Abstract
Chronic stress is a risk factor for depression and is characterized by elevated levels of brain monoamine oxidase A (MAOA). Mounting evidence has shown that MAOA is a biochemical link between stress and depression. Apigenin (API), a natural flavonoid, as demonstrated in vitro inhibitory effect on MAOA, is suggestive of antidepressant-like activity. However, the in vivo inhibitory effect of API on MAOA and how it affects depression still remain unclear. Here, we report the probable mechanisms of action of API in chronic unpredictable mild stress (CUMS)-induced depression in mice. Treatment with API reversed anhedonia, and reduced anxiety and immobility time in behavioral studies. API reduced brain corticosterone and malondialdehyde (MDA) levels but increased brain levels of glutathione and superoxide dismutase. Furthermore, interleukin-6 and tumor necrosis factor-α were attenuated by API. It also restored cell loss and inhibited the activity of MAOA in the hippocampal brain regions and prefrontal cortex. Comparative binding affinity of API for MAOA (-7.7 kcal/mol) through molecular docking studies was greater than that of reference compound, clorgyline (-6.8 kcal/mol). Favorable hydrophobic interactions important to API binding at MAOA binding cavity was revealed to include conventional hydrogen bond (Cys323 and Tyr444), π-Sulfur (Cys323), π-π Stacked (Tyr407), π-π T-shaped (Phe208), π-lone pair and π-alkyl (Ile335, Ile180) interactions. These results suggest that API is a potent, selective, reversible inhibitor of MAOA with capability of attenuating CUMS-induced depression via inhibiting MAOA enzyme activity and altering other pathomechanisms.
Keywords: Depression, Apigenin, Chronic unpredictable mild stress, Monoamine oxidase A enzyme, Molecular docking
Graphical abstract
Highlights
-
•
CUMS increased oxidative stress and reduced antioxidants status in depressed mice.
-
•
CUMS increased inflammation, stress, and brain MAOA levels in depressed mice.
-
•
Oxidative stress, inflammation, stress, and brain MAOA levels were reversed by API.
-
•
Comparative binding affinity of API for MAOA was greater than clorgyline.
-
•
API is a potent, selective, reversible inhibitor of MAOA.
1. Introduction
Chronic stress is one risk factor for psychiatric disorders such as depression, which is characterized by elevated levels of brain MAOA (Grunewald et al., 2012). The MAOA is the enzyme that is responsible for the deamination of monoamine neurotransmitters, and is also a biochemical link for stress and depression (Grunewald et al., 2012).
Depression is a chronic multidimensional and psychiatric disorder with various symptoms, including changes in mood, anhedonia, tiredness or fatigue (McKenna et al., 2005). The World Health Organization (WHO) considers depression as the fourth leading cause of disability worldwide (World Health Organization, 2010). The pathophysiologic basis of depressive behavior has been hypothesized to be as a result of depletion of neurotransmitters (norepinephrine and/or 5-HT) within the synaptic space of neuronal cells, thereby leading to altered mood (Pitsillou et al., 2020). Moreover, the multidimensional nature of depression has also been linked with inflammatory processes and increased levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, which have been reported in earlier studies to be elevated in the brain and peripheral tissues of depressed patients (Pitsillou et al., 2020; Müller, 2014). Dysfunctional hypothalamic-pituitary-adrenal axis (HPA), as well as reduced antioxidant activity and elevated oxidative stress, have also been associated with the pathology (Hovatta et al., 2010; Leonard and Maes, 2012; Scapagnini et al., 2012).
The limbic system plays a functional role in the regulation of emotion (Dean and Keshavan, 2017), and studies have revealed a dysfunctional corticolimbic system in depressed patients (Joormann and Stanton, 2016; Park et al., 2019). The dorsolateral prefrontal cortex (PFC), ventrolateral prefrontal cortex, anterior cingulate cortex, amygdala and hippocampus are important neurological structures of the corticolimbic system affected by depression (Mayberg et al., 1999; Korgaonkar et al., 2013; Smith et al., 2018).
Monoamine oxidases are metabolizing enzymes that are mostly found in the brain and peripheral tissues (Thorpe et al., 1987). MAOA is a structural isoform involved in the degradation of monoamine neurotransmitters (Youdim et al., 2006). The enzyme has been well implicated in various psychiatric illnesses due to its action in the deamination of serotonin, norepinephrine and dopamine. Studies have reported increased levels of brain MAOA in live patients and following post-mortem of humans with depressive disorders (Meyer et al., 2006); Meyer et al. (2009); Johnson et al. (2011); and in mothers during postpartum depression (Sacher et al., 2010).
API is a 4′,5,7-trihydroxyflavone. It is a natural plant product that belongs to the flavone class and is widely found in fruits and vegetables. Previous studies have shown that API possesses significant antioxidant (Lee et al., 1998; Romanova et al., 2001), anti-inflammatory (Funakoshi-Tago et al., 2011; Jeong et al., 2009) and antitumor activities (Li et al., 2009; Cai et al., 2011). Synergistic effects of API and paclitaxel on apoptosis of cancer cells have been reported (Xu et al., 2011). The effect of API on lipopolysaccharride treated-mice has been reported (Li et al., 2015).
The search for new antidepressant agents has increased in recent times. Current first line treatments target the modulation of the monoamine system (D Rosenblat et al., 2015), and MAOA is a promising drug target for the treatment of depression and neurodegenerative disorders (McKenna et al., 2005). Studies have shown the in vitro inhibitory effect of API on recombinant human MAOA and MAOB (Chaurasiya et al., 2014), as well as preliminary screening on its antidepressant-like effect in forced swimming test (FST) and CUMS-induced serotonergic alterations in rats (Nakazawa et al., 2003; Yi et al., 2008). However, there is still a concern regarding the in vivo inhibitory effect of API on MAOA and its effect on depression. Herein, we report the possible mechanisms of action of API in CUMS-induced depression in mice. We also provide mechanistic insights by investigating the binding mode of API relative to a reference compound (clorgyline) in the binding cavity of MAO by computing the relative binding affinities of the compounds. Furthermore, important interactions responsible for the possible downstream antidepressant activity of API and implicated binding site residues were investigated.
Here, we provide compelling demonstrations that API could be a potent inhibitor of MAOA. In order to establish this, we proposed that since API has demonstrated potent in vitro inhibitory effects against MAOA in a previous study (Chaurasiya et al., 2014); subjecting the animals to the CUMS paradigm (a well validated test used in the context of chronic depression in animals) (Katz et al., 1981; Willner, 1997; Willner et al., 1987), as well as treating the animals with API for a longer time, could establish this. Our results showed that API attenuated CUMS-induced depression, and that this effect could be mediated through the inhibition of MAOA enzyme activity, reducing oxidative stress, inflammation and neuroendocrine signaling pathways in mice. Furthermore, since selective MAOA inhibitors are very good in the management of depression (Etukudo, 2003); we chose clorgyline (a selective inhibitor of MAOA), and decided to establish at the molecular level the effect of MAOA binding affinity of API compared to clorgyline through molecular docking techniques and compared the binding site amino acid residues. This result showed that API (-7.7 kcal/mol) has a better binding affinity to MAOA compared to clorgyline (-6.8 kcal/mol). The molecular docking technique used in this study could be useful in providing insights into further investigations towards understanding the underlying molecular mechanisms at the gene levels and other pathways involved in the activity of API as a potent antidepressant. Nevertheless, this study provides strong evidence that API can be used as a potent inhibitor of MAOA and could be a potential therapeutic candidate in the management of depression.
2. Materials and methods
2.1. Animals
A total of 40 male Swiss mice of ten (10) animals per group (20–25 g; 8 weeks old) were used for the study. The animals were purchased from the Central Animal House, Ekiti State University, Ado-Ekiti, Ekiti State, Nigeria. The animals were kept in plastic cages at room temperature under good hygienic conditions, and had unrestricted access to rodent pellets and portable water except as dictated by the experimental design. Each experimental procedure was performed in compliance with the National Research Council's Guide for the Care and Use of Laboratory Animals (2011) of the National Academy of Sciences, United States of America. It also complied with ARRIVE 2.0 guidelines (https://arriveguidelines.org/arrive-guidelines/sample-size). Ethical approval (ref: AB/EC/014/09/369), was obtained from the Ethical Committee of Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria for the usage of laboratory animals.
2.2. Chronic unpredictable mild stress (CUMS)
The procedure for CUMS was performed as previously described by (Jiang et al., 2013) with some modifications. Mice were subjected to CUMS for two weeks. Fourteen different stressors were randomly administered to the animals at designated time of the day. Experimental animals were exposed to two different types of stressors each day in a manner unpredictable to them (Table 1). These stressors were randomly scheduled in the first week and repeated the second week. All the stressors were applied individually and regularly during the day and at night. The control animals were not disturbed in their home cages except during regular cage cleaning and weighing of animals.
Table 1.
Schedule of stressors in chronic unpredictable mild stress in mice.
| Days | Stressors and duration |
|---|---|
| 1 | Damp beddings (2 h); shaking the cages vigorously (1.5 h) |
| 2 | Food deprivation (12 h); tail pinch (5 min) |
| 3 | 45°C cage tilting (2 h); cat noise (1 h) |
| 4 | Overnight illumination (12 h); sawdust free cage (1 h) |
| 5 | Food and water deprivation (12 h); change of cage mates (30 min) |
| 6 | Water deprivation (12 h); exposure to predator odor (30 min) |
| 7 | Sawdust free cage + 120 ml of water (2 h); background noise (30 min) |
| 8 | Tail pinch (5 min); food deprivation (12 h) |
| 9 | Shaking the cages vigorously (1.5 h); damp beddings (2 h) |
| 10 | Cat noise (1 h); 45°C cage tilting (2 h) |
| 11 | Sawdust free cage (1 h); overnight illumination (12 h) |
| 12 | Change of cage mates (30 min); food and water deprivation (12 h) |
| 13 | Exposure to predator odor (30 min); water deprivation (12 h) |
| 14 | Background noise (30 min); sawdust free cage + 120 ml of water (2 h) |
2.3. Experimental design
Mice were randomly assigned to 4 groups of (where n = 10 animals per cage or group). Group 1 received no treatment and served as the control group; group 2 (stressed group) was exposed to CUMS alone; group 3 received API 12.5 mg/kg/day [intraperitoneally (i. p.)] + CUMS; group 4 received API 25 mg/kg/day (i. p.) + CUMS. Animals were treated daily for fourteen days. The treatment schedule for API as well as doses were chosen from a previous study (Nakazawa et al., 2003).
2.4. Weight measurement
Body weights of the mice were measured and recorded on the first day (Day 1 of CUMS- before starting the CUMS procedure) of the experiment and on the 14th day (the last day of CUMS paradigm). However, the animals were weighed every three days during the 14 days CUMS procedure, using an appropriate weighing balance.
2.5. Behavioral studies
The behavioral tests were carried out in a closed area with proper illumination and sound controlled behavioral analysis room. The tests were carried out to assess anhedonia, anxiety and depressive-like behaviors. The tests were performed on the mice in the following order: sucrose splash test (SST), elevated plus maze (EPM), forced swim test (FST) and tail suspension test (TST).
The SST was carried out as described previously by (Sadeghi et al., 2016) with some modifications. The test was performed before the start of the CUMS procedure (on day 1), and at the end of the CUMS procedure (on day 14). The SST is a test used in assessing motivational behavior in mice, which is a primary symptom of depression. However, the test was modified to assess anhedonic behavior in this study. The splash test used in this study involved spurting a 10% sucrose solution over the dorsal coat of a mouse in its home cage. After administering the sucrose solution, the total time spent grooming/licking was recorded for 5 min. Duration of grooming behavior, which included nose/face grooming, head washing, and body grooming, was observed by another observer (David et al., 2009).
