Abstract
Ferroptosis, a programmed cell death, has been identified and associated with cancer and various other diseases. Ferroptosis is defined as a reactive oxygen species (ROS)-dependent cell death related to iron accumulation and lipid peroxidation, which is different from apoptosis, necrosis, autophagy, and other forms of cell death. However, accumulating evidence has revealed a link between autophagy and ferroptosis at the molecular level and has suggested that autophagy is involved in regulating the accumulation of iron-dependent lipid peroxidation and ROS during ferroptosis. Understanding the roles and pathophysiological processes of autophagy during ferroptosis may provide effective strategies for the treatment of ferroptosis-related diseases. In this review, we summarize the current knowledge regarding the regulatory mechanisms underlying ferroptosis, including iron and lipid metabolism, and its association with the autophagy pathway. In addition, we discuss the contribution of autophagy to ferroptosis and elucidate the role of autophagy as a ferroptosis enhancer during ROS-dependent ferroptosis.
Subject terms: Autophagy, Stress signalling, Cancer, Cell death, Mitophagy
Facts
Ferroptosis is a non-apoptotic form of regulated cell death.
Ferroptosis is dependent on iron accumulation and ROS generation.
Oxidative stress contributes to ferroptosis.
Questions
Does autophagy contribute to ferroptosis?
What are the molecular mechanisms underlying the regulation of ferroptosis by autophagy?
How do ROS and iron accumulation increase ferroptosis on a molecular level?
Ferroptosis
Cell death is critical in the development of multiple human diseases and is closely linked to biological growth. Ferroptosis is a new type of cell death discovered in the past decade. Initially, ferroptosis has been discovered as a novel iron-dependent form of non-apoptotic regulated cell death. Ferroptosis is associated with the accumulation of iron, ROS, lipid peroxidation, and insufficient capacity to eliminate lipid peroxidation [1–4]. Moreover, ferroptosis is closely associated with the pathophysiology of several diseases including tumors [2], degenerative diseases [5], ischemia-reperfusion injury [6], kidney injury [1], and blood disorder [7], and has a tumor suppressor function that can be used in anti-cancer [2, 8]. Particularly, studies have shown that targeting ferroptosis could be a potential strategy in different cancer cell types. A study has shown that AML cells with mutations in the IDH1 or IDH2 genes are particularly sensitive to ferroptosis-inducing agents and that this sensitivity is mediated by the accumulation of the oncometabolite 2-hydroxyglutarate (2-HG) [9]. In addition, inhibiting ferroptosis in liver cancer cells promoted tumor growth and metastasis, while inducing ferroptosis inhibited tumor growth and reduced metastasis [2]. Another study reported that ferroptosis is involved in the regulation of tumor immune response in melanoma, a type of skin cancer [10].
Ferroptosis pharmacological modulation has emerged as a research focus for the treatment and prognosis of related diseases. While ferroptosis has been shown to have anti-tumor effects in some contexts, it can also promote tumor growth and survival in other contexts. As an anti-tumor effects, a classical ferroptosis inducer is associated with the System Xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, which plays a central role in the regulation of the antioxidant system and lipid peroxidation [11, 12]. In addition to oncogenic RAS (Rat sarcoma virus oncogene)-selective lethal small molecules, erastin, RAS-selective-lethal compound 3 (RSL3), and other chemical compounds, such as sulfasalazine, sorafenib, artemisinin, and 1, 2-dioxolane (FINO2), have been confirmed to induce ferroptosis [11, 13]. For example, in certain subtypes of cancer, such as glioblastoma and acute myeloid leukemia (AML), the induction of ferroptosis has been shown to have anti-tumor effects [14].
As opposite effects on tumor cell survival and growth, ferroptosis inhibitors may be used as treatment strategies in certain types of diseases with enhanced ferroptosis. Ferroptosis has been shown to promote tumor growth and survival in cancers such as pancreatic cancer [15]. In these cases, the use of ferroptosis inhibitors could potentially be a viable adjuvant treatment strategy to inhibit tumor growth. Targeting the key enzymes and molecules involved in the ferroptosis process is one potential approach to developing ferroptosis inhibitors for cancer treatment. For example, inhibitors of the lipid peroxidation enzyme, 15-lipoxygenase (15-LOX), have been shown to inhibit ferroptosis in cancer cells, and may have therapeutic potential for certain types of cancer [16].
Initially, the oncogenic RAS-selective lethal small molecule erastin-induced ferroptosis was described as autophagy-independent cell death in fibrosarcoma HT-1080 cells [17]. However, studies have demonstrated that autophagy plays a role in regulating iron content, ROS congestion, and lipid peroxidation during ferroptotic cell death [18, 19]. Thus, understanding autophagy and ferroptosis regulation could help treat tumors, inflammatory diseases, and fibrosis. Despite increasing evidence, the role and pathophysiology of autophagy during ferroptosis remain unclear [20]. In this review, we provide an overview of autophagy and ferroptosis and a comprehensive review of the molecular mechanisms of autophagy-dependent ferroptosis and its regulation.
Mechanisms of iron accumulation during ferroptosis
Iron is an essential component of biological systems and exists in two biologically relevant oxidation states, ferric iron (Fe3+) and ferrous iron (Fe2+), and functions as a cofactor for several proteins and enzymes that can readily undergo redox cycling. Specifically, excessive amounts of iron initiate several cytotoxic mechanisms that disrupt redox homeostasis and cause cell death, including ferroptosis [21].
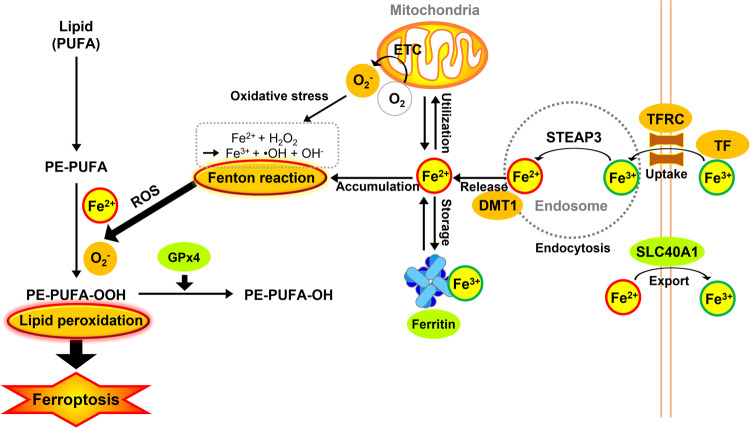
As iron regulates ROS production and enzyme activity in lipid peroxidation, iron homeostasis maintenance via iron metabolism, including iron uptake, storage, utilization, and export, provides a unified network to determine ferroptosis sensitivity [12]. Most biological iron is derived from nutritional sources and hemoglobin, a red blood cell component, and is directly absorbed into the bloodstream through intestinal mucosal cells, and is partly stored in ferritin [22]. Transferrin (TF), delivered as a glycoprotein, binds to Fe3+ and transports it through the blood to various tissues, such as the liver, spleen, and bone marrow. TF-bound ferric iron from the serum is recognized by the transferrin receptor (TFRC/TfR1) followed by internalization of the TF-TFRC complex by receptor-mediated endocytosis (Fig. 1). Increased iron uptake by TFRC overexpression enhances sensitivity to ferroptosis, and TFRC knockdown ameliorates erastin-induced ferroptosis. Thus, TFRC may serve as a biomarker of ferroptosis sensitivity [12, 22].
Fig. 1. Molecular metabolism of iron in ferroptosis.
