Abstract
We report a case of pathologically confirmed ALK-rearranged metastatic lung adenocarcinoma with emergence of EGFR L858R mutation on disease progression after two lines of treatment with ALK inhibitors. At initial diagnosis, tumoral ALK expression was detected without EGFR mutation by standard allele-specific polymerase chain reaction. There was sustained partial response to both first-line crizotinib and subsequent brigatinib. On disease progression to brigatinib, result of a liquid biopsy with circulating tumor DNA revealed only EGFR L858R, which was confirmed by tumor rebiopsy on the supraclavicular lymph node. The patient was then treated initially with pemetrexed and carboplatin, and erlotinib was subsequently added after two cycles of chemotherapy. The combination treatment has resulted in very good partial response and mild adverse effects. The overall clinical course would suggest the initial presence of two separate tumor clones, with ALK dominance at diagnosis. The subsequent breakthrough disease progression after initial response to brigatinib was related to uncontrolled growth of the EGFR-mutated tumor subpopulation. The implication on defining molecular mechanism of acquired resistance and treatment strategy would be discussed.
Keywords: Lung adenocarcinoma, Epidermal growth factor receptor, Anaplastic lymphoma kinase, Targeted therapy, Case report
Introduction
The co-existence of common driver oncogenes (e.g., EGFR and ALK) in advanced lung adenocarcinoma is rarely reported. Here, we report a case of metastatic-stage, ALK-driven lung adenocarcinoma with good prior response to two ALK inhibitors and subsequent failure related to the likely undiagnosed concomitant EGFR-mutated tumor clones. Consent was obtained from the patient for this case report.
Case Presentation
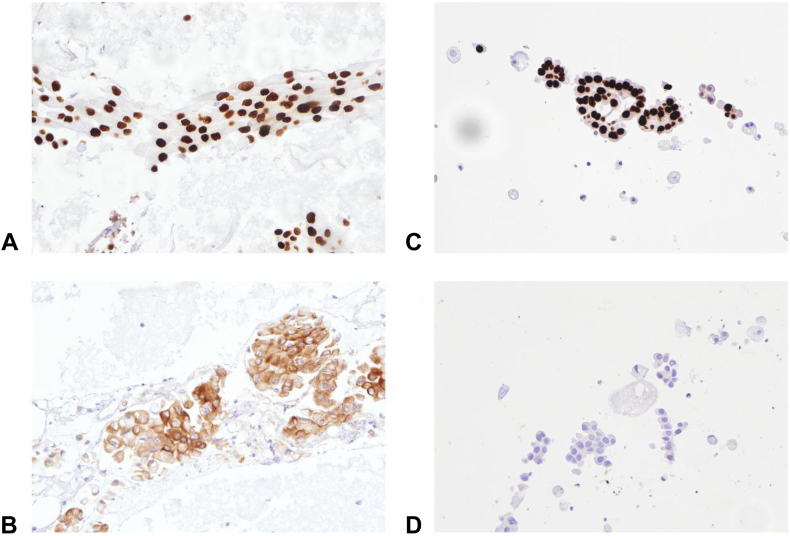
A 65-year-old man, with chronic smoking of 40 pack-years, was diagnosed stage IV lung adenocarcinoma in the right lower lobe with right supraclavicular lymph node and bilateral intrapulmonary metastases in January 2019. On presentation, result of fine-needle aspiration (FNA) of the supraclavicular lymph node revealed adenocarcinoma that was diffusely and strongly positive for TTF-1 and negative for p40 on immunostaining, compatible with pulmonary origin (Fig. 1A). Molecular tests were then done revealing no EGFR mutation by allele-specific polymerase chain reaction (Cobas EGFR Mutation Test version 2, Roche Diagnostics) but positive ALK immunocytochemical staining result (using both D5F3 clone in the Ventana system and 5A4 clone) (Fig. 1B). ROS1 was not detected using immunocytochemistry with D4D6 clone.
Figure 1.
Immunostaining of FNA samples of the right supraclavicular lymph node (H&E stain 200× magnification). (A, C) Positive TTF-1 staining on FNA specimen. (B) FNA specimen with ALK stain positive. (D) FNA specimen with ALK stain negative. (A) and (B) were taken on January 2, 2019, and (C) and (D) were taken on December 8, 2020. FNA, fine-needle aspiration; H&E, hematoxylin and eosin.
