Abstract
Okra is a commercially important vegetable crop that grows in tropical, subtropical, and warm temperate parts of the world, but its productivity is hindered by a lack of improved cultivars and delayed and erratic seedling emergence in the field. An experiment was conducted to evaluate the effect of seed priming treatments on okra genotypes' seedling emergence and fruit yield. In this experiment, Clemson spineless, Arka Anamika, SOH701, 240,207, and 240,586 okra genotypes were primed with tap water, 50% cow urine, 200 ppm GA3, and 0.5% KH2PO4 as treatments, and dry seed of each genotype was used as a control. The experiment was conducted in Dire Dawa by irrigation in a randomized complete block design with three replicates. GenStat software was used to analyse all the data collected in this experiment. Genotype and seed priming treatments significantly affected phenology, growth, fruit yield, and yield-related traits, and their interactions affected the above traits, except for days to seedling emergence and fruit number per plant. Genotype Clemson spineless (5.13 days) and seed primed with GA3 (4.6 days) had the shortest days to 50% seedling emergence, and genotype 240,586 primed with KH2PO4 produced the highest fruit yield per hectare (37.78 t ha−1). So, farmers in the study area are advised to use genotype 240,586 with KH2PO4 seed priming to increase fruit yield. However, research conducted at one site should be repeated at multiple sites in order to make recommendations that are relevant to the country.
Keywords: Fruit yield, Genotype, Okra, Pre-sowing treatment, Seedling emergence
1. Introduction
Okra [Abelmoschus esculentus (L.) Moench] is a warm-season annual herbaceous vegetable crop and a member of the Malvaceae family [1]. It originated in Ethiopia and Sudan in northeastern Africa [2,3]. Okra is now grown in warm temperate, tropical, and subtropical climates around the world [4,5]. According to FAOSTAT, okra covers 2.53 million hectares of land worldwide, with 10.5 million tons of fruit produced in the 2020 crop production year [6]. Okra stands out among fruits and vegetables, especially in developing countries like Ethiopia, due to its numerous nutritional, medicinal, exportability, and adaptability benefits [[7], [8], [9]]. Okra fruits are a good source of mucilage, fats, fibres, minerals, ascorbic acid, carotene, vitamins [10], proteins, carbohydrates [11], and edible oil [12,13]. Fresh okra leaves and immature green fruits are used in salads, soups, and stews [14], and dry okra seeds are used to make vegetable curd or as a coffee ingredient, especially in Africa [15].
Okra can grow in the majority of Ethiopia's agroecosystems, both in home gardens and commercially [16]. However, okra has traditionally been grown from landraces in the eastern, southwestern, western, and northwest regions of Ethiopia, and it has historically been a small-scale crop in the country [17]. Hence, there is no complet data on okra production and productivity in Ethiopia [18]. Nevertheless, okra has a potential to ensure food security, overcome malnutrition, meet demand for vegetables, and be exported, in Ethiopia and its fruit is already available at a premium price in urban areas of the country [19]. Okra fruit is also accessible in bars and restaurants in Europe as a boiled and fried vegetable salad, and it has gained popularity as a new alternative in market diversification [[20], [21], [22]]. As a result, in line with the goals of food security, meeting vegetable demand, and exporting okra fruit, the Ministry of Agriculture and Natural Resources (MOANR) has paid close attention to increasing okra production and productivity in Ethiopia, and recently private companies have started cultivating okra in the country for export purposes [16]. However, okra production is limited due to the lack of improved cultivars and prominently delayed and erratic seed germination and seedling emergence in the field due to seed dormancy [23], which has an impact on other management practices such as post-emergence weed control, fertilizer application, and consistent harvesting [24].
Effective production of agricultural and horticultural crops, including okra, depends on rapid, reliable, and complete germination and seedling emergence in the field. Successful seed germination and seedling establishment and development are critical steps in the crop's life cycle to ensure vigorous growth and high productivity [23,25]. However, the main problems in okra cultivation are extended germination, long seedling emergence times, non-uniformity, and poor seedling emergence and development [26]. Therefore, this crop requires trustworthy pre-sowing seed treatment methods that can increase seedling emergence, establish quickly and uniformly with vigorous crop stands, and improve production efficiency.
Seed priming is a low-cost, risk-free pre-sowing seed treatment in which the seeds are partially hydrated by soaking them in a specific solution so that almost all seed metabolic processes take place before germination and then redried to restore the original seed moisture content [27,28]. Seed priming treatments improve seed root emergence, germination speeds, seed vigor, seedling vigor, and uniform seedling establishment in the field by altering numerous physiological activities in seeds, such as protein synthesis, mitochondrial repair, or the synthesis of new mitochondria, and enhancing a-amylase activity and soluble sugar content [27,29,30]. Previous studies have shown that various seed priming treatments increases germination percentage, reduces germination and seedling emergence time, and improves seedling establishment in many agricultural and horticultural crops [[31], [32], [33]]. But the effectiveness of seed priming is dependent on a number of physical and chemical elements, including priming agents, water potential, crop species, and the quality of the seed [34]. Therefore, it is worth investigating suitable priming techniques for specific plant species such as okra to promote seedling emergence, establishment and productivity under different environmental conditions. Numerous studies on various seed priming methods have revealed improved seed germination and seedling vigor under controlled conditions for many crops [31], including okra [35]. However, relatively limited research has sought to evaluate the impact of different priming methods on okra across the entire growth season at field level. Furthermore, no research has been conducted on different seed priming techniques for okra genotypes' seedling emergence, growth, and fruit yield in Ethiopian conditions. Therefore, the objective of the current study was to examine the effects of various seed priming methods on seedling emergence, growth, and fruit yield performance of okra genotypes in order to determine the most suitable priming treatments for okra genotypes.
2. Materials and methods
2.1. Description of the study area
The research was conducted in Dire Dawa (Tony Farm) in 2019–2020 under irrigation conditions. Dire Dawa is 40, 66, and 518 km away from Haramaya University, Harar, and Addis Ababa, respectively. The climate of Dire Dawa is warm, dry, and has a low amount of precipitation. It is located between latitudes 9° 36′ 3.15″ north and longitudes 41° 51′ 0.5112″ east. The elevation of Dire Dawa is 950–1260 m above sea level [36]. About 25.4 °C is Dire Dawa's average yearly temperature, and the average maximum temperature is 31.4 °C, and the average minimum is 18.2 °C. The yearly average total rainfall is 604 mm, while the average annual humidity is 41.82%. Loamy sand soil is the major soil type in the study area [37].
2.2. Experimental materials and priming treatments
Five different okra genotypes were used in this experiment. Two promising genotypes (240,586 and 240,207) are from Ethiopia; one variety (Clemson spineless) was American; and two others (SOH701 and ArkaAnamika) were brought over from India and are now registered as commercial varieties in Ethiopia. The seeds of these five genotypes were harvested in October 2019 at Dire Dawa and kept in canvas bags at room temperature in the Horticulture Department of Haramaya University. Seed priming with tap water, 50% cow urine, 200 ppm GA3, and 0.5% KH2PO4 solutions were used to soak the five okra genotypes for 24 h, and unprimed seeds of each of the five genotypes were used as a control treatment. Therefore, there were 25 total treatment combinations.
