Abstract
Cognitive impairment related to major depressive disorder (MDD) is highly prevalent, debilitating and is lacking in effective treatments; dysregulated inflammatory physiology is a putative mechanism and may represent a therapeutic target. In depressed individuals exhibiting a pro-inflammatory phenotype who were enrolled in a 12-week randomized placebo-controlled trial of 3 doses of omega-3 polyunsaturated fatty acids (ω-3-FA), we examined: (i) the relationship between dysregulated inflammatory physiology and baseline cognitive impairment; (ii) improvement in cognitive impairment following treatment; and (iii) the association between baseline inflammatory biomarkers and change in cognitive impairment for those receiving treatment. We randomized 61 unmedicated adults aged 45.50 years (75% female) with DSM-5 MDD, body mass index >25 kg/m2, and C-reactive protein (CRP) ≥3.0 mg/L to three doses of ω-3-FA (1, 2, or 4 g daily) or matching placebo. Analyses focused on 45 study completers who had inflammatory biomarkers assessed [circulating CRP, interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα) as well as lipopolysaccharide (LPS)-stimulated concentrations of IL-6 and TNFα in peripheral blood mononuclear cells (PBMC)] and on the highest dose ω-3-FA (4 g daily; n = 11) compared to placebo (n = 10). Impairment in motivational symptoms (e.g., alertness, energy, enthusiasm) and higher-order cognitive functions (e.g., word-finding, memory) were assessed by a validated self-report measure. Among all 45 participants at baseline, lower concentrations of IL-6 in LPS-stimulated PBMC were associated with greater impairment in higher-order cognitive functions (r = −0.35, p = .02). Based on hierarchical linear modeling, individuals receiving 4 g/day of ω-3-FA reported significant improvement in motivational symptoms compared to placebo (B = −0.07, p = .03); in the 4 g/day group, lower baseline concentrations of TNFα in LPS-stimulated PBMC were associated with significant improvement in motivational symptoms (Ρ = .71, p = .02) following treatment. In this exploratory clinical trial, daily supplementation with 4 g of ω-3-FA improves motivational symptoms in depressed individuals exhibiting an inflammatory phenotype.
Highlights
-
•
Lower LPS-induced PBMC response was linked with impairment in higher-order cognition.
-
•
Motivational symptoms improved for depressed individuals receiving 4 g/day of omega-3.
-
•
Motivational symptoms improved most for those with lowest LPS-induced PBMC response.
1. Introduction
Depression affects 300 million individuals globally and is often characterized by an early onset and recurrent course – all contributors to its severe disease burden (Erskine et al., 2015; Burcusa and Iacono, 2007; Kessler et al., 2003; Liu et al., 2020). A significant subset of individuals with depression exhibit cognitive impairment (e.g., poor memory, attention problems, slow processing speed) (Rock et al., 2014), which is frequently present at the first onset of depression, persists even when depression is in remission, and worsens with repeated depressive episodes (Rock et al., 2014; Lee et al., 2012; Ahern and Semkovska, 2017; Semkovska et al., 2019; Hammar and Ardal, 2009). Effective treatments for cognitive impairment in depression are lacking (McIntyre et al., 2014; Legemaat et al., 2021) despite cognitive impairment being a clinically significant aspect of depression, as it is associated with worse treatment response, higher relapse rates, elevated risk for a neurodegenerative disorder, and greater social/occupational functional impairment (Lam et al., 2014; Withall et al., 2009). Thus, identification of the mechanisms underpinning cognitive impairment in depression is needed to develop efficacious treatments.
There is compelling evidence to suggest that inflammation plays a causal role in the etiology of some forms of depression (Miller and Raison, 2016; Dantzer et al., 2008), particularly frontostriatal-dependent symptoms of depression (e.g., anhedonia, fatigue, psychomotor slowing) (Lee et al., 2020; Treadway, 2016; Bekhbat et al., 2021; Miller and Raison, 2022). A substantial subset of depressed individuals (approximately 30%) exhibit a pro-inflammatory phenotype (Osimo et al., 2019; Brydges et al., 2022) and circulating levels of cytokines [e.g., interleukin-6 (IL-6), tumor necrosis factor-α (TNFα)] and acute phase proteins [e.g., C-reactive protein (CRP)] are elevated in individuals with depression compared to controls (Dowlati et al., 2010; Mac Giollabhui et al., 2021a). Likewise, enumerative (e.g., lymphopenia, neutrophilia) and functional [e.g., suppressed cytokine production/proliferative response in mitogen-stimulated peripheral blood mononuclear cells (PMBC), natural killer cell cytotoxicity] cellular abnormalities are observed in depression (Zorrilla et al., 2001; Herbert and Cohen, 1993a; Miller, 2010; Stein et al., 1991). For instance, individuals with depression exhibit lower production of cytokines in mitogen-stimulated PBMCs – typically interpreted as evidence of decreased immune cell competence because robust production of pro-inflammatory cytokines by immune cells following exposure to a pathogen is critical to fighting infection (Murphy and Weaver, 2017) – when compared to healthy controls (Lin et al., 2018; Herbert and Cohen, 1993b; Cyranowski et al., 2007). Thus, there is good reason to believe that higher levels of inflammatory biomarkers (e.g., TNFα) and decreased functional competence of immune cells (e.g., lower PBMC cytokine production following immune challenge) is associated with depressive symptomatology.
Beyond its associations with major depression in a broad sense, mounting evidence implicates inflammation specifically with cognitive impairment in depression (Carvalho et al., 2014). Empirical studies have shown that inflammation is associated with impaired cognition in medical (Crisan et al., 2014; Huang et al., 2016; Li et al., 2014), population-representative (Noble et al., 2010; Baune et al., 2008; Reichenberg et al., 2001; Singh-Manoux et al., 2014), and depressed samples (Krogh et al., 2014; Chang et al., 2012; Goldsmith et al., 2016; Grassi-Oliveira et al., 2011; Cullen et al., 2017; Mac Giollabhui et al., 2021b). Further, inflammation has been shown to disrupt multiple neuronal processes involved in components of cognition (Carvalho et al., 2014; McAfoose and Baune, 2009). For instance, inflammation may inhibit neurogenesis via decreases in neuronal proliferation and cell survival, which has been linked with structural and functional abnormalities in the hippocampus as well as deficits in both verbal abilities and memory (Chesnokova et al., 2016). Inflammation also reduces neural activation in the striatum in response to reward, and is linked to dysregulated frontrostriatal circuity as well as behavioral correlates of striatal dysfunction: psychomotor slowing, amotivation, and fatigue (Goldsmith et al., 2023; Eisenberger et al., 2010; Brydon et al., 2008; Harrison et al., 2015). The precise mechanisms and the nature of the causal relationship between inflammation and cognitive impairment in depression, however, is not well understood.