The effect of API on CUMS-induced anxiety behavior was assessed in the experimental mice according to the procedure described by (Lister, 1987). Each mouse was placed individually at the middle of the EPM with its head facing an open arm and was allowed to freely explore the maze for 5 min. The parameters measured were the frequency and duration of arm entries. An entry was scored when the four paws of the animals were completely into one arm of the EPM. Ethanol solution (70%) was used to clean the EPM after each test. The results were stated as time spent in arms and percentage of number of entries in arms (mean ratio of entries in an arm to total entries in both open and closed arms).
The FST was carried out as described previously by (Porsolt, 1981). The test is also known as the behavioral despair test. The test is used in assessing depressive-like behavior in both mice and rats. Mice were put into a Plexiglas cylinder (25 cm height, diameter 10 cm containing water to a height of 10 cm at 25°C and observed for 6 min. A mouse was judged immobile if it floated in the water in an upright position and made only slight movements to prevent sinking. The total duration of immobility was recorded during the last 4 min of the 6 min test. Decrease in immobility or increase in mobility time indicates an antidepressant-like effect.
The TST was carried out as described previously by (Porsolt et al., 1978). Mice were suspended on the edge of a table, 50 cm above the floor with the help of an adhesive tape placed approximately 1 cm from the tip of the tail. In this test, a mouse suspended by its tail was dangling freely in the air. Immobility time was recorded during a 6 min period. An animal was considered immobile when there was no sign of body movement.
2.6. Biochemical assays
At the end of the behavioral studies, animals were sacrificed under ether anesthesia and their brains harvested and kept in the refrigerator with ice block for 30 min. Then, the brain samples were weighed and homogenized with 10% w/v phosphate buffer (0.1 M, pH 7.4), using a mechanical homogenizer with a glass teflon. The homogenates were centrifuged at 4°C in a refrigerated centrifuge at a speed of 10,000 rpm for 10 min to obtain the supernatants. The supernatants were aliquoted and refrigerated at -20°C. Each brain supernatant was separated into various portions for the specific biochemical assays.
2.6.1. Estimation of brain levels of malondialdehyde (MDA)
MDA levels in the brain were estimated as an index of lipid peroxidation using the assay of thiobarbituric reacting substances (TBARS) according to the method of (Nagababu et al., 2010). A portion (100 μL) of each supernatant was diluted twenty times in 0.15 M Tris-KCl buffer and later deproteinized with 500 μL of trichloroacetic acid (30%). The mixture was centrifuged at 4000 rpm for 10 min at room temperature. Thereafter, an Eppendorf tube was used to put 200 μL of the supernatant. Then, 200 μL of thiobarbituric acid (0.75%) was later added; then, the mixture was heated at 80°C for 1 h. The tubes were cooled by placing them on the ice after which 200 μL was removed into microtitre plate and the absorbance was measured at 532 nm. Results were calculated using an index of absorption for MDA (molar extinction coefficient 1.56 × 105/M/cm). The concentration of TBARS in the tissues were expressed as ηmol MDA/mg protein.
2.6.2. Determination of reduced glutathione (GSH) levels
Reduced glutathione (GSH), a non-enzymic antioxidant marker, was measured in the supernatant of the brain tissues, using the method of (Jollow et al., 1974). Briefly, 100 μL of supernatant was diluted twenty times in 0.15 M Tris-KCl buffer and deproteinized with 500 μL trichloroacetic acid (30%). The mixture was centrifuged at 4000 rpm for 10 min at room temperature. Thereafter, 100 μL of the deproteinized supernatant was mixed with 100 μL of 5,5′ -dithios-nitrobenzoic acid (DTNB, 0.0006 M) in a microplate plate. The absorbance was read within 5 min at 405 nm in a microplate reader (MICRO READ 1000, Belgium). The GSH concentration was got from a standard curve of GSH (0–200 μM) and expressed as a μM GSH/mg protein.
2.6.3. Estimating brain levels of superoxide dismutase (SOD)
The brain levels of SOD activity were determined by the method of (Misra and Fridovich, 1972). SOD activity was estimated based on the inhibitory effect of the enzyme on autoxidation of epinephrine in sodium carbonate buffer solution (pH 10.7). Briefly, 50 μL of 2X diluted supernatant was added into a microliter plate which contained 150 μL of carbonate buffer. The reaction started by adding 30 μL of freshly prepared 0.3 mM epinephrine to the mixture. Blank was prepared, using 50 μL of distilled water. The increase in absorbance at 495 nm was monitored every 60 s for 240 s. The SOD activity was expressed as U/mg protein.
2.6.4. Estimation of brain levels of pro-inflammatory mediators (IL-6 and TNF-α)
Animals were anesthesized with ether and decapitated. Their brains were carefully removed and kept in a functional refrigerator with ice block for approximately 30 min. Thereafter, the mouse whole brain was homogenized in a glass test tube containing 10% w/v phosphate buffer (0.1 M, pH 7.4). Brain TNF-α (Cat. No: 430904) including IL-6 (Cat. No: 431304) were determined in line with the prescribed protocol of the ELISA kit manufacturer (Biolegend®, USA) specific to the cytokines of interest, with sensitivity limit of 4 pg/mL. All measurements were performed at normal room temperature based on Biolegend® guidelines, using microplate reader with 450 nm filter. The concentrations of IL-6 and TNF-α from the brain tissues were got from the standard curves of IL-6 and TNF-α standards included in the assay kits and expressed as pg/mL.
2.6.5. Estimation of serum corticosterone levels
Blood sample (1 ml) was obtained through cardiac puncture from the animals under ether anesthesia for the determination of serum corticosterone levels. The serum corticosterone concentration (ng/ml) was measured using an ELISA kit (Bioassay Laboratory Technology, China, Cat. No: EA0036Ra) according to the manufacturer's instructions. The blood sample was centrifuged at 3000 rpm for 15 min and the serum was collected for estimation of corticosterone levels. All samples, standard, and cortisol- Horse radish peroxidase (HRP) conjugate, were added to a micro-plate coated with mAb and to cortisol; thus, they were incubated at room temperature for 1 h. The bound cortisol-HRP was estimated using tetramethylbenzidine (TMB) substrate. The TMB (150 l) substrate was well added to each and incubated at room temperature for 30 min. The reading was taken at 650 nm using the Spectramax M-5® (Molecular Devices, Sunnyvale, USA) multifunctional plate reader equipped with SoftmaxPro v5.4® (SMP 5.4), and a 5-parameter sigmoid minus curve fit determined unknown concentrations.
2.7. Histology of the prefrontal cortex and hippocampal regions
Brain tissues of animals for histology were stored in 10% formaldehyde solution. Thereafter, the tissues fixed with paraffin wax and transverse sections (5–6 μm thick) were obtained by microtomy. These tissues were further processed by the routine methods, leading to embedment of sections on glass slides. The slides were then stained, using hematoxylin and counter stained with eosin. Thereafter, the hippocampal regions (cornus ammonus 1 (CA1), cornus ammonus 3 (CA3) and dentate gyrus, including the prefrontal cortex of mice brains, were examined and evaluated under a binocular light microscope (OPTO-EDU, China) which had an attached industrial digital camera for photomicrographs.
The population of healthy brain tissue cells of CUMS-treated mice was determined by counting viable neurons of the hippocampal pyramidal CA1, CA3, sub-granular zone of dentate gyrus, as well as the pre-frontal cortex, utilizing ImageJ software. Viable cells were counted within a specified area of a circular view in a section, using the eyepiece of microscope (OPTO-EDU, China) at × 800 magnification and graticule. Six separate measurements were made on each section, and a mean of the summation of each dimension obtained was then calculated. Viable cells were seen as round-shaped cytoplasmic cells with intact membrane devoid of any form of nuclear condensation and distorted features.
2.8. Immunohistochemistry
After the behavioral assessments, two mice from each group were sacrificed under ether anesthesia for immunohistochemical staining. The hearts were perfused with normal saline intracardially followed by 10% formaldehyde. Sections were mounted on gelatin-coated slides, dehydrated and cover-slipped.
The expressions of positive cells of MAOA in mice brains were estimated using immunohistochemistry kit (Elabscience, USA with Cat. No: E-AB-60293 MAOA Polyclonal antibody) in conformity with the manufacturer's instructions. Antigen retrieval solution (citric acid water) of 95 g of citric acid was put in 1 L of distilled water. The pH was regulated to 6.0 and the solution heated at 95°C. The slide was dropped into the solution and heated for 15 min then allowed to cool. The tissue was circled with hydrophobic pen to prevent water. Normal goat serum (ready to use) was added and then incubated at 37°C for 30 min before shaking to remove any excess liquid. Mouse/rabbit primary antibody was added with proper dilution ratio then incubated at 37°C for 2 h. The solution was washed 3 times with phosphate buffer solution (PBS) for 2 min after which the section was dried with absorbent paper. Polyperoxidase anti mouse/rabbit IgG was added and incubated at room temperature for 20 min and then washed 3 times with PBS for 2 min. One drop (approximately 50 μL) of 3, 3-diaminobenzidine (DAB) concentrate was added into each 1 ml of DAB substrate and mixed fully. The mixed reagent was the DAB working solution. The prepared DAB working solution was valid for 4 h and unused solution was abandoned. Control of the DAB coloration period was taken and the color of tan or brownish yellow showed a positive signal. The section was washed with deionized water, and the chromogenic reaction was terminated. The procedures for counterstaining were operated with hematoxylin for 60 s), dehydrated, transparentized and sealed. Images were acquired with the aid of an Optronics Digital Camera linked to a computer interface (MagnaFire) and an Olympus BX-51 binocular research microscope. These images were analyzed using Image J software (NIH, USA).
2.9. System preparation and molecular docking
The 3D structure of human MAOA, possessing total amino acid sequence of 513 residues and co-crystallized with harmine, a reversible MAOA inhibitor was retrieved from Protein Data Bank, PDB ID: 2Z5X (Son et al., 2008). Preprocessing of the protein was carried out using the Graphical User Interface (GUI) of UCSF Chimera (Pettersen et al., 2004, Pettersen et al., 2004) by removing all non-standard residues, ions and crystal water. Also, 2D structures of API (investigational molecule) and clorgyline (a selective MAOA inhibitor) were retrieved from PubChem (Kim et al., 2019), a web-based chemistry database. These ligands were converted to the 3D variant using Avogadro (Hanwell et al., 2012), auto-optimization, interconnective bond modifications and steric clashes detachment.
Further processing sequel to the initial preprocessing of the MAOA protein was carried out by removing hydrogen using UCSF Chimera (Pettersen et al., 2004). For the preprocessed ligands (API and clorgyline), hydrogen was added and thereafter Gasteiger charges. Co-crystallized harmine was removed from the binding cavity of prepared MAOA while coordinates defined as 41.0592, 27.1035, -14.6851 for center and 9.68149, 8.60456, 7.31293 for size were used in active site description preparatory to ligand docking. Molecular docking was implemented with UCSF Chimera-integrated AutoDock Vina (Pettersen et al., 2004; Trott and Olson, 2010; Feinstein and Brylinski, 2015) binding scores and poses with shape complementarily illustrating best ranked protein-ligand conformation, as well as lowest binding free energies were then selected for evaluation and visualization.
2.10. Statistical analysis
Data were analyzed statistically by utilizing one-way analysis of variance (ANOVA) and Tukey multiple comparision as post hoc test. Data were presented as mean ± standard deviation. Nested one-way ANOVA was used to analyze the weight in animals. The Shapiro-Wilk normality test was used to assess the normality and variance homogeneity of the data. The analysis was performed using GraphPad® prism software version 9. P < 0.05 was judged as statistically significant.
