Abstract
The objective of this research was to investigate the variation of water quality parameters at different depths of the Gilgel Gibe I reservoir in Oromia Jimma zone during wet seasons. Five stations within the reservoir were selected, and water quality parameters were determined at four different depths (surface, 5 m, 10 m, and 15 m). Water quality parameters were analyzed on-site using a HACH, HQ40d portable multi-meter, and turbidity was measured using Wag tech turbidity meter and in the laboratory using the standard method. Comparison of water quality parameters among depths were conducted using one-way ANOVA and Tukey's pairwise comparisons with 5% significance level. The probable contributing source of the investigated physicochemical water quality parameters at different depth was identified using Principal components analysis. The results show that depth wise except for total suspended solids (TSS), total dissolved solids (TDS), total phosphorus (TP) and soluble reactive phosphorus (SRP) the rest showed statistically significant difference at p < 0.05 level. Negative correlations were found between depth and dissolved oxygen (DO), water temperature, pH, nitrate (N03-) and chlorophyll a (Chl.a) while positive correlations were found between electrical conductivity (EC), biochemical oxygen demand (BOD5), turbidity and N03-. The study revealed that the release of nutrients associated with increased concentration of BOD5 at the bottom depth caused low concentration of dissolved oxygen due to oxygen consumption. This was further aggravated through the decomposition of organic matter, indicating organic pollution resulting from runoff from the catchment. The presence of dense masses of blue-green algae in the pelagic zone of Gilgel Gibe I reservoir suggested the presence of ample nutrients for its blooming and significant reduction of water quality, indicating possible eutrophic conditions. Therefore, catchment management is required to protect aquatic life and the reservoir function as a whole from reservoir water quality degradation.
Keywords: Organic matter, Chlorophyll a, Eutrophication, Physicochemical, Gilgel gibe
1. Introduction
Eutrophication of lakes and reservoirs is a severe hazard to the environment, the economy, and society worldwide [1]. The quality of water varies in nature and is often determined by calculating the rate of degradation and irregularity of physicochemical parameters [2]. Changes in water quantity and quality are influenced by types of aquatic habitats and natural elements including climate, terrain, and catchment geology [3]. The contribution of tributaries and differential water retention time greatly influence these changes [4].
Reservoirs can be damaged by high rates of sedimentation, an abundance of organic nutrients, and erosive loss of minerals from the catchments [5]. Additionally, compartments in reservoir ecosystems experience significant seasonal changes [6] that can harm the physicochemical and biological properties of reservoir water [7].
In reservoirs like Gilgel Gibe I Reservoir, where anthropogenic activity is flowing down to it and making it prone to water quality degradation, the problem of water quality deterioration is also a prevalent one. The aquatic system is experiencing a major eutrophication and water quality crisis as a result of anthropogenic activity [8].
Agriculture is frequently attributed as the main cause of surface water eutrophication. Ineffective practices cause substantial nutrient surpluses to be transmitted to water bodies through runoff and leaching [9]. Terrestrial organic matter also plays a significant role in riverine runoff [10]. Intense rainfall has a substantial correlation with the potential for eutrophication [11]. Fertilizers and pesticides are washed into bodies of water by runoff from agriculture. The risk of pollution and eutrophication is intimately related to drainage from agricultural land because concentrations of most water quality measures were highest in incoming water during the wet season [12].
Temperature, pH, DO, EC and alkalinity are some of the water quality parameters that regulate various aspects of the aquatic ecosystem [13,14]. Among these parameters, DO is considered to be the most important because it determines the health of the ecosystems [15]. The primary cause of oxygen depletion in a water body is from excessive algae and phytoplankton growth driven by high levels of phosphorus and nitrogen. Dung droppings also increase the organic content of the reservoir [16], which consumes dissolved oxygen. This can result in insufficient amounts of dissolved oxygen available for fish and other aquatic life. The prediction of DO concentrations is vital for fisheries and for aquatic managers responsible for maintaining ecosystem health [17]. Severe oxygen depletion can lead to fish kills and deformities in fish larvae [18] as well as changes in community composition and lake trophic state [19,20].
Vertical variability in the water column is a natural occurrence that is influenced by temperature gradients and light attenuation in the water column. These factors in turn influence biological activities and water chemistry [21]. Therefore, to recognize and protect the ecology of natural aquatic systems [22], it is imperative to describe vertical profiles of water physicochemical parameters at different depths of water. This helps in understanding the biochemical cycles and nutrient dynamics of an aquatic ecosystem [23].
Due to conservation concerns from existing anthropogenic activities and potential future developments in Gilgel Gibe I Reservoir, it is crucial to assess vertical water quality to ensure a suitable environment for the aquatic community. Although different studies have been carried out, vertical variation and distribution of physicochemical parameters of reservoir water column have not been well documented.
The objective of this study was to assess variability of the water quality at different depths in Gilgel Gibe I Reservoir.
2. Materials and method
2.1. Study area
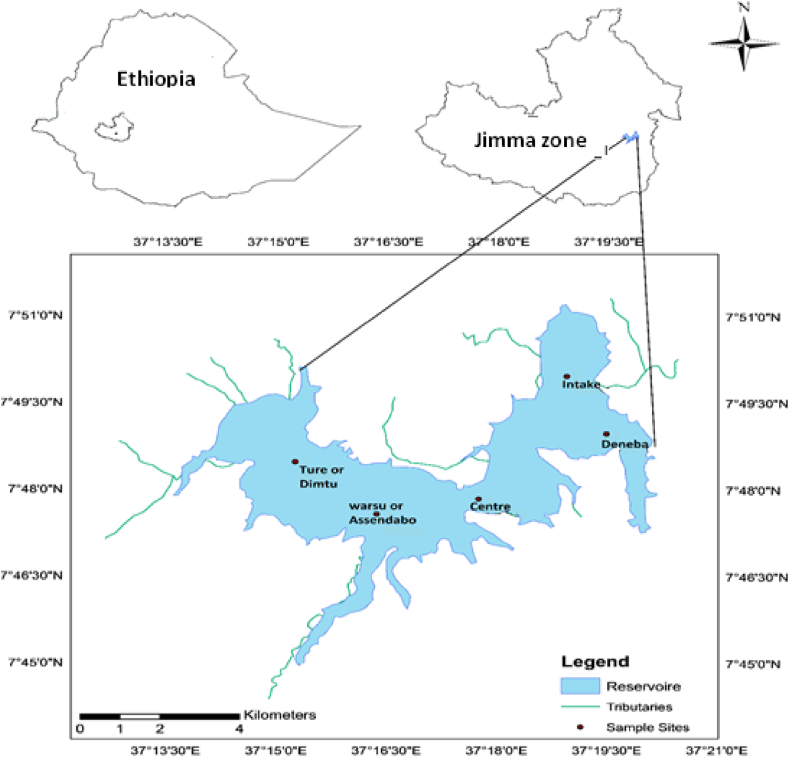
The Gilgel Gibe I Reservoir is situated in the Gibe - Omo River Basin in Oromiya regional state of Jimma zone (Fig. 1). The climate of the study area is classified as tropical humid and belongs to the high altitude cool tropic area of Ethiopia. The area experiences a unimodal pattern of seasonal rainfall distribution with up to 60% of the rainfall falling during the rainy season [24]. According to unpublished data from the Ethiopian National Meteorological Agency, from 1968 to 2015, the average annual rainfall in Jimma stations was 243 mm recorded in August. In terms of rainfall variability by river basins, west-flowing rivers such as Abay, Baro-Akobo, Omo-Gibe and Tekeze receive much rainfall [25].
