Abstract
All types of cranioplasty techniques restore the morphology of the skull and affect patient aesthetics. Safe and easy techniques are required to enhance patients’ recovery and the rehabilitation process. We propose a new method of cranioplasty. The 3-dimensional (3D) reconstruction of a thin-layer computed tomography (CT) scan of the skull was used to reflect the intact side onto the defect and subtract the overlapping points from one another. In this way, a 3D model of the planned implant can be built in the required shape and size. The precise fit of the implant can be checked by printing the defective part of the skull in case it can be modified. A sterilisable silicone mould based on the finalized model was created afterwards. Polymethyl methacrylate implants were prepared directly in an aseptic environment in the operating room during surgery. Between 2005 and 2020, we performed 54 cranioplasties on 52 patients whose craniotomies were performed previously for indications of traumatic brain injury, stroke or tumour surgeries. No technical problems were noted during the operations. In 2 cases, septic complications that occurred were not connected to the technique itself, and the implants were removed and later replaced. Our proposed technique based on 3D-printed individual silicone moulds is a reliable, safe, easily reproducible and low-cost method to repair different skull defects.
Subject terms: Biomedical engineering, Neurology, Fracture repair
Introduction
Various techniques have been developed to repair and precisely reconstruct skull defects. The challenges of these techniques are based on individual cases, and the institutionally developed methods can be insufficient for certain patients1–3. Manual moulding with polymethyl methacrylate (PMMA) is the simplest method to cover defects. This method is still applicable to smaller defects with nearly even surfaces at easily reached locations. To cover larger surfaces, different titanium implants can be used, but the complication rate in the long term can obscure the indication in cases where plastic reconstruction of the skin is needed.
The increase in availability of 3-dimensional (3D) printing was a turning point in the area of individual osteoplasty and particularly for cranioplasty4–6. Operations after traumatic brain injury and the widespread use of decompression in stroke patients have increased the demand for cranioplasty. Easy, surgeon-friendly, reproducible and low-cost techniques are necessary to develop. A cranioplasty procedure based on a special 3D printing method, well suited even for complicated geometries, has been in use at the University of Debrecen since 2005. In this procedure, first we print a sample that matches the shape and size of the intended replacement; then, based on this cast, we produce a silicone mould that in turn can be used to fabricate a replacement made of PMMA during surgery.
Methods and materials
We performed 54 cranioplasties on 52 patients. The male/female ratio was 2.46, and the mean age was 40.2 years (SD ± 13.41). The youngest patient was 17 years old at the time of cranioplasty while the oldest was 65 years old. The mean implant volume was 52.19 cm3 (SD ± 27.37). The mean implant surface area was 218.8 cm2 (SD ± 91.04). All of the operations were performed 3 months after craniectomy. All methods were performed in accordance with the relevant guidelines and regulations.
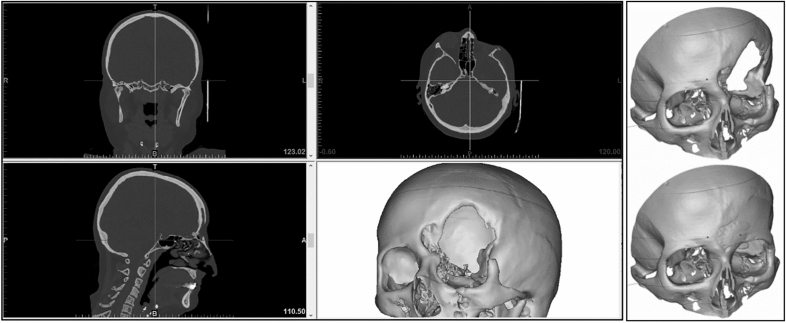
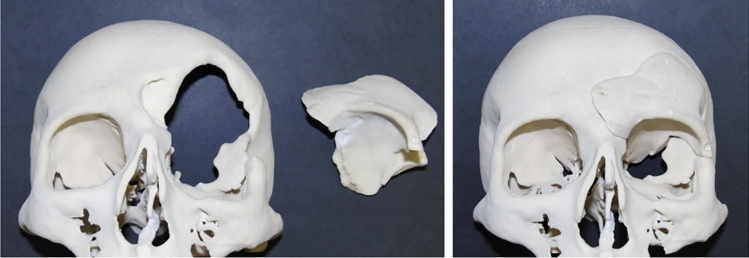
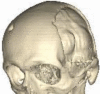
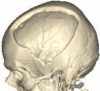
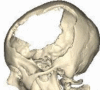
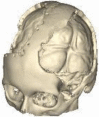
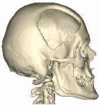
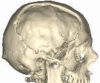
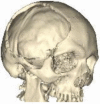
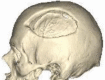
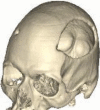
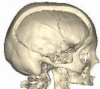
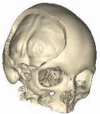
