Abstract
Fundamental questions in bacterial gene regulation concern how multiple regulatory proteins interact with the transcription apparatus at a single promoter and what are the roles of protein contacts with RNA polymerase and changes in DNA conformation. Transcription of the Escherichia coli uhpT gene, encoding the inducible sugar phosphate transporter, is dependent on the response regulator UhpA and is stimulated by the cyclic AMP receptor protein (CAP). UhpA binds to multiple sites in the uhpT promoter between positions −80 and −32 upstream of the transcription start site, and CAP binds to a single site centered at position −103.5. The role in uhpT transcription of portions of RNA polymerase Eς70 holoenzyme which affect regulation at other promoters was examined by using series of alanine substitutions throughout the C-terminal domains of RpoA (residues 255 to 329) and of RpoD (residues 570 to 613). Alanine substitutions that affected in vivo expression of a uhpT-lacZ transcriptional fusion were tested for their effect on in vitro transcription activity by using reconstituted holoenzymes. Consistent with the binding of UhpA near the −35 region, residues K593 and K599 in the C-terminal region of RpoD were necessary for efficient uhpT expression in response to UhpA alone. Their requirement was overcome when CAP was also present. In addition, residues R265, G296, and S299 in the DNA-binding surface of the C-terminal domain of RpoA (αCTD) were important for uhpT transcription even in the presence of CAP. Substitutions at several other positions had effects in cells but not during in vitro transcription with saturating levels of the transcription factors. Two DNase-hypersensitive sites near the upstream end of the UhpA-binding region were seen in the presence of all three transcription factors. Their appearance required functional αCTD but not the presence of upstream DNA. These results suggest that both transcription activators depend on or interact with different subunits of RNA polymerase, although their role in formation of proper DNA geometry may also be crucial.
Transcription activation, a common form of gene regulation in bacteria, can occur through formation of specific contacts between a DNA-bound activator protein and RNA polymerase (RNAP) holoenzyme. These protein contacts can recruit the binding of RNAP to weak promoter sequences, stimulate its isomerization from a closed complex to an open complex, or affect the rate of promoter clearance by the transcription elongation complex (reviewed in reference 32). Activator proteins can induce changes in DNA conformation to increase promoter effectiveness. Several portions of the RNAP subunits have been identified as having roles in promoter activation. The C-terminal domain of ς70 (ςCTD) contains a putative helix-turn-helix motif (region 4.2, residues 570 to 613) which recognizes the −35 promoter element (15) and some of the transcription activators that bind near the −35 region, including λ cI, PhoB, Ada, cyclic AMP (cAMP) receptor protein (CAP)–activating region-3 (AR3), FNR, and phage Mu Mor proteins (1, 21, 23–25). The C-terminal domain of the α subunit (αCTD) interacts with A+T-rich UP (upstream activating region) elements in the −60 to −40 region (31) and with many transcription regulators that bind upstream of the RNAP-binding site, including CAP, OmpR, OxyR, Ada, and BvgA (4, 5, 10, 23, 38).
CAP provides a prime example of activation through multiple RNAP contacts. When complexed with cAMP, CAP binds to a specific DNA target sequence in promoters of more than 100 genes in Escherichia coli (reviewed in references 6 and 22). CAP can directly activate transcription when it binds to sites that are centered between bp −41.5 and −92.5 upstream of the transcription start site and have proper helical phasing relative to the binding site for RNAP (14, 42). Different surfaces of CAP contact different parts of RNAP, depending on the location of the CAP-binding site in the promoter. Contact between a surface loop around residue 157 in CAP, called activating region-1 or AR1, and αCTD is important for CAP action at any effective site. A second surface of CAP, AR2, contacts the amino-terminal domain of RpoA when CAP is bound at position −41.5, adjacent to the site of the RNAP (29). A third surface element of CAP, AR3, is normally silent but can allow activation in place of AR1 or AR2 when altered by mutation. The AR3 region contacts ςCTD (5).
We address here the involvement of RNAP subunits in transcription activation at the uhpT promoter. Expression of the UhpT sugar phosphate transporter is induced by external glucose 6-phosphate (Glu6P) through the action of an atypical two-component system (20). The uhpT promoter lacks a −35 element and depends absolutely on the response regulator UhpA (11, 44). UhpA is activated by its phosphorylation on aspartate-54 by the membrane-bound UhpBC sensor kinase complex in response to extracellular Glu6P. UhpA binds to a dyad element centered at position −64, and phosphorylated UhpA can then bind to low-affinity sites that extend to position −32 (7). Catabolite repression of uhpT transcription results from the 10- to 15-fold stimulation caused by the binding of CAP-cAMP to a DNA site centered at position −103.5 (27). Since CAP cannot independently activate transcription from so far upstream (2, 42), it was of interest to test whether UhpA or CAP action was related to specific contacts with RNAP.