3. Results
3.1. Effect of apigenin on mice weight after exposure to CUMS
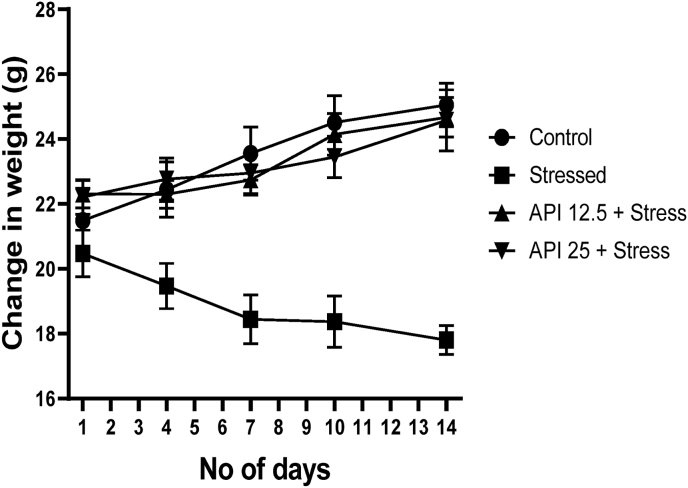
CUMS model of depression relies on repeated exposure to stress (Bolton et al., 2018), and a significant weight loss is one characteristic symptoms associated with depression (Diagnostic, 2013). To this end, the weights of the animals were measured on day 1 (before exposure to CUMS), and subsequently on day 4, 7, 10 and 14 (after exposure to CUMS). The weights of the mice were measured during these days in order to explore whether exposure to repeated stress could confer some weight changes in the animals. Results from this study show that there were no significant changes in the weights of the animals compared to the control on day 1 of the experiment (Fig. 1). One-way ANOVA showed that the weights of animals treated with API (12.5–25 mg/kg/day) and stress were significantly increased [F(3, 36) = 11.49, P < 0.0001] on day 4, 7, 10 and 14 compared to the stressed animals (Fig. 1). These data suggest that administering API to animals exposed to repeated stress may fully restore the weights of depressed animals.
Fig. 1.
Apigenin restored weight loss to normal in CUMS-induced mice. (n = 10). Data expressed as mean ± SD. One way analysis of variance followed by Tukey's multiple comparison test.
3.2. Apigenin relieved mice from CUMS-induced behavioral damage
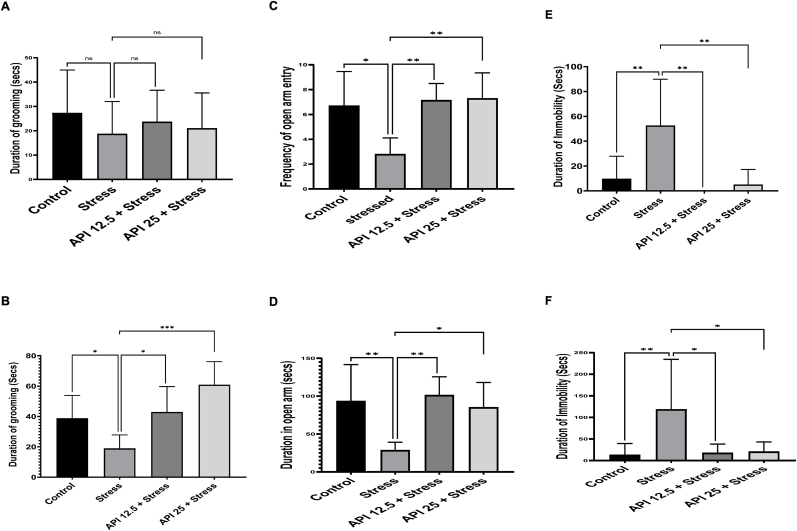
CUMS is an experimental model used to mimic negative life occurrences that happen to humans which may result in anhedonia in rats (Willner, 1997). In this present study, SST, EPM, FST and TST were utilized to evaluate the effects of API on the behavioral performances of CUMS-induced mice. One-way ANOVA result of SST (Fig. 2 A) shows that no significant differences [F(3, 36) = 0.6301, P = 0.6003] in the duration of grooming were observed in the stressed and the API-treated groups (API 12.5 and 25 mg/kg/day) compared to the control group on day 1. However, after mice exposure to CUMS for 14 days, one-way ANOVA result showed that the duration of grooming in mice was significantly decreased (Fig. 2 B) in the stressed group compared to the control group [F(3,25) = 9.423, P = 0.0435]; whereas administering API (12.5 and 25 mg/kg/day) for 14 days to the stressed animals showed a significant increase in the duration of grooming in the API 12.5 mg/kg/day compared to the stressed group [F(3,25) = 9.423, P = 0.0281], and an increase in the duration of grooming in the API 25 mg/kg/day compared to the stressed group [F(3,25) = 9.423, P = 0.0001] (Fig. 2 B). This increase in grooming time observed in the API-treated mice indicates that API alleviated anhedonic-like behavior in the depressed mice.
Fig. 2.
Effects of Apigenin on CUMS-induced depression in mice. (A) Effect of API on duration of grooming in mice on day 1 of the sucrose splash test. (B) Effect of API on duration of grooming on day 14 of the sucrose splash test. (C) API increases frequency of open arm entry in the elevated plus maze test. (D) API increases duration of open arm entry in the elevated plus maze test. (E) API shortened duration of immobility in the forced swimming test. (F) API shortened duration of immobility in the tail suspension test. (n = 10, *P < 0.05 and **P < 0.01 v control; *P < 0.05, ***P < 0.01 and ***P < 0.001 v stress). Data expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
The ability of an animal to remain in the closed arm for a longer time may be indicative of anxiety. Therefore, we assessed whether API administration was associated with the tendency to avoid open places, which is a typical anxiety behavior. The result obtained from the EPM (Fig. 2 C) shows that the frequency of arm entry in mice exposed to CUMS was significantly decreased in the stressed group compared to the control [F(3,25) = 5.592, P = 0.0120]. However, one-way ANOVA result showed that frequency of open arm entry in the treatment groups API (12.5 and 25 mg/kg/day) was significantly increased in the API 12.5 mg/kg/day compared to the stressed group [F(3,25) = 5.592, P = 0.0086], and in the API 25 mg/kg/day compared to the stressed group [F(3,25) = 5.592, P = 0.0065] (Fig. 2 C). Also, one-way ANOVA result showed that the duration of the stressed mice in the open arm was significantly decreased in the stressed group compared to the control group [F(3,27) = 6.662, P = 0.0037] (Fig. 2 D). However, administering API 12.5 mg/kg/day significantly increased the duration of mice in the open arm compared to the stressed group [F(3,27) = 6.662, P = 0.0026], and also administering API 25 mg/kg/day significantly increased the duration of mice in the open arm compared with the stressed group [F(3,27) = 6.662, P = 0.0229] (Fig. 2 D).
The FST test is used to assess behavioral despair in mice. We explored this in the experimental mice and the results obtained showed that mice exposure to CUMS significantly [F(3,25) = 8.254, P = 0.0029] increased duration of immobility in the stressed group compared to the control group (Fig. 2 E). However, treatment with API 12.5 mg/kg/day significantly decreased the duration of immobility in mice compared to the stressed animals [F(3,25 = 8.254; P = 0.0013], and also decreased the duration of immobility in mice treated with API 25 mg/kg/day compared to the stressed animals [F(3,25) = 8.254; P = 0.0035] (Fig. 2 E).
Similar results obtained from the TST showed that mice exposure to CUMS significantly increased duration of immobility in the stressed group compared to the control group [F(3,25) = 5.017, P = 0.0085] (Fig. 2 F). However, treatment with API 12.5 mg/kg/day significantly decreased the duration of immobility in stressed mice compared to the stressed group [F(3,25) = 5.017, P = 0.0303], and also decreased the duration of immobility in the API 25 mg/kg/day group compared to the stressed group [F(3,25) = 5.017, P = 0.0365) (Fig. 2 F).
3.3. Apigenin attenuated oxidative stress and enhanced antioxidant status in stressed mice
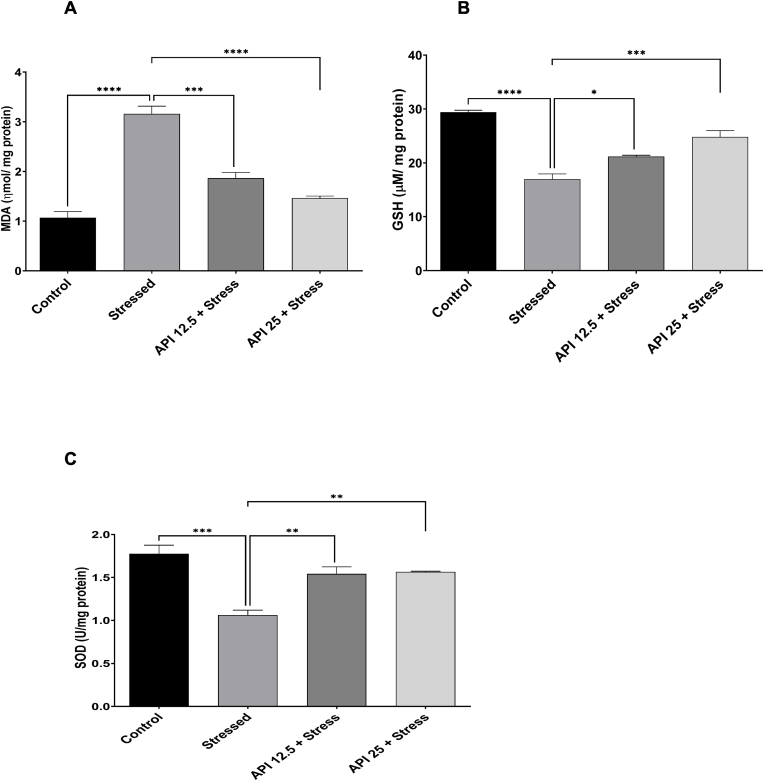
Exposure to chronic stress has been reported to be contributory to the formation of oxidative stress in some parts of the brain that take part in the development of depression (Juszczyk et al., 2021). More so, altered levels of SOD, MDA, and other oxidative stress markers have been reported in depressive disorders (Ozcan et al., 2004). In order to explore this, we estimated the levels of MDA, SOD and GSH in mice brains. Our results show a significant rise in MDA levels in the stressed group compared to the control group [F(3,8) = 57.55, P < 0.0001] (Fig. 3 A). However, administering API 12.5 mg/kg/day significantly reduced MDA levels in mice exposed to CUMS compared to the stressed group [F(3,8) = 57.55, P = 0.0003], and also administering API 25 mg/kg/day significantly reduced MDA levels in mice exposed to CUMS compared to the stressed group [F(3,8) = 57.55, P < 0.0001) (Fig. 3 A). This suggests that API has the potential to attenuate lipid peroxidation in mice exposed to chronic stress.
Fig. 3.
