Abstract
This research aimed to evaluate the oxidative stability and rheological properties of dark chocolates with the addition of essential oils (EO) of Cymbopogon citratus, Pimpinella anisum, and Mintostachys mollis. For this purpose, before the inclusion in chocolates, the EO were chemically characterized to identify the most important volatile compounds. We added essential oils of P. anisum, C. citratus and M. mollis to dark chocolates (cocoa 70%) at doses of 10, 12 and 14 μL per 500 g, separately. These chocolates were evaluated for oxidative activity, hardness, microstructure, rheological and melting properties and antioxidant capacity. It was found that C. citratus EO (10 μL/500 g of chocolate) improve the oxidative stability of the chocolates at 90 days of storage at 25 °C (230 meq O2/kg), while higher concentrations promote lipid oxidation. The incorporation of essential oils improves the antioxidant capacity, likewise, it changes the rheological, thermal, and microstructural properties. Therefore, essential oils can improve the physicochemical characteristics of dark chocolates allowing greater stability in oxidative fat and thus increase the shelf life.
Keywords: Cymbopogon citratus, Pimpinella anisum, Mintostachys mollis, Antioxidants, Thermic properties, Chocolate microstructure
1. Introduction
Chocolate is the main product of cocoa and is one of the most famous sweets worldwide, being consumed by people of different ages for its organoleptic properties (taste, aroma, and texture), nutritional values, and various benefits to the consumer [1]. In the last decade, the demand for quality of chocolate by consumers has increased. Considering this, the chocolate industry has been developing various techniques for continuous improvements in processes and formulas to guarantee the needs and satisfaction of customers [2]. However, processing of these chocolates is complex owing to the interaction of their ingredients (cocoa, cocoa butter and sugar) in the formation of a complex emulsion and their production stages such as: mixing, refining, conching, tempering, molding and packaging [1,3].
Conching, though, is one of the critical stages of the chocolate making process, playing a crucial role in reducing unwanted aroma compounds and moisture in addition to implying a change in the texture of the chocolate [4]. Next, the tempering operation implies a thermodynamic stability of the small fat crystals and their homogeneous dispersion to stabilize the polymorphic transitions V of the cocoa fat at temperatures of 32–34 °C. In the same way, this process generates the shine, softness, malleability, and characteristic color during the storage period [1,3,5]. However, the monounsaturated fats present in chocolate are the most sensitive and rapidly degradable by oxidation when exposed to light, oxygen, temperature, and air [6]. In addition, chocolate properties such as hardness and viscosity are the result of the interaction between the continuous crystallized fat phase and the dispersed solid particles [7], which are influenced by the production process, conching, tempering (size, shape, surface), incorporation of new additives, and the storage conditions [[7], [8], [9]].
Chocolate has high-quality polyphenol and fat content, thus considered a “superfood” [9,10]. Nevertheless, fats in chocolate are particularly susceptible to oxidation during processing and storage [11]. These groups of compounds are attributed beneficial properties for human health, mainly in the prevention of cardiac problems [12]. Moreover, the fats in chocolates determine not only their nutritional quality, but also their industrial quality [13]. The prevention of their deterioration due to the technology used in processing could determine the quality of the product.
It's important to highlight that, the use of essential oils as additives in food processing is becoming more frequent due to their bioactive properties of their volatile compounds and enhancing of sensory attributes. Unsurprisingly, they can improve flavor, aroma, and present antimicrobial and antioxidant properties. Unlike other additives, they do not leave any residue on the applied product; however, the safety of their application depends on their source, amount, and extraction method [14,15]. Even though, there is a great diversity of plant sources of essential oils, those extracted from Pimpinella anisum, Cimbopogon citratus, and Minthostachys mollis could be incorporated into chocolate formulations to improve their composition and aroma.
P. anisum is a herbaceous medicinal plant with a very similar aroma to fennel [16]. It is also widely used in food, cosmetics, and pharmaceuticals due to their aromatic compounds such as terpenes, anisaldehyde and estragole [17]. C. citratus, known as lemongrass, is popularly used in infusions to treat and prevent fever and gastric disorders [18]. The essential oil of C. citratus has a range of bioactive compounds [19] in which citral and geraniol stand out as the most pharmacologically and physiologically important constituent [20,21]. Furthermore, its antioxidant properties have been demonstrated to be harnessed to prevent spoilage of fat-rich foods, such as chocolate [22]. M. mollis is a wild plant that grows throughout most of the Andean region. It is commonly used by local populations in food and medicine to treat cardiovascular diseases, blood disorders, insect bites, and as an insect repellent [23]. It has also been shown to have high antioxidant power compared to vitamin C and other commercial antioxidants [24,25]. The essential oils from aromatic herbs are an excellent technological alternative to improve the quality and seek health benefits from the consumption of chocolate [26].
In this context, no work has been reported within the field of study, that allows the enrichment chocolates with essential oils of P. anisum, C. citratus and M. mollis, evaluating the effect that this incorporation can generate on the rheological and textural characteristics and in the antioxidant properties. Therefore, the objective of the present work was to evaluate the effect of the essential oils of these three species on the chemical, rheological, textural, microstructural, and antioxidant properties and on lipid oxidation prevention of dark chocolate at different storage temperatures.
2. Methodology
2.1. Chemicals and standards
Hexane HPLC grade, chloroform (99%), pure glacial acetic acid (99%), sodium thiosulphate (99%), methanol HPLC grade, 2,2-diphenyl-1-picrylhydrazyl (DPPH), sodium hydroxide (≥98%), potassium iodide (≥96%), Folin-Ciocalteu's phenol, sodium carbonate (99.5%), and the alkane standard solution (mixture of C10 – C40) were purchased from Sigma-Aldrich.
2.2. Study location
The study was carried out at the Coffee and Cocoa Quality Control Laboratory of the National University Toribio Rodríguez de Mendoza de Amazonas. This laboratory is located in the city of Chachapoyas at Latitude 6°14′0.83 “S, Longitude 77°51′12.14 “W and has an average annual temperature of 15.60 °C [27].
2.3. Extraction of essential oils
For the extraction of the essential oils of C. citratus, P. anisum and M. mollis, 20 kg of plant samples from each species were used. The plant samples were obtained from the Central Market of the city of Chachapoyas - Amazonas - Peru and taken to the Laboratory of the Faculty of Engineering and Agricultural Sciences of the National University Toribio Rodríguez de Mendoza of Amazonas in order to carry out the extraction process. In the laboratory, the essential oils were extracted by steam distillation in a semi-industrial stainless steel distiller, following the methodology described by Andrade et al. [28]. After extraction, the essential oils were packaged in amber glass bottles and stored in a refrigerator at 8 °C.