To investigate the action of UhpA and CAP, we developed an in vitro transcription system that reproduces the dependence on UhpA and the stimulation by CAP that is seen in vivo (30). Here, this in vitro expression system was used in combination with in vivo expression systems to evaluate the effect on uhpT transcription of alanine substitutions throughout αCTD and ςCTD. Key residues in an activator response segment of ςCTD and in the DNA-binding surface of αCTD were found to be important for uhpT transcription. ςCTD contributes to the action of UhpA alone, but not to that of UhpA plus CAP, whereas αCTD is important for CAP action. In addition, αCTD is important for protein-dependent changes in promoter DNA conformation that may combine with protein-protein contacts to enhance promoter folding around RNAP (30).
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 strains and plasmids used in this work are listed in Table 1. Plasmid-bearing cells were grown in the presence of ampicillin (200 μg/ml). Plasmids encoding ς70 alanine substitution mutants with mutations at residues 574 and 590 through 613 were described by Lonetto et al. (25) and are variants of pGEX2 in which the coding region of glutathione S-transferase (GST) is fused to RpoD residues 8 to 613 and expressed from the tac promoter. Plasmids encoding ς70 alanine substitution mutants with mutations at residues 570 and 575 were obtained from P. Landini, University of Birmingham (23). For overexpression and purification of these RpoD variants, the rpoD coding sequence was cloned into the vector pGEM-His6 so that the coding region for a His6 tag is fused to RpoD residues and expressed from the tac promoter. Plasmids encoding αCTD alanine substitution mutants with mutations between residues 255 and 329 were described by Savery et al. (34) and encode derivatives of RpoA without or with a His6-coding region inserted between codons 1 and 2.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Reference(s) or source |
|---|---|---|
| Strains | ||
| MC4100 | ΔlacU169 araD139 deoC1 flb5301 ptsF25 rbsR rpsL150 | 37 |
| RK1309 | MC4100 λRZ5(Km) uhpT-lacZ | 27 |
| XE65.2 | Δcrp39 ΔlacX74 rpsL thi | R. Ebright |
| TM37 | XE65.2::λRZ5(Km) uhpT-lacZ | T. Merkel |
| CAG20154 | galK thi-1 thr-1 leu-6 lacY1 tonA21 supE44 Ω(Cm) Ptrp-rpoD | 25 |
| IO697 | TM37 Ω(Cm) Ptrp-rpoD | P1(CAG20154) × TM37 |
| Plasmids | ||
| pRJK10 | ApruhpABCT | 43 |
| pLAW2 | AprrpoA | 47 |
| pLAD256 | AprrpoA-256 (encoding RNAP α subunit deleted at codon 256) | 16 |
| pHTf1α (and derivatives) | AprrpoA (derivatives carrying αCTD alanine substitutions at positions 255 to 271) | 12, 41 |
| pREIIα (and derivatives) | AprrpoA (derivatives carrying αCTD alanine substitutions at positions 273 to 329) | 3, 12, 34 |
| pHTT7f1NHα | AprrpoA (derivatives carrying αCTD alanine substitutions with His6-coding region, T7 promoter) | 12 |
| pGEX-2Tς70 (and derivatives) | AprrpoD (derivatives carrying RNAP ς70 subunit alanine substitutions at positions 574, 590 to 613) | 9, 25 |
| pVRς (and derivatives) | AprrpoD (derivatives carrying RNAP ς70 subunit alanine substitutions at positions 570 and 575) | 23 |
| pGEM-His6 (and derivatives) | AprrpoD (derivatives carrying His6-RNAP ς70 subunit alanine substitutions, T7 promoter) | P. Landini |
| pMKSe2 | AprrpoB, lacP control | R. Gourse |
| pT7β′ | AprrpoC, T7 promoter control | 45 |
| pLHN12s | AprrpoD, T7 promoter control | 34 |
Screening of rpoD mutants in vivo.
Two host strains were used to screen for rpoD mutant alleles affecting UhpA-dependent transcription of uhpT. A plasmid library carrying mutations resulting in single alanine substitutions at 19 positions between residues 570 and 613 of the C-terminal region of ς70 (obtained from C. A. Gross, University of California, San Francisco [25], or from P. Landini [23]) was introduced by transformation into strains RK1309 and IO697. Both strains are single lysogens of λRZ5 uhpT-lacZ. Transformants of RK1309 were grown in minimal medium A supplemented with 1% glycerol, 0.5% Casamino Acids, and 200 μg of ampicillin per ml. Transformants of IO697 were grown in minimal medium A supplemented with 1% glycerol, 20 μg of l-tryptophan per ml, 0.5% Casamino Acids, and 500 μg of ampicillin per ml. β-Galactosidase levels were measured after induction with 0.25 mM Glu6P for 40 min for RK1309 or 2 h for IO697.