One outstanding question is whether inflammation directly disrupts specific higher-order cognitive functions (e.g., episodic memory, word-finding abilities) or whether inflammation disrupts global cognitive functioning via modulation in motivational symptoms (e.g., interest, energy, anhedonia). There is growing appreciation of a critical role played by motivation symptoms in a range of higher-order cognitive abilities, such as working memory, attention, episodic encoding and decision-making – reviewed by Braver et al. (2014). Further, experimental administration of a purified endotoxin is associated with time-limited, low-level activation of immune physiology (CRP rises to approximately 5 mg/L 6 h post-endotoxin) (Engler et al., 2017) that is reliably associated with worsening fatigue but not higher-order neurocognitive abilities (e.g., episodic memory) (Schedlowski et al., 2014). These findings align with convergent animal and human experimental studies indicating that acute immune activation leads to onset of motivational symptoms (e.g., fatigue, low energy, amotivation) via modulation of frontostriatal circuitry (Miller and Raison, 2016; Felger and Miller, 2020; Haroon et al., 2016; Miller et al., 2013).
This paper examines the relationship between inflammation and two dimensions of cognitive function (motivational symptoms and higher-order cognitive functions) in a sample of 45 individuals with depression and exhibiting a pro-inflammatory phenotype (defined as CRP ≥3 mg/L and body mass index >25) who were enrolled in a 12-week exploratory randomized placebo-controlled trial (RCT) of 3 doses of omega-3 polyunsaturated fatty acids (ω-3-FA). Supplementation with ω-3-FA has been shown to be beneficial for depression (Nemets et al., 2002; Su et al., 2003; Mischoulon et al., 2009; Hallahan et al., 2016; Mocking et al., 2016) (as well as other psychiatric conditions) (Amminger et al., 2010) and the anti-inflammatory effect of ω-3 FA is well documented (Calder, 2017). Further, emerging evidence indicates that ω-3 FA may be most effective for those who exhibit a pro-inflammatory phenotype (Rapaport et al., 2016). Longitudinal analyses compare placebo (n = 10) to the group receiving 4 g of ω-3-FA daily (n = 11) because the 4 g group was the one in which clinical efficacy was demonstrated (Mischoulon et al., 2022).
We hypothesize that:
-
1
At baseline, higher levels of inflammatory biomarkers (circulating CRP, IL-6, TNFα) and lower production of IL-6/TNFα in lipopolysaccharides (LPS)-stimulated peripheral blood mononuclear cells (indexing decreased immune cell incompetence) are associated with greater impairment in higher-order cognitive functions (e.g., attention, memory) and/or more severe motivational symptoms (e.g., alertness, energy) in depressed individuals who exhibit a pro-inflammatory phenotype.
-
2
Motivational symptoms and higher-order cognitive functions improve for individuals with depression exhibiting a pro-inflammatory phenotype who are randomized to receive 4 g of ω-3-FA daily compared to matching placebo.
-
3
Baseline inflammatory biomarkers are associated with treatment response (i.e., improvement in motivational symptoms and higher-order cognitive functions) in individuals randomized to receive 4 g of ω-3-FA daily.
2. Methods
2.1. Participants and procedure
This parallel, double-blind RCT was conducted at Massachusetts General Hospital and Emory University. Institutional Review Board approval was obtained at both sites and the study was registered at ClinicalTrials.gov (NCT02553915). The primary aims of this exploratory RCT were to evaluate whether a dose-response relationship existed between three doses of ω-3-FA (1, 2, and 4 g daily) and change in inflammatory biomarkers and depression severity when compared to placebo. Patients (n = 147) were recruited between April 2016 and July 2018 through psychiatric programs and practices, weight management and bariatric centers, primary care offices, and advertisements. All subjects provided written informed consent during enrollment; 61 were randomized to an intervention and 45 completed the study with adequate adherence to key clinical and biomarker data (for details on adherence as well as adverse event, please see the published primary outcomes paper) (Mischoulon et al., 2022). Cross-sectional analyses focus on 45 individuals at baseline who completed the study and longitudinal analyses compare placebo (n = 10) to the group receiving 4 g of ω-3-FA daily (n = 11) because the 4 g group was the one in which clinical efficacy was demonstrated (Mischoulon et al., 2022).
Male and female outpatients aged 18–80 years were enrolled who: met criteria for a current Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Major Depressive Disorder (MDD) (American Psychiatric Association, 2013), without psychotic symptoms; had a body mass index >25 kg/m2 and plasma CRP ≥3 mg/L; an Inventory of Depressive Symptomatology, Clinician-Rated version, total score ≥25 at screening and baseline. Exclusion criteria included: lifetime neurocognitive disorder, psychotic or bipolar disorder, or anorexia nervosa; substance use disorder ≤3 months prior to screening; current obsessive compulsive disorder or bulimia nervosa; serious suicidal or homicidal risk; serious or unstable medical illness; malignancy not in remission for at least one year; active autoimmune disorder or inflammatory bowel disease; insulin-dependent diabetes mellitus; breastfeeding or pregnant women; failure to respond during current major depressive episode to >4 adequate antidepressant trials; concomitant psychotropic agents within 2 weeks of baseline visit (except for prescription hypnotics, diphenhydramine, or stable daily benzodiazepine); medications that might confound biomarker findings within 1 week of baseline visit and during the trial; consuming >3g/day of ω-3-FA, or >3 meals/week of fatty fish (assessed by a food diary). Eligible patients were randomized at baseline visit and seen biweekly to assess pill compliance, depressive symptoms, and adverse events until study completion at week 12. Complete inclusion/exclusion criteria have previously been published (Mischoulon et al., 2022).
Omega-3 polyunsaturated fatty acids capsules and matching placebos were provided by Nordic Naturals (Watsonville, California). Each medication capsule contained approximately 823 mg ω-3-FA fatty acids, with an eicosapentaenoic acid (EPA):docosahexaenoic acid (DHA) ratio of 3.9:1 (about 590 mg of EPA, 152 mg DHA), verified by an independent laboratory (Lichtenstein et al., 2006). Matched placebos contained soybean oil (about 54% omega-6 and 6% ω-3-FA, no EPA or DHA). Patients were randomized equally (permuted blocks of 4 or 8) to one of four arms: 1) EPA 1.18 g/day, 254 mg/day DHA; 2) EPA 2.36 g/day, 508 mg/day DHA; 3) EPA 4.72 g/day, 1.16 g/day DHA; or 4) placebo. Participants took four capsules twice daily (8 capsules/day).