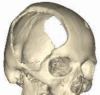
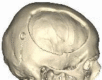
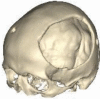
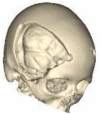
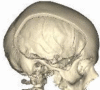
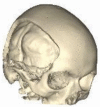
When cranioplasty was indicated, high-resolution CT scans were obtained with a slice thickness of 1 mm. The 3D reconstruction from DICOM files was performed with the Mimics® (Materialise, Belgium) software system. As a next step, a geometric form was generated that precisely fit the defect area and reproduced the original contours. This was mostly done based on the symmetry of the skull by mirroring its intact half through the midsagittal plane. To minimize the time needed for the calculations, we removed those parts of the models that are not essential according to the case (Fig. 1). The form of the implant of the cranioplasty was obtained by Boolean subtraction of the model with the defect from the reflected model representing the intact skull. If the defect cuts the symmetry plane or the replacement cannot be generated by mirroring due to other circumstances, consultation with the neurosurgeon facilitated the planning process. In 2 cases, when bilateral fronto-temporo-basal and bifronto-temporo-occipital decompression had to be reconstructed, the precompression CT scans were used in the planning process. Not only the replacement but also the defective part can be printed out to check the perfect fit before surgery. Printing was performed on Connex 260 (Stratasys, USA) equipment utilizing Objet technology. If necessary, the printed model can be fitted even more precisely by cutting, milling and grinding (Fig. 2). It is also possible to add blind holes that can lead during the operation the drill if needed. After finalizing the shape of the model, we fabricated a silicone mould using Protosil RTV 245 (Antropol, Germany), a two-component silicone material that becomes biologically inert after solidification. The mould is transparent, heat resistant up to 200 °C, and easily sterilisable; in addition, solidified PMMA does not adhere to it. Considering the hardness of the silicone 40A Shore used, the thickness of the silicon should be at least 12 mm surrounding the sample to avoid deformation while forming the low-viscosity bone cement mixture into the mould. After moulding, the silicone is kept at a temperature of 50 °C for 12 h to achieve complete solidification. All the moulds that conform to the General Data Protection Regulation (GDPR) (Fig. 3) are labelled. Through the lateral cut, the model of the defect is removed. The cut allows the silicone mould to be opened and closed like a book, paying special attention to a precise fit. To validate the process, PMMA is formed into the mould and inserted into the previously printed model containing the defect. No macroscopical gaps were observed in either case. The final implant is created at the operation theatre under sterile circumstances. PMMA is formed into the mould (Fig. 4). The forming of the bone cement must begin immediately after proper mixing to ensure that the viscosity is low enough to avoid any deformity. During the moulding process, special attention must be paid to the uniform distribution of the cement in the mould. The formation of air bubble inclusions can be prevented by using the proper technique. After polymerization for at least 10 min, the mould is opened through a lateral cut, and the implant is easily removed without any adhesion to silicone. As the last step of the procedure, the implant is fixed to the nearby bones with small plates or transosseous sutures through small bur holes. The main steps of the procedure are presented on Fig. 5.
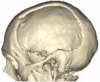
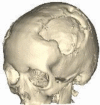
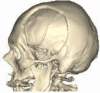
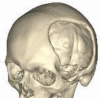
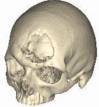
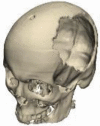
Figure 1.