The plasmid-borne rpoD alleles are expressed from an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter. However, addition of IPTG, even to cells expressing wild-type RpoD, resulted in marked inhibition of uhpT induction and of cell growth, perhaps owing to nonspecific inhibition of RNAP assembly or function (25). Hence, only basal-level expression of rpoD was used, which results in levels of protein comparable to those with expression from the chromosomal rpoD+ gene. The effect of the RpoD substitutions on transcription in RK1309 was expected to be less prominent than that in IO697 or in the studies described by Lonetto et al. (25), because the latter strains have reduced competition from the chromosome-encoded wild-type RpoD protein, which is expressed from a tryptophan-repressible trp promoter.
Screening of rpoD and rpoA mutants in vivo.
To screen for rpoA mutants affecting uhpT transcription, a plasmid library of rpoA mutants expressing alanine substitutions at 69 positions between residues 255 and 329 of αCTD (obtained from T. Gaal and R. Gourse, University of Wisconsin) (12) under lac control was screened in strain RK1309 or TM37 in a similar manner as described above, except that 1 mM IPTG was present throughout the growth of the culture for induction of rpoA gene expression.
β-Galactosidase assays.
As described previously (28), cells were grown in minimal medium A supplemented with 1% glycerol and 0.5% Casamino Acids, without or with 0.25 mM Glu6P for the indicated times. Cells were permeabilized with CHCl3-sodium dodecyl sulfate. The time course of hydrolysis of o-nitrophenyl-β-d-galactopyranoside was measured at 415 nm in a 96-well microplate reader (Molecular Devices) at 37°C and was normalized for culture density (optical density at 660 nm). Each assay was performed in triplicate.
Protein purification and reconstitution of RNAP with mutant subunits.
For reconstitution of RNAP with wild-type ς70 subunit or the alanine substitution mutants, His6-tagged proteins were purified using Ni-nitrilotriacetic acid columns (Qiagen, Valencia, Calif.), and GST-tagged proteins were purified on glutathione agarose columns (8). Purified ς factors were added at a 4:1 ratio to core RNAP (Epicentre Technologies, Madison, Wis.) (23) for the His-tagged subunits and at 10:1 for the GST fusion proteins prior to in vitro transcription assays.
N-terminal His-tagged wild-type and mutant α subunits were expressed from plasmid pHTT7f1-NHα (40) or derivatives constructed by gene replacement of the EcoRI-BamHI fragments encoding the desired alanine substitutions (12). Purification of α subunits by Ni2+ affinity chromatography; preparation, solubilization, and renaturation of inclusion bodies of β, β′, and ς70 subunits; and reconstitution of RNAP holoenzyme were carried out essentially as described previously (12, 39).
Cells carrying plasmids pMKSe2, pT7β′, and pLHN12ς were induced for expression of RpoB, RpoC, and RpoD, respectively. Wild-type E. coli UhpA protein was purified as described previously (7). CAP was purified by cAMP affinity chromatography (46). Both proteins were >95% homogeneous.
DNase I protection.
DNase I footprinting reactions were performed by the method of Galas and Schmitz (13) as described previously (30). The 5′-32P-end-labeled linear DNA fragments containing the uhpT promoter region were obtained by PCR with pRJK10 DNA as a template (43). The primers used were IOPT +50 (positions +50 to +26), IOPT-250 (−250 to −226) (30), IOPT-179, and IOPT-121; the sequences of these primers are available upon request. Primer IOPT+50 was 5′ end labeled by using T4 polynucleotide kinase and [γ-32P]ATP (3,000 Ci/mmol). PCR products were purified by using the QIAquick PCR purification kit (Qiagen). Promoter-containing DNA fragment (50,000 cpm) at a final concentration of 1 nM was incubated with the specified amounts of purified UhpA, CAP, and RNAP in 20 μl of TXN buffer, which comprises 40 mM Tris-HCl (pH 8.0), 50 mM KCl, 10 mM MgCl2, 10 mM dithiothreitol, and 200 μM cAMP. After 30 min at 37°C, DNase I digestion was begun by addition of 2 μl of TXN buffer containing 25 mM CaCl2, 25 mM MgCl2, and 0.5 μg of DNase I per ml. After 30 s at room temperature, 4 μl of stop solution {0.18 M EDTA, 0.34 μg of poly[d(I:C)] per ml, 30% glycerol} was added. DNA was precipitated and washed with 70% ethanol, dried under vacuum, dissolved in loading buffer, and resolved by electrophoresis on sequencing gels (5% polyacrylamide–7 M urea gels in 1× Tris-borate-EDTA buffer) (33). Radioactive DNA fragments were detected with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
In vitro transcription.
Transcription assays were performed as described by Olekhnovich et al. (30) at 25°C in TXN buffer (see above). The indicated amounts of the DNA template fragment, UhpA, and CAP were incubated for 10 min in 10 μl of TXN buffer prior to the addition of RNAP (5 μl). After 3 min at 25°C, 5 μl of TXN buffer containing nucleoside triphosphate (NTP) substrates and heparin was added to yield the following final concentrations: 1 nM DNA template, 220 nM UhpA; 20 nM CAP; 5 nM RNAP; 50 μg of heparin per ml, 200 μM (each) NTPs (ATP, CTP, and GTP), and 40 μM [α-32P]UTP (2.5 Ci/nmole). After 10 min, the reaction was terminated by addition of 5 μl of transcription stop solution (7 M urea, 0.1 M EDTA, 0.4% sodium dodecyl sulfate, 40 mM Tris-HCl [pH 8.0], 0.5% bromphenol blue, and 0.5% xylene cyanol). After electrophoresis in sequencing gels, the products were analyzed by autoradiography or PhosphorImager analysis with the ImageQuant program for quantitative comparisons.
