Abstract
Myocardial ischemia-reperfusion (MI/R) injury is a common and serious complication following reperfusion treatment for myocardial infarction (MI). Increasing evidence has verified the crucial role of circular RNAs (circRNAs) in the MI/R injury processes. The objective of this study was to investigate the effects and potential regulatory mechanisms of circHMGA2 on MI/R injury. Hypoxia/reoxygenation (H/R) models were established using human cardiac myocytes (HCMs) and mice models were induced by MI/R. The level of circHMGA2 was detected by RT-qPCR. Myocardial function was evaluated by the hemodynamic parameters, the activity of serum myocardial enzymes, HE staining and TUNEL assays. Cell proliferation was measured by CCK-8 assay. The ferrous ion (Fe2+) level was determined with an iron assay kit. Ferroptosis- and pyroptosis-related proteins were determined using western blotting. The levels of oxidative stress and inflammatory factors were analyzed using DCFH-DA staining or ELISA assays. CircHMGA2 was upregulated in H/R-induced HCMs and myocardial tissues of MI/R mice. In vitro, circHMGA2 knockdown attenuated the proliferation inhibition, restrained the ferroptosis and pyroptosis in H/R-induced HCMs. This regulatory mechanism may be associated with the suppression of NLRP3 pathway. In vivo, circHMGA2 depletion attenuated myocardial tissue damage of MI/R mice through inhibiting the oxidative stress and pyroptosis. Taken together, CircHMGA2 enhanced MI/R injury via promoting ferroptosis and pyroptosis, providing new insights into the treatment of MI/R injury.
Keywords: CircHMGA2, Myocardial ischemia-reperfusion, Ferroptosis, Pyroptosis, Oxidative stress
1. Introduction
Myocardial infarction (MI) is a cardiovascular diseases (CVD) that poses a significant threat to human health and life, which is usually caused by myocardial ischemia reperfusion injury resulting in cell necrosis due to insufficient blood supply to the heart [1]. Thrombolytic therapy or percutaneous coronary intervention (PCI) is an effective treatment for MI [2]. However, if the duration of the ischemic is prolonged before revascularization, the tissue injury becomes more severe and may result in irreversible damage. This process is known as myocardial ischemia-reperfusion (MI/R) injury [3]. MI/R injury has seriously impacts the prognosis of patients with ischemic CVD, and its mechanisms are complex and multifactorial, involving inflammation, oxidative stress and different types of cell necrosis [4,5]. Therefore, it is crucial to clarify the pathogenesis of MI/R injury and explore novel therapeutic targets.
Circular RNAs (circRNAs) are a kind of single-stranded and closed circular RNAs formed by reverse cleavage of precursor mRNA (pre-mRNA). They are widely present in the cytoplasm and are resistant to recognition and degradation by Ribonuclease R (RNase R) [6]. It is reported that circRNA exerts a vital regulatory effect in various organic diseases, including heart disease [7]. Some studies have reported that differential expressions of circRNAs are detected in the MI/R injury progression, suggesting that circRNAs may be associated with MI/R injury processed [8,9]. For instance, circRNA ACR is found to be lowly expressed in the MI/R mice and has been shown to mitigate MI/R injury by modulating Pink1/FAM65B pathway [10]. Moreover, circRNA TLK1 has been implicated in aggravating myocardial injury by targeting miR-214 through regulating TNF pathway [11]. CircHMGA2 is a newly discovered oncogenic RNA, arises from the back-splicing of high mobility group AT-hook2 (HMGA2) on chromosome 12:66221780–66232349 + and has been extensively studied for its tumor-promoting effects in various of tumors, including lung adenocarcinoma [12,13]. However, the roles and regulatory mechanisms of circHMGA2 in diseases other than cancer, especially in MI/R injury, have not been well investigated.
Pyroptosis and ferroptosis are the main forms of programmed cell death in cardiomyocytes [14,15], and several studies have demonstrated that these two forms of cell death also exist in the procession of MI/R injury [16]. Pyroptosis is a proinflammatory cell death activated by NLRP3 inflammasome and exerts a vital role in inflammatory and immunological responses [17]. Ferroptosis is a cell death caused by iron overload and accumulation of lipid peroxides dependent on reactive oxygen species (ROS) [18]. As two distinct forms of cell death, there is a certain association between pyroptosis and ferroptosis. In cancer, iron acts as a sensitizer amplifying ROS signaling to induce melanoma cell pyroptosis [19]. In type 2 diabetic cardiomyopathy, CD74 ablation inhibits cardiac dysfunction via pyroptosis-evoked regulation of ferroptosis [20]. However, few researches were performed on the regulation role of pyroptosis and ferroptosis in MI/R injury.
In this research, we successfully established both in vivo and in vitro models of MI/R injury and evaluated the impacts of circHMGA2 in the progression of MI/R. For the first time we identified that circHMGA2 exacerbated MI/R injury by upregulating NLRP3 expression and promoting the occurrence of ferroptosis and pyroptosis. These results shed light on the potential use of circHMGA2 as a novel biomarker for the diagnosis of MI/R injury.
2. Results
2.1. CircHMGA2 was overexpressed in H/R-induced HCM model and MI/R-induced mice model
To verify the functional roles of circHMGA2 in MI/R injury processes, we constructed H/R-induced HCM model in vitro and MI/R-induced mouse model in vivo. The expression of circHMGA2 were detected by RT-qPCR. PCR results manifested that the expression of circHMGA2 in H/R-induced HCMs was markedly than that in control HCMs (Fig. 1A). Moreover, circHMGA2 expression in myocardial tissues of MI/R mice was remarkable elevated than that in sham group (Fig. 1B). The substantially heightened expression of circHMGA2 in the model of myocardial hypoxic injury denoted that circHMGA2 may related with the MI/R injury progression.
Fig. 1.
CircHMGA2 was overexpressed in H/R-induced HCM model and MI/R-induced mice model. (A) The expression level of circHMGA2 in the H/R-induced HCMs were measured by RT-qPCR. ***p < 0.001 vs. con. (B) The expression level of circHMGA2 were measured by RT-qPCR in the myocardial tissues of MI/R mice. ***p < 0.001 vs. sham.