2.4. Chemical characterization of essential oils
The chemical composition of the essential oils (EOs) was determined following the methodology described by Adams et al. [29] with some modifications. For that, we used a gas chromatograph (model 7890 B GC System) coupled with a mass detector (model 5977 B MSD). The compounds were separated on a DB-5MS UI capillary column (60 m × 0.25 mm × 1.0 μm). The injector temperature was 220 °C, 0.5 μL of EO diluted composed of 5 μL of essential oil +995 μL of hexane was injected in splitless mode. Helium was used as a carrier gas with a flow rate of 1 mL/min. The transfer line and ionization source temperatures were 240 and 280 °C, respectively. The oven temperature was set from 60 to 246 °C, at 3 °C/min, held for 8 min, then raised to 300 °C at 5 °C/min and held for 4.2 min. The mass range scanned was from 40 to 600 amu. The mass spectra were compared with those from the NIST 2017 library, and to ensure the identities of the compounds, the linear retention index was calculated through injection of the homologous hydrocarbon series (C10 – C40).
2.5. Obtaining chocolate
Dry fermented cocoa beans with 7% moisture were used. They were cleaned and roasted at 120 °C for 25 min in an oven (Venticell Ecoline), following the methodology established by the chocolate industries. The roasted cocoa beans were then crushed in a bean sheller (AYZ) to remove the shells and obtain cocoa nibs. The cocoa nibs were ground (Prosol SAC, Tritur-50) to obtain cocoa liquor, and the refining process was carried out for 3 h in two-roller refiners with granite stones (Premier) of 3 kg capacity.
The base formulation for the preparation of dark chocolates 70% was 65% of cocoa paste and 5% of cocoa butter plus 30% of sugar with the addition of essential oils. The conching and refining process lasted 19 h following the methodology described in Leite et al. [30]. After this process, EO (10, 12, and 14 μL/500 g of chocolate) were added at 38 °C, these concentrations were determined in previous trials. The tempering was done manually with temperature variations from 48 °C to 28 °C, which allowed the crystals in the cocoa mass to settle properly and to finally be molded at 32 °C. The chocolates were then molded in polycarbonate molds for 14 × 12 × 6 cm tablets of 50 g and 1 × 1 × 0.5 cm tablets. All formulations were made in triplicate. The chocolates were wrapped in aluminum foil and stored at two experimental temperatures, an average of 18 °C, which was measured with a thermohygrometer (Boeco brand, Model SH-110), and 25 °C, controlled in an incubator (Binder, Germany), for 90 days until further analysis. The experimental process using the dark chocolate with EO can be seen in Fig. 1.
Fig. 1.
Summary of the experimental process.
2.6. Oxidative stability
The oxidative stability of the chocolates was measured using the oxidative deterioration method described by Chavez et al. [24] and Vargas-Arispuro et al. [31] at 18 °C and 25 °C during a 90-days test with 10-day periodic evaluations.
The peroxide index was determined using AOAC method 28.022 [32]; for this, 200 mg of sample was weighed in an analytical balance (VWR-503R2) and placed in a 50 mL Erlenmeyer flask. To the solution, 2 mL of chloroform 99% was added and shaken for 1 min, then 5 mL of 99% pure glacial acetic acid and 0.20 mL of 90% saturated potassium iodide solution were added. It was left to stand in a dark chamber for 10 min, then 15 mL of distilled water was added. To the mixture, it was incorporated 1% starch until it changed a blue color. Titration with 0.01 N sodium thiosulphate continued until the blue coloring disappeared, the flow rate was measured, and a blank assay was performed for the corresponding calculations using equation (1).
| (1) |
Where PV: Peroxide value, S: Spent 0.01 N thiosulfate solution, B: Spent 0.01 N thiosulfate solution in the blank, N: Normality of the 0.01 N thiosulfate solution and W: Weight of the analyzed sample (g).
2.7. Determination of hardness
The hardness of the chocolate samples was measured at 12 months of production on a CTX texture analyzer (AMETEK, Brookfield with Textura Pro 1.0.19 software). Equipped with a 30° conical probe and a 100 kg load cell, the test was performed at a test speed of 10 mm/s with an initial speed of 5 mm/s and a penetration distance of 0.8 mm. The hardness of the specimens was recorded as the maximum force at the defined penetration distance. Each determination was performed in triplicate following the methodology described by Lillah et al. [33].
2.8. Rheological properties
Rheological measurements of the chocolate suspensions were performed using a modular compact rheometer (Anton Paar, Model MCR 302e), equipped with a CC27 concentric cylinder geometry. The samples were melted at 40 °C in an oven (Venticell Ecoline, Germany) for 60 min. The rheometer cup was filled with the suspension at 40 °C. The temperature was controlled by a Peltier device.
Measurements started with preconditioning at 40 °C for 60 s, followed by a shear rate of 5 s−1 for 100 s. The shear rate was increased from 2 to 50 s−1. The data were processed by the equipment software (RheoCompass vs s 1.30) following the methodology described by Glicerina et al. [34] and Analytical Method 46 [35]. The flow curves were adjusted to the Casson model Equation (2), which is used to describe the rheological behavior of the yield stress fluid.
| (2) |
where σ (Pa) is the shear stress, σ0 is the Casson yield stress (Pa), K1 is the consistency index (Pa. s) and γ (s−1) is the shear rate.
2.9. Microstructure analysis
To observe the microstructure of the chocolate samples, 1 g of sample was melted in an oven (Venticell Ecoline, Germany) at 55 °C for 20 min. Then 10 μL of the melted material was placed on a glass slide (7 × 2.5 cm) and covered with a coverslip parallel to the plane of the slide, trying to make the sample dispersion homogeneous. The images of the chocolate microstructure were observed with an inverted fluorescence microscope (IX83, Olympus, Japan) at 40 × magnification and micrographs were captured with a digital camera (DP74, Olympus, Japan) following the described methodology by Afoakwa et al. [36].
2.10. Melting properties
The thermal behavior of the chocolate treatments was evaluated following the procedure described by Calva-Estrada et al. [37] using differential scanning calorimetry (DSC) with a DSC-60 plus equipment (DSC-60 plus). To obtain the thermal spectra, the equipment was left switched on for 30 min to stabilize the electronic system and the furnace. Then the samples were weighed on an analytical balance, between 2.5 ± 0.5 mg, placed in hermetically sealed aluminum vials and subjected to the DSC furnace, working with an empty sealed aluminum cuvette that served as a blank, with a nitrogen flow rate of 25 mL/min at normal pressure. The samples were melted at a temperature of 60 °C, for 20 min. For each peak present in the thermogram obtained, the onset temperature (T onset), end temperature (T end) and enthalpy (ΔH) were calculated [38].
2.11. Antioxidant capacity
Chocolate defatting was performed following the methodology described by Suazo et al. [39]. Five grams of chocolate was crushed in a Bosch bean grinder (TSM6A013 B) and sieved in a No. 45 sieve in order to remove large particles. Five hundred milligrams of chocolate were weighed, and 3 mL of hexane 99% was added and stirred for 10 min at a speed of 3000 rpm in an orbital shaker (Lauda, Germany BS150). Then the solution was centrifuged at 4830 rpm for 20 min to remove the supernatant (the process was repeated twice) using a centrifuge (PrO-Analytical, Britain). Finally, the samples were left to dry in a gas extraction cabinet (Labconco, USA) to eliminate the excess hexane.