During the process of ferroptosis induction, the increase of cytoplasmic instability of iron is observed. The overall process of iron metabolism, including iron uptake, storage, utilization, and efflux is involved in the regulation of ferroptosis. Fe3+ forms a complex with circulating transferrin (TF) and then, a Fe3+-TF complex binds to the transferrin receptor (TFR) and is transported inside the cell to the endosome. After endocytosis, Fe3+ is detached from TF and converted to Fe2+ via redox reaction by STEAP3 and then exported into the cytosol by DMT-1. Unbound Fe2+ in the cytosol can be transported to the mitochondrion and labile iron are stored by ferritin. Excess Fe2+ is exported out of the cell through SLC40A1, known as ferroportin-1 maintaining systemic iron homeostasis. Labile iron displays high chemical reactivity and cytotoxic potential. Fe2+ plays an important role in the development of ferroptosis via enzymatic lipid peroxidation by iron-containing enzymes, such as ALOXs or nonenzymatic lipid peroxidation by Fenton reaction catalyzing the formation of hydroxyl radicals (OH·) [25, 118].
After uptake by TFRC, endosomal iron is released from TF and is reduced from Fe3+ to Fe2+ by STEAP3 metalloreductase [23]. Iron is then released from the endosomal compartment into the cytoplasm by the divalent metal transporter DMT1 (DCT1, Nramp2, SLC11A2; solute carrier family 11 member 2). DMT1 (SLC11A2) sensitizes cells to ferroptosis by increasing cytosolic iron levels [24].
Unbound cytosolic Fe2+ can be transported to the mitochondria or form a labile iron pool that is stored by ferritin. Fe2+ regulates multiple processes, such as oxygen transport and iron-sulfur (Fe-S) assembly in mitochondria [22, 25]. Ferritin takes up labile Fe2+ and stores it in a stable unreactive Fe3+-oxide/hydroxide form [26]. Ferritin consists of two subunits, heavy chain 1 (FTH1) and light chain (FTL). Ferritin degradation can occur via ferritinophagy, a selective autophagy of ferritin, and is a prerequisite for iron release. Therefore, reducing iron storage via the knockdown of ferritin protein or induction of ferritinophagy increases ferroptosis [27]. The efflux of intracellular Fe2+ across the cell membrane requires the iron efflux pump ferroportin-1 (SLC40A1). Iron efflux by SLC40A1 alleviates iron overload and decreases ferroptosis [28].
Excess Fe2+ accumulates and reacts with hydrogen peroxide (H2O2) via the Fenton reaction, a metal-catalyzed reduction of hydrogen peroxide, to form highly toxic hydroxyl free radicals (•OH). Fe2+ acts as a catalyst, facilitating the conversion of H2O2 into •OH and hydroxide ions (OH-) [26, 29, 30]. The generated •OH can bring about damage to DNA, proteins, lipid membranes, and other biomolecules [26, 31].
Molecular mechanisms of lipid peroxidation during ferroptosis
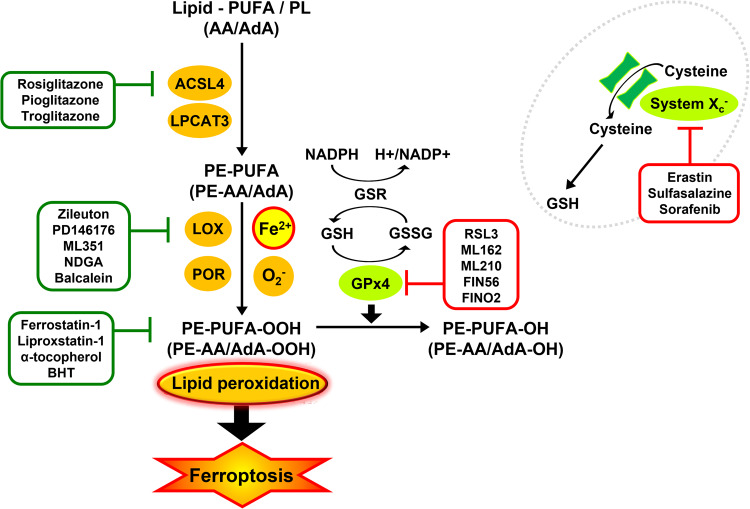
Excessive lipid peroxidation leads to cell death through ferroptosis. Lipid peroxidation is the process to generate lipid hydroperoxides (LOOHs) through which intermediate of peroxyl radicals formatted by ROS combines with lipids (ex-PUFA/PL;phospholipid) [32]. During ferroptosis, Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) take charge of most PUFAs production and promote the esterification of PUFA (AA and AdA) into phosphatidylethanolamine (PE) [33, 34]. Thus, ACSL4 inhibitors such as rosiglitazone, pioglitazone, and troglitazone decrease ferroptosis by regulating cellular sensitivity [32, 35].
Among biological membrane phospholipids, phosphatidylethanolamine (PE) and phosphatidylcholine (PC) including arachidonic acid (AA)- and adrenic acid (AdA) are principal targets for lipid peroxidation [33]. The peroxidation of PE-AA/AdA is enzymatically catalyzed by lipoxygenases (LOXs) and cytochrome P450 oxidoreductase (POR), or non-enzymatically by cellular free iron via the Fenton reaction [33, 36]. LOX inhibitors, such as Zileuton, PD146176, ML351, and NDGA, inhibit ferroptosis (Fig. 2) [37].
Fig. 2. Summary of lipid peroxidation mechanisms and signaling pathway.
The pathway regulating sensitivity during the generation of lipid peroxidation. PUFA is a double-sided, and its peroxidation contributes to cell damage. The production of PUFA derivatives requires the activation of the ACSL4-LPCAT3 enzymes pathway. PE-PUFA oxidation could occur either enzymatically via the action of Lox, or non-enzymatically via autoxidation to form PE-PUFA-OOH, both of which after all trigger ferroptosis. ROS produced by the Fenton reaction directly promotes lipid peroxidation and iron may increase the activity of ALOX, which are iron-containing enzymes. The toxic phospholipid hydroperoxides (R-OOHs) are detoxified to nontoxic phospholipid alcohol (R-OH) by GPX4 by consuming two glutathione (GSH) molecules as an electron donor. The ferroptosis inhibitors are shown in Green line, while the ferroptosis inducers are shown in Red line [48, 52].
Dysregulation of membrane lipid peroxidation is induced by loss of the lipid detoxification enzymatic GPX4, and subsequent accumulation of lipid-based ROS, particularly lipid hydroperoxides [3, 38]. The GSH/GPX4 axis is the most often targeted pathway for causing ferroptosis [32]. GPX4 reduces lipid peroxide (PE-AA/AdA-OOH) to lipid alcohol (PE-AA/AdA-OH) by oxidizing the GSH synthesized from cysteine, thereby defending cells from ferroptosis under normal conditions. Loss of GPX4 activity or depletion of GSH and inhibition of cysteine uptake leads to increased lipid peroxidation and ferroptosis [33]. Thus, GPX4 inhibitors, such as RSL3, ML162, ML210, FIN56, and FINO2 are classic ferroptosis inducers [37]. Furthermore, inhibitors of system Xc− as cystine transporters, such as erastin, sulfasalazine, and sorafenib, trigger ferroptosis [39]. Moreover, lipid peroxide (R-OOH) is suppressed by scavengers of lipid peroxidation involving ferrostatin-1, liproxstatin-1, Vitamin E (α-tocopherol), butylated hydroxytoluene (BHT) (Fig. 2) [40].
Molecular mechanisms of oxidative stress induction and ferroptosis
Ferroptosis is an iron-dependent oxidative cell death pathway characterized by the oxidative modification of phospholipid membranes via lipid peroxidation caused by ROS from the Fenton reaction [41, 42]. Iron-dependent oxidative stress can damage lipids and exacerbate lipid peroxidation [41, 43, 44]. Extensive oxidation of PUFA-containing phospholipids alters the membrane’s phase structure and permeability, resulting in plasma membrane rupture in response to lipid-ROS accumulation, such as lipid hydroperoxides [45].
