit (Agilent Technologies) to determine the library dilution required for template preparation. Ion Chef™ System (Thermo Fisher) was used for automated templated Ion Sphere Particles preparation and chip loading. Ion 540 chips (Thermo Fisher) were sequenced using Ion GeneStudio S5 Plus System (Thermo Fisher).
Raw sequence reads were processed using the small-RNA plugin available within the Torrent Suite Software version 5.10 (Thermo Fisher). The reads were aligned to mature miRs using the bowtie2 alignment software26, bundled with the plugin. Unmapped reads were further aligned to the whole‑genome to rescue miRbase unaligned reads and count other RNA molecules (tRNAs, rRNAs, mRNAs). miR raw counts were generated using the featureCounts27 software from the Subread package 1.5.3. miRs with mean raw counts larger than 20 were selected for further analyses.
To retrieve differentially expressed miR ratios, ratios between the raw counts of all filtered miRs were computed. For any miR pairs with identical count profiles (either due to being clustered or to one miR having two different names), one was removed and a unique identifier was assigned to represent the two miRs. Some miRs with the same name but different chromosomal origin showed different count profiles and were therefore considered as separate. Nevertheless, because the mature sequences of the same miR originating from different genomic loci are the same, in the RT-qPCR validation such occurrences were considered as one single mature miR.
RT-qPCR validation
The expression of selected miRs was evaluated by RT-qPCR on a CFX-96 machine (Bio-Rad) with TaqMan probes. Four µL of RNA from each sample were reverse transcribed using TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher), with a custom pool of selected miR primers (Thermo Fisher; miR assay IDs: 00546, 002304, 000408, 000390, 002277, 002245, 000468, 000391, 000377, 000382, 000398, 000396, 000407, 001090, 002253, 000420, 002619, 000389, 000580, 000397, 002248, 000442, 000439, 000399). Then 2.5 µL of reverse transcription reaction product were pre-amplified with TaqMan PreAmp Master Mix (Thermo Fisher) and a second pool of selected miR primers (Thermo Fisher). Preamplified samples were diluted with TE buffer and stored at −20 °C for up to one week. A volume of 0.10 µL of diluted preamplified sample was mixed with PCR Master Mix (Thermo Fisher) and water and then transferred in a well of the miR plate. Custom 96 well plates (Thermo Fisher) with 24 miR assays spotted in triplicate were used, allowing for the analysis of one sample per plate.
From the obtained triplicates the mean Cycle threshold (Ct) was calculated. Non-detects were replaced with the Ct value of 40. If a replicate within the triplicate was one standard deviation away from the mean it was excluded and a new Ct mean was calculated. In order to calculate the ratio between miR X and miR Y, the following equation was used as explained by Deng and colleagues in 201928: Ctmean(Y) − Ctmean(X).
Statistical analysis
On small-RNA sequencing data, Mann–Whitney U test was performed to compare miR ratios between cases and controls and p-values were corrected using the Benjamini–Hochberg method. The fold change was calculated based on the median in cases and controls. Significantly different ratios between cases and controls (Mann–Whitney U test adjusted p-value ≤ 0.01) with a fold change > 2 or < 0.5, were selected as strategy 1, while ratios with a coefficient of variation < 0.5 within controls and significantly different between cases and controls, without setting any criteria on fold change, were selected as strategy 2. The ratios from the two strategies were further analysed by a Least Absolute Shrinkage and Selection Operator (LASSO) logistic regression29. Five-fold cross-validation was used to preliminarily assess the performance of the model-selected ratios, separately for the two strategies defined above. Thus, the sample was randomly divided into five groups, called folds, and the LASSO logistic model was trained on five minus one folds. Then, the performances of the resulting model were evaluated on the remaining part of the data. This procedure was repeated for each fold and the performances obtained each time averaged. The following performance measures were considered: calibration intercept, Cox’s measure of spread (often called “calibration slope”)30, scaled Brier score, and area under the receiver operating characteristic curve (AUC). The first three measures mainly relate to the agreement between the observed outcomes and the outcomes predicted from the model. For the intercept and scaled Brier, ideal values should be as close to zero as possible, whereas for the Cox calibration slope close to one. The AUC refers to the model's ability to discriminate between individuals with a different outcome and the ideal values should be close to one. Cross-validation was performed mainly to select promising ratios to validate by RT-qPCR.
Using standard logistic regression, univariate odds ratios (OR) and corresponding 95% confidence intervals (CIs) were obtained on RT-qPCR data. The linearity assumption between a continuous predictor and the logit of risk was inspected through the Locally Weighted Scatterplot Smoothing (LOWESS) and restricted cubic splines, whereas for ordinal variables the Cochran-Armitage trend test was used to assess the presence of a linear trend. To derive a ratio-based signature as well as to preliminarily investigate the potential added value of miR ratios over more conventional BC risk factors and their potential independent role in predicting BC risk, the LASSO logistic regression was used. Three models were then fitted: one using miR ratios only, one combining the ratios with other potential BC risk factors and one on BC risk factors alone. To select BC-associated factors for inclusion in the model together with the miR ratios, we assessed the association between BC detection and other factors such as PRS, demographic, family, reproductive and screening history, life-style, and breast density information, as well as any interaction between them relevant to BC. The discriminatory ability of the models was assessed using AUC (with reported 95% CIs), whereas the Youden index was used as the criterion to derive a cut-off point on the predicted probabilities and compute sensitivity and specificity. The paired Delong test was used to compare the discrimination among different models.
The association between demographic, life-style, anthropometric and reproductive factors as well as cancer characteristics and the selected ratios were performed using the Mann–Whitney U test or the Kruskal Wallis test, as appropriate, for categorical covariates and using the Spearman correlation coefficient for continuous covariates. Continuous variables were reported by mean ± standard deviation (sd) or median and I and III quartiles, as appropriate, whereas categorical variables were reported as natural frequency and percentage. All analyses were performed using the open-source statistical computing environment R31. The main packages used were: glmnet32 for LASSO, ROCR33 and OptimalCutpoints34 for plotting ROC curves and cut-off search.
Target enrichment analysis
Target and functional enrichment analyses were performed on the miRs making up the ratio signature associated with BC detection using the Mienturnet online software35. In all the mentioned analyses the miRTarBase database was used as it includes experimentally validated miRs.
Results
Population characteristics
After RNA extraction from the plasma of 70 cases and 70 controls and library preparation, nine samples were excluded due to poor quality. Thus, the final cohort consisted of 65 cases and 66 controls (Fig. 1). The general characteristics of the study population are reported in Table 1. The only variables that showed a significant association with BC detection in this cohort were: BMI, breast density and WCRF score. The characteristics of cases are reported in Table 2, separately for invasive and in situ tumours. Cases were diagnosed on average 3 ± 2 months after blood collection. Fifty-five women were diagnosed with invasive breast tumours and eight with in situ lesions. The most frequent histotype was ductal (56.0% of invasive and 37.5% of in situ BCs) and the majority of cancers were stage IA (87.5%), Her2 negative (86.5%) and Ki-67 negative (76.5%). Further, DNA isolated from buffy coat was used to calculate the 77 SNP PRS, which was not found to be significantly associated with BC in our cohort (Table 1).