For the preparation of the methanolic solution (80%) of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, the methodology described by Jonfia-Essien et al. [40]. One hundred milligrams of defatted chocolate sample were taken and mixed with 10 mL of 80% methanol solution, stirred at 3000 rpm for 15 min, then the mixture was centrifuged at 4830 rpm for 30 min, and filtered on filter paper (Whatman N° 40–2.5 μm). The supernatant was stored at −20 °C until further analysis.
The antioxidant activity of chocolate extracts with EOs was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. A 0.1 mM methanolic solution of DPPH was prepared. Two milliliters of this solution were placed in a test tube and 200 μL of the extract was added. The mixture was shaken and incubated at 18 °C in dark. The absorbance of the solution was measured with a spectrophotometer (T 9200 PEAK Instruments, USA) at a wavelength of 517 nm. The percentage inhibition was calculated with equation (3).
| (3) |
Where: A0: Absorbance of DPPH solution, AS: Absorbance of methanol, AT: Absorbance of sample.
To determine the antioxidant capacity (mmol TE/L), the linear equation of the calibration curve of five dilutions of Trolox standard were used.
2.12. Statistical analysis of data
To compare the oxidative stability, rheological properties, hardness, microstructure, melting properties and antioxidant capacity of dark chocolates with the addition of essential oils of C. citratus, P. anisum and M. mollis, an analysis of variance and Tukey's multiple comparisons were performed, looking for fits to linear, quadratic, and exponential models, with the statistical software SPSS v.26.
3. Results and Discussion
3.1. Chemical composition of the essential oils
Table 1 describes the chemical composition of the 48 volatile substances identified in the EOs studied. Analysis by gas chromatography-mass spectrometry revealed the presence of 48 chemical compounds in the essential oils M. mollis, P. anisum y C. citratus. In the essential oil of M. mollis, 28 chemical compounds were identified, with linalool being the most abundant compound at 15.30%. This compound has essential biological functions, such as antibacterial, anticancer, anti-inflammatory, anxiolytic and contraceptive effects [41]. In P. anisum EO, 12 compounds were identified. The 2-nitro-propane was the most abundant compound with 91.82%, which presents anti-inflammatory functions [42]. On the other hand, 20 compounds were identified in the EO of C. citratus, where geraniol (15.76%) was the most abundant, followed by linalool (11.41%). The geraniol is within the group of monoterpenes which has chemo preventive and chemotherapeutic properties [43].
Table 1.
Percentage chemical composition of the essential oils.
| Retention Time | Compound name | Calculated Retention Index | Library Retention Index | M. mollis* | P. Anisum* | C. Citratus* |
|---|---|---|---|---|---|---|
| 8.27 | Propanoic acid | 691 | 700 | 0.05 | ND | 4.03 |
| 23.89 | 5-Hepten-2-one, 6-methyl- | 983 | 986 | ND | ND | 10.02 |
| 23.91 | Bicyclo [3.1.0]hexane, 4-methylene-1-(1-methylethyl)- | 984 | 974 | 3.36 | ND | ND |
| 23.95 | 3-Octanone | 995 | 986 | 0.49 | ND | ND |
| 24.27 | beta.-Myrcene | 990 | 991 | 1.14 | ND | ND |
| 24.47 | Cyclohexane, 1-methylene-4-(1-methylethenyl)- | 994 | 1004 | 4.42 | ND | ND |
| 24.53 | 3-Octanol | 995 | 994 | 5.72 | ND | ND |
| 26.74 | trans-.beta.-Ocimene | 1037 | 1049 | ND | ND | 5.57 |
| 27.02 | d-Limonene | 1042 | 1018 | 14.57 | 0.04 | ND |
| 27.39 | Eucalyptol | 1049 | 1032 | 0.74 | ND | 0.96 |
| 29.89 | Geraniol | 1255 | 1255 | ND | ND | 15.76 |
| 30.34 | Linalool | 1104 | 1099 | 15.30 | ND | 11.41 |
| 30.44 | Furan, 3-(4-methyl-3-pentenyl)- | 1106 | 1101 | ND | ND | 1.20 |
| 31.01 | 3-Octanol, acetate | 1116 | 1123 | 2.03 | ND | ND |
| 32.69 | 6-Octenal, 7-methyl-3-methylene- | 1221 | 1147 | ND | ND | 1.89 |
| 34.37 | (1R,2R,5S)-5-Methyl-2-(prop-1-en-2-yl)cyclohexanol | 1179 | 1196 | 0.54 | ND | ND |
| 34.54 | 3,6-Octadienal, 3,7-dimethyl- | 1182 | 1184 | ND | ND | 10.26 |
| 34.77 | Isoneral | 1164 | 1170 | ND | ND | 7.14 |
| 35.03 | Cyclohexanone, 5-methyl-2-(1-methylethenyl)-, trans- | 1215 | 1177 | 2.18 | ND | ND |
| 35.98 | L-.alpha.-Terpineol | 1211 | 1190 | 1.98 | ND | 0.15 |
| 36.19 | Cyclohexanone, 2-methyl-5-(1-methylethenyl)-, trans- | 1215 | 1201 | 0.62 | ND | ND |
| 36.34 | alpha.-Thujenal | 1218 | 1190 | 0.06 | ND | ND |
| 36.50 | 6-Octen-1-ol, 7-methyl-3-methylene- | 1222 | 1195 | ND | ND | 0.39 |
| 36.84 | Citronellol | 1228 | 1228 | ND | ND | 3.13 |
| 36.99 | 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | 1231 | 1228 | ND | ND | 7.84 |
| 37.03 | 8,9-Dehydrothymol | 1232 | 1221 | 0.79 | ND | ND |
| 37.15 | 3,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | 1234 | 1240 | ND | ND | 0.99 |
| 38.14 | Phenol, 4-(2-propenyl)- | 1255 | 1255 | ND | 1.22 | ND |
| 38.73 | Anethole | 1267 | 1286 | ND | 0.40 | ND |
| 38.90 | 7-Oxabicyclo [4.1.0]heptan-2-one, 6-methyl-3-(1-methylethyl)- | 1271 | 1256 | 11.73 | ND | ND |
| 39.09 | Benzaldehyde, 4-methoxy- | 1275 | 1251 | ND | 0.89 | ND |
| 39.55 | (−)-Neomenthylacetate | 1284 | 1304 | 3.07 | ND | ND |
| 39.65 | Propane, 2-nitro- | 653 | 675 | ND | 91.82 | ND |
| 40.07 | Thymol | 1296 | 1291 | 2.45 | 0.06 | ND |
| 40.48 | Bicyclo [2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1 S-endo)- | 1304 | 1284 | 0.57 | ND | ND |
| 41.22 | 2-Cyclohexen-1-one, 2-hydroxy-3-methyl-6-(1-methylethyl)- | 1319 | 1302 | 0.67 | ND | ND |
| 41.72 | 6-Hydroxycarvotanacetone | 1329 | 1318 | 2.15 | ND | ND |
| 41.95 | 2-Cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-, acetate | 1336 | 1336 | 0.69 | ND | ND |
| 43.50 | Eugenol | 1366 | 1357 | 0.38 | 0.02 | ND |
| 45.24 | Copaene | 1403 | 1376 | 11.12 | ND | ND |
| 46.04 | Benzene, 1,4-dimethoxy-2-methyl-5-isopropyl- | 1416 | 1422 | ND | 0.64 | ND |
| 49.38 | 2-Tridecanone | 1499 | 1497 | ND | ND | 5.41 |
| 49.87 | alpha.-Farnesene | 1511 | 1508 | ND | 1.46 | ND |
| 51.28 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1 S-cis)- | 1544 | 1524 | 10.66 | 0.55 | 0.51 |
| 51.57 | Thymyl isobutyrate | 1486 | 1480 | ND | 0.34 | ND |
| 53.50 | 2-Undecanone | 1297 | 1294 | ND | ND | 4.88 |
| 56.15 | Selin-6-en-4.alpha.-ol | 1666 | 1636 | ND | ND | 5.29 |
| 56.77 | (1R,7S,E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol | 1683 | 1695 | 0.45 | ND | ND |
| Other compounds | 2.08 | 2.57 | 3.18 | |||
Note. *The chemical composition of M. mollis, P. anisum, and C. citratus is expressed in percentage.
