Abstract
The past decade has seen a revolution in our understanding of how the cellular environment is organized, where an incredible body of work has provided new insights into the role played by membraneless organelles. These rapid advancements have been made possible by an increasing awareness of the peculiar physical properties that give rise to such bodies and the complex biology that enables their function. Viral infections are not extraneous to this. Indeed, in host cells, viruses can harness existing membraneless compartments or, even, induce the formation of new ones. By hijacking the cellular machinery, these intracellular bodies can assist in the replication, assembly, and packaging of the viral genome as well as in the escape of the cellular immune response. Here, we provide a perspective on the fundamental polymer physics concepts that may help connect and interpret the different observed phenomena, ranging from the condensation of viral genomes to the phase separation of multicomponent solutions. We complement the discussion of the physical basis with a description of biophysical methods that can provide quantitative insights for testing and developing theoretical and computational models.
Keywords: biomolecular condensates, virus, condensation, phase separation
Introduction
Viruses exploit various cellular membrane-bound organelles in their life cycle as a means of viral entry, genome replication, assembly, and egress, as well as as a means of evading the host immune system.1–6 Often, these compartments are hijacked and undergo dynamic reorganization to aid viral proliferation.4–5 Efforts to understand the ability of viruses to utilize host organelles have led to the design of drugs that deliberately target these processes.7 During the past decade, it has been recognized that some viruses can also harness host-membraneless organelles and form new biomolecular condensates as a part of their life cycle, utilizing these compartments to mediate activities such as transcription, genome replication, and host immune system evasion. In normal contexts, biomolecular condensates often act as a molecular reservoir for specific proteins and RNA, while in aberrant contexts they can sequester key proteins needed for a specific cellular response.8 Sequestration could play a key role for viruses in the evasion of the cellular immune response by partitioning host cell immune-response factors into condensates that impede their function.9–11 Furthermore, understanding the permeability of viral condensates to immune response proteins such as pattern recognition receptors can offer novel ways of targeting viruses.12 The large constellation of phenomena emerging on the cellular level requires, on one hand, the adoption of a new set of concepts for describing and rationalizing experimental observations and, on the other hand, the development of new technologies for investigating mechanisms and testing existing models. Here, we provide a polymer physics perspective on the mechanisms controlling the condensation of proteins and nucleic acids, which can help to sort out and interpret some of the different phenotypes in biomolecular condensates related to viruses. We complement the theoretical description with a brief discussion of current enabling methodologies that provide access to fundamental observables to quantify and connect molecular interactions and mesoscopic observables, from singlemolecules to demixed solutions.
What is a Biomolecular Condensate?
According to the empirical definition introduced by Banani et al.13 and recently reprised by Mittag and Pappu,14 condensates are membraneless entities that concentrate a given set of components (e.g., proteins, nucleic acids, metabolites…) in nonstoichiometric complexes.15 While the term biomolecular condensate is often automatically linked to phase separation and liquid–liquid demixing, the observations of an increased concentration of components, in vitro or within the cell, does not necessarily imply a single mechanism of action or specific material properties.14 As such, the term “condensate” captures different emerging phenomena on the mesoscale (e.g, phase separation and percolation), which all give rise to condensation. As we will discuss below, part of the ambiguity is inherent to the fact that many of the underlying mechanisms are or may be interrelated. Identification of the specific mechanism at play requires a precise interrogation of the molecular properties of the object, with particular respect to the concentration and temperature dependence of its components, which are a common result of the thermodynamics of the system. Note that measurements of transport properties (as often quantified by recovery after photobleaching experiments) only highlight the kinetics within the object, which are indirectly linked to the equilibrium properties of the system and, as such, cannot prove a specific mechanism.14,16–17
A common question surrounding the formation of biomolecular condensates pertains to their ability to select for specific components and filter out others, controlling their overall internal composition and buffering the concentration of species outside. The role of viral components in biomolecular condensates brings this concept to the extreme, since the virus introduces extraneous viral components into the infected cell that may invade existing condensates or form new ones. Understanding the rules that control the mechanism of selection (or exclusion) of these components from the cellular environment can play an essential part in decoding how the virus succeeds in hijacking the cellular machinery.
Viruses and Biomolecular Condensates
Lacking their own complete replication machinery, viruses require the infection of a host cell to assist them in the replication of their genomic material. With few exceptions, the nature of the genome dictates the localization of replication, which for DNA-based viruses happens in the nucleus whereas for RNA-based viruses occurs in the cytosol. Viruses with a double-stranded DNA or RNA genome rely on host polymerase proteins or their own RNA-dependent RNA-polymerase (RdRp) to transcribe the viral genome. The same occurs for single-stranded negative-sense RNA genomes, which also requires the assistance of RdRps. In contrast, single-stranded positive-sense RNA viruses can directly harness the host cell ribosomes to translate viral protein components. Another essential step in the life cycle of the virus is the correct packaging of the viral genome after replication. Interestingly, this requires organization and often compaction of large nucleic acids into relatively small volumes.18 This can be further complicated, since some viral genomes consist of multiple independent nucleic acid molecules, which must be packaged within the same virion (“segmented”) or within distinct virions (“multipartite”).18–19
In the following sections, we summarize three notable cases that exemplify how viruses can harness biomolecular condensates for: i) viral genome replication, ii) hijacking the stress machinery of the cell, iii) packaging the viral genome.
Viral factories for genome replication
The Mononegavirales family are non-segmented single-stranded negative-sense RNA viruses, including rabies virus, Ebola virus, measles virus, mumps virus, and human respiratory syncytial virus.20 Infection of host cells with this family of viruses leads to the accumulation of cytoplasmic inclusion bodies within the cell. While some of these bodies have been known for a long time and even used as diagnostic markers (such as the Negri Bodies for rabies virus,21 only later was it noted that these compartments contained both viral RNAs and viral and host proteins.22–23 The presence of essential components for viral replication supports the idea that these inclusion bodies are bona fide “viral factories”. Among the components, there are the RdRp necessary for transcribing the viral genome and Nucleoproteins. Nucleoprotein coats and protects the viral genome and is among one of the highest expressed viral components after infection. The current hypothesis is that at low concentrations, the Nucleoproteins protect the genomic RNA and allows RdRp to transcribe subgenomic RNA; at sufficiently high concentrations, the Nucleoprotein promotes phase separation and, by stabilizing the polymerase, inhibits termination and favors synthesis of genome-long RNA.24 In other words, the phase separation of Nucleoproteins (also called antiterminator proteins) may play the key-role of enriching the protein concentration and enabling the switch between subgenomic and genome-long transcription of mRNA.
Viral factories have since been found in the host cell post infection for a plethora of viruses, including other single-stranded negative-sense RNA viruses (such as vesicular stomatitis virus) and double-stranded RNA viruses (such as rotaviruses).25–29 Indeed, as mentioned above, double-stranded RNA viruses face similar challenges as single-stranded negative RNA viruses, since they cannot directly interact with host cell ribosomes and require the intervention of a viral polymerase. Therefore, it is plausible that these biomolecular condensates may serve an analogous function.
Overall, the round shape of viral factories,28–30 their ability to fuse25,27–29,31 to deform against physical barriers,31 their response to osmotic stress,31 and their ability to exchange material with the surrounding milieu25,27–29,31 supports that these micron sized objects21–22,32 are likely the results of an intracellular phase separation. Interestingly, these inclusion bodies do not fuse with other stress granules found in the cell,31 suggesting that the viral components encode for precise compartmentalization of these “factories” from other condensates.
Hijacking stress granules
Viruses can also exploit existing intracellular condensates and their components, as in the case of stress granules. As implied by the name, stress granules regulate the translational machinery of the cell in response to various stress factors, harboring several ribonucleoprotein complexes (including arrested pre-initiation complexes), mRNAs, and related translational initiation factors.33–34 Therefore, it is not surprising to observe that their assembly and function is hijacked upon viral infection. For example, upon infection by mammalian orthoreovirus (double-stranded RNA virus), the host’s protein expression is shut-down and cellular mRNAs accumulate in stress granules, where they are maintained transcriptionally inactive.35 Interestingly, stress granules arise after viral disassembly (uncoating) inside the host cell, but independently from viral transcription.35 The accumulation of typical stress granule markers like G3BP1 and TIA-1 and the dose response to phosphorylation of eIF2α35 suggests that viral components trigger the assembly of these stress granules, repurposing their function. Indeed, when virus-induced stress granules are disassembled because they are no longer necessary to the virus, further formation of stress granules in the cell is inhibited, even under exposure of infected cells to stress factors such as arsenite.36
Similar observations are found also for single-stranded positive-sense RNA viruses such as the Semliki Forest virus,37 Polio virus,38–40 and Hepatitis C virus,41 though each exhibit different phenotypes regarding viral transcription and recruitment or exclusion of specific components.
Viral genome packaging
Packaging of the viral genome poses a key challenge for viruses, requiring the condensation of large nucleic acids or the combination of multiple segments. Various strategies have evolved to achieve this result and some intersect with the formation of and partitioning into biomolecular condensates. For example, the Influenza A Virus (single-stranded negative-sense RNA virus) has a segmented genome containing eight distinct elements that are unusually replicated in the host cell nucleus and then transported to the cytosol in the form of viral ribonucleoprotein complexes, each comprising a single segment of genome.42 These ribonucleoprotein complexes colocalize with intracellular foci43–45 that form near the endoplasmic reticulum exit sites and exhibit spherical shape, fusion and fission events, fast exchange of components with the surrounding cytosol and rapid response to environmental stimuli.44 While these biomolecular condensates do not appear to contribute to evasion of cellular innate antiviral response, they have been proposed to facilitate the organization of the different genome segments for proper assembly and export at the plasma membrane.44
Many proteins involved in viral packaging have been found to easily partition into membraneless organelles. After infection with the Measles virus (single-stranded negative-sense RNA virus), the nucleoprotein and phosphoprotein, which are responsible for packaging the viral genome, colocalize within puncta identified as transcription factories. In vitro experiments support that both the nucleoproteins and phosphoproteins can co-phase-separate in solution and, upon addition of RNA, facilitate the assembly of nucleocapsid-like particles at a significantly higher rate than the one observed in absence of phase separation.46 These observations pose an interesting question on which type of interactions facilitate the formation of nucleocapsid-like particles within the complex environment of a condensate.
Recent experiments on the SARS-CoV-2 nucleocapsid protein, which is responsible for coating and condensing the viral genome,18 also reported colocalization in cytosolic inclusions.47–48 While there is evidence that genomic transcription happens in membrane-bound compartments, the strong propensity for phase separation of nucleocapsid protein poses a question on how its recruitment to the correct nucleic acid is controlled and how phase separation of the viral genome in the cytoplasm is avoided. This does not necessarily imply that a phase separation mechanism should be at play; conversely it can be seen as the necessity of mechanisms in place to instead avoid or control the phase separation propensity of certain components.49
How viruses impart specificity to packaging their own genomic nucleic acids, while excluding host cell nucleic acids, and their own subgenomic nucleic acids has been a longstanding question. We will discuss some simple models below.
Further reading
While these are some notable cases, additional examples and further details on the functional role of biomolecular condensates upon viral infection can be found in the following recent reviews:.24,50–53
A Polymer Theory Framework
Understanding how different components can lead to the assembly of biomolecular condensates or alter the function of existing ones requires accounting for the mechanisms that can lead to solution demixing. Proteins and nucleic acids are biological polymers each composed of a specific set of monomers, their fundamental units (residues and nucleotides, respectively). Given their polymeric nature, the language of polymer physics offers a simple framework to explain the driving forces controlling solution demixing concepts. The framework we present here is through the lens of Flory-Huggins solution theory.54
Let’s start by considering the case of a homopolymer, such as a nucleic acid composed of one single type of nucleotide. While this is an obvious oversimplification of what happens in realistic protein and nucleic acid sequences, it is the simplest case scenario where we can define the key quantities of interest. Note that often homopolymer theories can be applied to capture important features of a heteropolymer sequence (like a “real” protein or nucleic acid) because the properties encoded in the heteropolymer averages out on sufficiently long distances.
What controls the dimensions of such a biomolecule in a solvent (for example, an aqueous buffer solution)? What causes a group of molecules to aggregate or segregate from others? It is reasonable to assume that the properties of a biomolecule in that solvent (dimensions, aggregation propensity, demixing, etc…) will be controlled by the set of interactions between the molecule and the solvent or between the molecule and another molecule. More precisely, the overall set of interactions controlling a polymeric molecule is encoded in each monomer. Therefore, in the case of a homopolymer, we can define specific contributions for the monomer-solvent interaction () and monomer–monomer interaction (). For comparison, it useful also to consider a parameter that accounts for solvent–solvent interactions (). The behavior of the homopolymer in the solution will be dictated by the balance across these interactions. Stronger monomer-solvent interactions than monomer–monomer interactions will favor the interaction of the homopolymer with the solvent; in the opposite case, with stronger monomer–monomer interactions than monomer-solvent, this will favor intrachain interactions. In polymer physics, there is a key-parameter that accounts for this balance and this is commonly referred as the interaction parameter:
| (1) |
where is the Boltzman constant and is the temperature.
This is the only equation within this review and up to this point, we have focused only on the contribution of interactions. However, one additional key factor is the homopolymer concentration.