Fig. 1.
Location of depth sampling sites at Gilgel Gibe I Reservoir.
The reservoir was built on the Gilgel Gibe river in 1998 with the primary purpose of hydroelectric power production. It is characterized by a large 40 m high dam with a storage capacity of 839 Mm3, and covers more than 54 km2 of land [26].
The site has been operational since 2004 and is currently generating 184 MW at full capacity. It has an annual average flow of 50.4 m3/s with an active storage capacity of 657 million m3 and a dead storage capacity of 182 million m3. According to unpublished data from the Ethiopian National Meteorological Agency from 2005/6 to 2014/15, the minimum reservoir water level was 1654 m.a.s.l while the maximum was 1671.2 m.a.s.l. The maximum and minimum depths were recorded as 35 m and 2 m respectively with an average depth of 20 m. The reservoir receives water from surrounding tributaries such as Nadaguda, Nedi, Yedi and Gilgel Gibe rivers. The total catchment area is approximately 4225 km2 while the watershed is highly agricultural serving as an important food source for the region [27].
2.2. Sample collection
In the pelagic zone of the reservoir, five sampling sites (stations) were identified spatially. These sites were named Assendabo or Warsu site, Dimtu or Ture site, Centre, Intake, and Deneba site. During the wet season in October 2018, water samples were collected from different sampling sites at different depths of the reservoir using a Van Dorn sampler. Water depth was determined using a calibrated string with a measuring tape weighted at one end. For laboratory analysis of nitrate, total suspended solid (TSS) and total dissolved solid (TDS), samples were sifted by Whatman filter paper with a pore size of 0.45 μm using the standard method [28].
2.3. Water quality analyses
Water quality parameters were analyzed both on-site and in the laboratory. A HACH HQ40d portable multi-meter was used to measure temperature, pH, conductivity, and dissolved oxygen on-site. Turbidity was measured on-site using a Wag Tech turbidity meter with model number wag-WT 3020. Chl.a was measured on-site using a portable fluorometer. Other physicochemical parameters including 5-day biochemical oxygen demand (BOD5), total suspended solids (TSS), total nitrogen (TN), total dissolved solids (TDS), nitrate (NO3−), total phosphorus (TP), and soluble reactive phosphorus (SRP) were investigated in the laboratory of Environmental Health Science and Technology at Jimma University.
