Abstract
Background
Racial and ethnic minoritized groups are disproportionately at risk for Alzheimer’s Disease (AD), but are not sufficiently recruited in AD neuroimaging research in the United States. This is important as sample composition impacts generalizability of findings, biomarker cutoffs, and treatment effects. No studies have quantified the breadth of race/ethnicity representation in the AD literature.
Methods
This review identified median race/ethnicity composition of AD neuroimaging US-based research samples available as free full-text articles on PubMed. Two types of published studies were analyzed: studies that directly report race/ethnicity data (i.e., direct studies), and studies that do not report race/ethnicity but used data from a cohort study/database that does report this information (i.e., indirect studies).
Results
Direct studies (n = 719) have median representation of 88.9% white or 87.4% Non-Hispanic white, 7.3% Black/African American, and 3.4% Hispanic/Latino ethnicity, with 0% Asian American, Native Hawaiian/Pacific Islander, and American Indian/Alaska Native, Multiracial, and Other Race participants. Cohort studies/databases (n = 44) from which indirect studies (n = 1745) derived are more diverse, with median representation of 84.2% white, 83.7% Non-Hispanic white, 11.6% Black/African American, 4.7% Hispanic/Latino, and 1.75% Asian American participants. Notably, 94% of indirect studies derive from just 10 cohort studies/databases. Comparisons of two time periods using a median split for publication year, 1994–2017 and 2018–2022, indicate that sample diversity has improved recently, particularly for Black/African American participants (3.39% from 1994–2017 and 8.29% from 2018-2022).
Conclusions
There is still underrepresentation of all minoritized groups relative to Census data, especially for Hispanic/Latino and Asian American individuals. The AD neuroimaging literature will benefit from increased representative recruitment of ethnic/racial minorities. More transparent reporting of race/ethnicity data is needed.
Subject terms: Alzheimer's disease, Diagnostic markers
Plain language summary
Members of some racial and ethnic minority groups in the USA are more likely to develop Alzheimer’s Disease than white people. However, they are often not included in research studies of Alzheimer’s Disease. We looked at the race/ethnicity composition of people evaluated in papers published describing Alzheimer’s Disease research studies based in the USA that used images of the brain. We found that all racial/ethnic minority groups were underrepresented in Alzheimer’s Disease research studies, especially Hispanic/Latino and Asian American individuals. It is important that studies include representatives of all populations both for the health of those populations and improved understanding of Alzheimer’s Disease in all people. Such studies should also improve efforts to understand and address racial/ethnic disparities in Alzheimer’s Disease diagnosis and treatment.
Lim et al. provide a systematic review of the race/ethnicity representation in USA based neuroimaging studies of those with Alzheimer’s Disease. Whilst sample diversity has increased in recent years, minoritized groups in the USA remain underrepresented and more transparent reporting of race/ethnicity data is needed.
Introduction
Note on terminology: Throughout this paper, we apply the race and ethnicity categories described in the 1997 document “Office of Management and Budget Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity”1. This includes Hispanic/Latino ethnicity, and race categories of white, Black/African American, Asian American, Native Hawaiian/Pacific Islander, American Indian/Alaska Native, Multiracial, and Other Race.
Alzheimer’s Disease (AD) is a neurodegenerative disorder with insidious onset and progressive impairment of behavioral and cognitive functions. AD can be biologically classified by three neuropathological markers. These include: (1) Aβ plaques as indicated by cortical amyloid PET ligand binding or low CSF AB42; (2) fibrillar tau as indicated by elevated CSF phosphorylated tau or cortical tau PET ligand binding; and (3) neurodegeneration or neuronal injury assessed as CSF T-tau, FDG PET hypometabolism, or MRI atrophy2,3. Symptoms of AD vary and are associated with the stage of the disease. The advanced stage of the Alzheimer’s clinical syndrome known as Alzheimer’s Dementia is characterized by impairment of memory, comprehension, language, attention, and executive function2,4. The estimated prevalence of AD was 1.6% of the US population in 2014, and is expected to double to 3.3% by 2060, when 13.9 million Americans are projected to have the disease5.
There are significant differences in AD incidence across racial/ethnic groups in the United States. Compared to Non-Hispanic white individuals, Black/African American individuals are twice as likely and Hispanic/Latino individuals are 1.5 times as likely to develop AD6. Among people 65 and older in the US, Black/African American individuals have the highest AD prevalence of all racial groups (13.8%), followed by Hispanic/Latino individuals (12.2%), non-Hispanic white individuals (10.3%), American Indian and Alaska Native individuals (9.1%), and Asian American and Pacific Islander individuals (8.4%)5. These racial and ethnic differences have multiple potential causes, including socioeconomic and educational disparities and associated stressors, healthcare access, medical comorbidities—particularly cardiovascular risk and disease, genetic risk variants, environmental exposures, and myriad other factors7–15.
The United States is also experiencing a demographic shift, with minority populations, classified by the US Census Bureau as racial and ethnic groups other than non-Hispanic white, projected to outpace the growth of non-Hispanic white individuals in the coming decades. An estimated 45% of the US population ≥65 years old will be from a minority group by 20605. The total population of non-Hispanic whites is estimated to increase by 75%, African Americans by 172%, Asian Americans and Pacific Islanders by 270%, American Indian and Alaska Natives by 274%, and Hispanic/Latinos by 391%16.
Despite the differential AD risk and changing demographics, studies have consistently indicated that ethnic and racial minoritized groups are underrepresented in AD research17–19. Reviews have suggested that possible contributing factors mostly focus on recruitment challenges and include minoritized populations’ distrust of scientific studies based on egregious historical experimentation on African American and Native American individuals10,20, lack of appropriate trust building and community networking, language and literacy barriers, and insufficient resources and training to develop culturally informed recruitment and retention strategies21. These barriers to participation frequently overlap and vary across geographic regions and cultures18, and recruitment challenges are identified as the primary barrier negatively impacting AD clinical research progress22.
The implications of under-recruiting minoritized groups are significant. Existing studies have repeatedly reported on the lack of racial/ethnic diversity in individual clinical trials and AD research assessment databases19,23,24, which can impact the generalizability of findings on AD processes, biomarker cutoffs for diagnosis and normative comparisons, and effectiveness of AD treatments. A growing number of neuroimaging and biomarker studies are addressing factors like race and ethnicity25,26, though not all studies have identified ethnic/racial differences in the relationships between brain cognition and function27,28. For example, community-dwelling Mexican American individuals experience neurodegeneration at significantly younger ages than Non-Hispanic white individuals, and neurodegeneration is correlated with different health factors in these two populations29. Some, but not all, studies report that Black/African American individuals have lower cerebrospinal fluid levels of tau-related biomarkers than Non-Hispanic white individuals, despite similar CSF levels of AB42, NfL, and hippocampal and white matter hyperintensity volume30–32, and AD correlates with increased CSF IL-9 in Black/African American individuals but not white individuals33. Black/African American patients with elevated beta-amyloid demonstrate smaller hippocampal volume and decreased cortical thickness than comparative Non-Hispanic white patients34,35. These two groups also exhibit opposite patterns of Default Mode Network resting-state connectivity in AD, potentially demonstrating race-specific AD trajectories that may contribute to differential rates of cognitive decline36. The few studies on American Indian individuals do not show evidence of neurodegenerative risk from Apolipoprotein (APOE) E4 on intracranial volume or cognitive testing37 and correlations between cognition and hippocampal volume appear to be similar to Non-Hispanic white individuals38. The Honolulu-Asia Aging Study has found that Hawaiian Japanese American men have lower neuropathological density relative to Non-Hispanic white individuals39,40. Notably, for race- and ethnicity-based comparisons, these differences remain significant after accounting for other demographic variables and comorbid medical conditions.
