Abstract
The tRNA of the miaB2508::Tn10dCm mutant of Salmonella typhimurium is deficient in the methylthio group of the modified nucleoside N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A37). By sequencing, we found that the Tn10dCm of this strain had been inserted into the f474 (yleA) open reading frame, which is located close to the nag locus in both S. typhimurium and Escherichia coli. By complementation of the miaB2508::Tn10dCm mutation with a minimal subcloned f474 fragment, we showed that f474 could be identified as the miaB gene, which is transcribed in the counterclockwise direction on the bacterial chromosome. Transcriptional studies revealed two promoters upstream of miaB in E. coli and S. typhimurium. A Rho-independent terminator was identified downstream of the miaB gene, at which the majority (96%) of the miaB transcripts terminate in E. coli, showing that the miaB gene is part of a monocistronic operon. A highly conserved motif with three cysteine residues was present in MiaB. This motif resembles iron-binding sites in other proteins. Only a weak similarity to an AdoMet-binding site was found, favoring the idea that the MiaB protein is involved in the thiolation step and not in the methylating reaction of ms2i(o)6A37 formation.
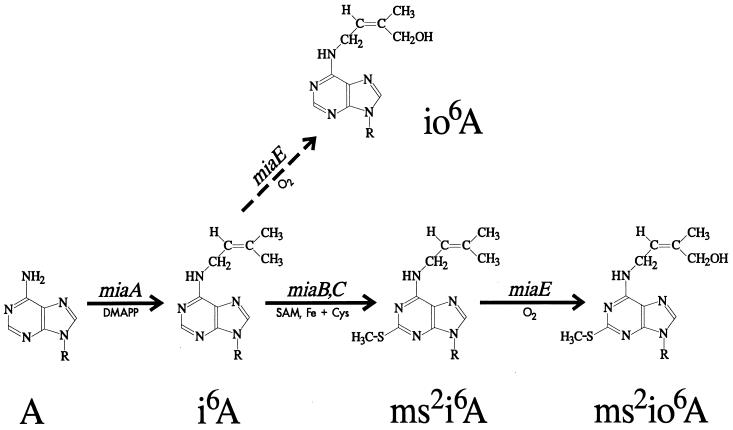
Isopentenylated adenine is frequently found at position 37 of eubacterial and some eukaryotic tRNAs that read codons with U in the first position. In Escherichia coli, 2-methylthio-N6-(isopentenyl)adenosine (ms2i6A) is found, whereas Salmonella typhimurium contains the hydroxylated derivative (ms2io6A) (9). The majority of the modified nucleosides (including ms2io6A) are synthesized after transcription of the tRNA has been completed (23, 53), and the formation of ms2io6A is thought to proceed according to the pathway depicted in Fig. 1. The MiaA enzyme catalyzes the first step, transfer of a dimethylallyl group from dimethylallyl diphosphate onto the adenine at position 37 of the tRNA (22, 35, 41). An iron-requiring methylthiolation then follows (8, 46, 61). Since a miaA mutant contains mainly A37 and not ms2A37 (18, 59), the enzyme(s) involved in the methylthiolation appears to recognize the isopentenyl group. This second step is believed to involve at least two distinct reactions, thiolation of i6A37 to s2i6A37 (1) and methyl transfer from S-adenosylmethionine (24), giving ms2i6A37. In S. typhimurium, but not in E. coli, the isopentenyl group is further modified by the addition of a hydroxyl group, resulting in ms2io6A37. This last step is catalyzed by the MiaE protein (42) and occurs only aerobically (6). The hydroxylating enzyme seems to recognize the ms2 group, since only small amounts of io6A are produced when methylthiolation is physiologically (6) or genetically (20) disturbed.
FIG. 1.
Postulated biosynthetic pathway for ms2i6A in E. coli and ms2io6A in S. typhimurium. The gene designations correspond to identified genetic loci or postulated (not yet identified) functions. Known cofactors and substrates are indicated. DMAPP, dimethylallyl diphosphate; SAM, S-adenosyl-l-methionine; R, the ribose moiety. The dashed arrow indicates that the reaction in question is used to a lesser extent. The hydroxylation reaction (mediated by MiaE, giving io6A and ms2io6A) does not occur in E. coli.
We previously described an S. typhimurium mutant (miaB2508::Tn10dCm) whose tRNA lacks the methylthiolated species and instead contains i6A37 (20). To gain further insight into the mechanism of the methylthiolation reaction, we have now identified the affected gene. Complementation analysis established that the f474 (yleA) open reading frames (ORFs) of E. coli and S. typhimurium encode a protein involved in the methylthiolation step of the biosynthesis of ms2i(o)6A [ms2i(o)6A denotes either ms2i6A or ms2io6A].
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) medium or glucose minimal medium (58). For growth of S. typhimurium cultures to be used in P22 transduction, NAA medium was used (20). Chloramphenicol, tetracycline, kanamycin, ampicillin, and carbenicillin were added to 12.5, 25, 50, 50, and 50 μg per ml, respectively, in LB medium. In minimal medium, 12.5 μg of chloramphenicol per ml or 6.25 μg of tetracycline per ml was used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 ΔlacU169 (φ80 lacZΔM15) | Bethesda Research Laboratories |
| CC104 | F′ lacI378 lacZ-GCG proB+/ara Δ(lac-pro)XIII | 14 |
| TX3325 | mutS::Tn10 miaB::Tn10dCm | This work |
| TX3346 | F′ lacI378 lacZ-GCG proB+/ara Δ(lac-pro)XIIImiaB::Tn10dCm | This work |
| S. typhimurium | ||
| GT522 | Wild type | 19 |
| GT907 | metA22 metE551 ilv-452 trpB2 xyl-404 rpsL120 flaA66 hsdL66 hsdA29 galE503 | This work |
| GT1825 | hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5/F′ del-14 lacIam 117 proAB+ | 20 |
| GT1975 | hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5 miaB2508::Tn10dCm/F′ del-14 lacIam 117 proAB+ | 20 |
| GT2176a | miaB2508::Tn10dCm phs | 20 |
| GT2734a | miaB2508::Tn10dCm | This work |
| GT3171 | miaB2508::Tn10dCm zbf-99::Tn10 | This work |
| GT3828 | miaB26 hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5/F′ del-14 lacIam117 proAB+ | This work |
| GT3830 | miaB27 hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5/F′ del-14 lacIam117 proAB+ | This work |
| GT3831 | miaB28 hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5/F′ del-14 lacIam117 proAB+ | This work |
| GT3833 | miaB29 hisO1242 hisD6404(Am) leuA414(Am) supF30 pro-688::Tn5/F′ del-14 lacIam117 proAB+ | This work |
| GT5441 | pUST138/miaB2508::Tn10dCm | This work |
| GT5442 | pBluescript SK/miaB2508::Tn10dCm | This work |
| GT5443 | zbf-99::Tn10 miaB26 | This work |
| GT5444 | zbf-99::Tn10 miaB27 | This work |
| GT5445 | zbf-99::Tn10 miaB28 | This work |
| GT5446 | zbf-99::Tn10 miaB29 | This work |
| GT5766 | pUST190/zbf-99::Tn10 miaB26 | This work |
| GT5767 | pUST190/zbf-99::Tn10 miaB27 | This work |
| GT5768 | pUST190/zbf-99::Tn10 miaB28 | This work |
| GT5769 | pUST190/zbf-99::Tn10 miaB29 | This work |
| MST1934 | galE496 mutL111::Tn10 metA22 metE55 rpsL120 xyl-404 fels2 HI-6 nml H2 enx hsdL-6 hsdA29 | S. R. Maloy |
| TT2342 | zbf-99::Tn10 supE(Su2) hisC527 leuA414 | J. R. Roth |
| Plasmids | ||
| pTX637 | pGEM3Z containing a 629-bp PCR fragment amplified from strain CC104 chromosome DNA with MiaBP1 and MiaBP2 primers | This work |
| pTX638 | pGEM3Z containing a 948-bp PCR fragment amplified from strain CC104 chromosome DNA with MiaBP1 and MiaBP2 primers | This work |
| pTX639 | pGEM3Z containing a 641-bp PCR fragment amplified from strain CC104 chromosome DNA with MiaBT1 and MiaBT2 primers | This work |
| pTX640 | pGEM3Z containing a 950-bp PCR fragment amplified from strain CC104 chromosome DNA with MiaBT1 and MiaBT3 primers | This work |
| pTX641 | Apr pBR322 vector containing a 2.4-kb chromosomal AatII insert from E. coli carrying the Tn10dCm inserted in the miaB gene | This work |
| pTX642 | Apr pKK223-3 vector containing a 1.55-kb E. coli miaB+ PCR fragment generated by amplification with the miaBSmaI and miaBPstI primers | This work |
| pUST135 | Cbr pT7Blue(R) vector containing a 1.3-kb fragment generated by PCR amplification of the GT3171 chromosome with the Tn10XbaI and T-L primers | This work |
| pUST136 | Kmr pLG339 vector containing a 5.5-kb chromosomal S. typhimurium Sau3A fragment including the miaB gene | This work |
| pUST137 | Cbr pBluescript SK vector carrying a 3.7-kb miaB+ NarI-ClaI fragment from pUST136 | This work |
| pUST138 | Cbr pBluescript SK vector carrying a 2.3-kb miaB+ fragment from pUST137 | This work |
| pUST190 | Strr pCL1921 vector carrying a 2.3-kb miaB+ NarI-NruI fragment from pUST136 | This work |
In reference 20, the genotype of strain GT2176 was reported as miaB2508::Tn10dCm. However, we found that this strain harbored an additional mutation. This second mutation, possibly located in the phs locus, rendered cells unable to produce H2S. This phenotype was not linked to the miaB mutation, and a new miaB2508::Tn10dCm strain (GT2734) was constructed by P22 transduction.