Promoter fragments for in vitro transcription reactions carried the uhpT sequence between positions −380 and +233, generated by PCR on pRK10 as template, or carried the lacUV5 promoter (34).
RESULTS
Effect of changes in ςCTD on uhpT-lacZ expression.
Binding of UhpA to the −32 region of the uhpT promoter appears to be important for its action. It was thus of interest to examine the role in uhpT transcription of residues 570 to 613 in the C-terminal end of ς70, which are involved both in the recognition of the −35 element and in the action of some activators that bind near position −40. Plasmids expressing RpoD or GST-RpoD proteins with the wild type or 19 mutants with alanine substitutions at residues 570, 574, and 575 and from residues 590 to 613 (kindly provided by C. A. Gross and P. Landini) (23, 25) were introduced into strain RK1309, carrying a single-copy uhpT-lacZ transcriptional fusion. The plasmid-coded RpoD proteins compete with the chromosome-encoded wild-type RpoD for assembly into RNAP holoenzyme. Decreased Glu6P-induced uhpT-lacZ expression should occur in cells expressing an RpoD variant that is defective in uhpT transcription. In this strain, none of the alanine substitutions affected expression, except for substitutions at residues 570 and 575, which affect action of the PhoB and Ada proteins and caused a 40% reduction in uhpT-lacZ expression (Fig. 1A).
FIG. 1.
Effect of alanine substitutions in ςCTD on uhpT-lacZ expression in vivo. (A) Results with host strain RK1309; (B) results with host strain IO607. Values of β-galactosidase activity are averages from three independent experiments and are presented as percentages of the wild-type (WT) values (270 and 9 Miller units, respectively). Residues 609 to 611 are alanines. Error bars indicate standard deviations.
Since transcription activation by AraC or FNR requires ςCTD only in the absence of their respective coactivators, CAP or NarL (25), we tested the effect of some alanine substitutions in ςCTD on uhpT-lacZ expression in the Δcrp strain TM37. Whereas expression of the RpoD-D570A or -E575A variant in the crp+ strain decreased uhpT-lacZ expression, these variants had little effect on the activity remaining in the Δcrp strain (Fig. 2A and B). Similar results were obtained with the R596A, K597A, H600A, S602A, and R603A variants (data not shown).
FIG. 2.
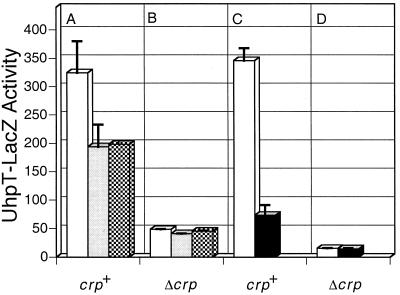
Changes in uhpT-lacZ expression in response to the presence of CAP and variations in RpoA and RpoD. Host strains were crp+ (A and C) and Δcrp (B and D). (A and B) Plasmids encoding wild-type RpoD (open bars), RpoD-E570A (shaded bars), and RpoD-D575A (cross-hatched bars). (C and D) Plasmids encoding wild-type RpoA (open bars) and RpoA-Δ256 (filled bars). Values are β-galactosidase activities in Miller units. Error bars indicate standard deviations.
Because the host strain background influences the phenotype of RpoD variants (25) and because the GST-RpoD variants might not compete effectively with wild-type RpoD, we introduced the panel of alanine substitution mutants into strain IO697. In this Δcrp uhpT-lacZ strain, the chromosomal rpoD+ allele is replaced with Ω(Cm)trpP-rpoD, which allows the effect of the RpoD variants to be tested with reduced competition from the wild-type protein and in the absence of the activator CAP. Expression of rpoD is repressed by addition of tryptophan, and cell growth is dependent on the tryptophan competitor indole-3-acrylic acid, unless a complementing plasmid-borne rpoD variant is present. In IO697, plasmids expressing the E591A, L595A, and L598A variants did not allow indole-3-acrylic acid-independent growth, indicating the inability of these variants to substitute for the wild-type RpoD. The Glu6P-induced β-galactosidase levels in the remaining strains were very low, at most about 3% of that in strain RK1309, owing to the absence of CAP function and the poor growth of the cells. The K593A and R599A variants of RpoD conferred a two- to threefold reduction in expression, whereas the D570A and E575A variants conferred expression comparable to that for the wild type and all other alanine substitution mutants. Thus, several residues in ςCTD appear to be important for UhpA activation.
Effect of changes in ςCTD on in vitro uhpT expression.