Apigenin attenuated oxidative stress and enhanced antioxidant status in stressed mice. (A) Apigenin attenuated malondialdehyde levels in mice exposed to CUMS. (B) Apigenin enhanced glutathione levels in mice exposed to CUMS. (C) Apigenin enhanced superoxide dismutase levels in mice exposed to CUMS. (n = 10, ***P < 0.001 and ****P < 0.0001 v control; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 v stress). Data expressed as mean ± SD. One way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
We further examined the antioxidant status of the mice after exposure to CUMS by evaluating the levels of SOD and GSH in their brains. Following the one-way ANOVA results obtained from the GSH test, the level of GSH in mice brains was significantly decreased in the stressed group compared to the control [F(3,8) = 43.88, P < 0.0001] (Fig. 3 B). However, treatment with API 12.5 mg/kg/day significantly increased GSH levels in the mice brains compared to the stressed [F(3,8) = 43.88, P = 0.0240], and also administering API 25 mg/kg/day significantly increased GSH levels in the mice brains compared to the stressed mice [F(3,8) = 43.88, P = 0.0005] (Fig. 3 B). In a similar manner, SOD levels were significantly decreased in the brains of the stressed mice relative to the control [F(3,8) = 17.54, P = 0.0005] (Fig. 3 C). However, treatment with API 12.5 mg/kg/day significantly increased SOD levels in the mice brains relative to the stressed group [F(3,8) = 17.54, P = 0.0066], and also administering API 25 mg/kg/day significantly increased SOD levels relative to the stress group [F(3,8) = 17.54, P = 0.0049] (Fig. 3 C).
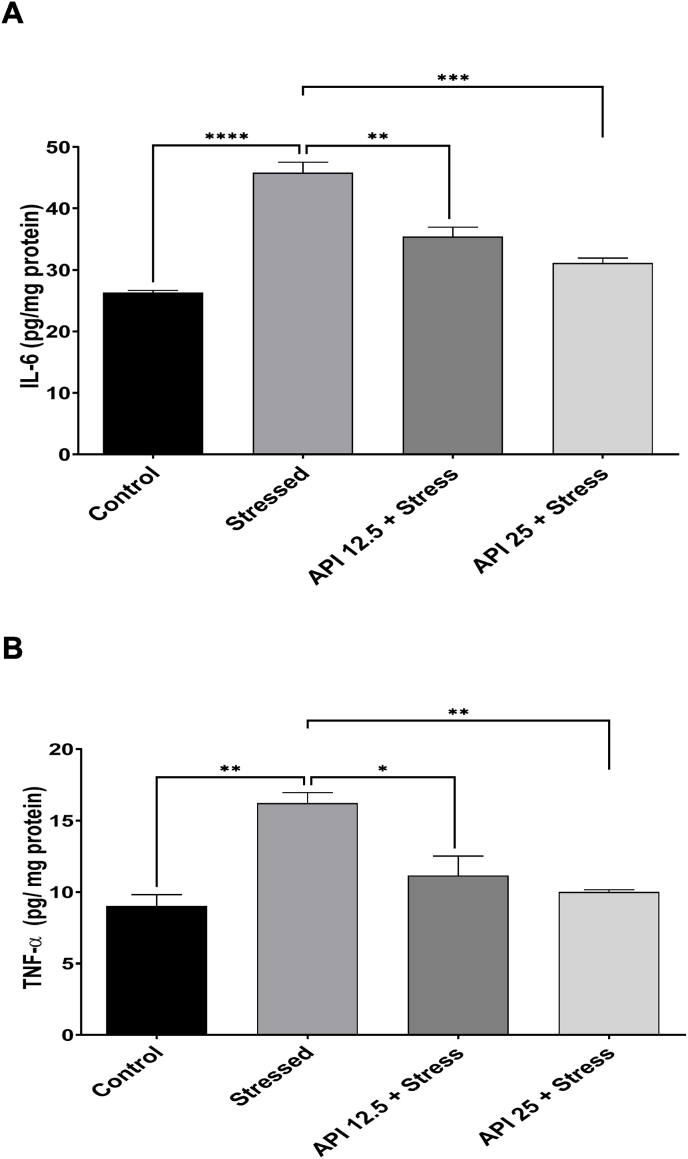
3.4. Apigenin attenuated IL-6 and TNF-α in the brains of CUMS-induced mice
Pathological changes in the brain that lead to neuropsychiatric disorders like depression, may be caused by an overproduction of pro-inflammatory cytokines such as TNF-α and IL-6 (Vaváková et al., 2015). In order to further investigate this correlation, we estimated the brain levels of IL-6 and TNF-α in the brains of the mice exposed to CUMS. The one-way ANOVA results show that the brain levels of IL-6 in the mice exposed to CUMS was significantly high in the stressed group compared to the control [F(3,8) = 44.02, P < 0.0001] (Fig. 4 A). However, treatment with API 12.5 mg/kg/day significantly decreased the elevated brain levels of IL-6 compared to the stressed group [F(3,8) = 44.02, P = 0.0017], and also administering API 25 mg/kg/day significantly decreased the elevated brain levels of IL-6 compared to the stressed group [F(3,8) = 44.02, P = 0.0002] (Fig. 4 A). Similarly, brain levels of TNF-α were evaluated and the one-way ANOVA results showed that the brain levels of TNF-α in mice exposed to CUMS was significantly increased in the stressed group compared to the control [F(3,8) = 13.04, P = 0.0019] (Fig. 4 B). However, treatment with API 12.5 mg/kg/day significantly decreased this high brain levels of TNF-α compared to the stressed group [F(3,8) = 13.04, P = 0.0156), and also significantly decreased high brain levels of TNF-α in mice administered 25 mg/kg/day [F(3,8) = 13.04, P = 0.0049] (Fig. 4 B).
Fig. 4.
Apigenin attenuated interleukin-6 and tumor necrosis factor-alpha in the brains of the CUMS-induced mice. (A) Apigenin attenuated interleukin-6 in the brains of the mice exposed to CUMS. (B) Apigenin attenuated tumor necrosis factor-alpha in the brains of the mice exposed to CUMS. (n = 10, **P < 0.01 and ****P < 0.0001 v control; *P < 0.05, **P < 0.01, ***P < 0.001 v stress). Data expressed as mean ± SD. One way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
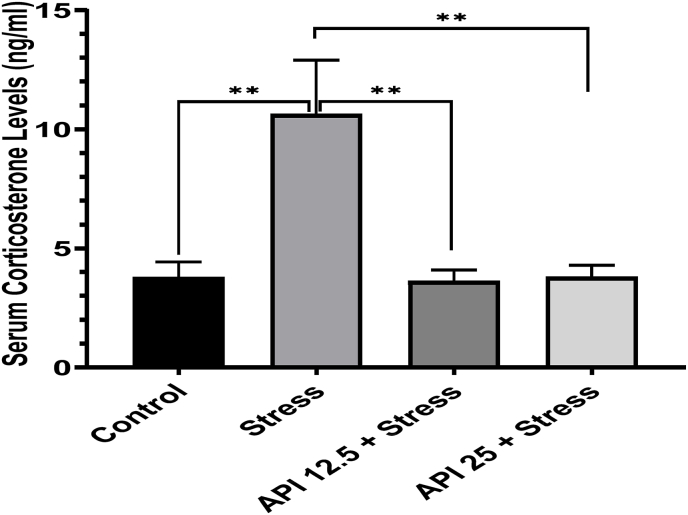
3.5. Apigenin downregulated corticosterone levels in the CUMS-induced mice
The hypothalamic–pituitary–adrenal (HPA) axis is a neuroendocrine system that regulates response to stress. In humans, a dysregulated HPA axis has been observed in depression; whereas, exposure to chronic corticosterone in rodent experiments has demonstrated depressive-like phenotype (Qin et al., 2019). Here, we evaluated serum corticosterone levels in the mice exposed to stress and observed an elevated corticosterone level in the stressed mice compared to the control [F(3,7) = 20.28, P = 0.0016] (Fig. 5). However, in the mice treated with API 12.5 mg/kg/day, the serum corticosterone level was significantly decreased relative to the control [F(3,7) = 20.28, P = 0.0014], and also significantly decreased corticosterone levels in mice treated with API 25 mg/kg/day [F3,7 = 20.28, P = 0.0031) relative to the control (Fig. 5).
Fig. 5.
Apigenin downregulated corticosterone levels in CUMS-induced mice. (n = 10, **P < 0.01 v control; **P < 0.01 v stress). Data expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
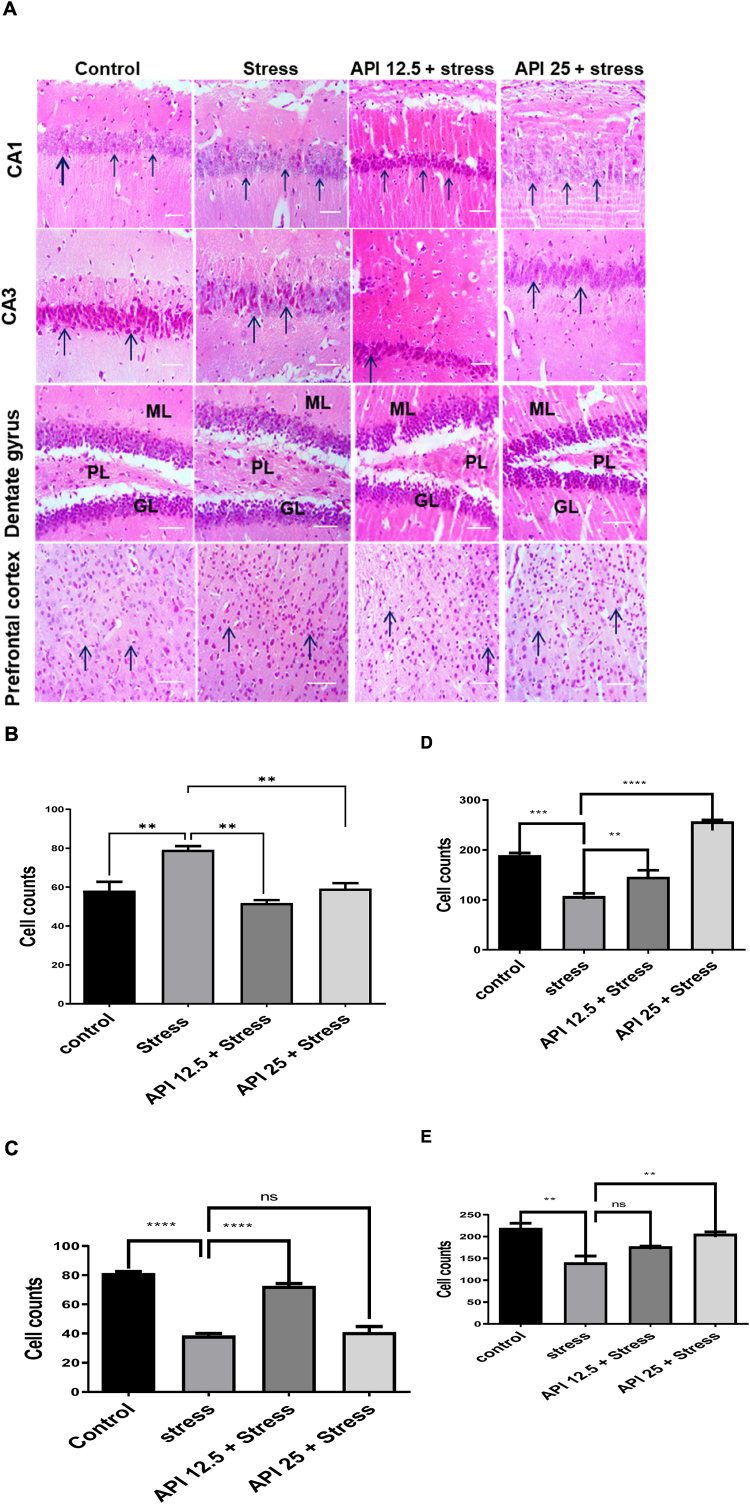
3.6. Apigenin reversed cell damage in the hippocampal brain regions and prefrontal cortex of CUMS-induced mice
Photomicrographs, demonstrating the effect of API on CUMS-induced histological changes in CA1, CA3, dentate gyrus of mice hippocampus and the prefrontal cortex, are shown in (Fig. 6 A). The control groups showed normal cell expressions of the CA1, CA3, dentate gyrus and the prefrontal cortex. The stressed group showed pyknotic and vacuolated cell expressions of the CA1, CA3 and dentate gyrus of the hippocampal regions, as well as the prefrontal cortex of the mice brains (Fig. 6 A). At a dose of API (12.5 mg/kg/day), normal cell expressions of the CA1, CA3 and dentate gyrus were observed, although few pyknotic and some normal distributions of cells were observed in the prefrontal cortex (Fig. 6 A). Recovery cells were observed in the CA1, dentate gyrus and the prefrontal cortex at a dose of API (25 mg/kg/day) while pyknotic cells were seen in the CA3 of the hippocampus at the same dose (Fig. 6 A).