2.3. Seed priming procedures
A 200 ppm gibberellic acid (GA3) solution was made by dissolving 0.2 g of GA3 in 1000 mL of distilled water. Similar to this, 5 g of potassium dihydrogen phosphate (KH2PO4) was dissolved in 1000 mL of distilled water to make a 0.05% KH2PO4 solution. Furthermore, local breed cow urine was collected and used to make a 50% cow urine solution by measuring 500 mL of fresh cow urine and blending it with 500 mL of distilled water to make a 1000 mL cow urine and distilled water solution. Prior to the application of priming treatments, all examined okra genotypes’ seeds were surface sterilized with a sodium hypochlorite 0.05% solution for 5 min and rinsed with distilled water to disinfect the seeds from pathogens. With the exception of the control treatment, the two hundred-sixteen surface-sterilized seeds of each okra genotype were then soaked in tap water, 0.5% KH2PO4, 200 ppm GA3, and 50% cow urine solutions for 24 h at room temperature. Following priming treatments applied, seeds were removed from each priming treatment and allowed to dry between sheets of paper per treatment in the shade to regain their original dry weight.
2.4. Experiment design and experimental procedures
A three-replicate randomized complete block (RCBD) design was used for this field experiment. In the experimental area, each treatment was randomly assigned to each replicate plot. Each plot was arranged in a single row consisting of 12 plants with a spacing of 0.6 m between plants. The plots and adjacent replicates were separated by 0.8 and 2 m, respectively. The land was prepared using a tractor and human labor. Furrows for irrigation were made by leveling the soil. Hand-made ridges were prepared in accordance with planting spacing. On December 28, 2019, three seeds per hill were sown at 5 cm depth and thinned to one plant per hill when plants reached the 3–4 leaf stage. Furrow irrigation was applied once per week at emergence and once every two weeks at flowering and pod development throughout the growing season. All other cultural practices, such as weeding, thinning, fertilization, and earthing up, were consistently applied as recommended for the crop. Fruit harvesting was started on March 4, 2020, by using a sharp knife when the fruit pod was bright green and fleshy and the seeds were small. Fruit harvesting was carried out eight times throughout the crop growth period.
2.5. Data collection
Data on quantitative traits were recorded using the International Plant Genetic Resources Institute [38] descriptor list for okra species. Ten plants per row were used to collect data for quantitative traits, leaving the two plants growing at either end of the row to serve as border plants. The data for crop phenology, plant growth traits, and tender fruit yield and yield-related traits were collected as described here below.
Count the days between seeding and 50% seedling emergence from the soil, 50% flowering, and the first tender fruit harvest for each plot on a per-plot basis. At the final harvest, the average number of branches and internodes per plant on ten plants from each plot was counted, and averages for ten plants were then determined. The number of tender fruits on ten plants in each plot at each harvest was counted and summed at the end of the harvest, and the average number of tender fruits per plant was then determined. Ten tender fruit lengths were measured per plot and for each harvest, from the base of the calyx to the tip of the fruit in centimeters, and the average was determined by dividing the sum of all tender fruit lengths by the total number of measured fruits. Ten tender fruits were used to measure the fruit length; they were also weighed using a sensitive balance for each plot and harvest, and the average fruit weight in grams was then determined. The weight of the tender fruits harvested on ten plants for each plot and each harvest was averaged to determine the tender fruit yield per plant (gram per plant), and the fruit yield per hectare (t ha−1) was determined by converting the tender fruit produced per plot to tons per hectare.
2.6. Data analysis
According to the statistical techniques given by Gomez and Gomez, the quantitative data was subjected to a two-way analysis of variance (ANOVA) using GenStat Software (version 16.1 by VSN International Ltd.) for the relevant experimental design [39]. Following the significance of mean squares, the comparisons of the mean performance of treatments were made using the Least Significant Difference (LSD) at the 5% probability level.
3. Results
Analysis of variance (ANOVA) results showed that there were significant variations between okra genotype and seed priming treatment in all crop phenology, growth, and yield traits, such as days to 50% seedling emergence, days to 50% flowering, days to fruit maturity, branches per plant, internodes per plant, fruits per plant, fruit length, average fruit weight, and fruit yield per plant and per hectare (Table 1). Except for days to 50% seedling emergence and fruit number per plant, the interaction of genotype and seed priming treatment significantly affected the days to 50% flowering, days to fruit maturity, branches per plant, internodes per plant, fruit length, average fruit weight, and fruit yield per plant and per hectare (Table 1).
Table 1.
Mean squares analysis value effect of seed priming treatment and okra genotype on crop phenology, growth, and fruit yield-related traits and yield in Dire Dawa in 2019–2020 by using F test.
| Traits | Genotype DF = 4 |
Seed Priming Df = 4 |
Genotype*Seed Priming DF = 16 |
|---|---|---|---|
| Days to 50% seedling emergence | 4.5867** | 34.5533** | 1.4617ns |
| Days to 50% flowering | 51.1133** | 109.2133** | 6.505** |
| Days to fruit maturity | 137.547** | 217.747** | 15.913* |
| Number of branches per plant | 13.88** | 8.659** | 2.987* |
| Number of internodes per plant | 73.25** | 492.27** | 32.07* |
| Number of fruits per plant | 413.875** | 33.393** | 2.301ns |
| Fruit length (cm) | 25.672** | 13.074** | 8.055** |
| Average fruit weight (g) | 713.26** | 252.77** | 60.72* |
| Fruit yield per plant (g) | 1,707,587** | 282,160** | 56,033* |
| Fruit yield per hectare (t) | 741.12** | 122.46** | 24.32* |
DF, Degree of freedom; ns, non-significance difference; *&**, significant level at 5% and 1%, respectively.
3.1. Effect of genotype and seed priming on crop phenology and growth traits
3.1.1. Days to 50% seedling emergence
Analysis of variance revealed that okra genotype and seed priming treatment had a significant effect on 50% day to seedling emergence. The interactions between the two factors, on the other hand, had no significant effect on 50% days to seedling emergence (Table 1). The results showed that all seed priming treatments reduced the number of days to seedling emergence in the field by 3–4 days compared to unprimed seeds. Okra seeds primed in GA3 had the lowest (4.6) number of days to 50% seedling emergence, which was statistically comparable to seeds primed in KH2PO4 (5.13 days). Days to 50% seedling emergence were somewhat lower for both seeds primed in cow urine (5.87 days) and tap water (5.73 days), which were statistically similar to each other. Unprimed okra seeds, in contrast, had the longest (8.53) day to 50% seedling emergence (Table 2). Regarding genotype, genotype Clemson spineless took fewer (5.13) days to reach 50% seedling emergence, which was not a statistically significant difference compared to genotype SOH701 (5.8 days). In contrast, genotype 240,586 had a significantly delayed time (6.6 days) to 50% seedling emergence, which was statistically similar to genotypes 240,207 (6.07 days) and Arka Anamika (6.27 days) (Table 2).
Table 2.
The effect of genotype and seed priming on days to 50% seed emergence of okra.
| Treatements | Days to 50% seedling emergence (day) |
|---|---|
| Genotypes | |
| Clemson spineless | 5.13c |
| Arka Anamika | 6.27ab |
| SOH701 | 5.80bc |
| 240,586 | 6.60a |
| 240,207 | 6.07ab |
| LSD (5%) | 0.696 |
| CV (%) | 15.9 |
| Seed priming | |
| Control | 8.53a |
| Tap water | 5.73bc |
| GA3 | 4.60d |
| KH2PO4 | 5.13cd |
| Cow urine | 5.87b |
| LSD (5%) | 0.696 |
| CV (%) | 15.9 |
Mean values within column in each main factor followed by the same letter (s) are not significantly different at 5% probability level; LSD, Least significant difference at 5% probability level; CV (%), coefficient of variation in percent; GA3, Gibberellic acid; KH2PO4, Potassium dihydrogen phosphate.