So while acknowledgement of racial and ethnic disparities in AD clinical outcomes and neuroimaging research has increased in recent years, with corresponding calls for better representation of ethnic/racial minoritized groups in AD research to ameliorate these disparities17,21,41, no studies have quantified the breadth of race/ethnicity representation in the neuroimaging literature, an important step in understanding the extent of minoritized group underrepresentation in a critical area of research in the field. This descriptive review addresses this gap by identifying mean and median race and ethnicity composition of AD-related neuroimaging research samples in published works based in the United States. We accessed this information from relevant publications indexed on the PubMed database. We also examined trends over time by investigating median representation over two time periods (1994–2017 and 2018–2022), delineated by median publication year for all included studies. Overall, we identified that all racial/ethnic minority groups were underrepresented in Alzheimer’s Disease research studies, especially Hispanic/Latino and Asian American individuals, and that sample diversity has been improving over time.
Methods
Search strategy
This review was conducted in accordance with the Preferred Reporting Item for Systematic Review and Meta-Analyses (PRISMA) guidelines42 and registered on the International Prospective Register of Systematic Reviews (PROSPERO) (ID CRD42022303573). We conducted an initial literature search in January 2022 using a PubMed database from a well-resourced university account, and articles were reviewed from January through September 2022. The primary inclusion search terms included “Alzheimers” and keyword variants related to neuroimaging, including “imaging” as well as specific neuroimaging techniques (e.g., “magnetic resonance imaging” or “positron emission tomography”) in the title or abstract. We were interested in publications with neuroimaging outcomes with research samples based in the United States, and therefore excluded studies with certain key terms in the title or abstract. First, search results were refined using PubMed’s filters, and were restricted to English journal articles, Human species, non-review- or meta-analysis-type articles, and available free full-text articles. We screened out non-quantitative studies using such terms as “qualitative”, “case study”, “commentary” and “study protocol”. As many animal studies were not filtered out by the Human Species filter, we excluded studies with key words such as “animals”, “rodent”, and “cell model”. Finally, we excluded studies primarily reporting histological or post-mortem data using such key words as “postmortem” “autopsy” and “histological”, as recruitment procedures and strategies for post-mortem and in-vivo neuroimaging studies can widely differ. We did, however, include studies for which living participants were recruited and imaged, and who subsequently agreed to a body donation for post-mortem research. The full search term and filters applied are available in Supplementary Table 1.
The remaining articles in the literature search were reviewed in full, as it was not always clear from abstract review whether a study recruited a sample based in the US, reported neuroimaging outcomes, or described a breakdown of race/ethnicity. Full-text articles were excluded at this stage if they did not meet the inclusion criteria, and reasons for exclusion are reported in the next section and in Fig. 1.
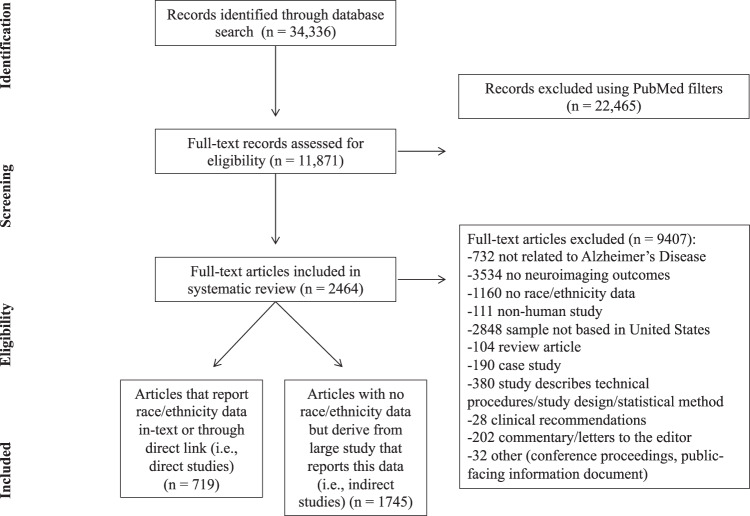
Fig. 1. Article selection flowchart.
Of 34,336 articles retrieved, 2464 articles were included in this review, divided into direct and indirect studies.
Eligibility criteria
Eligible articles for this review examined associations between AD processes and neuroimaging outcomes (e.g., from MRI, PET, CT) using quantitative methods/analysis, by means of experimental or observational study, included a sample based in the US, and reported race/ethnicity sample characteristics. To ensure that race/ethnicity comparisons were consistent across studies using US census categories, only participant samples that were based in the US were included. Multisite international studies with at least one US site that reported race/ethnicity categories consistent with the US census were also eligible for inclusion; only the US site reporting race/ethnicity data in such cases was specifically included in this review. AD was conceptualized broadly to expand the breadth of this review. For instance, reports examining health conditions as risk factors for AD, such as cardiovascular disease, obesity, depression, and Down syndrome were included so long as the connection to AD was explicitly tested or stated. Studies examining cognitively unimpaired individuals/healthy aging, sex differences and/or perimenopausal changes, both amnestic and non-amnestic MCI, or non-epidemiological risk factors (e.g., genetic polymorphisms) were also included so long as the connection to AD was explicitly tested or stated. Various Stage II and III clinical trials for pharmacological treatments and PET radiotracers for AD were eligible for inclusion as well. Some studies examined associations across multiple large datasets (e.g., Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Alzheimer’s Disease Cooperative Study (ADCS)) and presented race/ethnicity breakdowns for these databases separately. In these cases, each database was included as a separate study/row (e.g. one study that reports ADNI and ADCS data separately is counted as two studies, one for each dataset). Notably, studies comprised entirely of individuals with non-AD dementias (e.g., Lewy-Body/Parkinson’s Disease, frontotemporal dementia, vascular dementia) were excluded; studies examining multiple types of dementia were included if individuals along the AD spectrum (e.g., amnestic MCI) were also part of the study.
As data was formally requested from ADNI and in accordance with ADNI publishing policy: data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. For up-to-date information, see www.adni-info.org.
Using these eligibility criteria, a total of 11,871 full-text articles were reviewed. Articles excluded during this stage of review met at least one of the following criteria: Not related to AD (e.g., all participants had Parkinson’s Disease, study focused on another health condition); no neuroimaging outcomes (e.g., blood biomarkers or cognition outcomes only, histological studies); non-human study; non US-based study sample; review article/meta-analysis; case study; clinical recommendations; commentary or letters to the editor; or other miscellaneous articles (e.g., summary of conference proceedings, description of technical procedure/statistical technique, consumer-facing informational pamphlet).
Eligible studies
Studies that met all inclusion criteria fell into one of two groups. A portion of studies, hereafter referred to as “direct studies”, reported the race/ethnicity breakdown of their sample either within the text or to a direct link that had this information (e.g., to a clinicaltrials.gov link that reported the race/ethnicity composition of the sample). A second group of studies, hereafter referred to as “indirect studies”, did not report race/ethnicity information but instead described a larger study or database from which the study sample derived. For these larger studies/databases, we obtained demographic characteristics of the larger cited study/database using either formal data requests (e.g., Alzheimer’s Disease Neuroimaging Initiative), or recently published manuscripts using these datasets that reported race/ethnicity information. Use of such data for this review was consistent with consents and data access approvals from formal data requests.
Data extraction
The following study characteristics were extracted from eligible full-text articles: year of manuscript publication, total sample size, and direct/indirect study classification (i.e., whether race/ethnicity data was directly from the study/a proximal link or if it was generalized from a larger database). Sample sizes and percentages of total samples were also recorded for the following race/ethnicity categories based on the 1997 US Census: Hispanic/Latino ethnicity; white; Black/African American; American Indian/Alaska Native; Asian American; Native Hawaiian/Other Pacific Islander; Multiracial; Some Other Race; and Unknown Race43. Unknown race was included because the majority of studies did not elaborate upon race/ethnicity breakdowns for non-white categories.