2.2. Depletion of circHMGA2 suppressed ferroptosis in H/R-induced HCMs
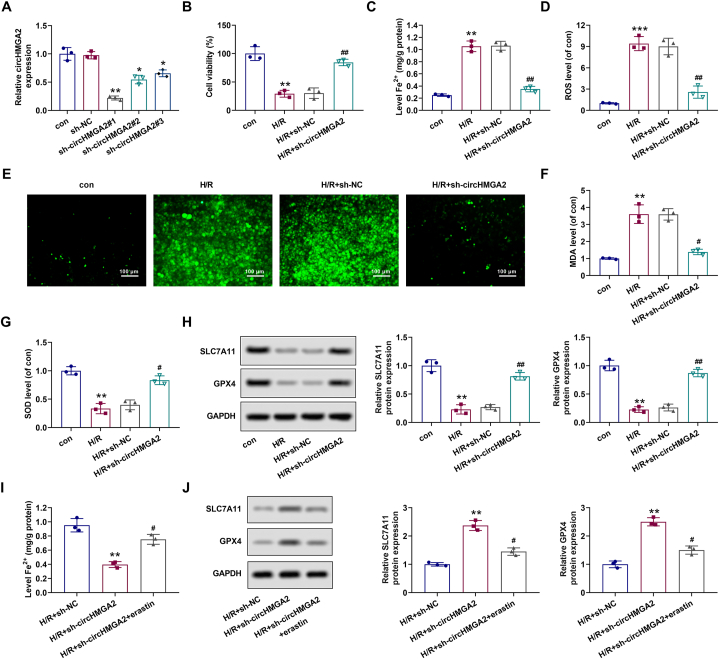
Next, we explored the regulatory effects of circHMGA2 on the biological functions on H/R-induced HCMs. Firstly, the expression of circHMGA2 in H/R-induced HCMs was decreased by the transfection of sh-circHMGA2#1, #2, #3. Compared with control group, circHMGA2 expression in sh-circHMGA2#1 group was the lowest. Therefore, the H/R-induced HCMs transfected with sh-circHMGA2#1 were used in the follow-up experiments (Fig. 2A). Then, cell proliferation, iron ion content, oxidative stress factors (ROS, SOD, MDA) and ferroptosis-related proteins (SLC7A11 and GPX4) were detected by CCK-8, iron kit, DCFH-DA staining, ELISA and Western blot assays. CCK-8 results showed that circHMGA2 knockdown effectively alleviated the inhibitory effects on the proliferative activity of H/R-induced HCMs (Fig. 2B). Results of iron assay kit showed that the circHMGA2 downregulation significantly attenuated the upregulation of Fe2+ level in H/R-induced HCMs (Fig. 2C). Results of DCFA-DA staining and ELISA assays showed that depletion of circHMGA2 led to a reduction in the upregulation of ROS and MDA, and the downregulation of SOD in H/R-induced HCMs (Fig. 2D–G). Western blotting showed that low circHMGA2 expression reversed the downregulation of SLC7A11 and GPX4 in H/R-induced HCMs (Fig. 2H). Furthermore, we observed that erastin reversed circHMGA2 knockdown-induced decreased Fe2+ level and upregulation of SLC7A11 and GPX4 in H/R-induced HCMs (Fig. 2I and H). In general, these above results suggest that knockdown of circHMGA2 mitigated the H/R-induced HCM cell injury by promoting cell proliferation, and inhibiting the levels of oxidative stress and ferroptosis.
Fig. 2.
Depletion of circHMGA2 suppressed ferroptosis in H/R-induced HCMs. (A) The efficiency of shRNA-mediated circHMGA2 knockdown (sh-circHMGA2#1, #2, #3) in H/R-induce HCMs was detected by RT-qPCR. *p < 0.05 and **p < 0.01 vs. sh-NC. (B) Effect of circHMGA2 knockdown on the proliferative ability in H/R-induced HCMs was detected by CCK-8 assay. **p < 0.01 vs. con; ##p < 0.01 vs. H/R + sh-NC. (C) Effect of circHMGA2 knockdown on the ferrous ion (Fe2+) content in H/R-induced HCMs was detected by iron assay kit. **p < 0.01 vs. con; ##p < 0.01 vs. H/R + sh-NC. (D–E) Effect of circHMGA2 knockdown on ROS levels in H/R-induced HCMs was detected by DCFH-DA staining. Quantifications were as described in (D) and the staining was shown in (E). ***p < 0.001 vs. con; ##p < 0.01 vs. H/R + sh-NC. (F–G) Effect of circHMGA2 knockdown on the levels of MDA (F) and SOD (G) in H/R-induced HCMs was detected by ELISA assays. **p < 0.01 vs. con; #p < 0.05 vs. H/R + sh-NC. (H) Effect of circHMGA2 knockdown on the levels of ferroptosis-related proteins (GPX4 and SLC7A11) in H/R-induced HCMs was detected by western blotting. **p < 0.01 vs. con; ##p < 0.01 vs. H/R + sh-NC. (I) Effect of erastin (ferroptosis inducer) on the ferrous ion (Fe2+) content in H/R-induced HCMs transfected with sh-circHMGA2 was detected by iron assay kit. **p < 0.01 vs. H/R + sh-NC; #p < 0.01 vs. H/R + sh-circHMGA2. (J) Effect of erastin on the levels of ferroptosis-related proteins (GPX4 and SLC7A11) was detected by western blotting in H/R-induced HCMs transfected with sh-circHMGA2. **p < 0.01 vs. H/R + sh-NC; #p < 0.01 vs. H/R + sh-circHMGA2.