Three main sources of lipid peroxidation during ferroptosis are hypothesized: (1) Fenton reaction-induced ROS caused by elevated intracellular iron levels increase iron-dependent oxidative stress and subsequent lipid peroxidation, (2) Mitochondrial iron accumulation and mtROS involved in lipid peroxidation, and (3) Antioxidant defense deficiency leading to lipid peroxidation [37, 42, 46].
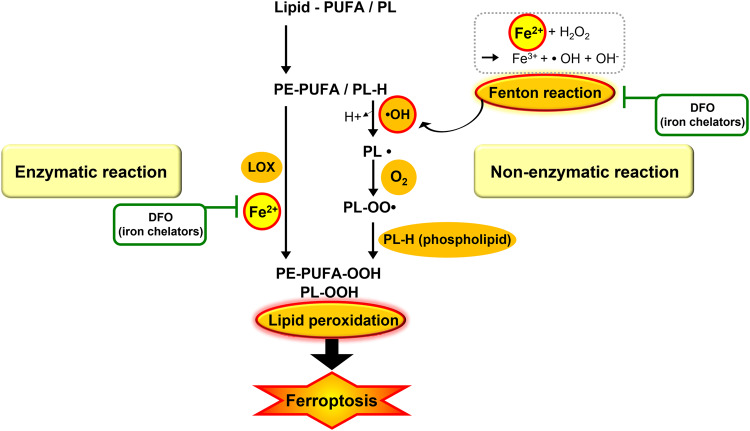
Abnormal iron metabolism may induce ferroptosis in at least two ways. First, ROS generation is mediated by iron via the Fenton reaction, and second, the activation of iron-containing enzymes, such as LOX (Fig. 3) [37]. Through the Fenton reaction, free iron reacts with H2O2, producing hydroxyl radicals, the most reactive radicals. The hydroxyl radical can nonenzymatically mediate the peroxidation of PE-FA. Hydroxyl radicals take hydrogen from PE-PUFAs or phospholipids to form a phospholipid carbon-centered radical. Subsequent oxygen addition to the peroxyl radical yields phospholipid hydroperoxide, which can promote ferroptosis [41, 47].
Fig. 3. The involvement of iron in enzymatic or non-enzymatic lipid peroxidation.
Labile iron has high chemical reactivity and cytotoxic potential. Fenton reaction as an oxidation process between Fe2+ and H2O2, is related to the non-enzymatic lipid peroxidation resulting in forming of a lipid radical (L•) via subtracting hydrogen (H) via providing hydroxyl radicals (OH·) to lipid (L-H). lipid radical (L•) binds to O2, thereby forming lipid peroxyl radical (LOO•), subsequently combine with hydrogen (H) exroted from nearby PUFA and form lipid hydroperoxides (LOOH) and a new lipid radical and triggers another oxidation reaction [48]. Phosphatidylethanolamines (PE); phospholipid (PL-H); phospholipid alkoxyl radical (PL-O·); phospholipid peroxyl radical (PL-OO·); phospholipid hydroperoxide (PL-OOH).
Furthermore, iron as a cofactor of LOX contributes to enzyme-mediated peroxidation [42, 48]. It should be noted that both cellular lipid hydroperoxides regulated by the LOXs enzyme and autoxidized peroxyl radical-mediated lipid peroxidation may promote ferroptosis initiation. Therefore, accumulated intracellular iron triggers ferroptosis, while iron chelators such as deferoxamine (DFO) reduce free radical production and delay ferroptosis (Fig. 3) [41, 42].
Unbound cytosolic Fe2+ can be taken up by mitochondria (Fig. 1), which use a consistent amount of cellular iron as a cofactor for several proteins involved in redox reactions of the respiratory chain [21]. Mitochondria have been proposed to regulate ferroptotic signaling pathways through control of ATP synthesis, ROS production, iron homeostasis, and redox status. Recent studies have demonstrated that several mitochondrial proteins, such as CDGSH iron sulfur domain 1 (CISD1) and Frataxin (FXN), also regulate ferroptotic cell death by mediating iron uptake and lipid peroxidation [21, 49].
GSH, a non-enzymatic antioxidant, and GPX4, a GSH-dependent antioxidant enzyme GSH peroxidase 4, play an important role in protecting cells against various oxidative stress. GPX4 converts GSH into GSSG and reduces cytotoxic lipid peroxides to their corresponding lipid alcohols. Loss of GPX4 activity and GSH depletion cause an imbalance in cellular antioxidant system, leading to the accumulation of lipid peroxides, which is a key feature of ferroptosis (Fig. 2) [2, 50].
Autophagy in ferroptosis
Autophagy is an evolutionarily conserved self-degradative process to maintain cellular homeostasis, mediated by the autophagosome as a double layer-membrane structure that undergoes maturation by fusing with lysosomes for degradation [51, 52]. Recent studies have suggested that autophagy plays an essential role in ferroptosis [20, 51, 53]. In normal cells, autophagy acts as a protective mechanism against the accumulation of damaged proteins and organelles, which can lead to cancer development. However, in some cases, autophagy can promote cancer cell survival and growth by providing them with the necessary nutrients and energy they require to maintain their metabolic requirements. Several studies have shown that autophagy plays a complex role in different subtypes of cancer. For example, a study found that autophagy promotes the survival of pancreatic cancer cells by protecting them from cell death induced by chemotherapy drugs [54]. Another study discovered that autophagy promotes the survival and metastasis of breast cancer cells by enhancing their ability to invade and migrate to other tissues [55]. Particularly, the relationship between autophagy and ferroptosis in cancer cells is complex and context-dependent. Autophagy can promote ferroptosis in cancer cells by degrading and recycling iron-storage proteins, such as ferritin, which can release iron and promote lipid peroxidation and oxidative stress. A study found that inducing autophagy in cancer cells increased the sensitivity of these cells to ferroptosis-inducing agents, such as erastin and RSL3 [11]. In contrast, autophagy can also inhibit ferroptosis in cancer cells by removing damaged or oxidized lipids that can trigger ferroptosis. A previous study reported that inhibiting autophagy in liver cancer cells increased the accumulation of oxidized lipids and enhanced their resistance to ferroptosis [56]. Therefore, the effect of autophagy on ferroptosis in cancer cells depends on the specific context and conditions of the tumor microenvironment.
There is evidence that certain gene mutations in cancer can affect the sensitivity to ferroptosis. For example, mutations in the Kras oncogene have been shown to promote resistance to ferroptosis in lung cancer cells [57]. Mutations that activate the PI3K/Akt/mTOR pathway, which is involved in cell survival and proliferation, have been associated with resistance to ferroptosis. For example, mutations in the PIK3CA gene, which encodes the p110α subunit of PI3K, have been shown to promote resistance to ferroptosis in breast cancer cells [58]. In contrast, mutations that activate the tumor suppressor gene p53 or its downstream targets, such as the BCL-2 family of proteins, have been associated with enhanced sensitivity to ferroptosis. For example, a previous study discovered that loss of the tumor suppressor gene LKB1, which leads to activation of the AMPK pathway and inhibition of mTOR signaling, enhances sensitivity to ferroptosis in lung cancer cells [59]. The role of autophagy in these cancers appears to be complex and context-dependent. Autophagy can promote either cell survival or cell death in response to ferroptosis-inducing agents, depending on the specific context. For example, a study reported that autophagy inhibition enhanced sensitivity to ferroptosis in pancreatic cancer cells, while a study showed that autophagy inhibition promoted ferroptosis-induced cell death in KRAS-mutant lung cancer cells [60]. Collectively, the interplay between gene mutations, ferroptosis, and autophagy in cancer is complex and requires further investigation.