For studies that only reported Ns of race/ethnicity categories, percentages for each category were calculated, while approximate Ns were calculated for studies that only reported percentages. Many studies reported Ns/% only for white participants. In these instances, as we could not determine the breakdown of other race/ethnic groups, the remaining non-white N/% was included as “Unknown” (e.g., 80% white, 20% Unknown). Participants who were classified as “Caucasian” in some studies were included in the “white” race category, consistent with historical usage of the term in medical literature44. Individuals who were classified as “European” or of European ancestry were coded in the Unknown race category, as race and ethnicity classifications do not consistently map onto genetic ancestry and particularly for European ancestry45. Studies differed in their approach to reporting Hispanic/Latino Ns/%. Multiple studies reported race and ethnicity separately, consistent with Census reporting of Hispanic/Latino ethnicity. Some studies reported Hispanic/Latino as a race category that summed with the other race categories to 100% (e.g., 34% white, 36% Black, 30% Hispanic/Latino, 0% Other). In the above example, it was therefore presumed that the white and Black individuals were non-Hispanic based on the sum total of 100%. Many studies reported race but no ethnicity data, while some studies with 100% white participants specifically stated that their white participants were non-Hispanic white; in the latter example, we therefore tabulated the study as 0% Hispanic/Latino. In ~2% of studies that reported race data, the reported sample size differed from the sum of Ns’ in race categories, even after accounting for cases in which Hispanic/Latino ethnicity was counted as race in some studies (e.g., reported total N = 1000, sum of race/ethnicity = 940). As total N was used to calculate percentages, a primary outcome in this review, we utilized the summed total race N (e.g., we used N = 940 rather than N = 1000 in the above hypothetical example) rather than the reported total N in these instances to more accurately reflect race/ethnicity percentages of individual studies.
Analyses
This review focuses on understanding race/ethnicity composition of research samples in US-based AD neuroimaging research. For direct studies, we therefore utilized scatterplots and histograms to understand: (1) distribution of race/ethnicity across relevant studies using median and/or mean; (2) racial/ethnic composition (i.e., % race/ethnicity) of study samples as a function of time (i.e., publication year). For this second aim, we utilized a median split on publication year for all studies. This yielded two time periods, 1994–2017 (n = 361 studies) and 2018–2022 (n = 358 studies) to compare race/ethnicity data for direct studies.
We separately examined the histograms and obtained mean/medians for larger databases cited in indirect studies that did not directly report race/ethnicity breakdowns of their samples. We also tabulated the number of times such larger databases were cited in indirect studies.
A risk of bias analysis within and across studies was considered, but race/ethnicity composition is not a modifiable outcome variable and it cannot be determined within the scope of this review whether studies or authors self-selected into reporting race/ethnicity compositions of study samples. The vast majority of studies also reported demographics for participants that met study-specific inclusion criteria rather than demographics of respective screening samples, and we therefore could not determine whether study-specific screening procedures influenced final race/ethnicity compositions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Literature search
A total of 2,464 articles were included after the full-text screening process. Of these, there were 719 direct studies reporting race/ethnicity data directly in-text or through a direct link, and 1745 indirect studies that did not report race/ethnicity data but derived from a larger database/cohort study that did report this data. Indirect studies were drawn from a total of 44 databases/studies.
Articles directly reporting race/ethnicity data
Among direct studies, there was variability in the type of race/ethnicity data reported (see Table 1). The vast majority of articles (n = 715/719) reported white race data, with fewer numbers of studies reporting non-white races/ethnicities (ns = 281 of 719 studies for Hispanic/Latinos to 467 of 719 studies for Black/African Americans). About 36% of studies reported Non-Hispanic white data. Similar patterns were observed for indirect studies derived from larger databases. These larger studies/databases reported white race data, but fewer studies/databases reported other racial/ethnic compositions (ns = 21 of 44 databases for Native/Hawaiian/Other Pacific Islanders to 30 of 44 databases for Black/African Americans), and less than half reported Non-Hispanic white data (n = 18/44). The median sample size was 231, with a range of 11 to 19,309 participants.
Table 1.
Race/Ethnicity of Alzheimer’s Disease neuroimaging studies directly (n = 719) and indirectly (n = 44 cohort studies) reporting this data.
| Race/Ethnicity | N(%) | Median % | Mean % |
|---|---|---|---|
| Direct Studies (n = 719) | |||
| Hispanic/Latino | 281(39%) | 3.39 | 14.87 |
| White | 715(99%) | 88.85 | 79.07 |
| Non-Hispanic White | 257(36%) | 87.40 | 71.44 |
| Black/African American | 467(65%) | 7.32 | 14.68 |
| American Indian/Alaska Native | 375(52%) | .00 | .70 |
| Asian American | 397(55%) | .00 | 2.87 |
| Native Hawaiian/Other Pacific Islander | 360(50%) | .00 | .04 |
| Multiracial | 360(50%) | .00 | .25 |
| Some Other Race | 357(50%) | .00 | .14 |
| Unknown | 717(99%) | .66 | 4.71 |
| Indirect Studies (n = 1745) | |||
| Hispanic/Latino | 25(57%) | 4.67 | 10.72 |
| White | 44(100%) | 84.18 | 78.92 |
| Non-Hispanic White | 17(39%) | 83.28 | 73.44 |
| Black/African American | 30(68%) | 11.59 | 14.29 |
| American Indian/Alaska Native | 22(50%) | .00 | .26 |
| Asian American | 29(66%) | 1.75 | 2.98 |
| Native Hawaiian/Other Pacific Islander | 21(48%) | .00 | .10 |
| Multiracial | 22(50%) | .00 | .66 |
| Some Other Race | 21(48%) | .00 | .11 |
| Unknown | 44(100%) | .77 | 4.04 |
Note. Indirect Studies (n = 1745) derive data from cohort studies/databases (n = 44) that are reported in this table. Race/Ethnicity categories derived from the 1997 United States Census. N represents the number of studies reporting respective race/ethnicity information. Median and Mean % represent the median and mean study’s percentage composition for each race/ethnicity category.
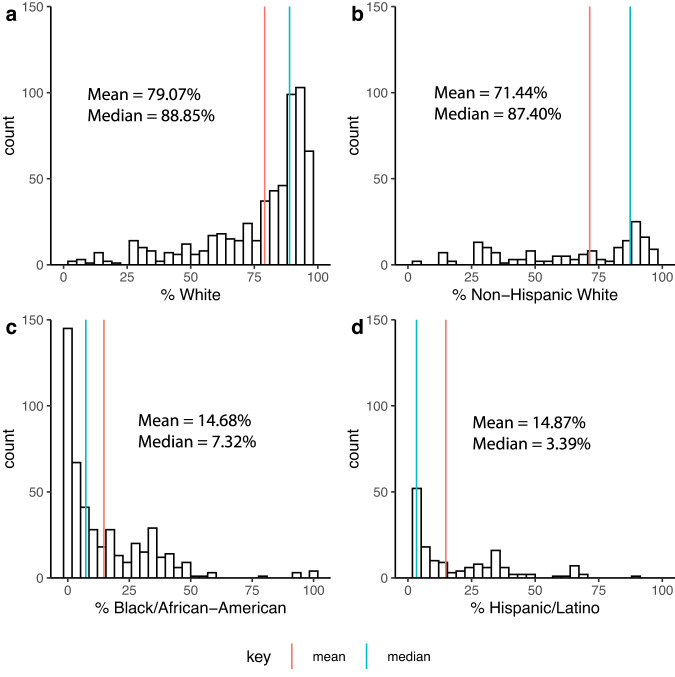
The distribution of race/ethnicity data reported across studies was skewed for all categories (see Fig. 2). Both mean and median % race/ethnicity for all studies was calculated, but median % is reported as the optimal measure of central tendency in light of this skew. Among direct studies, the median study was 88.9% white (87.4% Non-Hispanic white), 7.3% Black/African American, and 3.4% Hispanic/Latino ethnicity (Fig. 2). Asian American, American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, Multiracial, and Some Other Race are not depicted in these figures, but had median representation of 0.00%. Median Unknown race was 0.7%, and may be slightly elevated due to the number of studies that solely reported % white race. Notably, 54% of direct studies (n = 390/719) investigated race/ethnicity as a primary aim, and/or included statistical analyses of race/ethnicity (e.g., between diagnostic groups, as a covariate, or in stratified comparisons).