3.1.2. Days to 50% flowering and fruit maturity
Days to 50% flowering and fruit maturity were significantly influenced by genotype and seed priming treatment as well as by the interaction (Table 1). Genotype Clemson spineless primed in GA3 had the earliest (46.33) days to 50% flowering and days to first fruit maturity (64.330), while genotype SOH701 unprimed seed showed a delayed (60.67) days to 50% flowering, which was statistically equal to unprimed seed of genotype 240,207 (59.33 days). Similarly, unprimed seeds of genotype 240,207 delayed fruit maturation by 87.33 days in this study (Table 3).
Table 3.
The influence of genotype and seed priming on phenology and growth of okra.
| Treatments |
Traits |
||||
|---|---|---|---|---|---|
| Genotypes | Seed priming | Days to 50% flowering (day) | Days to fruit maturity (day) | Branches number per plant (number) | Internodes number per plant (number) |
| Clemson spineless | Control | 53.00hi | 74.00g-k | 3.80h | 14.47j |
| Tap water | 54.67d-g | 73.33ijk | 4.07gh | 15.67j | |
| GA3 | 46.33n | 64.33m | 6.00b-g | 29.65b-e | |
| KH2PO4 | 50.67kl | 67.00im | 6.67b-e | 17.73ij | |
| Cow urine | 51.33jkl | 73.67h-k | 7.27ab | 29.33b-f | |
| Arka Anamika | Control | 54.67d-g | 80.00bcd | 5.067d-h | 18.80hij |
| Tap water | 53.67fgh | 77.00c-i | 5.60b-h | 19.20g-j | |
| GA3 | 49.00m | 73.33ijk | 6.90a-d | 35.34ab | |
| KH2PO4 | 53.33gh | 75.33d-j | 5.87b-g | 23.00f-i | |
| Cow urine | 55.33cde | 76.33d-j | 7.41ab | 20.07g-j | |
| SOH701 | Control | 60.67a | 78.33b-h | 3.73h | 19.57g-j |
| Tap water | 54.00e-h | 81.33bc | 4.20fgh | 20.60g-j | |
| GA3 | 50.67kl | 73.33ijk | 4.80e-h | 32.25abc | |
| KH2PO4 | 54.00e-h | 74.67f-k | 5.27c-h | 23.93e-i | |
| Cow urine | 54.00e-h | 77.00c-h | 4.40fgh | 16.53j | |
| 240,586 | Control | 55.00def | 82.33b | 4.33fgh | 18.67hij |
| Tap water | 56.67c | 74.00g-k | 6.93a-d | 18.87hij | |
| GA3 | 50.00im | 70.33kl | 7.10abc | 31.13a-d | |
| KH2PO4 | 52.67hij | 73.00ijk | 5.13d-h | 19.33g-j | |
| Cow urine | 54.67d-g | 79.67b-e | 4.27fgh | 24.60e-h | |
| 240,207 | Control | 59.33ab | 87.33a | 6.13b−F | 25.00d-h |
| Tap water | 58.67b | 79.00b-f | 7.47ab | 20.00g-j | |
| GA3 | 51.67ijk | 72.00jk | 8.73a | 36.41a | |
| KH2PO4 | 55.67cd | 75.00e-k | 7.27ab | 25.40d-g | |
| Cow urine | 55.67cd | 78.67b-g | 5.93b-g | 28.87c-f | |
| LSD (5%) | 1.3876 | 4.686 | 1.963 | 6.46 | |
| CV (%) | 1.6 | 3.8 | 20.7 | 16.8 | |
Means followed by the same letters in the same column are not significantly different at P < 0.05 level of significance; LSD (5%), Least significant difference at 5% probability level; CV (%), coefficient of variation in percent; GA3, Gibberellic acid; KH2PO4, Potassium dihydrogen phosphate.
3.1.3. Number of branches per plant
Number of branches per plant was significantly affected by seed priming, genotype, and the interaction of the two factors (Table 1). Genotype 240,207 primed with GA3 (8.73), tap water (7.47), and KH2PO4 (7.27) had a higher number of branches per plant, and they displayed statistical similarity to genotype 240,586 primed in tap water (6.93) and GA3 (7.1), genotype Arka Anamika primed in GA3 (6.9) and cow urine (7.41), and genotype Clemson spineless primed in cow urine (7.27). Whereas genotype SOH701 without priming had a lower (3.73) number of branches per plant, with a non-significant difference between genotypes Clemson spineless (4.07), Arka Anamika (5.6), and SOH701 (4.2) primed in tap water, genotype SOH701 (4.8) primed in GA3, genotypes SOH701 (5.27 and 4.4) and 240,586 (5.13 and 4.27) primed in KH2PO4 and cow urine, and unprimed seeds of genotypes Clemson spineless (3.8), 240,586 (4.33), and Arka Anamika (5.07) (Table 3).
3.1.4. Number of internodes per plant
Genotype, seed priming, and their interactions all had a significant effect on the number of internodes per plant (Table 1). Genotype 240,207 primed with GA3 had the highest number of internodes per plant (36.41), which was statistically similar to genotypes 24,058 (31.13), Arka Anamika (35.34), and SOH701 (32.25) primed with GA3. Meanwhile, genotype Clemson spineless without priming treatment had the lowest (14.47) number of internodes per plant with a statistically non-significant difference from genotypes 240,586 (18.87), Arka Anamika (19.2), and SOH701 (20.6) primed in tap water, genotypes Clemson spineless (17.73) and 240,586 (19.33) primed in KH2PO4, genotypes Arka Anamika (20.07) and SOH701 (16.53) primed in cow urine, and unprimed seeds of genotypes 240,586 (16.53), Arka Anamika (18.8) and SOH701 (19.57) (Table 3).
3.2. Effect of genotype and seed priming on fruit yield and yield-related traits
3.2.1. Number of tender fruit per plant
Okra genotype and seed priming treatment had a substantial effect on the number of fruits per plant, while the number of fruits produced by a plant was not significantly affected by the interactions of genotype and seed priming treatment (Table 1). Totally, the results showed that the Ethiopian genotypes (240,586 and 240,207) produced more number of fruits per plant than the three introduced genotypes. Genotype 240,586 produced significantly more fruits per plant (30.36) than the other genotypes. However, genotype SOH701 had the lowest (18.06) number of fruits per plant (Table 4).
Table 4.
The effect of okra genotype and seed priming treatment on number of fruits per plant.
| Treatments | Number of fruits per plant (number) |
|---|---|
| Genotypes | |
| Clemson spineless | 21.99c |
| Arka Anamika | 19.28d |
| SOH701 | 18.06e |
| 240,586 | 30.36a |
| 240,207 | 27.20b |
| LSD (5%) | 1.058 |
| CV (%) | 6.2 |
| Seed priming | |
| Control | 21.08c |
| Tap water | 23.25b |
| GA3 | 23.60b |
| KH2PO4 | 25.23a |
| Cow urine | 23.72b |
| LSD (5%) | 1.058 |
| CV (%) | 6.2 |
Mean values within column in each main factor followed by the same letter (s) are not significantly different at 5% probability level; LSD, Least significant difference at 5% probability level; CV (%), coefficient of variation in percent; GA3, Gibberellic acid; KH2PO4, Potassium dihydrogen phosphate (see Fig. 1).