Biopolymers in Dilute Conditions
When the concentration of homopolymers is so low that interactions between different molecules can be neglected, we can consider the polymer to be under dilute conditions. The only interactions at play are intramolecular contacts and defines whether the polymer chain undergoes compaction or expansion. When favorable solvent-monomer interactions dominate over monomer–monomer interactions, the polymer maximizes its interaction with the solvent by adopting expanded configurations. Because the solvent interactions are favorable, the solvent is referred to as “a good solvent”. One practical example of biopolymers in good solvent is given by the conformations of a single-stranded nucleic acid, which, in the absence of ligands, adopts expanded conformations. The negative net charge of the chain (unfavorable monomer–monomer interactions) and the favorable interactions between nucleotides and aqueous buffer solution lead to expanded configurations. A similar behavior is seen for highly charged disordered proteins (the net charge of the chain disfavors monomer–monomer interactions) and for proteins in high concentrations of denaturants (denaturing solvents favors the interactions between residues and solvent).55–59
Viceversa, when monomer–monomer interactions dominate, the polymer minimizes its exposure to the solvent by adopting compact configurations. In this case, the solvent is defined as a “poor solvent”. Examples of biopolymers in poor solvent are disordered proteins dominated by hydrophobic interactions and folded domains.
It is important to note that according to this definition, the same solvent (e.g. an aqueous buffer) can be both a good and a poor solvent depending on the polymer that one is studying.
There is a third case to consider, which is the case where monomer-solvent interactions exactly counterbalance the contribution of solvent–solvent and monomer–monomer interactions. In this case, the parameter defined in Eq. (1) is equal to zero. This condition defines the transition limit between the good and poor solvent conditions. In this scenario, the solvent is considered a “theta solvent” or “ideal solvent”. Interestingly, many disordered proteins sit close to this interface, which makes them very sensitive to the surrounding environment.57 Therefore, by tuning the solvent quality (e.g. by altering the strength of interactions via temperature), a dilute molecule can undergo a coil-to-globule transition from compact configurations in poor solvent to expanded configurations in good solvent.60
In Figure 1 we exemplify the common result of Flory-Huggins model with a bead model of the polymer where we tune the strength of the interactions between each bead and the solvent. Notable examples of “tuning the strength of interactions” are ion screening of electrostatic interactions of nucleic acids and charged disordered proteins and the contribution of kosmotropic or chaotropic agents.55–56
Figure 1. Flory Huggins Theory expectations.
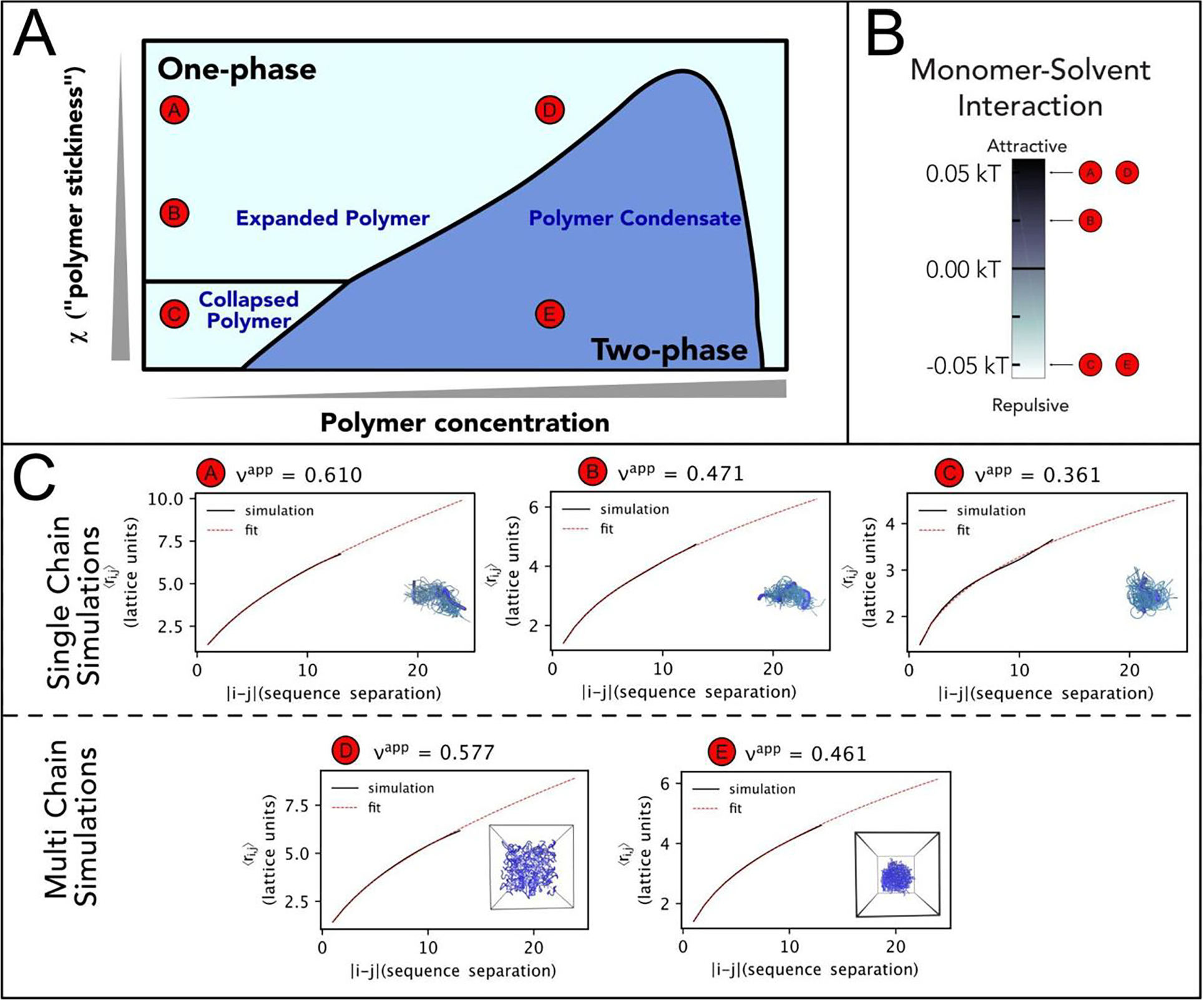
(A). Representation of the phase diagram proposed by Post and Zimm in their “Theory of DNA Condensation”.104 Here we compare it with the behavior of a 15-bead homopolymer in lattice-based PIMMS simulations67 and study how solution conditions affect single polymer conformations and multi-chain interactions. We report polymer concentration on the x-axis and “polymer stickiness” (as represented by χ, Eq. (1)) on the y-axis. At low concentrations, the polymer can adopt different degrees of collapse or expansions depending on the χ parameter. With increasing concentrations, depending on the polymer stickiness, the solution can undergo demixing and separate in two phases. (B). In coarse grained simulations, χ can be modulated by altering polymer–solvent interactions, whereas polymer–polymer and solvent–solvent (implicit) interactions remain fixed throughout all simulations. The graph depicts the corresponding pairwise interaction energy between each polymer bead (the monomer unit) and the solvent for a given solution condition. (C). We compare results obtained for single-chain and multi-chain. Single-polymer simulations show a change in the scaling exponent ν as a function of internal distance |i – j| and χ. Under solution conditions that favor monomer-solvent interactions, we observe a scaling exponent ν close to ~3/5, as expected for a polymer in good solvent. However, by making the monomer-solvent interactions more and more unfavorable, we observe a decrease in the scaling exponent till reaching the configuration of a collapsed globule, with a scaling exponent ν equal to ~1/3, consistent with poor solvent conditions. It is interesting to note that the theta-solvent condition (ν equal to ~½) is not achieved when the monomer-solvent interactions are equal to zero, but when they are favorably attractive. This reflects the inherent contribution of monomer–monomer and solvent–solvent interactions to the χ parameter. In multi-chain simulations, we observe two different scenarios. If solvent-monomer conditions are favorable, but monomer–monomer interactions are unfavorable, the homopolymer exists as a homogenous solution of overlapping polymers where each individual polymer has a certain degree of expansion. In the specific case simulated above, the scaling exponent ν is equal to 0.577. However, altering the delicate balance of monomer–monomer and solvent-monomer interactions can lead to solution demixing. Of note, the scaling factor has shifted from 0.361 to 0.461. As the polymer forms a condensate, it ‘solvates’ itself with other polymers. This result mimics expectations for the scaling exponent of a polymer in a melt, where the scaling exponent is ½. Interestingly, a similar result is expected also for the expanded chains at sufficiently high concentration.244–245
Biopolymers and Phase Separation
What happens when the homopolymers are no longer in dilute conditions? With increasing concentration of molecules, intermolecular interactions become relevant and monomer–monomer interactions can occur between different polymer chains. In this scenario, the solution can either remain well-mixed and contain an increasingly dense phase of polymers or undergo demixing (see Figure 1). Demixing occurs when the total free energy of the solution is better minimized by partitioning the polymer into a dilute and dense phase rather than maintaining a single well-mixed phase. In the case of a homopolymer, at a given constant temperature, the concentration of the dilute and dense phase are fixed (determined by the free energy and chemical potential of the polymer–solvent mixture) and only the relative fraction of each phase changes with increasing concentration of the polymer. This is a key expectation of a single homopolymer system and it is of relevance when characterizing phase separation of single components in vitro. In other words, when increasing the concentration within the demixing range, the homopolymer will partition in two phases, one with a lower and one with a higher concentration of homopolymer. The concentration of these two phases remains constant independently of the total concentration of homopolymer. Indeed, the concentration in the lower concentration phase is equivalent to the saturation concentration (i.e., the minimum concentration at which phase separation can occur), whereas the one in the higher concentration phase is equivalent to the maximum concentration at which phase separation can occur. While the concentrations remain constant, the relative abundance of each phase is dictated by the total concentration of homopolymer. Therefore, it is reasonable to expect a small volume of the dense phase when the total concentration of homopolymer in solution is close to the saturation concentration and vice versa.
If we further increase the concentration of molecules beyond the dense phase boundary, we will re-enter into a well-mixed phase that is now characterized by a concentrated solution of the polymer.
These three cases (dilute solution, demixed solution, concentrated solution) constitute the basic predictions of Flory-Huggins theory. It is interesting to note that all of these different conditions are the result of the same set of interactions and different phases emerge as a function of the total concentration of the polymer in the solution.
The theory also provides more insights on the conditions under which phase separation can occur. The Flory-Huggins theory identifies critical χ values at which phase separation occurs, which depends on the length of the polymer. However, there is no specific prescription regarding the exact strength of monomer–monomer, solvent–solvent, and monomer-solvent interactions besides the constraints imposed to satisfy at least this critical χ value. This is important since often weak interactions have been invoked in the context of biomolecular condensates as a prerequisite, but there is no such requirement for phase separation to happen; rather, the nature of the interaction (repulsive or attractive) and the balance between the polymer and solvent components favors or disfavors demixing.
One important element that emerges from the Flory-Huggins framework is that the same set of interactions controls the conformations of polymers under dilute conditions as well as their phase separation propensity. After determining conformational properties of a disordered region in isolation (via NMR and small-angle X-ray scattering) and corresponding phase separation boundaries (via absorbance and fluorescence correlation spectroscopy), Martin et al.61 used the Flory-Huggins theoretical model and simulations to extract the molecular interactions and confirm the interconnection between these two phenomena, including the role of valence and patterning of specific residues. Another notable example are elastin-like peptide sequences, whose single chain conformations and phase separation have been extensively characterized and compared.62–64 This intrinsic connection between the single chain and multi-chain behavior, which is encoded in the monomer interactions, has enabled the development of physics-driven models that capture the phase-separation propensity of disordered proteins and nucleic acids.61,65–69 Overall, these examples demonstrate that the implications of the Flory-Huggins model maintain validity when applied to biopolymers and can be deployed to rationalize the mechanism at action.
The Flory-Huggins model can be further complicated to account for the heterogeneous nature of the sequence, starting from the realization that specific monomers may be “stickier” than others. The theory of associative polymers proposed by Semenov and Rubinstein70 has offered a robust scaffold to conceptualize the role of “stickers” (elements that encode for associative interactions) and “spacers” (elements that do not contribute or contribute minimally to association)15,71 in controlling phase separation propensity. In the context of disordered proteins, distributed π-systems (e.g., π-π and cation-π interactions),61,72–81 oppositely charged residues,82–90 and hydrophobic aromatic and aliphatic residues85,91 have emerged as different typology of stickers. The nature of stickers encodes for context-dependent response of the phase behavior, enabling them to be modulated by environmental factors such as pH, ion screening, and post-translational modifications.71 The number and patterning of stickers contribute a further layer of tuning for the phase separation behavior.61,92–93 While spacers may not be directly involved in key associative interactions between chains that control phase-separation, they should not be regarded as intrinsically inert as they also encode for specific monomer–monomer and monomer-solvent interactions. Consequently, modulation of the spacer composition can alter the free energy of mixing and control phase behavior (concentrations, dynamics, and material properties).92,94–96 Importantly, addition of adhesive contacts between the monomers can give rise to percolation networks through the solution,70 where percolation represents a “networking transition” compared to the “density transition” observed in phase separation14: as such, the two phenomena can be disjointed (giving rise to distinct phases) or coupled (creating networks within condensates and modulating viscoelastic properties of the material).15,97–101 While these concepts have been extensively explored in the context of disordered proteins, nothing precludes their application to entire protein domains, with folded domains acting as sticker elements and disordered regions flanking the folded domains acting as spacers.102
Viral Genome Condensation: One vs Many
We have previously mentioned the problem of viral genome condensation and the phase separation propensity of specific components such as the viral nucleoproteins or nucleocapsids. The problem of nucleic acid condensation and the interplay between single chain condensation and multichain phase separation has been longly discussed in literature.103 The Flory-Huggins polymer framework suggests a direct connection between the case of a single polymer condensation (a necessary step in the packaging of viral genome) and the phase separation of many polymers. In this respect, it is interesting to look back to the work of Post and Zimm published in 1982.104 At that time, direct measurements of single nucleic acid conformations were not possible and the authors embraced expectations from Flory-Huggins theory of a polymer solution and proposed to quantify the association of multiple nucleic acid molecules (phase separation) as a way to measure intrachain interactions. As they stated, “It is not, of course possible to measure the behavior of a single polymer molecule experimentally; therefore, one must consider the consequences of intermolecular associations on the free energy of the system, recognizing the possibility of an aggregated polymer state.” Indeed, in the framework of Flory-Huggins theory, the same interactions control the intermolecular association of multiple chains and the intramolecular interactions leading to a single chain expansion or collapse. Post and Zimm added an important hypothesis to the Flory-Huggins theory, incorporating in the χ parameter the interactions of other molecules that control the condensation of the large polymer. In this respect the phase diagram in Figure 1(A) acquires a new meaning, where changes in the -parameter are now modulated by ligand concentrations and specificity, favoring or disfavoring single chain compaction or multi-chain phase separation.