Fig. 2. Histogram of the racial/ethnic composition of Alzheimer’s Disease neuroimaging studies directly reporting race/ethnicity data.
Mean and median % representation of (a) white; (b) Non-Hispanic white; (c) Black/African American; and (d) Hispanic/Latino participants for studies directly reporting race/ethnicity data (n = 719). Count represents the number of studies reporting a given %.
Race/ethnicity over time
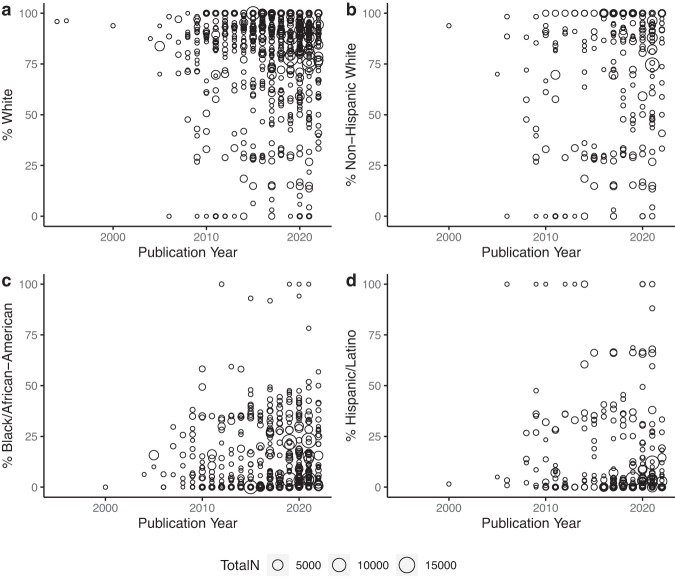
A visualization of race/ethnicity data over time is shown in Fig. 3, with a summarized depiction in Supplementary Fig. 1. While formal analyses were not conducted due to likely violations of multiple tests’ assumptions (i.e. non-independent observations and lack of information about study-specific nested data), at minimum there has been an increase in the number of studies in which Non-Hispanic white participants comprised <50% of the sample; the first such AD neuroimaging study in this review was published in 2006, and 13 such studies were published in 2021. Approximately half of the 90 articles with <50% white composition from 1994–2022 were published since 2017. Median race and ethnicity % using a median split for publication year are also shown in Table 2. Comparisons of the two periods from 1994–2017 and 2018–2022 indicated similar median % race and ethnicity percentages and interquartile ranges across the two time periods. The only notable change may be for median % Black/African American, which was 3.39% from 1994–2017 and 8.29% from 2018–2022.
Fig. 3. Study race/ethnicity composition by publication year for Alzheimer’s disease neuroimaging studies directly reporting race/ethnicity data.
Time by % representation of (a) white; (b) Non-Hispanic white; (c) Black/African American; and (d) Hispanic/Latino participants for studies directly reporting race/ethnicity data (n = 719). Each study represents a dot in this scatterplot. % Race refers to % of participants in each study. Total N (dot size) refers to the sample size of each study.
Table 2.
Median-split publication year by race/ethnicity for direct studies (n = 719).
| Race/Ethnicity | Time 1: 1994–2017 | Time 2: 2018–2022 | ||||
|---|---|---|---|---|---|---|
| Median % | N | Interquartile Range | Median % | N | Interquartile Range | |
| White | 89.45% | 360 | 71.84–96.06% | 88.53% | 355 | 71.54–94.18% |
| Non-Hispanic White | 86.40% | 128 | 31.40–100% | 87.50% | 129 | 50.05–99.15% |
| Hispanic/Latino | 3.39% | 133 | 0.00–28.34% | 3.37% | 148 | 0.00–18.39% |
| Black/African American | 5.56% | 229 | 0.00–25.83% | 8.29% | 238 | 2.08–27.73% |
| Asian American | 0.00% | 187 | 0.00–0.83% | 0.00% | 210 | 0.00–1.69% |
| American Indian/Alaska Native | 0.00% | 180 | 0.00% | 0.00% | 195 | 0.00% |
| Native Hawaiian/Other Pacific Islander | 0.00% | 175 | 0.00% | 0.00% | 185 | 0.00% |
| Multiracial | 0.00% | 176 | 0.00% | 0.00% | 184 | 0.00% |
| Some Other Race | 0.00% | 176 | 0.00% | 0.00% | 181 | 0.00% |
| Unknown | 0.58% | 360 | 0.00–6.54% | 0.67% | 357 | 0.00–6.58% |
Note. Median-split on publication year produces two time periods, 1994–2017 and 2018–2022. N represents the
number of studies reporting respective race/ethnicity information. Median % represent the median study’s percentage composition for each race/ethnicity category.
Indirect studies derived from larger databases
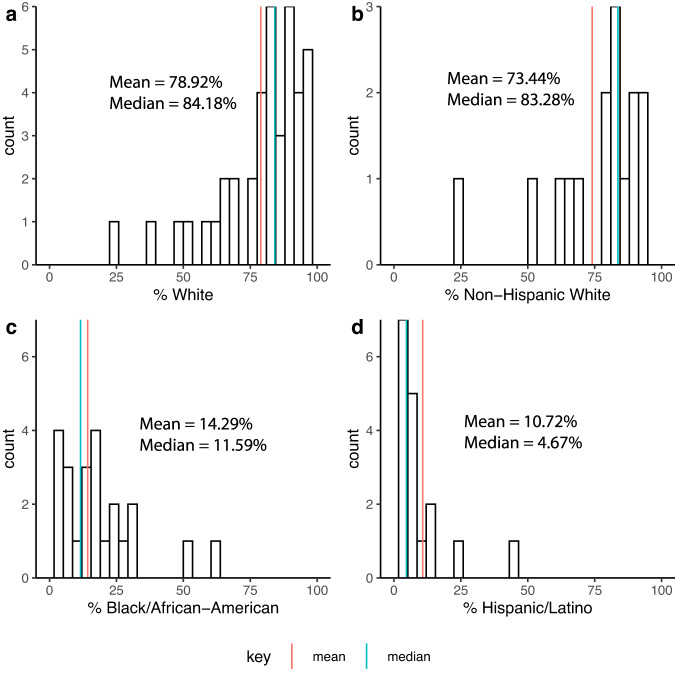
Indirect studies (n = 1745) derived from 44 larger databases/cohort studies and race/ethnicity was similarly skewed as direct studies (see Fig. 4). Among these 44 databases, median representation was 84.2% white (83.7% Non-Hispanic white), 11.6% Black/African American, 4.7% Hispanic/Latino, and 1.75% Asian American (Table 1; Fig. 4). American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, Multiracial, and Some Other Race had median representation of 0.00%. Median Unknown Race was 0.8%. The 44 databases/studies are shown in Table 3, with ~70% of the 1745 indirect studies drawing data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), and 94% drawing from 10 databases. The median sample size of these larger databases was 1575, with a range of 57 to 45,923 participants.