Table 1.
Demographic, family, reproductive and screening history, life-style, anthropometric measurements, education, breast density, PRS and 5-year absolute BC risk information is reported. Results of univariate logistic regression for each variable are also reported.
| Controls (n = 66) | Cases (n = 65) | OR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Age at enrollment, years | ||||||
| Mean ± sd | 57.82 ± 5.92 | 59.15 ± 6.00 | 1.04 (0.98–1.10) | 0.201 | ||
| Centre | ||||||
| Biella | 20 | (30.30) | 16 | (24.62) | 1 (ref) | |
| Torino | 46 | (69.70) | 49 | (75.38) | 1.33 (0.62–2.91) | 0.467 |
| Previous negative second-level screening rounds | ||||||
| 0 | 62 | (93.94) | 59 | (90.77) | 1 (ref) | |
| ≥ 1 | 4 | (6.06) | 6 | (9.23) | 1.58 (0.43–6.43) | 0.497 |
| Previous benign biopsies | ||||||
| 0 | 57 | (86.36) | 49 | (77.78) | 1 (ref) | |
| ≥ 1 | 9 | (13.64) | 14 | (22.22) | 1.81 (0.73–4.69) | 0.207 |
| Missing | 2 | |||||
| Education | ||||||
| Low | 21 | (32.31) | 22 | (34.38) | 1 (ref) | |
| Medium | 31 | (47.69) | 28 | (43.75) | 0.86 (0.39–1.90) | 0.712 |
| High | 13 | (20.00) | 14 | (21.88) | 1.03 (0.39–2.71) | 0.955 |
| Missing | 1 | 1 | ||||
| Nr. of first-degree relatives with BC | ||||||
| 0 | 58 | (87.88) | 56 | (88.89) | 1 (ref) | |
| ≥ 1 | 8 | (12.12) | 5 | (7.94) | 0.65 (0.19–2.06) | 0.469 |
| Missing | 2 | |||||
| Age at menarche, years | ||||||
| ≤ 11 | 22 | (33.33) | 19 | (29.69) | 1 (ref) | |
| 12–13 | 33 | (50.00) | 33 | (51.56) | 1.16 (0.53–2.54) | 0.713 |
| ≥ 14 | 11 | (16.67) | 12 | (18.75) | 1.26 (0.45–3.55) | 0.654 |
| Missing | 1 | |||||
| Age at first full pregnancy, years | ||||||
| Nulliparous | 19 | (28.79) | 11 | (16.92) | 0.39 (0.13 –1.13) | 0.087 |
| ≤ 19 | 3 | (4.55) | 1 | (1.54) | 0.22 (0.01–2.02) | 0.219 |
| 20–24 | 11 | (16.67) | 15 | (23.08) | 1 (ref) | |
| 25–29 | 21 | (31.82) | 16 | (24.62) | 0.51 (0.18–1.41) | 0.198 |
| ≥ 30 | 12 | (18.18) | 22 | (33.85) | 1.39 (0.47–4.13) | 0.545 |
| Contraceptive therapy | ||||||
| No OR use < 1 year | 29 | (45.31) | 31 | (48.44) | 1 (ref) | |
| 1–4 years | 10 | (15.62) | 5 | (7.81) | 0.47 (0.13–1.48) | 0.210 |
| ≥ 5 years | 25 | (39.06) | 28 | (43.75) | 1.04 (0.50–2.20) | 0.902 |
| Missing | 2 | 1 | ||||
| Breastfeeding | ||||||
| Nulliparous OR no breastf. OR breastf. < 6 months | 42 | (63.64) | 39 | (60.94) | 1 (ref) | |
| ≥ 6 months | 24 | (36.36) | 25 | (39.06) | 1.12 (0.55–2.29) | 0.751 |
| Missing | – | 1 | ||||
| Menopausal status | ||||||
| Not in menopause | 12 | (18.18) | 12 | (18.75) | 1 (ref) | |
| Menopause | 54 | (81.82) | 52 | (81.25) | 0.74 (0.30–1.78) | 0.504 |
| Missing | – | 1 | ||||
| HRT use | ||||||
| Not in menopause | 12 | (18.18) | 12 | (18.75) | 1 (ref) | |
| No HRT use OR HRT use < 1 year | 43 | (65.15) | 45 | (70.31) | 1.04 (0.42–2.60) | 0.921 |
| ≥ 1 year | 11 | (16.17) | 7 | (10.94) | 0.63 (0.18–2.18) | 0.475 |
| Missing | – | 1 | ||||
| Measured BMI, kg/m2 | ||||||
| Mean ± sd | 25.76 ± 5.15 | 28.02 ± 6.24 | 1.07 (1.01–1.15) | 0.029 | ||
| Waist circumference, cm | ||||||
| Mean ± sd | 88.00 ± 11.83 | 92.69 ± 17.55 | 1.02 (1.00–1.05) | 0.087 | ||
| Missing | 2 | 2 | ||||
| Level of occupational physical activity at age 30–39 years | ||||||
| Exclusively/maily sitting | 17 | (25.80) | 23 | (35.90) | 1 (ref) | |
| Standing or average | 43 | (65.20) | 34 | (53.10) | 0.58 (0.27–1.26) | 0.172 |
| Heavy or very heavy | 6 | (9.10) | 7 | (10.90) | 0.86 (0.24–3.12) | 0.817 |
| Missing | – | 1 | ||||
| Level of leisure time physical activity at 30–39 years | ||||||
| < 2 h/week | 35 | (53.00) | 34 | (53.10) | 1 (ref) | |
| ≥ 2 h/week | 31 | (47.00) | 30 | (46.90) | 1.00 (0.50—1.99) | 0.991 |
| Missing | 1 | 1 | ||||
| Alcohol habit | ||||||
| Never drinker or ex drinker | 16 | (24.24) | 18 | (29.69) | 1 (ref) | |
| Drinker, also occasionally | 50 | (75.76) | 45 | (70.31) | 0.76 (0.35–1.65) | 0.485 |
| Missing | – | 1 | ||||
| Smoking habit | ||||||
| Never smoker | 31 | (47.69) | 26 | (41.94) | 1 (ref) | |
| Ex-smoker | 19 | (29.23) | 25 | (40.32) | 1.57 (0.71–3.50) | 0.265 |
| Occasionally/Smoker | 15 | (23.08) | 11 | (17.74) | 0.87 (0.34–2.22) | 0.779 |
| Missing | 1 | 3 | ||||
| BI-RADS breast density | ||||||
| 1 | 21 | (31.82) | 21 | (32.31) | 1 (ref) | |
| 2 | 34 | (51.52) | 27 | (41.54) | 0.79 (0.36–1.75) | 0.566 |
| 3 or 4 | 11 | (16.67) | 17 | (26.15) | 1.55 (0.59–4.15) | 0.379 |
| TABAR breast density | ||||||
| 1 | 22 | (33.33) | 10 | (15.38) | 1 (ref) | |
| 2 | 25 | (37.88) | 23 | (35.38) | 2.02 (0.80–5.32) | 0.141 |
| 3 | 6 | (9.09) | 7 | (10.77) | 2.57 (0.69–10.01) | 0.162 |
| 4 or 5 | 13 | (19.70) | 25 | (38.46) | 4.23 (1.59–11.97) | 0.005 |
| 5-year absolute breast cancer risk estimate | ||||||
| Mean ± sd | 0.017 ± 0.008 | 0.020 ± 0.012 | 1.49 (0.71–3.23) | 0.296 | ||
| Missing | 2 | 4 | ||||
| WCRF/AICR score | ||||||
| Mean ± sd | 5.52 ± 0.98 | 5.12 ± 1.11 | 0.68 (0.47–0.96) | 0.034 | ||
| PRS | ||||||
| Mean ± sd | 0.98 ± 0.43 | 1.00 ± 0.41 | 1.09 (0.47–2.51) | 0.842 | ||
| Missing | 4 | – | ||||
OR odds ratio, CI confidence interval, sd standard deviation, HRT hormone replacement therapy, BI-RADS breast imaging reporting and database system: 1 almost fatty; 2 scattered fibroglandular densities; 3 heterogeneously dense; 4 extremely dense.