It is interesting to explore the implications of this model in a viral context. In the case of coronaviruses, compaction of the viral genome is driven by the interplay between a single-stranded nucleic acid and many copies of the Nucleocapsid protein. Our group and many others48,105–107 have observed a strong propensity of the SARS-CoV-2 nucleocapsid protein in promoting phase separation when interacting with nucleic acids, both in vitro and in living cells. The extreme phase separation promiscuity of this protein poses an interesting conceptual problem: how is the protein rescued and recruited on the correct nucleic acid? To address this question, we used a simple polymer bead model (the same described above in the Flory-Huggins paragraph) and asked how binding of a multivalent ligand (the nucleocapsid protein) to large polymers (the nucleic acids) can lead to phase separation of the solution.105 In absence of specific interactions, with increasing concentration of the multivalent ligand, we identified a set of conditions under which the solution undergoes phase separation (Figure 2). In other words, we observe a partitioning of the solution in a dilute and dense phase, the first one depleted of polymers and ligands, the second one enriched in both. This is largely consistent with the picture proposed by Post and Zimm104 and experimental observations for SARS-CoV-2 nucleocapsid protein in presence of non-specific nucleic acids.48,105–107 However, the viral genome encodes for specific interactions along its sequence and it has been hypothesized that particular binding sites with high affinity help in packaging the viral genome. These sites are often referred to as “packaging signals” and have been previously proposed in the context of retroviruses, beta-coronaviruses, mammalian C-type viruses, and influenza A virus.108–112 To understand the contribution of packaging signals, we tested the effect of introducing a high affinity site in the equivalent of the RNA sequence in our simulation.105 Interestingly, we found that a single high affinity site is sufficient to alter the phase separation propensity of the system, suppressing phase separation and facilitating condensation of individual chains.105 While the model is clearly simplistic, it suggests a possible mechanism of action by which nucleic acid with high affinity motifs can attract the nucleocapsid protein and avoid phase separation with nonspecific sequences (Figure 3(A)). In additon, phase separation propensity can be modulated by interactions with specific protein components,113 which could alter the nucleic acid and protein-component multivalence and possibly disfavor the demixing of the solution.49 Once the single genome is condensed, a further transition can occur, leading to a structured organization of the nucleic acid (helical, smectic, etc…) or inducing a more disordered arrangement similar to beads on a string, as in the case of SARS-CoV-2 genome114 (Figure 3(B)).
Figure 2. Phase separation with specific and non-specific packaging motif.

Adapted from Cubuk, Alston et al.105 (A). Overview of the coarse grained lattice based simulation model. The model uses a 61-bead homopolymer (brown) and a 2-bead binder species (blue). Beads are multivalent and can interact with all lattice sites based on the pairwise interactions in the adjacent interaction matrix. (B). Schematic representation of homopolymer and binder interactions, in absence of a high affinity binding site. (C). A concentration-dependent solution demixing occurs with enrichment of both homopolymer and binder within the condensate. Dashed line on the binder:polymer concentration plane denotes a qualitative estimate of the binodal. (D). Number of clusters observed as a function of polymer and binder concentration: a single condensate forms for almost all conditions where condensates are observed. (E). Schematics of the homopolymer with a single multivalent high affinity binding site (red bead) and binder species. (F). Condensate formation is suppressed in presence of a high affinity binding site. (G). The number of clusters observed (as a function of polymer and binder concentration) scales linearly with the number of homopolymers in the simulation (H). Snapshots of simulations of the homopolymer and binder in absence (left) and presence (right) of the high affinity binding site.
Figure 3. Competition model of nucleic acid condensation: single chains vs phase separation.
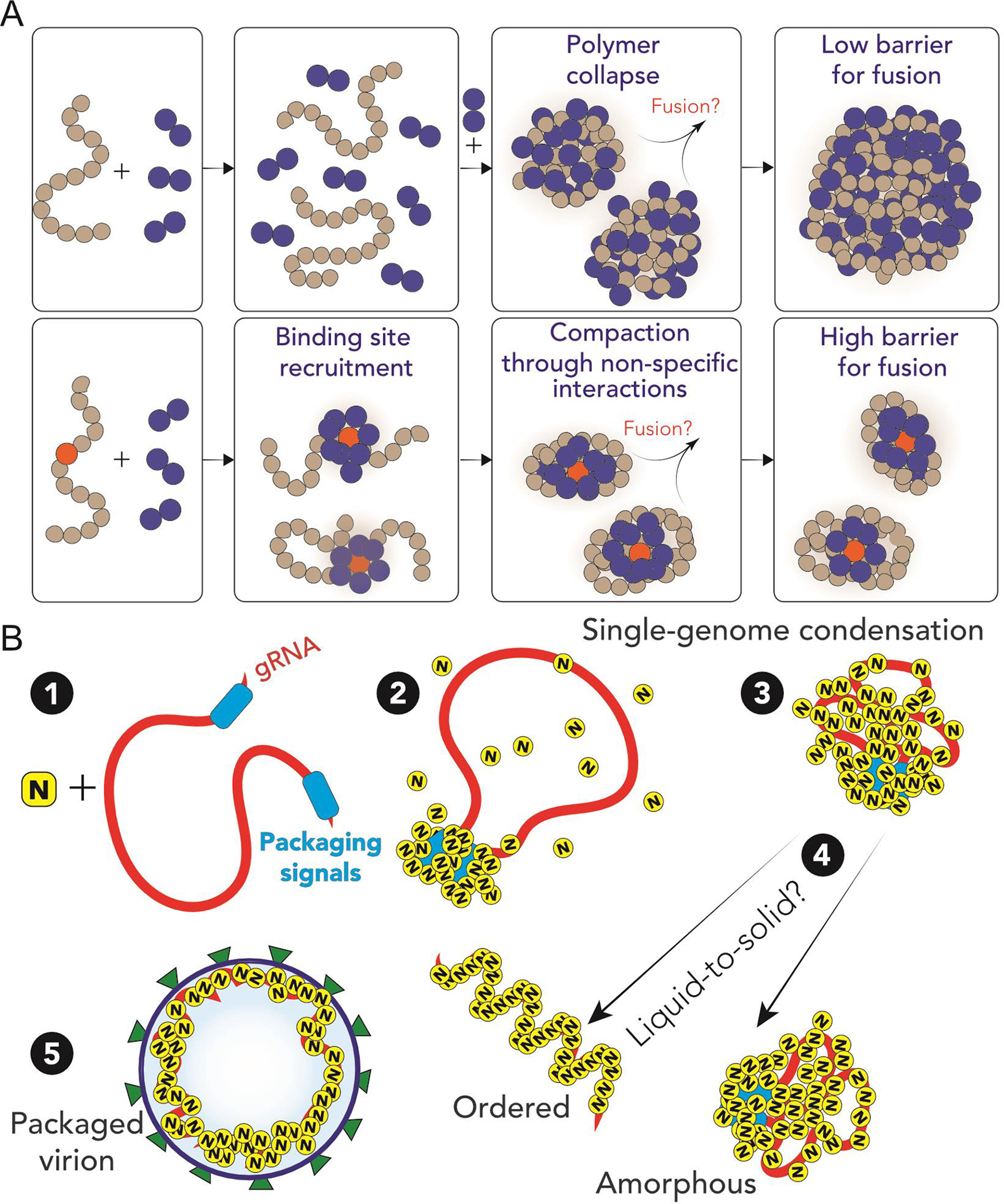
Adapted from Cubuk, Alston et al.105 (A). In absence of the high-affinity binding site, homopolymers collapse in presence of binder molecules and, under demixing conditions, can coalesce in larger condensates. Conversely, inclusion of a high affinity binding site leads to single polymer collapse with a high barrier for fusion. (B). Proposed model for SARS-CoV-2 genome packaging. (1–2) A simple overview of potential SARS-CoV-2 genome packaging mediated by nucleocapsid protein. Packaging signals could mediate clustering of N protein at specific loci, here depicted at the 5′ and 3′. (3) Clustering of nucleocapsid protein leads to condensation of the genome, enabling compaction of single genomes. (4) A liquid-to-solid transition of the single nucleocapsid protein-RNA condensate can lead to ordered or amorphous organization of the viral genome. The condensate is then able to be packaged via interaction with other SARS-CoV-2 structural proteins.
Multi-component Solution Demixing
The picture emerging from the Post and Zimm theory operates under a strong simplification by “hiding” the binding of the ligand within the interaction χ parameter. Indeed, the theory does not explicitly account for its dependence on the concentration of ligands. The explicit treatment of these effects requires expanding the Flory-Huggins theory to include multiple species.115–116 For the typical case of a ternary solution, including two homopolymers and a solvent, the model predicts different scenarios depending on the set of interactions and concentrations of each component. For example, if each polymer only phase separates on its own (i.e. obligate homotypic interaction), this reduces the problem to the case described above for a single type of homopolymer. Alternatively, as observed for the majority of protein and nucleic acid interactions, if the two polymers have favorable cross-interactions and demix together,the two-phase region of the phase diagram has a closed-loop topology (see Figure 4. Tie-lines identify the co-existing concentrations of the dilute and dense phase and all the intermediate concentrations at which the solution will partition at those specific concentrations. In the two-dimensional space identified by the concentrations of the two polymers, the slope of the tie lines indicates whether there is a preferential partitioning of a polymer with or versus the other. Tie lines parallel to one of the axes imply that one of the two polymers has no preferential partitioning between the two phases; positive slopes indicate an enrichment of both polymers in the dense phase, whereas a negative slope reveals segregation of one component with respect to the other (see Figure 5).
Figure 4. Examples of ternary phase diagrams comprising two polymers and a solvent.
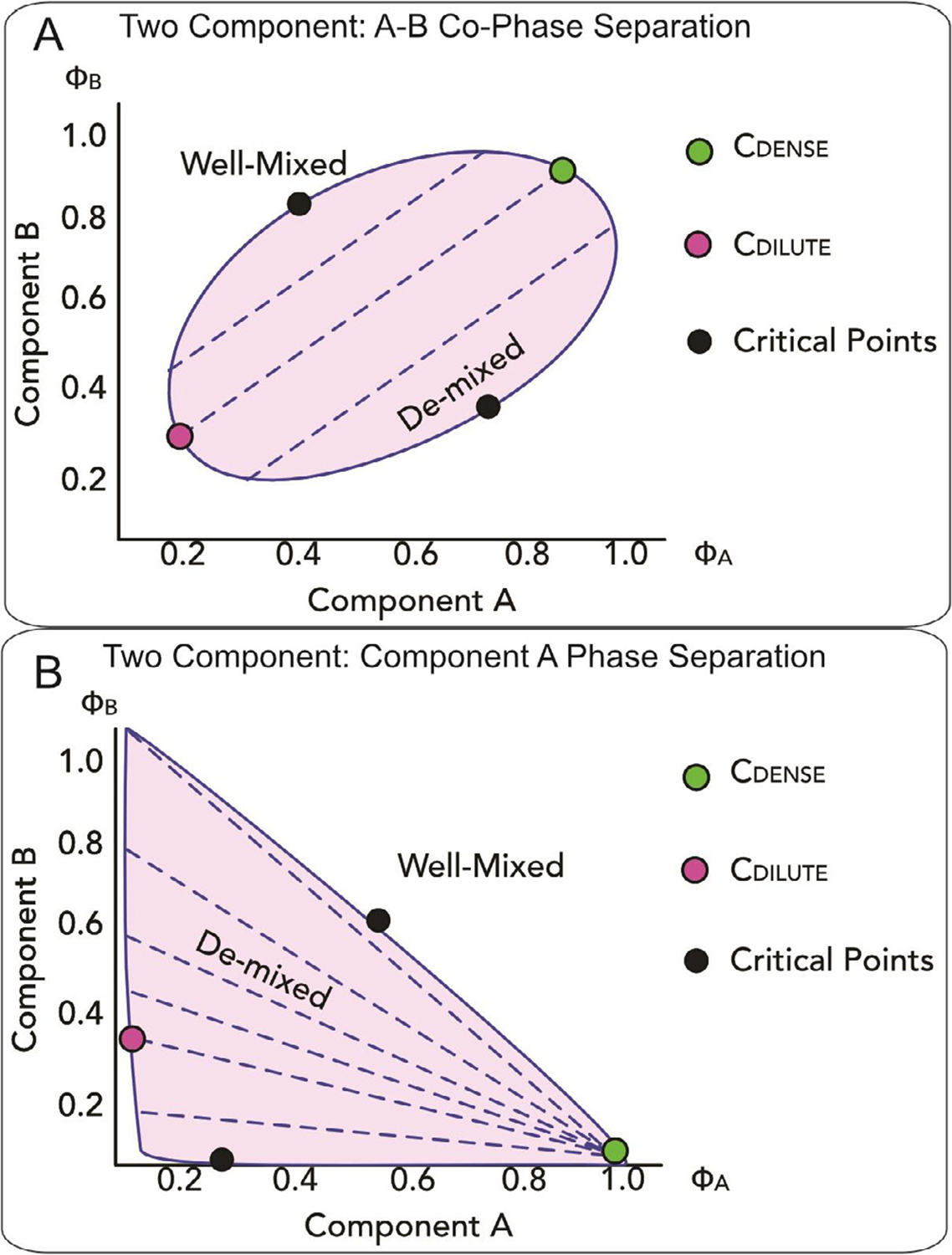
Phase diagrams as a function of the volume fraction ϕA and ϕB of the two polymer components A and B. In both panels, the shaded area indicates the region where the solution demixes, whereas black dots identify critical points. Dashed lines represent tie lines across which the concentrations in the dense (green dot) and dilute phase (magenta dot) remain constant. Note green and magenta dots are shown for only a single tie line, but the locus of all their points, i.e. all the light and dense phase concentrations adopted when the solution demixes, delimitates the phase boundaries. (A). In the first case, the two polymer components A and B co-phase separate. The positive slope of the tie lines indicates favorable interactions between the components and the two components are both enriched in the dense phase. (B). In the second case, component A undergoes phase separation and component B is excluded from the demixed solution.