Fig. 4. Histogram of the racial/ethnic composition of Alzheimer’s Disease neuroimaging studies indirectly reporting race/ethnicity data.
Mean and median % representation of (a) white; (b) Non-Hispanic white; (c) Black/African American; and (d) Hispanic/Latino participants for cohort studies/databases (N = 44) from which studies indirectly report race/ethnicity data (n = 1745). Count represents the number of cohort studies reporting a given %.
Table 3.
Number of studies that indirectly report race/ethnicity data (n = 1745) and cite larger databases.
| Study/Database | Abbreviation | # of Studies |
|---|---|---|
| Alzheimer’s Disease Neuroimaging Initiative | ADNI | 1219 |
| Mayo Clinic Study of Aging | MCSA | 103 |
| Harvard Aging Brain Study | HABS | 63 |
| Open Access Series of Imaging Studies | OASIS | 57 |
| Framingham Heart Study | FHS | 51 |
| Dominantly Inherited Alzheimer Network | DIAN | 46 |
| Wisconsin Registry for Alzheimers Prevention | WRAP | 43 |
| Religious Orders Study/Memory and Aging Project | ROSMAP | 24 |
| Baltimore Longitudinal Study of Aging | BLSA | 22 |
| National Alzheimer’s Coordinating Center—Uniform Data Set | NACC UDS | 14 |
| University of Kansas Brain Aging Project | UKBAP | 10 |
| Dallas Lifespan Brain Study | DLBS | 9 |
| Human Connectome Project—Aging | HCP-A | 9 |
| BIOCARD Study | BIOCARD | 7 |
| Cardiovascular Health Study | CHS | 7 |
| A4 Study | A4 | 6 |
| Atherosclerosis Risk in Communities—Neurocognitive Study | ARIC-NCS | 5 |
| Kronos Early Estrogen Prevention Study | KEEPS | 5 |
| Arizona APOE Cohort | AAC | 5 |
| Oregon Brain Aging Study | OBAS | 4 |
| Cache County Study on Memory in Aging | CCSMA | 4 |
| Central Control of Mobility in Aging | CCMA | 4 |
| Washington Heights Inwood Columbia Aging Project | WHICAP | 3 |
| The 90+ Study | – | 3 |
| Ginkgo Evaluation of Memory Study | GEM | 2 |
| Reference Ability Neural Network Study | RANN | 2 |
| EXPEDITION-3 | – | 1 |
| The Aging Brain Study | ABS | 1 |
| MISSION AD | MISSION AD | 1 |
| Investigation Into Delay to Diagnosis of Alzheimer’s Disease with Exelon | InDDEx | 1 |
| Dartmouth Memory and Aging Study | DMAS | 1 |
| Action to Control Cardiovascular Risk in Diabetes—Memory in Diabetes | ACCORD MIND | 1 |
| Einstein Aging Study | EAS | 1 |
| Chicago Health and Aging Project | CHAP | 1 |
| Vietnam Era Twin Study of Aging | VETSA | 1 |
| Coronary Artery Risk Development in Young Adults | CARDIA | 1 |
| UC Davis Aging Diversity Cohort | UCD ADC | 1 |
| Knight Alzheimer Disease Research Center | Knight ADRC | 1 |
| Imaging Dementia—Evidence for Amyloid Scanning | IDEAS | 1 |
| UCSF Hillblom Aging Network | UCSF HAN | 1 |
| Monongahela-Youghiogheny Healthy Aging Team | MYHAT | 1 |
| Intelligent Systems for Assessment of Aging Changes | ISAAC | 1 |
| Sacramento Area Latino Study on Aging | SALSA | 1 |
| Genomics Superstruct Project | GSP | 1 |
Discussion
This descriptive review of AD neuroimaging studies examined race/ethnicity composition of US-based research samples in a PubMed search of English, free full-text articles published until September 2022. We identified 2459 articles, of which 719 were direct studies reporting race/ethnicity data directly and 1745 were indirect studies that did not report this data, but instead derived from a cohort study/database that reported this information. Median representation of race/ethnicity data from direct studies was 88.9% white and 87.4% Non-Hispanic white, 7.3% Black/African American, and 3.4% Hispanic/Latino ethnicity, with 0% median representation of other race categories. The 44 databases from which 1745 indirect studies derived race/ethnicity data tended to be slightly more diverse, with the median database comprised of 84.2% white, 83.7% Non-Hispanic white, 11.6% Black/African American, 4.7% Hispanic/Latino, and 1.75% Asian American participants. Median representation of American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, and Multiracial were 0%.
Comparisons of this data with census information on US adults age 65 and older shed light on underrepresentation issues. The most recent census report on older Americans in 2020 indicated that 76% of older adults were Non-Hispanic white, 9% Black/African American, 9% Hispanic ethnicity, 5% Asian American, 0.6% American Indian/Alaska Native, 0.1% Native Hawaiian/Pacific Islander, and 0.8% multiracial46. Based on current older adult data, Hispanic/Latino and Asian American individuals demonstrate the greatest disparity between census representation and median AD neuroimaging study participant representation, followed closely by Black/African Americans, who demonstrate the highest risk of AD amongst all racial and ethnic groups5. Further, multiracial, American Indian/Alaska Native, and Native Hawaiians/Pacific Islander individuals essentially have no reported representation in AD neuroimaging study samples. Beyond comparisons with current cross-sectional race and ethnicity census data, the overall number of older adults and proportion of older adults who are ethnic/racial minorities has been and is expected to continue increasing in the US; approximately 13% of older adults were race/ethnic minorities in 1990, 15%, 20%, and 24% in 1999, 2009, and 2019, respectively, and this share is estimated to increase to 34% by 204046–48. On a basis of minority group composition, median race/ethnicity representation from studies in this review most closely approximate Census data from 1990 and 1999 for direct and indirect studies, respectively.
Racial/ethnic group representation in AD neuroimaging studies in the US is low but may be slowly improving over time. Comparing median sample compositions from 1994–2017 and 2018–2022 indicates that median % white (89.5% and 88.5%) and Non-Hispanic white (86.4% and 87.5%) remained relatively unchanged in direct studies, despite increased ethnic/racial diversity in older adult census data. The one promising representation change is that median % Black/African American participants in research samples may have improved slightly from 3.4% to 8.3% during these two time periods. Increasing representation of Black/African American individuals is particularly important, given elevated risks of AD among Black/African American populations and repeated calls for improving representation of this group in AD research49,50. The proportion of another minoritized group with high AD incidence, Hispanic/Latino individuals, remained static between these two time periods (3.4% and 3.4%), which further underscores severe underrepresentation of Hispanic/Latino individuals in AD neuroimaging research. Median representation of all other racial groups (Asian Americans, American Indian/Alaska Natives, Native Hawaiian/Other Pacific Islanders, and multiracial) has been consistently low, with median 0.00% representation across the two time periods. Overall, these trends appear to demonstrate that published studies retain similar levels of majority white samples over time, though the proportion of studies with more African-Americans may be increasing in recent years. Further, there is a small but growing number of studies that focus on minority recruitment and are composed of <50% white participants, with one such publication in 2006 and 13 in 2021.