The findings demonstrated that all seed priming treatments enhanced the number of fruits produced per plant over unprimed seeds. Okra plants grown from seeds primed with KH2PO4 produced the highest number (25.23) of fruits per plant compared to other seed priming treatments and the control. Plants grown from seeds primed with cow urine (23.72), GA3 (23.6), and tap water (23.25) showed no significant difference between them and increased the number of fruits per plant by about 11.6% over to the control treatment. While the lowest (21.08) number of fruits per plant was produced in the plants grown from unprimed seeds (Table 4).
3.2.2. Fruit length
Fruit length was significantly affected by genotype, seed priming treatment, and their interactions (Table 1). In comparison to other treatment combinations, the fruit of genotype 240,207 developed from GA3-primed seeds had significantly the longest (21.25 cm) length, as shown in Fig. 2. In contrast, unprimed seeds of genotype 240,586 had significantly shorter (11.87 cm) fruit length, as shown in Fig. 3. This was a non-significant difference between the same genotype primed with GA3 (14.27 cm), tap water (13.16 cm), cow urine (13.6 cm), and KH2PO4 (13.71 cm), genotype 240,207 primed with tap water (14.4 cm), cow urine (13.81 cm), and KH2PO4 (14.48 cm), and unprimed seeds of genotypes Arka Anamika (13.84 cm) and SOH701 (14.37 cm) (Table 5).
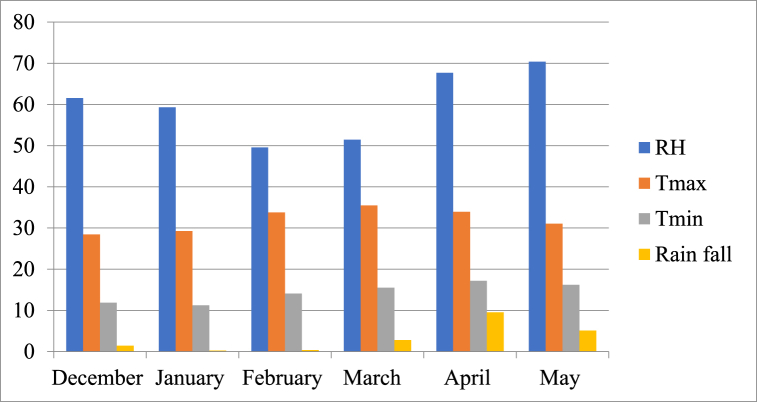
Fig. 1.
Climatic conditions during the crop-growing season from December 2019 to May 2020.
Fig. 2.

Harvested fruit of genotype 240,207 grown from GA3 treated seed.
Fig. 3.

Harvested fruit of genotype 240,586 grown from unprimed seeds.
Table 5.
The influence of genotype and seed priming treatment on fruit yield and yield related traits of okra.
| Treatments |
Traits |
||||
|---|---|---|---|---|---|
| Genotype | Seed priming | Fruit length (cm) | Average fruit weight (g) | Fruit yield per plant (g) | Fruit yield per hectare (t) |
| Clemson spineless | Control | 15.97b-h | 27.83efg | 582.4g-k | 12.13g-k |
| Tap water | 15.99b-g | 16.72i | 669.4f-i | 13.95f-i | |
| GA3 | 16.40b-g | 29.02efg | 627.2f-j | 13.07f-j | |
| KH2PO4 | 17.97bc | 41.28bc | 798.7efg | 16.64efg | |
| Cow urine | 16.87b-e | 28.01efg | 765.0e-h | 15.94e-h | |
| Arka Anamika | Control | 13.84f-i | 28.69efg | 270.0l | 5.63l |
| Tap water | 15.01d-h | 18.11hi | 346.4kl | 7.22kl | |
| GA3 | 16.41b-g | 22.96ghi | 584.6g-k | 12.18g-k | |
| KH2PO4 | 16.29b-g | 29.24efg | 581g-k | 12.10g-k | |
| Cow urine | 18.43b | 31.07efg | 518.6h-l | 10.80h-l | |
| SOH701 | Control | 14.37e-i | 29.36efg | 445.3i-l | 9.28i-l |
| Tap water | 15.50c-h | 30.01efg | 392.7ikl | 8.18jkl | |
| GA3 | 16.57b-f | 33.48c-f | 638.2f-j | 13.30f-i | |
| KH2PO4 | 16.86b-e | 40.96bcd | 566.6g-k | 11.80g-k | |
| Cow urine | 15.30c-h | 32.12def | 553.4g-k | 11.53g-k | |
| 240,586 | Control | 11.87i | 35.04b-e | 1214.5bcd | 25.30bcd |
| Tap water | 13.16hi | 27.43efg | 826.3efg | 18.22ef | |
| GA3 | 14.27e-i | 26.94e-h | 1271.9bc | 26.50bc | |
| KH2PO4 | 13.71ghi | 56.51a | 1813.4a | 37.78a | |
| Cow urine | 13.60ghi | 33.53c-f | 1317.6b | 27.45b | |
| 240,207 | Control | 17.23bcd | 35.21b-e | 681.5f-i | 14.20f-i |
| Tap water | 14.40e-i | 24.45f-i | 826.3efg | 17.21efg | |
| GA3 | 21.25a | 30.39efg | 876.1ef | 18.25ef | |
| KH2PO4 | 14.48d-i | 43.92b | 975.9de | 20.33de | |
| Cow urine | 13.81f-i | 35.15b-e | 1005.7cde | 20.95cde | |
| LSD (5%) | 2.824 | 9.05 | 273.3 | 5.694 | |
| CV (%) | 11 | 17.5 | 21.7 | 21.7 | |
Means followed by the same letters in the same column are not significantly different at P < 0.05 level of significance; LSD (5%), Least significant difference at 5% probability level; CV (%), coefficient of variation in percent; GA3, Gibberellic acid; KH2PO4, Potassium dihydrogen phosphate.
3.2.3. Average fruit weight, fruit yield per plant and per hectare
Okra genotype and seed priming treatment, as well as the interactions of the two factors, significantly affected the average fruit weight and fruit yield per plant and per hectare (Table 1). The largest (56.51 g) average fruit weight was produced by genotype 240,586 with KH2PO4-seed priming when compared to all other treatment combinations. However, genotype Clemson spineless primed with tap water had the lowest (16.72 g) average fruit weight, with no significant difference between genotype Arka Anamika primed with tap water (18.11 g), GA3 (22.96 g), and genotype 240,207 (24.45 g) primed with tap water (Table 5). Similarly, the fruit production of genotype 240,586 with KH2PO4 seed priming treatment was significantly the highest per plant (1813.4 g per plant) and per hectare (37.78 t ha−1), approximately 49.3% more than its unprimed counterpart of the same genotype. Contrarily, genotype Arka Anamika with unprimed seeds produced lower fruit per plant (270 g) and per hectare (5.63 t ha−1), with non-significant differences to the same genotype primed with tap water (346.4 g and 7.22 t ha−1) and cow urine (518.6 g and 10.8 t ha−1) and unprimed seeds of genotype SOH701 (445.3 g and 9.28 t ha−1) and primed with tap water (392.7 g and 8.18 t ha−1) (Table 5).