The BOD5 was assessed subsequent to DO incubation for a period of five days at a temperature of 20 °C in the absence of light. Gravimetric methodology [28] was employed to determine the TSS and TDS. Water samples were filtered through pre-weighed glass fiber filter paper to obtain TSS, which was then dried in an oven at 105 °C to eliminate any residual water. The resultant sample was placed in a desiccator, until it reached room temperature, and the change in weight was regarded as TSS. TDS was obtained by evaporating the filtrate to dryness in a pre-weighed dish, and subsequently drying it to a constant weight at 180 °C. The increase in the weight of the dish after drying was taken as the total dissolved solids. The ascorbic acid method was employed to analyse TP, with a direct reading on a spectrophotometer following persulfate oxidation [28]. One-way ANOVA and Tukey's pairwise comparisons with a 5% significance level were used to compare water quality parameters at different depths within the reservoir. Principal components analysis was used to identify the likely contributing source of the examined physicochemical water quality parameters at various depths.
2.4. Statistical analysis
The data analyses were conducted utilizing PAST for principal component analysis, as well as Statistica 8 software for both descriptive and correlation analyses. Pearson's correlation coefficient (r) was employed to exhibit the correlation among the variables, while the P-value was used to ascertain the statistical significance.
3. Results
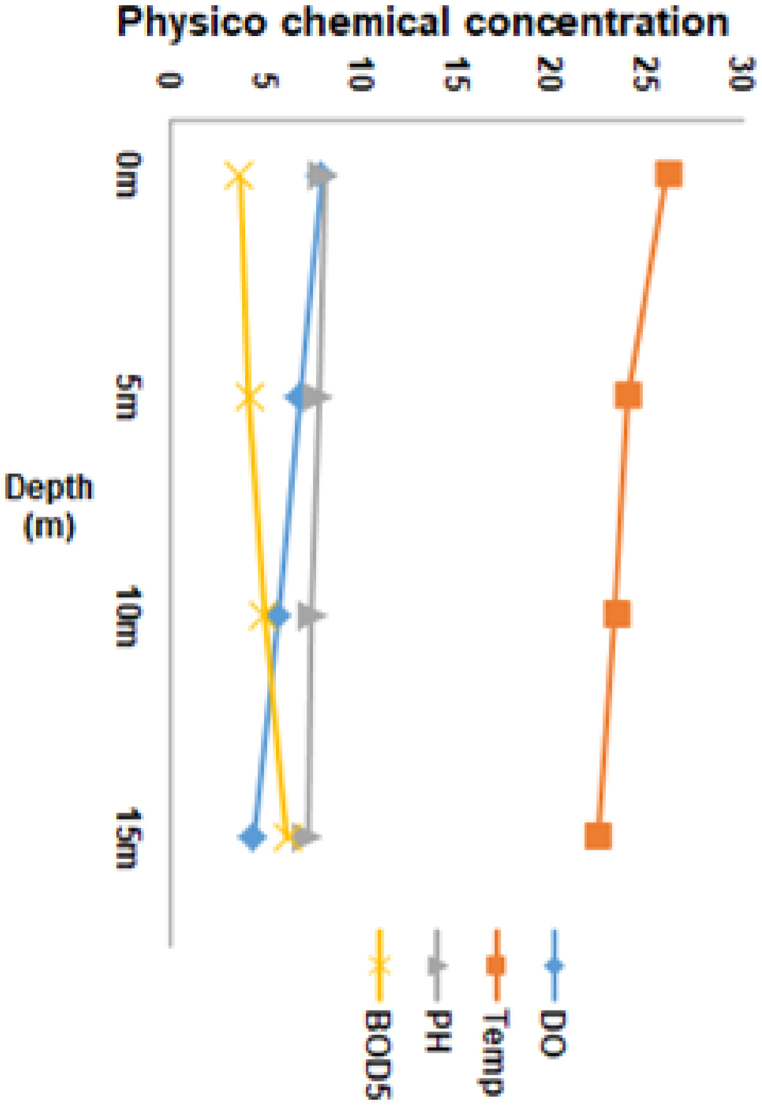
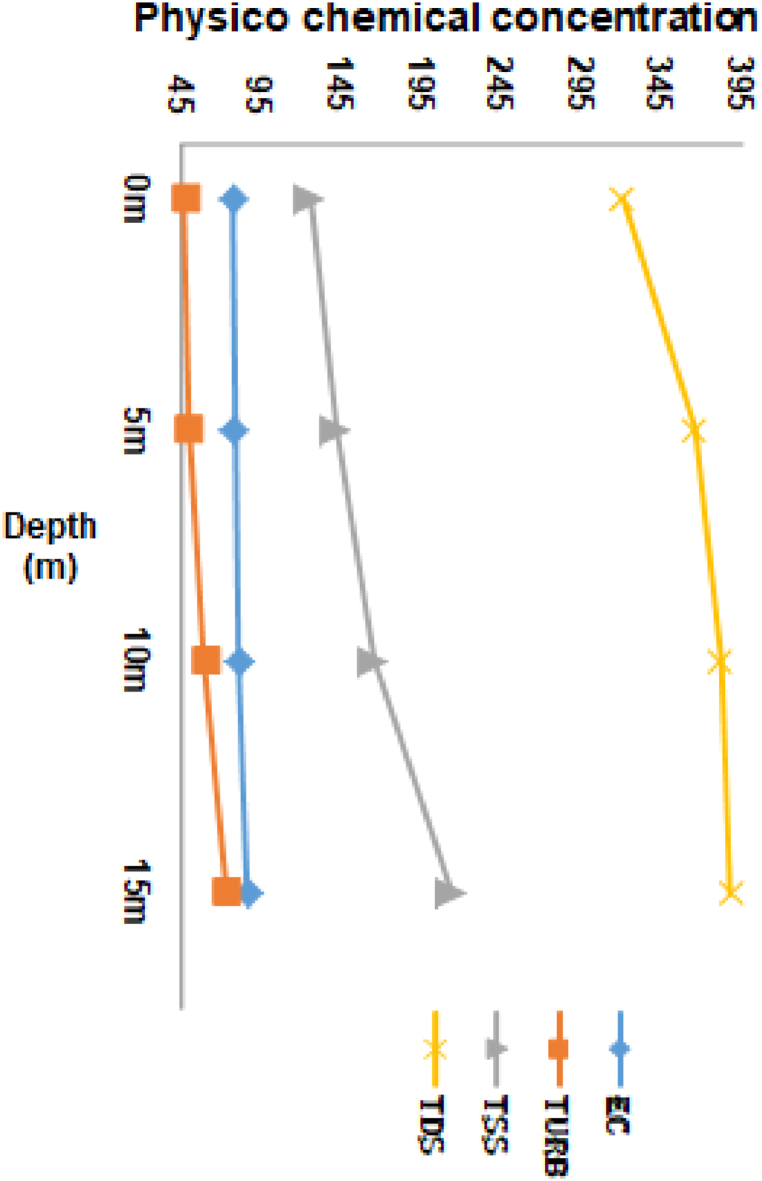
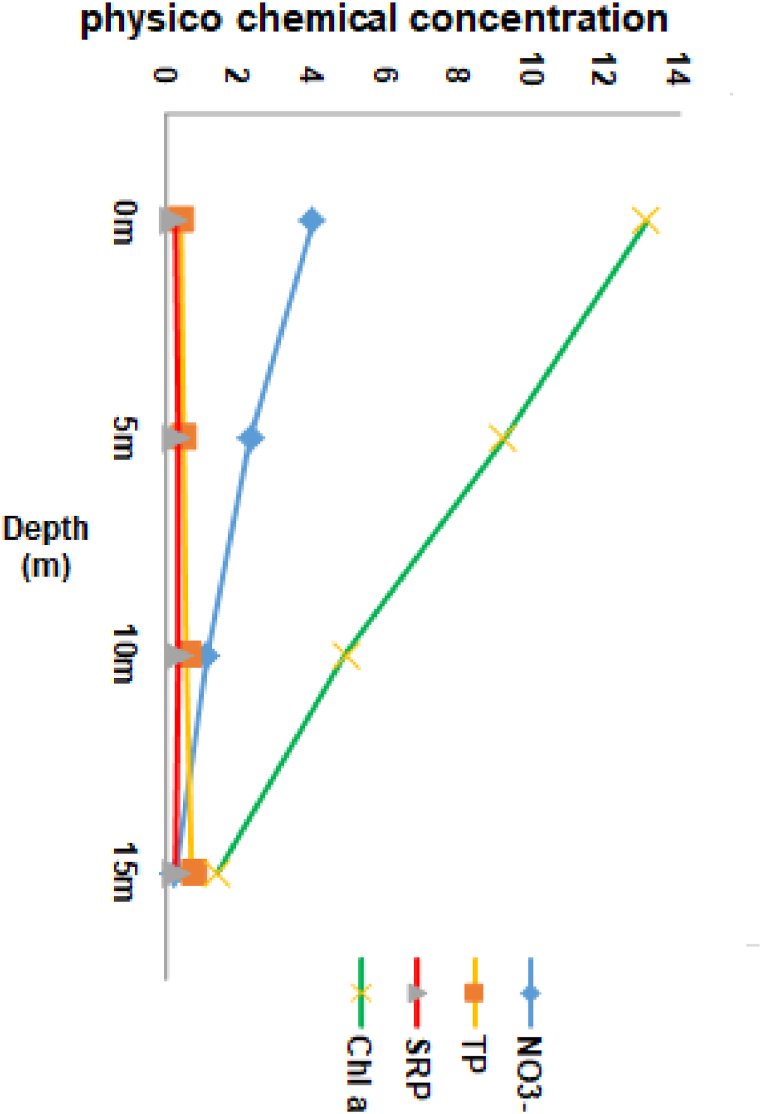
During the investigation period, the depth of reservoir sampling ranged from 1 m to 24 m. The distribution values for observed physicochemical parameters of reservoir water at different depths of the water column were presented in Fig. 2, Fig. 3, Fig. 4. Their correlations were presented in Table 1. A vertical analysis was performed to compare the depths of the studied reservoir with the physicochemical parameters of the water. The results indicated that statistically significant differences were observed at the p < 0.05 level for all parameters except TDS, TP, and SRP at various depths. Negative correlations were observed between depth and DO, water temperature, pH, nitrate, and Chl a at the p < 0.05 level. Positive correlations were observed between EC, BOD5, turbidity, and nitrate at the p < 0.05 level (Table 1).
Fig. 2.
Depth variation of water quality parameters based on median concentration (DO mg/L, Temperature °C, pH and BOD5 mg/L) at Gilgel Gibe I Reservoir. 0 m denotes surface.
Fig. 3.
Depth variation of water quality parameters based on median value (EC μS/cm, TDS mg/L, Turbidity NTU, and TSS mg/L) at Gilgel Gibe I Reservoir. 0 m denotes surface.
Fig. 4.
Depth variation of water quality parameters based on median concentration (NO3− mg/L, Chl.a mg/L, TP mg/L, and SRP mg/L) at Gilgel Gibe I Reservoir. 0 m denotes surface.
Table 1.