Overall trends in diversifying US research populations may also be reflected in the relatively greater diversity in indirect study databases relative to direct studies. These 44 databases appeared to have a lower median proportion of white participants relative to the 719 direct studies (84.2% white vs 88.9%; 83.7% Non-Hispanic white vs 87.4% Non-Hispanic white), and greater median numbers of Black (11.6% Black vs 7.3% Black), Hispanic/Latino (4.7% vs 3.4%) and Asian American (1.75% vs 0.00%) participants, though American Indian/Alaska Natives and Native Hawaiians/Other Pacific Islanders were again shown to have essentially zero representation. This greater relative diversity may be attributable to cohort studies’ active efforts to improve participant diversity in recent years, as race/ethnicity composition data was acquired for databases’ current participant numbers using formal data requests or recently published articles that contain this information. Large cohort studies of minority groups are increasingly recruiting participants, such as the Minority Aging Research Study51 and Latino CORE study at Rush University52, UC Davis Diversity Cohort53, the Health & Aging Brain among Latino Elders Study54, University of North Texas Health Science Center’s Black Alzheimer’s Brain Study (https://blackalzbrainstudy.com/), and the Study of Latinos—Investigation of Neurocognitive Aging (SOL-INCA)55. New initiatives in Alzheimer’s research participant registries target specific groups, such as the Collaborative Approach for Asian Americans and Pacific Islanders Research and Education Registry (https://www.alzheimers.gov/clinical-trials/care-registry-asian-americans-and-pacific-islanders-0). The African American Outreach Satellite has been successful in doubling African American enrollment as a part of Knight Alzheimer’s Disease Center at Washington University St. Louis56. These efforts include aspects of relationship-building within racial/ethnic communities and cultural institutions therein, multilingual materials and resources, focused advertisement and recruitment campaigns, hiring of diverse research staff, and sharing of research findings through public talks. Expanding such research efforts to strengthen and maintain relationships with such communities may be critical to the race/ethnicity representation improvements seen over time.
Increasing diversity recruitment efforts in ADNI may also reflect recent enrollment trends in large databases that will have impacts on AD research in the US. ADNI-1 was completed in 2010 and comprised of 90.4% Non-Hispanic white participants, while ADNI is 84.3% Non-Hispanic white across all phases from currently available baseline data (ADNI, 2022). ADNI4, the next phase of ADNI, aims to further improve on race/ethnicity composition by recruiting 50–60% of its new participants from underrepresented populations, including racial/ethnic minorities and those from lower socioeconomic backgrounds57. Further, the most recent ADNI publication indicates a total ADNI sample of 79.3% white, 11.5% Black, 5.6% Latinx, 2.7% Asian, 0.8% Native American, and 0.5% Other Race individuals57. Of note, approximately 70% of the 1745 indirect studies utilized ADNI data. If articles in this review reasonably reflect the US AD literature, large databases like ADNI have a responsibility to diversify samples not only to address historical underrepresentation, but also expand inquiry into mechanisms that increase AD vulnerability in these groups in light of how frequently ADNI data is published. ADNI has been critical to elucidating AD processes58, refining statistical techniques and predictive models59, and identifying targets for longitudinal treatment effects and biomarker thresholds60–62. ADNI data has been combined with other large population studies to examine genetic AD risk factors among Non-Hispanic white populations63. Expanding the number of racial/ethnic minorities in ADNI will provide rich datasets that allow for similar such investigations and cross-study comparisons across race/ethnicity groups in the US.
Improved recruitment of racial and ethnic minoritized groups is needed beyond large cohort databases in the US. Such large-scale volunteer databases are frequently used for complex data analyses that elicit conclusions assumed to be broadly applicable. In this review, 94% of indirect studies drew data from 10 such databases. The AD research community demonstrates significant density in social network structure64, and increasingly focuses on big data to advance AD research goals65. These volunteer databases are not frequently representative of general populations, however, in that participants are more likely to be female, less likely to live in socioeconomically deprived areas, are better educated, have higher incomes, and require fewer medications66–70. Large scale-volunteer databases require substantial resources and increasingly dominate funding and publication proportions across fields, but may not be well-equipped to address inequalities in health and aging71. Some in AD research have acknowledged these shortcomings72–74, and many existing epidemiologic US population-based cohorts that examine AD are not racially/ethnically diverse. Nationally representative surveys have identified race and ethnicity-specific risk factors for AD, including midlife obesity for American Indian and Alaska Native individuals, Black individuals, and white individuals; low years of education for Hispanic/Latino individuals, and physical inactivity for Asian American individuals14. AD neuroimaging studies may similarly benefit from diverse representation to examine race/ethnic disparities in risk factors and adverse experiences like stressful life events or medical comorbidities. This is especially relevant if adversity and other sequelae contribute to AD risk and impact the likelihood of receiving specialized AD treatment75,76. Representativeness may also be needed to understand heterogeneity in experiences within racial/ethnic groups contributing to differential AD outcomes. Efforts to improve representativeness of samples that contribute data to large-scale efforts like the US National Alzheimer’s Coordinating Center (NACC), in addition to smaller cohort samples that focus on representativeness can reduce potential bias by recruiting ethnic/racial minoritized individuals across a spectrum socioeconomic status, education, and health conditions.
AD neuroimaging studies in the US should also more openly publish race/ethnicity composition of research samples, and indicate data sources in secondary analyses. In this review, 719 articles reported this data directly and 1745 indirectly from a larger database, while an additional 1160 articles met study criteria but neither reported data directly nor cited a specific database from which the study derived. Approximately 20% of studies that met criteria for inclusion in this review therefore directly reported this information. Further, 64% of studies that directly reported race/ethnicity data did not report data for at least one race/ethnicity category, and 99.4% of direct studies reported data on white participants. Approximately a third of direct studies did not report where their data was sourced, and we therefore could not determine whether studies reflect potential geographic disparities in AD neuroimaging research representation. Transparency and reporting of this data using established guidelines44 is needed to understand the landscape of race and ethnic group representation in US AD neuroimaging research.
This descriptive review has strengths and limitations. Strengths include a review of all available articles with appropriate parameters on a well-indexed publication search, consideration of studies that directly report race/ethnicity and those that drew from larger databases that report this information, and examination of race/ethnicity composition over time. While race/ethnicity disparities in study representation have been acknowledged for decades, this is the first study to broadly quantify race/ethnicity representation in Alzheimer’s neuroimaging research in the United States. We believe the articles included in this review reasonably reflect the AD neuroimaging research literature in the United States. There are also important limitations. We specifically reviewed free full-text English language articles using a single database, PubMed, with a focus on US-based samples. Such a constrained search may not have captured all articles that met this review’s inclusion criteria, and limits the generalizability of these results. We did not search across multiple databases or scour references for additional relevant articles; it became clear after PROSPERO registration that PubMed was a more appropriate database for this review than PsycInfo given the biomedical nature of Alzheimer’s Disease. Further, the conclusions drawn from median percentages calculated from this review assumed race/ethnicity reporting is not biased; it is unknown if articles that did not report this data were more or less racially/ethnically diverse than articles that did report this data. Similarly, the data on 44 large databases likely underreports the number of existing AD neuroimaging databases, and may not fully capture data from large databases in this research literature. Considering missing information about data sources across studies that reported race/ethnicity data, data underlying direct studies in this review are likely, at least in part, nested. Due to the non-independent observations of percent race/ethnicity per study, a formalized statistical analysis of studies over time was not feasible. An inherent assumption in this review is that studies were consistent in how they captured participants’ race/ethnicity data. The United States Census has previously changed the form of the question inquiring about Hispanic ethnicity, which contributed to shifts in individuals’ responses, most notably between the 2000 and 2010 Census77. This review cannot determine whether questions inquiring about race/ethnicity were similar across studies. Not all studies reported race/ethnicity data for all categories, with most studies primarily reporting data from white participants. Increased transparency for future studies is needed to ensure fair and comparable percentages for race/ethnicity. Understanding representation of some minoritized groups may be limited due to the nature of US Census race/ethnicity categories. Middle-Eastern and North African (MENA) individuals are categorized as “white” on the US Census, though there is increasing evidence that a MENA label may more accurate to their lived experiences78. Hispanic/Latino individuals have historically self-reported as “Some Other Race” in research and population studies to expound on racial identities that do not conceptually align with US race taxonomy categories, with 45.3 million Hispanic/Latino individuals doing so in the 2020 Census79. Hispanic/Latino individuals may have an undercount rate of 4.99% from the 2020 Census, which further underscores Hispanic/Latino underrepresentation in AD neuroimaging research80. Future similar reviews across countries may be helpful to delineate race/ethnic diversity across countries and inform international collaborative AD research efforts.