However, the mechanisms of autophagy-dependent ferroptosis remain unknown, and further investigation of the mediators of autophagy induction during ferroptosis is required [20]. Understanding the relationship between ferroptosis and autophagy will help develop novel treatments for numerous diseases. Here, we outline the key role of the autophagy machinery in the promotion of lipid peroxidation and iron accumulation.
The role of autophagy in promoting iron accumulation
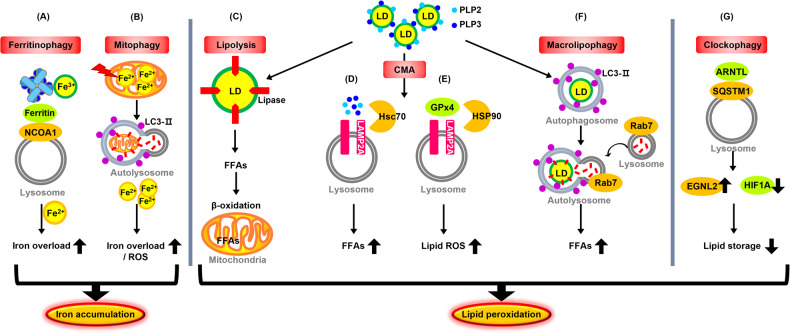
Previous studies have shown that excessive autophagy and lysosomal activity can promote ferroptotic cell death through iron accumulation. Ferritinophagy, the process of autophagic degradation of the iron-storage protein ferritin, reduces iron storage and promotes cellular iron accumulation through the release of free iron. Thus, ferritinophagy causes oxidative injury via the Fenton reaction and induces ferroptosis. Nuclear receptor coactivator 4 (NCOA4) as a selective cargo receptor responsible for autophagic ferritin degradation binds to ferritin through its C-terminal domain and delivers it to the nascent autophagosome [22, 61, 62].
However, the role of mitophagy, selective autophagy of mitochondria, in ferroptosis is complicated [63, 64]. During the early stages of iron overload, a significant amount of released iron is transported to the mitochondria as a buffer, and mitophagy may segregate iron into the mitophagosomes and decrease the origin of ROS for ferroptosis [65, 66]. However, mitochondrial damage by excessive iron overload induces further mitophagy, providing an additional source of iron for lipid peroxidation [67]. Eventually, extensive mitophagy releases iron, ROS, and peroxidated lipids from mitochondria at toxic levels, thereby activating various ROS-induced cell death pathways, including ferroptosis [37, 68].
Inhibition of the mitochondrial respiratory chain complex I by BAY 87-2243 induces the opening of mPTP and a loss of mitochondrial membrane potential (Δψ), thereby leading to the activation of ferroptosis via ROS increase by stimulated mitophagy [69, 70], while the knockdown of PINK1 inhibits BAY-induced ferroptosis [68]. Although several studies on mitophagy and ferroptosis are ongoing, little is known about how mitophagy-related mitochondrial proteins regulate ferroptosis, so further research is needed [49, 67, 71, 72].
Molecular mechanisms and the role of autophagy in promoting lipid peroxidation
The consumption of fat and lipids have been shown to impact autophagy and ferroptosis regulation in different diseases, especially in prostate and liver cancer cells [2, 73]. High-fat diets have been shown in their studies to decrease autophagy and increase susceptibility to ferroptosis in some types of cancer cells, which may contribute to cancer development and progression.
Specifically, de novo-synthesized fatty acids are stored in lipid droplets as storage sites for neutral lipids and in phospholipids in the membrane. Lipid droplets (LDs) act as potential ROS scavengers and inhibitors of PUFA oxidation [74–76]. Free fatty acids (FFAs) released by the breakdown of LDs generally serve as fuel for mitochondrial fatty acid β-oxidation for energy production and increase the peroxidation of PUFA and ferroptosis [77]. Therefore, LDs are emerging as modulators of lipid peroxidation and ferroptosis, whose breakdown promotes ferroptosis [78, 79].
LD degradation is mediated by lipolysis, a process mediated by lipases, and a selective autophagic mechanism called lipophagy (Fig. 4) [80, 81]. Lipolysis enables the highly regulated release of FFAs from triacylglycerol (TAGs). FFAs are oxidized by the beta-oxidation pathway and converted to Acetyl-CoA [81]. Lipophagy is a process through the lysosomal pathway mediated by autophagy in two forms: chaperone-mediated autophagy (CMA) and macrolipophagy [77]. CMA is a lysosome-dependent protein degradation process, during which LD coat proteins, perilipin 2 (PLP2) and perilipin 3 (PLP3), bounded by cytosolic heat shock-associated protein 70 (Hsc70), are imported into the lysosome through a lysosome-associated membrane protein (LAMP2A) and are degraded [82]. As a general event during ferroptosis, GPX4, which can directly diminish lipid hydroperoxide production, is degraded via CMA. GPX4 degradation during ferroptosis is enhanced by HSP90, which can be activated under oxidative stress and increase LAMP2A stability [22]. Macrolipophagy mediated by Rab7 induces autophagic degradation of lipid droplets by LC3-II-positive membranes via lysosomal transportation and degradation. Rab7, used as a marker of the endolysosomal pathway, is required for the fusion of autophagosomes with lysosomes and promotes the decomposition of LDs. Lipophagy-mediated LDs degradation increases FFA production and promotes lipid peroxidation and subsequent ferroptosis [63, 83].
Fig. 4. The role of autophagy in promoting ferroptosis.
A, B NCOA4-mediated ferritinophagy (A) and Mitophagy (B) promote iron accumulation in ferroptosis. C–G Lipolysis mediated by lipases (C) and Hsc70/HSP90 chaperone-mediated autophagy (CMA) (D, E) and RAB7A-mediated macrolipophagy (F), and the cargo receptor SQSTM1-mediated clockophagy (G) promote lipid peroxidation in ferroptosis.
Cargo receptor SQSTM1-mediated clockophagy promotes lipid peroxidation during ferroptosis. Clockophagy is recently discovered as a type of autophagic degradation of aryl hydrocarbon receptor nuclear translocator-like (ARNTL) during ferroptosis, induced by type 2 ferroptosis inducers (e.g., RSL3 and FIN56) [22, 63]. Degradation of ARNTL increases the expression of EGLN2, inhibiting HIF1A (hypoxia inducible factor 1, alpha subunit) activation. HIF1A restricts ferroptosis by increasing fatty acid uptake and lipid storage, and reducing fatty acid β-oxidation, thus minimizing peroxidation-mediated cytomembrane damage. Consequently, downregulation of HIF1A reduces HIF1A-dependent accumulation of lipid droplets and promotes lipid peroxidation [84].
Amplification of ferroptosis via autophagy and ROS feedback loop
There are evidences regarding feedback loop mechanisms in cancer that regulate autophagy and ferroptosis. These feedback loops involve signaling pathways that respond to changes in cellular metabolism and stress, and can either promote or inhibit autophagy and ferroptosis. One such feedback loop involves the p53 tumor suppressor protein, which is frequently mutated or lost in cancer cells. In normal cells, p53 can activate both autophagy and ferroptosis in response to cellular stress [85, 86]. However, in several cancer cells with p53 mutations, the loss of p53 function can lead to decreased autophagy and increased ferroptosis resistance. This can contribute to tumor growth and survival. Another feedback loop involves the NRF2-KEAP1 signaling pathway, which is involved in the regulation of oxidative stress and antioxidant responses in cells. In cancer cells, activation of the NRF2 pathway can lead to increased antioxidant defenses and resistance to ferroptosis [87]. However, NRF2 activation can also induce autophagy, which can promote ferroptosis in some contexts. In addition, other signaling pathways, such as the mTOR pathway and the unfolded protein response (UPR), can also regulate autophagy and ferroptosis in cancer cells through feedback mechanisms [88, 89]. These pathways respond to changes in nutrient availability, protein misfolding, and other stressors, and can either promote or inhibit autophagy and ferroptosis depending on the specific context. Collectively, the feedback loop mechanisms that regulate autophagy and ferroptosis in cancer cells are complex and context-dependent. Further research is needed to fully understand these mechanisms and their potential therapeutic implications for cancer treatment.