ND: not detected.
3.2. Oxidative stability
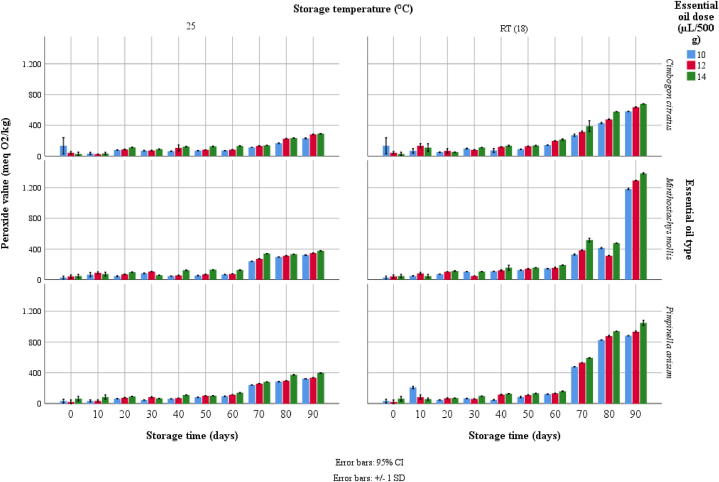
Fig. 2 shows that up to 60 days of storage, the lipid oxidation of the chocolates remains stable, but they slightly differ between the three essential oils in the two storage conditions. However, the remaining 30-day evaluation showed, with greater contrast, the effects of each natural additive used in the chocolates. Thus, the chocolates with the least lipid oxidation were the chocolates with C. citratus EO stored at 25 °C (values less than 300 meq O2/kg) and the least stable were the chocolates formulated with M. mollis EO. In fact, the chocolates with M. mollis EO stored at 18 °C had the highest lipid oxidation with values close to 1500 meq O2/kg. Moreover, in all the essential oil studied, a higher peroxide value was observed with increasing EO dosage, showing a significant pro-oxidant effect.
Fig. 2.
Effect of the incorporation of essential oils on the oxidative stability of dark chocolate during 90 days of storage.
Storage conditions are fundamental to guarantee the oxidative stability of the fats in dark chocolates. Temperature oscillations of 18 °C affected the protective and stabilizing activity of the essential oil studied. Oxidation of fats has been observed to be a factor of concern in the food industry due to quality loss in products, highlighting rancidity as a main concern [44]. In this research, we observed a positive effect of essential oils in preventing lipid oxidation of chocolate at a dose of 10 μL/500 g of chocolate, while the higher doses (12 and 14 μL/500 g of chocolate) promote the lipid oxidation [24] (Fig. 2).
C. citratus essential oil more effectively prevented fat oxidation under the two storage conditions. In contrast, the essential oil from M. mollis had less stabilizing effect in long term (90 days of storage). Studies made by Naseri et al. [45] found that controlled temperatures can reduce the formation of peroxides in edible oils.
As shown in Fig. 3 and Table 2, the addition of different types of essential oils modifies the hardness of dark chocolates. The higher the concentration of essential oil (14 μL/500 g of chocolate), the lower the level of hardness. Likewise, the storage conditions of the chocolates have a significant influence. At a temperature of 25 °C, the samples show higher hardness (2658–2391 g), while at the average temperature of 18 °C, the hardness is lower for all treatments (2475–2244 g).
Fig. 3.
Chocolate hardness with added EO from three different vegetal species stored at 25 °C and at 18 °C. The red lines correspond to the addition of 10 μL of OE to 500 g of chocolate, the brown lines to 12 μL and the black lines to 14 μL. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Hardness of the chocolate samples with essential oils.
| EO Type | EO Dose (μL/500 g) | Hardness (g) T° (25 °C) | Hardness (g) T° (18 °C) |
|---|---|---|---|
| C. citratus | 10 | 2658 ± 581c | 2475 ± 216 abc |
| 12 | 2511 ± 657c | 2342 ± 419 bc | |
| 14 | 2391 ± 275c | 2244 ± 178 bc | |
| P. anisum | 10 | 5044 ± 579a | 2903 ± 486 abc |
| 12 | 4168 ± 547b | 2371 ± 413 bc | |
| 14 | 3824 ± 645b | 2351 ± 209c | |
| M. mollis | 10 | 2790 ± 609c | 2513 ± 219a |
| 12 | 2677 ± 721c | 2311 ± 294 ab | |
| 14 | 2680 ± 471c | 2233 ± 146 ab |
a-c Equal letters in a column mean that there are no significant differences based on the Tukey test (p < 0.05).
The chocolate samples with higher concentrations of essential oils had lower hardness, which may be due to the solvent effect of the essential oils that influence the hardening and crystallization processes of the fat phase of 18 °C chocolate [46]. Furthermore, the texture of a chocolate changes as the bloom (fat and sugar) occurs. Therefore, despite working with the same variety of cocoa (Criollo), the same amount of fat, and the same type of sugar, this property tends to change [47]. Hence, tempering is essential for a chocolate to have a proper polymorphic shape and not modify color, hardness, handling, finish, and shelf life [48].
3.3. Rheological properties
Table 3 shows the plastic viscosity and Casson yield of the chocolates evaluated after 12 months of production. The chocolate with the addition of P. anisum essential oil (14 μL/500 g of chocolate) stored at 25 °C had the highest viscosity (3.50 Pa s) and its elastic limit was 17.474 Pa; in contrast to C. citratus (14 μL/500 g of chocolate), which had the lowest values. On the other hand, chocolate with the addition of M. mollis essential oil (14 μL/500 g of chocolate) stored of 18 °C average temperature reached higher viscosity (1.57 Pa s) and higher elastic limit (25.44 Pa), compared to C. citratus (14 μL/500 g of chocolate) which presented low values. The results show significant difference in all treatments (p > 0.05).