Plasmid constructions.
DNA inserts in plasmids pTX637 to pTX640, which were used in mRNA mapping (below), were amplified by PCR with the primers listed in Table 2. HindIII sites were introduced into primers MiaBP1, MiaBT2, and MiaBT3, and BamHI sites were introduced into primers MiaBP2, MiaBP3, and MiaBT1. PCR fragments amplified from strain CC104 chromosomal DNA with MiaBP1-MiaBP2, MiaBP1-MiaBP3, MiaBT1-MiaBT2, and MiaBT1-MiaBT3 were first cut with HindIII and BamHI and then cloned into pGEM3Z (Promega) (cut with HindIII and BamHI) to make plasmids pTX637, pTX638, pTX639, and pTX640, respectively.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea | Target site |
|---|---|---|
| MiaBP1 | 5′-ATAGCCAAGCTTCGGTAGCTTAAGTTC-3′ | E. coli o391 (nt 8697–8671 in accession no. AE000170) |
| MiaBP2 | 5′-TTCCAGGGATCCAACTGATGGAAGAC-3′ | E. coli miaB (nt 8032–8057 in AE000170) |
| MiaBP3 | 5′-GGTGTAAGGATCCACGCAGTAGGTGC-3′ | E. coli miaB (nt 7725–7750 in AE000170) |
| miaBPstI | 5′-AGGCCCACCGGAACTGCAGGCCTG-3′ | 4 bp downstream of the UAA codon of E. coli miaB (nt 6779–6802 in AE000170) |
| miaBSmaI | 5′-ATTTTTACCCC*GGGCTGATAACGCTCA-3′ | 105 bp upstream of E. coli miaB AUG start codon (nt 8336–8307 in AE000170) |
| MiaBT1 | 5′-AAGGTGGATCCGTTCCGGTTCGATATC-3′ | E. coli f359a (nt 6394–6420 in AE000170) |
| MiaBT2 | 5′-ATCATGAAGCTTTCCGGGCGTACGG-3′ | E. coli miaB (nt 7043–7019 in AE000170) |
| MiaBT3 | 5′-TCCGTAAGCTTCGTGCGGCGCGTCC-3′ | E. coli miaB (nt 7363–7339 in AE000170) |
| MotH | 5′-AGGGACGGCTGACTTCTTCACC-3′ | S. typhimurium miaB (nt 1165–1144 in AJ249116) |
| PCR1 | 5′-AATACCGAGACGACGTTCGAGCTG-3′ | S. typhimurium f359a (nt 2376–2353 in AJ249116) |
| PCR2 | 5′-AAATCCGCACGTCAGGCTGGCTG-3′ | S. typhimurium o391 (nt 334–356 in AJ249116) |
| Sekv14 | 5′-TACTCGTTCATCTGACAGCC-3′ | S. typhimurium miaB (nt 683–664 in AJ249116) |
| T-I | 5′-GACAAGATGTGTATCCACCTTAAC-3′ | IS10R, facing outward |
| T-L | 5′-ACCTTTGGTCACCAACGCTTTTCC-3′ | Tn10dTc, ∼70 bp from the left end, facing outward |
| Tn10XbaI | 5′-TAATCTAGAACCTCTTACGTGCC-3′ | Tn10dCm, 342 bp from the end, facing outward |
The underlined bases represent synthetic restriction sites. Base changes introduced to create the restriction sites are in boldface. The ∗ in miaBSmaI means that there are three additional bases (TAA) in this place in the E. coli chromosomal sequence.
Plasmid pTX641, which contains a 2.4-kb fragment covering the Tn10dCm transposon and the flanking chromosomal region, was constructed by AatII digestion of chromosomal DNA from E. coli TX3346. The digested DNA was ligated into the AatII site of plasmid pBR322 (5). The chromosomal DNA sequences flanking the transposon were 600 and 300 bp.
pTX642 contains the wild-type miaB gene of E. coli. It was constructed by PCR amplification from the genome of strain CC104 by using primers miaBSmaI and miaBPstI. The amplified 1.55-kb fragment was cloned into the SmaI-PstI-digested plasmid pKK223-3 (Pharmacia Biotech) by using the engineered restriction sites of the primers.
The miaB gene of S. typhimurium was subcloned from the 5.5-kb miaB+ chromosomal insert of plasmid pUST136 (see Fig. 2). A 3.7-kb NarI fragment, covering miaB (f474), f359a, and parts of the o391 and f155b ORFs, was ligated into the high-copy-number vector pBluescript SK (Stratagene, La Jolla, Calif.). This construct was denoted pUST137. A minimal miaB+ construct was made by digestion of pUST137 with NruI and SmaI. A 2.3-kb fragment was removed, and the remaining 5.2-kb miaB+ fragment was circularized by ligation, giving plasmid pUST138. A corresponding low-copy-number construct was made by isolating a 2.3-kb miaB+ NarI-NruI fragment from pUST136. The ends of the fragment were made blunt by filling in with Klenow DNA polymerase I (New England Biolabs, Beverly, Mass.). The fragment was ligated into the SmaI site of the pCL1921 vector (34), resulting in plasmid pUST190.
FIG. 2.
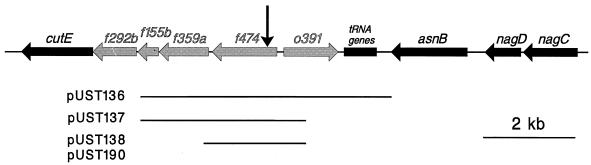
Genetic organization of the region between the cutE (lnt) gene and the nag operon at 14.8 to 15.1 min of the E. coli chromosome. Previously identified genes and their directions of transcription are indicated by solid arrows. The tRNA cluster is represented by a solid box. Shaded arrows indicate putative ORFs. (There is also an alternative nomenclature for the E. coli ORFs, where f474 is denoted yleA, o391 is yleB, f359a is ybeZ, and f155b is ybeY.) The vertical arrow indicates the point where the miaB::Tn10dCm transposon had been inserted. Horizontal bars represent the inserts of the S. typhimurium plasmids. Plasmids pUST138 and pUST190 are high-copy number and low-copy number versions, respectively, carrying the same insert.
The ability of putative miaB+ plasmids to complement the phenotypes of the S. typhimurium mutant was investigated by introducing the plasmids into the mutant strain and analyzing the tRNA modification pattern of the resulting strain. For the E. coli plasmid pTX642, complementation was studied by investigating the influence on the Su+3 suppressor tRNA-mediated readthrough of amber codons in a lacZ(UGA) miaB::Tn10dCm background. The LacZ expression was monitored with MacConkey lactose-chloramphenicol-ampicillin plates.