Figure 5. Overview of Techniques to Study Condensation and Phase Separation.

Graphical summary of techniques that can be employed to study nucleic acid condensation and phase separation of proteins and protein-nucleic acid mixtures.
An obvious implication of the ternary (or higher components) Flory-Huggins model is that, differently from the case of a single homopolymer and solvent, the concentration of polymers in the light and dense phase depends on the total concentration of each polymer. Indeed, if we keep the concentration of one polymer component constant and we increase the concentration of the other polymer within the phase-boundaries of demixing, we will cross many different tie lines, which implies that different concentrations of each polymer occur in the light and dense phase. While this may partially limit the buffering capacity of condensates,117 it is possible that condensate-driving components have evolved to conserve specific buffering capacity within the range of concentrations accessible in the cell. Similarly, viral invading components may be optimized to specifically favor the solution demixing of existing components. Note that in this case preservation of a specific function does not imply a strict amino acid sequence conservation, as has been shown already for functional evolution of disordered proteins.118–119 Therefore, a deeper understanding of the molecular grammar dictating assembly and emerging properties of condensates will provide predictive tools for classifying proteins and molecules that may favor or disfavor condensation.
It is important to remark how the original Flory-Huggins theory that we discussed does incorporate only preferential interactions between the monomeric components and does not account for chemical binding that may affect the conformational properties of components or lead to persistent contacts. This is partially addressed by the theory of associate polymers that we discussed above, where explicit attractive contacts can be introduced.120–121 The theory can easily be extended to account for distinct associative components. Recently, Nandi et al.122 have included explicit treatments that account for polynomial binding in the Flory-Huggins framework by constructing independent lattices of defined interaction for each component, with the model qualitatively reproducing experimental observations.
From a biological point of view, comparing these different models teaches us that different types of interactions (e.g monomer solubility and preferential interactions, binding, ion screening or ion condensation) contribute differently to the mixing free energy of the system. This can be particularly important in the context of testing and designing drugs for targeting condensates. Modulating solubility of the protein may modulate partitioning of small molecules of interest, whereas altering the physico-chemical properties of condensates (e.g by increasing crosslinking) may affect their function. Indeed, the recent finding that drugs can cause hardening of viral intracellular bodies and block viral replication suggests these as viable routes to identify therapeutic strategy.123
Accessing Condensation: From Single-molecules to Phase Separation
Forty years after the Post and Zimm paper,104 there is now a broad array of single-molecule methodologies that enables the investigation of single nucleic acid condensation. While single-molecule approaches can provide important insights on the molecular interactions at play, ensemble methods such as Nuclear Magnetic Resonance and small angle X-ray scattering (SAXS) also enable access to essential complementary information on protein and nucleic acid conformations and interactions. At the same time, the incredible interest for phase separation has led to the development of new methods to characterize phase boundaries as well as transport and rheological properties of condensates. Rather than a comprehensive review of each of the techniques, which is outside of the scope of this review, we decided to highlight the principal biophysical methods that can be used to study the interactions occurring at the level of single-molecules (as for the condensation of single nucleic acids) and the phase separation of many. In particular, we exemplify their application to investigate viral nucleic acid and, when this is not possible, discuss their potential application based on current experiments on other systems to study viruses moving forward.
Turbidity measurements
Turbidity experiments can generate a quantitative description of condensate phase diagram boundaries.124–126 This technique takes advantage of the light scattered by condensates when the system is above its saturation concentration, i.e. the concentration where the components separate into a dense and dilute phase. As such, turbidity is commonly measured at wavelengths where no absorption is expected from the sample and the decrease in light transmission reflects the opacity of the demixed solution. When paired with centrifugation of the sample, which enables separating the dense and dilute phases, it allows for careful measurements of the concentration in each phase and construction of tie lines.127 Phase separation of the SARS-CoV-2 nucleocapsid protein and corresponding phase boundaries have been studied using turbidity assays.48,105,107,128 A limitation of the approach pertains to the impossibility of distinguishing phase separation from aggregation or percolation and inspection of the sample via imaging is required to confirm the underlying assembly process.
Imaging via light microscopy
Light microscopy imaging provides one of the most common methods for identifying the formation of biomolecular condensates, both in vitro and in living cells. Macroscopic demixing in vitro can often be visualized without any specific labeling. However, the identification of specific components participating in the condensate, particularly within the cells, is helped by fluorescent labels, which are either covalently attached to a protein and/or nucleic acid or genetically encoded. The condensate formation is then studied as a function of solution conditions: ion concentration, pH, crowding agents, temperature, and obviously molecular concentration.129 To access qualitative phase boundaries, images are taken as conditions are perturbed and the presence (or absence) of condensates is visualized to quantify entrance into and exit from the two-phase regime.130–132 Microscopy can also provide insights into the partitioning of protein and nucleic acid components within the condensates, as in the case of the partitioning of SARS-CoV-2 nucleocapsid protein into stress granules.133
Fluorescence recovery after photobleaching (FRAP)
A common approach that is combined with fluorescence microscopy is the assessment of molecular mobilities within biomolecular condensates and with the surrounding milieu via fluorescence recovery after photobleaching (FRAP).17,134–135 In typical FRAP experiments, fluorescently labeled molecules are photobleached using a high-power laser. The mobility of molecules within the bleached spot is quantified by monitoring the change in fluorescence signal before and after photobleaching and by following its recovery, which can be even just partial. Though it provides one of the easiest approaches to assess transport properties within the condensate, a careful interpretation of FRAP results is required to identify the mechanisms at play on the molecular scale and disentangle diffusive effects from kinetically-trapped states.14,17,136
Fluorescence correlation spectroscopy (FCS)
FCS provides a method to determine both the concentration and the diffusion of molecules by measuring the fluctuations of the fluorescence signal within the confocal spot. As such, FCS enables quantifying both concentration and stoichiometry of species in the dilute and dense phase of a demixing solution as well as the mobility of molecules within and outside the condensates.17,61,137–138 The resolution of FCS and the concentration regime in which it can be applied makes FCS a perfect approach to connect properties of the phase separated solution to the properties of single nucleic acid compaction.139 Compaction of nucleic acids can be visualized by quantifying the change in hydrodynamic radius of fluorescently labeled molecules as a function of solution conditions, proteins, and crowders.140–142 Importantly, FCS can be easily performed both in vitro and in cell.143 In the context of nucleic acid condensation, Sabanayagam et al. used FCS to measure Bacteriophage T4 DNA packaging, utilizing changes in diffusion to assess translocation of bacteriophage DNA from the bulk solution to the interior of the bacteriophage prohead.144 Gopal et al. showed that viral RNAs are more compact on average than non-viral RNA, alluding to a potential evolutionary reduction in dimensions that could aid in packaging genomic RNA into relatively small virions.145 By treating RNA molecules as branched polymers, FCS measurements of the hydrodynamic radii of long RNAs have also been used to help predict RNA dimensions from secondary structure predictions.146
Förster resonance energy transfer (FRET)
FRET provides a ‘spectroscopic ruler’ to measure distance changes within the molecule of interest,147–148 taking advantage of the distance-dependent non-radiative energy transfer between the donor and acceptor fluorophores. Ensemble and single-molecule FRET methods have been largely used to study nucleic acid condensation and conformational changes of proteins upon binding to the nucleic acid.149 Like FCS, FRET can be performed both in vitro and in cell,150–151 including its single-molecule applications.152–153 FRET measurements can also be applied to proteins within biomolecular condensates.90,154–155 FRET has been applied to study DNA and RNA bending and folding in viruses: oftentimes, viral nucleocapsid proteins are highly positively charged and act as macromolecular counterions, facilitating charge screening and enabling proper folding of the nucleic acid.156–157 Taken further, these charged proteins could also be utilized to shift nucleic acid protein-solvent interactions and facilitate condensation and viral genome packaging.
Single-molecule force spectroscopy
Another class of techniques that offers robust characterization of nucleic acid compaction are single-molecule micromanipulation techniques which include optical and magnetic tweezers as well as atomic force microscopy. These three techniques enable not only quantitative observables of nucleic acid compaction but also report on the forces that enable compaction.158–160 These single-molecule micromanipulation techniques have been used to measure nucleic acid compaction as a function of pH and ionic strength,161–165 protein induced condensation,166–168 and crowding agent effects.169 In particular, the combination of fluorescence and force spectroscopy enabled quantifying the forces generated in protein:nucleic acid co-condensates and determining how those forces can act on non-condensate localized nucleic acids to mediate DNA condensation.167,170 In regards to viral nucleic acid compaction, Gien et al. were able to understand how the HIV-1 nucleocapsid protein mediates viral genomic DNA compaction using a combination of optical tweezers and atomic force microscopy.168 Optical trapping techniques can also be used to study the microrheology of in-vitro reconstituted condensates, which can provide quantitative insights into the condensate material properties.171 Indeed, optical traps enable the physical manipulation of individual droplets and the study of droplet fusion, providing insights on surface tension properties of different biomolecular condensates and kinetics of fusion.131,171–175 In addition, as demonstrated by Jawert et al.,174 optical traps can be harnessed to measure the viscoelastic moduli within a single droplet. This is realized by trapping two polystyrene beads within the condensate and by monitoring the perturbation experienced by one bead when displacing the second bead at different frequencies.174
Nucleic acid curtains
Curtains enable high throughput single-molecule measurements of nucleic acid compaction.176–179 This approach requires the attachment of long fluorescently labeled nucleic acids to a surface (e.g. by using supported lipid bilayers) within a microfluidics flow cell. The flow stretches the nucleic acid, while other components (protein or cations) can complex with the nucleic acid. Labeling of the components enables direct visualization of the effects of solution conditions or protein concentrations on nucleic acid compaction. Calcines-Cruz et al. utilized DNA curtains to develop viro-mimetic scaffolds and programmable bioinspired nanomaterials, which can be used for studying how sequence specificity enables packaging of viral nucleic acids.180
Nuclear Magnetic Resonance (NMR) spectroscopy
NMR spectroscopy enables the interrogation of intermolecular interactions with atomistic resolution. By probing the interactions of atomic nuclei with a magnetic field, information on the chemical environment being experienced by each nucleus can be determined.181 Due to its ability to capture the structures of conformationally dynamic and heterogeneous molecules, in both physiological and non-physiological conditions, NMR has been used extensively to study the structure of viral nucleic acid elements182–189 viral proteins,190–191 and the role of disordered regions.192–193 The amino acid residues involved in nucleic acid binding can be determined by chemical shift perturbation,194–196 giving insight into residues that may be important for phase separation or nucleic acid condensation.48 Furthermore, NMR spectra of biomolecules within condensates94,197–199 offer the opportunity to understand how condensate environments affect proteins and nucleic acids.
Small-angle X-ray scattering (SAXS)
SAXS reports on the size, shape, conformation, and molecular weight of molecules in solution.200–201 Its ability to measure compaction and expansion of molecules makes it a useful technique for studying nucleic acid condensation, and has been applied particularly for studying the effects of RNA folding,202–205 DNA compaction,206–210 and RNA compaction.211 It can also be used to study the conformations of proteins undergoing phase separation.212
Computational methods
When it comes to interrogating the molecular driving forces for nucleic acid condensation and phase separation, simulations can provide insights that may be challenging to extract from experimental measurements. Simulations rely on a representation scheme of the biomolecules of interest and of its interactions.213–214 These are encoded by a force field that either describes each component at atomic resolution (all-atom) or as a coarse-grained model that approximates the scale of observation. For simulating macroscopic behaviors, such as peptide- and protein-induced nucleic acid condensation or phase separation, coarse grained models are typically favored over all atom representations because of the decreased computational effort,215 though extensive all-atom simulations may provide details on parameters that are difficult to coarse-grain, such as the contribution of ion screening and counterion adsorption.216–221 Recent years have seen the development of coarse-grained force-fields aimed to describing biomolecular condensates. At the ultra coarse-grained level, there are lattice-based models such as LASSI68,222 and PIMMS,223 where multiple residues or even entire domains are described as single beads: both have been successfully used to describe the phase separation of mixtures of nucleic acids and proteins. Less coarse grained models utilize one bead per nucleotide or residue representations of biomolecules. These include the hydropathy scale (HPS) model,69,86,172,215,224–229 the Kim/Hummer (KH) model,215 and the multi-scale π-π (Mpipi) model.65 The HPS, KH, and Mpipi models each contain bonded, electrostatic, and short range pairwise-interaction terms. For the HPS model amino acid specific pairwise interactions are encoded by a hydrophobicity scale224,230 and parametrized against SAXS and FRET measurements of intrinsically disordered regions. The KH model is based on amino acid pairwise interactions, which are derived from experimental data (second virial coefficient of lysozyme and binding affinity of the ubiquitin-CUE complex).231 Mpipi differs in that its sequence specific pairwise potentials are based on potential of mean force calculations of all-atom simulations of amino acid and amino acid: nucleic acid pairs in explicit solvent with ions. It also accounts for π-π interactions based on the frequency of π-π interactions that occur in a set of PDB structures.232 Mpipi quantitatively predicts the radius of gyration of disordered proteins and was able to recapitulate experimental observables such as multiphasic droplets of polyarginine, polylysine, and polyU RNA.65,233
The ability to recapitulate experimental findings with simulations provides the ability to systematically interrogate sequence features of disordered proteins and nucleic acids that may drive phase separation and/or nucleic acid compaction. Development of robust models tested and supported by experimental observations may enable better understanding of the sequence to function relationship between condensate components.