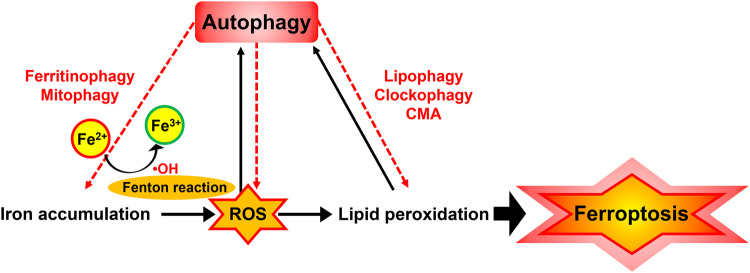
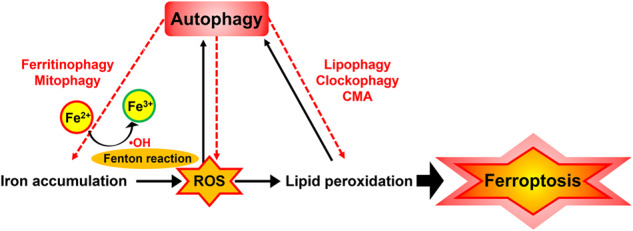
The interrelationship between autophagy and ferroptosis has attracted increasing attention, providing a novel concept regarding cell death regulation [90]. Increasing evidence suggests that autophagy serves to ferroptotic cell death at least under certain conditions [22]. Here, we summarize the key perspectives on the impact of autophagy as an enhancer of ROS-dependent ferroptosis. Furthermore, autophagy is considered a reinforcer that promotes ROS-dependent ferroptosis as a synergistic effect via disruption of the redox balance. In ferroptosis, ROS triggers autophagy, resulting in ROS-mediated autophagy promoting ROS generation, and this feedback loop contributes to ferroptosis activation (Fig. 5).
Fig. 5. Autophagy as an enhancer of ferroptosis induced by iron accumulation-mediated ROS and lipid-based ROS.
Autophagy is triggered by ROS in ferroptosis, caused by excess iron or lipid-dependent ROS accumulation, and then promotes iron and ROS accumulation, and this feedback loop contributes to the acceleration of ferroptosis. Ferroptosis is induced by increased ROS levels caused by elevated intracellular iron concentration, even without autophagy activation. In addition, autophagy alone without ROS generation could not induce ferroptosis. Thus, it cannot be concluded that ferroptosis is autophagic cell death and it is considered that autophagy enhances ferroptosis only under certain conditions.
Activation loop of autophagy by ROS during ferroptosis
Classically, ferroptosis is an iron-dependent oxidative cell death caused by ROS and has been considered autophagy-dependent ferroptosis [22, 91]. However, in the absence of ROS generation, autophagy activation could inhibit chemical-induced ferroptosis in cancer cells. In these studies, autophagy activation was able to attenuate FIN56 or erastin-induced ferroptosis, even in the absence of ROS generation. The authors suggested that autophagy inhibited ferroptosis by reducing lipid peroxidation and iron accumulation [92–94].
In contrast, some additional studies explained the pathways and mechanisms accounting for ROS-initiated autophagy during ferroptosis [11, 20, 95]. A study investigated the role of ROS in regulating autophagy during ferroptosis [20]. They revealed that ROS generated during ferroptosis activated the AMPK pathway, which in turn phosphorylated and activated the ULK1 complex, a key initiator of autophagy. Another study investigated the role of the Nrf2 pathway in regulating autophagy during ferroptosis [11]. The authors showed that Nrf2, could also activate autophagy in response to ROS generated during ferroptosis. They suggested that Nrf2-mediated autophagy activation was a protective mechanism against ferroptosis-induced cell death. In addition, investigated the role of the MAPK pathway in regulating autophagy during ferroptosis [95]. They revealed that activation of the MAPK pathway by ROS generated during ferroptosis could activate the transcription factor TFEB, which in turn induced the expression of genes involved in lysosomal biogenesis and autophagy. These studies suggest that ROS generated during ferroptosis can activate various signaling pathways, such as AMPK, Nrf2, and MAPK, which in turn activate autophagy as a protective mechanism against ferroptosis-induced cell death.
Specifically, the activation of autophagy by ROS has been suggested in several studies. Iron accumulation-mediated ROS play an essential role in the induction of lipid peroxidation and activation of autophagy in ferroptosis. Liu et al. demonstrated that radiation-induced iron accumulation by the release of lysosomal iron generates ROS via the Fenton reaction and increases autophagy in a time-dependent manner [96]. In other studies, erastin, an inducer of ferroptosis, has been associated with the regulation of the antioxidant system through several molecules, including system Xc- [97]. In this case, erastin-induced ROS triggers autophagy, and activated autophagy leads to iron-dependent ferroptosis by the degradation of ferritin and induction of transferrin receptor 1 (TfR1) expression [98, 99].
ROS-producing agents such as hydrogen peroxide and 2-methoxyestradiol (2-ME), cause autophagy and autophagic cell death. Blocking ROS generation through overexpression of MnSOD (manganese-superoxide dismutase) or using ROS scavengers also effectively blocked autophagy and ferroptosis [53, 91, 100]. In addition, acid sphingomyelinase (ASM), a key enzyme in sphingolipid metabolism, regulates autophagy by activating nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase)-derived ROS in erastin-induced ferroptosis [101]. Beclin1, a critical protein associated with autophagy initiation, is upregulated by the ROS/p53 signaling pathway activated by ginsenosides, such as Rh4, during ferroptosis. Subsequently, the ROS scavenger N-acetyl-cysteine (NAC) reversed the inhibitory effect of Rh4 on cancer cell proliferation [102].
Subsequent accumulation of lipid-based ROS and loss of activity of the lipid repair enzyme, GPX4, induces lipid peroxidation and ferroptosis [103]. Previous studies have shown that lipid peroxidation can lead to autophagy activation through ROS induction. The products of lipid peroxidation activated by ROS induce autophagy [91, 104]. The signaling pathways of lipid peroxidation-driven autophagy are variable. Unsaturated lipid peroxidation-derived aldehydes such as 4-hydroxy-trans-2-nonenal (HNE) promote autophagy by a JNK-dependent mechanism [105, 106]. SIN-1-induced lipid peroxidation is associated with autophagy induction by TP53INP1, which interacts with LC3 and ATG8-family proteins [107, 108]. Lipid-soluble antioxidants such as resveratrol and vitamin E and the decrease in iron-mediated ROS via NAC and overexpression of superoxide dismutase 2 (SOD2) attenuated autophagy [96, 109, 110]. Therefore, autophagy is triggered by ROS, including iron accumulation-mediated and lipid-based ROS, which are the major causes of ferroptosis.
Amplification loop of ROS by autophagy during ferroptosis
The autophagic mechanisms maintaining the balance between cell protection and cell death during ROS induction remain unclear [111]. Under normal environments, ROS-induced autophagy alleviates oxidative stress to protect cells from damage. For example, autophagy can protect cells by removing ROS to conserve mitochondrial integrity, avoid apoptosis, and promote antigen presentation. However, excessive ROS-induced autophagy can cause autophagic cell death and amplify ferroptosis by promoting higher ROS levels under certain circumstances [91, 100].