TABAR Tabàr’s breast density classification system; as I (balanced proportion of all components of breast tissue), II (predominance of fat tissue), III (predominance of fat tissue with retroareolar residual fibrous tissue), IV (predominantly nodular densities), V (predominantly fibrous tissue).
WCRF/AICR world cancer research fund/American institute for cancer research.
Education categories: Low (Secondary school or lower), Medium (High school/technical school), High (bachelor’s degree or higher).
PRS polygenic risk score.
Table 2.
Histological and molecular subtype characteristics of invasive and in situ breast cancer cases (n = 65).
| Invasive (n = 57) | In situ (n = 8) | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Histotype | Histotype | ||||
| Ductal NOS | 30 | 56.0 | Ductal NOS | 3 | 37.5 |
| Lobular | 8 | 18.0 | Solid | 1 | 12.5 |
| Tubular | 4 | 8.0 | Micropapillary | 1 | 12.5 |
| Other | 9 | 18.0 | Papillary | 1 | 12.5 |
| Missing | 6 | Other | 2 | 25.0 | |
| Grade | Grade | ||||
| I | 18 | 34.7 | I | 2 | 25.0 |
| II | 25 | 51.0 | II | 2 | 25.0 |
| III | 6 | 14.3 | III | 4 | 50.0 |
| Missing | 8 | Tumour size [I27], mm | |||
| pT | 1–10 | 4 | 50.0 | ||
| 1a-1b-1mic | 25 | 48.1 | 11–20 | 2 | 25.0 |
| 1c | 24 | 42.6 | 21 + | 2 | 25.0 |
| 2 + | 5 | 9.3 | |||
| Missing | 3 | ||||
| Tumour size, mm | |||||
| 1–10 | 25 | 46.3 | |||
| 11–20 | 24 | 44.4 | |||
| 21 + | 5 | 9.3 | |||
| Misssing | 3 | ||||
| Stage | |||||
| IA | 42 | 87.5 | |||
| IIA | 3 | 6.3 | |||
| IIIC | 2 | 4.2 | |||
| IV | 1 | 2.1 | |||
| Missing | 9 | ||||
| Molecular subtypes | |||||
| ER | |||||
| Negative | 8 | 15.1 | |||
| Positive (> 10%) | 45 | 84.9 | |||
| Missing or undetermined | 4 | ||||
| PgR | |||||
| Negative | 17 | 32.1 | |||
| Positive (> 10%) | 36 | 69.9 | |||
| Missing or undetermined | 4 | ||||
| Her2 | |||||
| Negative | 45 | 86.5 | |||
| Positive | 7 | 13.5 | |||
| Missing or undetermined | 5 | ||||
| Ki-67 | |||||
| Negative | 39 | 76.5 | |||
| Positive (> 20%) | 12 | 23.5 | |||
| Missing or undetermined | 6 | ||||
| Intrinsic subtype | |||||
| Luminal A-like | 27 | 52.9 | |||
| Luminal B-like (HER2 negative) | 13 | 25.5 | |||
| Luminal B-like (HER2 positive) | 5 | 9.8 | |||
| HER2 positive (non luminal) | 2 | 3.9 | |||
| Triple negative | 4 | 7.8 | |||
| Missing | 6 | ||||
NOS not otherwise specified, pT pathologic evaluation of tumour size, ER estrogen receptor, PgR progesterone receptor, Her2 human epidermal growth factor receptor 2.
Identification of circulating miR ratios through small-RNA sequencing
Out of 1340 circulating miRs detected by small-RNA sequencing, 104 had a mean count larger than 20 across the 131 samples, resulting in 97 unique miRs and 4656 miR ratios (Fig. 1). Based on the Mann–Whitney U test, 886 ratios were differentially expressed between cases and controls in the first set and 67 in the second. miR ratios with less than twofold modulation were removed from the first set, leaving 246 ratios. Fitting a LASSO logistic model separately on each set of ratios obtained from the two strategies, resulted in 9 and 12 ratios associated with BC risk (Fig. 1 and Table S.1 in Additional file 1), respectively. The AUCs of the selected ratios, computed on the original sample, are reported in Table 3 and ranged from 0.66 to 0.81. The overall performance of the two LASSO models as assessed by five-fold cross-validation, is also reported in Table 3. The calibration intercept, which is an assessment of calibration-in-the-large, had a target value of 0, whereas the Cox slope slightly deviated from its target value of 1. In particular, for strategy 1, the model including the 9 ratios tended to produce risk estimates that were too moderate whereas, for strategy 2, the model including the 12 ratios produced estimates that were too extreme, that is, that were too high for women at high risk and too low for women at low risk. For both strategies, the Brier score suggested an absence of disagreement between the observed outcome and the prediction. Discrimination, as measured by the AUC, was 0.80 and 0.79 for the first and second strategy, respectively.
Table 3.