2.2. Measures
2.2.1. Inflammatory biomarkers
Phlebotomy for fasting biomarkers was performed at baseline, week 4, week 8 and week 12 between 7:30–11:00 a.m., after 30 min of rest, with avoidance of anti-inflammatory medications ≤24 h before draws. Plasma CRP was assessed with a high sensitivity turbidimetric assay (Raison et al., 2013), with assay sensitivity 0.18 mg/L, range of measure 0.2–80 mg/L, and functional sensitivity (at 20% CV) 0.2 mg/L. All samples from each subject were assayed together to reduce inter-assay variability. Peripheral blood mononuclear cells (PBMC) were isolated from fasting blood collected in sodium citrate Vacutainer Cell Preparation Tubes (Becton Dickinson, NJ). Cells were re-suspended in cell culture medium (RPMI-1640, 10% fetal bovine serum, 100 U penicillin, 100 μg streptomycin) and plated in 12-well plates at the density of 2 × 106 cell/well. Two wells were treated as control and two wells were treated with 10 ng/ml lipopolysaccharide (LPS). After 5 h incubation, cells were scraped and cell pellet and cell culture medium were separately collected after centrifugation. Plasma IL-6 and TNF from PBMC culture supernatant were measured with fluorokine MAP assays (R&D Systems, Minneapolis, MN) (Felger et al., 2016).
2.2.2. Cognitive functioning
The Cognitive and Physical Functioning Questionnaire (CPFQ), a reliable and validated scale, was administered at baseline, week 4, week 8 and week 12. The CPFQ is sensitive to change among persons with clinical depression and was designed to assess clinically relevant impairment in motivational symptoms and higher-order cognitive functions that are typically dysregulated in depression (Fava et al., 2006, 2009). The CPFQ includes 7 questions related to: i) motivation/interest/enthusiasm; ii) wakefulness/alertness; iii) energy; iv) ability to focus/sustain attention; v) ability to remember/recall information; vi) ability to find words; and vii) sharpness/mental acuity. Each question is rated on a 6-point scale: 1 = “greater than normal,” 2 = “normal,” 3 = “minimally diminished,” 4 = “moderately diminished,” 5 = “markedly diminished,” and 6 = “totally absent.” Two subscales of the CPFQ were identified in clinical samples: 1) motivational symptoms (items 1–3); higher-order cognitive functions (items 4–7) (Baer et al., 2014). Total score and subscales scores were calculated based on the sum of the item ratings. Test-retest reliability in the current sample was r = 0.57 (baseline to week 4), r = 0.87 (week 4 to week 8), and r = 0.84 (week 8 to week 12).
2.3. Analyses
Analyses were conducted in R 4.2.2 and multilevel models with random intercepts and fixed slopes were estimated using lmer4 (Bates et al., 2015). Inflammatory biomarkers were log-transformed to impose a normal distribution upon these variables; estimates of skewness and kurtosis improved for all transformed variables and were subsequently ≥ −1 and ≤ 1. The analyses conducted in this manuscript were not pre-specified primary or secondary aims of the RCT and should be considered exploratory in nature.
First, cross-sectional analyses examined the associations of immune biomarkers with motivational and higher-order cognitive subscales of the CPFQ in 45 individuals with depression at baseline (i.e., pre-randomization); associations were estimated using Pearson's correlation and immune biomarkers were log-transformed. Second, demographic and clinical characteristics for the placebo and 4 g/day of ω-3-FA group were compared (independent sample t-tests, Kruskal-Wallis, and chi-squared tests of difference were used to estimate whether groups differed based on means, medians, and cell counts, respectively) and descriptive statistics provide means, standard deviations, and effect sizes (Cohen's d, including a 95% confidence interval) for motivational/higher-order cognitive subscales of the CPFQ by randomization group (placebo vs. 4 g/day of ω-3-FA) over time (baseline, week 4, week 8, and week 12). Hierarchical linear models (random intercept/fixed slope), using the default unstructured variance-covariance structure, estimated whether i) randomization status (placebo vs. 4 g/day of ω-3-FA), ii) time in treatment (week), and iii) their combination (interaction of randomization status by time treatment) predicted motivational/higher-order cognitive subscales of the CPFQ. Third, Spearman's rho was used to estimate the association between baseline immune biomarkers (not log-transformed) and change in motivational/higher-order cognitive subscales from baseline to week 12.
3. Results
3.1. Cross-sectional analyses: Association between immune biomarkers and cognition in 45 depressed individuals
As hypothesized, lower concentrations of IL-6 in LPS-stimulated PBMCs at baseline were associated with greater impairment on the higher-order cognitive functioning subscale (r = −0.35, p = .02) of the CPFQ and lower concentrations of TNFα in LPS-stimulated PBMCs were (non-significantly) associated with greater impairment on the higher-order cognitive functioning subscale (r = −0.28, p = .06). Baseline IL-6 and TNFα in LPS-stimulated PBMCs were not associated with motivational symptoms. There were no significant associations of baseline circulating CRP, IL6 or TNFα with motivational symptoms or higher-order cognitive functioning. All associations are reported in Supplementary Table 1, Section A.
3.2. Longitudinal analyses: Effect of 4 g of omega-3 on cognition compared to placebo over 12 weeks of treatment
Treatment groups (placebo vs. 4 g/day) did not differ by demographic or clinical variables at baseline (see Table 1). Means, standard deviations, and standardized mean differences for the motivational and higher-order cognitive functions subscales of the CPFQ are presented in Table 2 for the placebo vs. 4 g/day ω-3-FA at baseline, week 4, 8, and 12 (data for the 1 g/day and 2 g/day groups are presented in Supplementary Table 2). Notably, the placebo group exhibited significantly higher levels of impairment on the higher-order cognitive functions subscale at week 4 (p = .04) and non-significantly higher levels of impairment at baseline (p = .07), indicative of randomization failure for this variable.
Table 1.