Genetic techniques.
Transductions with phage P22 HT105/1 (int-201) (48) were performed as described elsewhere (15). Generalized transduction with phage P1vir was performed as described previously (49). To avoid the degradation of plasmids originally grown in E. coli, plasmids were first introduced into the restriction-deficient and modification-proficient S. typhimurium GT907 by transformation (39). The plasmids were thereafter moved into the relevant S. typhimurium genetic background by P22 transduction.
DNA manipulations.
Procedures for DNA restriction digestions, agarose gel electrophoresis, DNA ligation, and transformation of competent E. coli cells were performed essentially as described by Sambrook et al. (47). Restriction enzymes and DNA ligase was purchased from Boehringer (Mannheim, Germany), Promega (Madison, Wis.), and New England Biolabs (Beverly, Mass.). Medium-scale plasmid preparations were done with Bio-Rad (Hercules, Calif.) Quantum or Qiagen (Hilden, Germany) midiprep kits. Small preparations of plasmid DNA were made with the Qiagen or Promega miniprep kits. Chromosomal DNA was isolated with the Genome kit from Bio 101 Inc, Vista, Calif.
Amplification of DNA by PCR.
PCR amplification was performed with Taq DNA polymerase (Boehringer) or Vent (exo+) DNA polymerase (New England Biolabs) by using the buffers supplied with the enzymes. For amplification of the S. typhimurium miaB mutant alleles, a high-salt buffer containing 0.6 M KCl and 20 mM MgCl2 was used. Routinely, 15 pmol of the appropriate primers (Table 2) and about 100 ng of template DNA were added to the reaction mixture. In some cases, cell suspensions were used instead of pure DNA. The cell suspension was obtained by resuspending one colony in 50 μl of distilled water, incubating it at room temperature for 30 min, and then using 1 to 2 μl of the suspension in the PCR mixture. Cycling was performed in a PC-960G microplate gradient thermal cycler (Corbett Research, Sydney, Australia) or in a PTC-100 programmable thermal controller (MJ Research, Waterstown, Mass.). The first cycle was preceded by a 3- to 5-min 95°C denaturation step, and the reaction temperature cycle was 95°C for 1 min, 50 to 68°C (depending on the primers used) for 1 min, and 72°C for 3 min. The cycle was repeated 25 to 30 times and was followed by a 5-min extension at 72°C. The PCR products were visualized on 0.9 to 1.5% agarose gels. The fragments were either purified directly from the PCR mixture by using a Promega PCR clean-up kit or GenElute PCR DNA Purification kit (Supelco, Bellefonte, PA) or isolated from the agarose gel and then purified with the GeneClean III kit from Bio 101.
Southern hybridization.
For colony hybridization, bacterial colonies were lifted onto a Hybond N+ membrane (Amersham, Little Chalfont, England) by replica plating, and the cells were lysed in situ with 0.5 M NaOH (28). The released DNA was immobilized on the membrane by UV illumination. For screening of plasmids, 1 to 2 μg of DNA was digested with suitable restriction enzymes. The digests were separated on 0.7% agarose gels and blotted onto the membrane by capillary transfer (51). Hybridizations were carried out as specified by the manufacturer (Amersham). The probe used was a ∼350-bp [α-32P]dATP-labeled miaB+ fragment generated by PCR from plasmid pUST135 by using primers T-I and MotH.
DNA sequencing and sequence analysis.
Double-stranded plasmid DNA containing the miaB+ region of S. typhimurium was sequenced with an ABI Prism cycle-sequencing kit (Perkin-Elmer, Norwalk, Conn.) in an ABI Prism 377 DNA sequencer. Gel-purified PCR fragments were used as templates for sequencing mutant alleles of S. typhimurium miaB. Two or three PCR products from separate PCR runs of the same strain were mixed before being sequenced. Sequencing of the E. coli miaB mutant was performed at the Core facility of the Department of Microbiology and Molecular Genetics, University of Texas Houston Medical School. The sequences were analyzed by using the University of Wisconsin Genetics Computer Group programs and the BLAST server at the National Center for Biotechnology Information (2).
Localized mutagenesis of miaB with hydroxylamine.
A P22 phage stock (0.5 ml; titer of 4.7 × 1010 PFU per ml) propagated on strain TT2342 was treated with 0.4 M hydroxylamine (32). The mutagenesis was performed at 37°C and was interrupted after 39 h of incubation. The mutagenized phage lysate (108 PFU per ml) was thereafter used to transduce strain GT1825. Transductants, which had received a portion of the zbf-99::Tn10dTc region, which is 83% linked to miaB (20), were selected by plating the transduction mixture on TYS plates (42) containing 15 μg of tetracycline per ml and 10 mM EGTA. The level of amber suppression in the lacI-lacZ gene of the F′ in the Tcr transductants was then screened by printing on minimal plates containing histidine, leucine, and 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per ml. Strain GT1825 gives blue colonies on such a plate at 41°C because of efficient function of the supF30 amber suppressor tRNA. In contrast, strain GT1975, containing the miaB2508::Tn10dCm allele, is light blue at this temperature (20). Colonies which exhibited a reduced level of LacI-LacZ fusion protein (as judged by the color of the colony) compared to GT1825 were chosen for further studies.
HPLC analysis of the contents of modified nucleosides in tRNA.
tRNA was isolated either as described by Connolly and Winkler (11) or by the procedure described by Buck et al. (7). tRNA was digested to nucleosides with nuclease P1 and alkaline phosphatase (25). The resulting hydrolysate was analyzed by high-performance liquid chromatography (HPLC) by the method of Gehrke and Kuo (26) on a Supelcosil LC-18S column (Supelco) with a Waters or Shimadzu HPLC system.
Determination of the transcriptional start point by primer extension.
Total cellular RNA from S. typhimurium cells grown in LB medium at 37°C to a cell density of about 7 × 108 cells per ml was isolated by extraction with hot phenol (60). The oligonucleotide primers Sekv14 and PCR2 were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase. The labeled primers were purified from unincorporated nucleotides by chromatography on a Quick Spin Sephadex G-25 column (Boehringer Mannheim). Approximately 0.1 pmol of labeled primer was mixed with 20 μg of RNA. The mixture was then denatured at 95°C and subjected to annealing and primer extension with avian myeloblastosis virus reverse transcriptase (Pharmacia Biotech) as described previously (27). To estimate the sizes of the reaction products, dideoxy sequencing reaction products obtained with plasmid pUST137 and primers Sekv14 and PCR2, respectively, were electrophoresed along with the primer extension reaction products on a standard 6% urea–polyacrylamide sequencing gel.
RNase T2 protection assays of chromosomal transcripts.
RNA from E. coli CC104 was purified from cells grown in LB medium to mid-exponential phase (approximately 5 × 108 cells per ml). RNA was prepared by adding portions of bacterial cultures directly to lysis solutions without intervening steps as described previously (56). RNase T2 protection assays of transcripts from the bacterial chromosome were completed as described previously (56). RNA probes 1 to 4 and the corresponding complementary probes 1C to 3C for mapping of the miaB region (see Fig. 4) were synthesized by using the following phage RNA polymerase and linearized plasmid templates: probe 1 (T7; pTX638 with HindIII); probe 1C (SP6; pTX638 with BamHI); probe 2 (T7; pTX637 with HindIII); probe 2C (SP6; pTX637 with BamHI); probe 3 (T7; pTX640 with HindIII); probe 3C (SP6; pTX640 with BamHI); probe 4 (T7; pTX639 with HindIII). A series of labeled, undigested RNA molecules of known lengths were used as size standards to determine the lengths of the protected fragments (standard errors ≈ 5 to 10%) (56). Each hybridization reaction mixture contained 40 μg of total RNA.
FIG. 4.