To test the effect of the alanine substitutions in RpoD which affected cellular expression, the His6-tagged D570A and E575A RpoD proteins and the GST-tagged E591A, K593A, L598A, and R599A proteins were purified. The purified proteins were reconstituted with core RNAP to form Eς70 holoenzymes. The reconstituted RNAPs were used for single-round in vitro transcription reactions with a linear uhpT promoter DNA template (positions −380 to +233) (1 nM) and the purified UhpA (220 nM) and CAP (20 nM) proteins (Fig. 3). Transcription from the CAP-independent lacUV5 promoter was used to measure the RNAP activities of the reconstituted enzymes. DNA templates were preincubated at 25°C with UhpA in the absence and presence of CAP at concentrations previously found to be maximally stimulatory for uhpT transcription (30). The preincubated templates were then incubated with the RNAP holoenzymes for 3 min prior to addition of the NTP substrates and heparin (50 μg/ml) to block further transcription initiation. Both RpoD and RNAP core enzyme were required for transcription from either promoter (data not shown). Expression from the lacUV5 promoter was decreased by the E591A and L598A substitutions, suggesting a general defect. In the former case, this decrease was associated with reduced amounts of the RpoD protein, perhaps as a result of protein instability.
FIG. 3.
Transcription of the uhpT and lacUV5 promoters by wild-type (WT) RNAP and mutant RNAPs carrying RpoD variants E570A, D575A, E591A, K593A, L598A, and R599A. Reactions were carried out in the absence and presence of 20 nM CAP, as indicated. (A) The ratios of the amounts of the uhpT and the lac transcripts were determined by PhosphorImager quantitation. Transcription of uhpT was measured in the presence of UhpA. (B) uhpT transcription (Txn) in the presence of UhpA without and with CAP as indicated, calculated relative to the amount of transcription produced by the wild-type RNAP in the presence of UhpA.
By normalizing uhpT transcription in the absence of CAP to that of lacUV5, it was seen that the D570A and E575A variants showed no defect in uhpT-specific transcription (Fig. 3A, lanes 1 to 3), suggesting that the defect seen in the corresponding β-galactosidase expression was an indirect effect. The E591A variant showed uhpT transcription that was reduced to almost the same degree as was lac transcription (Fig. 3A, lanes 4 and 5), consistent with a general defect. In contrast, the K593A, L598A, and R599A variants were strongly impaired for uhpT transcription relative to lac (Fig. 3A, lanes 6 to 8).
The effect of CAP on uhpT transcription was tested (Fig. 3B). Under these assay conditions, CAP stimulated in vitro expression by 3.2-fold, in comparison to the 10- to 15-fold stimulation of uhpT-lacZ expression by CAP. Although UhpA-dependent transcription by the reconstituted holoenzymes containing RpoD-K593A, -L598A, and -R599A was strongly reduced by 70 to 90%, it was strongly stimulated by CAP. In comparison to the 3.5-fold stimulation with wild-type RpoD, CAP stimulated uhpT transcription by these variant holoenzymes by 7- to 18-fold, up to levels approaching that of the wild-type. These results show that the K593A and R599A substitutions interfere with UhpA-dependent transcription activation but are only moderately impaired for transcription stimulated by UhpA plus CAP. Note that CAP alone does not allow any uhpT transcription in the absence of UhpA.
Effect of changes in αCTD on uhpT-lacZ expression.
The involvement of αCTD in uhpT expression was examined. First, plasmids expressing RpoA+ or RpoA-Δ256, in which αCTD is deleted from residue 256, were introduced into crp+ and Δcrp cells. Glu6P-induced expression of the uhpT-lacZ fusion was measured after induction of the variant subunits with IPTG (Fig. 2C and D). Expression of RpoA-Δ256 resulted in a fivefold reduction of uhpT expression in the crp+ strain, indicating a key role of αCTD in uhpT transcription. Expression of either RpoA+ or RpoA-Δ256 caused a similar 20-fold decrease in uhpT expression in the absence of CAP. This result suggested that overexpressed RpoA interferes with uhpT expression and that αCTD participates in transcription activation by CAP but perhaps not by that of UhpA alone.
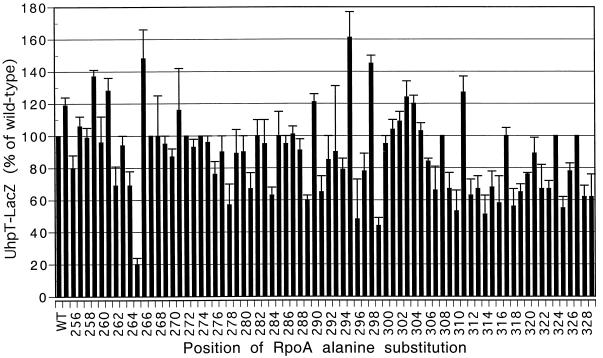
To localize the sites in αCTD important for uhpT transcription, we examined the effect of expression of RpoA variants carrying alanine substitutions throughout αCTD from residues 255 to 329 (provided by T. Gaal and R. Gourse). Alanine substitutions at numerous positions in αCTD affected uhpT expression (Fig. 4). Three RpoA mutants, R265A, G296A, and S299A, had the strongest effects and reduced expression by 2.5- to 5-fold. Less pronounced reductions resulted from other substitutions, including those at residues 262, 264, 278, 281, 284, 289, and 291 and at many sites distal to residue 307. Some substitutions resulted in elevated expression.