4. Discussion
Uniform germination, seedling emergence, seedling vigor, plant growth, and maturity are the most crucial factors to ensure effective crop establishment and yield [25,40]. Seed priming treatments improve germination, seedling emergence, and crop establishment in the field by breaking seed dormancy and improving seed vigor [27,41]. Rapid and uniform emergence and development of seedlings in the field are critical steps in all crop life cycles to ensure vigorous growth and high productivity [40]. Many research reports have shown that seed priming techniques increase the speed and consistency of seedling field emergence and seedling development in the field [42]. In a similar trend, our findings demonstrated that all seed priming techniques reduced the number of days to field emergence compared to unprimed seeds. The reduction of the number of days to seedling emergence in the field can be achieved through all seed priming treatments that enhances the physiological activity of the seed embryo by overcoming seed dormancy, encouraging embryo growth, and increasing seed vigor [43]. Apart from this, okra seeds primed with GA3 resulted in the emergence of seedlings from the soil earlier than other seed priming techniques. So the field emergence response of okra seedlings was more pronounced with GA3 than with other seed priming techniques. Because GA3 enhances seed hydrolase activity and catalyzes the breakdown of preserved food materials into sugars and amino acids to improve germination and seedling emergence over the three remaining seed priming techniques. GA3 is also widely used to break seed dormancy and promote seed germination by promoting embryonic growth and softening the seed coat to improve germination and seedling emergence both in laboratory and field conditions [44]. The results confirmed by Ref. [43] found that okra seeds primed with 50 ppm GA3 had significantly shorter days to seedling emergence over the other priming treatments and the control. Furthermore [45], reported that primed okra seedlings emerged from the soil more quickly than unprimed seeds.
The present findings demonstrated that okra genotypes significantly influenced seedling emergence in the field. As we explain in the result section, in comparison to genotypes 240,586, 240,207, and Arka Anamika, genotypes Clemson spineless and SOH701 took a shorter days to attain 50% seedling field emergence. This is because of the inherent range of okra genotypes, genetic differences contributed to the variances in okra seedling emergence. Based on a previous research result reported by Ref. [35], the Clemson spineless and SOH701 genotypes had a lower percentage of hard seeds than genotype 240,586 and 240,207 due to genetic differences in the laboratory conditions. As a result, seedlings from genotypes Clemson spineless and SOH701 emerged quickly from the soil, whereas those from hard-seeded genotypes 240,586 and 240,207 showed irregular and delayed seedling emergence in the field for this study. Therefore, genotypes Clemson spineless and SOH701 are crucial resources for plant breeding programs aiming for earlier seedling emergence variety releases. The findings are in line with those of [19,46], who found that okra genotypes significantly affected seedling emergence.
Improved field emergence, vigorous plants that are more tolerant of stressful environments, early flowering and maturity, earlier harvesting, and increased production can all be the result of direct seed priming effects in almost all crops [47,48,55,56]. In the current study, we found that the seed priming treatments accelerated flowering and fruit maturity as well as improved growth and fruit yield-related traits and yields of okra genotypes. The majority of okra genotypes, with the exception of genotype 240,207, respond to all seed priming techniques for early flowering and fruit maturation. However, genotypes' responses to seed priming treatments differed for flowering and fruit maturity, which may be due to the genetic heterogeneity of the genotypes. Okra genotypes grown from all seed priming treatments had the quickest flowering and fruit maturity over unprimed seeds due to early seedling emergence and vegetative growth [49]. Genotype Clemson spineless primed with GA3 had the shortest days to 50% flowering and the earliest first fruit maturity when compared to other genotypes and seed priming treatments. Over all, GA3-primed seeds of all genotypes showed better response to germination and fruit maturation than the other seed priming treatments. The beneficial effects of gibberellins have been revealed in a variety of crops by showing shortened juvenile phases and improved early flowering and fruit development [50]. The findings are consistent with those of the authors [43], who reported that GA3 primed seeds reduced the number of days to 50% flowering, fruit setting, and maturity as compared to unprimed okra seed. Furthermore, the current research is supported by Ref. [51], who revealed that plants of a chili variety grown from GA3-primed seeds took fewer days to reach 50% flowering and fruit maturity than unprimed seeds. However, the current research results are contrary to Ref. [52], who reported that okra seed primed with hot and normal water as well as a 3% NaCl solution increased the number of days to flowering and fruit maturity compared to unprimed seed.
As reported in the result section of this study, the number of branches and internodes per plant and fruit length of okra genotypes were significantly influenced by seed priming treatments. The genotypic responses of okra genotypes to each seed priming treatment were roughly different for the number of branches and internodes per plant and fruit length. Genotype 240,207 primed in GA3, tap water, and KH2PO4 had the heighest branches per plant, followed by genotype 240,586 primed in GA3 and tap water, genotype Arka Anamika primed in GA3 and cow urine, and genotype Clemson spineless primed in cow urine. Genotype 240,207, grown from seeds primed in GA3, and all other genotypes primed in GA3 except genotype Clemson spineless, had a higher number of internodes per plant. Similar to this, the highest fruit lengths were recorded from genotype 240,207 with seeds primed in GA3. The highest number of branches, internodes per plant, and fruit length from GA3 primed seeds may be GA3 attribute that promotes seedling establishment and increased meristematic cell activity in seedlings of genotypes, which is essential for plant vigor growth and fruit length [53]. Gibberellins play a crucial role in regulating a variety of plant developmental processes, such as seed germination and seedling emergence, stem lengthening, and leaf elongation. The advantageous benefits of GA3 have been demonstrated in a number of crops via increased seed germination and seedling establishment [54,55], leaf expansion and development, and stem elongation [56,57]. Genetic diversity in okra may be the cause of the differences in responses to GA3 seed priming and the other priming treatments in the current investigation. The findings are consistent with those of the authors [58], who found that primed okra seeds with 100 ppm GA3 produced more branches per plant than the control. Moreover, the outcome is consistent with [43], who reported that okra fruit length and average fruit weight were significantly influenced by 50 ppm GA3-primed seeds compared to unprimed seeds.
The number of branches and internodes per plant as well as the length of the fruit were also increased in the current study for some specific okra genotypes using tap water, cow urine, and KH2PO4 seed priming, which may be related to the early field emergence of plant seedlings. In addition to encouraging early seedling emergence and growth, cow urine and KH2PO4 had effects on the increased number of branches and internodes per plant and fruit length by providing nutrients. Seed treatment with cow urine improved growth traits and fruit length due to the presence of a variety of phytonutrients, including N, P, K, and micronutrients, which are essential plant nutrients that promote plant growth [59]. Hence, cow urine is considered an alternative fertilizer in crops cultivation [32,60], and cow urine also provides plant growth hormones to enhance plant growth [61]. Similar to seed priming with cow urine, seed priming with KH2PO4 can also provide phytonutrients such as K and P to promote seedling and whole plant growth [62]. This result is supported by Ref. [63], who reported that the application of cow urine concentration led to an increased number of branches and leaves per okra plant over the control [64]. also found that seed priming with cow urine improved maize growth traits over unprimed seed. Similarly, in accordance with the current findings [32], in wheat reported that seeds primed with cow urine improved field emergence and growth performance. Furthermore, the current results are supported by the findings of [65], who revealed that okra seeds primed with distilled water, diammonium phosphate, and single superphosphate for 24 h produced more internodes, branches, and leaves per plant than dry seeds.
The number of fruits per plant showed significant differences among five okra genotypes, which may be due to genetic factors. According to them, the genotypes 240,586 and 240,207 that originated in Ethiopia produced more fruits per plant than the newly introduced genotypes. The higher number of fruits per plant in this study from the genotypes collected in Ethiopia could be due to the genotypic factor. Furthermore, Ethiopian genotypes might be more locally adapted than introduced genotypes to contribute an increased number of fruits per plant. Okra genotypes that originated in Ethiopia are therefore essential resources for breeding initiatives that aim for an increased number of fruits per plant. The current findings are consistent with [66] report that okra genotypes collected in Ethiopia produced more fruit per plant than commercial okra cultivars introduced in other countries [19]. also reported that the genotypes of okra originating from Ethiopia had more fruits per plant than the imported commercial cultivars from other countries. Whereas, the current finding is in contrast to Ref. [52], who reported that the number of fruits per plant was non-significant between okra varieties.