Recent Advances on Protein-Nucleic Acid Coacervation
An increasing number of studies has turned to address the role of protein-nucleic acid condensates in regulating the cell machinery, in particular exploring nuclear organization.89,132,179,234–236 While many of these studies do not concern viral components, they well exemplify the relevant interactions that will drive phase separation of viral proteins. Here, we briefly summarize some of the biophysical concepts that have emerged from the recent study of protein-RNA condensates, focusing on general concepts that can be readily applied to viral components.
Because of the negatively charged nature of nucleic acids, one of the obvious driving forces of phase separation is the interaction with positively charged proteins. The phase separation of oppositely charged polymers in polymer physics is commonly referred as “complex coacervation”237–239 and concerns the electrostatic attractions between oppositely charged groups as much as the role of ions condensing on the highly charged polymers. The specific base of the nucleic acid has a direct impact on the morphology of the condensate, with poly-rG sequences leading to amorphous condensates compared to other liquid-like condensates obtained for other homooligonucleotides.240 Transient interactions due to base stacking also modulate dynamics of exchange within the coacervate.240 When mediating the interaction with protein, the length dependence of the interaction (short range if involving cation-pi interactions, long range if purely electrostatic) further contribute in modulating the phase boundaries.241 The balance of interactions in phase transition of RNA – protein complexes can lead even to ordered hollow condensates.83,172 The stoichiometry and length of the nucleic acid further modulate the surface tension of the condensate242 and its internal rearrangements.49 Analogously, the pattern of charged residues in the sequence alters the viscoelastic properties of coacervates.243
Conclusions
While the past decade has seen growing evidence that viral infections harness intracellular condensates, the challenge that lies ahead is in understanding the rules controlling the participation of viral components to specific condensates and uncovering the mechanisms related to the emerging phenotypes. The role of viral proteins and nucleic acids in modulating cell condensates provides an intriguing perspective on how “external” components can modulate phase separation propensity, shifting phase boundaries of protein and nucleic acids in the cytoplasm or nucleus. It may also provide important insights into how evolution has encoded for robustness in cellular compartmentalization and whether viral genomes that are highly mutated conserve specific properties related to phase separation. At the same time, the large promiscuity identified for specific components such as the nucleocapsid protein of SARS-CoV-2 poses the complementary challenge of understanding which cellular or biochemical factors may suppress or limit the phase separation propensity of viral proteins.
Bridging the molecular and mesoscopic scales and decoding the phase separation grammar encoded in proteins and nucleic acids will require both top–bottom and bottom-up approaches. Top-bottom approaches where condensates are studied within the cellular complexity are essential to ensure that findings are pertinent to the function of the compartments, but are limited in granting access to molecular details. Rigorous investigation of intracellular puncta are necessary to understand the nature of the observed objects and the role of each component. Bottom-up approaches may be limited in testing biomolecular function, but are essential for precise measurements of the interactions at play. Direct measurement of interactions in both well-mixed and dense phases are necessary for precise models that account for different modes of binding and stoichiometries, in particular if disordered regions are involved.
Undoubtedly, the complexity of the cellular environment highly complicates the simplified models presented here, with multiple components coexisting and demixing at the same time. However, a better understanding of the interactions connecting intra- and inter-molecular chain interactions will help validate theories and computational models of phase separation. This is particularly important since a direct test of all possible condensate components may be impracticable, but theoretical predictions and computational models may offer a strategy to explore buffering capacity and response to extraneous components.
Condensates are more than the sum of the parts and determination of the collective properties of viral condensates (e.g., surface tension, viscoelasticity, etc…) may provide important insights on how these components alter the normal function of these intracellular bodies. At the same time, investigation of viral condensates will enable development of new therapeutics aimed to target these specific components during the virus life cycle.
Acknowledgements
The contribution of J.J.A and A.S. to this work is supported by the NIH National Institute on Allergic and Infectious Diseases (Soranno, R01AI163142) and the National Cancer Institute (Alston, F99CA264413). We thank Alex Holehouse and Melissa D Stuchell Brereton for insightful discussions.
Footnotes
DECLARATION OF COMPETING INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Jhullian J. Alston: Software, Visualization, Writing – original draft, Writing – review & editing. Andrea Soranno: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
DATA AVAILABILITY
Data will be made available on request.
References
- 1.Netherton CL, Wileman T, (2011). Virus factories, double membrane vesicles and viroplasm generated in animal cells. Curr. Opin. Virol. 1, 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff G, Melia CE, Snijder EJ, Bárcena M, (2020). Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 28, 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Sastre A, (2017). Ten Strategies of Interferon Evasion by Viruses. Cell Host Microbe. 22, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale-Costa S, Amorim MJ, (2016). Recycling endosomes and viral infection. Viruses 8, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Castro IF, Volonté L., Risco C., (2013). Virus factories: biogenesis and structural design. Cell. Microbiol. 15, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández de Castro I, Tenorio R, Risco C, (2016). Virus assembly factories in a lipid world. Curr. Opin. Virol. 18, 20–26. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Liang R, Gao Y, Li Y, Deng X, Xiang R, Zhang Y, Ying T, et al. , (2019). Development of small-molecule inhibitors against zika virus infection. Front. Microbiol. 10, 2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin Y, Brangwynne CP, (2017). Liquid phase condensation in cell physiology and disease. Science 357 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- 9.Vandelli A, Vocino G, Tartaglia GG, (2022). Phase separation drives SARS-CoV-2 replication: A hypothesis. Front Mol Biosci. 9, 893067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Dai T, Qin Z, Pan T, Chu F, Lou L, Zhang L, Yang B, et al. , (2021). Targeting liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein promotes innate antiviral immunity by elevating MAVS activity. Nat. Cell Biol. 23, 718–732. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Zhang L, Shen J, Zhai Y, Jiang Q, Yi M, Deng X, Ruan Z, et al. , (2021). The STING phase-separator suppresses innate immune signalling. Nat. Cell Biol. 23, 330–340. [DOI] [PubMed] [Google Scholar]
- 12.Said EA, Tremblay N, Al-Balushi MS, Al-Jabri AA, Lamarre D, (2018). Viruses Seen by Our Cells: The Role of Viral RNA Sensors. J. Immunol. Res. 2018, 9480497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banani SF, Lee HO, Hyman AA, Rosen MK, (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittag T, Pappu RV, (2022). A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell. 82, 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J-M, Holehouse AS, Pappu RV, (2020). Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 49, 107–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soranno A, (2019). The Trap in the FRAP: A cautionary tale about transport measurements in biomolecular condensates. Biophys. J. 117, 2041–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor NO, Wei M-T, Stone HA, Brangwynne CP, (2019). Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J. 117, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flint SJ, Racaniello VR, Rall GF, Hatziioannou T, Skalka AM, (2020). Principles of Virology: Molecular Biology. Wiley. [Google Scholar]
- 19.Varsani A, Lefeuvre P, Roumagnac P, Martin D, (2018). Notes on recombination and reassortment in multipartite/segmented viruses. Curr. Opin. Virol. 33, 156–166. [DOI] [PubMed] [Google Scholar]
- 20.Nevers Q, Albertini AA, Lagaudrière-Gesbert C, Gaudin Y, (2020). Negri bodies and other virus membrane-less replication compartments. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Negri A (1905). Contributo allo studio dell’eziologia della rabbia. Tipografia e Legatoria Cooperativa. [Google Scholar]
- 22.Lahaye X, Vidy A, Pomier C, Obiang L, Harper F, Gaudin Y, Blondel D, (2009). Functional characterization of Negri bodies (NBs) in rabies virus-infected cells: Evidence that NBs are sites of viral transcription and replication. J. Virol. 83, 7948–7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahaye X, Vidy A, Fouquet B, Blondel D, (2012). Hsp70 protein positively regulates rabies virus infection. J. Virol. 86, 4743–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagan SM, Weber SC, (2022). Let’s phase it: viruses are master architects of biomolecular condensates. Trends Biochem. Sci.. 10.1016/j.tibs.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Su JM, Samuel CE, Ma D, (2019). Measles Virus Forms Inclusion Bodies with Properties of Liquid Organelles. J. Virol. 93 10.1128/JVI.00948-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma D, George CX, Nomburg JL, Pfaller CK, Cattaneo R, Samuel CE, (2018). Upon Infection, Cellular WD Repeat-Containing Protein 5 (WDR5) Localizes to Cytoplasmic Inclusion Bodies and Enhances Measles Virus Replication. J. Virol. 92 10.1128/JVI.01726-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinrich BS, Maliga Z, Stein DA, Hyman AA, Whelan SPJ, (2018). Phase Transitions Drive the Formation of Vesicular Stomatitis Virus Replication Compartments. MBio 9 10.1128/mBio.02290-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiger F, Acker J, Papa G, Wang X, Arter WE, Saar KL, Erkamp NA, Qi R, et al. , (2021). Liquid-liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J. 40, e107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caragliano E, Bonazza S, Frascaroli G, Tang J, Soh TK, Grünewald K, Bosse JB, Brune W, (2022). Human cytomegalovirus forms phase-separated compartments at viral genomes to facilitate viral replication. Cell Rep. 38, 110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlier CM, Wu Y-J, Allart S, Malnou CE, Schwemmle M, Gonzalez-Dunia D, (2013). Analysis of borna disease virus trafficking in live infected cells by using a virus encoding a tetracysteine-tagged p protein. J. Virol. 87, 12339–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudrière-Gesbert C, Gaudin Y, Blondel D, (2017). Negri bodies are viral factories with properties of liquid organelles. Nat. Commun. 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménager P, Roux P, Mégret F, Bourgeois J-P, Le Sourd A-M, Danckaert A, Lafage M, Préhaud C, et al. , (2009). Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog. 5, e1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolozin B, Ivanov P, (2019). Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20, 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcelo A, Koppenol R, de Almeida LP, Matos CA, Nóbrega C, (2021). Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation? Cell Death Dis. 12, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Q, Hastings C, Miller CL, (2009). Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J. Virol. 83, 11090–11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin Q, Carroll K, Hastings C, Miller CL, (2011). Mammalian orthoreovirus escape from host translational shutoff correlates with stress granule disruption and is independent of eIF2alpha phosphorylation and PKR. J. Virol. 85, 8798–8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljeström P, (2005). Importance of eIF2α phosphorylation and stress granule assembly in alphavirus translation regulation. MBoC. 16, 3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazroui R, Sukarieh R, Bordeleau M-E, Kaufman RJ, Northcote P, Tanaka J, Gallouzi I, Pelletier J, (2006). Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2α phosphorylation. MBoC. 17, 4212–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White JP, Cardenas AM, Marissen WE, Lloyd RE, (2007). Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2, 295–305. [DOI] [PubMed] [Google Scholar]