FIG. 4.
Effect of alanine substitutions in αCTD on uhpT-lacZ expression in vivo. Values of β-galactosidase activity are averages from three independent experiments and are plotted as percentages of the wild-type (WT) value (370 Miller units). Error bars indicate standard deviations.
Effect of changes in αCTD on expression in vitro.
We examined the in vitro transcription activity and the degree of CAP stimulation by using 12 reconstituted RNAP holoenzymes. Wild-type RpoA and variants with alanine substitutions at 11 positions which affected expression in vivo were purified and mixed with the other wild-type components to form RNAP Eς70 holoenzymes. Each reconstituted RNAP holoenzyme was used in single-round runoff transcription reactions of the uhpT and lacUV5 promoter fragments, as described above.
The reconstituted RNAP carrying wild-type RpoA showed basal and CAP-stimulated transcription similar to those of commercially obtained enzyme (Fig. 5 and data not shown). All of the reconstituted RNAPs carrying variant RpoA subunits had about the same activity as the wild-type enzyme for transcription of the lacUV5 promoter (Fig. 5). Consistent with the in vivo results, RNAP carrying RpoA-R265A was very defective for uhpT transcription and was not detectably stimulated by CAP. RNAP carrying RpoA-G296A was markedly impaired in vitro and did not respond to the presence of CAP, whereas RpoA-S299A was strongly impaired but was stimulated by CAP. The other seven RpoA alanine substitution mutants were not substantially impaired in the in vitro assay, although the degree of CAP stimulation of the RpoA variants L281A, K291A, and L307A was different from that for the wild-type enzyme. The impaired level of transcription by the G296A and S299A variants was partially restored by prolonged incubation of the holoenzyme with the template DNA prior to initiation of the transcription assay (data not shown). Taken together, these results show that certain residues in αCTD are required for effective uhpT transcription but that most are not. It is also clear that some changes in αCTD were more detrimental to transcription than was the absence of CAP, indicating that the effect of αCTD extends beyond its effect on CAP action alone.
FIG. 5.
Transcription of the uhpT and lacUV5 promoters by wild-type (WT) RNAP and mutant RNAPs carrying the indicated alanine substitutions in RpoA. The relative amounts of the uhpT transcript (Txn level), determined by PhosphorImager quantitation, are presented relative to that for the wild-type RNAP in the presence of UhpA.
Changed DNA geometry depends on αCTD but not upstream DNA.
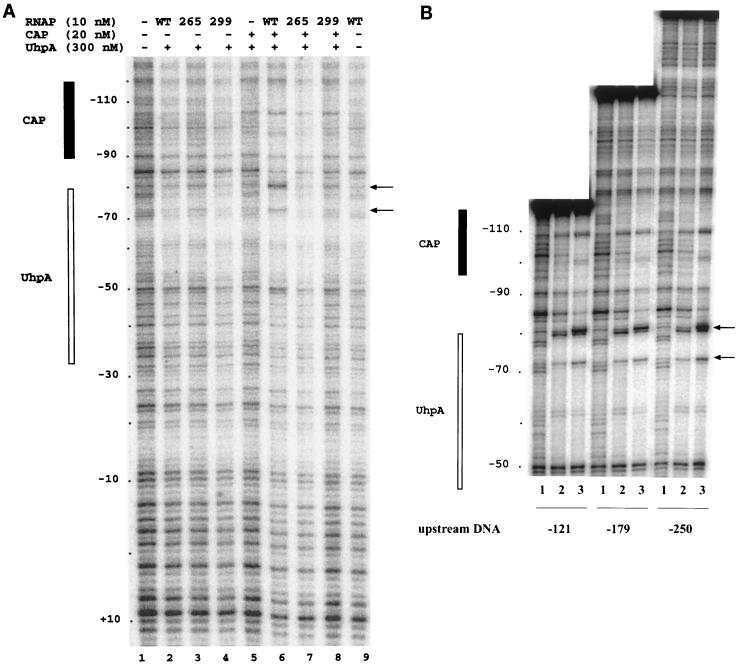
To look for the sites of RNAP binding at the uhpT promoter and for possible changes in DNA geometry caused by the binding of CAP and αCTD, we examined the DNase I digestion pattern of the uhpT promoter fragment (positions −250 to +50) in the presence of the three transcription factors (Fig. 6A). As seen previously (7, 30), UhpA protected sites in the −80 to −32 region, and CAP protected or enhanced cleavage between positions −114 to −93. Surprisingly, no footprint could be assigned to RNAP, whether alone or with UhpA and CAP. The lack of strong binding by RNAP is consistent with the lability of the open complex to dissociation by polyanions (30).