Depth correlation water quality parameters in Gilgel Gibe I Reservoir p < 0.05 level.
| Depth | DO | Temp | EC | pH | BOD | Turb | TSS | TDS | N0-3 | TP | SRP | Chl. a | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth | 1 | ||||||||||||
| DO | −0.94 | 1 | |||||||||||
| Temp | −0.78 | 0.82 | 1 | ||||||||||
| EC | 0.82 | −0.72 | −0.58 | 1 | |||||||||
| pH | −0.91 | 0.97 | 0.85 | −0.66 | 1 | ||||||||
| BOD | 0.61 | −0.65 | −0.35 | 0.59 | −0.51 | 1 | |||||||
| Turb | 0.87 | −0.86 | −0.59 | 0.71 | −0.83 | 0.74 | 1 | ||||||
| TSS | 0.49 | −0.48 | −0.37 | 0.78 | −0.40 | 0.38 | 0.31 | 1 | |||||
| TDS | 0.48 | −0.56 | −0.72 | 0.51 | −0.56 | 0.32 | 0.46 | 0.39 | 1 | ||||
| No−3 | −0.72 | 0.81 | 0.74 | −0.57 | 0.81 | −0.48 | −0.62 | −0.37 | −0.50 | 1 | |||
| TP | 0.45 | −0.45 | −0.33 | 0.11 | −0.48 | 0.25 | 0.59 | −0.05 | 0.36 | −0.16 | 1 | ||
| SRP | 0.09 | −0.13 | 0.01 | 0.15 | −0.11 | 0.47 | 0.42 | 0.06 | 0.37 | 0.013 | 0.58 | 1 | |
| Chl. a | −0.96 | 0.93 | 0.83 | −0.79 | 0.92 | −0.65 | −0.84 | −0.49 | −0.55 | 0.75 | −0.33 | −0.12 | 1 |
3.1. Temperature
The difference between the surface and bottom temperature was in the average of 1.7 °C. Regarding the vertical distribution, there existed a positive correlation with pH, nitrate, and Chl. a, at a statistical significance level of p < 0.05, whereas an inverse correlation with EC, turbidity, and TDS, also at a significance level of p < 0.05 (Table 1). Through the use of the Kruskal-Wallis test, a statistically significant dissimilarity was proven to exist between the surface and a depth of 15 m at a P < 0.05 level.
3.2. Dissolved oxygen
The concentration of dissolved oxygen in the surface water of the reservoir remained relatively consistent. The uppermost layer of the water column was characterized by well-oxygenated water. At a depth of 15 m, the dissolved oxygen level reached its lowest point (Fig. 2). Vertically, a notable positive correlation was observed between dissolved oxygen and temperature, pH, NO3−, and Chl a, while a negative correlation was observed with EC, BOD5, turbidity, TDS, and TP at the p < 0.05 level (Table 1).
3.3. PH
The range of the median vertical pH value spans from 8.1 in the surface water column to 7.2 in the bottom. The alkaline pH distribution of the surface waters exhibited a consistent or even concentration (Fig. 2). In contrast, the pH values in the deep water column were low. Statistical analysis indicated a significant difference (P < 0.05) between surface versus 15 m, surface versus 10 m, and 5 m versus 15 m depths. Moreover, a significant positive correlation was observed between pH and NO3− as well as Chl a, whereas a negative correlation was found between pH and BOD5, turbidity, and TDS at the p < 0.05 level, (Table 1).
3.4. BOD5
The concentration of BOD5 at the surface was observed to be lower in comparison to the concentration at the lower depth, and a decrease in DO was noted (Fig. 2). A statistically significant difference between the BOD5 concentrations at the depth of 1 m and 15 m was observed with a significance level of P < 0.05. Furthermore, it was observed that the concentration of BOD5 was negatively correlated with Chl. a, and positively correlated with turbidity at the significance level of p < 0.05 (Table 1).
3.5. Electrical conductivity
The data presented in Fig. 3 indicates that the values of EC exhibit an increasing trend with depth. Statistical analysis reveals a significant difference (P < 0.05) between the values of surface versus 15 m, surface versus 10 m, and 5 m versus 15 m. Further analysis shows that EC has a positive correlation with BOD5, turbidity, TSS, and TDS as depth increases, while exhibiting a negative correlation with pH, NO3−, and chl a at the p < 0.05 level (Table 1).
3.6. Total dissolved solids
Though the concentration value of TDS increases with depth (Fig. 3), no significant differences were detected among the monitored depths. It has a negative correlation with NO3− and chl.a at the p < 0.05 (Table 1).
3.7. Turbidity
The phenomenon of turbidity concentration elevates as the depth increases. The Kruskal-Wallis test has revealed a statistically significant distinction between the surface and depth of 10 m, and 15 m, 5 m versus 15 m (P < 0.05). The study has also established a positive correlation between TP and turbidity concentration while a negative correlation between NO3− and Chl. a. was observed at the p < 0.05 level (Table 1).
3.8. Total suspended solids
Total suspended solids increased slightly with depth (Fig. 3), and the concentration at 15 m was significantly different from the other depths. It had no correlation with any of the water quality parameters. The Kruskal-Wallis test showed a statistically significant difference (P < 0.05) between surface and 15 m and between surface and 10 m.
3.9. Nutrient
In the Gilgel Gibe I Reservoir, it is evident that nitrate is considerably more abundant than soluble reactive phosphorus (Fig. 4). Nitrate levels exhibit a significant decrease as depth increases, while SRP displays a slight increment in concentration with increasing depth (Fig. 4). TP and SRP demonstrate negligible concentrations, exhibiting a nearly uniform distribution throughout the water column (Fig. 4). Notably, the surface water column displays lower median values of TP and SRP compared to nitrate. Furthermore, TP has a positive correlation with SRP at the p < 0.05 level. The Kruskal-Wallis test reveals a statistically significant difference (P < 0.05) between the surface and 15 m depth. Nevertheless, no significant differences are detected for TP and SRP among the observed depths.
The highest concentrations of TP and SRP were observed at a depth of 15 m (Fig. 4). There is a statistically significant difference between surface and 15 m depth for nitrate concentrations at the P < 0.05.
3.10. Chlorophyll a and Secchi disc
Surface Chl. a concentration ranges from 13.69 mg/L – 12.53 mg/L, with the median value of 13. Water transparency in the reservoir as measured by the Secchi disc had an overall median of 0.26 cm, with a minimum value of 0.24 cm and a maximum of 0.29 cm.
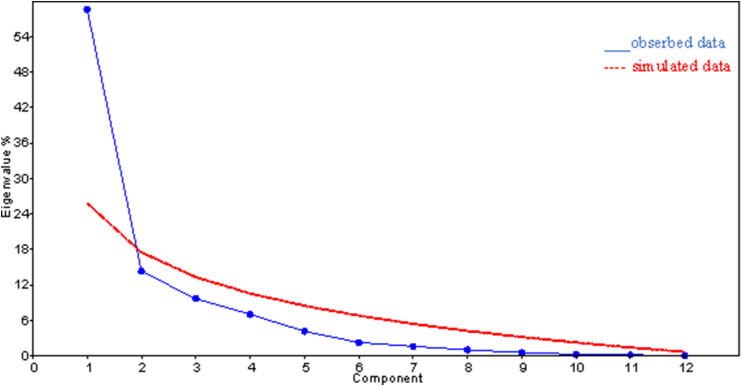
3.11. Scree plot
The scree plot reveals the eigenvalues arranged from large to small as a function of the principal component numbers. It helps to determine the number of important components. After the second component (Fig. 5) the other components cannot be considered, because the curve starts to tilt downward.
Fig. 5.
Scree plot showing the components to be considered.
It shows the eigenvalues on the y-axis and the number of factors on the x-axis. Eigenvector categorized the twelve water physicochemical parameters in to two groups, or components. The PCA analysis extracted two PCs with eigenvalues >1, and explained 73% of the total variance in the data set.