- 40.White JP, Lloyd RE, (2011). Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. J. Virol. 85, 12442–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ariumi Y, Kuroki M, Kushima Y, Osugi K, Hijikata M, Maki M, Ikeda M, Kato N, (2011). Hepatitis C virus hijacks P-body and stress granule components around lipid droplets. J. Virol. 85, 6882–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisfeld AJ, Neumann G, Kawaoka Y, (2015). At the centre: influenza A virus ribonucleoproteins. Nat. Rev. Microbiol. 13, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakdawala SS, Wu Y, Wawrzusin P, Kabat J, Broadbent AJ, Lamirande EW, Fodor E, Altan-Bonnet N, et al. , (2014). Influenza a virus assembly intermediates fuse in the cytoplasm. PLoS Pathog. 10, e1003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alenquer M, Vale-Costa S, Etibor TA, Ferreira F, Sousa AL, Amorim MJ, (2019). Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat. Commun. 10, 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou Y-Y, Heaton NS, Gao Q, Palese P, Singer RH, Lionnet T, (2013). Colocalization of different influenza viral RNA segments in the cytoplasm before viral budding as shown by single-molecule sensitivity FISH analysis. PLoS Pathog. 9, e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guseva S, Milles S, Jensen MR, Salvi N, Kleman J-P, Maurin D, Ruigrok RWH, Blackledge M, (2020). Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci Adv. 6, eaaz7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu S, Ye Q, Singh D, Villa E, Cleveland DW, Corbett KD, (2020). The SARS-CoV-2 Nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat. Commun.. 10.1101/2020.07.30.228023.2020.07.30.228023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savastano A, Ibáeñz de Opakua A, Rankovic M, Zweckstetter M, (2020). Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nat. Commun. 11, 6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soranno A, Incicco JJ, De Bona P, Tomko EJ, Galburt EA, Holehouse AS, Galletto R, (2022). Shelterin Components Modulate Nucleic Acids Condensation and Phase Separation in the Context of Telomeric DNA. J. Mol. Biol. 434, 167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etibor TA, Yamauchi Y, Amorim MJ, (2021). Liquid Biomolecular Condensates and Viral Lifecycles: Review and Perspectives. Viruses 13 10.3390/v13030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoca V, Di Nunzio F, (2021). Membraneless organelles restructured and built by pandemic viruses: HIV-1 and SARS-CoV-2. J. Mol. Cell Biol. 13, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brocca S, Grandori R, Longhi S, Uversky V, (2020). Liquid-liquid phase separation by intrinsically disordered protein regions of viruses: roles in viral life cycle and control of virus-host interactions. Int. J. Mol. Sci. 21 10.3390/ijms21239045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolnik O, Gerresheim GK, Biedenkopf N, (2021). New perspectives on the biogenesis of viral inclusion bodies in negative-sense RNA virus infections. Cells. 10 10.3390/cells10061460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinstein M, Colby RH, (2003). Polymer Physics. Oxford University Press, New York. [Google Scholar]
- 55.Murphy MC, Rasnik I, Cheng W, Lohman TM, Ha T, (2004). Probing single-stranded DNA conformational flexibility using fluorescence spectroscopy. Biophys. J. 86, 2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Müller-Späth S., Soranno A, Hirschfeld V, Hofmann H, Rüegger S, Reymond, Nettels D, Schuler B, (2010). Charge interactions can dominate the dimensions of intrinsically disordered proteins. Proc. Natl. Acad. Sci. U. S. A. 107, 14609–14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann H, Soranno A, Borgia A, Gast K, Nettels D, Schuler B, (2012). Polymer scaling laws of unfolded and intrinsically disordered proteins quantified with single-molecule spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 109, 16155–16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohn JE, Millett IS, Jacob J, Zagrovic B, Dillon TM, Cingel N, Dothager RS, Seifert S, et al. , (2004). Random-coil behavior and the dimensions of chemically unfolded proteins. Proc. Natl. Acad. Sci. 101, 12491–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holehouse AS, Pappu RV, (2018). Collapse transitions of proteins and the interplay among backbone, sidechain, and solvent interactions. Annu. Rev. Biophys. 47, 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soranno A, (2020). Physical basis of the disorder-order transition. Arch. Biochem. Biophys. 685, 108305. [DOI] [PubMed] [Google Scholar]
- 61.Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, Bremer A, Grace CR, Soranno A, et al. , (2020). Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quiroz FG, Chilkoti A, (2015). Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 14, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varanko AK, Su JC, Chilkoti A, (2020). Elastin-like polypeptides for biomedical applications. Annu. Rev. Biomed. Eng. 22, 343–369. [DOI] [PubMed] [Google Scholar]
- 64.Zeng X, Holehouse AS, Chilkoti A, Mittag T, Pappu RV, (2020). Connecting coil-to-globule transitions to full phase diagrams for intrinsically disordered proteins. Biophys. J. 119, 402–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joseph JA, Reinhardt A, Aguirre A, Chew PY, Russell KO, Espinosa JR, Garaizar A, Collepardo-Guevara R, (2021). Physics-driven coarse-grained model for biomolecular phase separation with near-quantitative accuracy. Nat Comput Sci. 1, 732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joseph JA, Espinosa JR, Sanchez-Burgos I, Garaizar A, Frenkel D, Collepardo-Guevara R, (2021). Thermodynamics and kinetics of phase separation of protein-RNA mixtures by a minimal model. Biophys. J. 120, 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holehouse AS. & Pappu RV. (2019). PIMMS (0.24 pre-beta), 10.5281/zenodo.3588456. [DOI] [Google Scholar]
- 68.Choi J-M, Dar F, Pappu RV, (2019). LASSI: A lattice model for simulating phase transitions of multivalent proteins. PLoS Comput. Biol. 15, e1007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dignon GL, Zheng W, Kim YC, Mittal J, (2019). Temperature-controlled liquid-liquid phase separation of disordered proteins. ACS Cent. Sci. 5, 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semenov AN, Rubinstein M, (1998). Thermoreversible gelation in solutions of associative polymers. 1. statics. Macromolecules 31, 1373–1385. [Google Scholar]
- 71.Ginell GM, Holehouse AS, (2023). An introduction to the stickers-and-spacers framework as applied to biomolecular condensates. In: Zhou H--X., Spille J--H, Banerjee PR. (Eds.), Phase-Separated Biomolecular Condensates: Methods and Protocols. Springer, US, New York, NY, pp. 95–116. [DOI] [PubMed] [Google Scholar]
- 72.Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, et al. , (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP, (2015). Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, et al. , (2018). A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16. 10.1016/j.cell.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bremer A, Farag M, Borcherds WM, Peran I, Martin EW & Pappu RV et al. (n.d.). Deciphering how naturally occurring sequence features impact the phase behaviors of disordered prion-like domains. bioRxiv [Preprint](2021), 10.1101/2021.01.01.425046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han TW, Kato M, Xie S, Wu LC, Mirzaei H, Pei J, Chen M, Xie Y, et al. , (2012). Cell-free Formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779. 10.1016/j.cell.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, et al. , (2012). Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767. 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frey S, Richter RP, Görlich D, (2006). FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314, 815–817. [DOI] [PubMed] [Google Scholar]
- 79.Yoshizawa T, Ali R, Jiou J, Fung HYJ, Burke KA, Kim SJ, Lin Y, Peeples WB, et al. , (2018). Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. Cell 173, 693–705.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, Phillips EC, Miyashita A, et al. , (2018). FUS Phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell 173, 720–734.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H-R, Chiang W-C, Chou P-C, Wang W-J, Huang J-R, (2018). TAR DNA-binding protein 43 (TDP-43) liquid–liquid phase separation is mediated by just a few aromatic residues. J. Biol. Chem. 293, 6090–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Y-H, Forman-Kay JD, Chan HS, (2016). Sequence-specific polyampholyte phase separation in membraneless organelles. Phys. Rev. Lett. 117, 178101. [DOI] [PubMed] [Google Scholar]
- 83.Banerjee PR, Milin AN, Moosa MM, Onuchic PL, Deniz AA, (2017). Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets. Angew. Chem. Int. Ed Engl. 56, 11354–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang L-W, Lytle TK, Radhakrishna M, Madinya JJ, Vélez J, Sing CE, Perry SL, (2017). Sequence and entropy-based control of complex coacervates. Nat. Commun. 8, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pak CW, Kosno M, Holehouse AS, Padrick SB, Mittal A, Ali R, Yunus AA, Liu DR, et al. , (2016). Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell. 63, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaur T, Raju M, Alshareedah I, Davis RB, Potoyan DA, Banerjee PR, (2021). Sequence-encoded and composition-dependent protein-RNA interactions control multiphasic condensate morphologies. Nat. Commun. 12, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Y-H, Chan HS, (2017). Phase separation and single-chain compactness of charged disordered proteins are strongly correlated. Biophys. J. 112, 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cummings CS, Obermeyer AC, (2018). Phase separation behavior of supercharged proteins and polyelectrolytes. Biochemistry 57, 314–323. [DOI] [PubMed] [Google Scholar]
- 89.Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA, et al. , (2016). Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. Elife 5 10.7554/eLife.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galvanetto N, Ivanović MT, Chowdhury A, Sottini A, Nüesch MF, Nettels D, Best RB, Schuler B, (2022). Ultrafast molecular dynamics observed within a dense protein condensate. bioRxiv. 10.1101/2022.12.12.520135.2022.12.12.520135. [DOI] [Google Scholar]
- 91.Schmidt HB, Barreau A, Rohatgi R, (2019). Phase separation-deficient TDP43 remains functional in splicing. Nat. Commun. 10, 4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holehouse AS, Ginell GM, Griffith D, Böke E, (2021). Clustering of aromatic residues in prion-like domains can tune the formation, state, and organization of biomolecular condensates. Biochemistry 60, 3566–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiner BG, Pyo AGT, Meir Y, Wingreen NS, (2021). Motif-pattern dependence of biomolecular phase separation driven by specific interactions. PLoS Comput. Biol. 17, e1009748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murthy AC, Dignon GL, Kan Y, Zerze GH, Parekh SH, Mittal J, Fawzi NL, (2019). Molecular interactions underlying liquid-liquid phase separation of the FUS low-complexity domain. Nat. Struct. Mol. Biol. 26, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harmon TS, Holehouse AS, Pappu RV, (2018). Differential solvation of intrinsically disordered linkers drives the formation of spatially organized droplets in ternary systems of linear multivalent proteins. New J. Phys. 20, 045002. [Google Scholar]
- 96.Ranganathan S, Shakhnovich EI, (2020). Dynamic metastable long-living droplets formed by sticker-spacer proteins. Elife 9 10.7554/eLife.56159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi J-M, Hyman AA, Pappu RV, (2020). Generalized models for bond percolation transitions of associative polymers. Phys Rev E. 102, 042403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohan MC, Pappu RV, (2020). Making the case for disordered proteins and biomolecular condensates in bacteria. Trends Biochem. Sci. 45, 668–680. [DOI] [PubMed] [Google Scholar]
- 99.Harmon TS, Holehouse AS, Rosen MK, Pappu RV, (2017). Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. Elife 6 10.7554/eLife.30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhandari K, Cotten MA, Kim J, Rosen MK, Schmit JD, (2021). Structure-function properties in disordered condensates. J. Phys. Chem. B. 125, 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmit JD, Bouchard JJ, Martin EW, Mittag T, (2020). Protein network structure enables switching between liquid and gel states. J. Am. Chem. Soc. 142, 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, et al. , (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teif VB, Bohinc K, (2011). Condensed DNA: condensing the concepts. Prog. Biophys. Mol. Biol. 105, 208–222. [DOI] [PubMed] [Google Scholar]
- 104.Post CB, Zimm BH, (1982). Theory of DNA condensation: collapse versus aggregation. Biopolymers 21, 2123–2137. [DOI] [PubMed] [Google Scholar]
- 105.Cubuk J, Alston JJ, Jeremías Incicco J, Singh S, Stuchell-Brereton MD, Ward MD, Zimmerman MI, Vithani N, et al. , (2020). The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. bioRxiv 12 10.1101/2020.06.17.158121.2020.06.17.158121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iserman C, Roden CA, Boerneke MA, Sealfon RSG, McLaughlin GA, Jungreis I, Fritch EJ, Hou YJ, et al. , (2020). Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol. Cell. 80, 1078–1091.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu S, Ye Q, Singh D, Cao Y, Diedrich JK, Yates JR 3rd, Villa E, Cleveland DW, et al. , (2021). The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein. Nat. Commun. 12, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adam MA, Miller AD, (1988). Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J. Virol. 62, 3802–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dornburg R, Temin HM, (1990). Presence of a retroviral encapsidation sequence in nonretroviral RNA increases the efficiency of formation of cDNA genes. J. Virol. 64, 886–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mansky LM, Krueger AE, Temin HM, (1995). The bovine leukemia virus encapsidation signal is discontinuous and extends into the 5’ end of the gag gene. J. Virol. 69, 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vile RG, Ali M, Hunter E, McClure MO, (1992). Identification of a generalised packaging sequence for Dtype retroviruses and generation of a D-type retroviral vector. Virology 189, 786–791. [DOI] [PubMed] [Google Scholar]
- 112.Ding P, Kharytonchyk S, Waller A, Mbaekwe U, Basappa S, Kuo N, Frank HM, Quasney C, et al. , (2020). Identification of the initial nucleocapsid recognition element in the HIV-1 RNA packaging signal. Proc. Natl. Acad. Sci. U. S. A. 117, 17737–17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bessa LM, Guseva S, Camacho-Zarco AR, Salvi N, Maurin D, Perez LM, Botova M, Malki A, et al. , (2022). The intrinsically disordered SARS-CoV-2 nucleoprotein in dynamic complex with its viral partner nsp3a. Sci Adv. 8, eabm4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klein S, Cortese M, Winter SL, Wachsmuth-Melm M, Neufeldt CJ, Cerikan B, Stanifer ML & Boulant S et al. (n.d.). SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography, 10.1101/2020.06.23.167064. [DOI] [PMC free article] [PubMed]