Performance of the selected miR ratios on small-RNA sequencing data.
| Strategy 1 | Strategy 2 | |
|---|---|---|
| AUC in the original sample | ||
| miR-335-5p_let-7f.-5p-2 | 0.76 | |
| miR-199a-3p-2_let-7a-5p-2 | 0.71 | |
| miR-199a-3p-2_let-7f.-5p-2 | 0.72 | |
| let-7a-5p-2_miR-22-3p | 0.81 | |
| let-7a-5p-2_miR-320a | 0.80 | |
| let-7f.-5p-1_miR-19b-3p-1 | 0.79 | |
| miR-27a-3p_miR-122-5p | 0.71 | |
| let-7f.-5p-2_miR-146a-5p | 0.78 | |
| miR-15b-5p_miR-16-5p-1 | 0.71 | |
| miR-26b-5p_miR-142-5p | 0.68 | |
| let-7a-5p-2_miR-106b-5p | 0.77 | |
| let-7f.-5p-1_miR-103a-1 | 0.76 | |
| let-7f.-5p-2_miR-103a-2 | 0.74 | |
| miR-93-5p_miR-19b-3p-1 | 0.69 | |
| miR-22-3p_miR-19b-3p-2 | 0.67 | |
| miR-101-3p-2_miR-19b-3p-1 | 0.68 | |
| miR-30d-5p_miR-20a-5p | 0.68 | |
| let-7b-5p_miR-19b-3p-1 | 0.74 | |
| miR-15a-5p_miR-16-5p-2 | 0.66 | |
| miR-20a-5p_miR-19b-3p-1 | 0.77 | |
| miR-21-5p_miR-23a-3p | 0.68 | |
| Performance of the strategies by fivefold cross-validation | ||
| Calibration intercept | 0.051 | 0.024 |
| Calibration slope | 1.269 | 0.840 |
| Scaled brier | 0.060 | 0.060 |
| AUC | 0.797 | 0.791 |
AUC: area under the ROC curve.
Cross-platform validation
By combining strategy 1 and strategy 2, a total of 20 ratios, which included 24 unique miRs, were further analysed by RT-qPCR on 130 samples (Fig. 1). One ratio (let-7f-5p-2_miR-103a-3p-2) was removed as miR-103a-3p-2 and miR-103a-3p-1, found in the let-7f-5p-1_miR-103a-3p-1 ratio, had identical counts in all but two samples (with negligible difference) and their ratio partners had identical mature miR sequences. In addition, one control sample had to be excluded from the RT-qPCR step due to insufficient plasma volume. Based on the median ratio values, small-RNA sequencing and RT-qPCR yielded overall concordant values in cases and controls. However, four ratios showed an opposite trend (Fig. S.1 in Additional file 2—comparing corresponding miR ratios between the two platforms). Seven ratios had a significantly positive Spearman rank correlation coefficient (p-value < 0.01) between the two platforms (miR-26b-5p_miR-142-5p, miR-101-3p_miR-19b-3p, let-7b-5p_miR-19b-3p, let-7f-5p_miR-19b-3p, let-7a-5p_miR-320a, miR-27a-3p_miR-122-5p, miR-199a-3p_let-7a-5p), with the coefficients ranging from 0.23 to 0.34 (Table S.2 in Additional file 1). Albeit not significantly correlated, 9 ratios had positive correlation coefficients < 0.20 and four had negative coefficients between the compared platforms. AUCs for each ratio as well as the univariable logistic regression results are reported in Table 4. Overall, the individual accuracy ranged from 0.48 to 0.65 and three ratios were associated with BC at a nominal 5% level of significance: miR-26b-5p_miR-142-5p, let-7a_miR-22-3p, and miR-199a-3p_let-7a-5p.
Table 4.
Results from standard univariable logistic regression and AUCs on RT-qPCR data.
| miR ratio | Expression in cases | Expression in controls | OR | (95% CI) | P | AUC | ||
|---|---|---|---|---|---|---|---|---|
| Median | [IQ range] | Median | [IQ range] | |||||
| miR-26b_miR-142-5p | 5.84 | [5.08–6.08] | 6.05 | [5.61–6.44] | 0.48 | (0.28–0.77) | 0.005 | 0.65 |
| let-7a_miR-22-3p | 5.48 | [3.54–7.81] | 6.94 | [4.08–9.89] | 0.85 | (0.73–0.98) | 0.026 | 0.63 |
| miR-199a-3p_let-7a-5p | 1.66 | [1.08–2.21] | 1.24 | [0.92–1.90] | 1.64 | (1.05–2.63) | 0.033 | 0.61 |
| miR-93-5p_miR-19b-3p | − 3.07 | [− 3.45 to − 2.79] | − 3.29 | [− 3.52 to − 3.04] | 2.05 | (1.00–4.50) | 0.059 | 0.61 |
| miR-199a-3p_let-7f.-5p | 3.65 | [2.97–4.15] | 3.20 | [2.74–3.93] | 1.41 | (0.97–2.08) | 0.077 | 0.58 |
| miR-15a-5p_miR-16-5p | − 19.10 | [− 20.43 to − 16.51] | − 17.41 | [− 18.50 to − 16.96] | 0.82 | (0.65–1.02) | 0.087 | 0.63 |
| miR-22-3p_miR-19b-3p | − 11.04 | [− 13.85 to − 9.74] | − 12.53 | [− 15.49 to − 10.03] | 1.12 | (0.98–1.30) | 0.113 | 0.60 |
| let-7b-5p_miR-19b-3p | − 3.19 | [− 3.61 to − 2.69] | − 2.83 | [− 3.38 to − 2.54] | 0.74 | (0.47–1.13) | 0.176 | 0.59 |
| let-7f.-5p_miR-146a-5p | − 7.82 | [− 8.60 to − 7.42] | − 7.57 | [− 8.41 to − 6.71] | 0.83 | (0.62–1.10) | 0.210 | 0.59 |
| miR-27a-3p_miR-122-5p | 0.78 | [− 0.75–2.06] | 0.65 | [− 0.80–1.48] | 1.12 | (0.93–1.36) | 0.241 | 0.54 |
| let-7a-5p_miR-106b-5p | − 0.17 | [− 0.68–0.38] | 0.07 | [− 0.36–0.70] | 0.78 | (0.50–1.20) | 0.270 | 0.59 |
| miR-15b-5p_miR-16-5p | − 10.13 | [− 10.92 to − 8.80] | − 10.12 | [− 10.88 to − 9.32] | 1.12 | (0.92–1.38) | 0.276 | 0.52 |
| miR-335-5p_let-7f.-5p | 1.44 | [0.78–2.27] | 1.03 | [0.46–2.03] | 1.18 | (0.86–1.64) | 0.319 | 0.58 |
| miR-20a-5p_miR-19b-3p | − 0.50 | [− 0.69 to − 0.14] | − 0.48 | [− 0.76 to − 0.21] | 0.78 | (0.45–1.28) | 0.351 | 0.48 |
| let-7f.-5p_miR-19b-3p | − 7.79 | [− 8.36 to − 7.08] | − 7.49 | [− 8.16 to − 6.66] | 0.87 | (0.64–1.16) | 0.355 | 0.57 |
| let-7f.-5p_miR-103a-3p | − 1.23 | [− 1.88 to − 0.79] | − 1.19 | [− 1.66 to − 0.82] | 0.86 | (0.61–1.20) | 0.390 | 0.53 |
| let-7a-5p_miR-320a | − 4.30 | [− 4.94 to − 3.49] | − 4.08 | [− 4.63 to − 3.16] | 0.88 | (0.65–1.17) | 0.399 | 0.57 |
| miR-30d-5p_miR-20a-5p | − 4.27 | [− 4.79 to − 4.04] | − 4.29 | [− 4.62 to − 4.03] | 0.97 | (0.63–1.50) | 0.905 | 0.50 |
| miR-21-5p_miR-23a-5p | 4.34 | [3.93–4.91] | 4.30 | [3.80–4.95] | 1.02 | (0.66–1.58) | 0.917 | 0.52 |
| miR-101-3p_miR-19b-3p | − 8.09 | [− 8.63 to − 7.65] | − 8.17 | [− 8.64 to − 7.69] | 0.99 | (0.70–1.41) | 0.969 | 0.49 |
OR odds ratio, CI confidence interval, AUC area under the ROC curve.