Baseline Demographic and Clinical Characteristics For Individuals Randomized to Receive 4 g a day of Omega-3 and Placebo.
| Demographic | Placebo |
4g/day Omega 3 |
|
|---|---|---|---|
| (n = 10) | (n = 11) | ||
|
51.50 (13.13) | 43.45 (14.74) | |
|
Female | 8, 80% | 7, 64% |
| Male | 2, 20% | 4, 36% | |
|
Black/African-American | 5, 50% | 5, 46% |
| Asian | 0, 0% | 1, 9% | |
| Caucasian | 4, 40% | 4, 36% | |
| Unknown/Not Reported | 1, 10% | 1, 9% | |
|
Hispanic | 3, 30% | 1, 9% |
| Not Hispanic | 7, 70% | 10, 91% | |
|
High School or Less | 2, 20% | 1, 9% |
| Some College or More | 8, 80% | 10, 91% | |
|
Married/Living Together | 1, 10% | 3, 28% |
| Separated/Divorced/Widowed | 5, 50% | 4, 36% | |
| Never Married | 4, 40% | 4, 36% | |
| Clinical Characteristics | |||
| IDS-C30 Score [Mean (SD)] | 36.60 (10.54) | 31.55 (4.97) | |
| CRP mg/L [Median (IQR Range)]ⴕ | 6.9 (4.55) | 3.86 (4.71) | |
| Body Mass Index [Median (IQR) Range] | 40.78 (7.02) | 35.14 (4.97) | |
| CPFQ: Lower-order Cognitive Subscale | 11.30 (2.63) | 11.73 (1.95) | |
| CPFQ: Higher-order Cognitive Subscale | 14.10 (3.51) | 11.64 (2.42) | |
ⴕ = difference in median values estimated using Kruskal Wallis Test; CPFQ = Cognitive Physical Functioning Questionnaire.
Table 2.
Comparison of Placebo (n = 10) vs. 4 g a day Omega 3 (n = 11) for Study Completers at Baseline, Week 4, Week 8, and Week 12 on the motivational symptoms and higher-order cognitive function subscales of the Cognitive Physical Functioning Questionnaire (CPFQ).
| CPFQ: Motivational Symptoms (3 items) |
CPFQ: Higher-Order Cognitive Function (4 items) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | 4 g/d EPA | p | Cohen's D [95% CI] | Placebo | 4 g/d EPA | p | Cohen's D [95% CI] | ||
| Baseline | Mean (SD) | 11.30 (2.63) | 11.73 (1.95) | .67 | −.20 [-1.05, .67] | 14.10 (3.51) | 11.64 (2.42) | .07 | .87 [-.04, 1.76] |
| Week 4 | Mean (SD) | 10.20 (2.10) | 8.73 (3.85) | .29 | .49 [-.38, 1.36] | 12.20 (2.57) | 9.91 (2.21) | .04 | 1.01 [.08, 1.91] |
| Week 8 | Mean (SD) | 9.30 (3.06) | 7.36 (2.77) | .14 | .70 [-.19, 1.58] | 11.20 (3.29) | 9.27 (2.57) | .15 | .69 [-.20, 1.57] |
| Week 12 | Mean (SD) | 9.10 (2.51) | 7.00 (3.03) | .10 | .79 [-.11, 1.67] | 10.70 (2.91) | 9.18 (1.94) | .17 | .65 [-.24, 1.53] |
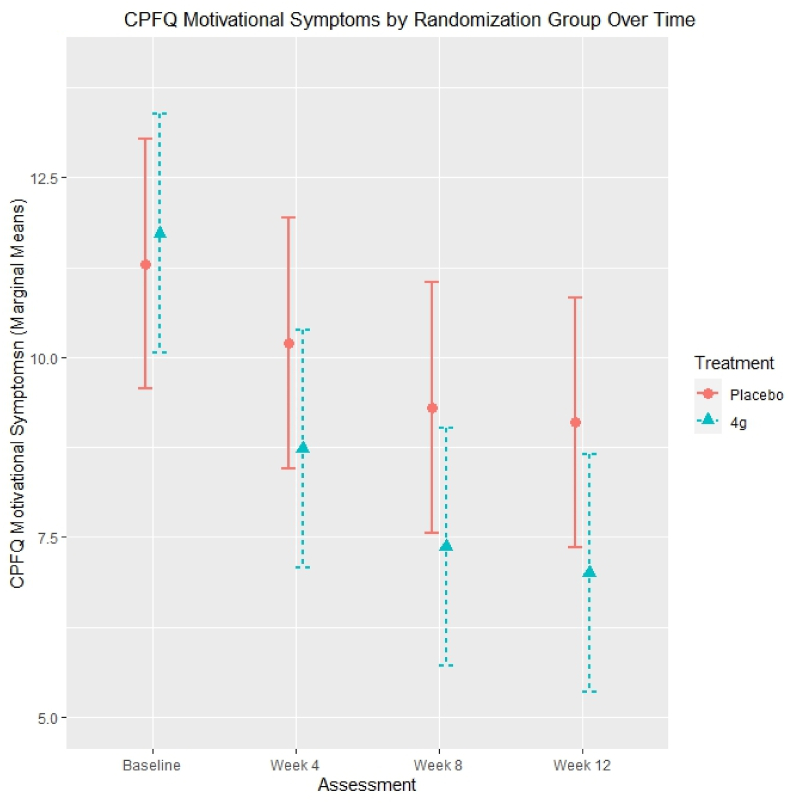
Hierarchical linear modelling demonstrated that individuals who completed treatment and who were randomized to 4 g/day of ω-3-FA reported significantly reduced impairment on the motivational subscale of the CPFQ compared to placebo. Marginal means are estimated based on hierarchical linear models and are visualized in Fig. 1. Within a hierarchical linear model predicting motivational symptoms, ‘time in treatment’ was associated with decreasing impairment in motivational symptoms (B = −0.19, p = .006), ‘randomization status’ was not significantly associated with motivational symptoms (B = −0.02, p = .96), and the interaction of ‘time in treatment’ by ‘randomization status’ was significantly associated with decreasing impairment in motivational symptoms (B = −0.07, p = .03). Within a hierarchical linear model predicting higher-order cognitive functions, ‘time in treatment’ (B = −0.28, p < .001) and ‘randomization status’ (B = −0.84, p = .03) were significantly associated with decreasing impairment in higher-order cognitive functions; however, the interaction of ‘time in treatment’ by ‘randomization status’ was not significantly associated with higher-order cognitive functions (B = 0.03, p = .41).
Fig. 1.
CPFQ motivational symptoms by randomization group over time.