Overall, this review demonstrates that racial/ethnic minoritized groups in AD neuroimaging research in the United States have been historically underrepresented, but diversity may be slowly improving in recent years, particularly for Black/African American participants. Understanding the landscape of Alzheimer’s Disease representation in studies is necessary to contextualize progress in correcting these imbalances and historical failures.
Supplementary information
Acknowledgements
The authors gratefully acknowledge Shaneen Upal and Catherine Kim for their expert technical assistance in completing this manuscript. This work was supported by the National Institute on Aging [grant numbers K24AG081325, 1R01AG055430, and 1RF1AG068166 to S.D.H., T32AG000037 to A.C.L., 5R01AG056405 to L.L.B., R01AG062711 to M.L., K01AG064986 to A.L.N.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the named funders. Data were provided in part by OASIS: OASIS-3: Longitudinal Multimodal Neuroimaging: Principal Investigators: T. Benzinger, D. Marcus, J. Morris; NIH P30 AG066444, P50 AG00561, P30 NS09857781, P01 AG026276, P01 AG003991, R01 AG043434, UL1 TR000448, R01 EB009352. AV-45 doses were provided by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. OASIS-3_AV1451: Principal Investigators: T. Benzinger, J. Morris; NIH P30 AG066444, AW00006993. AV-1451 doses were provided by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly. OASIS-4: Clinical Cohort: Principal Investigators: T. Benzinger, L. Koenig, P. LaMontagne Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author contributions
S.D.H. conceived of and supervised the current review. A.C.L. conducted the literature review, collated and analyzed data, and contributed to the manuscript. L.L.B., G.H.W., M.L., A.L.N., L.F., and J.H. contributed to and edited the manuscript. All authors read and approved the manuscript.
Peer review
Peer review information
Communications Medicine thanks Theodore (D) Cosco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Data used in this manuscript and used to plot the figures can be accessed from: https://github.com/aaronclim/adreview81. (10.5281/zenodo.8096807)
Code availability
Code for generating figures can be accessed from: https://github.com/aaronclim/adreview81. 10.5281/zenodo.8096807
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-023-00333-6.
References
- 1.The White House. Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. https://obamawhitehouse.archives.gov/omb/fedreg_1997standards (2022).
- 2.Jack CR, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CRJ, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020;25:E5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews KA, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures: Special Report: Race, Ethnicity and Alzheimer’s in America. https://www.alz.org/media/Documents/alzheimers-facts-and-figures-special-report-2021.pdf (2021).
- 7.Tang MX, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 8.Connell CM, Scott Roberts J, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2009;23:110–116. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta VK, et al. Disparities in age-associated cognitive decline between African-American and Caucasian populations: the roles of health literacy and education. J. Am. Geriatr. Soc. 2016;64:1716–1723. doi: 10.1111/jgs.14257. [DOI] [PubMed] [Google Scholar]
- 10.Ighodaro ET, et al. Challenges and considerations related to studying dementia in blacks/African Americans. J. Alzheimers Dis. 2017;60:1–10. doi: 10.3233/JAD-170242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorpe CT, et al. Racial and ethnic differences in initiation and discontinuation of antidementia drugs by medicare beneficiaries. J. Am. Geriatr. Soc. 2016;64:1806–1814. doi: 10.1111/jgs.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Vorst IE, Koek HL, Stein CE, Bots ML, Vaartjes I. Socioeconomic disparities and mortality after a diagnosis of dementia: results from a nationwide registry linkage study. Am. J. Epidemiol. 2016;184:219–226. doi: 10.1093/aje/kwv319. [DOI] [PubMed] [Google Scholar]
- 13.Hinton L, Franz C, Friend J. Pathways to dementia diagnosis: evidence for cross-ethnic differences. Alzheimer Dis. Assoc. Disord. 2004;18:134–144. doi: 10.1097/01.wad.0000127444.23312.ff. [DOI] [PubMed] [Google Scholar]
- 14.Nianogo RA, et al. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 2022;79:584–591. doi: 10.1001/jamaneurol.2022.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younan D, et al. Racial/ethnic disparities in Alzheimer’s disease risk: role of exposure to ambient fine particles. J. Gerontol. A Biol. Sci. Med. Sci. 2021;77:977–985. doi: 10.1093/gerona/glab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau. Projections of the Size and Composition of the U.S.https://www.census.gov/library/publications/2015/demo/p25-1143.html (2015).
- 17.Barnes LL. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat. Rev. Neurol. 2022;18:56–62. doi: 10.1038/s41582-021-00589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indorewalla, K. K., O’Connor, M. K., Budson, A. E., Guess, (DiTerlizzi) C. & Jackson, J. Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer’s disease research. J. Alzheimers Dis. 80, 927–940 (2021). [DOI] [PMC free article] [PubMed]
- 19.Shin J, Doraiswamy PM. Underrepresentation of African-Americans in Alzheimer’s trials: a call for affirmative action. Front. Aging Neurosci. 2016;8:123. doi: 10.3389/fnagi.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence J. The Indian health service and the sterilization of Native American women. Am. Indian Q. 2000;24:400–419. doi: 10.1353/aiq.2000.0008. [DOI] [PubMed] [Google Scholar]
- 21.Babulal GM, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15:292–312. doi: 10.1016/j.jalz.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fargo KN, Carrillo MC, Weiner MW, Potter WZ, Khachaturian Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement. 2016;12:1113–1115. doi: 10.1016/j.jalz.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Besser L, et al. Version 3 of the National Alzheimer’s coordinating center’s uniform data set. Alzheimer Dis. Assoc. Disord. 2018;32:351–358. doi: 10.1097/WAD.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doody RS, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 25.Khan MJ, Desaire H, Lopez OL, Kamboh MI, Robinson RAS. Why inclusion matters for Alzheimer’s disease biomarker discovery in plasma. J. Alzheimers Dis. 2021;79:1327–1344. doi: 10.3233/JAD-201318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleason CE, et al. Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: a contextualized review of the evidence. Alzheimer’s Dement. 2022;18:1545–1564. doi: 10.1002/alz.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCarli C, et al. Brain behavior relationships among African Americans, whites, and Hispanics. Alzheimer Dis. Assoc. Disord. 2008;22:382–391. doi: 10.1097/WAD.0b013e318185e7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sencakova D, et al. Hippocampal atrophy correlates with clinical features of Alzheimer disease in African Americans. Arch. Neurol. 2001;58:1593–1597. doi: 10.1001/archneur.58.10.1593. [DOI] [PubMed] [Google Scholar]
- 29.O’Bryant SE, et al. Neurodegeneration from the AT(N) framework is different among Mexican Americans compared to non-Hispanic Whites: a Health & Aging Brain among Latino Elders (HABLE) Study. Alzheimers Dement. (Amst) 2022;14:e12267. doi: 10.1002/dad2.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brickman AM, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17:1353–1364. doi: 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howell JC, et al. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimers Res. Ther. 2017;9:88. doi: 10.1186/s13195-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajan KB, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann. Neurol. 2020;88:1065–1076. doi: 10.1002/ana.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wharton W, et al. IL-9 alterations linked to Alzheimer’s disease in African Americans. Ann. Neurol. 2019;86:407–418. doi: 10.1002/ana.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonough IM. Beta-amyloid and cortical thickness reveal racial disparities in preclinical Alzheimer’s disease. Neuroimage Clin. 2017;16:659–667. doi: 10.1016/j.nicl.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeker KL, et al. Socioeconomic status mediates racial differences seen using the AT(N) framework. Ann. Neurol. 2021;89:254–265. doi: 10.1002/ana.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misiura MB, et al. Race modifies default mode connectivity in Alzheimer’s disease. Transl. Neurodegener. 2020;9:8. doi: 10.1186/s40035-020-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suchy-Dicey. A., Howard, B., Longstreth, W. T., Reiman, E. M. & Buchwald, D. APOE genotype, hippocampus, and cognitive markers of Alzheimer’s disease in American Indians: Data from the Strong Heart Study. Alzheimers Dement. 10.1002/alz.12573 (2022). [DOI] [PMC free article] [PubMed]
- 38.Suchy-Dicey A, et al. Cognitive correlates of MRI-defined cerebral vascular injury and atrophy in elderly American Indians: the cerebrovascular disease and its consequences in American Indians study. J. Int. Neuropsychol. Soc. 2020;26:263–275. doi: 10.1017/S1355617719001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovitch H, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology. 2001;57:226–234. doi: 10.1212/WNL.57.2.226. [DOI] [PubMed] [Google Scholar]
- 40.White LR, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia aging studies. Neurology. 2016;86:1000–1008. doi: 10.1212/WNL.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Census Bureau. Population Estimates Program.https://www.census.gov/programs-surveys/popest.html (2010).