Identification of robust miR ratios for BC detection
To obtain the most promising miR ratios for BC detection and test their added value compared with other variables associated with BC (Table 1), three LASSO logistic regression models were fitted (see “Statistical analysis” section). The first model selected 6 ratios with non-zero coefficients: miR-199a-3p_let-7a-5p, miR-26b-5p_miR-142-5p, let-7b-5p_miR-19b-3p, miR-101-3p_miR-19b-3p, miR-93-5p_miR-19b-3p, let-7a-5p_miR-22-3p (Table 5). The corresponding AUC was 0.73 (95% CI 0.64–0.82); Youden’s optimal cut-off was 0.51 with corresponding sensitivity and specificity of 65.2% and 75.0%, respectively. The second model included the abovementioned ratios as well as miR-21-5p_miR-23a-3p, Tabar’s breast density classification and WCRF scores (as continuous variables), BMI (≥ 30 vs. < 30), menopausal status (yes vs. no) and an interaction term between the last two variables. Menopause and the interaction term were included due to the known different effects of BMI in pre- and post-menopausal women36. The AUC associated with this model was 0.79 (95% CI 0.71–0.87); the Youden’s cut-off was 0.50 and the associated sensitivity and specificity were 71.9% and 76.6%, respectively (Fig. 2). For comparison, the model including the abovementioned non-molecular factors only had an associated AUC of 0.72 (95% CI 0.63–0.81); at the optimal cut-off value of 0.51, sensitivity and specificity were 68.8% and 70.3%, respectively. The Delong test on the model with non-molecular variables and the model which additionally included the miR ratios revealed a significant difference between their AUCs (Z = − 2.0857, p = 0.03701). The other model comparisons yielded insignificant AUC differences.
Table 5.
Results from LASSO logistic regression for the risk of BC on RT-qPCR performed on the miR ratio only (Model 1) and also including non-molecular variables (Model 2).
| Model 1 | Model 2 | |
|---|---|---|
| Coefficients | Coefficients | |
| Intercept | 1.32 | 1.14 |
| miR-199a-3p_let.7a-5p | 0.16 | 0.16 |
| miR-26b-5p_miR-142-5p | − 0.16 | − 0.13 |
| let-7b-5p_miR-19b-3p | − 0.18 | − 0.27 |
| miR-101-3p_miR-19b-3p | − 0.07 | − 0.10 |
| miR-93-5p_miR-19b-3p | 0.47 | 0.57 |
| let-7a-5p_miR-22-3p | − 0.04 | − 0.05 |
| miR-21-5p_miR-23a-3p | 0.02 | |
| Menopausal status | − 0.03 | |
| BMI | 0.36 | |
| Menopausal status*BMI‡ | 0.15 | |
| WCRF/AICR score | − 0.16 | |
| TABAR | 0.27 |
TABAR tabàr’s breast density classification system, WCRF/AICR world cancer research fund/American institute for cancer research.
‡Interaction between menopausal status and BMI.
Figure 2.
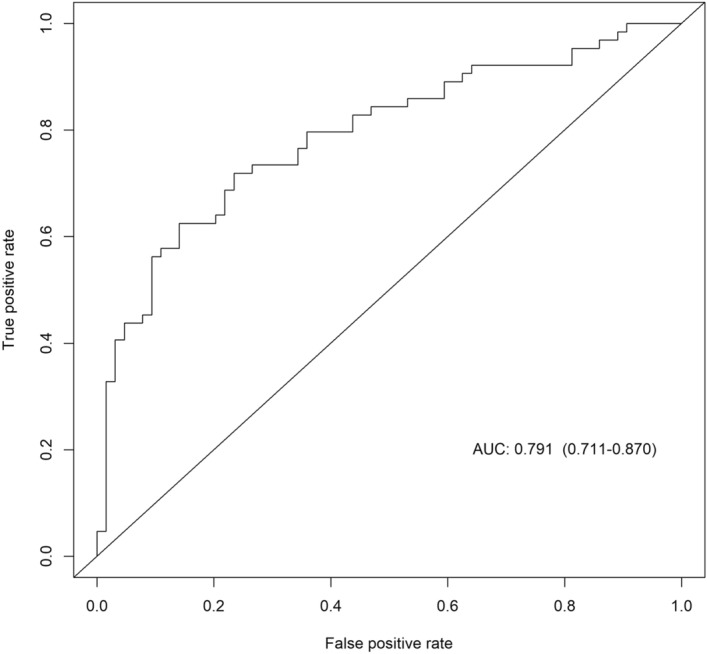
ROC curve of the multivariable model on 7 miR ratios and other non-molecular variables.
Five of the seven miR ratios in the final model had significant associations with clinicopathological characteristics based on the RT-qPCR data (Fig. S.2 in Additional S.2). Namely, miR-93-5p_miR-19b-3p was found to be lower in ER+ compared to ER− invasive BC patients (p = 0.037). miR-26b-5p_miR-142-5p was lower in ki-67+ compared to ki-67− invasive BCs (p = 0.048). Interestingly, miR-21-5p_miR-23a-3p was higher in ER+ than in ER− invasive BC patients (p = 0.030), in PgR+ versus PgR− (p = 0.036) as well as in ki-67− in contrast to ki-67+ BC invasive patients (p = 0.033). Lastly, let-7a-5p_miR-22-3p was lower in ductal compared to other BC histotypes (p = 0.050).