He received crizotinib 250 mg twice daily under a clinical trial protocol since February 2019 with partial response. His disease progressed in November 2019 with enlarging left upper lobe lung nodule on computed tomography of the thorax and a new 5-mm subcortical lesion at the right occipital lobe on magnetic resonance imaging (MRI) of the brain. Systemic treatment was then switched to second-line brigatinib 180 mg daily (after the first week of 90 mg daily) under another clinical trial since February 2020, which achieved disease stabilization on interval tumor scans and resolution of the brain lesion on MRI. Disease progression was noted since October 2020 with an enlarging right supraclavicular lymph node. A commercially available plasma next-generation sequencing molecular profiling (Guardant360) was performed, revealing only EGFR L858R mutation without ALK alterations or other co-mutations. A repeat FNA of the right supraclavicular lymph node in December 2020 confirmed adenocarcinoma with EGFR exon 21 L858R mutation (by allele-specific polymerase chain reaction) but undetectable ALK protein immunostaining (Fig. 1C and D). The absence of EGFR mutation from his previous FNA specimen in 2019 was reviewed and verified by the pathologist. Another plasma cell-free DNA for EGFR mutations also revealed L858R (allelic fraction at 1.3%).
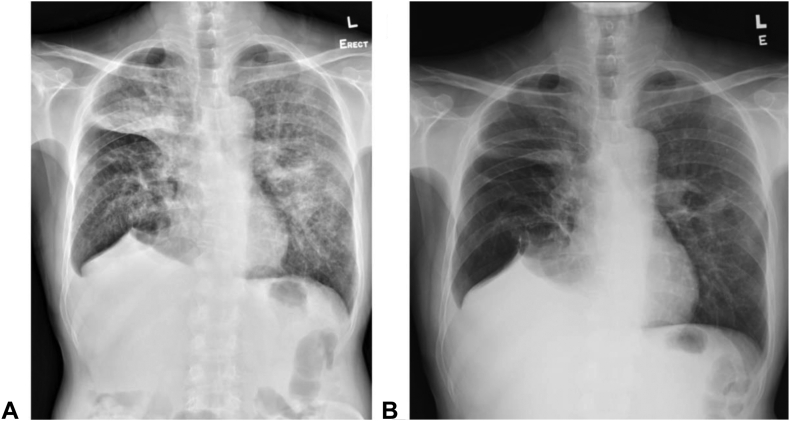
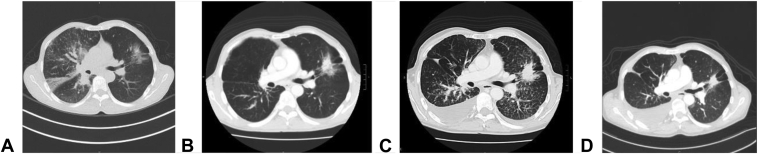
After confirming the emergence of EGFR-mutated adenocarcinoma of the lung on brigatinib treatment for his previous ALK-mutated adenocarcinoma of the lung, pemetrexed (500 mg/m2) and carboplatin (area under the curve = 5) were started for this patient on December 8, 2020 but brigatinib was stopped as per clinical trial protocol. Erlotinib (150 mg daily) (instead of osimertinib for financial constraint) was added in the combination with chemotherapy since February 9, 2021. To date, he has completed six cycles of doublet chemotherapy and now on maintenance pemetrexed (19th cycle) with erlotinib. Serial chest radiographs are found in Figure 2A and B. Reassessment computed tomography scan of the thorax revealed good partial response, with interval reduction in size of the left upper lobe lung mass and lymphangitic changes (Fig. 3A–D). MRI brain result also revealed no metastasis. The combination treatment was well tolerated.
Figure 2.
Serial chest radiographs. (A) At disease progression after brigatinib. (B) After six cycles of doublet chemotherapy with erlotinib.
Figure 3.
Temporal sequence of representative CT scans. (A) At diagnosis on November 30, 2018. (B) Before brigatinib on February 14, 2020. (C) Before chemotherapy on November 23, 2020. (D) After chemotherapy plus erlotinib on April 1, 2021. CT, computed tomography.