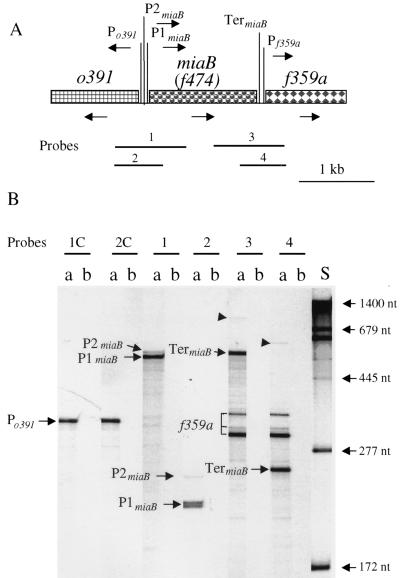
(A) Structure and transcription of the region surrounding the miaB gene at 15 min in the chromosome of E. coli K-12. The figure is drawn to scale. The orientations of the ORFs of o391, miaB, and f359a are indicated by arrows. Locations of the putative Po391, P1miaB, P2miaB, and Pf359a promoters and the Rho-independent TermiaB terminator were determined as described in the text. Horizontal lines represent RNA probes 1 to 4 for mapping in vivo transcripts by the RNase T2 protection assay (shown in panel B). (B) RNase T2 protection assays of transcripts from the o391-miaB-f359a region of the E. coli K-12 chromosome. The positions of RNA probes 1 to 4 used for this assay are indicated in panel A. Probes 1 to 4 correspond to the miaB and f359a noncoding strand and hybridized with miaB and f359a transcript. Probes 1C and 2C were complementary to probe 1 and 2, respectively, and hybridized to o391 transcripts. Lanes: a, RNA probes hybridized with 40 μg of total cellular RNA; b, RNA probes hybridized with 50 μg of tRNA (self-hybridization control); S, RNA size standards. Po391, P1miaB, and P2miaB are transcripts initiated from the putative promoters Po391, P1miaB, and P2miaB, respectively; TermiaB is the transcript terminated at the TermiaB terminator; f359a indicates f359a transcripts.
Nucleotide sequence accession number.
The S. typhimurium DNA sequence described in this work was deposited in the EMBL database under accession no. AJ249116.
RESULTS
Identification of the insertion point of the Tn10dCm transposon of the miaB2508::Tn10dCm mutant.
The miaB2508::Tn10dCm mutation, which gives rise to undermodification of ms2io6A tRNA in S. typhimurium, is located close to the nag locus at 16 min on the S. typhimurium chromosome (20). To establish the exact location of Tn10dCm in this mutant, we took advantage of the fact that there is another transposon (zbf-99::Tn10dTc) located 2 to 3 kb from the miaB2508::Tn10dCm insertion (20). We intended to amplify the region between these two transposons by PCR by using as template a lysate from strain GT3171 (miaB2508::Tn10dCm zbf-99::Tn10dTc). However, we did not detect a PCR product of the expected size (2 to 3 kb). Instead, we found a fragment of approximately 1.3 kb (data not shown) when the Tn10XbaI and T-L primers were used. The obtained fragment was ligated into the pT7Blue T vector (Novagen), yielding plasmid pUST135. Sequencing of the insert revealed that one end of the fragment consisted of Tn10dCm DNA while the other end was the result of the T-L primer binding to a nontransposon sequence. A search for sequence homologies showed that the amplified fragment was homologous to the f474 (yleA) ORF of E. coli identified by Blattner et al. (4). This ORF lies (together with four other ORFs) between the cutE (lnt) gene and a tRNA cluster located close to the nag operon of E. coli (Fig. 2). This demonstrates that the Tn10dCm transposon of the S. typhimurium miaB mutant had been inserted in the f474 (yleA) gene, which is hereafter called miaB.
Construction of a miaB::Tn10dCm mutant of E. coli.
An E. coli miaB::Tn10dCm mutant is useful for further studies of the effects of ms2i6A tRNA undermodification on spontaneous mutagenesis (11, 12) and bacterial physiology (20). Since galE mutants of S. typhimurium can be infected by the E. coli-specific phage P1 (40), we first moved the miaB2508::Tn10dCm mutation of S. typhimurium GT2176 into a galE background (strain MST1934) by P22 transduction. Integration of S. typhimurium DNA into the E. coli chromosome can be achieved by using a mutS or mutL E. coli recipient (44). The miaB2508::Tn10dCm allele was therefore moved first into an E. coli mutS strain by P1vir transduction (resulting in strain TX3325) and thereafter into a Su+3 lacZ(UGA) strain of E. coli to verify the antisuppressor activity of the miaB mutation (20). We also moved the miaB mutation into strain CC104 and showed that in such a background, the mutation did not influence the growth rate. This was in accordance with earlier observations of the S. typhimurium miaB2508::Tn10dCm mutant (20). Sequencing of a plasmid (pTX641) carrying chromosomal DNA from strain TX3346 verified that the DNA flanking the transposon was homologous to the f474 ORF, thereby confirming the location of miaB. The tRNA from the E. coli miaB strains showed an accumulation of i6A, whereas ms2i6A was absent (data not shown). These results confirmed the construction of the miaB::Tn10dCm mutant of E. coli.
Construction and properties of minimal miaB+ plasmids.
We isolated a plasmid containing the miaB+ wild-type gene of S. typhimurium from an S. typhimurium genomic library (29), which was introduced into E. coli DH5α. Colony hybridization with a miaB+ (f474+) probe (see Materials and Methods) followed by Southern hybridization identified one plasmid, pUST136, which gave a positive signal. Subcloning from pUST136 resulted in a smaller construct (pUST137), which complemented the ms2 deficiency of the miaB2508::Tn10dCm mutant (data not shown). The insert in this plasmid was sequenced and was shown to be homologous to the E. coli ORFs f155b, f359a, f474, and o391 (Fig. 2). Thus, this region of the S. typhimurium chromosome is very similar to its counterpart in E. coli.
Since the f474 ORF is the first of three on the pUST137 plasmid that would be transcribed in the same direction (Fig. 2), it is possible that the mutant phenotype of the miaB2508::Tn10dCm strain is due to polar effects on downstream transcription. To rule out this possibility, a smaller plasmid construct, pUST138, was made in which the only intact ORF was f474. Introduction of this plasmid into S. typhimurium GT2734 (miaB2508::Tn10dCm) showed that this plasmid could complement the miaB2508::Tn10dCm mutation-induced modification-deficient phenotype as judged by HPLC analysis of tRNA (Table 3). We therefore conclude that the S. typhimurium ORF corresponding to E. coli f474 is indeed the miaB gene.
TABLE 3.
Presence of the different i6A derivatives in the tRNA of S. typhimurium carrying wild-type and mutant miaB alleles
| Strain | Relevant genotypea | Plasmid | Growth temp (°C) | Ratio of i6A derivativeb to m2A
|
|||
|---|---|---|---|---|---|---|---|
| i6A | io6A | ms2i6A | ms2io6A | ||||
| GT2734 | miaB2508::Tn10dCm | 37 | 0.55 | 0.068 | — | — | |
| GT5441 | miaB2508::Tn10dCm | pUST138 | 37 | 0.008 | 0.006 | 0.12 | 0.48 |
| GT5442 | miaB2508::Tn10dCm | pBluescript SK | 37 | 0.45 | 0.057 | — | — |
| GT1825 | miaB+ | 37 | 0.007 | 0.007 | 0.19 | 0.48 | |
| 41 | 0.012 | 0.029 | — | 0.83 | |||
| GT3828 | miaB26 | 37 | 0.060 | 0.007 | 0.21 | 0.37 | |
| 41 | 0.36 | 0.008 | — | 0.075 | |||
| GT3830 | miaB27 | 37 | 0.45 | — | — | — | |
| 41 | 0.42 | — | — | — | |||
| GT3831 | miaB28 | 37 | 0.44 | — | — | — | |
| 41 | 0.43 | 0.010 | — | — | |||
| GT3833 | miaB29 | 37 | 0.43 | 0.013 | — | — | |
| 41 | 0.41 | — | — | — | |||
| TT2342 | miaB+ zbf-99::Tn10 | 41 | 0.006 | 0.010 | 0.14 | 0.54 | |
| GT5443 | miaB26 zbf-99::Tn10 | 41 | 0.36 | 0.008 | 0.070 | 0.077 | |
| GT5444 | miaB27 zbf-99::Tn10 | 41 | 0.39 | 0.023 | — | — | |
| GT5445 | miaB28 zbf-99::Tn10 | 41 | 0.37 | 0.025 | — | — | |
| GT5446 | miaB29 zbf-99::Tn10 | 41 | 0.44 | 0.018 | — | — | |
| GT5766 | miaB26 zbf-99::Tn10 | pUST190 | 41 | — | — | 0.21 | 0.44 |
| GT5767 | miaB27 zbf-99::Tn10 | pUST190 | 41 | — | — | 0.21 | 0.46 |
| GT5768 | miaB28 zbf-99::Tn10 | pUST190 | 41 | — | — | 0.23 | 0.44 |
| GT5769 | miaB29 zbf-99::Tn10 | pUST190 | 41 | — | — | 0.19 | 0.45 |
See Table 1 for complete genotypes.