3D reconstruction based on CT scans and the design process using the mirroring technique. Image was created by the authors with Materialise Mimics 21.0 software (URL: https://www.materialise.com/en/healthcare/mimics-innovation-suite).
Figure 2.
The 3D-printed skull segment and replacement. Photos were taken by the authors.
Figure 3.
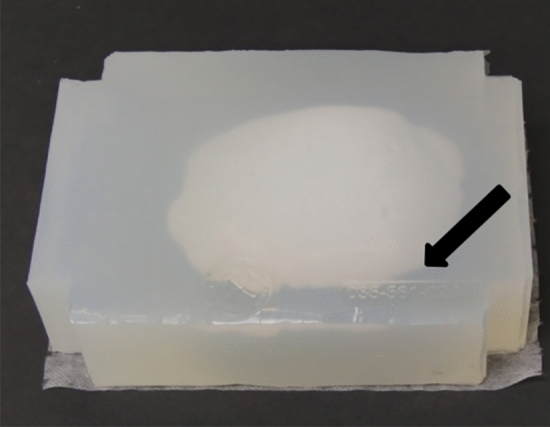
The silicone mould with the identification number. Photos were taken by the authors.
Figure 4.
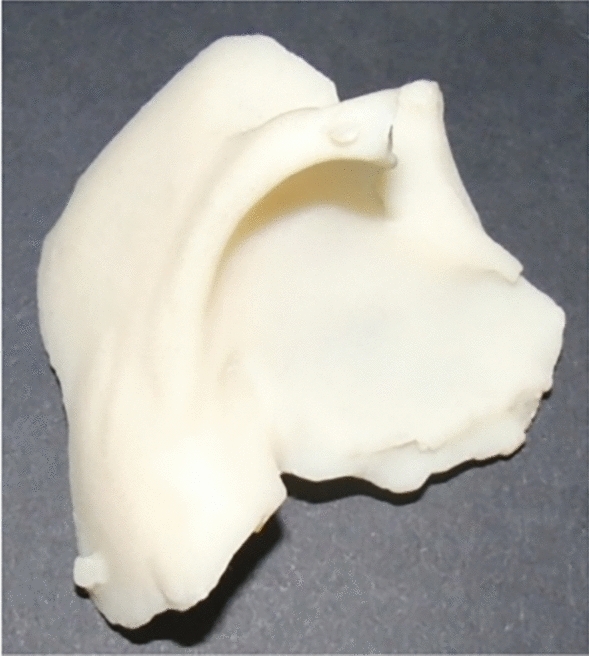
The replacement made with PMMA. Photos were taken by the authors.
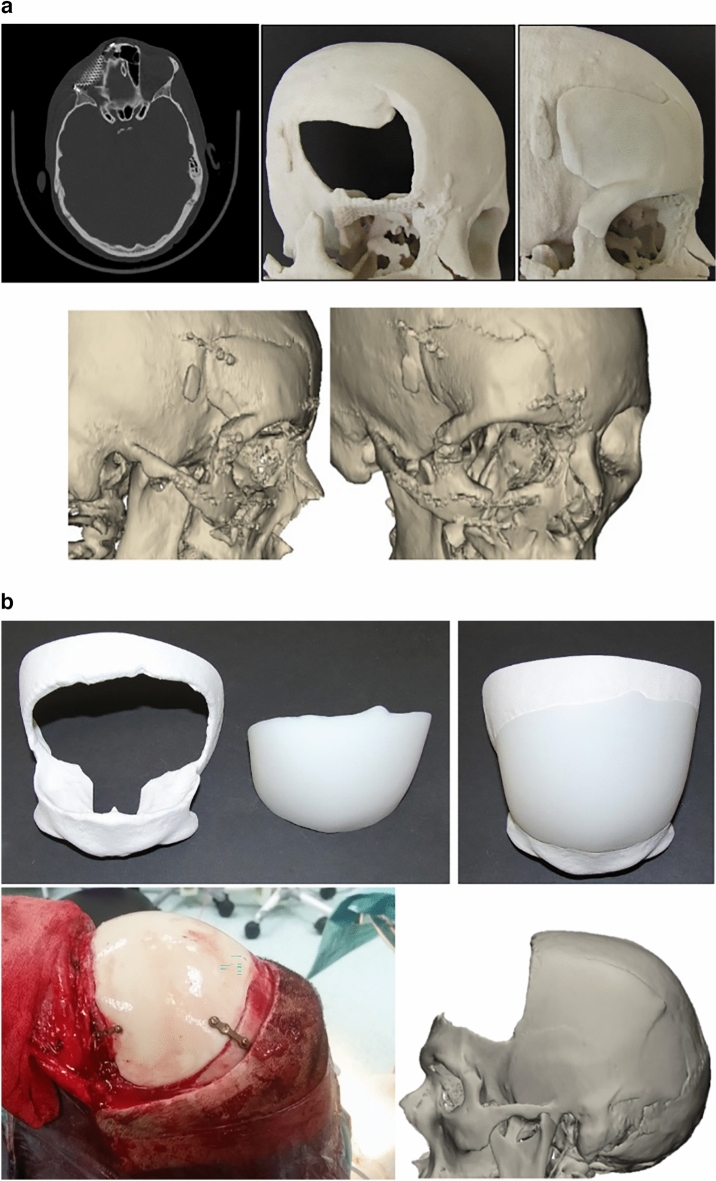
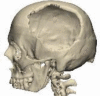
Figure 5.
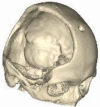
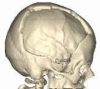
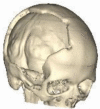
a and b Preoperative and postoperative pictures from different patients treated using our method. Photos were taken by the authors. Image was created by the authors with Materialise Mimics 21.0 software (URL: https://www.materialise.com/en/healthcare/mimics-innovation-suite).
Ethics approval
The experimental protocol was approved by the Regional and Institutional Ethics Committee, Clinical Center of the University of Debrecen (DE RKEB.IKEB 6371-2023).
Informed consent
Informed consent was obtained from all individual participants included in the study at the moment of hospitalization for surgery.
Results
In all cases, the technique was successfully used during the operations. Thirty-four implantations were performed poststroke, 4 were performed after tumour removal and 16 were performed after traumatic brain injury. Two patients had bilateral implants, one of them was composed of one piece after bifronto-temporo-basal craniectomy. Two patients from the trauma group underwent 2 operations because of wound infections that necessitated the removal of the implant. In these 2 cases, the same silicone mould was used to create the implant 3 months later (Table 1). The fittings were adequate, and postoperative cosmetics were comforting in all cases. In one patient where part of the frontal cranial base and frontal sinus had to be reconstructed after several years of open craniocerebral injury, a short period of epidural pneumocephalus developed after 2 weeks when he blew his nose. The air was absorbed within days, but another event occurred after another 3 weeks with a similar course. The reported complications were not related to the technology itself.
Table 1.
Data of the patients treated by our newly developed method.