2.3. Depletion of circHMGA2 attenuated pyroptosis in H/R-induced HCMs
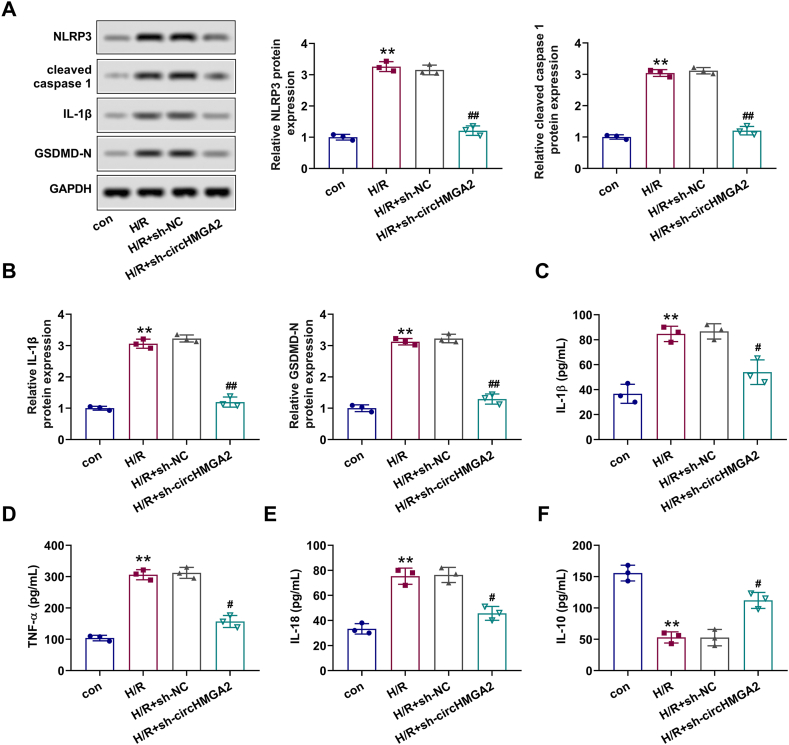
Given the strong correlation between H/R injury and inflammation [21], we conducted further investigations into the relationship between circHMGA2 and pyroptosis in H/R-induced HCMs. Western blot and ELISA assays were adapted to measure the expression of pyroptosis-related proteins (NLRP3, cleaved caspase-1, IL-1β and GSDMD-N) and inflammatory cytokines (TNF-α, IL-1β, IL-10 and IL-18) in each cell group. Results presented that the expression levels of NLRP3, cleaved caspase-1, IL-1β and GSDMD-N in H/R-induced HCMs were significantly increased, which could be mitigated by circHMGA2 knockdown (Fig. 3A and B). Furthermore, as expected, circHMGA2 downregulation attenuated the suppressive effects on pro-inflammatory cytokines (TNF-α, IL-1β and IL-18) levels and enhanced the levels of anti-inflammatory cytokine (IL-10) levels in H/R-induced HCMs (Fig. 3C–F). Taken together, these findings indicate that circHMGA2 knockdown could effectively alleviate the pyroptosis in H/R-HCMs.
Fig. 3.
Depletion of circHMGA2 attenuated pyroptosis in H/R-induced HCMs. (A) Effect of circHMGA2 knockdown on the expression of pyroptosis-related proteins (NLRP3, cleaved caspase-1, IL-1β and GSDMD-N) was detected by western blotting. (B) Effect of circHMGA2 knockdown on the expression of inflammatory factors (TNF-α, IL-1β, IL-10 and IL-10) H/R-induced HCMs was detected by western blotting. (C–F) Effect of circHMGA2 knockdown on the expression of inflammatory factors, including IL-1β (C), TNF-α (D), IL-18 (E) and IL-10 (F) was detected by ELISA assays in H/R-induced HCMs. **p < 0.01 vs. H/R; #p < 0.05 and ##p < 0.01 vs. H/R + sh-NC.
2.4. CircHMGA2 knockdown repressed the ferroptosis and pyroptosis in H/R-induced HCMs through downregulating NLRP3 expression
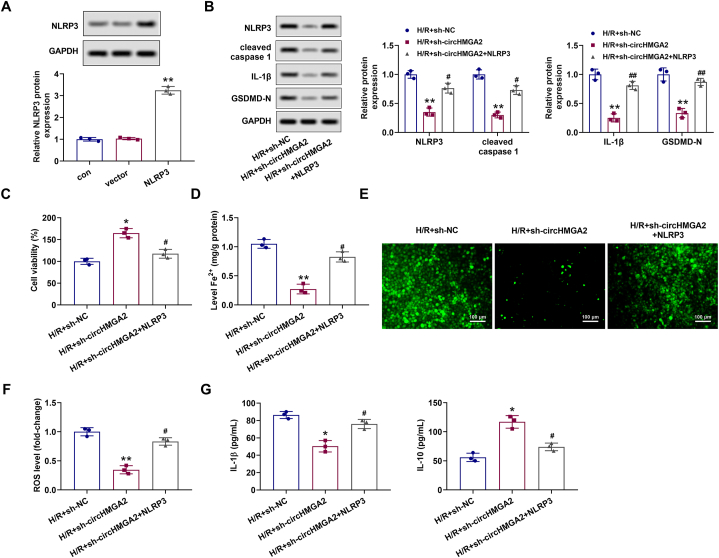
Based on the aforementioned results, we continued to explore the regulatory mechanism of circHMGA2 on ferroptosis and pyroptosis in H/R-induced HCMs. Initially, we constructed the NLRP3 overexpression plasmid (pcDNA-NLRP3) to enhance NLRP3 expression in H/R-induced HCMs. Western blotting showed that the expression of NLRP3 in H/R-induced HCMs transfected with pcDNA-NLRP3 was significantly increased (Fig. 4A). In the subsequent cell function experiments, in H/R-induced HCMs, we once again found that circHMGA2 knockdown could alleviate the upregulation of pyroptosis-related proteins (cleaved caspase-1, NLRP3, IL-1β and GSDMD-N) (Fig. 4B), the decrease of cell proliferative activity (Fig. 4C), the increase of Fe2+ content (Fig. 4D), the increase of ROS level (Fig. 4E and F), the increase levels of IL-1β and the decrease levels of IL-10 (Fig. 4G). However, these aforementioned alterations were reversed by NLRP3 overexpression (Fig. 4B–G). Consequently, these findings collectively suggested that circHMGA2 knockdown may alleviate the H/R injury of HCMs by inhibiting NLRP3-related pathway.
Fig. 4.
CircHMGA2 knockdown repressed the ferroptosis and pyroptosis in H/R-induced HCMs through downregulating NLRP3 expression. (A) The efficiency of pcDNA-mediated NLRP3 overexpression (pcDNA-NLRP3) in H/R-induced HCMs was detected by RT-qPCR. **p < 0.01 vs. vector. (B) Effect of circHMGA2 knockdown on the expression of pyroptosis-related proteins (NLRP3, cleaved caspase-1, IL-1β and GSDMD-N) was detected by western blotting in H/R-induced HCMs transfected with pcDNA-NLRP3. **p < 0.01 vs. H/R + sh-NC; #p < 0.05 and ##p < 0.01 vs. H/R + sh-circHMGA2. (C) Effect of circHMGA2 knockdown on the proliferative activity was detected by CCK-8 assay in H/R-induced HCMs transfected with pcDNA-NLRP3. *p < 0.05 vs. H/R + sh-NC; #p < 0.05 vs. H/R + sh-circHMGA2. (D) Effect of circHMGA2 knockdown on the ferrous ion (Fe2+) content in H/R-induced HCMs transfected with pcDNA-NLRP3 was detected by iron assay kit. **p < 0.01 vs. H/R + sh-NC; #p < 0.05 vs. H/R + sh-circHMGA2. (E–F) Effect of circHMGA2 knockdown on ROS levels in H/R-induced HCMs transfected with pcDNA-NLRP3 was detected by DCFH-DA staining. The staining was shown in (E) and quantifications were as described in (F). **p < 0.01 vs. H/R + sh-NC; #p < 0.05 vs. H/R + sh-circHMGA2. (G) Effect of circHMGA2 knockdown on the expression of inflammatory factors, including IL-1β and IL-10 was detected by ELISA assays in H/R-induced HCMs transfected with pcDNA-NLRP3. *p < 0.05 vs. H/R + sh-NC; #p < 0.05 vs. H/R + sh-circHMGA2.