Table 3.
Rheological properties of chocolates with essential oils.
| EO Type | EO Dose (μL/500 g) | Storage conditions | Casson plastic viscosity (Pa. s) | Casson yield strength (Pa) |
|---|---|---|---|---|
| C. citratus | 10 | 25 °C | 2.896 ± 0.017 bc | 14.952 ± 0.571d |
| 12 | 2.700 ± 0.052 bcd | 17.167 ± 0.716 cd | ||
| 14 | 2.667 ± 0.091 bcd | 15.253 ± 2.072d | ||
| P. anisum | 10 | 25 °C | 3.001 ± 0.011 ab | 14.592 ± 0.437d |
| 12 | 3.151 ± 0.044 ab | 17.393 ± 0.603 cd | ||
| 14 | 3.501 ± 0.120a | 17.474 ± 1.445 cd | ||
| M. mollis | 10 | 25 °C | 3.464 ± 0.012a | 17.347 ± 0.396 cd |
| 12 | 2.758 ± 0.050 bcd | 14.080 ± 0.589d | ||
| 14 | 2.403 ± 0.004 cde | 16.078 ± 0.047d | ||
| C. citratus | 10 | 18 °C | 1.674 ± 0.064f | 23.9281 ± 1.347 ab |
| 12 | 1.674 ± 0.064f | 23.929 ± 1.346 ab | ||
| 14 | 1.239 ± 0.292g | 19.832 ± 0.019 bcd | ||
| P. anisum | 10 | 18 °C | 1.522 ± 0.011f | 25.890 ± 7.247a |
| 12 | 1.536 ± 0.044f | 24.711 ± 0.010 ab | ||
| 14 | 1.542 ± 0.356f | 23.149 ± 1.347 abc | ||
| M. mollis | 10 | 18 °C | 1.636 ± 0.001f | 24.705 ± 0.001 ab |
| 12 | 1.650 ± 0.543f | 23.073 ± 2.827 abc | ||
| 14 | 1.556 ± 0.047f | 25.440 ± 0.470 ab |
a-g Equal letters in a column mean that there are no significant differences based on the Tukey test (p < 0.05).
From the rheological point of view, chocolates have a complex behavior. For example, they have an apparent elastic limit and a plastic viscosity that depends on the manufacturing process [10]. High viscosity chocolates have a pasty sensation that lasts longer in the mouth [49] and have low consumer acceptability. However, in this research it was found that Casson's plastic viscosity and yield strength decreased significantly as the dosage of the essential oils increases. Other research [7,50] showed similar results, as the higher the dose of cinnamon essential oil added, the lower the viscosity and yield strength of the chocolates.
3.4. Microstructure
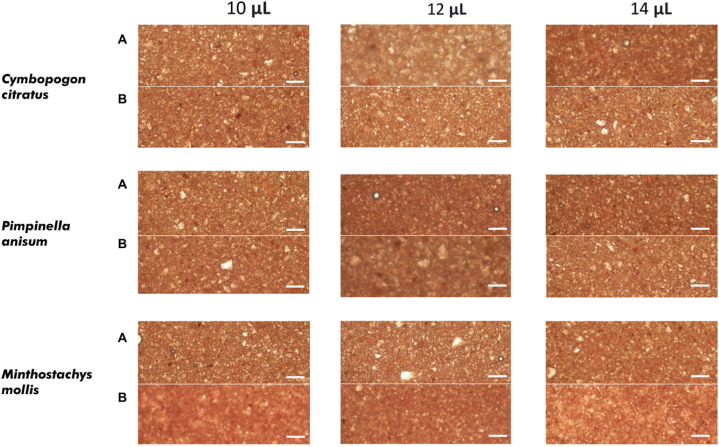
In Fig. 4, the micrographs show an increase in the dispersion of the crystal structure of the chocolates. The higher the concentrations of essential oil added, the greater the dispersion of chocolate particles. Nevertheless, the variation of the average storage temperature of 18 °C affected the food matrix by dispersing the particles and increasing the granulometry.
Fig. 4.
Microstructure of chocolate with the addition of EO at 25 °C (A) and 18 °C (B). Scale bars = 2 mm.
3.5. Melting properties
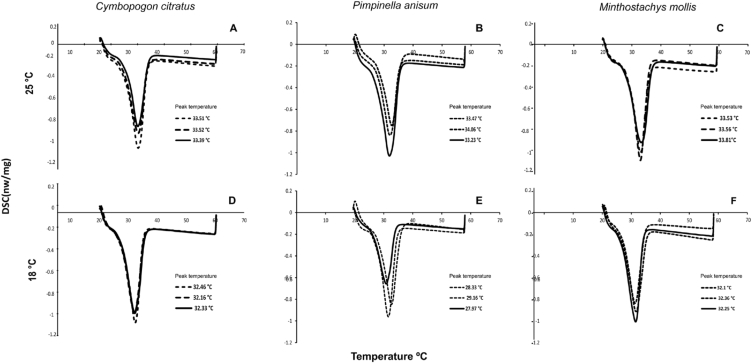
Fig. 5 shows the melting profiles obtained for each treatment. Chocolates stored at 25 °C presented a maximum melting temperature between 33 and 34 °C, with a ΔH of 31.97–48.92 J/g, whereas chocolates stored at average temperature of 18 °C reached a maximum temperature of 32 °C with a ΔH of 38.64–62.63 J/g. All treatments showed significant difference (p > 0.05).
Fig. 5.
DSC thermograms of chocolate samples. The dotted lines correspond to the addition of 10 μL of OE to 500 g of chocolate, the dashed lines to 12 μL and the continuous lines to 14 μL.
The size of crystals present in chocolates is generally related to the formation of fat blooms, which causes a lot of dispersion on the chocolate surface [51]. Recent studies have shown that the higher the variation of storage temperatures, the higher the number of surface crystals [52]. In this study, however, higher doses of EO (14 μL/500 g of chocolate) resulted in greater dispersion of the particles which could facilitate the processes of fat bloom in the matrix. Ali et al. [53] found that the chocolate filled with palm mid fraction and desiccated coconut stored at 18 °C presented the slower migration and changes in the chemical composition and polymorphic stability.
The calorimetric properties of chocolate also depend on the processing methods such as tempering and cooling [47]. Taking into account that during chocolate production the crystalline state and the amount of fat determine the melting properties of the product, and that there are 6 different polymorphic forms recognized by Roman numerals (I-VI) or Greek letters (γ, α, β2′, β1′, β2 and β1) [54]. Structure VI reaches a high melting temperature (34–36 °C) making the chocolate have an undesirable sandy texture; importantly, the melting point of all treatments is found in the polymorphic form V (32–33 °C) which coincides with the mouth temperature. Moreover, in the chocolate industry the crystallization of form V is preferred because it provides brightness and a fine texture to the product [55]. Nonetheless, it should be considered that temperature fluctuations in storage would favor the fat bloom phenomenon macroscopically observable by the presence of a white layer on the surface of the chocolate [56].