The i6A derivative present in the largest amount in each strain is shown in bold letters. —, not detected. Compounds present at amounts lower than 0.005 (less than 1% of the total amount of i6A-derivatives) cannot be detected.
In addition, a 1.55-kb PCR fragment covering the wild-type miaB+ gene of E. coli CC104 was cloned into the pKK223-3 vector. This construct (pTX642) was shown by complementation (see Materials and Methods) to contain a functional miaB+ gene. Taken together, the data from both S. typhimurium and E. coli firmly identifies f474 as the miaB gene. The minimal E. coli miaB+ clone also shows that the elements sufficient for adequate expression of the MiaB peptide are present within this minimal 1.5-kb fragment.
Analysis of MiaB sequences.
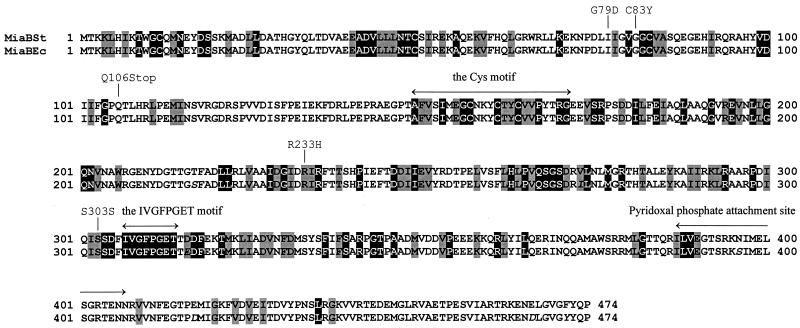
A search for amino acid sequence homologies in the Swiss-Prot and GenBank databases was performed by using the BLAST utility at the National Center for Biotechnology Information server. The polypeptides showing homology to MiaB from S. typhimurium and E. coli could be divided into three groups. One consists of polypeptides highly homologous to MiaB, in organisms presumably containing the ms2i(o)6A modification. The second comprises polypeptides of high homology but from organisms presumably not containing the ms2i(o)6A modification. The polypeptides in the first two groups have more than 35% similarity to the S. typhimurium MiaB peptide. The entries in the third group showed less overall similarity (<35%) but had high homology within two of the conserved motifs in MiaB. These two motifs, which were present in almost all of the MiaB homologues, are indicated in Fig. 3. A few proteins of known function were found within this group, but no conclusion about the roles of these two motifs could be drawn based on these enzymes, since they constitute a seemingly heterogeneous group. It is likely that these two conserved motifs are involved in some fundamental type of interaction exhibited by a variety of enzymes.
FIG. 3.
Amino acid sequences of the MiaB polypeptides of S. typhimurium and E. coli as deduced from the DNA sequences. The E. coli sequence differs from the S. typhimurium sequence at only eight positions, which are shown in italics in the figure. The solid boxes indicate amino acids that are conserved in at least 50% of the MiaB homologues (that is, polypeptides giving E values of 10−9 or less in BLAST). Shaded boxes represent amino acids conserved in 50% of the MiaB homologues if amino acids with similar properties also were considered.
A search for matches to protein patterns in the PROSITE database (3) was performed with by the Genetics Computer Group program. The MiaB protein sequence was analyzed by using the BLOCKS service at the Fred Hutchinson Cancer Research Center Blocks WWW server. The protein motifs that were regarded as relevant are described in Discussion.
Characterization of point mutations in the miaB gene of S. typhimurium.
Point mutations in miaB were identified to provide information about domain function and to further rule out polarity effects. The S. typhimurium chromosome was mutagenized with hydroxylamine (see Materials and Methods) to obtain missense mutations. Since the miaB2508::Tn10dCm null mutation reduces the efficiency of the suppressor tRNACUATyr at least twofold (20), it was possible to detect other mutations in miaB by monitoring amber suppression of the lacI-lacZ hybrid gene. Of about 5,500 colonies, 26 were picked that exhibited a reduced level of suppression. An analysis of the modified nucleosides in their tRNA showed that four of these 26 isolates contained i6A instead of ms2io6A when grown at 41°C (Table 3). The miaB26 mutant (GT3828) contained ms2io6A when grown at 37°C, while the other three were deficient in the ms2 group at both temperatures.
To determine the location of the point mutations in these four mutant strains (GT3828, GT3830, GT3831 and GT3833), fragments containing the miaB allele of these strains were amplified by PCR with the PCR1 and PCR2 primers. The PCR resulted in fragments of about 2 kb, which corresponds well to the predicted 2,043 bp. Sequencing of the fragments revealed that two of the mutations (Fig. 3), G79D in strain GT3830 (miaB27) and C83Y in strain GT3831 (miaB28), were located in a motif that vaguely resembles an AdoMet-binding site. The third mutation, R233H of strain GT3828 (miaB26), which gave rise to a MiaB− phenotype only at elevated temperature, was located further downstream. The temperature-sensitive phenotype indicates that this amino acid may be involved in maintaining the structure of the protein. Last, in strain GT3833 (miaB29), codon 106 was changed from Gln to a stop codon (UAA), resulting in a peptide whose size corresponds to only about one-quarter of the full-length protein. This mutant also contained a second, silent mutation (Ser to Ser) located at codon 303, which is downstream of the nonsense mutation at codon 106.
To confirm that the mutant phenotypes of these four mutants were not due to additional mutations outside the miaB gene, the ability of the mutants to be complemented by the minimal MiaB+ plasmid (pUST190) was tested. The various miaB alleles were moved into a wild-type background (strain GT522) by cotransduction with the zbf-99::Tn10 allele. MiaB− transductants were identified by HPLC analysis of the tRNA (data not shown). The pUST190 plasmid was then introduced, and all four mutants proved to have ms2io6A in their tRNA when the plasmid was present (Table 3). This indicates that these point mutations give rise to the modification-deficient phenotype of the four mutants.
Transcriptional organization of the miaB gene.
To investigate whether transcription of miaB is linked to the adjacent genes (Fig. 4A), the E. coli miaB transcript was mapped by RNase T2 protection assays with RNA probes 1 to 4 and the complementary probes 1C to 3C (Fig. 4B).
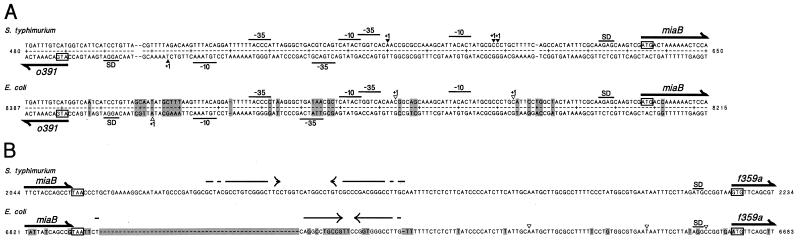
Putative promoters Po391 and PmiaB were located within the intercistronic region of o391 and miaB, respectively. Hybridization to probes 1C and 2C, which correspond to the o391 noncoding strand and cover the 5′ regions of o391 and miaB, showed a band of 315 nucleotides (nt). This band was mapped to be an o391 transcript with a 5′ end at approximately 22 nt before the AUG codon of the o391 reading frame. Hybridization of the complementary probes 1 and 2 corresponding to the miaB noncoding strand showed a dark band of 540 nt with probe 1 and a doublet of 212 and 214 nt with probe 2. The doublet probably represents end nibbling by RNase T2. These bands (P1miaB) (Fig. 4) originated upstream from miaB and place the start of transcription of the major miaB promoter approximately 33 nt upstream of the miaB AUG start codon. Two lighter bands of 570 and 243 nt were also detected by probes 1 and 2, respectively. These lighter bands probably correspond to a weaker upstream miaB promoter (P2miaB) (Fig. 4), whose start of transcription is approximately 63 nt from the miaB AUG start codon. The relative amount of transcript corresponding to the putative P2miaB promoter was only about 11% of that from the major P1miaB promoter.