2.5. CircHMGA2 knockdown inhibited myocardial injury in MI/R mice
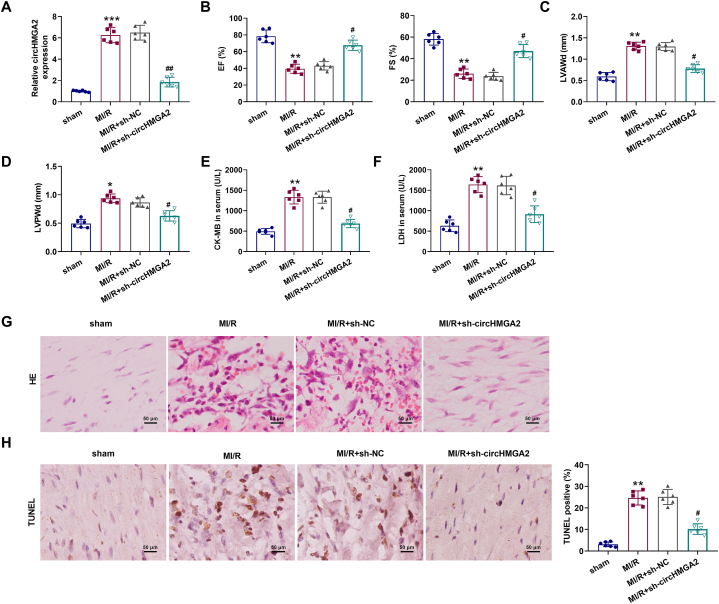
In addition to in vitro cell function verification, we also constructed the MI/R mice model in mice bearing HCMs stably transfected with circHMGA2 shRNA lentivirus or control shRNA lentivirus. PCR results revealed a significant increase in circHMGA2 expression in the myocardium of MI/R mice, while the additionally stably transfection of sh-circHMGA2 inhibited the expression of circHMGA2 in the myocardium of MI/R mice (Fig. 5A). Subsequently, the extent of myocardial injury in MI/R mice was assessed using echocardiography, HE staining and ELISA assay. Echocardiographic results showed that circHMGA2 knockdown alleviated the decrease of ejection fraction (EF) and fraction shortening (FS) levels in MI/R mice (Fig. 5B), and circHMGA2 knockdown alleviated the increase of left ventricular anterior wall diastole (LVAWd) and left ventricular posterior wall diastole (LVPWd) levels in MI/R mice (Fig. 5C and D). In addition, ELISA assay showed that circHMGA2 knockdown reduced the upregulation of serum myocardial injury markers (CK-MB and LDH) in MI/R mice (Fig. 5E and F). HE staining assay revealed that circHMGA2 knockdown attenuated the inflammatory cell infiltration and rupture dissolution of myocardial tissues in MI/R mice (Fig. 5G). TUNEL assay showed that circHMGA2 downregulation inhibited the elevated levels of apoptosis in myocardial of MI/R mice (Fig. 5H). Overall, these above results confirmed that circHMGA2 knockdown could effectively protected myocardial injury in vivo.
Fig. 5.
CircHMGA2 knockdown inhibited myocardial injury in MI/R mice. (A) The expression of circHMGA2 was detected by RT-qPCR in the myocardial tissues of MI/R mice injected with HCMs stably expressing sh-circHMGA2. (B) The levels of EF and FS was detected by echocardiography in MI/R mice injected with HCMs stably expressing sh-circHMGA2. (C–D) The levels of LVAWd (C) and LVPWd (D) were detected by echocardiography in MI/R mice injected with HCMs stably expressing sh-circHMGA2. (E–F) The serum myocardial injury markers, including CK-MB (E) and LDH (F) was detected by ELISA assays in MI/R mice injected with HCMs stably expressing sh-circHMGA2. (G) The degree of myocardial injury in the myocardial tissues was detected by HE staining in MI/R mice injected with HCMs stably expressing sh-circHMGA2. (H) Apoptosis of myocardial tissues was detected by TUNEL staining in MI/R mice injected with HCMs stably expressing sh-circHMGA2. **p < 0.01 and ***p < 0.001 vs. sham; #p < 0.05 and ##p < 0.01 vs. MI/R + sh-NC.
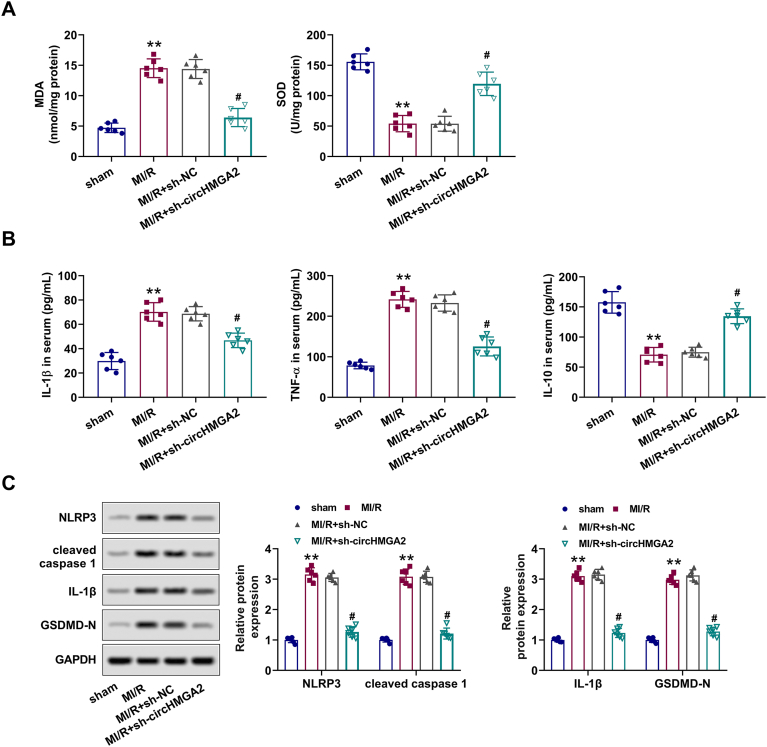
2.6. CircHMGA2 knockdown alleviated myocardial injury in MI/R mice by inhibiting oxidative stress and pyroptosis
Finally, we explored the regulatory roles of circHMGA2 on MI/R injury in vivo. ELISA assay results showed that transfection of sh-circHMGA2 significantly alleviated the upregulation of MDA and downregulation of SOD in serum of MI/R mice (Fig. 6A). Furthermore, circHMGA2 downregulation inhibited the increase of serum TNF-α and IL-1β levels, and restored the decreased IL-10 levels in myocardial of MI/R mice (Fig. 6B). Western blotting revealed that circHMGA2 knockdown reversed the increased expression of pyroptosis-related proteins (cleaved caspase-1, NLRP3, IL-1β and GSDMD-N) in the myocardium of MI/R mice (Fig. 6C). To sum up, circHMGA2 deletion may protect the myocardium ischemic injury in MI/R mice via inhibiting oxidative stress and pyroptosis.