FIG. 6.
DNase I footprinting at the uhpT promoter region. (A) UhpA, CAP, and RNAP variants were present as indicated on the top. The positions protected by UhpA and CAP are indicated on the left, and the positions of the DNase hypersensitive sites are indicated on the right. RNAP samples contained a 5 nM concentration of the wild-type (WT), 265A, or 299A form of RpoA, as indicated. (B) Three DNA fragments whose upstream ends were at positions −121, −179, and −250, as indicated, were used. For each DNA fragment, the sample in lane 1 contained DNA, that in lane 2 contained DNA, 220 nM UhpA, and 80 nM CAP, and that in lane 3 contained DNA, UhpA, CAP, and 10 nM WT RNAP.
DNase I hypersensitivity occurred at positions −80 and −74 only in the presence of all three factors, i.e., RNAP, UhpA, and CAP (Fig. 6A, lane 6). These sites are near the most-upstream UhpA-binding region, close to the CAP-binding site. Footprinting was carried out with the reconstituted RNAP carrying the wild-type or the R265A or S299A form of RpoA. The formation of the hypersensitive sites in the ternary complex did not occur with the R265A variant and was markedly reduced with the S299A variant (compare lanes 6 to 8 in Fig. 6A). Finally, we tested whether the appearance of the DNase-hypersensitive sites required the presence of upstream DNA. Footprinting was carried out with three DNA fragments whose upstream ends were at positions −121, −179, and −250 (Fig. 6B). DNase digestion was carried out with free DNA (lanes 1) or in the presence of UhpA plus CAP (lanes 2) or UhpA plus CAP plus RNAP (lanes 3). All three DNA fragments displayed comparable amounts of both hypersensitive sites in the presence of all three protein components.
DISCUSSION
UhpA is absolutely required for uhpT transcription in vivo and in vitro (30, 36). CAP is required for efficient transcription, but maximal in vitro transcription can occur in the absence of CAP with longer incubation of the template with UhpA and RNAP. CAP has multiple effects on the formation of the open complex; namely, it enhances the binding of UhpA and RNAP to the promoter, accelerates open complex formation, and slows open complex dissociation (30). The instability of the open complex at the uhpT promoter is indicated both by the susceptibility of the transcription complex to polyanions (30) and by the absence of a DNase protection footprint.
The present study identified protein contact sites important for transcription at the uhpT promoter. Since UhpA binds near the −35 region, we expected that UhpA might contact the CTD of RpoD, as found for some transcription activators that bind in a similar position. The structure of the ςCTD may resemble that of the C-terminal helix-turn-helix DNA-binding domain of NarL and UhpA (25). Portions of one helix recognize the −35 element and are preceded and followed by residues that affect activation by proteins that bind near −35, such as activation of λPRM by cI protein (24), of the ada and aidB promoters by Ada (23), of the pho regulon promoters by PhoB (21), and of the phage Mu middle promoter by Mor protein (1). Alanine substitutions for K593, K597, R599, and H600 reduced activation by CAP in vivo and in vitro (19, 25) but only when CAP action depended on AR3. Alanine substitutions for E591, K593, and R596 had moderate effects on AraC-dependent transcription but only in the absence of CAP (25). Residues 593, 596, 597, and 600 affected transcription stimulation by Fnr at the narG promoter but only in the absence of the activator P-NarL.
Owing to its importance for transcription, in vivo assays of the effect of alanine substitutions in ςCTD are difficult. Overexpression of RpoD is toxic, but the mutant forms insufficient amounts to compete with the wild type for incorporation into the RNAP holoenzyme. Indeed, we found no effect of the alanine substitutions in ςCTD on uhpT-lacZ expression in a wild-type strain, except for the indirect action of the 570A and 575A variants not seen in vitro. Although uninduced expression of the plasmid-coded GST-RpoD proteins is equivalent to the expression of the chromosomal allele, these proteins are probably unable to compete effectively for incorporation into the holoenzyme. The requirement for ςCTD residues 593 and 599 was clearly seen only in cells lacking crp and with repression of the chromosomal rpoD allele by excess tryptophan. The role of these residues was verified by in vitro transcription assays, showing that residues 593, 598, and 599 were required for UhpA activation as well as other transcription activators. As seen at other promoters, these ςCTD residues are needed for the action of the transcription factor bound near position −35, in this case UhpA. This promoter was strongly activated by UhpA plus CAP even though UhpA-dependent transcription was greatly impaired. Because CAP cannot activate uhpT transcription by itself, UhpA must activate transcription in two ways, one involving interaction with ςCTD and the other involving interaction with CAP.