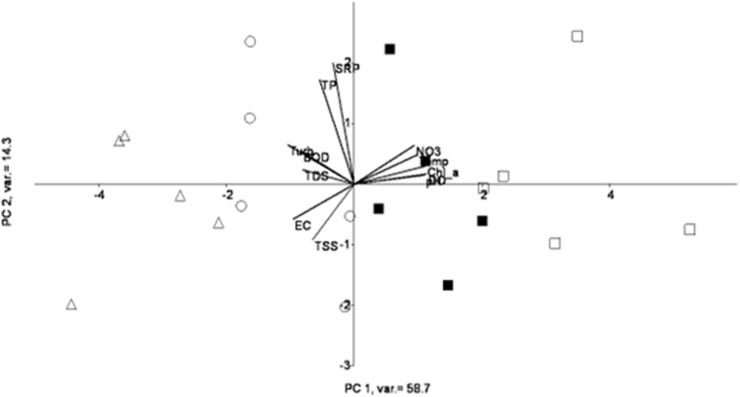
Principal component analysis was used to identify the probable depth characteristic for the investigated water physicochemical parameters. The first PC which explained 58.7% of the variance and was positively associated with DO, pH, temperature, chl.a and nitrate. (Fig. 6), accounts for high loadings at 1 m and 5 m depth, while being negatively associated with turbidity, BOD5 DO, TDS, EC, and TSS (Table 2) and are the characteristics of 10 m and 15 m depth (Fig. 6). Similarly, PC 2 which explains 14.3% of the total variance, associated with positive loadings of SRP and TP, was found to be the characteristic of 5 m depth (Fig. 6). Chl.a was among the variables that explain 58.7% of PC 1 (Fig. 6), with the loading value of 0.948 (Table 2).
Fig. 6.
PCA depth analysis of water quality parameters for Gilgel Gibe I Reservoir (the different depths, 0 m (surface), 5 m, 10 m and 15 m represented by square, dark square, circle, and triangle respectively).
Table 2.
The factor loadings values and explained variance of water quality. Positive and negative strong correlations are marked bold.
| Parameters | PC 1 58.7% | PC 2 14.3% |
|---|---|---|
| DO | 0.962 | 0.070 |
| Temp | 0.835 | 0.202 |
| EC | −0.814 | −0.242 |
| pH | 0.936 | 0.063 |
| BOD5 | −0.697 | 0.227 |
| Turb | −0.888 | 0.273 |
| TSS | −0.552 | −0.385 |
| TDS | −0.665 | 0.100 |
| NO−3 | 0.800 | 0.269 |
| TP | −0.461 | 0.726 |
| SRP | −0.281 | 0.843 |
| Chl.a | 0.948 | 0.126 |
5. Discussion
This study showed significant depth variability in the distribution of water physicochemical parameters. Only TDS, TP and SRP did not show a significant difference among different depths. The other water quality parameters (DO, Temperature, pH, EC, BOD5, Turb, TSS, NO3, and chl.a) had a significant difference across different depths.
The increase in depth exhibited a decrease in temperature, pH, and dissolved oxygen. Aquatic ecosystems heavily rely on water temperature as a key physical characteristic, impacting various water quality parameters [29]. The measurement of dissolved oxygen serves as an indicator for disturbances to these competitive processes, and defines the habitat for organisms that require oxygen for their metabolic activities [30]. The oxygen conditions in a lake are crucial for the composition of its aquatic communities, as most organisms are dependent on oxygen. As adaptations of aquatic life exist to accommodate a wide range of oxygen conditions, some species are limited in their vertical distribution due to insufficient oxygen in deep waters, while others can survive prolonged periods without oxygen [31].
Decreases in pH values and dissolved oxygen with increasing water depth are similar to those reported in the Environmental Research and Design report, which were common due to increased production of CO2 from sediment decomposition processes [32]. Aeration and aquatic plants may explain high surface water column oxygen concentrations. According to Ref. [33], aeration plays a key role in the distribution of oxygen at depths near the surface of water. During daylight, aquatic plants engage in photosynthesis, resulting in the removal of carbon dioxide from the surrounding environment. This phenomenon can be attributed to the increased levels of dissolved oxygen and reduced levels of carbonic anhydride, ultimately leading to an increase in pH value [8,34]. As a result, it can be inferred that elevated pH values are closely associated with high organic content and eutrophic conditions [35].
The concentration of dissolved oxygen did not decline below 3 mg/L. However, reduced concentrations of dissolved oxygen, when combined with the presence of toxic substances, may lead to stress responses in aquatic ecosystems because the toxicity of certain elements is increased by low concentrations of dissolved oxygen [30].
The correlation between an elevation in pH levels within surface water and an increase in temperature may be due to the fact that warm waters tend to exhibit heightened pH levels, which is a result of the conversion of CO2 to organic carbon through photosynthesis [36]. Concentration of pH is also influenced by photosynthesis, respiration, and nitrogen assimilation. For instance, assimilation of nitrate (NO3-) requires the removal of an equal quantity of hydrogen ions [31]. Accordingly, the elevated levels of organic matter within the lower water column are likely responsible for the lower values of pH [37]. Nonetheless, it should be noted that the pH range examined within this study falls within the acceptable range of water quality (6.5–8.5) and conforms to the guidelines for ambient environmental standards for fish production in Ethiopia (6.5–9.0) [38,39].
The bottom sediment of reservoirs is comprised of particulate matter delivered by a river, slope runoff, and the precipitation of detritus through sedimentation. The aquatic environment is subject to the discharge of a diverse range of contaminants, which accumulate in sediments [40]. Organic matter is a pivotal constituent of bottom sediments, exerting significant influence on the physical, chemical, and biological characteristics of the sediment. At greater depths of the sediment bed, an increased release of nutrients is correlated with an increased concentration of BOD5, which consequently leads to a diminished quantity of dissolved oxygen owing to the oxygen consumption (Fig. 2). This observation is concordant with the research conducted by Ref. [41], and he suggested that this increment of BOD5 was aggravated through the decomposition of organic matter, and by chemical reactions in bottom water column. Aquatic systems contain organic matter that is generated through two distinct means, either internally produced (autochthonous organic matter) or transported from the terrestrial environment (allochthonous organic matter) [42]. The decomposition of organic matter at the sedimentary layer of water bodies provides inorganic nutrients, such as nitrogen and phosphorus, which facilitate the growth of algae in tropical lakes and reservoirs [35]. Owing to the high decomposition rate at the bottom layer in tropical regions, the production of oxygen through photosynthesis is outweighed by its consumption [43]. It is noteworthy that the BOD5 levels studied exceeded the maximum permissible limit set by the European Union (3.0–6.0 mg/L) [44].
Turbidity, a parameter that assesses the presence of suspended particles in the water column, is influenced by organic sources such as algae, organic matter, colloids, and sediment materials [30,32]. The increase in turbidity and total suspended solids with depth aligns with the findings of [45,46], who suggested that settling and re-suspension of inorganic solid particles are the likely causes. Therefore, the rise in BOD5, turbidity, and TSS with depth could be attributed to the aforementioned factors mentioned by ERD. Moreover, it may be inferred that the Gilgel Gibe I Reservoir has high productivity, and the bottom oxygen level is low due to the decomposition of organic matter. In water bodies with low productivity, most of the organic matter undergoes decomposition before it reaches the bottom water, thus not depleting the oxygen level there [31]. In our study, the dissolved oxygen (DO) was found to be inversely proportional with depth.
The presence of Total solids within suspended particles and their subsequent settling has significant impacts on aquatic ecosystems. While suspended, Total solids reduce light penetration, ultimately affecting primary production. Upon settling, benthic organisms and their habitats can be devastated. Additionally, fish may experience adverse effects as a result of mechanical and abrasive impairment of their gills [30]. An increasing trend in Electrical Conductivity has been observed as depth increases, as noted by Ref. [45]. This phenomenon is attributed to the inorganic dissolved solids stemming from decaying submerged biomass and sediment [47]. suggested that an increase in conductivity values indicates a propensity towards higher levels of eutrophication. Furthermore [8], contends that the adsorption of dissolved salts on the surface of suspended particles, which are carried by water floods and discharged to bottom sediments, contributes to this trend.