Primer extension analysis of the o391 and miaB genes of S. typhimurium confirmed the presence of transcriptional start sites at 24 nt upstream of the o391 start codon and 37 and 38 nt upstream of the miaB AUG codon (Fig. 5 and 6). A second 5′-end was detected 64 nt upstream of the miaB start codon, indicating that the expression of the miaB gene of S. typhimurium was also probably governed by at least two promoters. However, in contrast to E. coli miaB, expression from the two miaB promoters was nearly equal in S. typhimurium. A striking feature of this second promoter is that the −35 region overlaps the divergent o391 promoter. The transcription of this region might thus be quite intricate.
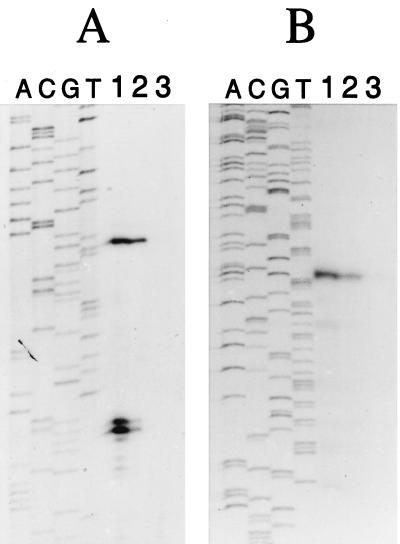
FIG. 5.
Mapping of the 5′ ends of the miaB (A) and the o391 (B) mRNA by primer extension analyses with RNA isolated from S. typhimurium grown at 37°C. The Sekv14 primer was used in both sequencing and extension reactions for mapping of the miaB transcripts, while the PCR2 primer was used in mapping of the o391 transcriptional start point. Lanes A, C, G, and T represent the DNA-sequencing ladder. Lanes: 1, pUST138 plasmid in GT522 background; 2, pUST137 plasmid in GT522 background; 3, GT522 without any plasmid.
FIG. 6.
(A) Nucleotide sequences of the E. coli and S. typhimurium miaB promoter region. Included are the sequences encoding the N-terminal parts of the MiaB and o391 peptides. Putative −35 and −10 promoter sequences and potential Shine-Dalgarno sequences (SD) are indicated. The solid triangles represent the mRNA 5′ ends identified in primer extension analyses, and the open triangles indicate mRNA 5′ ends detected in RNase T2 protection assays. Ends detected by RNase T2 assays are accurate to only about 10 nt. Shaded boxes indicate positions in the E. coli sequence (accession no. AE000170), which differs from the S. typhimurium sequence (accession no. AJ249116). (B) Nucleotide sequence of the terminator regions of the miaB gene of E. coli and S. typhimurium. Included are the sequences encoding the C-terminal part of the MiaB peptide and the N-terminal part of the F359a peptide. The inverted repeats of the putative terminator stem are indicated by arrows.
Probes 3 and 4 were used to map the transcripts from the miaB-f359a junction. Hybridization with these two probes showed three common bands of 295, 310, and 340 nt, representing f359a transcripts with the 5′ end at approximately 7, 22, and 52 nt before the AUG codon. The shortest band (295 nt) made up 68% of the f359a transcript and probably represented a transcript from a putative promoter, Pf359a. Bands of 600 and 246 nt were detected with probes 3 and 4, respectively, and represented miaB transcripts terminated at a predicted Rho-independent termination site, TermiaB (Fig. 6). A very small amount (4% of the miaB transcript) of the miaB-f359a cotranscript (arrows) was present in hybridization with either probe 3 or 4 and probably represented incomplete termination of the miaB transcript at TermiaB. No bands were detected in hybridization reactions with the complementary probes 3C (data not shown), indicating that there is no antisense transcription of miaB or f359a.
DISCUSSION
The genetic organization of tRNA modification genes does not seem to conform to a common theme (62). Among the tRNA modification genes whose genetic organizations have been resolved, only one, the trmA gene (36), has hitherto been shown to be a monocistronic operon. The queA and tgt genes, which are both required for biosynthesis of Q34, are grouped in a polycistronic operon (43, 45). tRNA modification genes can also be found in complex operons, either with seemingly unrelated genes, as in the case with the miaA gene (12, 55, 57), or, like the trmD gene (10), together with genes encoding other components involved in translation. In this study, we identified the miaB gene of S. typhimurium and E. coli, which is involved in the methylthiolation step in the biosynthesis of the modified nucleoside ms2i(o)6A37 in tRNA. Our experiments revealed that the S. typhimurium homologue to the f474 (yleA) ORF of E. coli had been truncated by the Tn10dCm insertion in the miaB2508::Tn10dCm mutant. The f474 ORF is located between the cutE (lnt) gene and the nag operon at 14.9 min on the E. coli chromosome. Sequencing of a 3.7-kb chromosomal fragment from S. typhimurium covering the miaB (f474) gene showed that the genetic organization in this area is highly homologous to that in the E. coli counterpart (Fig. 2). The only major difference is a 51-bp insertion almost immediately downstream of the miaB UAA stop codon in S. typhimurium (Fig. 6B). Putative Rho-independent transcription termination signals were identified in this area between the miaB gene and the downstream ORF f359a (Fig. 6B). In E. coli, the 3′ end of the miaB transcript coincides with this possible secondary RNA structure. This, together with the fact that more than 95% of the E. coli miaB transcripts ended at this terminator and that the downstream f359a ORF was transcribed from its own promoter, suggests that the miaB gene is transcribed as a monocistronic unit in E. coli (and most probably in S. typhimurium as well). Thus, we present the second case of a tRNA modification gene being transcribed as a monocistronic unit.
Putative FIS-binding sites have been observed in the promoter regions of several tRNA modification genes (trmA [29], queA-tgt [50], and miaA [57]; reviewed in reference 62). However, no sequence similar to a FIS consensus binding site (33) is present in the miaB promoter. Neither did the miaB promoters contain the discriminator sequence associated with stringent control (54). These two observations indicate that the expression of the MiaB protein is probably not regulated in the same manner as that of its tRNA substrate. It is known that the MiaB-mediated methylthiolation reaction requires iron (8, 46, 61) and that the concentration of iron in the cell is tightly regulated by Fur, which is a repressor for many iron-regulated operons (13). However, no putative Fur-binding site (16, 21) is present in the promoter of miaB.
miaB seems to be transcribed from two upstream promoters in E. coli and S. typhimurium cells grown in LB medium (Fig. 4 and 5). However, in E. coli, transcripts from the putative promoter proximal to the miaB translation start codon are present in excess over those from the upstream promoter, whereas in S. typhimurium, the numbers of transcripts from the two putative promoters are about equal. Further work is needed to determine whether this expression pattern represents differential transcription initiation or transcript processing in these two bacterial species and whether miaB transcription is differently regulated. Whether the overlap of the o391 and upstream miaB promoters has regulatory significance also remains to be determined.