Fig. 6.
Apigenin reversed cell damage in the hippocampal brain regions and prefrontal cortex of CUMS-induced mice. (A) Effect of apigenin on the expressions of healthy cells on the histoarchitecture of the CA1, CA3, dentate gyrus and the prefrontal cortex of CUMS-induced mice after staining with H and E. (B) The population of healthy cells in the CA1 of mice brains after pretreatment with apigenin in CUMS-induced mice (C) The population of healthy cells in the CA3 of mice brains after pretreatment with apigenin in CUMS-induced mice (D) Population of healthy cells in the dentate gyrus of mice brains after pretreatment with apigenin in CUMS-induced mice (E) The population of healthy cells in the prefrontal cortex of mice brains after pretreatment with apigenin in CUMS-induced mice. Scale bar = 320 μm (n = 10, **P < 0.01, ***P < 0.01 and ****P < 0.0001 v control; **P < 0.01, ****P < 0.0001 v stress). Data expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
Following the results obtained from counting the population of viable cells using ImageJ software, the stressed group in the CA1, CA3, dentate gyrus and the prefrontal cortex of the mice brains showed significant [F(3,8) = 14.47, P = 0.0061] (Fig. 6 B); [F(3,8) = 74.82, P < 0.0001] (Fig. 6 C); [F(3,8) = 64.60, P = 0.0004] (Fig. 6 D); [F(3,8) = 11.71, P = 0.0027] (Fig. 6 E) reductions respectively in the population of healthy cells relative to the control. However, the administration of API 12.5 mg/kg/day significantly [F(3,8) = 14.47, P = 0.0012] (Fig. 6 B); [F(3,8) = 74.82, P < 0.0001] (Fig. 6 C); [F(3,8) = 64.60, P = 0.0366] (Fig. 6 D) increased the population of healthy cells in the CA1, CA3 and dentate gyrus respectively relative to the stressed group. Observationally, administering API (12.5 mg/kg/day) to the stressed mice did not affect the population of healthy cells in the prefrontal cortex as an increase in the population of viable cells in the prefrontal cortex was observed at this dose, although this increase was not statistically significant [F(3,8) = 11.71, P = 0.1238] (Fig. 6 E). Furthermore, administering API at a dose of 25 mg/kg/day markedly [F(3,8) = 14.47, P = 0.0082] (Fig. 6 B); [F(3,8) = 64.60, P < 0.0001] (Fig. 6 D); [F(3,8) = 11.71, P = 0.0082] (Fig. 6 E) increased the population of healthy cells in the CA1, dentate gyrus and the prefrontal cortex respectively relative to the stressed group. However, API administration at this same dose (25 mg/kg/day) did not affect the population of healthy cells in the CA3, as there was no significant difference [F(3,8) = 74.82, P = 0.9107] (Fig. 6 C) between the population of healthy cells in this brain region compared to the stressed group. Although an increase in the population of viable cells in the CA3 (25 mg/kg/day) was observed. This increase was not statistically significant (Fig. 6 C).
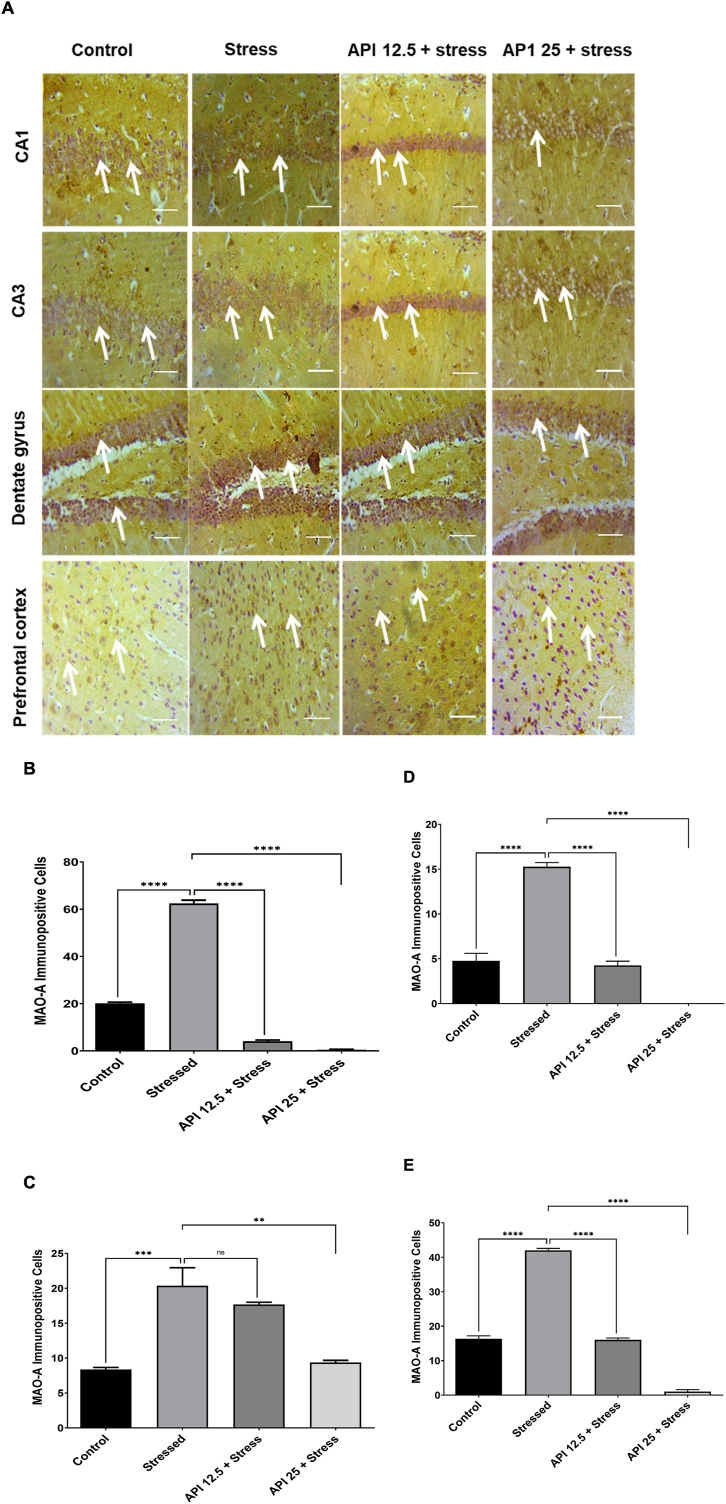
3.7. Apigenin inhibited monoamine oxidase a inhibitory enzymatic activity in the hippocampal brain regions and prefrontal cortex of CUMS-induced mice
Photomicrographs showing the inhibitory effect of API on MAOA inhibitory effect on the CA1, CA3, dentate gyrus and the prefrontal cortex of the brains of CUMS-induced mice are shown in (Fig. 7 A). MAOA immunoreactive positive cells are represented by a brownish coloured morphology. The control groups showed few expressions of MAOA immunoreactive positive cells in the CA1, CA3, dentate gyrus and prefrontal cortex of the mice brains (Fig. 7 A). The stressed group showed dense expressions of the MAOA immunoreactive positive cells in the CA1, CA3 and dentate gyrus of the hippocampal regions, including the prefrontal cortex of the mice brains (Fig. 7 A). At a dose of API (12.5 mg/kg/day), scanty expressions of MAOA immunoreactive positive cells in the CA1, CA3, dentate gyrus, as well as the prefrontal cortex of the mice brains, were observed (Fig. 7 A). More so, at a dose of API (25 mg/kg/day), very scanty expressions of MAOA immunoreactive positive cells in the CA1, CA3, dentate gyrus, as well as the prefrontal cortex of the mice brains, were seen (Fig. 7 A).
Fig. 7.
Apigenin inhibited monoamine oxidase A inhibitory enzymatic activity in the hippocampal brain regions and prefrontal cortex of CUMS-induced mice (A) Inhibitory effect of apigenin on the expressions of MAOA immunoreactive positive cells in the CA1, CA3, dentate gyrus and prefrontal cortex of CUMS-induced mice after immunohistochemistry (B) Inhibitory effect of apigenin on the density of MAOA immunoreactive positive cells in the CA1 of CUMS-induced mice (C) Inhibitory effect of apigenin on the density of MAOA immunoreactive positive cells in the CA3 of CUMS-induced mice (D) Inhibitory effect of apigenin on the density of MAOA immunoreactive positive cells in the dentate gyrus of CUMS-induced mice (E) Inhibitory effect of apigenin on the density of MAOA immunoreactive positive cells in the prefrontal cortex of CUMS-induced mice. Scale bar = 320 μm (n = 10, ***P < 0.001, ****P < 0.0001 v control; **P < 0.01, ****P < 0.0001 v stress). Data expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
The results obtained from counting the density of MAOA immunoreactive positive cells using ImageJ software showed that the cells in the stressed group was significantly increased [F(3,8) = 1119, P < 0.0001] (Fig. 7 B); [F(3,8) = 20.14, P = 0.0010] (Fig. 7 C); [F(3,12) = 141.7, P < 0.0001] (Fig. 7 D); [F(3,8) = 651.8, P < 0.0001] (Fig. 7 E) in the CA1, CA3, dentate gyrus and prefrontal cortex respectively, compared to the control. However, administration of API (12.5 mg/kg/day) significantly reduced [F(3,8) = 1119, P < 0.0001] (Fig. 7 B); [F(3,8) = 141.7, P < 0.0001] (Fig. 7 D); [F(3,8) = 651.8, P < 0.0001] (Fig. 7 E) the density of MAOA immunoreactive positive cells in the CA1, dentate gyrus, including the prefrontal cortex respectively relative to the CUMS group. Nevertheless, it was observed that administering API at a dose of 12.5 mg/kg/day to the stressed mice did not show any significant difference [F(3,8) = 20.14, P = 0.5252] (Fig. 7 B) in the density of MAOA immunoreactive positive cells in the CA3 sub region of the hippocampus compared to the stressed group. In addition, a slight decrease in MAOA immunoreactive positive cells was observed in the CA3 at this same dose, but this decrease was not statistically significant (Fig. 7 B). Again, administering API at a dose of 25 mg/kg/day markedly deceased [F(3,8) = 1119, P < 0.0001] (Fig. 7 B); [F(3,8) = 20.14, P < 0.0017] (Fig. 7 C); [F(3,12) = 141.7, P < 0.0001] (Fig. 7 D); [F(3,8) = 651.8, P < 0.0001] (Fig. 7 E) the density of MAOA immunoreactive positive cells in the CA1, CA3, dentate gyrus and prefrontal cortex respectively, relative to the stressed group.
3.8. Comparative binding mode of apigenin and its favorable binding affinity at MAOA binding cavity
Having shown the beneficial effects of API in attenuating CUMS-induced depression through the various experimental investigations we employed earlier, we proceeded to delineate the mechanistic effect of MAOA inhibition of API in comparison with a reversible inhibitor, clorgyline. The protein-inhibitor complex structures of MAOA with API and clorgyline were predicted by performing molecular docking, using AutoDock Vina integrated UCSF Chimera (Pettersen et al., 2004; Trott and Olson, 2010) Molecular docking technique is widely used in the drug design process as a predictive tool in binding affinity, binding mode, and binding orientation as well as for target fishing and profiling (Tanwar et al., 2017; Liu et al., 2018).