Fig. 6.
CircHMGA2 knockdown alleviated myocardial injury in MI/R mice by inhibiting oxidative stress and pyroptosis. (A) The expression of oxidative stress factors (SOD and MDA) was detected by ELISA assays in the serum of MI/R mice injected with HCMs stably expressing sh-circHMGA2. (B) The expression of inflammatory cytokine (TNF-α, IL-1β and IL-10) was detected by ELISA assays in the serum of MI/R mice injected with HCMs stably expressing sh-circHMGA2. (C) The expression of pyroptosis-related proteins (NLRP3, cleaved caspase-1, IL-1β and GSDMD-N) was detected by ELISA assays in the myocardial tissues of MI/R mice injected with HCMs stably expressing sh-circHMGA2. **p < 0.01 vs. sham; #p < 0.05 vs. MI/R + sh-NC.
3. Discussion
MI is a leading cause of disability and death worldwide, with MI/R injury being a major contributor to death in patients with MI [22]. CircRNA has complex biological functions and exerts critical roles in processed of multiple heart diseases [23]. In this research, we verify that circHMGA2 was enrichment in the ischemia-reperfusion model in vivo and in vitro, and circHMGA2 knockdown could alleviate the MI/R injury by suppressing ferroptosis and pyroptosis. These findings highlight the importance of circHMGA2 in the pathogenesis of MI/R injury and provide insights into potential therapeutic strategies for managing this condition.
The regulatory effect of circRNAs on ischemia-reperfusion injury has been confirmed by extensively studied. Previous reports have demonstrated that circRbms1 inhibited ischemia-reperfusion injury by regulating miR-92a/BCL2L11 pathway in H/R-induced H9c2 cells and MI/R mice [24], circ-NNT aggravated H/R-induced myocardial injury through upregulating USP46 expression by targeting miR-33a-5p [25], and circFoxo3 attenuated MI/R injury by modulating autophagy and HMGB1 expression in mice [26]. In our present study, we have, for the first time, confirmed that circHMGA2 could exacerbate ischemia-reperfusion injury in vivo and in vitro. CircHMGA2 is a kind of RNA with less research, and previous related researches were limited to the field of oncology. For example, circHMGA2 exacerbated the malignant proliferation of lung adenocarcinoma cells by promoting epithelial-mesenchymal transition (EMT) and metastasis [12]. In addition, in non-small cell lung cancer (NSCLC) cells, circHMGA2 enhanced tumor progression by acting as a sponge for miR-331-3p and regulating glycolysis [13]. Notably, circHMGA2 could promote the growth of malignant tumors through miR-129-5p/CLDN1 axis in thyroid papillary carcinoma [27]. However, until now, no studies have investigated the role of circHMGA2 in viscera injury processes. In this study, we found significantly elevated expression of circHMGA2 in H/R-induced HCMs and the myocardium of MI/R mice. Knockdown of circHMGA2 effectively inhibited the decreased proliferative activities of H/R-induced HCMs and myocardial injury of MI/R mice. These above findings underscored the potential significance of circHMGA2 as a key factor involved in the regulation of MI/R injury.
Then we delved into the regulatory effects of circHMGA2 on MI/R injury, with a specific focus on ferroptosis, which has been implicated in previous studies as closely associated with MI/R injury [28,29]. Iron metabolism disorder and lipid peroxidation are fundamental mechanisms underlying ferroptosis. Most iron binds closely to the transferrin or ferritin under the normal physiological conditions, but during the period time of ischemia, a large amount of iron is released from ferritin, resulting in iron overload [30]. A Clinical trial reported that infusion of deferoxamine in patients undergoing coronary artery bypass grafting could effectively control iron overload and reduce MI/R injury [31]. Iron overload, in turn, contributes to elevated levels of ROS, thereby exacerbating MI/R injury. High expression of iron and ROS in HFR gene knockout mice were more likely to be MI/R injury and cardiomyocyte apoptosis [32]. Therefore, how to effectively alleviate ferroptosis during MI/R injury process may be a potential treatment strategy [33]. To date, no research has yet reported the regulatory effects of circRNAs on ferroptosis in MI/R injury. In this study, we found that circHMGA2 knockdown resulted in reduced Fe2+ content in H/R-induced HCMs. Meanwhile, the downregulation of circHMGA2 reduced the upregulation of ROS and MDA, and the downregulation of SOD in H/R-induced HCMs. Additionally, as central regulators of ferroptosis, GPX4 and SLC7A11 are always considered indicators of ferroptosis, and suppression of GPX4 and SLC7A11 causes ferroptosis [34,35]. Consistent with expectations, our results confirmed that circHMGA2 knockdown suppressed the downregulation of GPX4 and SLC7A11 in H/R-induced HCMs. Collectively, these results proved that circHMGA2 may exacerbate HCM cell ischemic injury by promoting ferroptosis.