The ecological significance of nutrient concentrations in water cannot be understated, as the existence of sufficient nutrients may lead to the proliferation of phytoplankton. Upon comparing water depth and quality parameters, it was discovered that nitrate ion displays a positive correlation, consistent with the findings of [48]. In contrast, other nutrients such as TP and SRP were distributed uniformly and showed a positive correlation, with the release of SRP from organically-bound phosphorus likely being the cause [44]. Available nutrients, represented by nitrate, decrease with depth, with the formation of available nutrients from organic matter and total nutrients being predominant near the bottom of the water body, likely due to the regeneration of particulate matter sedimentation from the epilimnion [49]. This results in a significant reduction in water quality (Eutrophication), which is often due to contamination by nitrate and phosphate [8]. The less concentration of nutrient on the surface water column may be associated with phytoplankton consumption during photosynthesis [34]. Our study recorded surface water column nitrate concentration ranges 1.8–6.4 mg/L hence concentrations of nitrate in excess of 0.2 mg/L tend to stimulate algal growth and indicate possible eutrophic conditions [44,50] also suggested that the appearance of dense or floating algal blooms can be signs that eutrophication may be taking place.
The effects of a particular factor are contingent upon other factors, as evidenced by the effect of nutrient loading on phytoplankton abundance in ecosystems, which is dependent on variables such as light availability and sedimentation [51]. Factors such as nutrient levels, phosphorus content, pH, water flow, and temperature have been shown to exert an influence on chlorophyll content [52].
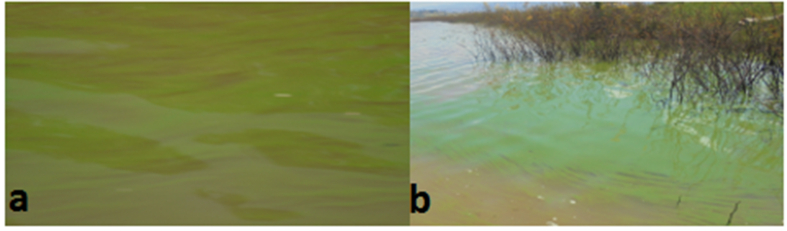
Land use categories within the catchment area are indicative of water pollution, as per reference [53]. Agricultural activities are commonly practiced within the catchment area of the Gilgel Gibe reservoir I. Run-off from agricultural land exerts a significant impact on the process of lake eutrophication as noted in Ref. [54]. The pollutant load in the run-off flow exhibits a considerable variation, being quite low during the dry season and rising to very high levels during the rainy season, as per references [55,56]. The increase in pollutant load is directly proportional to a rise in the degradation of the aquatic system. Water bodies that exhibit high nutrient content (eutrophic) are characterized by high levels of chlorophyll (ranging from 5 to 140 μg l-1) as per reference [44]. The presence of Chl a may be used as an indicator of the pollution level of a given water body, as noted in Ref. [30]. Our investigation revealed dense masses of blue-green algae in the pelagic zone and along the shoreline swept by the winds (Fig. 7a & b). The occurrence of such blue-green algae in the Gilgel Gibe I Reservoir suggests an ample supply of nutrients to support their blooming. Chlorophyll-a serves as an indirect measure of the amount of algal biomass in the water column, and can therefore be employed to evaluate the overall health of aquatic systems, as noted in Ref. [32].
Fig. 7.
Dense masses of Chl.a at Gilgel Gibe Reservoir I.
Nutrients alone cannot indicate whether a water body actually has a nuisance plant problem, whereas increased chl.a in the water indicates that plants, algae or cyanobacteria are actually growing [30]. The occurrence of algal bloom can furnish pertinent information on both the water quality and degradation of reservoirs [57]. and has advantage in biomonitoring of aquatic environment because these organisms reflect the concentration of physicochemical parameters in the water ecosystem [58]. An understanding of the Chl.a enables researchers to draw conclusions about a water bodys’ health and ecological status. Chlorophyll a concentration is often used as a general indicator of plant biomass because all plants, algae and cyanobacteria contain about 1–2% (dry wt) chlorophyll a.
The correlation between chl a and surface and 5 m depth was found to be significant (Fig. 6) This finding elucidates the prevalence of chlorophyll a in the surface water column. It is postulated that the observed low water transparency during the study period may be attributed to this phenomenon, as well as increased surface runoff accompanied by sediment load from tributaries. Furthermore, the abundance of algae above 5 m could be attributed to the occurrence of vertical mixing, which can retain algae below the euphotic zone for extended periods, thereby resulting in relatively low populations of primary producers and associated zooplankton [59]. Water clarity, or visibility, is influenced by various factors, including suspended particles such as algae or inorganic matter [32]. The ideal water column transparency ranges from 1 m to 2 m Secchi disk depth, while aquatic systems with a Secchi disk depth of less than 1 m are considered to have poor water column quality [32]. It is noteworthy that the impact of one factor on water quality is contingent upon other factors. For instance, the impact of nutrient loading on phytoplankton abundance is subject to the availability of light and sedimentation rates, among other factors [51]. Additionally, chlorophyll content is influenced by various factors, including nutrients, phosphorus, pH, water flow, water temperature, and other variables [52].
6. Conclusion
The findings of the present investigation have demonstrated significant correlations among physicochemical factors in the water column of the reservoir. Moreover, pollution loads were found to be accumulated predominantly towards the bottom of the reservoir water. The reduction in dissolved oxygen, in conjunction with the increase in the concentration of EC, TSS, BOD5, and nutrient values, was associated with runoff, pollution load, and the decomposition of organic matter. In particular, the elevated value of BOD5 from the surface to the bottom (ranging from 0.76 Mg/L to 6.9 mg/L) that corresponded to the decomposition of organic matter, which consumed dissolved oxygen and exhibited high nutrient concentration, indicated the presence of organic pollution. The decline in pH concentration was linked to the release of carbonic anhydride upon utilization of dissolved oxygen. The water in the reservoir was characterized by an almost alkaline nature at the surface and a neutral pH at the bottom. The surface water temperature exhibited relatively higher values (30 °C) with variations down the water column. The implications of this research are related to the deterioration of the quality of reservoir water due to the observed dense masses of blue-green algae in the pelagic zone. This is indicative of eutrophic conditions in the reservoir, which could cause changes in the structure of the reservoir water ecosystem and other detrimental effects that may lead to a complete cessation of its functions and uses.
Author contribution statement
Bizuneh Woldeab: Conceived and designed the experiments; Performed the experiments;
Analyzed and interpreted the data; wrote the paper.
Argaw Ambelu: Analyzed and interpreted the data.
Zewdu Efrem: Performed the experiments.
Siyoum Deribe: Performed the experiments.
Moa Megersa: Performed the experiments and wrote the paper.
Tibebu Alemu: Analyzed and interpreted the data; Wrote the paper.
Seid Tiku Mereta: Conceived and designed the experiments.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors confirm that we have no conflicts of interest to disclose and all authors agreed for submission of this manuscript to the Journal. In addition, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors would like to thank Jimma University and Mada Walabu University of Ethiopia for financing. The authors also wish to thank the local community and Mr. Seyifu Mohamed, who helped us during fieldwork.