| Age | Sex | Vol cm3 | Area cm2 | Comp | |||
|---|---|---|---|---|---|---|---|
| 49 | Female | 58.2 | 270 | – |  |
 |
 |
| 32 | Male | 45.7 | 271.5 | – |  |
 |
 |
| 39 | Male | 48.3 | 234.1 | – |  |
 |
 |
| 27 | Female | 18.6 | 107.1 | – | 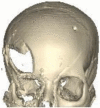 |
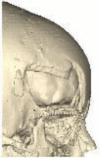 |
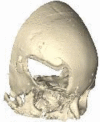 |
| 60 | Male | 52.4 | 252.7 | – | 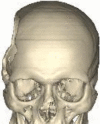 |
 |
 |
| 58 | Female | 108.3 | 355.4 | – |  |
 |
 |
| 37 | Male | 16.1 | 80.6 | – |  |
 |
 |
| 54 | Female | 35.7 | 156.1 | – | 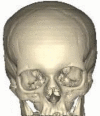 |
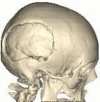 |
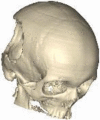 |
| 35 | Male | 20.4 | 115.2 | – | 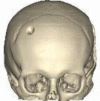 |
 |
 |
| 61 | Male | 39.2 | 231 | – |  |
 |
 |
| 21 | Male | 45.8 | 218 | – | 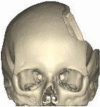 |
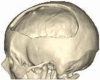 |
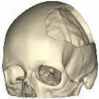 |
| 27 | Male | 62.8 | 298.7 | – | 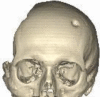 |
 |
 |
| 42 | Male | 63.59 | 236.6 | – | 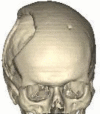 |
 |
 |
| 39 | Male | 11.9 | 53 | – |  |
 |
 |
| 33 | Male | 94.8 | 350.6 | – |  |
 |
 |
| 37 | Male | 65.3 | 197.3 | – |  |
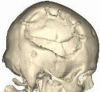 |
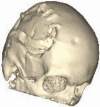 |
| 27 | Male | 69 | 288.3 | – | 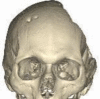 |
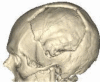 |
 |
| 21 | Male | 57.9 | 277.1 | – |  |
 |
 |
| 40 | Male | 54.8 | 233.2 | – |  |
 |
 |
| 49 | Female | 63.1 | 255.8 | – |  |
 |
 |
| 56 | Female | 10 | 58 | – |  |
 |
 |
| 42 | Male | 95.5 | 336.5 | Septic |  |
 |
 |
| 17 | Female | 64.6 | 243 | Septic |  |
 |
 |
| 17 | Female | 88.4 | 324 | Septic |  |
 |
 |
| 39 | Male | 53.9 | 202.4 | – | 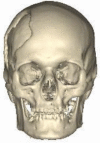 |
 |
 |
| 32 | Male | 4.8 | 38 | – |  |
 |
 |
| 52 | Male | 95.4 | 339.9 | – |  |
 |
 |
| 51 | Male | 37.3 | 149.3 | – | 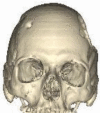 |
 |
 |
| 56 | Female | 101.1 | 339.2 | 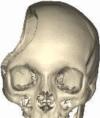 |
 |
 |
|
| 28 | Male | 34.3 | 136 | 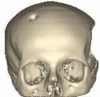 |
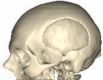 |
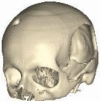 |
|
| 32 | Female | 63 | 248.9 | 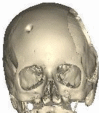 |
 |
 |
|
| 44 | Male | 79.4 | 277.7 |  |
 |
 |
|
| 57 | Female | 52.9 | 221.8 |  |
 |
 |
|
| 57 | Female | 65.9 | 236.2 |  |
 |
 |
|
| 61 | Male | 51.2 | 181.2 | 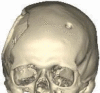 |
 |
 |
|
| 41 | Male | 105 | 51.5 |  |
 |
 |
|
| 31 | Male | 24.1 | 160.6 |  |
 |
 |
|
| 51 | Male | 36.2 | 180.5 |  |
 |
 |
|
| 42 | Male | 70 | 309.2 |  |
 |
 |
|
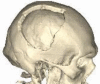
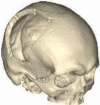
| 48 | Male | 35.4 | 168.8 | 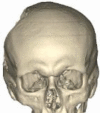 |
 |
 |
|
| 44 | Male | 80.8 | 338.5 | 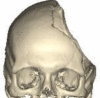 |
 |
 |
|
| 38 | Female | 27 | 165.8 |  |
 |
 |
|
| 54 | Female | 30.1 | 218.2 | 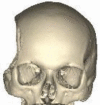 |
 |
 |
|
| 6 | Male | 25.1 | 179.9 |  |
 |
 |
|
| 32 | Male | 31.3 | 184.3 |  |
 |
 |
|
| 41 | Male | 91.1 | 412.9 |  |
 |
 |
|
| 45 | Female | 72.7 | 332.5 | 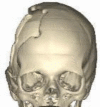 |
 |
 |
|
| 56 | Female | 73.7 | 317.1 |  |
 |
 |
|
| 45 | Male | 69.3 | 283.1 |  |
 |
 |
|
| 30 | Female | 34 | 140.5 |  |
 |
 |
|
| 65 | Male | 39.2 | 231 | 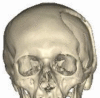 |
 |
 |
|
| 29 | Male | 12.5 | 81.4 |  |
 |
 |
|
| 22 | Male | 10 | 82.3 |  |
 |
 |
|
| 23 | Male | 21.3 | 165.8 | – | 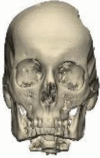 |
 |
 |
Discussion
Cranioplasty serves to protect the previously exposed neural structures and provides patients with cosmetic satisfaction. In addition, it improves the neuropsychological outcome. Studies show that earlier defect restoration results in progressive improvement in patients’ function7. Not only can the circulation return to normal conditions, but the cerebrospinal fluid circulation also recovers. Different techniques and materials have been used since ancient times to fulfil those purposes and have had various results8,9. From the technical side, neurosurgeons are expecting constant availability, reproducibility and easy handling in the operating room. From the patient side, the implanted material is the most important factor regarding the outcome.