HPLC analysis of the modified nucleosides in the tRNA of the miaB2508::Tn10dCm mutant (20) and of miaB point mutants (see Results) show that they contain i6A instead of ms2io6A. This suggest that the gene disrupted in these mutants encodes a protein involved in the addition of the thio part of the modification, by encoding either a thiotransferase or a bifunctional enzyme catalyzing both the thiolation and the methylation reaction. However, when Agris et al. (1) showed that the methylthiolation reaction occurs in two steps, they noticed that the s2i6A derivative was unstable. Therefore, we cannot rule out that we failed to detect s2i6A under the conditions used in our experiments. To address this issue, we extracted tRNA from a rel met strain grown in rich medium lacking methionine, analogous to the way Agris et al. (1) obtained methyl-deficient tRNA. In the HPLC analysis of isopentenylated nucleosides from this tRNA, we did not detect any s2i6A derivative as monitored by mass spectrometry; only i6A was present (data not shown). Thus, at present, we may be unable to detect s2i6A derivatives by these methods.
Two conserved motifs were found in almost all of the polypeptides homologous to MiaB. One is an array of three cysteine residues spaced by 3 and 2 amino acids (CxxxCxxC; positions 157 to 164 in MiaBSt [Fig. 3]), and the other is a highly conserved motif (IVGFPGET; positions 307 to 314 in MiaBSt [Fig. 3]) located in the C-terminal part of the proteins. The cysteine motif has previously been noticed and is denoted “uncharacterized protein family UPF0004 signature” (PS01278) in the PROSITE database. This stretch of amino acids in MiaB was also recognized when searching the BLOCKS 11.0 database (31). The protein block matching this cysteine motif is found in a group of enzymes known as radical activating enzymes, and their signature motif is designated PS01087 in PROSITE. The MiaB cysteines match the second half of the PS01087 motif, which is presumed to bind iron (52). The fact that the MiaB cysteine pattern only partly matches the radical activating enzyme signature probably indicates that both types of enzyme have active sites where an iron molecule is coordinated by the cysteines but that the rest of the active site is different due to differences in catalytic specificity. Since iron has been implicated in the methylthiolation of i6A (8, 46, 61), it is likely that the MiaB protein is a thiotransferase, assuming that the cysteine motif of MiaB really binds iron.
A potential binding site for pyridoxal phosphate (motif PS00595 in PROSITE) was also identified in the E. coli and S. typhimurium MiaB sequences (LVxxTSRKxxxxxxGxTxN; positions 388 to 402 in MiaBSt [Fig. 3]) and in several MiaB homologues. Lipsett and Peterkofsky (37) observed that incorporation of sulfur into tRNA was enhanced if pyridoxal phosphate was included in the reaction mixture. However, contradictory results have also been obtained (30), claiming that pyridoxal phosphate is not a cofactor for tRNA sulfur transferase activity in E. coli. Still, studies of the enzymatic reaction catalyzed by two purified enzymes involved in the biosynthesis of s4U showed that this reaction was dependent on pyridoxal phosphate (38). Thus, the presence of a putative pyridoxal phosphate-binding site in MiaB supports the suggestion that the MiaB enzyme is involved in a thiolation reaction.
The MiaB polypeptide contained only a weak similarity to the glycine-rich motif ΔΔ(D/E)ΔGxGxGxΔxxxΔΔ∧ (where Δ is a hydrophobic residue and ∧ is a charged or polar amino acid), which has been proposed to be involved in binding of S-adenosylmethionine (AdoMet) (63). Interestingly, two of the point mutations obtained in this study are located within this region (G79D and C83Y [Fig. 3]), indicating that the function of MiaB may be decreased by mutations in this region. However, the two glycine residues present in MiaB from S. typhimurium and E. coli are conserved in only two of the homologues from other organisms (YleA from Haemophilus influenzae and Yhe2 from Pseudomonas aeruginosa). Thus, the weak similarity to an AdoMet-binding site and the lack of conservation of these amino acids suggest that the miaB gene does not encode a methyltransferase. However, this possibility cannot be excluded, because Djordjevic and Stock (17) found that the AdoMet-binding site of different types of methyltransferases have common sizes and shapes, even though there is a lack of sequence identity. It is also possible that the structure of the MiaB enzyme is unique and that the structure of the AdoMet-binding site deviates from the ones observed in proteins which exhibit the consensus pattern mentioned above.
Finally, the MiaB polypeptide was compared with the amino acid sequence of the MiaA protein. This alignment revealed that these two peptides have only 16% identity and that there are no obvious shared motifs. If there is some common structure, for instance for recognition of the adenine moiety or the tRNA, it is thus probably reflected in overall structure and not in sequence similarity. Alternatively, the MiaB enzyme may recognize just the i6A modified base and not the tRNA molecule. The homology between MiaB and the MiaE protein was even lower (13% identity).
In summary, the presence of putative iron- and pyridoxal phosphate-binding sites favors the conclusion that MiaB is the thiotransferase required in ms2i(o)6A37 tRNA modification. The absence of an apparent AdoMet-binding site suggests that MiaB may not be bifunctional and carry out the methyltransferase reaction as well. However, we cannot rule out that MiaB carries out the methyltransfer by using a nonconsensus AdoMet-binding site. These hypotheses will be tested by future biochemical characterization of purified MiaB.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Cancer Foundation (project 680) and Swedish Natural Science Council (BU-2830) to G.R.B. and by grant MCB-9420416 from the National Science Foundation and grant CA77193 from the National Institutes of Health to M.E.W.
We thank Kerstin Jacobsson and Solveig Ericsson for skillful technical assistance and Yong Yang for help with PCR amplification and cloning.
REFERENCES
- 1.Agris P F, Armstrong D J, Schäfer K P, Söll D. Maturation of a hypermodified nucleoside in transfer RNA. Nucleic Acids Res. 1975;2:691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acids Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett 3 r d G, Bloch C A, Perna N T, Burland V, Riley M, Collado Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 6.Buck M, Ames B N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984;36:523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- 7.Buck M, Connick M, Ames B N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal Biochem. 1983;129:1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- 8.Buck M, Griffiths E. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982;10:2609–2624. doi: 10.1093/nar/10.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck M, McCloskey J A, Basile B, Ames B N. cis 2-Methylthio-ribosylzeatin (ms2io6A) is present in the transfer RNA of Salmonella typhimurium, but not Escherichia coli. Nucleic Acids Res. 1982;10:5649–5662. doi: 10.1093/nar/10.18.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byström A S, von Gabain A, Björk G R. Differentially expressed trmD ribosomal protein operon of Escherichia coli is transcribed as a single polycistronic mRNA species. J Mol Biol. 1989;208:575–586. doi: 10.1016/0022-2836(89)90149-6. [DOI] [PubMed] [Google Scholar]
- 11.Connolly D M, Winkler M E. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(Δ2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J Bacteriol. 1989;171:3233–3246. doi: 10.1128/jb.171.6.3233-3246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly D M, Winkler M E. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J Bacteriol. 1991;173:1711–1721. doi: 10.1128/jb.173.5.1711-1721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupples C G, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc Natl Acad Sci USA. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis R W, Botstein D, Roth J R. A manual for genetic engineering: advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 16.de Lorenzo V, Wee S, Herrero M, Neilands J B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djordjevic S, Stock A M. Crystal structure of the chemotaxis receptor methyltransferase CheR suggests a conserved structural motif for binding S-adenosylmethionine. Structure. 1997;5:545–558. doi: 10.1016/s0969-2126(97)00210-4. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg S P, Yarus M, Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979;135:111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- 19.Ericson J U, Björk G R. Pleiotropic effects induced by modification deficiency next to the anticodon of tRNA from Salmonella typhimurium LT2. J Bacteriol. 1986;166:1013–1021. doi: 10.1128/jb.166.3.1013-1021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esberg B, Björk G R. The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J Bacteriol. 1995;177:1967–1975. doi: 10.1128/jb.177.8.1967-1975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escolar L, Pérez-Martín J, de Lorenzo V. Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J Mol Biol. 1998;283:537–547. doi: 10.1006/jmbi.1998.2119. [DOI] [PubMed] [Google Scholar]