For seed priming, all seed priming methods increased the number of fruits produced per plant when compared to unprimed seeds. This is due to the fact that seed priming treatements enhance early seedling emergence and the vigor of plant growth, which encourages the efficient use of available plant nutrients during the growing season [67]. In this study, when compared to other seed priming treatments, plants established from seeds primed with KH2PO4 produced the highest number of fruits per plant. This is because the seeds primed with KH2PO4 not only stimulated seed vigor and improved seedling emergence and establishment but also increased the K and P phytonutrients for seedling growth, which can help mitigate K and P deficits in plant growth [62], which is important for increased fruit production per plant over other priming methods. The outcome is consistent with [65], who revealed that okra seeds primed with phosphate solution and distilled water produced more fruits per plant than the control. Moreover [52], also reported that okra seed primed with hot and normal water as well as a 3% NaCl solution increased the total number of fruits per plant than the control treatment.
Seed priming increases yields in many crops through directly enhancing seedling emergence, promoting earlier plant growth and flowering, and improving crop establishment [68,69]. In the current study, except for tap water and cow urine seed treatments, most okra genotypes responded better to almost all seed priming treatments over unprimed seeds for increased average fruit weight and fruit yield per plant and per hectare. Despite the fact that the efficacy of priming treatments for these traits varies among okra genotypes due to genetic interaction of the genotype with priming agents. The higher fruit yield in the present study could be attributed to active growth and net assimilation, which resulted in more branches, more fruit pods per plant, and higher fruit weight due to the direct effect of priming treatments over unprimed seeds. But in comparison to all treatment combinations, genotype 240,586 seeds primed with KH2PO4 produced the fruit with the highest average weight and fruit yield per plant and per hectare over the others. This is because seed priming with KH2PO4 increases K and P content in the seeds, which can stimulate seed vigor and improve earlier seedling emergence and establishment, as well as increase K and P plant nutrients for seedling growth, leading to increased growth and yield [62]. Vigorous seedling establishment and plant growth could possibly have improved fruit yield per plant and hectare by increasing fruit weight and number [70]. Furthermore, earlier crop establishment and vigorous growth reduce weed competition, allowing plants to absorb more water and nutrients, resulting in a higher yield [71]. The result is consistent with that of [72], who found that treating okra seeds with KH2PO4 in a 0.5% solution increased fruit yield per plant and per hectare in comparison to untreated seeds. Similar results had also been reported by Ref. [73], okra seeds treated with a 3% Na2HPO4 solution for 24 h produced significantly more fruit per hectare than untreated seeds. Besides [65], who reported that okra seeds soaked in a single superphosphate solution and distilled water for 24 h greatly enhanced the fruit yield [23]. also reported that okra seed primed by various priming agents for 24 h significantly increased the fruit yield and yield attributes of okra. Furthermore, according to Ref. [74], okra seed primed with 0.5% concentration of DAP increased fruit yield per hectare in comparison to other concentrations and control treatments.
5. Conclusions
The results of the current study revealed that various seed priming treatments had diverse effects on the phenology, growth, fruit yield, and yield-related traits of okra genotypes. Genotype Clemson spineless had the shortest days to 50% seedling emergence; okra seeds primed with GA3 had the shortest days to seediling emergence; and genotype Clemson spineless primed with GA3 had the quickest days to flowering and fruit maturity. In terms of average fruit weight, fruit yield per plant, and yield per hectare, genotype 240,586 primed with KH2PO4 showed the best performance. Farmers in the study area are advised to cultivate genotype 240,586 with KH2PO4 seed priming to increase okra fruit production. Farmers can also cultivate genotype 240,207 with cow urine seed priming, genotype Arka Anamika with GA3 or KH2PO4, and genotypes Clemson spineless and SOH701 with cow urine or KH2PO4 seed priming to enhance fruit production over dry seed of each genotype. However, the study, which was conducted at one site, will need to be repeated in multiple locations to provide significant recommendations for the country.
Author contribution statement
Mekuria Bereded: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data and Wrote the paper.
Wasu Mohammed: Conceived and designed the experiments and Analyzed and interpreted the data.
Kebede Woldetsadik and Edosa Atinku: Conceived and designed the experiments; Performed the experiments and Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Naveed A., Khan A.A., Khan I.A. Generation mean analysis of water stress tolerance in okra (Abelmoschus esculentus L.) Pakistan J. Bot. 2009;41(1):195–205. [Google Scholar]
- 2.Aladele S.E., Ariyo O.J., De Lapena R. Genetic relationships among West African okra (Abelmoschus caillei) and Asian genotypes (Abelmoschus esculentus) using RAPD. Afr. J. Biotechnol. 2008;7(10):1426–1431. [Google Scholar]
- 3.Santos B.M., Dittmar P.J., Olson S.M., Webb S.E., Zhang S. University of Florida IFAS extension; 2012. Okra Production in Florida; pp. 163–171. [Google Scholar]
- 4.Oyelade O.J., Ade-Omowaye B.I.O., Adeomi V.F. Influence of variety on protein, fat contents and some physical characteristics of okra seeds. J. Food Eng. 2003;57(2):111–114. [Google Scholar]
- 5.Yuan C.Y., Zhang C., Wang P., Chang S., Hu H.P., Xiao W.J., Jiang X.T., Lu S.B., Ye J.Z., Guo X.H. Genetic diversity analysis of okra (Abelmoschus esculentus L.) by inter-simple sequence repeat (ISSR) markers. Genet. Mol. Res. 2014;13(2):3165–3175. doi: 10.4238/2014.April.25.1. [DOI] [PubMed] [Google Scholar]
- 6.FAOSTAT (food and agriculture organization of the united nations) https://www.fao.org/faostat/en/#data/QCL/visualize Available online: accessed on 11 July 2022.