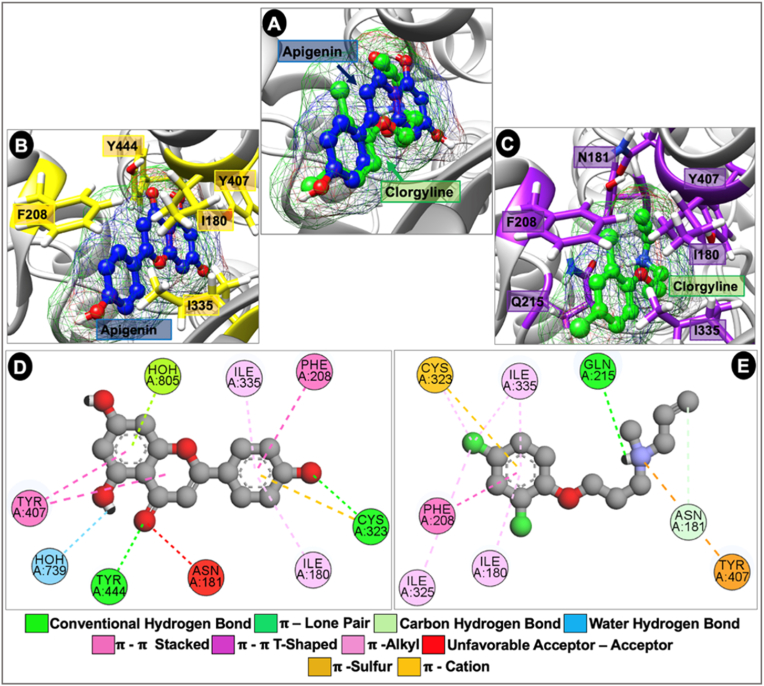
As depicted in Fig. 8 A, API occupies similar region and assumes similar binding orientation to clorgyline in the binding cavity of the MAOA receptor although with deeper reaching and reactive functional groups. Binding cavity hydrophobic residues such as I180, F208, C323, I335 and Y407 allowed for important intermolecular interactions with both API and clorgyline (Fig. 8 B. C). Further observations of the interactions of API with MAOA receptor revealed that the compound made important water hydrogen bond interactions with binding cavity water molecules unlike clorgyline and showed propensity for more favorable interactions than observed in the clorgyline-bound complex. Hydrogen bond interactions believed to be important in molecular recognition of the compounds were made through interactions with C323 and Y444 for API and Q215 for clorgyline (Fig. 8 D. E). Other important intermolecular interactions contributing to stability of API at the binding cavity include π-π T-shaped interaction with F208, π-π Stacked with Y407, π-lone pair and π-alkyl interactions with I180 and 1335. Taken together, possibly through better shape complementarity at the binding site, API showed higher binding affinity (-7.70 kcal/mol) to the MAOA receptor than the reversible inhibitor, clorgyline (-6.80 kcal/mol).
Fig. 8.
Computational modeling experiment to predict binding sites of apigenin and clorgyline on monoamine oxidase A enzyme. (A) Depicts the binding mode of investigational API (blue) and reference drug, clorgyline (green) in the binding cavity of MAO A receptor sequel to molecular docking. Both compounds are shown as ball and stick surface mesh representations. (B & C) depicts important binding site residues for API-bound (yellow) and clorgyline-bound (purple) complexes. (D & C) shows important intermolecular interactions in the API-bound (D) clorgyline-bound (E) and complexes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Exposure to different forms of stressors for a long time can negatively impact the brain and might be a factor that predisposes some persons to depression, as well as affect the brain levels of MAOA (Grunewald et al., 2012). This study has been able to show the probable mechanisms of action of API in CUMS-induced depression in mice. The study has shown that treating the animals with API reversed anhedonia, reduced anxiety and immobility time in behavioral studies; reduced brain corticosterone and MDA levels but increased brain levels of glutathione and superoxide dismutase. Additionally, IL-6 and TNF-α were attenuated by API. There was restoration of lost cells and inhibition of the activity of MAOA in the hippocampal brain regions and prefrontal cortex. Molecular docking studies showed that comparative binding affinity of API for MAOA was greater than that of reference compound, clorgyline.
It is quite interesting to understand the mechanisms underlying how API affects stress-induced depression. Of particular interest is that of MAOA enzyme because of its biochemical connection with stress and depression (Morilak and Frazer, 2004), coupled with the knowledge that the present remedies for depression target the monoamine system (D Rosenblat et al., 2015). We discovered in an earlier study that API inhibited MAOA in an in vitro study (Chaurasiya et al., 2014), including demonstrating some preliminary antidepressant-like effects in mice (Nakazawa et al., 2003; Yi et al., 2008). However, the in vivo inhibitory effect of API on MAOA and how it affects depression is still largely unknown. Therefore, it becomes important to further explore the possible mechanistic effects of API in CUMS-induced depression in mice. We also provided mechanistic basis by investigating the binding mode of API in comparison to a reference compound (clorgyline) in the binding cavity of MAOA. We, therefore, suggested that API is an inhibitor of MAOA with the potential of attenuating CUMS-induced depression via the inhibition of MAOA enzyme activity, attenuating the oxido-inflammatory pathways, and neuroendocrine signaling pathway in mice.
Findings from the study revealed that CUMS induction had a weight reducing effect on the mice while chronic administration of API significantly improved the weights of these animals. This loss of body weight in the mice could be related to the intensity of the stressors applied over a long time (Retana-Marquez et al., 2003).
Stress is very pivotal to the development of several psychiatric disorders, such as depressive disorders and anxiety disorders (Higuchi et al., 2017). The CUMS paradigm was used in this study to induce chronic depressive-like behavior in the animals. Anhedonia, a main symptom in mood disorder that is present in most psychiatric disorders and is associated with reduced interest in events known to be pleasurable to an individual (Diagnostic, 2013), was assessed in the mice by utilizing the SST. It was observed that the chronically stressed mice lost interest in grooming, which was very evident in the reduced grooming time recorded during the SST test. This actually reflects anhedonic-like condition. Meanwhile, it is good to note that the unpredictable nature of the stressors in the CUMS paradigm may have predisposed the animals to depression, resulting in anhedonic condition. This is because the CUMS paradigm mimics a chronic depressive-like state in response to stress in mice (Duman, 2010) and also reduces the likelihood of the mice to cope with a repeated stressor (Aguilera, 1998; Tannenbaum et al., 2002). More so, anhedonia in the CUMS model responds to chronic treatment with most antidepressant drugs (Papp et al., 1996; Krishnan et al., 2007). Similarly, the mice responded to anhedonia after API administration in this study, as treatment with API significantly increased grooming time in the mice.
Findings from the EPM test revealed that chronically stressed mice showed more preference for the closed arm of the maze over the open arm, while the API-treated mice demonstrated high preference for the open arm of the EPM. The EPM is a well-established model that assesses anxiety-like behavior by recording the number of entries in the open arms (Bild and Ciobica, 2013). A sign of insecurity and anxiety behavior is revealed when an animal prefers to enter the closed arms (Bourin, 2022).
This study has also demonstrated that immobility time was significantly increased in chronically stressed mice, in both the FST and TST. However, repeated treatment with API reduced immobility time in mice in both tests. The changes in an animal's activity and mood after being exposed to FST was described by Porsolt et al. as “behavioral despair” (Porsolt et al., 1979). Furthermore, Porsolt hypothesized that the immobile position in the mice during these tests reflects a state of lowered mood or hopelessness and predicted that the duration of this immobile position could be lowered by antidepressants (Porsolt et al., 1978). Due to their predictive validity, these behavioral screening tests are selectively sensitive to treatments that affect depression (Porsolt et al., 1978), and are also selective for monoamine-based mechanisms (Lucki, 1997; Weiss and Kilts, 1998). In this study, animals in the stressed group demonstrated this state of hopelessness and despair, which are manifestations of a depressed state but API was able to attenuate this condition. Therefore, our results have been able to demonstrate that API can reverse depressive-like behavior, and also suggest that API might be a potential antidepressant.
Although factors contributing to the development of depression are still unclear to a large extent, research has indicated that stressful situation causes an increase in the secretion of ROS (Juszczyk et al., 2021), and this could alter redox signaling and control, leading to biomolecular damage (Wojsiat et al., 2018). Thus, oxidative stress, a process associated with an imbalance between antioxidant defense mechanisms and free radical production (Betteridge, 2000) has been confirmed to occur in some brain structures that participate in the development of depression due to exposure to chronic stress (Sies et al., 2017). Moreover, a growing body of evidence has shown the association between increased levels of biomarkers of oxidative stress and development of depression (Milne et al., 2005; Black et al., 2015; Gold et al., 2015). The MDA-an important marker of cell damage caused by oxidative stress (Maes et al., 2011; Scapagnini et al., 2012) represents an end product of polyunsaturated fatty acids and amino acid peroxidation, thus reducing the body's antioxidant defense system (Juszczyk et al., 2021). It is known that an elevated amount of proinflammatory cytokines causes lipid peroxidation, which forms free radicals, including an increased breakdown of monoamines (Vaváková et al., 2015). A recent report has shown that elevated ROS activity increased the activity of prooxidant enzymes, as well as xanthine oxidase and MAO (Juszczyk et al., 2021). Furthermore, studies have shown that depression can alter the activity of oxidative stress-related enzymes most of which are increased in depressed patients (Maes et al., 2011; Scapagnini et al., 2012). The role of ROS in the pathological mechanism of depression has been associated with hyperactivity of the HPA-axis, and this stress-induced elevation of blood cortisol levels increases glucose metabolism and overproduction of ROS (Maes et al., 2011). In contrast, it is noteworthy that enzymatic endogenous antioxidants, such as SOD, catalase and the non-enzymatic antioxidants like glutathione, melatonin (Aguilar et al., 2016), have proven to be useful in preventing the destructive effect of free radicals on cells. Accordingly, natural products like API have demonstrated excellent antioxidant activities in previous studies (Romanova et al., 2001; Singh et al., 2004; Jung, 2014). Similarly, results obtained from this study have shown that API was able to attenuate lipid peroxidation by reducing the MDA levels, as well as increasing the brain levels of SOD and GSH in mice brains exposed to chronic stress. This suggests that API might be acting through the oxidative stress pathway to prevent depression, making it a potentially useful drug in ameliorating the destructive activities of free radicals, and also in attenuating depressive-like behaviors.
The impact, which chronic stress has on proinflammatory cytokines, has been demonstrated in several studies (Raison et al., 2006; Marketon and Glaser, 2008). These studies show that proinflammatory cytokines are overproduced during stressful situations (Raison et al., 2006; Marketon and Glaser, 2008). Moreover, when proinflammatory cytokines activate the corticotropin releasing hormone (CRH), this causes an upregulation of adrenocorticotropic hormone (ACTH) and cortisol. Most studies have proposed that overexpression of CRH is the key link between chronic stress and depression (Owens and Nemeroff, 1991). Meanwhile, depression has been reported to be correlated with increased production of proinflammatory cytokines, such that this increase may lead to pathological alterations in the brain that may result in depression and depressive states (Maes, 2011; Leonard and Maes, 2012). Inflammatory reaction in depressed and anxiety patients were caused by raised levels of pro-inflammatory cytokines like TNFα, IL-1α, IL-1β, IL-4, IL-5, IL-6 (Salim et al., 2012; Vaváková et al., 2015). Therefore, inhibiting these proinflammatory cytokines may improve the effectiveness of antidepressants and the state of depressed patients (Scapagnini et al., 2012; Maes et al., 2012). This study has been able to show that chronic stress altered the levels of proinflammatory cytokines (IL-6 and TNF-α) in mice brains which may have led to the manifestation of depressive-like behaviors observed in the animals. However, the administration of API reduced these high levels of cytokines in their brains, suggesting that API might be preventing depression through the inflammatory pathway, and making it a potential antidepressant that might be explored to prevent or delay depressive conditions.