- 115.Hsu CC, Prausnitz JM, (1974). Thermodynamics of polymer compatibility in ternary systems. Macromolecules 7, 320–324. [Google Scholar]
- 116.Koningsveld R, Staverman AJ, (1967). Liquid-liquid phase separation in multicomponent polymer solutions. Colloid Polym. Sci. 220, 31–40. [Google Scholar]
- 117.Deviri D, Safran SA, (2021). Physical theory of biological noise buffering by multicomponent phase separation. Proc. Natl. Acad. Sci. U. S. A. 118 10.1073/pnas.2100099118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown CJ, Johnson AK, Dunker AK, Daughdrill GW, (2011). Evolution and disorder. Curr. Opin. Struct. Biol. 21, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Holehouse AS, Das RK, Ahad JN, Richardson MOG, Pappu RV, (2017). CIDER: resources to analyze sequence-ensemble relationships of intrinsically disordered proteins. Biophys. J. 112, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Xu B, Weiner BG, Meir Y, Wingreen NS, (2021). Decoding the physical principles of two-component biomolecular phase separation. Elife 10 10.7554/eLife.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prusty D, Pryamitsyn V, Olvera de la Cruz M., (2018). Thermodynamics of associative polymer blends. Macromolecules 51, 5918–5932. [Google Scholar]
- 122.Nandi SK, Österle D, Heidenreich M, Levy ED, Safran SA, (2022). Affinity and valence impact the extent and symmetry of phase separation of multivalent proteins. Phys. Rev. Lett. 129, 128102. [DOI] [PubMed] [Google Scholar]
- 123.Risso-Ballester J, Galloux M, Cao J, Le Goffic R, Hontonnou F, Jobart-Malfait A, Desquesnes A, Sake SM, et al. , (2021). A condensate-hardening drug blocks RSV replication in vivo. Nature 595, 596–599. [DOI] [PubMed] [Google Scholar]
- 124.Van Lindt J, Bratek-Skicki A, Nguyen PN, Pakravan D, Durán-Armenta LF, Tantos A, Pancsa R, Van Den Bosch L, et al. , (2021). A generic approach to study the kinetics of liquid-liquid phase separation under nearnative conditions. Commun Biol. 4, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ceballos AV, McDonald CJ, Elbaum-Garfinkle S, (2018). Methods and strategies to quantify phase separation of disordered proteins. Methods Enzymol. 611, 31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li P, Zeng X, Li S, Xiang X, Chen P, Li Y, Liu B-F, (2022). Rapid determination of phase diagrams for biomolecular liquid-liquid phase separation with microfluidics. Anal. Chem. 94, 687–694. [DOI] [PubMed] [Google Scholar]
- 127.Milkovic NM, Mittag T, (2020). Determination of protein phase diagrams by centrifugation. In: Kragelund BB., Skriver K. (Eds.), Intrinsically Disordered Proteins: Methods and Protocols. Springer, US, New York, NY, pp. 685–702. [DOI] [PubMed] [Google Scholar]
- 128.Carlson CR, Asfaha JB, Ghent CM, Howard CJ, Hartooni N, Safari M, Frankel AD, Morgan DO, (2020). Phosphoregulation of phase separation by the SARS-CoV-2 N protein suggests a biophysical basis for its dual functions. Mol. Cell. 80, 1092–1103.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ganser LR, Myong S, (2020). Methods to study phase-separated condensates and the underlying molecular interactions. Trends Biochem. Sci. 45, 1004–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Henninger JE, Oksuz O, Shrinivas K, Sagi I, LeRoy G, Zheng MM, Andrews JO, Zamudio AV, et al. , (2021). RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaur T, Alshareedah I, Wang W, Ngo J, Moosa MM, Banerjee PR, (2019). Molecular crowding tunes material states of ribonucleoprotein condensates. Biomolecules 9 10.3390/biom9020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP, (2015). RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. U. S. A. 112, E5237–E5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J, Shi C, Xu Q, Yin H, (2021). SARS-CoV-2 nucleocapsid protein undergoes liquid-liquid phase separation into stress granules through its N-terminal intrinsically disordered region. Cell Discov. 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Z, Zhang G, Zhang H, (2019). Protocol for analyzing protein liquid–liquid phase separation. Biophysics Reports. 5, 1–9. [Google Scholar]
- 135.Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW, (1976). Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alberti S, Gladfelter A, Mittag T, (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peng S, Li W, Yao Y, Xing W, Li P, Chen C, (2020). Phase separation at the nanoscale quantified by dcFCCS. Proc. Natl. Acad. Sci. U. S. A. 117, 27124–27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guillén-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlüßler R, Kim K, Trussina IREA., et al. , (2020). RNA-Induced conformational switching and clustering of G3BP Drive stress granule assembly by condensation. Cell 181, 346–361.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Magde D, Elson E, Webb WW, (1972). Thermodynamic fluctuations in a reacting system--measurement by fluorescence correlation spectroscopy. Phys. Rev. Lett. 29, 705–708. [Google Scholar]
- 140.Kral T, Hof M, Jurkiewicz P, Langner M, (2002). Fluorescence correlation spectroscopy (FCS) as a tool to study DNA condensation with hexadecyltrimethylammonium bromide (HTAB). Cell. Mol. Biol. Lett. 7, 203–211. [PubMed] [Google Scholar]
- 141.Kral T, Hof M, Langner M, (2002). The effect of spermine on plasmid condensation and dye release observed by fluorescence correlation spectroscopy. Biol. Chem. 383, 331–335. [DOI] [PubMed] [Google Scholar]
- 142.Ramisetty SK, Langlete P, Lale R, Dias RS, (2017). In vitro studies of DNA condensation by bridging protein in a crowding environment. Int. J. Biol. Macromol. 103, 845–853. [DOI] [PubMed] [Google Scholar]
- 143.Hodges C, Meiners J-C, (2018). Fluorescence correlation spectroscopy on genomic DNA in living cells. In: Lyubchenko YL. (Ed.), Nanoscale Imaging: Methods and Protocols. Springer, New York, New York, NY, pp. 415–424. [DOI] [PubMed] [Google Scholar]
- 144.Sabanayagam CR, Oram M, Lakowicz JR, Black LW, (2007). Viral DNA packaging studied by fluorescence correlation spectroscopy. Biophys. J. 93, L17–L19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gopal A, Egecioglu DE, Yoffe AM, Ben-Shaul A, Rao ALN, Knobler CM, Gelbart WM, (2014). Viral RNAs are unusually compact. PLoS One 9, e105875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borodavka A, Singaram SW, Stockley PG, Gelbart WM, Ben-Shaul A, Tuma R, (2016). Sizes of long RNA molecules are determined by the branching patterns of their secondary structures. Biophys. J. 111, 2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schuler B, Lipman EA, Steinbach PJ, Kumke M, Eaton WA, (2005). Polyproline and the “spectroscopic ruler” revisited with single-molecule fluorescence. Proc. Nat. Acad. Sci. 102, 2754–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stryer L, Haugland RP, (1967). Energy transfer: a spectroscopic ruler. Proc. Natl. Acad. Sci. U. S. A. 58, 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Niaki AG, Sarkar J, Cai X, Rhine K, Vidaurre V, Guy B, Hurst M, Lee JC, et al. , (2020). Loss of Dynamic RNA Interaction and Aberrant Phase Separation Induced by Two Distinct Types of ALS/FTD-Linked FUS Mutations. Mol. Cell. 77, 82–94.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pelicci S, Diaspro A, Lanzanò L, (2019). Chromatin nanoscale compaction in live cells visualized by acceptor-to-donor ratio corrected Förster resonance energy transfer between DNA dyes. J. Biophotonics. 12, e201900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levchenko SM, Pliss A, Peng X, Prasad PN, Qu J, (2021). Fluorescence lifetime imaging for studying DNA compaction and gene activities. Light Sci Appl. 10, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.König I, Zarrine-Afsar A, Aznauryan M, Soranno A, Wunderlich B, Dingfelder F, Stüber JC, Plückthun A, et al. , (2015). Single-molecule spectroscopy of protein conformational dynamics in live eukaryotic cells. Nat. Methods. 12, 773–779. [DOI] [PubMed] [Google Scholar]
- 153.Schuler B, König I, Soranno A, Nettels D, (2021). Impact of in-cell and in-vitro crowding on the conformations and dynamics of an intrinsically disordered protein. Angew. Chem. Weinheim Bergstr. Ger.. 10.1002/ange.202016804. [DOI] [PubMed] [Google Scholar]
- 154.Wen J, Hong L, Krainer G, Yao Q-Q, Knowles TPJ, Wu S, Perrett S, (2021). Conformational expansion of tau in condensates promotes irreversible aggregation. J. Am. Chem. Soc. 143, 13056–13064. [DOI] [PubMed] [Google Scholar]
- 155.Ray S, Singh N, Patel K, Krishnamoorthy G, Maji SK, (2023). FRAP and FRET Investigation of α-Synuclein Fibrillization via Liquid-Liquid Phase Separation In Vitro and in HeLa Cells. Methods Mol. Biol. 2551, 395–423. [DOI] [PubMed] [Google Scholar]
- 156.Holmstrom ED, Liu Z, Nettels D, Best RB, Schuler B, (2019). Disordered RNA chaperones can enhance nucleic acid folding via local charge screening. Nat. Commun. 10, 2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, Musier-Forsyth K, Falk C, Barbara PF, (2013). Single-molecule spectroscopic study of dynamic nanoscale DNA bending behavior of HIV-1 nucleocapsid protein. J. Phys. Chem. B. 117, 4183–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bustamante CJ, Chemla YR, Liu S, Wang MD, (2021). Optical tweezers in single-molecule biophysics. Nat. Rev. Methods Primers. 1 10.1038/s43586-021-00021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fabian R Jr, Gaire S, Tyson C, Adhikari R, Pegg I, Sarkar A, (2021). A horizontal magnetic tweezers for studying single DNA molecules and DNA-binding proteins. Molecules 26 10.3390/molecules26164781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Butt H-J, Cappella B, Kappl M, (2005). Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 59, 1–152. [Google Scholar]
- 161.Wang Y, Gao T, Li S, Xia W, Zhang W, Yang G, (2019). Direct demonstration of DNA compaction mediated by divalent counterions. J. Phys. Chem. B. 123, 79–85. [DOI] [PubMed] [Google Scholar]
- 162.Gao T, Zhang W, Wang Y, Yang G, (2019). DNA compaction and charge neutralization regulated by divalent ions in very low pH solution. Polymers 11 10.3390/polym11020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van den Broek B, Noom MC, van Mameren J, Battle C, Mackintosh FC, Wuite GJL, (2010). Visualizing the formation and collapse of DNA toroids. Biophys. J. 98, 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baumann CG, Bloomfield VA, Smith SB, Bustamante C, Wang MD, Block SM, (2000). Stretching of single collapsed DNA molecules. Biophys. J. 78, 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baumann CG, Smith SB, Bloomfield VA, Bustamante C, (1997). Ionic effects on the elasticity of single DNA molecules. Proc. Natl. Acad. Sci. U. S. A. 94, 6185–6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nguyen T, Li S, Chang J-T-H, Watters JW, Ng H, Osunsade A, David Y, Liu S, (2022). Chromatin sequesters pioneer transcription factor Sox2 from exerting force on DNA. Nat. Commun. 13, 3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Renger R, Morin JA, Lemaitre R, Ruer-Gruss M, Jülicher F, Hermann A, Grill SW, (2022). Co-condensation of proteins with single- and double-stranded DNA. Proc. Natl. Acad. Sci. U. S. A. 119 e2107871119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gien H, Morse M, McCauley MJ, Kitzrow JP, Musier-Forsyth K, Gorelick RJ, Rouzina I, Williams MC, (2022). HIV-1 nucleocapsid protein binds double-stranded DNA in multiple modes to regulate compaction and capsid uncoating. Viruses 14 10.3390/v14020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xi B, Ran S-Y, (2017). Formation of DNA pearl-necklace structures on mica surface governed by kinetics and thermodynamics. J. Polym. Sci. B Polym. Phys. 55, 971–979. [Google Scholar]
- 170.Quail T, Golfier S, Elsner M, Ishihara K, Murugesan V, Renger R, Jülicher F, Brugue s, J, (2021). Force generation by protein–DNA co-condensation. Nat. Phys. 17, 1007–1012. [Google Scholar]
- 171.Alshareedah I, Kaur T, Banerjee PR, (2021). Methods for characterizing the material properties of biomolecular condensates. Methods Enzymol. 646, 143–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alshareedah I, Moosa MM, Raju M, Potoyan DA, Banerjee PR, (2020). Phase transition of RNA–protein complexes into ordered hollow condensates. Proc. Nat. Acad. Sci. 117, 15650–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gui X, Luo F, Li Y, Zhou H, Qin Z, Liu Z, Gu J, Xie M, et al. , (2019). Structural basis for reversible amyloids of hnRNPA1 elucidates their role in stress granule assembly. Nat. Commun. 10, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jawerth LM, Ijavi M, Ruer M, Saha S, Jahnel M, Hyman AA, Jülicher F, Fischer-Friedrich E, (2018). Salt-dependent rheology and surface tension of protein condensates using optical traps. Phys. Rev. Lett. 121, 258101. [DOI] [PubMed] [Google Scholar]
- 175.Jawerth L, Fischer-Friedrich E, Saha S, Wang J, Franzmann T, Zhang X, Sachweh J, Ruer M, et al. , (2020). Protein condensates as aging Maxwell fluids. Science 370, 1317–1323. [DOI] [PubMed] [Google Scholar]
- 176.Greene EC, Wind S, Fazio T, Gorman J, Visnapuu M-L, (2010). Chapter 14 - DNA curtains for high-throughput single-molecule optical imaging. In: Walter NG. (Ed.), Methods in Enzymology. Academic Press, pp. 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schaub JM, Zhang H, Soniat MM, Finkelstein IJ, (2018). Assessing protein dynamics on low-complexity single-stranded DNA curtains. Langmuir 34, 14882–14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zuo L, Zhang G, Massett M, Cheng J, Guo Z, Wang L, Gao Y, Li R, et al. , (2021). Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat. Commun. 12, 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Keenen MM, Brown D, Brennan LD, Renger R, Khoo H, Carlson CR, Huang B, Grill SW, et al. , (2021). HP1 proteins compact DNA into mechanically and positionally stable phase separated domains. Elife 10 10.7554/eLife.64563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Calcines-Cruz C, Finkelstein IJ, Hernandez-Garcia A, (2021). CRISPR-guided programmable self-assembly of artificial virus-like nucleocapsids. Nano Lett. 21, 2752–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Murthy AC, Fawzi NL, (2020). The (un)structural biology of biomolecular liquid-liquid phase separation using NMR spectroscopy. J. Biol. Chem. 295, 2375–2384. 10.1074/jbc.rev119.009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Summers MF, South TL, Kim B, Hare DR, (1990). High-resolution structure of an HIV zinc fingerlike domain via a new NMR-based distance geometry approach. Biochemistry 29, 329–340. [DOI] [PubMed] [Google Scholar]