The autograft appears to be the best material for cranioplasty, but in open craniocerebral injuries, it is not possible to spare the bone. Moreover, temporal subcutaneous implantation can lead to substantial resorption. Deep freezing might overcome that issue, but after reimplantation, resorption is still noted10.
Metal implants have advantages in terms of durability and infection control. They are precise, reliable, and suitable for everyday routine. Metal printing processes can be performed only in special laboratories at an extremely high cost. Direct fabrication of the implant can be performed by various techniques, such as selective laser or electron beam melting, but this method is practically available only for a few selected institutes11.
Custom prostheses made from hydroxyapatite have a low complication rate. The cancellous ultrastructure resembles a diploe, but the high cost restricts their use in general6.
Klammert et al.12, in their cadaver pilot experiments, used calcium phosphate for powder-based 3D printing that could, in principle, be suitable for direct implantation, but due to infection problems, the use of this method in living organisms is not yet feasible.
Kim et al.4 printed a special mould coated with plastic, allowing the fabrication of replacement during surgery; however, applying that coating on the mould and securing asepsis can be complicated.
Small defects with hidden locations, e.g., after neurovascular decompression from the retromastoid approach, can be filled with manual moulding by administering PMMA. The custom-made techniques allow more precise replacement for more extended areas and in more problematic locations. 3D planning and preoperative printing simply disclose the possibility of poor fitting5,6,12. No cactus effect, such as when mesh is implanted8,9, is possible. Any mismatch occurs at the surface, and the edges can be easily blurred. The implant can be fixed with miniplates or any commercially available devices.
The heat that is released during the polymerization of the PMMA warms the silicone, thus completely eliminating the risk of harmful effects of the heat production in the affected region. If any technical problem arises, implant fabrication can be repeated during surgery, and if the mould is kept, it can be reused for replantation if necessary. Another advantage of polymerizing on a mould is that fewer residual monomers will contact the dura mater directly.
The technique of the 3D-printing based custom-made cranioplasty using silicone moulding with PMMA is easy and reliable. More silicone moulding studies may be explored in further studies. Thin slice CT scans are easily obtainable. The computer-assisted design of the implant results in excellent cosmetics. Planning includes symmetrical mirroring across the midsagittal plane of the skull and follows the contours of the exposed bone. The 3D printing of the whole skull with the prefabricated implant allows the cosmetic result to be checked preoperatively.
To cover cranial defects, PMMA is a widely used material in neurosurgery. It is sterile and available in a less viscous form; thus, the least complicated defects can also be filled with it. The 3D-printed silicone mould can easily be sterilised in a standard fashion in hospitals. Quality control is manageable since the sterilisation of preoperatively mixed and produced PMMA implants raises several technical standards that cannot be followed in labs with smaller case caseloads. The operation time is no longer than that when other techniques are used, since the moulding procedure needs only the original operation time, during which PMMA is expected to solidify, and the procedure can be done by a nurse.