The response to stress is mainly controlled by the HPA axis. Hyperactivity of HPA axis and MAOA are notable stress responses (Ruiz et al., 2018). During stressful situations, the HPA axis is activated by neuropeptides, corticotropin releasing factor, and arginine-vasopressin which leads to the synthesis and release of ACTH and, in turn, stimulates the release of glucocorticoids (cortisol in humans and corticosterone in rodents) from the adrenal gland (Ruiz et al., 2018). Activated HPA axis has a strong impact on the brain. It controls neuronal survival, neurogenesis, hippocampal size, acquiring new memories, and in affective states (Herbert et al., 2006). Considering the role of the HPA axis in controlling stress and brain functioning, it is reasonable to state that the HPA axis may become dysfunctional in neuropsychiatric disorders like major depression. Accordingly, increased levels of cortisol have been discovered in the saliva, plasma and urine of depressed patients (Nemeroff and Vale, 2005). By exposing animals to unpredictable stressors, the levels of glucocorticoids could be elevated which, in turn, could reduce body weight gain and elevate corticosterone levels (Herman et al., 1995; Cullinan and Wolfe, 2000). These changes in the HPA axis, coupled with the high circulating levels of cortisol, explain many of the characteristics of depression. A number of antidepressants alter the HPA axis (Mason and Pariante, 2006; Thomson and Craighead, 2008; Surget et al., 2011). In this study, we evaluated brain corticosterone levels in mice, and found a significant increase in corticosterone in the mice brains exposed to chronic stress, which supports previous observations on the HPA axis (Nemeroff and Vale, 2005). However, administering API to the experimental animals reduced the elevated corticosterone levels significantly, showing that API could have some promising effect in stress-induced depression, and that it could also be acting through the HPA axis in bringing about this effect.
Chronic stress plays a pivotal role in the development of abnormalities in the hippocampus and prefrontal cortex. Several neural pathways have been associated with the development of depression. The hippocampus and prefrontal cortex have been most implicated in the pathology and progress of the disease (McKinnon et al., 2009; Treadway et al., 2015). Chronic stress has been shown to cause volumetric reductions in the hippocampus and prefrontal cortex (Wise et al., 2017; Belleau et al., 2019). Studies have revealed that exposure to chronic stress can trigger some neurotoxic processes such as dysregulated HPA axis, inflammation, oxidative stress, and neurotransmitter alterations that interact and may lead to the development of major depression which, in turn, is associated with significant volumetric reductions in the hippocampus and prefrontal cortex (Belleau et al., 2019; Saaltink and Vreugdenhil, 2014; Slavich and Irwin, 2014; Filipović et al., 2017; Holmes et al., 2018; Cole et al., 2011). However, notable increases in the volumes of the hippocampus and prefrontal cortex have been observed (Cole et al., 2011; Kempton et al., 2011) when antidepressants were administered to patients with major depressive and anxiety disorders (van Tol et al., 2010). Similarly, the present study has demonstrated that chronic stress caused cell damage in the hippocampus and the prefrontal cortex of mice brains, as well as reduced the population of viable cells found in these brain regions. In addition, we observed that chronic stress triggered a dysregulated HPA axis, inflammation and oxidative stress in the mice brains which might have triggered cell damage in the hippocampal regions and prefrontal cortex. But, after administering API to the experimental mice, promising changes were observed. API was able to reverse cell damage and also increase the density of viable cells in the brains of chronically stressed mice, particularly in the hippocampus and prefrontal cortex.
Meyer and colleagues discovered high density of MAOA in different parts of the brain (prefrontal cortex, hippocampus, and midbrain) of depressed patients (Meyer et al., 2006, 2009). They also reported a link between the concentrations of MAOA in the anterior cingulate cortex and the prefrontal cortex, and how this link is associated with the recurrence of symptoms of depression (Meyer et al., 2009). MAO inhibitors have been noted for their ability to inhibit MAO activity, and Sacher and Wilson (Sacher et al., 2010) had reported that MAOA inhibitors reduced MAOA density in different parts of the brain, such as the anterior cingulate cortex, the prefrontal cortex, hippocampus, midbrain and others. Accordingly, the current study has discovered high density of MAOA in the hippocampal brain regions (CA1, CA3, dentate gyrus), and the prefrontal cortex of chronically stressed mice. However, through the administration of API, this high density of MAOA was attenuated in stressed mice. However, at 25 mg/kg/day dose of API, this effect on MAOA inhibitory activity was not quite significant in the CA3 brain region of the hippocampus. Therefore, it is very reasonable to state that API might be acting in similar manner like other MAO inhibitors in attenuating MAOA activity in these parts of the brain in depressive conditions. This further indicates that the mechanism could be through the monoamine oxidase pathway.
Results obtained from the mechanistic effect of MAOA inhibition of API in comparison with a reversible inhibitor, clorgyline, showed that API possesses better inhibitory capacity against MAOA than clorgyline and it achieves this by assuming better shape complementarity at the receptor binding site and engaging in important intermolecular interactions through its aromatic rings with the binding cavity residues such as I180, F208, C323, I335, Y407 and Y444. Important interactions pertinent to its inhibitory activity include hydrogen bond formation with binding cavity residues, as well as water hydrogen bond formation with binding cavity water molecules. Other hydrophobic interactions include π-Sulfur, π-π Stacked, π-π T-shaped, π-lone pair and π-alkyl interaction making its binding affinity and thus its inhibitory capacity to be relatively higher than observed in clorgyline.
5. Conclusions
Although MAOA inhibitors are less commonly used in the management of depression due to their side effects, MAOA is still utilized as a biomarker in human brain imaging studies of psychiatric diseases, as well as depression. This study has been able to show that API is a reversible inhibitor of MAOA, and that it might be attenuating CUMS-induced depression through the inhibition of MAOA enzyme activity, reducing oxidative stress, inflammation and neuroendocrine signaling pathways in mice. More so, these novel pharmacological targets discovered for API in this study might be helpful in designing treatment strategies for the future.
CRediT authorship contribution statement
Juliet N. Olayinka: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization, Project administration. Oluwole B. Akawa: Writing – review & editing, Formal analysis, Data curation. Emmanuela K. Ogbu: Investigation, Resources, Project administration. Anthony T. Eduviere: Visualization, Resources, Writing – review & editing. Raymond I. Ozolua: Writing – review & editing, Methodology, Resources. Mahmoud Soliman: Writing – review & editing, Resources, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the efforts of Mr Fafure Damola who assited in conducting the histology and the immunohistochemistry.
Contributor Information
Juliet N. Olayinka, Email: julietolayinka@abuad.edu.ng.
Oluwole B. Akawa, Email: akawaob@abuad.edu.ng.
Emmanuela K. Ogbu, Email: ogbuemmanuella456@gmail.com.
Anthony T. Eduviere, Email: taeduviere@delsu.edu.ng.
Raymond I. Ozolua, Email: ozolua@uniben.edu.
Mahmoud Soliman, Email: soliman@ukzn.ac.zn.
Data availability
Data will be made available on request.
References
- Aguilar T.A.F., Navarro B.C.H., Pérez J.A.M. Endogenous antioxidants: a review of their role in oxidative stress. A master Regul. oxidative Stress. 2016:3–20. Transcr. factor nrf2. [Google Scholar]
- Aguilera G. Corticotropin releasing hormone, receptor regulation and the stress response. Trends Endocrinol. Metabol. 1998;9:329–336. doi: 10.1016/s1043-2760(98)00079-4. [DOI] [PubMed] [Google Scholar]
- Belleau E.L., Treadway M.T., Pizzagalli D.A. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol. Psychiatr. 2019;85:443–453. doi: 10.1016/j.biopsych.2018.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge D.J. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Bild W., Ciobica A. Angiotensin-(1–7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J. Affect. Disord. 2013;145:165–171. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Black C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W.J.H. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Bolton J.L., Molet J., Regev L., Chen Y., Rismanchi N., Haddad E., Yang D.Z., Obenaus A., Baram T.Z. Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol. Psychiatr. 2018;83:137–147. doi: 10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin. Neurosci. 2022;17:295–303. doi: 10.31887/DCNS.2015.17.3/mbourin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Zhao X.-L., Liu A.-W., Nian H., Zhang S.-H. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18:366–373. doi: 10.1016/j.phymed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Chaurasiya N.D., Ibrahim M.A., Muhammad I., Walker L.A., Tekwani B.L. Monoamine oxidase inhibitory constituents of propolis: kinetics and mechanism of inhibition of recombinant human MAO-A and MAO-B. Molecules. 2014;19:18936–18952. doi: 10.3390/molecules191118936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J., Costafreda S.G., McGuffin P., Fu C.H.Y. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Cullinan W.E., Wolfe T.J. Chronic stress regulates levels of mRNA transcripts encoding β subunits of the GABAA receptor in the rat stress axis. Brain Res. 2000;887:118–124. doi: 10.1016/s0006-8993(00)03000-6. [DOI] [PubMed] [Google Scholar]
- D Rosenblat J., S McIntyre R., S Alves G., N Fountoulakis K., F Carvalho A. Beyond monoamines-novel targets for treatment-resistant depression: a comprehensive review. Curr. Neuropharmacol. 2015;13:636–655. doi: 10.2174/1570159X13666150630175044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.J., Samuels B.A., Rainer Q., Wang J.-W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.-P. Neurogenesis-dependent and-independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J., Keshavan M. The neurobiology of depression: an integrated view. Asian J. Psychiatr. 2017;27:101–111. doi: 10.1016/j.ajp.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Diagnostic A.P.A. 2013. Statistical Manual of Mental Health Disorders. DSM-5. 5. [Google Scholar]
- Duman C.H. Models of depression. Vitam. Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Etukudo I. Lippincott Williams & Wilkins; 2003. Ethnobotany. Conventional and Traditional Uses of Plants. [Google Scholar]
- Feinstein W.P., Brylinski M. Calculating an optimal box size for ligand docking and virtual screening against experimental and predicted binding pockets. J. Cheminf. 2015;7 doi: 10.1186/s13321-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović D., Todorović N., Bernardi R.E., Gass P. Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive-and anxiety-like symptoms. Brain Struct. Funct. 2017;222:1–20. doi: 10.1007/s00429-016-1218-9. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M., Nakamura K., Tago K., Mashino T., Kasahara T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharm. 2011;11:1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015 doi: 10.1155/2015/581976. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M., Johnson S., Lu D., Wang Z., Lomberk G., Albert P.R., Stockmeier C.A., Meyer J.H., Urrutia R., Miczek K.A. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanwell M.D., Curtis D.E., Lonie D.C., Vandermeersch T., Zurek E., Hutchison G.R. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminf. 2012;4:1–17. doi: 10.1016/j.aim.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J., Goodyer I.M., Grossman A.B., Hastings M.H., De Kloet E.R., Lightman S.L., Lupien S.J., Roozendaal B., Seckl J.R. Do corticosteroids damage the brain? J. Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Adams D., Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Soga T., Parhar I.S. Regulatory pathways of monoamine oxidase A during social stress. Front. Neurosci. 2017;11:604. doi: 10.3389/fnins.2017.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S.E., Hinz R., Conen S., Gregory C.J., Matthews J.C., Anton-Rodriguez J.M., Gerhard A., Talbot P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatr. 2018;83:61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Hovatta I., Juhila J., Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010;68:261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Jeong G.-S., Lee S.-H., Jeong S.-N., Kim Y.-C., Kim E.-C. Anti-inflammatory effects of apigenin on nicotine-and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int. Immunopharm. 2009;9:1374–1380. doi: 10.1016/j.intimp.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Jiang P., Zhang W.-Y., Cai H.-L., Liu Y.-P., Chen L.-Y. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38:2091–2098. doi: 10.1016/j.psyneuen.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Johnson S., Stockmeier C.A., Meyer J.H., Austin M.C., Albert P.R., Wang J., May W.L., Rajkowska G., Overholser J.C., Jurjus G., Dieter L., Johnson C., Sittman D.B., Ou X.-M. The reduction of R1, a novel repressor protein for monoamine oxidase A, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Joormann J., Stanton C.H. Examining emotion regulation in depression: a review and future directions. Behav. Res. Ther. 2016;86:35–49. doi: 10.1016/j.brat.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Jung W.-W. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int. J. Mol. Med. 2014;33:1327–1334. doi: 10.3892/ijmm.2014.1666. [DOI] [PubMed] [Google Scholar]