In addition to ferroptosis, pyroptosis has been confirmed to be associated with MI/R injury by multiple reports recently [16,36]. As we all know, apoptosis is considered to be the main way of MI/R injury-mediated programmed cell death. With the deepening of research, inflammatory cell death mediated by NLRP3/caspase-1 could compete with the classical apoptosis pathway mediated by caspase-8/-9 to induce cell death, which is called pyroptosis [37]. Unlike non-inflammatory cell death seen in apoptosis, pyrolysis could induce the formation of NLRP3 inflammasome which consisted of NLRP3, pro-caspase-1 and apoptosis-related speck-like protein (ASC). Afterwards, cleave pro-caspase-1 promoted the secretion of downstream cytokines including TNF-α, IL-1β and IL-18. Additionally, it triggers the expression of Gasdermin D (GSDMD) in the cytoplasm, resulting in sterile inflammation [38]. Excessive inflammation can further induce cardiomyocyte death in the early stage of MI/R injury, and long-term inflammatory cell infiltration can also cause tissue fibrosis and aggravate the deterioration of cardiac function [37]. Sandanger et al. reported that knockdown of caspase-1 or NLRP3 reduced early mortality of MI mice, while inhibiting inflammatory response could protect the left ventricular function, suggesting that caspase-1 and NLRP3-mediated pyroptosis may play a crucial role in MI/R injury [39]. In addition, Qiu et al. found that compared with normal rats, the expression levels of NLRP3 and caspase-1 in MI/R injury rats were significantly increased, accompanied by more extensive inflammatory cell infiltration [40]. In this study, we found that circHMGA2 knockdown inhibited oxidative stress and inflammatory factor-mediated pyroptosis in H/R-induced HCMs and MI/R mice, which was characterized by the downregulation of pyroptosis-related proteins (cleaved caspase-1, NLRP3, IL-1β and GSDMD-N) and pro-inflammatory cytokines (TNF-α, IL-1β and IL-18), and upregulation of anti-inflammatory cytokines IL-10. Furthermore, NLRP3 overexpression partially reversed the protective effect of circHMGA2 knockdown on H/R-induced HCM cell injury, and other mechanisms need to be further explored. One last point that deserves explicit mention is that transfection of pcDNA-NLRP3 plasmid enhances NLPR3 expression through the expected transcription and translation pathway. But the interference of NLPR3 expression caused by sh-circHMGA2 plasmid transfection suggests the involvement of additional mechanisms, such as post-transcriptional regulation. These mechanisms could involve interactions with transcription factors, formation of RNA-RNA complexes and so on, all contributing to the modulation of NLRP3 expression. However, the specific mechanisms require to be further investigated. Overall, these findings highlight the potential role of circHMGA2 in modulating pyroptosis and its contribution to the pathogenesis of MI/R injury.
This is the first study to partly disclose the regulatory roles of circHMGA2 in MI/R injury. Of note, the limitations of our study should not be overlooked. First, the competitive endogenous RNA (ceRNA) network based on the effects of circHMGA2 in MI/R injury model was not explored. Second, the clinical implication of circHMGA2 expression has not been summarized. These issues should be clarified in the further work. Finally, given the experimental focus, we primarily concentrate on the role of circHMGA2 in myocardial injury and did not cover the signaling pathways of pyroptosis or ferroptosis. We intend to explore the specific signaling pathways that circHMGA2 may be involved in future studies.
In conclusion, our study provides evidence that circHMGA2 knockdown effectively mitigates MI/R injury in vivo and in vitro by inhibiting ferroptosis, pyroptosis and oxidative stress, which may be tied with the suppression of NLRP3-related pathway. Based on our findings, circHMGA2 holds promise as a potential diagnostic marker for MI therapy. Further investigations are warranted to explore the underlying mechanisms and evaluate the therapeutic potential of targeting circHMGA2 in the treatment of MI/R injury.
4. Materials and methods
4.1. Cardiomyocytes culture and treatment
From the Shanghai Academy of Chinese Sciences, we obtained primary human cardiac myocytes (HCMs). HCMs were cultured in DMEM high-glucose medium (BeNa, China) added with 20% FBS (BeNa, China) at the 37 °C incubator supplemented with 5% CO2. To induce ischemia/reperfusion (I/R) model, HCMs were subjected to hypoxia under 37 °C. Specifically, these cells were cultured in DMEM without serum or glucose, and transferred to a hypoxic chamber containing 0.1% O2, 5% CO2, and 95% N2 for 7 h. Subsequently, cells were reoxygenated for 2 h in complete DMEM high-glucose medium under normoxic conditions.
4.2. Cell transfection
Short hairpin RNAs (shRNAs) against HMGA2 (sh-circHMGA2#1, sh-circHMGA2# 2 and sh-circHMGA2#3), scrambled control (sh-NC), NLRP3 overexpression plasmid (pcDNA-NLRP3) and its corresponding control (pcDNA-NC) were all purchased by Geneseed (Guangzhou, China). For transfection, these oligonucleotides (50 nM) were introduced into HCMs using Lipofectamine 3000 (Invitrogen, USA). After transfection for 24 h, the expressions of circHMGA2 were checked by RT-qPCR to determine the transfection efficiency. Erastin, ferroptosis inducer, was obtained from Selleck (Shanghai, China) for in vitro experiments. In H/R-induced HCMs, erastin (25 μM) or free erastin was administered at 37 °C for subsequent experimentation 48 h after transfection.
4.3. RT-qPCR
Total RNAs from H/R-induced HCMs cells in different groups was extracted using Trizol reagent (Cwbio, China). With the use of PrimeScriptTM RT Master Mix (Takara, Japan), cDNA was synthesized from equal amount of total RNA, followed by PCR quantitative analysis using QuantiNova SYBR Green Kit (QIAGEN) according to the instruction. GAPDH was loaded as a normalization control for circRNA. The PCR amplification consisted of 40 cycles at 94 °C for 30 s (denaturation), 56 °C for 45 s (annealing) and 73 °C for 1 min (extension). The Ct value was obtained from QuantStudio 6 System (ABI, USA) and we used the 2−ΔΔCt method to analyze data. Sequences of primers used were as follows: circHMGA2, 5′-GACCTCCTCCTCTGTTGCAG-3′ (forward) and 5′-AGACCAGGGGTCACACAA-3′ (reverse); GAPDH, 5′-CGACCACTTTGTCAAGCTCA-3′ (forward) and 5′-AGGGGTCTACATGGCAACTG-3′ (reverse).
4.4. CCK-8 assay
Cultured cells were maintained at 96-well plates (2 × 103 cells/well) for the indicated time. After 72 h, the cells were treated with CCK-8 reagent (Sigma-Aldrich, USA) at a volume of 10 μL per well and incubated for an additional 2 h. The absorbance of cells in each hole at 450 nm was examined using the microplate reader (Tecan, Germany).
4.5. Measurement of ferrous ion (Fe2+) levels
The levels of iron ion in H/R-induced HMCs were measured using iron assay kit (Beyotime, China) following the manufacturer's instructions. Briefly, in the 5-10 X volumes of iron assay buffer, H/R-induced HMCs were homogenized. Cell supernatant were then added with iron reducer and incubated for 20 min, and the absorbance at 593 nm of each group was assessed by the microplate reader.