- 183.South TL, Blake PR, Hare DR, Summers MF, (1991). C-terminal retroviral-type zinc finger domain from the HIV-1 nucleocapsid protein is structurally similar to the N-terminal zinc finger domain. Biochemistry 30, 6342–6349. [DOI] [PubMed] [Google Scholar]
- 184.Cheong HK, Cheong C, Choi BS, (1996). Secondary structure of the panhandle RNA of influenza virus A studied by NMR spectroscopy. Nucleic Acids Res. 24, 4197–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee M-K, Bae S-H, Park C-J, Cheong H-K, Cheong C, Choi B-S, (2003). A single-nucleotide natural variation (U4 to C4) in an influenza A virus promoter exhibits a large structural change: implications for differential viral RNA synthesis by RNA-dependent RNA polymerase. Nucleic Acids Res. 31, 1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lu K, Heng X, Garyu L, Monti S, Garcia EL, Kharytonchyk S, Dorjsuren B, Kulandaivel G, et al. , (2011). NMR detection of structures in the HIV-1 5’-leader RNA that regulate genome packaging. Science 334, 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brown JD, Kharytonchyk S, Chaudry I, Iyer AS, Carter H, Becker G, Desai Y, Glang L, et al. , (2020). Structural basis for transcriptional start site control of HIV-1 RNA fate. Science 368, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wacker A, Weigand JE, Akabayov SR, Altincekic N, Bains JK, Banijamali E, Binas O, Castillo-Martinez J, et al. , (2020). Secondary structure determination of conserved SARS-CoV-2 RNA elements by NMR spectroscopy. Nucleic Acids Res. 48, 12415–12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun Y-T, Varani G, (2022). Structure of the dengue virus RNA promoter. RNA 28, 1210–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang C, Ndassa Y, Summers MF, (2002). Structure of the N-terminal 283-residue fragment of the immature HIV-1 Gag polyprotein. Nat. Struct. Biol. 9, 537–543. [DOI] [PubMed] [Google Scholar]
- 191.Shen Q, Cho J-H, (2019). The structure and conformational plasticity of the nonstructural protein 1 of the 1918 influenza A virus. Biochem. Biophys. Res. Commun. 518, 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Whitehead RD 3rd, Teschke CM, Alexandrescu AT, (2019). NMR mapping of disordered segments from a viral scaffolding protein enclosed in a 23 MDa procapsid. Biophys. J. 117, 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schiavina M, Pontoriero L, Uversky VN, Felli IC, Pierattelli R, (2021). The highly flexible disordered regions of the SARS-CoV-2 nucleocapsid N protein within the 1–248 residue construct: sequence-specific resonance assignments through NMR. Biomol. NMR Assign. 15, 219–227. 10.1007/s12104-021-10009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dinesh DC, Chalupska D, Silhan J, Koutna E, Nencka R, Veverka V, Boura E, (2020). Structural basis of RNA recognition by the SARS-CoV-2 nucleocapsid phosphoprotein. PLoS Pathog. 16, e1009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Redzic JS, Lee E, Born A, Issaian A, Henen MA, Nichols PJ, Blue A, Hansen KC, et al. , (2021). The Inherent dynamics and interaction sites of the SARS-CoV-2 nucleocapsid N-terminal region. J. Mol. Biol. 433, 167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pontoriero L, Schiavina M, Korn SM, Schlundt A, Pierattelli R, Felli IC, (2022). NMR reveals specific tracts within the intrinsically disordered regions of the SARS-CoV-2 nucleocapsid protein involved in RNA encountering. Biomolecules 12 10.3390/biom12070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Burke KA, Janke AM, Rhine CL, Fawzi NL, (2015). Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell. 60, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Emmanouilidis L, Esteban-Hofer L, Damberger FF, de Vries T, Nguyen CKX, Ibáñez LF, Mergenthal S, Klotzsch E, et al. , (2021). NMR and EPR reveal a compaction of the RNA-binding protein FUS upon droplet formation. Nat. Chem. Biol. 17, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Emmanouilidis L, Esteban-Hofer L, Jeschke G, Allain F-H-T, (2021). Structural biology of RNA-binding proteins in the context of phase separation: What NMR and EPR can bring? Curr. Opin. Struct. Biol. 70, 132–138. [DOI] [PubMed] [Google Scholar]
- 200.Pollack L, (2011). SAXS studies of ion-nucleic acid interactions. Annu. Rev. Biophys. 40, 225–242. [DOI] [PubMed] [Google Scholar]
- 201.Kikhney AG, Svergun DI, (2015). A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins. FEBS Lett. 589, 2570–2577. [DOI] [PubMed] [Google Scholar]
- 202.Lake JA, (1967). Yeast transfer RNA: a small-angle x-ray study. Science 156, 1371–1373. [DOI] [PubMed] [Google Scholar]
- 203.Russell R, Millett IS, Tate MW, Kwok LW, Nakatani B, Gruner SM, Mochrie SGJ, Pande V, et al. , (2002). Rapid compaction during RNA folding. Proc. Natl. Acad. Sci. U. S. A. 99, 4266–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Caliskan G, Hyeon C, Perez-Salas U, Briber RM, Woodson SA, Thirumalai D, (2005). Persistence length changes dramatically as RNA folds. Phys. Rev. Lett. 95, 268303. [DOI] [PubMed] [Google Scholar]
- 205.Welty R, Pabit SA, Katz AM, Calvey GD, Pollack L, Hall KB, (2018). Divalent ions tune the kinetics of a bacterial GTPase center rRNA folding transition from secondary to tertiary structure. RNA 24, 1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Koltover I, Wagner K, Safinya CR, (2000). DNA condensation in two dimensions. Proc. Natl. Acad. Sci. U. S. A. 97, 14046–14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qiu X, Andresen K, Lamb JS, Kwok LW, Pollack L, (2008). Abrupt transition from a free, repulsive to a condensed, attractive DNA phase, induced by multivalent polyamine cations. Phys. Rev. Lett. 101, 228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Baker MAB, Tuckwell AJ, Berengut JF, Bath J, Benn F, Duff AP, Whitten AE, Dunn KE, et al. , (2018). Dimensions and global twist of single-layer DNA origami measured by small-angle X-ray scattering. ACS Nano 12, 5791–5799. [DOI] [PubMed] [Google Scholar]
- 209.Ober MF, Baptist A, Wassermann L, Heuer-Jungemann A, Nickel B, (2022). In situ small-angle X-ray scattering reveals strong condensation of DNA origami during silicification. Nat. Commun. 13, 5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Souza BBS, Lourenço TC., Gerbelli BB., Oseliero Filho PL., Oliveira CLP., Miranda., da Silva ER, (2022). A biophysical study of DNA condensation mediated by histones and protamines. J. Mol. Liq. 368, 120745. [Google Scholar]
- 211.Li L, Pabit SA, Meisburger SP, Pollack L, (2011). Double-stranded RNA resists condensation. Phys. Rev. Lett. 106, 108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Martin EW, Harmon TS, Hopkins JB, Chakravarthy S, Incicco JJ, Schuck P, Soranno A, Mittag T, (2021). A multi-step nucleation process determines the kinetics of prion-like domain phase separation. Nat. Commun. 12, 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Best RB, (2022). Atomistic Force Fields for Proteins. Methods Mol. Biol. 2019, 3–19. [DOI] [PubMed] [Google Scholar]
- 214.Alston JJ, Soranno A, Holehouse AS, (2021). Integrating single-molecule spectroscopy and simulations for the study of intrinsically disordered proteins. Methods 193, 116–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dignon GL, Zheng W, Kim YC, Best RB, Mittal J, (2018). Sequence determinants of protein phase behavior from a coarse-grained model. PLoS Comput. Biol. 14, e1005941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Young MA, Jayaram B, Beveridge DL, (1997). Intrusion of counterions into the spine of hydration in the minor groove of B-DNA: fractional occupancy of electronegative pockets. J. Am. Chem. Soc. 119, 59–69. [Google Scholar]
- 217.Giambasu GM, Luchko T, Herschlag D, York DM, Case DA, (2014). Ion counting from explicit-solvent simulations and 3D-RISM. Biophys. J. 106, 883–894. 10.1016/j.bpj.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Savelyev A, MacKerell AD Jr, (2015). Competition among Li(+), Na(+), K(+), and Rb(+) monovalent ions for DNA in molecular dynamics simulations using the additive CHARMM36 and Drude polarizable force fields. J. Phys. Chem. B. 119, 4428–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yoo J, Aksimentiev A, (2012). Competitive binding of cations to duplex DNA revealed through molecular dynamics simulations. J. Phys. Chem. B. 116, 12946–12954. [DOI] [PubMed] [Google Scholar]
- 220.Pasi M, Maddocks JH, Lavery R, (2015). Analyzing ion distributions around DNA: sequence-dependence of potassium ion distributions from microsecond molecular dynamics. Nucleic Acids Res. 43, 2412–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xi K, Wang F-H, Xiong G, Zhang Z-L, Tan Z-J, (2018). Competitive binding of Mg2+ and Na+ Ions to nucleic acids: from helices to tertiary structures. Biophys. J. 114, 1776–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kar M, Dar F, Welsh TJ, Vogel LT, Kühnemuth R, Majumdar A, Krainer G, Franzmann TM, et al. , (2022). Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc. Natl. Acad. Sci. U. S. A. 119 e2202222119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cubuk J, Alston JJ, Incicco JJ, Singh S, Stuchell-Brereton MD, Ward MD, Zimmerman MI, Vithani N, et al. , (2021). The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 12, 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ashbaugh HS, Hatch HW, (2008). Natively unfolded protein stability as a coil-to-globule transition in charge/hydropathy space. J. Am. Chem. Soc. 130, 9536–9542. [DOI] [PubMed] [Google Scholar]
- 225.Ashbaugh HS, (2009). Tuning the globular assembly of hydrophobic/hydrophilic heteropolymer sequences. J. Phys. Chem. B. 113, 14043–14046. [DOI] [PubMed] [Google Scholar]
- 226.Dignon GL, Zheng W, Mittal J, (2019). Simulation methods for liquid–liquid phase separation of disordered proteins. Curr. Opin. Chem. Eng. 23, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Regy RM, Dignon GL, Zheng W, Kim YC, Mittal J, (2020). Sequence dependent phase separation of protein-polynucleotide mixtures elucidated using molecular simulations. Nucleic Acids Res. 48, 12593–12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tejedor AR, Garaizar A, Ramírez J, Espinosa JR, (2021). ‘RNA modulation of transport properties and stability in phase-separated condensates. Biophys. J. 120, 5169–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tesei G, Schulze TK, Crehuet R & Lindorff-Larsen K (n.d.). Accurate model of liquid-liquid phase behaviour of intrinsically-disordered proteins from optimization of single-chain properties, 10.1101/2021.06.23.449550. [DOI] [PMC free article] [PubMed]
- 230.Kapcha LH, Rossky PJ, (2014). A simple atomic-level hydrophobicity scale reveals protein interfacial structure. J. Mol. Biol. 426, 484–498. [DOI] [PubMed] [Google Scholar]
- 231.Kim YC, Hummer G, (2008). Coarse-grained models for simulations of multiprotein complexes: application to ubiquitin binding. J. Mol. Biol. 375, 1416–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Vernon RM, Chong PA, Tsang B, Kim TH, Bah A, Farber P, Lin H, Forman-Kay JD, (2018). Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7 10.7554/eLife.31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fisher RS, Elbaum-Garfinkle S, (2020). Tunable multiphase dynamics of arginine and lysine liquid condensates. Nat. Commun. 11, 4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, et al. , (2016). Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gibson BA, Doolittle LK, Schneider MWG, Jensen LE, Gamarra N, Henry L, Gerlich DW, Redding S, et al. , (2019). Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jack A, Kim Y, Strom AR, Lee DSW, Williams B, Schaub JM, Kellogg EH, Finkelstein IJ, et al. , (2022). Compartmentalization of telomeres through DNA-scaffolded phase separation. Dev. Cell. 57, 277–290.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Overbeek JTG, Voorn MJ, (1957). Phase separation in polyelectrolyte solutions. Theory of complex coacervation. J. Cell. Comp. Physiol. 49, 7–26. 10.1002/jcp.1030490404. [DOI] [PubMed] [Google Scholar]
- 238.Sing CE, (2017). Development of the modern theory of polymeric complex coacervation. Adv. Colloid Interface Sci. 239, 2–16. [DOI] [PubMed] [Google Scholar]
- 239.Sing CE, Perry SL, (2020). Recent progress in the science of complex coacervation. Soft Matter 16, 2885–2914. [DOI] [PubMed] [Google Scholar]
- 240.Boeynaems S, Holehouse AS, Weinhardt V, Kovacs D, Van Lindt J, Larabell C, Van Den Bosch L, Das R, et al. , (2019). Spontaneous driving forces give rise to protein–RNA condensates with coexisting phases and complex material properties. Proc. Nat. Acad. Sci. 116, 7889–7898. 10.1073/pnas.1821038116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Alshareedah I, Kaur T, Ngo J, Seppala H, Kounatse L-A-D, Wang W, Moosa MM, Banerjee PR, (2019). Interplay between short-range attraction and long-range repulsion controls reentrant liquid condensation of ribonucleoprotein-RNA complexes. J. Am. Chem. Soc. 141, 14593–14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Laghmach R, Alshareedah I, Pham M, Raju M, Banerjee PR, Potoyan DA, (2022). RNA chain length and stoichiometry govern surface tension and stability of protein-RNA condensates. iScience 25, 104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Alshareedah I, Moosa MM, Pham M, Potoyan DA, Banerjee PR, (2021). Programmable viscoelasticity in protein-RNA condensates with disordered sticker-spacer polypeptides. Nat. Commun. 12, 6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cubuk J, Soranno A, (2022). Macromolecular crowding and intrinsically disordered proteins: A polymer physics perspective. ChemSystemsChem.. 10.1002/syst.202100051. [DOI] [Google Scholar]
- 245.Soranno A, Koenig I, Borgia MB, Hofmann H, Zosel F, Nettels D, Schuler B, (2014). Single-molecule spectroscopy reveals polymer effects of disordered proteins in crowded environments. Proc. Natl. Acad. Sci. U. S. A. 111, 4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