- 7.Kumar S., Dagnoko S., Haougui A., Ratnadass A., Pasternak N., Kouame C. Okra (abelmoschus spp.) in west and central Africa: potential and progress on its improvement. Afr. J. Agric. Res. 2010;5:350–3598. [Google Scholar]
- 8.Thirupathi Reddy M., Hari Babu K., Ganesh M., Chandrasekhar Reddy K., Begum H., Purushothama Reddy B., Narshimulu G. Genetic variability analysis for the selection of elite genotypes based on pod yield and quality from the germplasm of okra (Abelmoschus esculentus L. Moench) Journal of Agricultural Technology. 2012;8(2):639–655. [Google Scholar]
- 9.Temam N., Mohamed W., Aklilu S. Agro morphological characterization and evaluation of okra [Abelmoschus esculentus (L.) Moench] genotypes for yield and other variability components at Melkassa, Central Ethiopia. MOJ Ecology & Environmental Sciences. 2020;5(2):80–87. [Google Scholar]
- 10.Benchasri S. Okra (Abelmoschus esculentus (L.) Moench) as a valuable vegetable of the world. Ratarstvo i povrtarstvo. 2012;49(1):105–112. [Google Scholar]
- 11.Kumar S., Yadav Y.C. Correlation coefficient and path analysis studies in okra [Abelmoschus esculentus (L.) Moench] Ann. Hortic. 2009;2(2):166–170. [Google Scholar]
- 12.Saifullah M., Rabbani M.G. Evaluation and characterization of okra (Abelmoschus esculentus L. Moench.) genotypes. Saarc J. Agric. 2009;7(1):92–99. [Google Scholar]
- 13.Fajinmi A.A., Fajinmi O.B. Incidence of okra mosaic virus at different growth stages of okra plants (Abelmoschus esculentus (L.) Moench) under tropical condition. J. Gen. Mol. Virol. 2010;2(1):28–31. [Google Scholar]
- 14.Salameh N.M. Genetic diversity of okra (Abelmoschus esculentus L.) landraces from different agro-ecological regions revealed by AFLP analysis. Am Eur J Agric Environ Sci. 2014;14(2):155–160. [Google Scholar]
- 15.Moekchantuk T., Kumar P. vol. 56. 2004. (Export Okra Production in Ailand, Intercountry Programme for Vegetable IPM in South & SE Asia Phase II Food and Agriculture Organization of the United Nations Bangkok’ailand). [Google Scholar]
- 16.MoANR (Ministry of Agriculture and Natural Resources) 2016. Crop Variety Registers Issue. 19; p. 211. Ethiopia: Addis Abeba. [Google Scholar]
- 17.Mihretu Y., Weyessa G., Adugna D. Variability and association of quantitative characters among okra (Abelmoschus esculentus (L.) Moench) collection in south western Ethiopia. J. Biol. Sci. 2014;14(5):336–342. [Google Scholar]
- 18.Binalfew T., Alemu Y. Characterization of okra (Abelmoschus esculentus (L.) Moench) germplasms collected from Western Ethiopia. International Journal of Research in Agriculture and Forestry. 2016;3(2):11–17. [Google Scholar]
- 19.Demelie M., Mohamed W., Gebre E. Genetic diversity of Ethiopian okra collections through multivariate analysis at were, rift valley of Ethiopia. The International Journal of Science and Technoledge. 2015;3(8):186–193. [Google Scholar]
- 20.Possingham J.V. Under-exploited wild species that have potential for horticulture. Under-exploited wild species that have potential for horticulture. Advance Horticulture Science. 1990;4:1000–1007. [Google Scholar]
- 21.Dantas T.L., Alonso Buriti F.C., Florentino E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants. 2021;10(8):1683. doi: 10.3390/plants10081683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabianová J., Šlosár M., Kopta T., Vargová A., Timoracká M., Mezeyová I., Andrejiová A. Yield, antioxidant activity and total polyphenol content of okra fruits grown in Slovak Republic. Horticulturae. 2022;8(10):966. [Google Scholar]
- 23.Sharma A.D., Rathore S.V.S., Srinivasan K., Tyagi R.K. Comparison of various seed priming methods for seed germination, seedling vigour and fruit yield in okra (Abelmoschus esculentus L. Moench) Sci. Hortic. 2014;165:75–81. [Google Scholar]
- 24.Mohammadi G., Aval M.B. Differential responses for harvesting times and storage on hardness of different varieties of okra. Not. Sci. Biol. 2011;3(4):117–122. [Google Scholar]
- 25.Finch-Savage W.E., Bassel G.W. Seed vigour and crop establishment: extending performance beyond adaptation. J. Exp. Bot. 2016;67(3):567–591. doi: 10.1093/jxb/erv490. [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi G., Khah E.M., Honarmand S.J., Shirkhani A., Shabani G. Effects of seed hardness breaking techniques on okra (Abelmoschus esculentus L.) germination. Intl. J. Agric. Crop Sci. 2012;4(6):264–273. [Google Scholar]
- 27.Paparella S., Araújo S.S., Rossi G., Wijayasinghe M., Carbonera D., Balestrazzi A. Seed priming: state of the art and new perspectives. Plant Cell Rep. 2015;34:1281–1293. doi: 10.1007/s00299-015-1784-y. [DOI] [PubMed] [Google Scholar]
- 28.Chunthaburee S., Sanitchon J., Pattanagul W., Theerakulpisut P. Alleviation of salt stress in seedlings of black glutinous rice by seed priming with spermidine and gibberellic acid. Not. Bot. Horti Agrobot. Cluj-Napoca. 2014;42(2):405–413. [Google Scholar]
- 29.Eisvand H.R., Shahrosvand S., Zahedi B., Heidari S., Afrougheh S. Effects of hydro‐priming and hormonal priming by gibberellin and salicylic acid on seed and seedling quality of carrot (Daucus carota var. sativus). Iran. J. Plant Physiol. 2011;1:233–239. [Google Scholar]
- 30.Pawar V.A., Laware S.L. Seed priming a critical review. Int. J. Sci. Res. Biol. Sci. 2018;5:94–101. [Google Scholar]
- 31.Ibrahim E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016;192:38–46. doi: 10.1016/j.jplph.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Wolie A.W., Zewudie D.A., Feleke T.T. Evaluation of seed priming and coating on emergence, yield and yield components of bread wheat (Triticum aestivum L.) in Northwest Ethiopia. Ethiopian Journal of Science and Technology. 2017;10(2):123–136. [Google Scholar]
- 33.Tsegay B.A., Andargie M. Seed priming with gibberellic acid (GA 3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum var. Abyssinicum A. Braun and lathyrus sativus L. Journal of Crop Science and Biotechnology. 2018;21:261–267. [Google Scholar]
- 34.Hussain S., Zheng M., Khan F., Khaliq A., Fahad S., Peng S., Nie L. Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep08101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bereded Sheferie M. Effect of seed priming methods on seed quality of okra (Abelmoschus esculentus (L.) Moench) genotypes. Advances in Agriculture. 2023;2023:1–9. [Google Scholar]
- 36.Tilahun K., Merkel B.J. Estimation of groundwater recharge using a GIS-based distributed water balance model in Dire Dawa, Ethiopia. Hydrogeol. J. 2009;17(6):1443–1457. [Google Scholar]
- 37.Tilahun K. The characterisation of rainfall in the arid and semi-arid regions of Ethiopia. WaterSA. 2006;32(3):429–436. [Google Scholar]
- 38.PGRC Ethiopia: country report to the FAO international technical conference on plant genetics resources (Leipzig). Plant Genetic resources Center, Addis Ababa, Ethiopia, April, 1995. 1996. http://www.fao.org/fileadmin/templates/agphome/documents/PGR/SoW1/Africa/ETHIOPIA.pdf
- 39.Gomez K.A., Gomez A.A. second ed. John wiley & sons; New York, USA: 1984. Statistical Procedures for Agricultural Research. [Google Scholar]