4.6. DCFH-DA staining
Intracellular ROS levels using DCFH-DA (Invitrogen, USA). The radicals inside H/R-induced HCMs were reacted with dye through deacetylation, resulting in its conversion into its fluorescent byproduct DCF. After 24 h, H/R-induced HCMs were resuspended in PBS for further analysis. Subsequently, a solution of 10 μM DCFH-DA was added into the suspension (3 × 105 cells/ml) and keep incubation at 37 °C for 15 min. Cells were cleansed and the fluorescence of cells were measured at 490–545 nm using SpectraMax M2 microplate reader (Molecular Devices, USA).
4.7. ELISA assay
As specified in the kit instructions, all steps were followed strictly. Expression levels of oxidative stress factors (SOD and MDA), as well as pyroptosis-related proteins (GPX4 and SLC7A11), and inflammatory cytokines (TNF-α, IL-1β, IL-10 and IL-10) in H/R-induced HCMs and serum of MI/R mice, were detected by the respective ELISA assay kits (all purchased Beyotime).
4.8. TUNEL staining
All operations strictly follow the instructions of the TUNEL staining kit (Mannheim, Germany). Briefly, myocardial tissues were sliced into slices and then antigen repair was performed for 5 min. Subsequently, the slices were treated with proteinase K (15 mg/ml) and incubated them with equilibration buffer for 10 min. Next, PBS and alkaline phosphatase were added to rinse the slices, followed by incubation. These slices were then stained with TUNEL regent and DAPI (Invitrogen, USA), and TUNEL-stained slices were observed under the fluorescence microscope (Olympus BX51, Zeiss, USA).
4.9. Establishment of MI/R mice model
Male C57BL/6 nude mice aged 8 weeks old (18–20 g) were procured from Vital River Laboratories (Beijing, China). The mice were acclimatized and randomly divided into four groups: sham, MI/R and MI/R + sh-NC and MI/R + sh-circHMGA2 (n = 5 per group). Lentivirus harboring sh-circHMGA2 and sh-NC were constructed by GenePharma (Shanghai, China). HCMs (1 × 106 cells) stably transfected with sh-circHMGA2 or sh-NC were injected subcutaneously into the axilla of mice. To establish the MI/R mice model, a left anterior descending (LAD) ligation was performed with 6–0 silk suture on mice in the treatment group. During the I/R procedure, these mice experienced 1 h of ischemia followed by 8 h of reperfusion. Subsequently, oligonucleotides were injected into the mice in the treatment group, while mice in the sham group were not received any treatment. All mice were euthanized by intraperitoneal injection of 3% sodium pentobarbital at a dose of 50 mg/kg, and myocardial tissues of mice were collected for the next analysis. All animal experimental protocols were authorized by the animal ethics committee of the Second Affiliated Hospital of Air Force Medical University.
4.10. Echocardiographic assessment
One week after the ligation surgery, a two-dimensional echocardiogram was conducted using a 20 MHz probe and GE Vivid 7.0 electrocardiogram machine (GE Company, Schenectady, NY), a two-dimensional electrocardiogram was performed. Five consecutive cardiac cycles were used to calculate all measured values. Computer algorithms were used to calculate the ejection fraction (EF), fraction shortening (FS), left ventricular end-diastolic anterior wall thickness (LVAWd) and left ventricular end-diastolic posterior wall thickness (LVPWd).
4.11. HE staining
The morphology of the myocardial tissues was examined using HE staining. Myocardial tissues were fixed in 10% formalin, dehydrated in a series of graded ethanol solutions, and embedded in paraffin to obtain continuous 5 μm-thick slices. Following staining with hematoxylin and eosin, slices were examined under a microscope to observe the tissue morphology.
4.12. Western blotting
Total proteins were extracted using RIPA reagent (Beyotime), and their concentrations were determined using BCA kit (Beyotime), proteins were loaded to 10% SDS-PAGE for band separation. Separated bands were transferred to PVDF membranes and then blocked with 5% skim milk at 37 °C for 1 h. After that, these membranes containing protein bands were exposed to the primary antibodies overnight at 4 °C environment, such as anti-GPX4 (ab262509, 1:1000; Abcam, USA), anti-SLC7A11 (ab275411, 1:1000), anti-NLRP3 (ab263899, 1:1000), anti-cleaved caspase-1 (ab25901, 1:1000), anti-IL-1β (ab254360, 1:1000), anti-GSDMD-N (ab215203, 1:1000) and anti-GAPDH (ab8245, 1:1000). Next, these membranes were exposed to the corresponding secondary antibody conjugated with horseradich peroxidase (ab205718, 1:2000) at 37 °C for 2 h. Finally, the protein signaling was checked using the enhanced chemiluminescence (ECL) reagent (Beyotime, China), and the intensity of the protein bands was quantified using Image J software 10.0 (Bethesda, USA).
4.13. Statistical analysis
Data from three independently repeated experiments were expressed as the mean ± standard deviation (SD) and analyzed using GraphPad Prism 8.0 (GraphPad, USA). Statistical difference in different groups was compared using Student's t-test (for two groups) or one-way ANOVA with Tukey's test (for multiple groups). A p-value of less than 0.05 was considered statistically significant.
Ethics committee approval and patient consent
This present study was performed based on the principles expressed in the Declaration of Helsinki. All animal experiments were approved by the Ethics Committee of the Second Affiliated Hospital of Air Force Medical University and conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Author contribution statement
Pin Feng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yi Chu: Performed the experiments; Analyzed and interpreted the data.
Jun Li, Jingyi Dang, Jianghong Chen: Performed the experiments.
Wei Zhang: Conceived and designed the experiments.