Unregulated iron homeostasis can result in excessive iron accumulation and subsequently lead to damage to proteins, lipids, and DNA through the generation of free radicals and oxidative stress [112]. Several studies have demonstrated that ferritinophagy, a type of selective autophagy, contributes to ferroptosis by inducing iron-dependent ROS production by mediating the degradation of ferritin. Ferritin degradation eventually results in the cytosolic release of chelated iron, thereby inducing oxidative stress [20, 113]. Knockout or knockdown of ATG5 and ATG7 inhibited ferroptosis induced by erastin with diminished intracellular Fe2+ levels, and lipid peroxidation [20, 51]. A dithiocarbamate derivative, 2-pyridylhydrazone dithiocarbamate s-acetic acid (PdtaA), decreased GSH and increased lipid peroxidation and ferritinophagy-mediated ROS production. Autophagy inhibition using the autophagy inhibitor 3-methyladenin (3-MA) counteracted the effect of PdtaA on ferritinophagy and ferroptosis induction via downregulation of GPx4 and xCT [113].
The relationship between autophagy and ferroptosis has a complicated interaction during oxidative stress. Several studies have demonstrated that ROS-mediated autophagy promotes ROS-induced lipid peroxidation and cell death [91]. Blocking ROS-induced autophagosome accumulation through the autophagy inhibitor 3-MA or knocking down autophagy genes, including beclin-1, ATG-5, and ATG-7, effectively blocked ROS generation and oxidative stress-induced cell death [53, 100]. Autophagy mediated by ASM, a key enzyme in sphingolipid metabolism, plays a critical role in GPX4 degradation via ferroptosis inducers and ferroptosis activation. During the activation of ASM by erastin, autophagy inhibitors such as Bafilomycin A1, hydroxychloroquine (HCQ), and ammonium chloride (NH4Cl) inhibit erastin-induced ROS generation and ferroptosis [101]. Furthermore, autophagy induction following GSH depletion plays a key role in the increase of oxidative stress and lipid peroxidation in ferroptosis by GSH depletion [98].
Some additional studies could explain the pathways and mechanisms accounting for autophagy-dependent generation of ROS during ferroptosis. Yang and colleagues investigated the role of the autophagy protein p62/SQSTM1 in regulating ROS generation during ferroptosis. The authors showed that p62/SQSTM1 could bind to and stabilize the ferroptosis regulator NCOA4, which in turn promoted the transfer of iron from ferritin to the lipid peroxidation system, generating ROS and promoting ferroptosis. The authors suggested that p62/SQSTM1-mediated ROS generation was a positive feedback loop that amplified ferroptosis [114]. Another study investigated the role of the autophagy protein ATG5 in regulating ROS generation during ferroptosis. ATG5-deficient cells had reduced levels of ROS and were resistant to ferroptosis, while ATG5-overexpressing cells had increased levels of ROS and were more susceptible to ferroptosis. The authors suggested that ATG5-mediated ROS generation was a positive feedback loop that amplified ferroptosis [51]. In addition, the role of autophagy in regulating ROS generation during ferroptosis induced by the compound FIN56. Autophagy activation increased ROS levels and sensitized cancer cells to FIN56-induced ferroptosis, while autophagy inhibition reduced ROS levels and protected cells from ferroptosis. The authors suggested that autophagy-mediated ROS generation was a positive feedback loop that amplified ferroptosis [93]. These studies suggest that autophagy can amplify ROS generation during ferroptosis by regulating key pathways involved in iron metabolism and lipid peroxidation, such as the p62/SQSTM1-NCOA4 axis and ATG5-mediated signaling.
Therefore, excessive autophagy stimulated by oxidized lipids and prolonged iron-mediated ROS plays a critical enhancer role in ferroptosis via the promotion of oxidative stress through enhancement of iron accumulation by ferritinophagy and lipid peroxidation by lipophagy, clockophagy, and CMA [22, 115]. Thus, even if autophagy alone does not lead to ferroptosis, it may act as an amplifier during ferroptosis, and we can conclude that ferroptosis is autophagic cell death under specific conditions.
Perspectives
Ferroptosis is a cell death process driven by iron-dependent lipid peroxidation [41, 42]. Recently, studies have expanded on the molecular mechanisms underlying ferroptosis and revealed a complex regulatory mechanism [22]. Ferroptosis has been shown to play an important regulatory role in the occurrence and development of several diseases [2, 116]. Direct control of ferroptosis via modulation of lipid peroxidation influences disease therapy, however, the sources of ferroptosis induction are diverse and ferroptosis is also implicated in disease development. Highly metastatic and resistant cancers are extremely vulnerable to ferroptosis inducing treatment; however, the pathophysiology of neurological diseases including neurodegeneration, stroke, and neurotrauma, are associated with ferroptosis [50, 117]. Therefore, elucidating the mechanisms underlying ferroptosis can improve our understanding of human diseases and provide potential prevention and treatment interventions for various diseases [117].
In this review, we discussed the role of autophagy as a ferroptosis enhancer via the ROS amplification loop. We summarized that autophagy is triggered by ROS including iron accumulation-mediated ROS and lipid-based ROS in ROS-dependent ferroptosis and then acts to amplify iron accumulation and lipid peroxidation and subsequently can induce autophagic cell death under specific conditions. Accordingly, therapeutic strategies targeting the crosstalk between autophagy, iron, and ROS in ferroptosis could provide novel promising directions for the treatment of diseases and improve therapeutic options [93]. In addition, various types of autophagy such as ferritinophagy, mitophagy, lipophagy, clockophagy, contribute to ferroptosis. However, the type of autophagy that contributes to ferroptosis remains unclear, and further studies are needed to identify novel target molecules that regulate autophagy-dependent ferroptosis. Therefore, additional research on the relationship between autophagy and ferroptosis mechanisms in pathological pathways can provide insights into disease development. This review would pave the way for understanding the role of autophagy in the connection between oxidative stress, autophagy, and ferroptosis.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education and the KIST Institutional Program and the KRIBB Research Initiative Program (NRF-2021R1A2C1094382 to SWC; 2E31700-22-P005 and KGM5322321 to S-JL).
Author contributions
Conceptualization, SWC, S-JL; Validation, SL, NH, BGS, Sangguk Lee. and S-JL, SWC; Investigation, SWC, SL, NH, BGS; Writing Original Draft Preparation, SWC, SL; Writing Review & Editing, SWC, SL, NH, BGS., Sangguk Lee.; Visualization, SL; Supervision, SWC; Project Administration, SWC; Funding Acquisition, SWC., S-JL.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education and the KIST Institutional Program and the KRIBB Research Initiative Program (NRF-2021R1A2C1094382 to SWC; 2E31700-22-P005 and KGM5322321 to S-JL).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All aspects of this study were approved by the Institutional Research Ethics Committee of University of Ulsan.
Consent for publication
This paper has been read and approved for publication by all of its participating authors.
Footnotes
Edited by Professor Gian Maria Fimia
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Seunghee Lee, Narae Hwang, Byeong Geun Seok.