- 22.Fittler F, Kline L K, Hall R H. N6-(Δ2-isopentenyl)adenosine: biosynthesis in vitro by an enzyme extract from yeast and rat liver. Biochem Biophys Res Commun. 1968;31:571–576. doi: 10.1016/0006-291x(68)90516-0. [DOI] [PubMed] [Google Scholar]
- 23.Fleissner E, Borek E. A new enzyme of RNA synthesis: RNA methylase. Proc Natl Acad Sci USA. 1962;48:1199–1203. doi: 10.1073/pnas.48.7.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gefter M L. The in vitro synthesis of 2′-omethylguanosine and 2-methylthio 6N (γ,γ-dimethylallyl) adenosine in transfer RNA of Escherichia coli. Biochem Biophys Res Commun. 1969;36:435–441. doi: 10.1016/0006-291x(69)90583-x. [DOI] [PubMed] [Google Scholar]
- 25.Gehrke C W, Kuo K C, McCune R A, Gerhardt K O, Agris P F. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr. 1982;230:297–308. [PubMed] [Google Scholar]
- 26.Gehrke C W, Kuo K C. Ribonucleoside analysis by reversed-phase high performance liquid chromatography. In: Gehrke C W, Kuo K C T, editors. Chromatography and modification of nucleosides. A. Analytical methods for major and modified nucleosides. Journal of Chromatography Library. Amsterdam, The Netherlands: Elsevier; 1990. pp. A3–A71. [Google Scholar]
- 27.Göransson M, Sondén B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin B E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990;344:682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- 28.Grunstein M, Hogness D S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA. 1975;72:3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson C, Lindström P H, Hagervall T G, Esberg K B, Björk G R. The trmA promoter has regulatory features and sequence elements in common with the rRNA P1 promoter family of Escherichia coli. J Bacteriol. 1991;173:1757–1764. doi: 10.1128/jb.173.5.1757-1764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayward R S, Eliceiri G L, Weiss S B. Ribonucleic acid sulfur-transferase activity in extracts from Escherichia coli. Cold Spring Harbor Symp Quant Biol. 1966;31:459–464. doi: 10.1101/sqb.1966.031.01.059. [DOI] [PubMed] [Google Scholar]
- 31.Henikoff S, Henikoff J G. Protein family classification based on searching a database of blocks. Genomics. 1994;19:97–107. doi: 10.1006/geno.1994.1018. [DOI] [PubMed] [Google Scholar]
- 32.Hong J S, Ames B N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci USA. 1971;68:3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hübner P, Arber W. Mutational analysis of a prokaryotic recombinational enhancer element with two functions. EMBO J. 1989;8:577–585. doi: 10.1002/j.1460-2075.1989.tb03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerner C G, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;18:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung H C, Chen Y, Winkler M E. Regulation of substrate recognition by the MiaA tRNA prenyltransferase modification enzyme of Escherichia coli K-12. J Biol Chem. 1997;272:13073–13083. doi: 10.1074/jbc.272.20.13073. [DOI] [PubMed] [Google Scholar]
- 36.Lindström P H, Stüber D, Björk G R. Genetic organization and transcription from the gene (trmA) responsible for synthesis of tRNA (uracil-5)-methyltransferase by Escherichia coli. J Bacteriol. 1985;164:1117–1123. doi: 10.1128/jb.164.3.1117-1123.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipsett M N, Peterkofsky A. Enzymatic thiolation of E. coli sRNA. Proc Natl Acad Sci USA. 1966;55:1169–1174. doi: 10.1073/pnas.55.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipsett M N. Biosynthesis of 4-thiouridylate. Participation of a sulfurtransferase containing pyridoxal 5′-phosphate. J Biol Chem. 1972;247:1458–1461. [PubMed] [Google Scholar]
- 39.MacLachlan P R, Sanderson K E. Transformation of Salmonella typhimurium with plasmid DNA: differences between rough and smooth strains. J Bacteriol. 1985;161:442–445. doi: 10.1128/jb.161.1.442-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mojica T. Transduction by phage P1CM clr-100 in Salmonella typhimurium. Mol Gen Genet. 1975;138:113–126. doi: 10.1007/BF02428116. [DOI] [PubMed] [Google Scholar]
- 41.Moore J A, Poulter C D. Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: a binding mechanism for recombinant enzyme. Biochemistry. 1997;36:604–614. doi: 10.1021/bi962225l. [DOI] [PubMed] [Google Scholar]
- 42.Persson B C, Björk G R. Isolation of the gene (miaE) encoding the hydroxylase involved in the synthesis of 2-methylthio-cis-ribozeatin in tRNA of Salmonella typhimurium and characterization of mutants. J Bacteriol. 1993;175:7776–7785. doi: 10.1128/jb.175.24.7776-7785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogliano K J, Beckwith J. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J Bacteriol. 1994;176:804–814. doi: 10.1128/jb.176.3.804-814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rayssiguier C, Thaler D S, Radman M. The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature. 1989;342:396–401. doi: 10.1038/342396a0. [DOI] [PubMed] [Google Scholar]
- 45.Reuter K, Slany R, Ullrich F, Kersten H. Structure and organization of Escherichia coli genes involved in biosynthesis of the deazaguanine derivative queuine, a nutrient factor for eukaryotes. J Bacteriol. 1991;173:2256–2264. doi: 10.1128/jb.173.7.2256-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg A H, Gefter M L. An iron-dependent modification of several transfer RNA species in Escherichia coli. J Mol Biol. 1969;46:581–584. doi: 10.1016/0022-2836(69)90197-1. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 49.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 50.Slany R K, Kersten H. The promoter of the tgt/sec operon in Escherichia coli is preceded by an upstream activation sequence that contains a high affinity FIS binding site. Nucleic Acids Res. 1992;20:4193–4198. doi: 10.1093/nar/20.16.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Eliasson R, Pontis E, Andersson J, Buist G, Sjöberg B M, Reichard P. Generation of the glycyl radical of the anaerobic Escherichia coli ribonucleotide reductase requires a specific activating enzyme. J Biol Chem. 1995;270:2443–2446. doi: 10.1074/jbc.270.6.2443. [DOI] [PubMed] [Google Scholar]
- 53.Svensson I, Boman H G, Eriksson K G, Kjellin K. Transfer of methyl groups from methionine to soluble RNA from E. coli. Acta Chem Scand. 1963;17:868–869. doi: 10.1016/s0022-2836(63)80006-6. [DOI] [PubMed] [Google Scholar]
- 54.Travers A A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984;12:2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsui H C, Feng G, Winkler M E. Transcription of themutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Eς32-specific promoters during heat shock. J Bacteriol. 1996;178:5719–5731. doi: 10.1128/jb.178.19.5719-5731.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsui H-C, Pease A J, Koehler T M, Winkler E M. Detection and quantitation of RNA transcribed from bacterial chromosomes. Methods Mol Genet. 1994;3:179–204. [Google Scholar]
- 57.Tsui H C, Winkler M E. Transcriptional patterns of the mutL-miaA superoperon of Escherichia coli K-12 suggest a model for posttranscriptional regulation. Biochimie. 1994;76:1168–1177. doi: 10.1016/0300-9084(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 58.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 59.Vold B S, Lazar J M, Gray A M. Characterization of a deficiency of N6-(Δ2-isopentenyl)-2-methylthioadenosine in the Escherichia coli mutant trpX by use of antibodies to N6-(Δ2-isopentenyl)adenosine. J Biol Chem. 1979;254:7362–7367. [PubMed] [Google Scholar]
- 60.von Gabain A, Belasco J G, Schottel J L, Chang A C, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wettstein F O, Stent G S. Physiologically induced changes in the property of phenylalanine tRNA in Escherichia coli. J Mol Biol. 1968;38:25–40. doi: 10.1016/0022-2836(68)90126-5. [DOI] [PubMed] [Google Scholar]
- 62.Winkler M E. Genetics and regulation of base modification in the tRNA and rRNA of prokaryotes and eukaryotes. In: Grosjean H, Benne R, editors. Modification and editing of RNA. Washington, D.C.: ASM Press; 1998. pp. 441–469. [Google Scholar]
- 63.Wu G, Williams H D, Zamanian M, Gibson F, Poole R K. Isolation and characterization of Escherichia coli mutants affected in aerobic respiration: the cloning and nucleotide sequence of ubiG. Identification of an S-adenosylmethionine-binding motif in protein, RNA, and small-molecule methyltransferase. J Gen Microbiol. 1992;10:2101–2112. doi: 10.1099/00221287-138-10-2101. [DOI] [PubMed] [Google Scholar]