- 40.Singh H., Jassal R.K., Kang J.S., Sandhu S.S., Kang H., Grewal K. Seed priming techniques in field crops-A review Agricultural Reviews. 2015;36(4):251–264. [Google Scholar]
- 41.Rao M.J., Hussain S., Anjum M.A., Saqib M., Ahmad R., Khalid M.F., Sohail M., Nafees M., Ali M.A., Ahmad N., Zakir I. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants. 2019. Effect of seed priming on seed dormancy and vigor; pp. 135–145. [Google Scholar]
- 42.Ali S., Alam M., Basir A., Adnan M., Malik M.F.A., Shah A.S., Ibrahim M. Effect of seed priming on germination performance and yield of okra (Abelmoschus esculentus L.) Pakistan J. Agric. Res. 2016;29(3):250–259. [Google Scholar]
- 43.Sanodiya K., Pandey G., Kacholli P., Dubey A.K. Effect of growth regulator on growth, yield and seed quality parameters of okra (Abelmoschus esculentus L.): cv.. Utkal Gaurav. Int J Curr Microbiol App Sci. 2017;6(10):3551–3556. [Google Scholar]
- 44.Pallaoro D.S., Avelino A.C.D., Camili E.C., Guimarães S.C., Albuquerque M.C.D.F. Condicionamento de sementes de milho com reguladores vegetais. Journal of Seed Science. 2016;38:227–232. [Google Scholar]
- 45.Shafi G., Ara N., Khan F.U. The effect of seed priming and soaking durations with Di Ammonium Phosphate (DAP) on seedling emergence and morphological traits in okra (Hibiscus esculentus L.) International Journal of Environment. 2014;3(3):113–125. [Google Scholar]
- 46.Marsh L. Emergence and seedling growth of okra genotypes at low temperatures. Hortscience. 1993;27(12):1310–1312. [Google Scholar]
- 47.Khan H.A., Ayub C.M., Pervez M.A., Bilal R.M., Shahid M.A., Ziaf K. Effect of seed priming with NaCI on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009;28(1):81–87. [Google Scholar]
- 48.Sadeghian S.Y., Yavari N. Effect of water‐deficit stress on germination and early seedling growth in sugar beet. J. Agron. Crop Sci. 2004;190(2):138–144. [Google Scholar]
- 49.Rehman H.U., Basra S., Ahmed M., Farooq M. Field appraisal of seed priming to improve the growth, yield, and quality of direct seeded rice. Turk. J. Agric. For. 2011;35(4):357–365. [Google Scholar]
- 50.Wilkie J.D., Sedgley M., OlesenT Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008;59(12):3215–3228. doi: 10.1093/jxb/ern188. [DOI] [PubMed] [Google Scholar]
- 51.Chandiniraj A., Holebasappa K., Hore J.K., Chattopdyay N. Growth and yield of chili (Capsicum annuum L.) as influenced by different growth regulators. J. Life Sci. 2016;11:385–388. [Google Scholar]
- 52.Tania S.S., Rhaman M.S., Hossain M.M. Hydro-priming and halo-priming improve seed germination, yield and yield contributing characters of okra (Abelmoschus esculentus L.) Tropical Plant Research. 2020;7(1):86–93. [Google Scholar]
- 53.Mahmood T., Basra S.M. Invigoration of low vigor sunflower hybrids by seed priming. Int. J. Agric. Biol. 2009;11:521–528. [Google Scholar]
- 54.Lee J.W., Kim Y.C., Kim J.U., Jo I.H., Kim K.H., Kim D.H. Effects of gibberellic acid and alternating temperature on breaking seed dormancy of Panax ginseng CA Meyer. Korean. Journal of Medicinal Crop Science. 2016;24(4):284–293. [Google Scholar]
- 55.Urbanova T., Leubner‐Metzger G. Gibberellins and seed germination. Annual Plant Reviews. 2016;49:253–284. [Google Scholar]
- 56.Oh W., Kim K.S. Light intensity and temperature regulate petiole elongation by controlling the content of and sensitivity to gibberellin in Cyclamen persicum. Horticulture, Environment, and Biotechnology. 2014;55:175–182. [Google Scholar]
- 57.Oh W., Kim J., Kim Y.H., Lee I.J., Kim K.S. Shoot elongation and gibberellin contents in Cyclamen persicum are influenced by temperature and light intensity. Horticulture, Environment, and Biotechnology. 2015;56:762–768. [Google Scholar]
- 58.Gadade S.B., Shinde V.S., Deosarkar D.B., Shinde S.S. Effect of plant growth regulators on growth and yield of okra (Abelmoschus esculentus L.) Plant Archives. 2017;17(1):177–180. [Google Scholar]
- 59.Ambika S., Balakrishnan K. Enhancing germination and seedling vigour in cluster bean by organic priming. Sci. Res. Essays. 2015;10(8):298–301. [Google Scholar]
- 60.Sridevi G., Srinivasamurthy C.A. Source separated anthropogenic liquid waste (Human Urine)-A potential plant nutrients for banana cultivation. Biores Bulletin. 2011;1:52–57. [Google Scholar]
- 61.Chaudhary S., Kushwaha M., Seema P.S., Sodani R., Kumar S. Cow urine: a boon for sustainable agriculture. Int. J. Curr. Microbiol. App. Sci. 2017;6(2):1824–1829. [Google Scholar]
- 62.M'Sehli W., Kallala N., Jaleli K., Bouallegue A., Mhadhbi H. Monopotassium phosphate (KH2PO4) and salicylic acid (SA) as seed priming in Vicia faba L. and Vicia sativa L. Biosci. j. Uberlândia (Online) 2020;36(6):2078–2091. [Google Scholar]
- 63.Jandaik S., Thakur P., Kumar V. Efficacy of cow urine as plant growth enhancer and antifungal agent. Advances in Agriculture. 2015;2:1–7. [Google Scholar]
- 64.Kumar M. Influence of seed priming with urine, phosphorus and zinc on maize (Zea mays L.) yield in an acid soil of Northeast India. Indian J. Hill Farming. 2014;27(1):132–137. [Google Scholar]
- 65.Shah A.R., Sajid M., Abdur-Rab Ara N., Ahmad M., Wahid F., Shafi G. Response of germination, growth and yield of okra (Abelmoschus esculentus) to seed priming duration and p-sources in Northwest Pakistan. Afr. J. Plant Sci. 2011;5(11):663–670. [Google Scholar]
- 66.Mohammed J., Mohammed W., Shiferaw E. Performance and genetic variability of okra (Abelmoschus esculentus (L.) Moench) genotypes in Ethiopia for agromorphology and biochemical traits. Advances in Agriculture. 2022;2022:1–8. [Google Scholar]
- 67.Muhammad I., Kolla M., Volker R., Günter N. Impact of nutrient seed priming on germination, seedling development, nutritional status and grain yield of maize. J. Plant Nutr. 2015;38(12):1803–1821. [Google Scholar]
- 68.Harris D., Joshi A., Khan P.A., Gothkar P., Sodhi P.S. On-farm seed priming in semi-arid agriculture: development and evaluation in maize, rice and chickpea in India using participatory methods. Exp. Agric. 1999;35(1):15–29. [Google Scholar]
- 69.Harris D., Hollington P.A. On-farm’seed priming an update. Tropical Agriculture Association (UK) Newsletter. 2001;21(4):7. [Google Scholar]
- 70.Sisodia A., Padhi M., Pal A.K., Barman K., Singh A.K. Advances in Seed Priming, Springer; Singapore: 2018. Seed Priming on Germination, Growth and Flowering in Flowers and Ornamental Trees; pp. 263–288. [Google Scholar]
- 71.Gebreegziabher B.G., Qufa C.A. Plant physiological stimulation by seeds salt priming in maize (Zea mays): prospect for salt tolerance. Afr. J. Biotechnol. 2017;16(5):209–223. [Google Scholar]
- 72.Vijaykumar A., Dharmalingam C., Sambandamurathi S. Effect of pre- sowing treatment seed yield and quality in okra. South Indian Hortic. 1988;6:118–120. [Google Scholar]
- 73.Amal A. Influence of seed priming on growth and seed yield of okra (Abelmoschus esculentus) Egyptian. J. Agric. Res. 2014;92(1) [Google Scholar]
- 74.Shafi G., Ara N., Khan F.U., Jamal Y., Basir A. Influence of DAP seed priming and soaking durations on yield and yield associated traits in Okra. Pure and Applied Biology (PAB) 2021;4(3):389–397. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.