References
- 1.Han C, Liu Y, Dai R, Ismail N, Su W, Li B. Ferroptosis and its potential role in human diseases. Front Pharm. 2020;11:239. doi: 10.3389/fphar.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12:34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou M, Jiang Y, Ji Y, Zhou Q, Du Z, Zhu H, et al. Role and mechanism of ferroptosis in neurological diseases. Mol Metab. 2022;61:101502. doi: 10.1016/j.molmet.2022.101502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H-F, Tuo QZ, Yin Q-Z, Lei P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res. 2020;41:220–30. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Jiang J, Fu N, Chen L. Targetting ferroptosis for blood cell-related diseases. J Drug Target. 2022;30:244–58. doi: 10.1080/1061186X.2021.1971237. [DOI] [PubMed] [Google Scholar]
- 8.Stockwell BR, Friedmann Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang TX, Liang JY, Zhang C, Xiong Y, Guan KL, Yuan HX. The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis. Cell Death Dis. 2019;10:755. doi: 10.1038/s41419-019-1984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talty R, Bosenberg M. The role of ferroptosis in melanoma. Pigment Cell Melanoma Res. 2022;35:18–25. doi: 10.1111/pcmr.13009. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Shen Y, Chen C, Sui X, Yang J, Wang L, et al. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Cancer Biol Med. 2019;16:630–46. doi: 10.20892/j.issn.2095-3941.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Yu C, Kang R, Tang D. Iron metabolism in ferroptosis. Front Cell Dev Biol. 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648–57. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin X, Ping J, Wen Y, Wu Y. Mechanism of ferroptosis and applications in tumor treatment. Front Pharm. 2020;11:1061. doi: 10.3389/fphar.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Xu J, Zhang B, Tan Z, Meng Q, Hua J, et al. Ferroptosis: At the crossroad of gemcitabine resistance and tumorigenesis in pancreatic cancer. Int J Mol Sci. 2021;22:10944. doi: 10.3390/ijms222010944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, et al. Lipoxygenase‐mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–94. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–83. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. doi: 10.1038/s41419-019-2064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron overload, oxidative stress, and ferroptosis in the failing heart and liver. Antioxid (Basel) 2021;10:1864. doi: 10.3390/antiox10121864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc Natl Acad Sci USA. 2008;105:7410–5. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolff NA, Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thévenod F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep. 2018;8:211. doi: 10.1038/s41598-017-18584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onukwufor JO, Dirksen RT, Wojtovich AP. Iron dysregulation in mitochondrial dysfunction and Alzheimer’s disease. Antioxid (Basel) 2022;11:692. doi: 10.3390/antiox11040692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bystrom LM, Guzman ML, Rivella S. Iron and reactive oxygen species: friends or foes of cancer cells? Antioxid Redox Signal. 2014;20:1917–24. doi: 10.1089/ars.2012.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9:1505. doi: 10.3390/cells9061505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang N, Ma H, Li J, Meng C, Zou J, Wang H, et al. HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J Mol Cell Cardiol. 2021;150:65–76. doi: 10.1016/j.yjmcc.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Carocci A, Catalano A, Sinicropi MS, Genchi G. Oxidative stress and neurodegeneration: the involvement of iron. Biometals. 2018;31:715–35. doi: 10.1007/s10534-018-0126-2. [DOI] [PubMed] [Google Scholar]
- 30.Liu JL, Fan YG, Yang ZS, Wang ZY, Guo C. Iron and Alzheimer’s disease: from pathogenesis to therapeutic implications. Front Neurosci. 2018;12:632. doi: 10.3389/fnins.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan J, Ren H, Wang J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol. 2019;4:93–95. doi: 10.1136/svn-2018-000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrad M, Kagan VE, Bayir H, Pagnussat GC, Head B, Traber MG, et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018;32:602–19. doi: 10.1101/gad.314674.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Kim WK, Bae KH, Lee SC, Lee EW. Lipid metabolism and ferroptosis. Biol (Basel) 2021;10:184. doi: 10.3390/biology10030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang D, Kroemer G. Peroxisome: the new player in ferroptosis. Signal Transduct Target Ther. 2020;5:273. doi: 10.1038/s41392-020-00404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kung Y-A, Chiang H-J, Li ML, Gong YN, Chiu HP, Hung CT, et al. Acyl-coenzyme A synthetase long-chain family Member 4 is involved in viral replication organelle formation and facilitates virus replication via ferroptosis. mBio. 2022;13:e0271721. doi: 10.1128/mbio.02717-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu H, Wang F, Ta N, Zhang T, Gao W. The multifaceted regulation of mitochondria in ferroptosis. Life (Basel) 2021;11:222. doi: 10.3390/life11030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol. 2020;8:586578. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Patel D, Kharkar PS, Gandhi NS, Kaur E, Dutt S, Nandave M. Novel analogs of sulfasalazine as system x c – antiporter inhibitors: insights from the molecular modeling studies. Drug Dev Res. 2019;80:758–77. doi: 10.1002/ddr.21557. [DOI] [PubMed] [Google Scholar]
- 40.Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7:193. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren JX, Li C, Yan XL, Qu Y, Yang Y, Guo ZN. Crosstalk between oxidative stress and Ferroptosis/Oxytosis in ischemic stroke: possible targets and molecular mechanisms. Oxid Med Cell Longev. 2021;2021:6643382. doi: 10.1155/2021/6643382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 44.Razayian M, Niknam V, Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol Rep. 2019;6:1309–13. doi: 10.1016/j.toxrep.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbaradei A, Wang Z, Malmstadt N. Oxidation of membrane lipids alters the activity of the human serotonin 1A receptor. Langmuir. 2022;38:6798–807. doi: 10.1021/acs.langmuir.1c03238. [DOI] [PubMed] [Google Scholar]
- 46.Zhou R-P, Chen Y, Wei X, Yu B, Xiong ZG, Lu C, et al. Novel insights into ferroptosis: implications for age-related diseases. Theranostics. 2020;10:11976–97. doi: 10.7150/thno.50663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan H-F, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology, and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–8. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun Y, Kim J. Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 2018;7:278. doi: 10.3390/cells7120278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma S, Dielschneider RF, Henson ES, Xiao W, Choquette TR, Blankstein AR, et al. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLOS ONE. 2017;12:e0182921. doi: 10.1371/journal.pone.0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–76. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mowers EE, Sharifi MN, Macleod KF. Autophagy in cancer metastasis. Oncogene. 2016;36:1619–30. doi: 10.1038/onc.2016.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang ZL, Yao Z, Wang L, Ding H, Shao JJ, Chen AP, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Müller F, Lim JKM, Bebber CM, Seidel E, Tishina S, Dahlhaus A, et al. Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ. 2023;30:442–56. doi: 10.1038/s41418-022-01096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117:31189–97. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatt V, Lan T, Wang W, Kong J, Lopes EC, Wang J, et al. Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors. Cell Death Dis. 2023;14:61. doi: 10.1038/s41419-023-05592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharm Res. 2021;166:105466. doi: 10.1016/j.phrs.2021.105466. [DOI] [PubMed] [Google Scholar]
- 62.Santana-Codina N, Mancias JD. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals. 2018;11:114. doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan B. Mitochondrial regulation of ferroptosis. J Cell Biol. 2021;220:e202105043. doi: 10.1083/jcb.202105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 66.Lane DJR, Merlot AM, Huang ML-H, Bae D-H, Jansson PJ, Sahni S, et al. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta. 2015;1853:1130–44. doi: 10.1016/j.bbamcr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Yu F, Zhang Q, Liu H, Liu J, Yang S, Luo X, et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 2022;8:40. doi: 10.1038/s41421-022-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S, Zhang J, Liu C, Wang Q, Yan J, Hui L, et al. The role of mitophagy in regulating cell death. Oxid Med Cell Longev. 2021;2021:6617256. doi: 10.1155/2021/6617256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basit F, van Oppen LMPE, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schofield JH, Schafer ZT. Mitochondrial reactive oxygen species and mitophagy: a complex and nuanced relationship. Antioxid Redox Signal. 2021;34:517–30. doi: 10.1089/ars.2020.8058. [DOI] [PubMed] [Google Scholar]