3.3. Longitudinal analyses: Association of baseline immune biomarkers with change in cognition from baseline to week 12 in depressed individuals randomized to receive 4 g/day of ω-3-FA (n = 11)
Within the 4 g/day group, subjects with lower concentrations of TNFα in LPS-stimulated PBMCs experienced significantly greater change in motivational symptoms (Ρ = .71, p = .02). Lower concentrations of IL-6 in LPS-stimulated PBMCs were (non-significantly) associated with greater change in motivational symptoms (Ρ = 0.59, p = .06) on the CPFQ. No other significant associations were observed; however, numerically sizeable (but non-significant) associations were observed for CRP (Ρ = −0.34, p = .31) and IL-6 (Ρ = −0.30, p = .37) with change in motivational symptoms, such that subjects with higher circulating levels of CRP and IL-6 at baseline were those who experienced greater improvements in motivational symptoms. All associations are reported in Supplementary Table 1, Section B.
4. Discussion
At baseline, lower concentrations of cytokines in LPS-stimulated PBMCs were associated with greater impairment in higher-order cognitive functions (e.g., memory, word-finding) in individuals with depression and an inflammatory phenotype. Compared to those randomized to placebo, participants randomized to receive 4 g/day of omega-3 (ω-3-FA) reported improved motivational symptoms (e.g., interest/enthusiasm, alertness, energy). Moreover, individuals exhibiting lower concentrations of cytokines in LPS-stimulated PBMCs (indicative of decreased immune cell competence) at baseline reported the largest improvements in motivational symptoms following 4 g/day of ω-3-FA.
4.1. Inflammation and higher-order cognitive functions at baseline
Lower cytokine concentrations in LPS-stimulated PBMCs at baseline were associated with greater impairment in higher-order cognitive functions at baseline. Lower concentrations of cytokines in LPS-stimulated PBMCs are interpreted as decreased immune cell competence because robust production of pro-inflammatory cytokines by immune cells following exposure to a pathogen is critical to fighting infection (Murphy and Weaver, 2017). Lower concentrations of cytokines in LPS-stimulated PBMCs are typically associated with higher levels of circulating pro-inflammatory proteins; indeed, it has long been recognized that individuals with depression exhibit lower concentrations of LPS-stimulated PBMCs compared to healthy controls (Lin et al., 2018; Herbert and Cohen, 1993b; Cyranowski et al., 2007). It is possible that the baseline association of lower cytokine concentrations in LPS-stimulated PBMCs with greater impairment in higher-order cognitive functions reflects deleterious effects of chronic immune dysregulation on brain structure and function over time, potentially via disrupted long-term potentiation, neurogenesis, and apoptosis (Mac Giollabhui et al., 2020, 2021b, 2021c; Amor et al., 2010; Mac Giollabhui, 2021) – associations that may be more difficult to detect in circulating cytokines and acute phase reactants, which vary substantially over time (Walsh et al., 2023). This finding is aligned with prior work demonstrating that a dysregulated immune system is associated with structural and functional brain abnormalities (Yin et al., 2019; Han and Ham, 2021) as well as worse performance on neuropsychological tests in medical (Crisan et al., 2014; Huang et al., 2016; Li et al., 2014), population-representative (Noble et al., 2010; Baune et al., 2008; Reichenberg et al., 2001; Singh-Manoux et al., 2014), and depressed samples (Krogh et al., 2014; Chang et al., 2012; Goldsmith et al., 2016; Grassi-Oliveira et al., 2011; Cullen et al., 2017; Mac Giollabhui et al., 2021b).
4.2. The effect of omega-3 FA supplementation on cognition in depression
This is the first study to demonstrate beneficial effects on cognition of high dose ω-3-FA supplementation in an inflammatory subtype of depression. Over the last twenty years, there has been intense focus on the potential benefit of ω-3-FA in clinical populations, particularly MDD and attention-deficit/hyperactivity disorder (ADHD). Overall, evidence suggests that ω-3-FA alleviate inattentive symptoms and depression in these populations, respectively; however, significant heterogeneity has been observed and it is likely that these associations are dependent on dose, composition of preparation, duration of treatment, and patient characteristics (e.g., socioeconomic status, low ω-3-FA levels) (Chang et al., 2018; Frensham et al., 2012). Accumulating data also indicate that ω-3-FA may exert beneficial effects on memory and executive function in healthy adults, particularly those experiencing cognitive impairment (Alex et al., 2020; Mazereeuw et al., 2012). However, very little research has examined a potential effect of ω-3-FA supplementation on cognitive impairment in depression (Solé et al., 2015). One study of subclinical depression found that ω-3-FA supplementation was unrelated to improved cognitive performance on standardized neuropsychological measures, when compared to placebo (Rogers et al., 2008). Similar results were observed in a separate study reporting that ω-3-FA supplementation was associated with improved performance on emotionally valenced [e.g., where negative emotions are elicited on a cognitive task by using negatively valenced words, for example “failure”) rather than neutral words] cognitive testing when compared to placebo, but not on neutral cognitive assessments (Antypa et al., 2012). Our results point to the potential of ω-3-FA to rapidly alleviate motivational deficits, but not higher-order cognitive functions, within a brief time frame (i.e., within 12 weeks of treatment) (Braver et al., 2014). Divergent results across studies may be due to differences in inclusion criteria resulting in the analysis of different clinical phenotypes. Prior observational research has found that cognitive difficulties are more pronounced in individuals with higher inflammatory biomarkers and higher BMI (Fard et al., 2020). Further research is needed to determine whether the beneficial effect of ω-3-FA observed in this study is dependent on higher levels of CRP and BMI. Specific improvement in motivational symptoms is in agreement with recent research demonstrating that chronic inflammation is associated with a specific symptom profile (i.e., anhedonia, psychomotor slowing) in depression (Miller and Raison, 2022; Frank et al., 2021; White et al., 2017; Fried et al., 2019; Suneson et al., 2023).
4.3. Baseline immune dysfunction as a predictor of treatment response
In this study, individuals who exhibited attenuated functional responses of PBMCs to an immune challenge were the ones who reported the greatest improvement in motivational symptoms. Similarly, although non-significant, the size of the association between decreases in other pro-inflammatory biomarkers and improvement in motivational symptoms was substantial for CRP and IL-6 (Ρs ≥0.30). These data suggest that, even in a study specifically recruiting individuals with chronic, low-grade inflammation (CRP ≥3 mg/L), individuals with greater levels of inflammation or immune dysfunction show the strongest response. Such findings are consistent with a prior RCT in which individuals with CRP ≥5 mg/L were found to benefit most from administration of a TNF antagonist (Raison et al., 2013). Further, the association of lower cytokine concentrations in LPS-stimulated PBMCs with greater improvement in motivational symptoms (e.g., enthusiasm, alertness, energy) rather than higher order cognitive functions may indicate acute, state-dependent effects of inflammation that are alleviated when inflammation is reduced. These findings are in agreement with experimental research demonstrating that low levels of immune activation (typically via administration of vaccine or endotoxin) are reliably associated with motivational symptomatology (e.g., fatigue, alertness) but not higher-order neurocognitive abilities (e.g., episodic memory) (Schedlowski et al., 2014) as well as considerable work detailing the sensitivity of frontostriatal circuitry to immune activation (Miller and Raison, 2016; Felger and Miller, 2020; Haroon et al., 2016; Miller et al., 2013).