The slightly rough surface of PMMA results perfect adhesion to the skin. Complications may occur by several factors, but skull replacement does not increase the risk.13–17. To prevent surgical complications, general principles should be followed. To prevent further complications, the bony edges should be exposed completely since the implant is planned accordingly. It is very important that the skin should be handled with respect. During the first intervention decompression should also be preplanned, presuming that the patient will need reconstruction of the skull18. In our cases, we meticulously paid attention to infection prevention. If the skin was broken or presumably weak, we postponed the operation. As an institutional standard, cranioplasty is generally indicated 3 months after craniectomy. Patients receive a bolus of preventive intravenous antibiotics at the induction of the anaesthesia that can be repeated after 4 h if needed to prevent infection. If cranioplasty is presumed, closure of the skin is performed with monofilament materials. The closure of the dura can be a problem in open trauma cases, and in decompressive craniectomy, the dura should be left open as a rule. During cranioplasty, the newly formed encephalomyosynangioses should be preserved as much as possible to prevent bleeding, CSF leakage or delay of expected recovery in already diminished cortical functions. If we pay attention and eliminate all the possible known factors that can lead to complications, the hazard of any operation, such as cranioplasty, can be minimized.
The technique can be used as a secondary option, e.g., in cases where conventional cranioplasty has been performed with complications. In-home sterilisation of the silicone mould and intraoperative preparation of PMMA from the original packaging both minimize the risk of infection. We have not yet used the technique in the paediatric population, but the results are encouraging for implementation.
Acknowledgements
György Falk: The authors thank for the technical support in 3D printing for the Varinex Zrt.
Author contributions
L.C.: 3D modelling, 3D printing. Z.C.: Initiation of conception, supervising. L.N.: Clinical Neurosurgeon using the cranioplasties for the cranial reconstructions. V.Z.K.: Clinical Neurosurgeon using the cranioplasties for the cranial reconstructions. Á.É.K.: 3D reconstructions of the CT scans, Silicon moulding. H.S.H.: 3D reconstructions of the CT scans, Silicon moulding. S.M.: 3D modelling, 3D printing.
Funding
Open access funding provided by University of Debrecen. The project was supported by the National Research, Development and Innovation Office – NKFIH K113180 and a grant from the Hungarian Ministry of Finance and the European Union (GINOP 2.3.2-15-2016-00022).
Data availability
All institutions involved in those interventions meets the GDPR data management and archiving guidelines. All of the requested raw datas like anonymized CT scans and 3D models available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alkhaibary A, Alharbi A, Alnefaie N, Oqalaa Almubarak A, Aloraidi A, Khairy S. Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020;139:445–452. doi: 10.1016/j.wneu.2020.04.211. [DOI] [PubMed] [Google Scholar]
- 2.Arnaoutakis D, Bahrami A, Cohn JE, Smith JE. Cranioplasty using a mixture of biologic and nonbiologic agents. JAMA Fac. Plast. Surg. 2018;20:9–13. doi: 10.1001/jamafacial.2017.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris DA, Fong AJ, Buchanan EP, Monson L, Khechoyan D, Lam S. History of synthetic materials in alloplastic cranioplasty. Neurosurg. Focus. 2014;36:E20. doi: 10.3171/2014.2.FOCUS13560. [DOI] [PubMed] [Google Scholar]
- 4.Kim BJ, Hong KS, Park KJ, Park DH, Chung YG, Kang SH. Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J. Korean Neurosurg. Soc. 2012;52:541–546. doi: 10.3340/jkns.2012.52.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales-Gómez JA, Garcia-Estrada E, Leos-Bortoni JE, Delgado-Brito M, Flores-Huerta LE, De La Cruz-Arriaga AA, Torres-Díaz LJ, De León ÁRM. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J. Neurosurg. 2018 doi: 10.3171/2017.12.jns172574. [DOI] [PubMed] [Google Scholar]