- 44.Flanagin A, Frey T, Christiansen SL. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326:621–627. doi: 10.1001/jama.2021.13304. [DOI] [PubMed] [Google Scholar]
- 45.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum. Genom. 2015;9:1. doi: 10.1186/s40246-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Administration for Community Living. 2020 Profile of Older Americans. https://acl.gov/sites/default/files/aging%20and%20Disability%20In%20America/2020Profileolderamericans.final_.pdf (2021).
- 47.AARP Public Policy Institute. Racial and Ethnic Differences Among Older Adults in Long-Term Care Ser. https://assets.aarp.org/rgcenter/il/fs119_ltc.pdf (2022).
- 48.U.S. Census Bureau. The Older Population in the United States. https://www.census.gov/content/dam/Census/library/publications/2000/demo/p20-532.pdf (2000).
- 49.Barnes LL, Bennett DA. Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff. (Millwood) 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power MC, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic black and white individuals in the United States, 2000–2016. JAMA Neurol. 2021;78:275–284. doi: 10.1001/jamaneurol.2020.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The minority aging research study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr. Alzheimer Res. 2012;9:734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquez DX, et al. Representation of older Latinxs in cohort studies at the Rush Alzheimer’s disease center. Neuroepidemiology. 2020;54:404–418. doi: 10.1159/000509626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan ML, et al. APOE effects on late life cognitive trajectories in diverse racial/ethnic groups. J. Int. Neuropsychol. Soc. 2022;4:1–10. doi: 10.1017/S1355617722000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Bryant S, et al. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: Results from the HABLE study. Alzheimers Dement. 2022;18:240–250. doi: 10.1002/alz.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González HM, et al. A research framework for cognitive aging and Alzheimer’s disease among diverse US Latinos: design and implementation of the Hispanic Community Health Study/Study of Latinos—Investigation of Neurocognitive Aging (SOL-INCA) Alzheimer’s Dement. 2019;15:1624–1632. doi: 10.1016/j.jalz.2019.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams MM, Meisel MM, Williams J, Morris JC. An interdisciplinary outreach model of African American recruitment for Alzheimer’s disease research. Gerontologist. 2011;51:S134–S141. doi: 10.1093/geront/gnq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiner, M. W. et al. Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimer’s Dement.18, 307–317 (2023). [DOI] [PMC free article] [PubMed]
- 58.Petersen RC, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI) Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venugopalan J, Tong L, Hassanzadeh HR, Wang MD. Multimodal deep learning models for early detection of Alzheimer’s disease stage. Sci. Rep. 2021;11:3254. doi: 10.1038/s41598-020-74399-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jutten RJ, et al. Finding treatment effects in Alzheimer trials in the face of disease progression heterogeneity. Neurology. 2021;96:e2673–e2684. doi: 10.1212/WNL.0000000000012022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer PF, Pichet Binette A, Gonneaud J, Breitner JCS, Villeneuve S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated Tau and AV1451 positron emission tomography. JAMA Neurol. 2020;77:508–516. doi: 10.1001/jamaneurol.2019.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaw LM, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neu SC, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer’s disease. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbash S. Dynamics of social network structure for Alzheimer and lymphoma scientific communities. Biol. Direct. 2015;10:6. doi: 10.1186/s13062-015-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang R, Simon G, Yu F. A review of big data promises. Int. J. Med. Inform. 2017;106:48–56. doi: 10.1016/j.ijmedinf.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaziano JM, et al. Million veteran program: a mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fry A, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pirastu N, et al. Genetic analyses identify widespread sex-differential participation bias. Nat. Genet. 2021;53:663–671. doi: 10.1038/s41588-021-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor AE, et al. Exploring the association of genetic factors with participation in the Avon longitudinal study of parents and children. Int. J. Epidemiol. 2018;47:1207–1216. doi: 10.1093/ije/dyy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brayne C, Moffitt TE. The limitations of large-scale volunteer databases to address inequalities and global challenges in health and aging. Nat. Aging. 2022;2:775–783. doi: 10.1038/s43587-022-00277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashford, M.T. et al. Screening and enrollment of underrepresented ethnocultural and educational populations in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 10.1002/alz.12640 (2022). [DOI] [PMC free article] [PubMed]
- 73.National Alzheimer’s Coordinating Center. About NACC Data. https://naccdata.org/requesting-data/nacc-data (2022).
- 74.Rabin JS, et al. Interactive associations of vascular risk and β-Amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard aging brain study. JAMA Neurol. 2018;75:1124. doi: 10.1001/jamaneurol.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murchison, C. F., Kennedy, R. E., McConathy, J. E. & Roberson, E. D. Racial differences in Alzheimer’s disease specialist encounters are associated with usage of molecular imaging and dementia medications: an enterprise-wide analysis using i2b2. J. Alzheimers Dis. 79, 543–557 (2021). [DOI] [PMC free article] [PubMed]
- 76.Zuelsdorff M, et al. Stressful life events and racial disparities in cognition among middle-aged and older adults. J. Alzheimers Dis. 2020;73:671–682. doi: 10.3233/JAD-190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.U.S. Census Bureau. Factors That Influence Change in Hispanic Identification: Evidence from Linked Decennial Census and American Community Survey Data. https://www2.census.gov/ces/wp/2018/CES-WP-18-45.pdf (2018).
- 78.Maghbouleh N, Schachter A, Flores RD. Middle Eastern and North African Americans may not be perceived, nor perceive themselves, to be White. Proc. Natl Acad. Sci. USA. 2022;119:e2117940119. doi: 10.1073/pnas.2117940119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Census Bureau National Advisory Committee on Racial, Ethnic, and Other Populations. What 2020 Census Results Tell Us About Persisting Problems with Separate Questions on Race and Ethnicity in the Decennial Census.https://www2.census.gov/about/partners/cac/nac/meetings/2022-05/presentation-what-2020-census-results-tell-us.pdff (2022).
- 80.U.S. Census Bureau. Census Bureau Releases Estimates of Undercount and Overcount in the 2020 Census (U.S. Department of Commerce, 2022).
- 81.Lim, A. C. AD Review 1.0. 10.5281/zenodo.8096807 (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this manuscript and used to plot the figures can be accessed from: https://github.com/aaronclim/adreview81. (10.5281/zenodo.8096807)
Code for generating figures can be accessed from: https://github.com/aaronclim/adreview81. 10.5281/zenodo.8096807