Identifying treatments for cognitive impairment in depression is a critical unmet need. Yet, only one pharmacological treatment – Trintellix – has compelling data supporting pro-cognitive effects in a subset of depressed individuals (McIntyre et al., 2014). Moreover a recent meta-analysis of cognitive training interventions for people with MDD found that the acute benefits diminished after treatment ended (Legemaat et al., 2021). Our study suggests that the motivational difficulties which contribute to cognitive impairment in MDD may be treated using high dose ω-3-FA. As inflammation is associated with progressive cognitive decline and the long-term benefit (i.e., when taken for longer than twelve weeks of treatments) of ω-3-FA on higher-order cognitive functioning is unknown, this is a promising avenue for future investigation (Amor et al., 2010). Future work would benefit from better evaluating the degree to which improved motivational symptoms contribute to real-world neuropsychological functioning (i.e., patient performance in their actual day-to-day life, rather than self-report or laboratory-based performance measures) as well as gains in social, occupational, and academic functioning.
4.4. Limitations
First, this modestly powered exploratory trial was not designed to produce definitive results; thus these findings should be considered preliminary and requiring replication in an adequately powered larger study. Second, it is unlikely that the sample was representative of the population from which it was recruited; thus, results observed here, although true to this sample, may not generalize more broadly to depressed individuals as a whole. Finally, although the CPFQ is reliable and validated instrument that is sensitive to change and has been associated with performance-based neurocognitive measures among patients with depression, the degree to which it corresponds to real-world cognitive functioning is unknown.
4.5. Conclusions
In this exploratory RCT of individuals with depression and a pro-inflammatory phenotype randomized to receive daily supplementation of omega-3 (or matching placebo), baseline dysfunction in LPS-stimulated PBMCs was associated with greater impairment in higher-order cognitive functions. It was motivational symptoms, however, that improved for individuals receiving 4 g per day of omega-3 polyunsaturated fatty acids over 12 weeks, compared to placebo and, further, it was those with more severe immune dysfunction who benefited most. Although findings need to be replicated in larger samples, this study suggests that motivational symptoms that accompany chronic inflammation can respond to anti-inflammatory treatments, such as omega-3 supplementation, within a brief time period.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Naoise Mac Giollabhui: No conflict of interest. David Mischoulon: Dr. Mischoulon has received research support from Nordic Naturals and Heckel Medizintechnik GmbH. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, Peerpoint Medical Education Institute, LLC, and Harvard blog. He also works with the MGH Clinical Trials Network and Institute (CTNI), which has received research funding from multiple pharmaceutical companies and NIMH. Boadie Dunlop: Dr. Dunlop has received research support from Boehringer-Ingelheim, Compass, NIMH, and Usona Institute, and has served as a consultant to Biohaven, Cerebral Therapeutics, Department of Defense, Myriad Neuroscience, NeuroRx, Inc., Otsuka, and Sage. Becky Kinkead: No conflict of interest. Pamela Schettler: No conflict of interest. Richard Liu: Dr. Liu has received research support from the National Institute of Mental Health, the American Foundation for Suicide Prevention, and American Psychological Foundation,. He has served as a consultant for Relmada Therapeutics. Olivia Okereke: Dr. Okereke has received royalties from Springer Publishing for a book on late-life depression prevention, meeting honoraria and/or travel support from the AARP Global Council on Brain Health, and research support from the NIH. Stefania Lamon-Fava: No conflict of interest. Maurizio Fava: All disclosures can also be viewed online by navigating to: https://mghcme.org/maurizio-fava-bio-disclosure. Mark Rapaport: No conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100666.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Available upon reasonable request.
References
- Ahern E., Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology. 2017;31(1):52. doi: 10.1037/neu0000319. [DOI] [PubMed] [Google Scholar]
- Alex A., Abbott K.A., McEvoy M., Schofield P.W., Garg M.L. Long-chain omega-3 polyunsaturated fatty acids and cognitive decline in non-demented adults: a systematic review and meta-analysis. Nutr. Rev. 2020;78(7):563–578. doi: 10.1093/nutrit/nuz073. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Amminger G.P., Schäfer M.R., Papageorgiou K., et al. Long-chain ω-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatr. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Amor S., Puentes F., Baker D., Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antypa N., Smelt A.H., Strengholt A., Van der Does A.W. Effects of omega-3 fatty acid supplementation on mood and emotional information processing in recovered depressed individuals. J. Psychopharmacol. 2012;26(5):738–743. doi: 10.1177/0269881111424928. [DOI] [PubMed] [Google Scholar]
- Baer L., Ball S., Sparks J., et al. Further evidence for the reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire (CPFQ) Ann. Clin. Psychiatr. 2014;26(4):270–281. [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2015;67(1):1–48. [Google Scholar]
- Baune B., Ponath G., Golledge J., Varga G. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol. Aging. 2008;29(6):937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bekhbat M., Goldsmith D.R., Woolwine B.J., Haroon E., Miller A.H., Felger J.C. Transcriptomic signatures of psychomotor slowing in peripheral blood of depressed patients: evidence for immunometabolic reprogramming. Mol. Psychiatr. 2021:1–9. doi: 10.1038/s41380-021-01258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Krug M.K., Chiew K.S., et al. Mechanisms of motivation–cognition interaction: challenges and opportunities. Cognit. Affect Behav. Neurosci. 2014;14:443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges C.R., Bhattacharyya S., Dehkordi S.M., et al. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain Behav. Immun. 2022;102:42–52. doi: 10.1016/j.bbi.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L., Harrison N.A., Walker C., Steptoe A., Critchley H.D. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol. Psychiatr. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcusa S.L., Iacono W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem. Soc. Trans. 2017;45(5):1105–1115. doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- Carvalho A.F., Miskowiak K.K., Hyphantis T.N., et al. Cognitive dysfunction in depression - pathophysiology and novel targets. CNS Neurol. Disord.: Drug Targets. 2014;13(10):1819–1835. doi: 10.2174/1871527313666141130203627. [DOI] [PubMed] [Google Scholar]
- Chang H.H., Lee I.H., Gean P.W., et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav. Immun. 2012;26(1):90–95. doi: 10.1016/j.bbi.2011.07.239. [DOI] [PubMed] [Google Scholar]