3.6. Antioxidant capacity
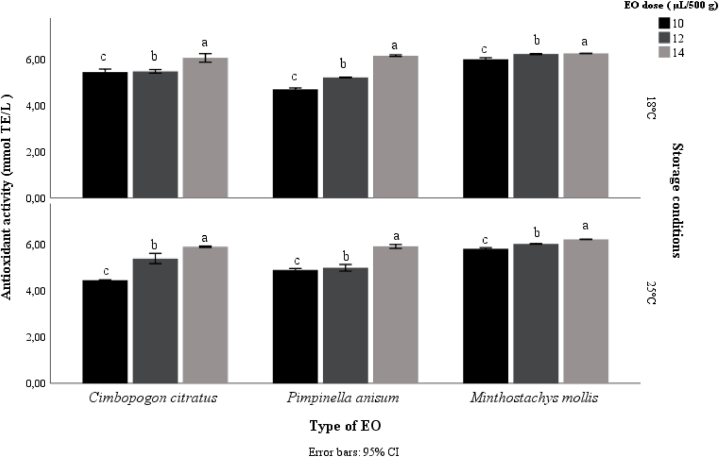
Fig. 6 shows the effects of the essential oil of M. mollis, P. anisum and C. citratus on the antioxidant activity of the chocolate samples. It is observed that, as the dose of essential oil increases, the antioxidant activity also increases for both storage conditions. However, the samples with the highest antioxidant content are chocolates with M. mollis essential oil with values ranging between 5.8 and 6.2 mmol TE/L.
Fig. 6.
Antioxidant capacity of chocolates with EO stored for 90 days at two conditions.
According to the results, essential oils have great potential as an alternative to synthetic additives in foods to enhance their antioxidant activity, as observed with S. chamaecparissus essential oil which can increase the antioxidant activity of dark and white chocolates [7,57,58].
4. Conclusions
The essential oils of C. citratus, P. anisum and M. mollis stabilized lipid oxidation in dark chocolates over a 60-day storage period. At longer storage, such as 90 days, the effects may vary depending on the additive used. Moreover, the stabilizing effect of C. citratus, P. anisum, and M. mollis essential oils in dark chocolate varies according to the storage conditions, as fluctuating storage temperatures promotes lipid oxidation, while controlled temperature conditions improve the oxidative stability of fats. However, the 10 μL of C. citratus in 500 g of chocolate stored at 25 °C showed a higher oxidation stability.
The results of this study reveal that the essential oils used may have great potential as natural additives to prevent oxidation and increase the antioxidant capacity as well as improve textural, calorimetric, microstructural in rheological properties of dark chocolates. Therefore, this should serve as a basis for future studies to confirm the favorable effect of the use of oils in the chocolate industry.
Author contribution statement
Luz Quispe-Sanchez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Marilu Mestanza, Aline C. Caetano, Tony Chuquizuta: Performed the experiments; Wrote the paper. Manuel Oliva-Cruz: Contributed reagents, materials, analysis tools or data. Nelson Rimarachin: Performed the experiments. Malluri Goñas, Elizabeth Renee Ambler Gill: Analyzed and interpreted the data; Wrote the paper. Segundo G. Chavez: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper. Data availability statement: Data included in article/supp. Material/referenced in article. Declaration of interest's statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the funding of CONCYTEC & World Bank, through its executing unit Prociencia, through the Project with Contract N° 026-2016 “Círculo de Investigación para la Innovación y fortalecimiento de la cadena de valor del cacao nativo fino de aroma en el nororiente peruano-CINCACAO”, the subprojects "Análisis metagenómico y técnicas cromatográficas para la obtención de un cultivo iniciador para mejorar la calidad del chocolate nativo fino de aroma en el nororiente peruano" - METACACAO, under Contract N° 008-2020-FONDECYT-BM, likewise “Chocolates finos elaborados utilizando cacao de denominación de origen “Cacao Amazonas Perú” - "CHOCOINDES", under Contract N° 012-2018-FONDECYT-BM-IADT-AV. Furthermore, they would also like to thank the SNIP Project No. 352641 “CEINCACAO".
References
- 1.Ibrahim S.F., Ezzati N.S., Dalek M., Firdaus Q.A., Raffie M., Ain M.R.F. Quantification of physicochemical and microstructure properties of dark chocolate incorporated with palm sugar and dates as alternative sweetener. Mater. Today Proc. [Internet] 2020;31:366–371. doi: 10.1016/j.matpr.2020.06.235. [DOI] [Google Scholar]
- 2.Aprotosoaie A.C., Luca S.V., Miron A. Flavor chemistry of cocoa and cocoa products-an overview. Compr. Rev. Food Sci. Food Saf. 2016;15(1):73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- 3.Lim P.Y., Wong K.Y., Thoo Y.Y., Siow L.F. Effect of Inulin, Fructo-Oligosaccharide, Trehalose or Maltodextrin (M10 and M30) on the Physicochemical and Sensory Properties of Dark Compound Chocolate. Lwt [Internet] 2021;149 doi: 10.1016/j.lwt.2021.111964. [DOI] [Google Scholar]
- 4.Engeseth N.J., Ac Pangan M.F. Current context on chocolate flavor development — a review. Curr. Opin. Food Sci. [Internet] 2018;21:84–91. doi: 10.1016/j.cofs.2018.07.002. [DOI] [Google Scholar]
- 5.Guckenbiehl Y., Martin A., Ortner E., Rothkopf I., Schweiggert-Weisz U., Buettner A., et al. Aroma-active volatiles and rheological characteristics of the plastic mass during conching of dark chocolate. Food Res. Int. [Internet] 2022;162(PB) doi: 10.1016/j.foodres.2022.112063. [DOI] [PubMed] [Google Scholar]
- 6.Khosh manzar M., Pirouzifard M.K., Hamishehkar H., Pirsa S. Cocoa butter and cocoa butter substitute as a lipid carrier of Cuminum cyminum L. essential oil; physicochemical properties, physical stability and controlled release study. J Mol. Liq. [Internet] 2020 Sep;314 doi: 10.1016/j.molliq.2020.113638. [DOI] [Google Scholar]
- 7.Bölek S., Tosya F., Akçura S. Effects of Santolina chamaecyparissus essential oil on rheological, thermal and antioxidative properties of dark chocolate. Int. J. Gastron. Food Sci. 2022;27 [Google Scholar]
- 8.Afoakwa E.O., Paterson A., Fowler M. Factors influencing rheological and textural qualities in chocolate - a review. Trends Food Sci. Technol. 2007;18(6):290–298. [Google Scholar]
- 9.Glicerina V., Balestra F., Dalla Rosa M., Romani S. Microstructural and rheological characteristics of dark, milk and white chocolate: a comparative study. J. Food Eng. [Internet] 2016;169:165–171. doi: 10.1016/j.jfoodeng.2015.08.011. [DOI] [Google Scholar]