- Juszczyk G., Mikulska J., Kasperek K., Pietrzak D., Mrozek W., Herbet M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer's disease: the role of antioxidants in prevention and treatment. Antioxidants. 2021;10:1439. doi: 10.3390/antiox10091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R.J., Roth K.A., Carroll B.J. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci. Biobehav. Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Salvador Z., Munafò M.R., Geddes J.R., Simmons A., Frangou S., Williams S.C.R. Structural neuroimaging studies in major depressive disorder: meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatr. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kim Sunghwan, Chen Jie, Cheng Tiejun, Gindulyte Asta, Jia He S.H., Li Qingliang, Shoemaker Benjamin A., Thiessen Paul A., Bo Yu L.Z., Bolton J.Z., E E. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47:D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar M.S., Grieve S.M., Etkin A., Koslow S.H., Williams L.M. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–871. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Han M.-H., Graham D.L., Berton O., Renthal W., Russo S.J., LaPlant Q., Graham A., Lutter M., Lagace D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Mbwambo Z.H., Chung H., Luyengi L., Gamez E.J., Mehta R.G., Kinghorn A.D., Pezzuto J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Leonard B., Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Li Z., Hu X., Wang Y., Fang J. Apigenin inhibits proliferation of ovarian cancer A2780 cells through Id1. FEBS Lett. 2009;583:1999–2003. doi: 10.1016/j.febslet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Li R., Zhao D., Qu R., Fu Q., Ma S. The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci. Lett. 2015;594:17–22. doi: 10.1016/j.neulet.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu S., Yosief H.O., Dai L., Huang H., Dhawan G., Zhang X., Muthengi A.M., Roberts J., Buckley D.L., Perry J.A., Wu L., Bradner J.E., Qi J., Zhang W. Structure-guided design and development of potent and selective dual bromodomain 4 (BRD4)/Polo-like kinase 1 (PLK1) inhibitors. J. Med. Chem. 2018;61:7785–7795. doi: 10.1021/acs.jmedchem.8b00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997 doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maes M. An intriguing and hitherto unexplained co-occurrence: depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2011;35:784–794. doi: 10.1016/j.pnpbp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Maes M., Fišar Z., Medina M., Scapagnini G., Nowak G., Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates—nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- Marketon J.I.W., Glaser R. Stress hormones and immune function. Cell. Immunol. 2008;252:16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Mason B.L., Pariante C.M. The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News Perspect. 2006;19:603–608. doi: 10.1358/dnp.2006.19.10.1068007. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Liotti M., Brannan S.K., McGinnis S., Mahurin R.K., Jerabek P.A., Silva J.A., Tekell J.L., Martin C.C., Lancaster J.L. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatr. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKenna M.T., Michaud C.M., Murray C.J.L., Marks J.S. Assessing the burden of disease in the United States using disability-adjusted life years. Am. J. Prev. Med. 2005;28:415–423. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- McKinnon M.C., Yucel K., Nazarov A., MacQueen G.M. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Meyer J.H., Ginovart N., Boovariwala A., Sagrati S., Hussey D., Garcia A., Young T., Praschak-Rieder N., Wilson A.A., Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatr. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Meyer J.H., Wilson A.A., Sagrati S., Miler L., Rusjan P., Bloomfield P.M., Clark M., Sacher J., Voineskos A.N., Houle S. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Psychiatr. 2009;66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- Milne G.L., Musiek E.S., Morrow J.D. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10:10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Morilak D.A., Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Müller N. Immunology of major depression. Neuroimmunomodulation. 2014;21:123–130. doi: 10.1159/000356540. [DOI] [PubMed] [Google Scholar]
- Nagababu E., Rifkind J.M., Boindala S., Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 2010;610:165–180. doi: 10.1007/978-1-60327-029-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Yasuda T., Ueda J., Ohsawa K. Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003;26:474–480. doi: 10.1248/bpb.26.474. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Vale W.W. The neurobiology of depression: inroads to treatment and new drug discovery. J. Clin. Psychiatry. 2005;66:5. [PubMed] [Google Scholar]
- Owens M.J., Nemeroff C.B. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- Ozcan M.E., Gulec M., Ozerol E., Polat R., Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int. Clin. Psychopharmacol. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Papp M., Moryl E., Willner P. Pharmacological validation of the chronic mild stress model of depression. Eur. J. Pharmacol. 1996;296:129–136. doi: 10.1016/0014-2999(95)00697-4. [DOI] [PubMed] [Google Scholar]
- Park C., Rosenblat J.D., Lee Y., Pan Z., Cao B., Iacobucci M., McIntyre R.S. The neural systems of emotion regulation and abnormalities in major depressive disorder. Behav. Brain Res. 2019;367:181–188. doi: 10.1016/j.bbr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Pettersen Eric F., Goddard Thomas D., Conrad C., Huang, Couch Gregory S., Greenblatt Daniel M., Elaine C., Meng T.E.F. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pitsillou E., Bresnehan S.M., Kagarakis E.A., Wijoyo S.J., Liang J., Hung A., Karagiannis T.C. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol. Biol. Rep. 2020;47:753–770. doi: 10.1007/s11033-019-05129-3. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D. Antidepressants: Neurochemical, Behavioural and Clinical Perspectives. Raven Press; 1981. Behavioural despair. [Google Scholar]
- Porsolt R.D., Anton G., Blavet N., J M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Bertin A., Blavet N., Deniel M., Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur. J. Pharmacol. 1979;57:201–210. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- Qin D., Li Zhifei, Li Zhaoxia, Wang L., Hu Z., Lü L., Wang Z., Liu Y., Yin Y., Li Zhaofu. Chronic glucocorticoid exposure induces depression-like phenotype in rhesus macaque (Macaca Mulatta) Front. Neurosci. 2019;13:188. doi: 10.3389/fnins.2019.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retana-Marquez S., Bonilla-Jaime H., Vazquez-Palacios G., Dominguez-Salazar E., Martinez-Garcia R., Velazquez-Moctezuma J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology. 2003;28:207–227. doi: 10.1016/s0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Romanova D., Vachalkova A., Cipak L., Ovesna Z., Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48:104–107. [PubMed] [Google Scholar]
- Ruiz N.A.L., Del Ángel D.S., Olguín H.J., Silva M.L. Neuroprogression: the hidden mechanism of depression. Neuropsychiatric Dis. Treat. 2018;14:2837. doi: 10.2147/NDT.S177973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaltink D.-J., Vreugdenhil E. Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? Cell. Mol. Life Sci. 2014;71:2499–2515. doi: 10.1007/s00018-014-1568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J., Wilson A.A., Houle S., Rusjan P., Hassan S., Bloomfield P.M., Stewart D.E., Meyer J.H. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch. Gen. Psychiatr. 2010;67:468–474. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- Sadeghi M., Peeri M., Hosseini M.-J. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol. Behav. 2016;163:177–183. doi: 10.1016/j.physbeh.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Salim S., Chugh G., Asghar M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- Scapagnini G., Davinelli S., Drago F., De Lorenzo A., Oriani G. Antioxidants as antidepressants. CNS Drugs. 2012;26:477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. 2017 doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- Singh J.P.V., Selvendiran K., Banu S.M., Padmavathi R., Sakthisekaran D. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140:774. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Alkozei A., Killgore W.D.S., Lane R.D. Nested positive feedback loops in the maintenance of major depression: an integration and extension of previous models. Brain Behav. Immun. 2018;67:374–397. doi: 10.1016/j.bbi.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Son S.Y., Ma J., Kondou Y., Yoshimura M., Yamashita E., Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: the control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A., Tanti A., Leonardo E.D., Laugeray A., Rainer Q., Touma C., Palme R., Griebel G., Ibarguen-Vargas Y., Hen R. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatr. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum B., Tannenbaum G.S., Sudom K., Anisman H. Neurochemical and behavioral alterations elicited by a chronic intermittent stressor regimen: implications for allostatic load. Brain Res. 2002;953:82–92. doi: 10.1016/s0006-8993(02)03273-0. [DOI] [PubMed] [Google Scholar]
- Tanwar H., Sneha P., Thirumal Kumar D., Siva R., Walter C.E.J., George Priya Doss C. Advances in Protein Chemistry and Structural Biology. first ed. Elsevier Inc; 2017. A computational approach to identify the biophysical and structural aspects of methylenetetrahydrofolate reductase (MTHFR) mutations (A222V, E429A, and R594Q) leading to schizophrenia. [DOI] [PubMed] [Google Scholar]
- Thomson F., Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem. Res. 2008;33:691–707. doi: 10.1007/s11064-007-9518-3. [DOI] [PubMed] [Google Scholar]
- Thorpe L.W., Westlund K.N., Kochersperger L.M., Abell C.W., Denney R.M. Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J. Histochem. Cytochem. 1987;35:23–32. doi: 10.1177/35.1.3025289. [DOI] [PubMed] [Google Scholar]
- van Tol M.-J., van der Wee N.J.A., van den Heuvel O.A., Nielen M.M.A., Demenescu L.R., Aleman A., Renken R., van Buchem M.A., Zitman F.G., Veltman D.J. Regional brain volume in depression and anxiety disorders. Arch. Gen. Psychiatr. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Waskom M.L., Dillon D.G., Holmes A.J., Park M.T.M., Chakravarty M.M., Dutra S.J., Polli F.E., Iosifescu D.V., Fava M. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatr. 2015;77:285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. Autodock vina: improving the speed and accuracy of docking. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334.AutoDock. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaváková M., Ďuračková Z., Trebatická J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/898393. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.M., Kilts C.D. Animal models of depression and schizophrenia. Textb. Psychopharmacol. 1998:88–123. [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wise T., Radua J., Via E., Cardoner N., Abe O., Adams T.M., Amico F., Cheng Y., Cole J.H., de Azevedo Marques Perico C. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatr. 2017;22:1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojsiat J., Zoltowska K.M., Laskowska-Kaszub K., Wojda U. Oxidant/antioxidant imbalance in Alzheimer's disease: therapeutic and diagnostic prospects. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/6435861. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization World Health organization - WHO (2010) mental health. 2010. http://www.who.int/topics/mental_health/en [WWW Document]. IOP sPublishing PhysicsWeb. URL. accessed 9.19.17.
- Xu Y., Xin Y., Diao Y., Lu C., Fu J., Luo L., Yin Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0029169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L.-T., Li J.-M., Li Y.-C., Pan Y., Xu Q., Kong L.-D. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008;82:741–751. doi: 10.1016/j.lfs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Youdim M.B.H., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.