- 6.Staffa, G., Nataloni, A., Compagnone, C. & Servadei, F. Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir. (Wien)149, 161–170 (2007); discussion 170. [DOI] [PubMed]
- 7.Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G, Ahmad FU. Early cranioplasty is associated with greater neurological improvement: A systematic review and meta-analysis. Neurosurgery. 2018;82:278–288. doi: 10.1093/neuros/nyx182. [DOI] [PubMed] [Google Scholar]
- 8.Sahoo NK, Tomar K, Thakral A, Kumar S. Failures in cranioplasty—a clinical audit & review. J. Oral. Biol. Craniofac. Res. 2021;11:66–70. doi: 10.1016/j.jobcr.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeap MC, Tu PH, Liu ZH, Hsieh PC, Liu YT, Lee CY, Lai HY, Chen CT, Huang YC, Wei KC, Wu CT, Chen CC. Long-term complications of cranioplasty using stored autologous bone graft, three-dimensional polymethyl methacrylate, or titanium mesh after decompressive craniectomy: A single-center experience after 596 procedures. World Neurosurg. 2019;128:e841–e850. doi: 10.1016/j.wneu.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Park SP, Kim JH, Kang HI, Kim DR, Moon BG, Kim JS. Bone flap resorption following cranioplasty with autologous bone: Quantitative measurement of bone flap resorption and predictive factors. J. Korean Neurosurg. Soc. 2017;60:749–754. doi: 10.3340/jkns.2017.0203.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Hajje A, Kolos EC, Wang JK, Maleksaeedi S, He Z, Wiria FE, Choong C, Ruys AJ. Physical and mechanical characterisation of 3D-printed porous titanium for biomedical applications. J. Mater. Sci. Mater. Med. 2014;25:2471–2480. doi: 10.1007/s10856-014-5277-2. [DOI] [PubMed] [Google Scholar]
- 12.Klammert U, Gbureck U, Vorndran E, Rödiger J, Meyer-Marcotty P, Kübler AC. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Craniomaxillofac. Surg. 2010;38:565–570. doi: 10.1016/j.jcms.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Frassanito P, Fraschetti F, Bianchi F, Giovannenze F, Caldarelli M, Scoppettuolo G. Management and prevention of cranioplasty infections. Childs Nerv. Syst. 2019;35:1499–1506. doi: 10.1007/s00381-019-04251-8. [DOI] [PubMed] [Google Scholar]
- 14.Henry J, Amoo M, Murphy A, O'Brien DP. Complications of cranioplasty following decompressive craniectomy for traumatic brain injury: Systematic review and meta-analysis. Acta Neurochir. (Wien) 2021;163:1423–1435. doi: 10.1007/s00701-021-04809-z. [DOI] [PubMed] [Google Scholar]
- 15.Hirschmann D, Kranawetter B, Tomschik M, Wais J, Winter F, Frischer JM, Millesi M, Herta J, Roessler K, Dorfer C. New-onset seizures after cranioplasty—a different view on a putatively frequently observed phenomenon. Acta Neurochir. (Wien) 2021;163:1437–1442. doi: 10.1007/s00701-021-04720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocque BG, Agee BS, Thompson EM, Piedra M, Baird LC, Selden NR, Greene S, Deibert CP, Hankinson TC, Lew SM, Iskandar BJ, Bragg TM, Frim D, Grant G, Gupta N, Auguste KI, Nikas DC, Vassilyadi M, Muh CR, Wetjen NM, Lam SK. Complications following pediatric cranioplasty after decompressive craniectomy: a multicenter retrospective study. J Neurosurg Pediatr. 2018;22:225–232. doi: 10.3171/2018.3.PEDS17234. [DOI] [PubMed] [Google Scholar]
- 17.Shih FY, Lin CC, Wang HC, Ho JT, Lin CH, Lu YT, Chen WF, Tsai MH. Risk factors for seizures after cranioplasty. Seizure. 2019;66:15–21. doi: 10.1016/j.seizure.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Veldeman M, Geiger M, Clusmann H. How I do it-the posterior question mark incision for decompressive hemicraniectomy. Acta Neurochir. (Wien) 2021;163:1447–1450. doi: 10.1007/s00701-021-04812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All institutions involved in those interventions meets the GDPR data management and archiving guidelines. All of the requested raw datas like anonymized CT scans and 3D models available from the corresponding author on reasonable request.