- 10.Medina-Mendoza M., Rodriguez-Pérez R.J., Rojas-Ocampo E., Torrejón-Valqui L., Fernández-Jeri A.B., Idrogo-Vásquez G., et al. Rheological, bioactive properties and sensory preferences of dark chocolates with partial incorporation of Sacha Inchi (Plukenetia volubilis L.) oil. Heliyon. 2021;7(2) doi: 10.1016/j.heliyon.2021.e06154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolatowska-Żebrowska K., Ostrowska-Ligęza E., Wirkowska-Wojdyła M., Bryś J., Górska A. The oxidative stability of fat in three dark chocolates at different stages of manufacturing process. Proceedings [Internet] 2020;4:1–5. www.mdpi.com/journal/proceedings [Google Scholar]
- 12.Davinelli S., Corbi G., Righetti S., Sears B., Olarte H.H., Grassi D., et al. Cardioprotection by cocoa polyphenols and ω-3 fatty acids: a disease-prevention perspective on aging-associated cardiovascular risk. J. Med. Food. 2018;21(10):1060–1069. doi: 10.1089/jmf.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chire-Fajardo G.C., Ureña-Peralta M.O., Hartel R.W. Fatty acid profile and solid fat content of peruvian cacao for optimal production of trade chocolate. Rev. Chil. Nutr. 2020;47(1):50–56. [Google Scholar]
- 14.Bajpai V.K., Baek K.H. Biological efficacy and application of essential oils in foods-A review. J. Essent. Oil-Bearing Plants. 2016;19(1):1–19. [Google Scholar]
- 15.Mihai A.L., Popa M.E. Essential oils utilization in food industry - a literature review. Sci. Bull. 2013;XVII:187–192. [Google Scholar]
- 16.Das S., Kumar Singh V., Kumar Dwivedy A., Kumar Chaudhari A., Deepika, Kishore Dubey N. Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chem. 2021;344 doi: 10.1016/j.foodchem.2020.128574. [Internet] [DOI] [PubMed] [Google Scholar]
- 17.Anastasopoulou E., Graikou K., Ganos C., Calapai G., Chinou I. Pimpinella anisum seeds essential oil from Lesvos island: effect of hydrodistillation time, comparison of its aromatic profile with other samples of the Greek market. Safe use. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110875. [Internet] [DOI] [PubMed] [Google Scholar]
- 18.Mendes Hacke A.C., Miyoshi E., Marques J.A., Pereira R.P. Anxiolytic properties of Cymbopogon citratus (DC.) stapf extract, essential oil and its constituents in zebrafish (Danio rerio) J. Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.113036. [Internet] [DOI] [PubMed] [Google Scholar]
- 19.Lawal O.A., Ogundajo A.L., Avoseh N.O., Ogunwande I.A. Medicinal Spices and Vegetables from Africa [Internet] Elsevier; 2017. Cymbopogon citratus; pp. 397–423. [DOI] [Google Scholar]
- 20.Ekpenyong C.E., Akpan E., Nyoh A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015;13(5):321–337. doi: 10.1016/S1875-5364(15)30023-6. [Internet] [DOI] [PubMed] [Google Scholar]
- 21.Yoplac I., Hidalgo A., Vargas L. Antimicrobial biofilms with microencapsulated citral and sodium caseinate to extend the shelf life of fresh cheese. Food Packag. Shelf Life. 2022;34 doi: 10.1016/j.fpsl.2022.100932. [Internet] [DOI] [Google Scholar]
- 22.Cheel J., Theoduloz C., Rodríguez J., Schmeda-Hirschmann G. Free radical scavengers and antioxidants from lemongrass (Cymbopogon citratus (DC.) Stapf.) J. Agric. Food Chem. 2005;53(7):2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 23.Gómez Ticerán D., Martínez Portugués B., Jáuregui Jí Condado, Ramón Quispe G.N., Quinteros Gómez J.M., Albán Castillo J.A. Caracterización de patrones de variación morfológica de la Mynthostachys mediante métodos estadísticos multivariante. Pesquimat. 2014;10(2) http://revistasinvestigacion.unmsm.edu.pe/index.php/matema/article/view/9440 [Internet] [Google Scholar]
- 24.Chavez S.G., Gómez N.A., Mestanza M. Efecto del aceite esencial de Minthostachys mollis Kunth en la estabilidad oxidativa de aceite de sachainchi (Plukenetia hayllabambana) Rev. Chil. Nutr. 2022;49(2):173–180. [Google Scholar]
- 25.Solis-Quispe L., Tomaylla-Cruz C., Callo-Choquelvica Y., Solís-Quispe A., Rodeiro I., Hernández I., et al. Chemical composition, antioxidant and antiproliferative activities of essential oil from Schinus areira L. and Minthostachys spicata (Benth.) Epl. grown in Cuzco, Peru. J. Essent. Oil Res. 2016;28(3):234–240. [Google Scholar]
- 26.Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J.A. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2011;51(1):13–28. doi: 10.1080/10408390903044271. [DOI] [PubMed] [Google Scholar]
- 27.Rascón J., Gosgot Angeles W., Quiñones Huatangari L., Oliva M., Barrena Gurbillón M.Á. Dry and wet events in andean populations of northern Peru: a case study of Chachapoyas, Peru. Front. Environ. Sci. 2021;9:1–13. [Google Scholar]
- 28.Andrade K.C.R., Martins D.H.N., Barros D. de A., de Souza P.M., Silveira D., Fonseca-Bazzo Y.M., et al. Essential oils of Cordia species, compounds and applications: a systematic review. Bol. Latinoam. y del Caribe. Plantas. Med. y Aromat. 2022;21(2):156–175. [Google Scholar]
- 29.Adams Robert P. Carol Stream. Allured publishing; 2017. Identification of essential oil components by gas chromatography/mass spectrometry. Ed. 4.1. [Google Scholar]
- 30.Leite P.B., Bispo E. da S., de Santana L.R.R. Sensory profiles of chocolates produced from cocoa cultivars resistant to Moniliophtora perniciosa. Rev. Bras. Frutic. 2013;35(2):594–602. [Google Scholar]
- 31.Vargas-Arispuro I., Sanz B.I., Martínez-Téllez M.A., Primo-Yúfera E. Actividad antioxidante de compuestos aislados del residuo no-volátil del aceite esencial de naranja. Grasas Aceites. 1998;49(2):159–164. [Google Scholar]
- 32.International A. Assoc Off Agric Chem; Washington, DC: 1980. AOAC: Official Methods of Analysis; p. 552. [Google Scholar]
- 33.Lillah Asghar A., Pasha I., Murtaza G., Ali M. Improving heat stability along with quality of compound dark chocolate by adding optimized cocoa butter substitute (hydrogenated palm kernel stearin) emulsion. Lwt [Internet] 2017;80:531–536. doi: 10.1016/j.lwt.2017.02.042. [DOI] [Google Scholar]
- 34.Glicerina V., Balestra F., Rosa M.D., Romani S. Rheological, textural and calorimetric modifications of dark chocolate during process. J. Food Eng. [Internet] 2013;119(1):173–179. doi: 10.1016/j.jfoodeng.2013.05.012. [DOI] [Google Scholar]