Target enrichment analysis results
Functional target enrichment analysis was performed on miRs making up the ratio signature in the model with non-molecular variables. Due to the software limitation of possible number of miRs in a single functional enrichment analysis, we excluded let-7b-5p as it has a very similar mature sequence and function to let-7a-5p, which was included in the analysis. Functional enrichment on the 10 miRs revealed their general involvement in cancer and breast cancer pathways, PI3K—Akt signaling pathway as well as the ATM-dependent DNA damage response (Wikipathways). Additionally, their targets were involved in androgen receptor signalling and EGF/EGFR signalling pathways (Supplementary Fig. S.3). Messenger RNAs of 12 genes were commonly targeted by at least 5 of the 10 analysed miRs, with the most targeted genes being the tumour suppressor phosphatase and tensin homolog (PTEN) (7 miRs) followed by Nuclear FMR1 Interacting Protein 2 (NUFIP2) (6 miRs).
Discussion
Mammography is currently the gold standard examination for BC screening, and organised population screening programs have significantly reduced BC mortality. However, BC screening has known limitations (i.e. false positives, overdiagnosis and interval cancers) that could be overcome with accurate non-invasive markers capable of complementing or tailoring mammographic screening37. Although they have been proposed for many different cancers, including BC, in diagnostic/prognostic contexts, to our knowledge, no studies focused on the potential role of circulating miRs in asymptomatic women undergoing general mammographic screening. In this study, we aimed to identify new circulating biomarkers associated with BC in plasma samples from a nested case–control study within a large cohort of women attending BC screening15. Importantly, BC status of cases and controls was not known before blood sampling as blood collection occurred before obtaining the first-level mammography result and none of the participants experienced any symptoms which might indicate BC. Small-RNA sequencing was first applied and, to facilitate subsequent validation by RT-qPCR (where no suitable normalisers are available) as well as cross-platform comparison, miR ratios in place of the individual miRs were considered throughout the analyses. A total of 20 ratios, made up of 24 unique miRs, were obtained as potential biomarker candidates based on the small-RNA sequencing data. The 24 miRs were then further tested with RT-qPCR on the same initial cohort. To assess the diagnostic ability of the candidate miR biomarkers and make a comparison to other non-molecular variables associated with BC in our cohort, three diagnostic models were built: a model on non-molecular variables only, a model on miR ratios only and a model with miR ratios and non-molecular variables combined. In the multivariable model which included five non-molecular variables, a signature of 7 miR ratios consisting of 11 unique miRs was identified. Four of the seven ratios were found to be associated with clinicopathological characteristics among cases, such as the ER or ki-67 status, implying a possibly direct function of the miRs making up the ratios in cancer formation and progression. The target enrichment analysis of the miRs making up the 7 ratios revealed that the genes they target are involved in cancer pathways including BC. Importantly, all 10 analysed miRs were enriched in the PI3-Akt signalling pathway, which is relevant to tumour progression and endocrine resistance in BC38. The genes commonly targeted by the majority of the 10 miRs were PTEN and NUFIP2. PTEN is a known tumour suppressor blocking the PI3K signalling39, while NUFIP2 is an RNA-binding protein40.
Five of the unique 11 miRs, from the model including miR ratios and non-molecular variables, were previously detected as potential diagnostic circulating biomarkers in other BC studies whose TNM stage distribution of cases also roughly matched the distribution of stages observed in BC screening programs41. The 5 miRs are: let-7a-5p42, miR-19b-3p43–45, let-7b-5p43,46, miR-93-5p43,47 and miR-21-5p48. Barring miR-19b-3p and miR-21-5p, the mentioned miRs are believed to be tumour suppressors or to have a protective role in BC tissue49–51. For instance, let-7a is believed to suppress BC cell migration by downregulating the CC chemokine receptor 752. Moreover, through IL-8 regulation, let-7b suppresses the cancer-promoting nature of BC-associated fibroblasts49. The diagnostic accuracy of the miR ratios (both alone and when combined with non-molecular variables) showed promising results with (AUCs of 0.73 and 0.79, respectively) which are comparable to what has been obtained in previous studies44,53–56. For example, Fang et al. 2019, which also utilised a plasma-based miR ratio model (5-ratios) by cross-platform validation on 131 samples, obtained a sensitivity and specificity of 71.7% and 78.2%, respectively56. The 5 ratios were made up of 7 unique miRs, of which none match the miRs in our final model. This might, in part, be due to the different reference populations or variations in experimental and analytical methods. For instance, unlike our study, Fang et al. 2019 did not calculate the pairwise ratios on the small-RNA sequencing data but only on RT-qPCR. Another study performed in 201544, conducted on a profiling (N = 86) and validation cohort (N = 196), reported an 8-miR model (miR-16, let-7d, miR-103, miR-107, miR-148a, let-7i, miR-19b, miR-22-5p) with a 91% sensitivity and 49% specificity and an AUC of 0.81. One of the miRs in their model, miR-19b, was included in three ratios obtained in our final models.
Among the majority of similar studies involving circulating cell-free miRs, controls usually come from healthy donors recruited in a separate setting from the cases, which were generally diagnosed before blood sampling57. Hence, the primary strength of our study is that all samples came from the screening setting and were prospectively sampled with the limitation of a relatively small sample size58. Additionally, most of the published studies on diagnostic cell-free circulating miRs were based on endogenous or exogenous miR normalisers10. However, an essential aspect of standardising the cell-free circulating miR analysis is the normalisation method10,59, and utilising miR ratio-based values is a good step forward to overcome the lack of optimal endogenous or exogenous normalisers28. Moreover, blood sampling before biopsy and before knowing the BC status might provide a higher chance of obtaining a real un-confounded circulating miR profile.
Conclusions
We identified candidate miR ratios which could assist, together with non-molecular parameters, in early BC detection in the setting of mammographic screening and can be measured through a widespread and low-cost technique. This is the first study reporting circulating miRs for BC detection in a screening setting. Considering the relatively small number of patients and lack of external validation, further evaluation of the presented miR biomarkers on a larger cohort is warranted.
Supplementary Information
Acknowledgements
We would like to thank the nurses, volunteers, administrative personnel, biologists and nutritionists involved in the ANDROMEDA project for their valuable contribution in the enrolment procedures. Our gratitude also goes to Professor Jaakko Kaprio, from the Institute for Molecular Medicine Finland (FIMM) at the University of Helsinki, for his precious suggestions and comments regarding the work. Finally, we would like to thank Dr. Alberto Costa for the initial spark leading to the conceptualisation of this study.