Funding statement
This study did not receive any specific funding or grant.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledges
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17849.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shepard D., VanderZanden A., Moran A., et al. Ischemic heart disease worldwide, 1990 to 2013: estimates from the global burden of disease study 2013. Circulation: Cardiovas. Qual. Outcomes. 2015;8(4):455–456. doi: 10.1161/CIRCOUTCOMES.115.002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson S.M., Ferdinandy P., Andreadou I., et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;73(1):89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 3.Bencsik P., Gömöri K., Szabados T., et al. Myocardial ischaemia reperfusion injury and cardioprotection in the presence of sensory neuropathy: therapeutic options. Br. J. Pharmacol. 2020;177(23):5336–5356. doi: 10.1111/bph.15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moens A.L., Claeys M.J., Timmermans J.P., et al. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int. J. Cardiol. 2005;100(2):179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Del Re, Amgalan D., Linkermann A., et al. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019;99(4):1765–1817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong D., He R., Dang Y., et al. The latest overview of circRNA in the progression, diagnosis, prognosis, treatment, and drug resistance of hepatocellular carcinoma. Front. Oncol. 2021;10 doi: 10.3389/fonc.2020.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Zheng Q., Bao C., et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai X., Niu R., Liu J., et al. Roles of noncoding RNAs in the initiation and progression of myocardial ischemia–reperfusion injury. Epigenomics. 2021;13(9):715–743. doi: 10.2217/epi-2020-0359. [DOI] [PubMed] [Google Scholar]
- 9.Huang F., Mai J., Chen J., et al. Non-coding RNAs modulate autophagy in myocardial ischemia-reperfusion injury: a systematic review. J. Cardiothorac. Surg. 2021;16(1):140. doi: 10.1186/s13019-021-01524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L.Y., Zhai M., Huang Y., et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019;26(7):1299–1315. doi: 10.1038/s41418-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y.F., Zhao L., Wang B.C., et al. The circular RNA TLK1 exacerbates myocardial ischemia/reperfusion injury via targeting miR-214/RIPK1 through TNF signaling pathway. Free Radic. Biol. Med. 2020;155:69–80. doi: 10.1016/j.freeradbiomed.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z., Zhu X., Li Y., et al. Circ-HMGA2 (hsa_circ_0027446) promotes the metastasis and epithelial-mesenchymal transition of lung adenocarcinoma cells through the miR-1236-3p/ZEB1 axis. Cell Death Dis. 2021;12(4):313. doi: 10.1038/s41419-021-03601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S., Zhao J., Wen S., et al. CircRNA High Mobility Group At-hook 2 regulates cell proliferation, metastasis and glycolytic metabolism of nonsmall cell lung cancer by targeting miR-331-3p to upregulate High Mobility Group At-hook 2. Anti Cancer Drugs. 2022;34(1):81–91. doi: 10.1097/CAD.0000000000001343. [DOI] [PubMed] [Google Scholar]
- 14.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Tseng H.H.L., Vong C.T., Kwan Y.W., et al. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 2016;6(1):1–13. doi: 10.1038/srep35016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J., Liu D., Zhao L., et al. Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management. Exp. Ther. Med. 2022;23(6):1–11. doi: 10.3892/etm.2022.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu P., Zhang X., Liu N., et al. Pyroptosis: mechanisms and diseases. Signal Transduct. Targeted Ther. 2021;6(1):128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Cao F., Yin H., et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B., Zhang J., Liu X., et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28(12):1171–1185. doi: 10.1038/s41422-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L., Yin Z., Qin X., et al. CD74 ablation rescues type 2 diabetes mellitus-induced cardiac remodeling and contractile dysfunction through pyroptosis-evoked regulation of ferroptosis. Pharmacol. Res. 2022;176 doi: 10.1016/j.phrs.2022.106086. [DOI] [PubMed] [Google Scholar]
- 21.Algoet M., Janssens S., Himmelreich U., et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trend. Cardiovas. Med. 2022 doi: 10.1016/j.tcm.2022.02.005. S1050-1738(22)00029-9. PMID: 35181472. [DOI] [PubMed] [Google Scholar]
- 22.Ferraro R., Latina J.M., Alfaddagh A., et al. Evaluation and management of patients with stable angina: beyond the ischemia paradigm: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;76(19):2252–2266. doi: 10.1016/j.jacc.2020.08.078. [DOI] [PubMed] [Google Scholar]
- 23.Lin F., Zhao G., Chen Z., et al. circRNA-miRNA association for coronary heart disease. Mol. Med. Rep. 2019;19(4):2527–2536. doi: 10.3892/mmr.2019.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L., Zhang Y., Jiang Y., et al. Circular RNA Rbms1 inhibited the development of myocardial ischemia reperfusion injury by regulating miR-92a/BCL2L11 signaling pathway. Bioengineered. 2022;13(2):3082–3092. doi: 10.1080/21655979.2022.2025696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X., Hang Y., Lu Y., et al. CircRNA circ-NNT mediates myocardial ischemia/reperfusion injury through activating pyroptosis by sponging miR-33a-5p and regulating USP46 expression. Cell Death Discover. 2021;7(1):370. doi: 10.1038/s41420-021-00706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun G., Shen J.F., Wei X.F., et al. Circular RNA Foxo3 relieves myocardial ischemia/reperfusion injury by suppressing autophagy via inhibiting HMGB1 by repressing KAT7 in myocardial infarction. J. Inflamm. Res. 2021;14:6397. doi: 10.2147/JIR.S339133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Zhou Q., Jiang J. Circ_0027446 induces CLDN1 expression to promote papillary thyroid cancer cell malignancy by binding to miR-129–5p. Pathol. Res. Pract. 2022;238 doi: 10.1016/j.prp.2022.154095. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W., Zhou Y., Xu T., et al. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021:2021. doi: 10.1155/2021/9929687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W., Li W., Leng Y., et al. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39(2):210–225. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 30.Ma X., Liu H., Foyil S.R., et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125(25):3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paraskevaidis I.A., Iliodromitis E.K., Vlahakos D., et al. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur. Heart J. 2005;26(3):263–270. doi: 10.1093/eurheartj/ehi028. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez M., Lokan J., Leung C., et al. A critical evaluation of the role of iron overload in fatty liver disease. J. Gastroenterol. Hepatol. 2022;37(10):1873–1883. doi: 10.1111/jgh.15971. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y., Wang X., Liu X., et al. Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants. 2022;11(11):2196. doi: 10.3390/antiox11112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardoso B.R., Hare D.J., Bush A.I., et al. Glutathione peroxidase 4: a new player in neurodegeneration? Mol. Psychiatr. 2017;22(3):328–335. doi: 10.1038/mp.2016.196. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Zhuang L., Gan B. BAP1 suppresses tumor development by inducing ferroptosis upon SLC7A11 repression. Mol. Cell. Oncol. 2019;6(1) doi: 10.1080/23723556.2018.1536845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen S.C., He F., Cheng C., et al. Uric acid aggravates myocardial ischemia–reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110990. [DOI] [PubMed] [Google Scholar]
- 37.Toldo S., Mauro A.G., Cutter Z., et al. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018;315(6):H1553–H1568. doi: 10.1152/ajpheart.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ø Sandanger, Ranheim T., Vinge L.E., et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 2013;99(1):164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 40.Qiu Z., Lei S., Zhao B., et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/9743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.