- Chang J.P., Su K.P., Mondelli V., Pariante C.M. Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder: a systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology. 2018;43(3):534–545. doi: 10.1038/npp.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V., Pechnick R.N., Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016;58:1–8. doi: 10.1016/j.bbi.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan A.F., Oancea C., Timar B., Fira-Mladinescu O., Crisan A., Tudorache V. Cognitive impairment in chronic obstructive pulmonary disease. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen A.E., Tappin B.M., Zunszain P.A., et al. The relationship between salivary C-reactive protein and cognitive function in children aged 11–14years: does psychopathology have a moderating effect? Brain Behav. Immun. 2017;66:221–229. doi: 10.1016/j.bbi.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski J.M., Marsland A.L., Bromberger J.T., Whiteside T.L., Chang Y., Matthews K.A. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav. Immun. 2007;21(2):229–237. doi: 10.1016/j.bbi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., et al. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatr. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H., Brendt P., Wischermann J., et al. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Mol. Psychiatr. 2017;22(10):1448–1454. doi: 10.1038/mp.2016.264. [DOI] [PubMed] [Google Scholar]
- Erskine H.E., Moffitt T.E., Copeland W.E., et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol. Med. 2015;45(7):1551–1563. doi: 10.1017/S0033291714002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard M., Cribb L., Nolidin K., Savage K., Wesnes K., Stough C. Is there a relationship between low-grade systemic inflammation and cognition in healthy people aged 60–75 years? Behav. Brain Res. 2020;383 doi: 10.1016/j.bbr.2020.112502. [DOI] [PubMed] [Google Scholar]
- Fava M., Graves L.M., Benazzi F., et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. J. Clin. Psychiatry. 2006;67(11):1754–1759. doi: 10.4088/jcp.v67n1113. [DOI] [PubMed] [Google Scholar]
- Fava M., Iosifescu D.V., Pedrelli P., Baer L. Reliability and validity of the Massachusetts general hospital cognitive and physical functioning questionnaire. Psychother. Psychosom. 2009;78(2):91–97. doi: 10.1159/000201934. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Miller A.H. Identifying immunophenotypes of inflammation in depression: dismantling the monolith. Biol. Psychiatr. 2020;88(2):136–138. doi: 10.1016/j.biopsych.2020.04.024. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatr. 2016;21(10):1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank P., Jokela M., Batty G.D., Cadar D., Steptoe A., Kivimäki M. Association between systemic inflammation and individual symptoms of depression: a pooled analysis of 15 population-based cohort studies. Am. J. Psychiatr. 2021;178(12):1107–1118. doi: 10.1176/appi.ajp.2021.20121776. [DOI] [PubMed] [Google Scholar]
- Frensham L.J., Bryan J., Parletta N. Influences of micronutrient and omega-3 fatty acid supplementation on cognition, learning, and behavior: methodological considerations and implications for children and adolescents in developed societies. Nutr. Rev. 2012;70(10):594–610. doi: 10.1111/j.1753-4887.2012.00516.x. [DOI] [PubMed] [Google Scholar]
- Fried E.I., Von Stockert S., Haslbeck J., Lamers F., Schoevers R., Penninx B. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol. Med. 2019:1–9. doi: 10.1017/S0033291719002770. [DOI] [PubMed] [Google Scholar]
- Goldsmith D.R., Haroon E., Woolwine B.J., et al. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav. Immun. 2016;56:281–288. doi: 10.1016/j.bbi.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D., Bekhbat M., Mehta N.D., Felger J.C. Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol. Psychiatr. 2023;93(4):405–418. doi: 10.1016/j.biopsych.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R., Bauer M.E., Pezzi J.C., Teixeira A.L., Brietzke E. Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuroendocrinol. Lett. 2011;32(4):540–544. [PubMed] [Google Scholar]
- Hallahan B., Ryan T., Hibbeln J.R., et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry. 2016;209(3):192–201. doi: 10.1192/bjp.bp.114.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar A., Ardal G. Cognitive functioning in major depression--a summary. Front. Hum. Neurosci. 2009;3:26. doi: 10.3389/neuro.09.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K.M., Ham B.J. How inflammation affects the brain in depression: a review of functional and structural mri studies. J. Clin. Neurol. 2021;17(4):503–515. doi: 10.3988/jcn.2021.17.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Fleischer C., Felger J.C., et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatr. 2016;21(10):1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Cercignani M., Voon V., Critchley H.D. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015;40(4):831–838. doi: 10.1038/npp.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert T.B., Cohen S. Depression and immunity: a meta-analytic review. Psychol. Bull. 1993;113(3):472. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Herbert T.B., Cohen S. Depression and immunity: a meta-analytic review. Psychol. Bull. 1993;113(3):472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Huang Y.S., Guilleminault C., Hwang F.M., et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine. 2016;95(41) doi: 10.1097/MD.0000000000004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krogh J., Benros M.E., Jørgensen M.B., Vesterager L., Elfving B., Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav. Immun. 2014;35:70–76. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Lam R.W., Kennedy S.H., McIntyre R.S., Khullar A. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can. J. Psychiatr. 2014;59(12):649–654. doi: 10.1177/070674371405901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Hermens D.F., Ma Porter, Redoblado-Hodge M.A. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J. Affect. Disord. 2012;140:113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Lee Y., Mansur R.B., Brietzke E., et al. Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav. Immun. 2020;88:631–639. doi: 10.1016/j.bbi.2020.04.063. [DOI] [PubMed] [Google Scholar]
- Legemaat A.M., Semkovska M., Brouwer M., et al. Effectiveness of cognitive remediation in depression: a meta-analysis. Psychol. Med. 2021;52(16):1–16. doi: 10.1017/S0033291721001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Robertson C.M., Yu X., Cheypesh A., Dinu I.A., Li J. Early postoperative systemic inflammatory response is an important determinant for adverse 2-year neurodevelopment-associated outcomes after the Norwood procedure. J. Thorac. Cardiovasc. Surg. 2014;148(1):202–206. doi: 10.1016/j.jtcvs.2013.07.079. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A.H., Matthan N.R., Jalbert S.M., Resteghini N.A., Schaefer E.J., Ausman L.M. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006;84(3):497–504. doi: 10.1093/ajcn/84.3.497. [DOI] [PubMed] [Google Scholar]