Abbreviations
- BC
Breast cancer
- SNP
Single nucleotide polymorphism
- miR
MicroRNA
- RT-qPCR
Quantitative reverse transcription polymerase chain reaction
- STROBE
The strengthening the reporting of observational studies in epidemiology
- BMI
Body mass index
- DM
Digital mammography
- WCRF
World cancer research fund
- AICR
American institute for cancer research
- BI-RADS
Breast imaging reporting and data system
- Ct
Cycle threshold
- LASSO
Least absolute shrinkage and selection operator
- AUC
Area under the ROC curve
- OR
Odds ratio
- CI
Confidence interval
- LOWESS
Locally weighted scatterplot smoothing
- sd
Standard deviation
- HRT
Hormone replacement therapy
- ER
Estrogen receptor
- PgR
Progesterone receptor
- Her2
Human epidermal growth factor receptor 2
Author contributions
The following authors were involved in Conceptualisation: G.C., N.S., L.G., E.P.; Data curation: G.C., E.P., E.S., V.V., A.O.; Formal analysis: P.O., E.P, E.S., E.R., E.C.; Funding acquisition: N.S., G.M., G.C.; Investigation: L.G.; Writing—original draft: G.C., E.P., E.S.; Writing—review: E.C., A.R., G.M., N.S, L.G.; Resources: G.C.; Methodology: E.B., T.V., I.G., M.M-G., E.S.; Project administration: Fe.G., Fr.G., L.G., G.C.; Software: A.O., E.P., E.S.; Supervision: G.C., L.G., N.S.; Validation: I.G., E.S.; Visualization: E.P., G.C., E.S. All authors read and approved the final manuscript.
Funding
The project was funded by an investigator grant from the Italian Association for Cancer Research (AIRC IG 2014 Ref No 15374) to N.S. and by the European Union’s Horizon 2020 Research and Innovation Programme, Marie Skłodowska-Curie (Grant Number 859860) to G.C. and E.S. G.M. was granted by the Ministero dell’Istruzione, dell’Università e della Ricerca–MIUR project “Dipartimenti di Eccellenza 2018–2022” (No. D15D18000410001) to the Department of Medical Sciences, University of Turin.
Data availability
The small-RNA sequencing data generated in this study, both raw and processed, has been deposited in the NCBI Gene Expression Omnibus public repository and is accessible through GEO Series accession number GSE210329 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210329). The RT-qPCR data is available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Giovanna Chiorino and Elisabetta Petracci.
These authors jointly supervised this work: Nereo Segnan and Livia Giordano.
Contributor Information
Emir Sehovic, Email: emir.sehovic@fondazionetempia.org.
Nereo Segnan, Email: nereo.segnan@cpo.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38886-0.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Heywang-Köbrunner SH, Hacker A, Sedlacek S. Advantages and disadvantages of mammography screening. Breast Care. 2011;6:2–2. doi: 10.1159/000329005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers ER, Moorman P, Gierisch JM, Havrilesky LJ, Grimm LJ, Ghate S, et al. Benefits and harms of breast cancer screening: A systematic review. JAMA. 2015;314:1615. doi: 10.1001/jama.2015.13183. [DOI] [PubMed] [Google Scholar]
- 4.Schünemann HJ, Lerda D, Quinn C, Follmann M, Alonso-Coello P, Rossi PG, et al. Breast cancer screening and diagnosis: A synopsis of the European breast guidelines. Ann. Intern. Med. 2020;172:46. doi: 10.7326/M19-2125. [DOI] [PubMed] [Google Scholar]
- 5.Allweis TM, Hermann N, Berenstein-Molho R, Guindy M. Personalized screening for breast cancer: Rationale, present practices, and future directions. Ann. Surg. Oncol. 2021;28:4306–4317. doi: 10.1245/s10434-020-09426-1. [DOI] [PubMed] [Google Scholar]
- 6.Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: A life-table model. JAMA Oncol. 2018;4:1504. doi: 10.1001/jamaoncol.2018.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am. J. Hum. Genet. 2019;104:21–34. doi: 10.1016/j.ajhg.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyng MB, Kodahl AR, Binder H, Ditzel HJ. Prospective validation of a blood-based 9-miRNA profile for early detection of breast cancer in a cohort of women examined by clinical mammography. Mol. Oncol. 2016;10:1621–1626. doi: 10.1016/j.molonc.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019;234:5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal V, Priyanka K, Tuli HS. Emergence of circulating MicroRNAs in breast cancer as diagnostic and therapeutic efficacy biomarkers. Mol. Diagn. Ther. 2020;24:153–173. doi: 10.1007/s40291-020-00447-w. [DOI] [PubMed] [Google Scholar]
- 11.Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA Gene Expression Deregulation in Human Breast Cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—The mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Bai X, Zeng X, Liu J, Liu F, Zhang Z. circRNA-miRNA-mRNA in breast cancer. Clin. Chim. Acta. 2021;523:120–130. doi: 10.1016/j.cca.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007;147:573. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 15.The Andromeda working group. Giordano L, Gallo F, Petracci E, Chiorino G, Segnan N. The ANDROMEDA prospective cohort study: predictive value of combined criteria to tailor breast cancer screening and new opportunities from circulating markers: study protocol. BMC Cancer. 2017;17:785. doi: 10.1186/s12885-017-3784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura L, Giorgi D, Giordano L, Frigerio A, Mantellini P, Zappa M, et al. Mammographic breast cancer screening in Italy: 2011–2012 survey. Epidemiol. Prev. 2015;39(3 Suppl 1):21–29. [PubMed] [Google Scholar]
- 17.Romaguera D, Vergnaud A-C, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with world cancer research fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am. J. Clin. Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 18.Karavasiloglou N, Hüsing A, Masala G, van Gils CH, Turzanski Fortner R, Chang-Claude J, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and risk of in situ breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. BMC Med. 2019;17:221. doi: 10.1186/s12916-019-1444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petracci E, Decarli A, Schairer C, Pfeiffer RM, Pee D, Masala G, et al. Risk factor modification and projections of absolute breast cancer risk. JNCI J. Natl. Cancer Inst. 2011;103:1037–1048. doi: 10.1093/jnci/djr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS) Radiol. Clin. North Am. 2002;40:409–430. doi: 10.1016/S0033-8389(01)00017-3. [DOI] [PubMed] [Google Scholar]
- 22.Gram IT, Funkhouser E, Tabár L. The Tabár classification of mammographic parenchymal patterns. Eur. J. Radiol. 1997;24:131–136. doi: 10.1016/S0720-048X(96)01138-2. [DOI] [PubMed] [Google Scholar]
- 23.Mavaddat N, Pharoah PDP, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. Prediction of breast cancer risk based on profiling with common genetic variants. JNCI J. Natl. Cancer Inst. 2015;107:djv036. doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mello-Grand M, Gregnanin I, Sacchetto L, Ostano P, Zitella A, Bottoni G, et al. Circulating microRNAs combined with PSA for accurate and non-invasive prostate cancer detection. Carcinogenesis. 2019;40:246–253. doi: 10.1093/carcin/bgy167. [DOI] [PubMed] [Google Scholar]
- 25.Hill AF, editor. Exosomes and Microvesicles: Methods and Protocols. Springer; 2017. [Google Scholar]
- 26.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Smyth GK, Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Zhu Y, Wang H, Khadka VS, Hu L, Ai J, et al. Ratio-based method to identify true biomarkers by normalizing circulating ncRNA sequencing and quantitative PCR data. Anal. Chem. 2019;91:6746–6753. doi: 10.1021/acs.analchem.9b00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibshirani R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996;58:267–288. [Google Scholar]
- 30.Cox DR. Two further applications of a model for binary regression. Biometrika. 1958;45:562–565. doi: 10.1093/biomet/45.3-4.562. [DOI] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing (2021).