Discussion
We have reported a rare case of EGFR-mutated lung adenocarcinoma that emerged on failing two ALK inhibitors for treating ALK-rearranged metastatic lung cancer. Among Asian population, EGFR and ALK are the two most often recognized actionable driver mutations in NSCLC. Concomitant EGFR and ALK mutations in different lesions had been reported in the literature, with 4.71% among 1059 patients with multifocal lung adenocarcinoma. In patients with unifocal lung adenocarcinoma, EGFR/ALK co-alteration was reported in 0.83%.1
Our case first presented with a metastatic lung adenocarcinoma that only had ALK rearrangement. After two lines of ALK-targeted therapy, however, his disease progressed with newly detected EGFR L858R mutation in the absence of ALK rearrangement on rebiopsy. The overall clinical course suggested the presence of two separate tumor clones with different molecular profiles, which was predominated by ALK rearrangement initially. On exposure to ALK-targeted therapy, the original minor EGFR-driven clone was selected to outgrow leading to subsequent disease progression. This phenomenon has rarely been reported but clinically relevant in choosing the most appropriate treatment options. Disease progression while on second-generation ALK inhibitors is more often associated with secondary resistance ALK mutations (e.g., G1202R) that may only be susceptible to the third-generation inhibitor lorlatinib.2, 3, 4, 5, 6 Our case report on the emergence of a different oncogenic driver at the time of acquired resistance, likely preexisting as a minor clone initially, would reinforce the need to perform tumor rebiopsy on disease progression while on ALK inhibitors. This not only can provide information on the known resistance mechanisms to ALK inhibitors, but it may also reveal unexpected findings such as another driver mutation (in the absence of the original ALK rearrangement), such as in our case, which has critical treatment implications. Instead of switching to another ALK inhibitor, systemic chemotherapy with or without alternative targeted therapy would be a more reasonable approach. Taking our case as an example, systemic chemotherapy can possibly control the residual ALK-driven tumor clones whereas combination of EGFR tyrosine kinase inhibitor and chemotherapy was found to have superior disease control and progression-free survival compared with tyrosine kinase inhibitor alone in EGFR-driven lung adenocarcinoma.7
Interestingly, this patient received brigatinib as the second-line treatment after crizotinib failure, which has dual inhibitory activities on EGFR and ALK mutants.8 Brigatinib has also been found to be effective against EGFR triple mutation (C797S/T790M/activating mutation)–harboring lung cancers both in vitro and in vivo.9, 10, 11 Nonetheless, the uncontrolled tumor growth with sensitizing EGFR mutant while on brigatinib in our case may suggest the lack of clinical activity of brigatinib monotherapy in EGFR-mutated lung adenocarcinoma. This is also in keeping with previous reports that clinically effective treatment strategy for EGFR-mutant lung cancer would involve combined anti-EGFR monoclonal antibody together with brigatinib, rather than brigatinib alone.10
Conclusions
Our case with EGFR L858R-mutated (without co-existing ALK rearrangement) metastatic lung adenocarcinoma that evolved on ALK-specific treatments for initially confirmed ALK-driven disease represents a rare yet clinically important presentation. Tissue rebiopsy should be advocated for investigating acquired resistance to targeted therapy as in this case with both diagnostic and therapeutic implications.
CRediT Authorship Contribution Statement
Jackson Ka Chun Leung: Writing—roles/writing—original draft, Writing—review and editing, Visualization.
Wang Chun Kwok: Writing—roles/writing—original draft, Writing—review and editing.
Arthur Chun Fung Leung: Writing—review and editing, Resources.
Po Tsui: Writing—review and editing, Resources.
James Chung-Man Ho: Conceptualization, Writing—review and editing, Supervision.
Acknowledgments
The patient involved in this case report gave his informed consent authorizing use and disclosure of his health information.
Footnotes
Disclosure: The authors declare no conflict of interest.
Cite this article as: Leung JKC, Kwok WC, Leung ACF, Tsui P, Ho JCM. Emerging EGFR-mutated subclones in a patient with metastatic ALK-rearranged lung adenocarcinoma treated with ALK-targeted therapy: a case report. JTO Clin Res Rep. 2023;4:100542.
References
- 1.Fan J., Dai X., Wang Z., et al. Concomitant EGFR mutation and EML4-ALK rearrangement in lung adenocarcinoma is more frequent in multifocal lesions. Clin Lung Cancer. 2019;20:e517–e530. doi: 10.1016/j.cllc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Z., Huang X., Xie B., Xie W., Huang M., Lin L. Primary resistance to brigatinib in a patient with lung adenocarcinoma harboring ALK G1202R mutation and LIPI-NTRK1 rearrangement. Onco Targets Ther. 2020;13:4591–4595. doi: 10.2147/OTT.S249652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hida T., Seto T., Horinouchi H., et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci. 2018;109:2863–2872. doi: 10.1111/cas.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J.J., Zhu V.W., Schoenfeld A.J., et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol. 2018;13:1530–1538. doi: 10.1016/j.jtho.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw A.T., Solomon B.J., Besse B., et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosomi Y., Morita S., Sugawara S., et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi: 10.1200/JCO.19.01488. [DOI] [PubMed] [Google Scholar]
- 8.Mezquita L., Planchard D. The role of brigatinib in crizotinib-resistant non-small cell lung cancer. Cancer Manag Res. 2018;10:123–130. doi: 10.2147/CMAR.S129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchibori K., Inase N., Araki M., et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun. 2017;8 doi: 10.1038/ncomms14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Yang N., Zhang Y., et al. Effective treatment of lung adenocarcinoma harboring EGFR-activating mutation, T790M, and cis-C797S triple mutations by brigatinib and cetuximab combination therapy. J Thorac Oncol. 2020;15:1369–1375. doi: 10.1016/j.jtho.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J., Zou M., Lv J., Han Y., Wang G., Wang G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. Onco Targets Ther. 2018;11:5545–5550. doi: 10.2147/OTT.S170358. [DOI] [PMC free article] [PubMed] [Google Scholar]