- 35.ICA . Analytical Method. vol. 46. CAOBISCO; Bruxelles, Belgium: 2000. Viscosity of cocoa and chocolate products. [Google Scholar]
- 36.Afoakwa E.O., Paterson A., Fowler M., Vieira J. Microstructure and mechanical properties related to particle size distribution and composition in dark chocolate. Int. J. Food Sci. Technol. 2009;44(1):111–119. [Google Scholar]
- 37.Calva-Estrada S.J., Utrilla-Vázquez M., Vallejo-Cardona A., Roblero-Pérez D.B., Lugo-Cervantes E. Thermal properties and volatile compounds profile of commercial dark-chocolates from different genotypes of cocoa beans (Theobroma cacao L.) from Latin America. Food Res. Int. [Internet] 2020;136 doi: 10.1016/j.foodres.2020.109594. [DOI] [PubMed] [Google Scholar]
- 38.Gloria H., Sievert D. Changes in the physical state of sucrose during dark chocolate processing. J. Agric. Food Chem. 2001;49(5):2433–2436. doi: 10.1021/jf0008240. [DOI] [PubMed] [Google Scholar]
- 39.Suazo Y., Davidov-Pardo G., Arozarena I. Effect of fermentation and roasting on the phenolic Concentration and antioxidant activity of cocoa from Nicaragua. J. Food Qual. 2014;37(1):50–56. [Google Scholar]
- 40.Jonfia-Essien W.A., West G., Alderson P.G., Tucker G. Phenolic content and antioxidant capacity of hybrid variety cocoa beans. Food Chem. 2008;108(3):1155–1159. doi: 10.1016/j.foodchem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhai R., Ma J., An Y., Wen Z., Liu Y., Sun Q., et al. Ultra-stable linalool/water Pickering emulsions: a combined experimental and simulation study. Colloids Surfaces A Physicochem. Eng. Asp. [Internet] 2022;654 doi: 10.1016/j.colsurfa.2022.130034. [DOI] [Google Scholar]
- 42.Wijaya M., Wiharto M., Auliah A. Characterization compound chemical from cocoa waste as acetic acid and phenol. J. Phys. Conf. Ser. 2021;(3):1918. [Google Scholar]
- 43.Silva G. dos S.e., Marques JN. de J., Linhares E.P.M., Bonora C.M., Costa É.T., Saraiva M.F. Review of anticancer activity of monoterpenoids: geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022;362 doi: 10.1016/j.cbi.2022.109994. [DOI] [PubMed] [Google Scholar]
- 44.Othón-Díaz E.D., Fimbres-García J.O., Flores-Sauceda M., Silva-Espinoza B.A., López-Martínez L.X., Bernal-Mercado A.T., et al. Antioxidants in oak (quercus sp.): potential application to reduce oxidative rancidity in foods. Antioxidants. 2023;12(4) doi: 10.3390/antiox12040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naseri S., Mahmoudian M.H., Yari A.R., Molaghen S., Mahmoodian Z. Evaluation of peroxide value and acid number of edible oils consumed in the sandwich and fast food shops of qom, Iran in 2016. Arch. Hyg. Sci. 2018;7(2):91–97. [Google Scholar]
- 46.Morgan J. Chocolate: a flavor and texture unlike any other. Am. J. Clin. Nutr. 1994 Dec;60(6):1065S–1067S. doi: 10.1093/ajcn/60.6.1065S. https://linkinghub.elsevier.com/retrieve/pii/S0002916523185868 [Internet] [DOI] [PubMed] [Google Scholar]
- 47.Ostrowska-Ligęza E., Marzec A., Górska A., Wirkowska-Wojdyła M., Bryś J., Rejch A., et al. A comparative study of thermal and textural properties of milk, white and dark chocolates. Thermochim. Acta. [Internet] 2019;671:60. doi: 10.1016/j.tca.2018.11.005. [DOI] [Google Scholar]
- 48.Ewens H., Metilli L., Simone E. Analysis of the effect of recent reformulation strategies on the crystallization behaviour of cocoa butter and the structural properties of chocolate. Curr. Res. Food Sci. [Internet] 2021;4:105. doi: 10.1016/j.crfs.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Sierra E., Benítez-Correa E., Villavicencio MN de, González J. Chocolate reducido en azúcar obtenido por tecnología de molinos de bolas. Cienc. Tecnol. Aliment. [Internet] 2019;29(2):17–23. [Google Scholar]
- 50.Garti N., Aserin A. Cocoa Butter and Related Compounds. AOCS Press; 2012. Effect of emulsifiers on cocoa butter and chocolate rheology, polymorphism, and bloom [internet] pp. 275–305. [DOI] [Google Scholar]
- 51.Chen H., Zhou P., Song C., Jin G., Wei L. An approach to manufacturing heat-stable and bloom-resistant chocolate by the combination of oleogel and sweeteners. J. Food Eng. [Internet] 2022;330 doi: 10.1016/j.jfoodeng.2022.111064. [DOI] [Google Scholar]
- 52.Jin J., Jin Q., Wang X., Akoh C.C. Improving heat and fat bloom stabilities of “dark chocolates” by addition of mango kernel fat-based chocolate fats. J. Food Eng. [Internet] 2019;246:33–41. doi: 10.1016/j.jfoodeng.2018.10.027. [DOI] [Google Scholar]
- 53.Ali A., Selamat J., Che Man Y., Suria A. Effect of storage temperature on texture, polymorphic structure, bloom formation and sensory attributes of filled dark chocolate. Food Chem. 2001;72(4):491–497. https://linkinghub.elsevier.com/retrieve/pii/S0308814600002715 [Internet] [Google Scholar]
- 54.Devos N., Reyman D., Sanchez-Cortés S. Chocolate composition and its crystallization process: a multidisciplinary analysis. Food. Chem. [Internet] 2021;342 doi: 10.1016/j.foodchem.2020.128301. [DOI] [PubMed] [Google Scholar]
- 55.Wille R.L., Lutton E.S. Polymorphism of cocoa butter. J. Am. Oil Chem. Soc. 1966;43(8):491–496. doi: 10.1007/BF02641273. [DOI] [PubMed] [Google Scholar]
- 56.Lonchampt P., Hartel R.W. Fat bloom in chocolate and compound coatings. Eur. J. Lipid. Sci. Technol. [Internet] 2004;106(4):241–274. https://onlinelibrary.wiley.com/doi/10.1002/ejlt.200400938 [Google Scholar]
- 57.Muhammad D.R.A., Lemarcq V., Alderweireldt E., Vanoverberghe P., Praseptiangga D., Juvinal J.G., et al. Antioxidant activity and quality attributes of white chocolate incorporated with Cinnamomum burmannii Blume essential oil. J. Food Sci. Technol. 2020;57(5):1731–1739. doi: 10.1007/s13197-019-04206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dwijatmoko M.I., Praseptiangga D., Muhammad D.R.A. Effect of cinnamon essential oils addition in the sensory attributes of dark chocolate. Nusant. Biosci. 2016;8(2):301–305. [Google Scholar]