- 71.Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liégeois M, et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324. doi: 10.1016/j.redox.2022.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierzynowska K, Rintz E, Gaffke L, Węgrzyn G. Ferroptosis and its modulation by autophagy in light of the pathogenesis of lysosomal storage diseases. Cells. 2021;10:365. doi: 10.3390/cells10020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL, et al. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8:e2593. doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Y, Xue Z, Huang T, Che X, Wu G. Lipid metabolism in ferroptosis and ferroptosis-based cancer therapy. Front Oncol. 2022;12:941618. doi: 10.3389/fonc.2022.941618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bailey AP, Koster G, Guillermier C, Hirst EMA, MacRae JI, Lechene CP, et al. Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell. 2015;163:340–53. doi: 10.1016/j.cell.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koizume S, Miyagi Y. Lipid droplets: a key cellular organelle associated with cancer cell survival under normoxia and hypoxia. Int J Mol Sci. 2016;17:1430. doi: 10.3390/ijms17091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang S, Peng X, Yang S, Li X, Huang M, Wei S, et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022;13:132. doi: 10.1038/s41419-022-04593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petan T. Lipid droplets in cancer. Rev Physiol Biochem Pharm. 2023;185:53–86. doi: 10.1007/112_2020_51. [DOI] [PubMed] [Google Scholar]
- 79.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, et al. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 80.Schott MB, Weller SG, Schulze RJ, Krueger EW, Drizyte-Miller K, Casey CA, et al. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. J Cell Biol. 2019;218:3320–35. doi: 10.1083/jcb.201803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215–27. doi: 10.1016/j.molcel.2022.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Losmanová T, Janser FA, Humbert M, Tokarchuk I, Schläfli AM, Neppl C, et al. Chaperone-mediated autophagy markers LAMP2A and HSC70 are independent adverse prognostic markers in primary resected squamous cell carcinomas of the lung. Oxid Med Cell Longev. 2020;2020:8506572. doi: 10.1155/2020/8506572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hansen TE, Johansen T. Following autophagy step by step. BMC Biol. 2011;9:39. doi: 10.1186/1741-7007-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Yang M, Kang R, Klionsky DJ, Tang D. Autophagic degradation of the circadian clock regulator promotes ferroptosis. Autophagy. 2019;15:2033–5. doi: 10.1080/15548627.2019.1659623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J, Zhang C, Wang J, Hu W, Feng Z. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci. 2020;21:8387. doi: 10.3390/ijms21218387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rahman MA, Park MN, Rahman MH, Rashid MM, Islam R, Uddin MJ, et al. p53 modulation of autophagy signaling in cancer therapies: perspectives mechanism and therapeutic targets. Front Cell Dev Biol. 2022;10:761080. doi: 10.3389/fcell.2022.761080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Song X, Long D. Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front Neurosci. 2020;14:267. doi: 10.3389/fnins.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J. KEAP1-NRF2 signaling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem J. 2015;469:347–55. doi: 10.1042/BJ20150568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lei G, Zhuang L, Gan B. mTORC1 and ferroptosis: regulatory mechanisms and therapeutic potential. Bioessays. 2021;43:e2100093. doi: 10.1002/bies.202100093. [DOI] [PubMed] [Google Scholar]
- 90.Liu J, Guo ZN, Yan XL, Huang S, Ren JX, Luo Y, et al. Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front Cell Neurosci. 2020;14:577403. doi: 10.3389/fncel.2020.577403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su L-J, Zhang J-H, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buccarelli M, Marconi M, Pacioni S, De Pascalis I, D'Alessandris QG, Martini M, et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018;9:841. doi: 10.1038/s41419-018-0864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun Y, Berleth N, Wu W, Schlütermann D, Deitersen J, Stuhldreier F, et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation, and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 2021;12:1028. doi: 10.1038/s41419-021-04306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li M, Wang X, Lu S, He C, Wang C, Wang L, et al. Erastin triggers autophagic death of breast cancer cells by increasing intracellular iron levels. Oncol Lett. 2020;20:57. doi: 10.3892/ol.2020.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen W, Yang W, Zhang C, Liu T, Zhu J, Wang H, et al. Modulation of the p38 MAPK pathway by anisomycin promotes ferroptosis of hepatocellular carcinoma through phosphorylation of H3S10. Oxid Med Cell Longev. 2022;2022:6986445. doi: 10.1155/2022/6986445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma S, Fu X, Liu L, Liu Y, Feng H, Jiang H, et al. Iron-dependent autophagic cell death induced by radiation in MDA-MB-231 breast cancer cells. Front Cell Dev Biol. 2021;9:723801. doi: 10.3389/fcell.2021.723801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao Yuechen LiY, Zhang R, Wang F, Wang T, Jiao Y. The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther. 2020;13:5429–41. doi: 10.2147/OTT.S254995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun Y, Zheng Y, Wang C, Liu Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018;9:753. doi: 10.1038/s41419-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, Li L, Li M, Luo Z. Autophagy-dependent ferroptosis as a therapeutic target in cancer. ChemMedChem. 2021;16:2942–50. doi: 10.1002/cmdc.202100334. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–82. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 101.Thayyullathil F, Cheratta AR, Alakkal A, Subburayan K, Pallichankandy S, Hannun YA, et al. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis. 2021;12:26. doi: 10.1038/s41419-020-03297-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu Y, Pi D, Chen Y, Zuo Q, Zhou S, Ouyang M. Ginsenoside Rh4 inhibits colorectal cancer cell proliferation by inducing ferroptosis via autophagy activation. Evid Based Complement Altern Med. 2022;2022:6177553. doi: 10.1155/2022/6177553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–76. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiang JG, Fukumoto R, Gorbunov NV. Lipid peroxidation after ionizing irradiation leads to apoptosis and autophagy. IntechOpen; 2012.
- 105.Haberzettl P, Hill BG. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J. 2008;410:525–34. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 107.Coliva G, Duarte S, Pérez-Sala D, Fedorova M. Impact of inhibition of the autophagy-lysosomal pathway on biomolecules carbonylation and proteome regulation in rat cardiac cells. Redox Biol. 2019;23:101123. doi: 10.1016/j.redox.2019.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saadi H, Seillier M, Carrier A. The stress protein TP53INP1 plays a tumor suppressive role by regulating metabolic homeostasis. Biochimie. 2015;118:44–50. doi: 10.1016/j.biochi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 109.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–60. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farrugia G, Balzan R. Oxidative stress and programmed cell death in yeast. Front Oncol. 2012;2:64. doi: 10.3389/fonc.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y-F, Liu H, Luo XJ, Zhao Z, Zou ZY, Li J, et al. The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol Hematol. 2017;112:21–30. doi: 10.1016/j.critrevonc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Yan N, Zhang J. Iron metabolism, ferroptosis, and the links with Alzheimer’s disease. Front Neurosci. 2019;13:1443. doi: 10.3389/fnins.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Longlong LiH, Li Y, Feng J, Guan D, Zhang Y, et al. Ferritinophagy-mediated ROS Production Contributed to proliferation inhibition, apoptosis, and ferroptosis induction in action of mechanism of 2-pyridylhydrazone dithiocarbamate acetate. Oxid Med Cell Longev. 2021;2021:5594059. doi: 10.1155/2021/5594059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang L, Ye F, Liu J, Klionsky DJ, Tang D, Kang R. Extracellular SQSTM1 exacerbates acute pancreatictis by activating autophagy-dependent ferroptosis. Autophagy. 2022;5:1–12. doi: 10.1080/15548627.2022.2152209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu J, Kong W, Xie Z. Expression and prognostic characteristics of ferroptosis-related genes in colon cancer. Int J Mol Sci. 2021;22:5652. doi: 10.3390/ijms22115652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren JX, Sun X, Yan XL, Guo ZN, Yang Y. Ferroptosis in neurological diseases. Front Cell Neurosci. 2020;14:218. doi: 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mao H, Zhao Y, Li H, Lei L. Ferroptosis as an emerging target in inflammatory diseases. Prog Biophys Mol Biol. 2020;155:20–28. doi: 10.1016/j.pbiomolbio.2020.04.001. [DOI] [PubMed] [Google Scholar]