References
- 1.Wagner T., Erickson L.E. Sustainable management of eutrophic lakes and reservoirs. J. Environ. Protect. 2017;8(4):436–463. [Google Scholar]
- 2.Logan B., Taffs K.H. Relationship between diatoms and water quality (TN, TP) in sub-tropical east Australian estuaries. J. Paleolimnol. 2013;50(1):123–137. [Google Scholar]
- 3.Zakeyuddin M.S., ASR M.S., Mohammad M.S., Mohd Fadzil N.F., Hashim Z.H., Wan Omar W.M. Spatial and temporal variations of water quality and trophic status in Bukit Merah Reservoir, Perak. Sains Malays. 2016;45(6):853–863. [Google Scholar]
- 4.Corgosinho P.H.C., Pinto-Coelho R.M. Zooplankton biomass, abundance and allometric patterns along an eutrophic gradient at Furnas Reservoir (Minas Gerais, Brazil) Acta Limnol. Bras. 2006;18(2):213–224. [Google Scholar]
- 5.El-Radaideh N. Water quality characteristics of zeklap reservoir water, Jordan. Int. J. Ecol. Dev. 2016;31(1):32–48. [Google Scholar]
- 6.Pagioro T.A., Thomaz S.M. Longitudinal patterns of sedimentation in a deep, monomitic subtropical reservoir (Itaipu, Brazil–Paraguay) Arch. Hydrobiol. 2002;154(3):515–528. doi: 10.1127/archiv-hydrobiol/154/2002/515. [DOI] [Google Scholar]
- 7.Gikas G.D., Tsihrintzis V.A., Akratos C.S., Haralambidis G. Water quality trends in polyphytos reservoir, aliakmon river, Greece. Environ. Monit. Assess. 2009;149:163–181. doi: 10.1007/s10661-008-0191-z. [DOI] [PubMed] [Google Scholar]
- 8.Panwar S., Malik D.S. Vertical variations in physicochemical characteristics of Bhimtal lake (Uttarakhand) Journal of Sustainable Environmental Research. 2014;3(2):187–193. [Google Scholar]
- 9.Ramos T., Darouich H., Gonçalves M., Brito D., Castelo Branco M., Martins J., Fernandes M., Pires F., Morais M., Neves R. An integrated analysis of the eutrophication process in the Enxoé reservoir within the DPSIR framework. Water. 2018;10(11):1576. [Google Scholar]
- 10.Bianchi T.S., Cui X., Blair N.E., Burdige D.J., Eglinton T.I., Galy V. Centers of organic carbon burial and oxidation at the land-ocean interface. Org. Geochem. 2018;115:138–155. doi: 10.1016/j.orggeochem.2017.09.008. [DOI] [Google Scholar]
- 11.Lin Jr-L., Arthur K., Ying M.H., Kang S.-F. Eutrophication factor analysis using Carlson trophic state index (CTSI) towards non-algal impact reservoirs in Taiwan. Sustainable Environment Research. 2022;32 [Google Scholar]
- 12.Woldeab B., Beyene A., Ambelu A., Buffam I., Mereta S.T. Seasonal and spatial variation of reservoir water quality in the south west of Ethiopia. Environ. Monit. Assess. 2018;190(3):1–13. doi: 10.1007/s10661-018-6527-4. [DOI] [PubMed] [Google Scholar]
- 13.Radhika C., Mini I., Gangadevi T. Studies on abiotic parameters of a tropical fresh water lakeVellayani Lake. Thiruvananthapuram Dist. Kerala. Poll. Res. 2004;23:49–63. [Google Scholar]
- 14.Camacho J., Reynaga E., Barcelo-Quintal E., Orozco-Guareño C., Alvarez-Bobadilla D., Gomez-Salazar S. Water quality assessment of a tropical Mexican lake using multivariate StatisticalTechniques. J. Environ. Protect. 2015;6:215–224. [Google Scholar]
- 15.Yang L., Lei K., Meng W., Fu G., Yan W. Temporal and spatial changes in nutrients and chlorophyll-a in a shallow lake, Lake Chaohu, China: an 11-year investigation. J. Environ. Sci. 2013;25:1117–1123. doi: 10.1016/s1001-0742(12)60171-5. [DOI] [PubMed] [Google Scholar]
- 16.Ambelu A., Lock K.L., Goethals P.L. Hydrological and anthropogenic influence in the Gilgel Gibe I reservoir (Ethiopia) on marcroinvertebrate assemblages. Lake Reservoir Manag. 2013;29(3):143–150. [Google Scholar]
- 17.Meding M.E., Jackson L.J. Biotic, chemical, and morphometric factors contributing to winter anoxia in prairie lakes. Limnol. Oceanogr. 2003;48:1633–1642. [Google Scholar]
- 18.Robarts R.D., Waiser M.J., Arts M.T., Evans M.S. Seasonal and diel changes of dissolved oxygen in a hypertrophic prairie lake. Lakes Reserv. Res. Manag. 2005;10:167–177. [Google Scholar]
- 19.Wetzel R.G. third ed. Academic Press; San Diego, CA: 2001. Limnology: Lake and River Ecosystems. [Google Scholar]
- 20.Ruuhijärvi J., Rask M., Vesala S., Westermark A., Olin M., Keskitalo J., Lehtovaara A. Recovery of the fish community and changes in the lower trophic levels in a eutrophic lake after a winter kill of fish. Hydrobiologia. 2010;646:145–158. [Google Scholar]
- 21.Anderson A.-M. Environmental Monitoring and Evaluation Branch Alberta Environment; 2002. Spatial Variability of Water Quality in Wabamun Lake.http://www3.gov.ab.ca/env/info/infocentre/publist.cfm [Google Scholar]
- 22.Patil P.N., Sawant D.V., Deshmukh R.N. Physicochemical parameters for testing of water: a review. Int. J. Environ. Sci. 2012;3:1194–1207. [Google Scholar]
- 23.Reimer A., Landmann G., Kempe S. Lake Van, eastern Anatolia, hydrochemistry and history. Aquat. Geochem. 2008;15:195–222. [Google Scholar]
- 24.Demissie T., Saathoff F., Seleshi Y., Gebissa A. Evaluating the effectiveness of best management practices in Gilgel Gibe basin watershed—Ethiopia. Journal of Civil Engineering and Architecture. 2013;7(10):1240–1252. [Google Scholar]
- 25.Berhanu B., Seleshi Y., Melesse A.M. In: Nile River Basin Ecohydrological Challenges, Climate Change and Hydropolitics. Melesse A.M., Abtew W., Setegn S.G., editors. Springer International Publishing; 2014. Surface water and groundwater resources of Ethiopia: potentials and challenges of water resources development; pp. 97–117. [Google Scholar]
- 26.Ethiopian Electric Power Corporation (EEPC) Hydropower for Sustainable Development; 2011. Gilgel Gibe Hydroelectric Project – Environmental Management Plan.www.h4sd.info/Contact-Us/H4SD_PRESS_Case-Study_GG-I_31-03 2011_Final.aspx. [Google Scholar]
- 27.Wakjira T., Tamene A., Dawud T. Land use land cover change analysis using multi temporal landsat data in Gilgel Gibe, Omo Gibe basin, Ethiopia. Int. J. Sci. Technol. 2016;5(7):309–323. [Google Scholar]
- 28.American Public Health Association (APHA) twentieth ed. American Public Health Association; Washington DC: 1999. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 29.Dirican S. Assessment of water quality using physico-chemical parameters of Çamlıgöze Dam Lake in Sivas, Turkey. Ecologia. 2015;5(1):1–7. [Google Scholar]