- 32.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: Visualizing classifier performance in R. Bioinformatics. 2005;21:3940–3941. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 34.López-Ratón M, Rodríguez-Álvarez MX, Suárez CC, Sampedro FG. OptimalCutpoints: An R package for selecting optimal cutpoints in diagnostic tests. J. Stat. Softw. 2014;61:1–36. doi: 10.18637/jss.v061.i08. [DOI] [Google Scholar]
- 35.Licursi V, Conte F, Fiscon G, Paci P. MIENTURNET: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019;20:545. doi: 10.1186/s12859-019-3105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Liu L, Zhou Q, Imam MU, Cai J, Wang Y, et al. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. 2017;17:936. doi: 10.1186/s12889-017-4953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: Implications for personalised screening. Br. J. Cancer. 2011;104:1656–1663. doi: 10.1038/bjc.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014;6:154–166. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller TW, Pérez-Torres M, Narasanna A, Guix M, Stål O, Pérez-Tenorio G, et al. Loss of phosphatase and tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bardoni B, Castets M, Huot M-E, Schenck A, Adinolfi S, Corbin F, et al. 82-FIP, a novel FMRP (fragile X mental retardation protein) interacting protein, shows a cell cycle-dependent intracellular localization. Hum. Mol. Genet. 2003;12:1689–1698. doi: 10.1093/hmg/ddg181. [DOI] [PubMed] [Google Scholar]
- 41.Toss A, Isca C, Venturelli M, Nasso C, Ficarra G, Bellelli V, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6:100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S, Luo Q, Peng H, Li J, Zhao M, Wang J, et al. A panel of serum noncoding RNAs for the diagnosis and monitoring of response to therapy in patients with breast cancer. Med. Sci. Monit. 2018;24:2476–2488. doi: 10.12659/MSM.909453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou X, Xia T, Li M, Wang T, Liu P, Zhou X, et al. MicroRNA profiling in serum: Potential signatures for breast cancer diagnosis. CBM. 2021;30:41–53. doi: 10.3233/CBM-201547. [DOI] [PubMed] [Google Scholar]
- 44.Frères P, Wenric S, Boukerroucha M, Fasquelle C, Thiry J, Bovy N, et al. Circulating microRNA-based screening tool for breast cancer. Oncotarget. 2016;7:5416–5428. doi: 10.18632/oncotarget.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Zhou Y, Xia T, Zhou X, Huang Z, Zhang H, et al. Circulating microRNAs from the miR-106a–363 cluster on chromosome X as novel diagnostic biomarkers for breast cancer. Breast Cancer Res. Treat. 2018;170:257–270. doi: 10.1007/s10549-018-4757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li M, Zou X, Xia T, Wang T, Liu P, Zhou X, et al. A five-miRNA panel in plasma was identified for breast cancer diagnosis. Cancer Med. 2019;8:7006–7017. doi: 10.1002/cam4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K, Schwarzenbach H. Deregulated serum concentrations of circulating cell-free MicroRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin. Chem. 2013;59:1489–1496. doi: 10.1373/clinchem.2013.205161. [DOI] [PubMed] [Google Scholar]
- 48.Yu X, Liang J, Xu J, Li X, Xing S, Li H, et al. Identification and validation of circulating MicroRNA signatures for breast cancer early detection based on large scale tissue-derived data. J. Breast Cancer. 2018;21:363. doi: 10.4048/jbc.2018.21.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Harbi B, Hendrayani S-F, Silva G, Aboussekhra A. Let-7b inhibits cancer-promoting effects of breast cancer-associated fibroblasts through IL-8 repression. Oncotarget. 2018;9:17825–17838. doi: 10.18632/oncotarget.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang Y, Liao X-H, Yu C-X, Yao A, Qin H, Li J-P, et al. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp. Cell Res. 2017;357:135–144. doi: 10.1016/j.yexcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Liu K, Zhang C, Li T, Ding Y, Tu T, Zhou F, et al. Let-7a inhibits growth and migration of breast cancer cells by targeting HMGA1. Int. J. Oncol. 2015;46:2526–2534. doi: 10.3892/ijo.2015.2949. [DOI] [PubMed] [Google Scholar]
- 52.Kim S-J, Shin J-Y, Lee K-D, Bae Y-K, Sung KW, Nam SJ, et al. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012;14:R14. doi: 10.1186/bcr3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heydari N, Nikbakhsh N, Sadeghi F, Farnoush N, Khafri S, Bastami M, et al. Overexpression of serum MicroRNA-140-3p in premenopausal women with newly diagnosed breast cancer. Gene. 2018;655:25–29. doi: 10.1016/j.gene.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Wang S, Cao X, Liu J. Analysis of circulating microRNA biomarkers for breast cancer detection: A meta-analysis. Tumor Biol. 2014;35:12245–12253. doi: 10.1007/s13277-014-2533-5. [DOI] [PubMed] [Google Scholar]
- 55.Peña-Cano MI, Saucedo R, Morales-Avila E, Valencia J, Zavala-Moha JA, López A. Deregulated microRNAs and adiponectin in postmenopausal women with breast cancer. Gynecol. Obstet. Invest. 2019;84:369–377. doi: 10.1159/000496340. [DOI] [PubMed] [Google Scholar]
- 56.Fang R, Zhu Y, Hu L, Khadka VS, Ai J, Zou H, et al. Plasma MicroRNA pair panels as novel biomarkers for detection of early stage breast cancer. Front. Physiol. 2019;9:1879. doi: 10.3389/fphys.2018.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sehovic E, Urru S, Chiorino G, Doebler P. Meta-analysis of diagnostic cell-free circulating microRNAs for breast cancer detection. BMC Cancer. 2022;22:634. doi: 10.1186/s12885-022-09698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Calster B, Van Smeden M, De Cock B, Steyerberg EW. Regression shrinkage methods for clinical prediction models do not guarantee improved performance: Simulation study. Stat. Methods Med. Res. 2020;29:3166–3178. doi: 10.1177/0962280220921415. [DOI] [PubMed] [Google Scholar]
- 59.Lee I, Baxter D, Lee MY, Scherler K, Wang K. The importance of standardization on analyzing circulating RNA. Mol. Diagn. Ther. 2017;21:259–268. doi: 10.1007/s40291-016-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The small-RNA sequencing data generated in this study, both raw and processed, has been deposited in the NCBI Gene Expression Omnibus public repository and is accessible through GEO Series accession number GSE210329 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE210329). The RT-qPCR data is available upon request from the corresponding author.