- Lin P., Ding B., Wu Y., Dong K., Li Q. Mitogen-stimulated cell proliferation and cytokine production in major depressive disorder patients. BMC Psychiatr. 2018;18(1):1–7. doi: 10.1186/s12888-018-1906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., He H., Yang J., Feng X., Zhao F., Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatr. Res. 2020;126:134–140. doi: 10.1016/j.jpsychires.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N. Inflammation and depression: research designs to better understand the mechanistic relationships between depression, inflammation, cognitive dysfunction, and their shared risk factors. Brain Behav Immun Health. 2021;15 doi: 10.1016/j.bbih.2021.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Swistun D., Murray S., et al. Executive dysfunction in depression in adolescence: the role of inflammation and higher body mass. Psychol. Med. 2020;50(4):683–691. doi: 10.1017/S0033291719000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Ng T.H., Ellman L.M., Alloy L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol. Psychiatr. 2021;26(7):3302–3314. doi: 10.1038/s41380-020-00867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Alloy L.B., Hartman C.A. Investigating whether depressed youth exhibiting elevated C reactive protein perform worse on measures of executive functioning, verbal fluency and episodic memory in a large, population based sample of Dutch adolescents. Brain Behav. Immun. 2021;94:369–380. doi: 10.1016/j.bbi.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Giollabhui N., Alloy L.B., Schweren L.J.S., Hartman C.A. Investigating whether a combination of higher CRP and depression is differentially associated with worse executive functioning in a cohort of 43,896 adults. Brain Behav. Immun. 2021;96:127–134. doi: 10.1016/j.bbi.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazereeuw G., Lanctot K.L., Chau S.A., Swardfager W., Herrmann N. Effects of omega-3 fatty acids on cognitive performance: a meta-analysis. Neurobiol. Aging. 2012;33(7):1482. doi: 10.1016/j.neurobiolaging.2011.12.014. e1417-1482. e1429. [DOI] [PubMed] [Google Scholar]
- McAfoose J., Baune B.T. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McIntyre R.S., Lophaven S., Olsen C.K. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int. J. Neuropsychopharmacol. 2014;17(10):1557–1567. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H. Depression and immunity: a role for T cells? Brain Behav. Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. Burning down the house: reinventing drug discovery in psychiatry for the development of targeted therapies. Mol. Psychiatr. 2022:1–8. doi: 10.1038/s41380-022-01887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D., Papakostas G.I., Dording C.M., et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. J. Clin. Psychiatry. 2009;70(12):1636–1644. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D., Dunlop B.W., Kinkead B., et al. Omega-3 fatty acids for major depressive disorder with high inflammation: a randomized dose-finding clinical trial. J. Clin. Psychiatry. 2022;83(5) doi: 10.4088/JCP.21m14074. [DOI] [PubMed] [Google Scholar]
- Mocking R., Harmsen I., Assies J., Koeter M., Ruhé H., Schene A. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry. 2016;6(3) doi: 10.1038/tp.2016.29. e756-e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Weaver C. ninth ed. Garland Science, Taylor & Francis Group; New York, New York: 2017. Janeway's Immunobiology. [Google Scholar]
- Nemets B., Stahl Z., Belmaker R. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatr. 2002;159(3):477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Noble J.M., Manly J.J., Schupf N., Tang M.X., Mayeux R., Luchsinger J.A. Association of C-reactive protein with cognitive impairment. Arch. Neurol. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo E.F., Baxter L.J., Lewis G., Jones P.B., Khandaker G.M. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol. Med. 2019;49(12):1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Rutherford R.E., Woolwine B.J., et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatr. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport M.H., Nierenberg A.A., Schettler P.J., et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol. Psychiatr. 2016;21(1):71–79. doi: 10.1038/mp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A., Yirmiya R., Schuld A., et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatr. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rogers P.J., Appleton K.M., Kessler D., et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br. J. Nutr. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- Schedlowski M., Engler H., Grigoleit J.-S. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav. Immun. 2014;35:1–8. doi: 10.1016/j.bbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Semkovska M., Quinlivan L., O'Grady T., et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatr. 2019;6(10):851–861. doi: 10.1016/S2215-0366(19)30291-3. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A., Dugravot A., Brunner E., et al. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83(6):486–493. doi: 10.1212/WNL.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé B., Jiménez E., Martinez-Aran A., Vieta E. Cognition as a target in major depression: new developments. Eur. Neuropsychopharmacol. 2015;25(2):231–247. doi: 10.1016/j.euroneuro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Stein M., Miller A.H., Trestman R.L. Depression, the immune system, and health and illness: findings in search of meaning. Arch. Gen. Psychiatr. 1991;48(2):171–177. doi: 10.1001/archpsyc.1991.01810260079012. [DOI] [PubMed] [Google Scholar]
- Su K.-P., Huang S.-Y., Chiu C.-C., Shen W.W. Omega-3 fatty acids in major depressive disorder: a preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2003;13(4):267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- Suneson K., Grudet C., Ventorp F., et al. An inflamed subtype of difficult-to-treat depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2023;125 doi: 10.1016/j.pnpbp.2023.110763. [DOI] [PubMed] [Google Scholar]
- Treadway M.T. 2016. Dynamics of Inflammation and its Blockade on Motivational Circuitry in Depression. December 30 - November 18, 2021. NCT03006393. [Google Scholar]
- Walsh C.P., Lindsay E.K., Grosse P., et al. A systematic review and meta-analysis of the stability of peripheral immune markers in healthy adults. Brain Behav. Immun. 2023;107:32–46. doi: 10.1016/j.bbi.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kivimäki M., Jokela M., Batty G.D. Association of inflammation with specific symptoms of depression in a general population of older people: the English Longitudinal Study of Ageing. Brain Behav. Immun. 2017;61:27–30. doi: 10.1016/j.bbi.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Withall A., Harris L., Cumming S. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol. Med. 2009;39(3):393–402. doi: 10.1017/S0033291708003620. [DOI] [PubMed] [Google Scholar]
- Yin L., Xu X., Chen G., et al. Inflammation and decreased functional connectivity in a widely-distributed network in depression: centralized effects in the ventral medial prefrontal cortex. Brain Behav. Immun. 2019;80:657–666. doi: 10.1016/j.bbi.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Luborsky L., McKay J.R., et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav. Immun. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon reasonable request.