- 30.Australia. National . Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand; 2000. Water Quality Management Strategy (ANWQMS), Australian and New Zealand Guidelines for Fresh and Marine Water Quality. [Google Scholar]
- 31.Lampert W., Sommer U. second ed. Oxford University Press; 2007. Limnoecology. The Ecology of Lakes and Streams. [Google Scholar]
- 32.Environmental Research and Design (ERD), Evaluation of Surface Water Quality Characteristics in Casselberry Lakes. Final Report. 2013. [Google Scholar]
- 33.Prokopkin I., Mooij W., Janse J., Degermendzhy A. A general one-dimensional vertical ecosystem model of Lake Shira (Russia, Khakasia): description, parametrization and analysis. Aquat. Ecol. 2010;44:585–618. [Google Scholar]
- 34.Sliva L., Williams D.D. Buffer zone versus whole catchment approaches to studying land use impact on river water quality. Water Res. 2001;35(14):3462–3472. doi: 10.1016/s0043-1354(01)00062-8. [DOI] [PubMed] [Google Scholar]
- 35.Kalff J. Prentice Hall; Upper Saddle River, N.J: 2002. Limnology: Inland Water Ecosystems. [Google Scholar]
- 36.Araoye P.A. The seasonal variation of pH and dissolved oxygen (DO2) concentration in Asa lake Ilorin, Nigeria. Int. J. Phys. Sci. 2009;4(5):271–274. [Google Scholar]
- 37.Al-Taani A.A., El-Radaideh N.M., Al Khateeb W.M. Status of water quality in king talal reservoir dam, Jordan. Water Resour. 2018;45(4):603–614. [Google Scholar]
- 38.Environmental Protection Authority (Epa) Environmental Protection Agency; 2001. Parameters of Water Quality: Interpretation and Standards; pp. 1–132. Published by EPA Ireland. [Google Scholar]
- 39.Environmental Protection Authority and The United Nations Industrial Development Organization (Epa and UNIDO) Addis Ababa; 2003. Guidline Ambient Environment Standards for Ethiopia. [Google Scholar]
- 40.Baran A., Tarnawski M., Urbański K., Klimkowicz-Pawlas A., Spałek I. Concentration, sources and risk assessment of PAHs in bottom sediments. Environ. Sci. Pollut. Control Ser. 2017;24(29):23180–23195. doi: 10.1007/s11356-017-9944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Obenour D.R., Scavia D., Johengen T.H., Michalak A.M. Spatial and temporal trends in Lake Erie hypoxia, 1987–2007. Environ. Sci. Technol. 2013;47(2):899–905. doi: 10.1021/es303401b. [DOI] [PubMed] [Google Scholar]
- 42.Deininger A., Frigstad H. Reevaluating the role of organic matter sources for coastal eutrophication, oligotrophication, and ecosystem health. Front. Mar. Sci. 2019;6:210. doi: 10.3389/fmars. 2019.00210. [DOI] [Google Scholar]
- 43.Makhlough A. MSc Thesis; 2008. Water Quality Characteristics of Mengkuang Reservoir Based on Phytoplankton Community Structure and Physico- Chemical Analysis. [Google Scholar]
- 44.Chapman D.V. 2nded. UNESCO/WHO/UNEP; New York: 1996. Water Quality Assessments: a Guide to the Use of Biota, Sediments and Water in Environmental Monitoring. [Google Scholar]
- 45.Ling T.Y., Nyanti L., Muan T., Grinang J., Sim S.F., Mujahid A. Physicochemical parameters of bakun reservoir in belaga, Sarawak, Malaysia,13 Months after reaching full supply level. Sains Malays. 2016;45(2):157–166. [Google Scholar]
- 46.Aris A.Z., Lim W.Y., Praveena S.M., Yusoff M.K., Ramli M.F., Juahir H. Water quality status of selected rivers in Kota Marudu, Sabah, Malaysia and its suitability for usage. Sains Malays. 2014;43(3):377–388. [Google Scholar]
- 47.Kaul V., Handoo J. Physicochemical characteristics of Nilnang a high altitude forest lake in Kashmir and its composition with the valley lakes. Proc. Indian. Nat. Sci. Acad. B. 1980;46:528–541. [Google Scholar]
- 48.Baatar B., Chuluun B., Tang S.L., Bayanjargal O., Oyuntsetseg B. Vertical distribution of physical–chemical features of water and bottom sediments in four saline lakes of the Khangai mountain region, Western Mongolia. Environ. Earth Sci. 2017;76:130. doi: 10.1007/s12665-017-6447-6. [DOI] [Google Scholar]
- 49.Baker D., Richards P. Context for re-evaluating agricultural source phosphorus loadings to the Great Lakes. J. Environ. Qual. 2006;31:96–108. [Google Scholar]
- 50.Schindler D.W., Hecky R.E., Findlay D.L., Stainton M.P., Parker B.R., Paterson M.J., Beaty K.G., Lyng M., Kasian S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA. 2008;105(32):11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson K., Jorgensen B.B. American Geophysical Union; 2013. Eutrophication: Definition, History and Effect Eutrophication in Coastal Marine Ecosystems; pp. 1–9. [Google Scholar]
- 52.Johan F.B., Jafri M.Z.B.M., San L.H., Omar W.M.W., Ho T.C. vol. 1083. IOP Publishing; 2018. August. Chlorophyll a concentration of fresh water phytoplankton analysed by algorithmic based spectroscopy. (Journal of Physics: Conference Series). 1. [Google Scholar]
- 53.Sobolewski W. Effect of agricultural land use on the water quality of Polish lakes: a regional approach. Pol. J. Environ. Stud. 2016;25(6):2705–2710. [Google Scholar]
- 54.Rahmani T., Poorbagher H., Javanshir A., Kamangar B.B. Effects of agriculture, tourism and the dam on eutrophic status of the Zerebar Lake, Iran. International Journal of Advanced Biological and Biomedical Research. 2013;1(4):403–420. [Google Scholar]
- 55.Ribeiro K.H., Favaretto N., Dieckow J., Souza L.C.P., Minella J.P.G., de Almeida L., Ramos M.R. Quality of surface water related to land use: a case study in a catchment with small farms and intensive vegetable crop production in southern Brazil. Soil Use Manag. 2014;38(2):656–668. [Google Scholar]
- 56.Hossain M.S. Impact of land use change on stream water quality: a review of modeling approaches. Journal of Research in Engineering and Applied Sciences. 2017;2(1):1–6. [Google Scholar]
- 57.Swaminathan M.S. Biodiversity: an effective safety net against environmental pollution. Environ. Pollut. 2003;126:287–291. doi: 10.1016/s0269-7491(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 58.Zbikowski R., Szefer P., Latala A. Comparison of green algae Cladophorasp. and Enteromorpha sp. as potential biomonitors of chemical elements in the Southern Baltic. Sci. Total Environ. 2007;387:320–332. doi: 10.1016/j.scitotenv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Morris G.L., Fan J. McGraw-Hill Book Co.; New York: 1998. Reservoir Sedimentation Handbook. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.