αCTD participates in the function of many transcriptional activators, including CAP (34), OmpR (38), SoxS (17), Ada (23), and BvgA of Bordetella pertussis (4), as well as in antitermination by λ N protein (35). Some residues in this domain, such as R265 and S299, are needed for the action of many of these transcription activators and for stimulation by the UP element. Other positions are needed only for function of specific activators. It is now apparent that αCTD interacts with both DNA and the activator proteins, that mutations affecting either function can interfere with transcription activation, and that αCTD can have positive and negative effects at certain promoters. The structure of αCTD (18) revealed that the residues necessary for the action of the UP element and many transcription factors comprise its DNA-binding face. The residues that are needed only for certain transcription factors lie on protein contact surfaces. Mutations that interfere with the DNA-binding activity of αCTD are usually more deleterious than those that affect the activator interaction surfaces.
Alanine substitutions at numerous sites between residues 255 and 329 affected Glu6P-induced uhpT-lacZ expression. The R265A change in the DNA-binding surface was the most severely affected both in vivo and in vitro, as seen on most αCTD-dependent activators. The degree of impairment of the G296A and S299A substitution mutants was substantially reduced by prolonged incubation with RNAP, a condition which also reduces the degree of stimulation by CAP. This result suggests that CAP participates with αCTD in the recruitment of RNAP to the promoter, rather than for open complex formation or promoter clearance. Although CAP can interact with αCTD at other promoters, it binds too far upstream to contact RNAP at the uhpT promoter (14, 42). In studies of an artificial promoter containing two CAP-binding sites, CAP bound at position −102.5 could stimulate transcription when another molecule of CAP was bound at position −41.5 (2). In this situation, both CAP dimers contacted αCTDs and promoted their binding to adjacent DNA. This result suggests that CAP sites that are too far upstream for effective contact with RNAP can be brought into position by the DNA-bending activity of the downstream CAP dimer. Perhaps UhpA plays a similar role at the uhpT promoter to alter DNA geometry and allow contact of RNAP and CAP. Our results show that both ςCTD and αCTD are important for optimal transcription at the uhpT promoter. Similar dependence on both RNAP subunits has been found for the action of Ada protein and the Mor protein of phage Mu (1, 23). Although it seems most likely that ςCTD contacts UhpA, the αCTD contacts are not yet known.
Because the effect of the removal of αCTD or the R265A substitution mutant was more deleterious than was the absence of CAP, it is likely that αCTD is needed for more than just allowing CAP to act. Two possibilities are that αCTD interacts directly with both CAP and UhpA or that αCTD binds to nonspecific DNA upstream of the CAP site which is brought into reach of RNAP by the DNA-bending activity of CAP. None of the changes in the ARs of CAP that affect transcription at other CAP-dependent promoters had any significant effect on uhpT transcription (26, 27), suggesting that CAP action may be through changes in DNA geometry rather than protein contacts.
Although RNAP did not produce a DNase footprint at the uhpT promoter, the requirement for all three proteins, i.e., RNAP, UhpA, and CAP, for maximal formation of the DNase-hypersensitive sites at positions −74 and −80 indicates that RNAP binds to the promoter along with UhpA and CAP. The DNA distortion leading to DNase hypersensitivity could result from the binding of αCTD to this region or from the production there of a sharp bend resulting from DNA looping that requires all three proteins. Because formation of the hypersensitive sites required αCTD but not upstream DNA, we favor the view that αCTD binds between the CAP and UhpA sites. Future experiments using affinity-directed DNA cleavage might provide evidence for this hypothesis.
The weak binding of RNAP may reflect the weak binding of UhpA to its target sites. It is likely that the weak yet specific binding of UhpA to the DNA is a general adaptation designed to allow genome-bound transcription factors to maintain communication with their cognate membrane-bound sensor kinase. Frequent dissociation of UhpA from the DNA can allow adjustment of its level of phosphorylation to reflect the current state of occupancy of the receptor for Glu6P and hence to allow efficient regulation.
ACKNOWLEDGMENTS
We are indebted to Carol Gross, Paolo Landini, Tamas Gaal, and Rick Gourse for providing the strains and advice that made this work possible. John Dahl contributed to initial stages of this work. We are especially indebted to the anonymous reviewers of the manuscript who insisted on more extensive analysis of the involvement of RpoD.
This work was supported by research grant GM38681 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Artsimovitch I, Murakami K, Ishihama A, Howe M M. Transcription activation by the bacteriophage Mu Mor protein requires the C-terminal regions of both α and ς70 subunits of Escherichia coli RNA polymerase. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 2.Belyaeva T A, Rhodius V A, Webster C L, Busby S J W. Transcription activation at promoters carrying tandem DNA sites for the Escherichia coli cyclic AMP receptor protein: organization of the RNA polymerase α subunits. J Mol Biol. 1998;277:789–804. doi: 10.1006/jmbi.1998.1666. [DOI] [PubMed] [Google Scholar]
- 3.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 4.Boucher P E, Murakami K, Ishihama A, Stibitz S. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J Bacteriol. 1997;179:1755–1763. doi: 10.1128/jb.179.5.1755-1763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busby S, Ebright R H. Transcription activation at class II CAP-dependent promoters. Mol Microbiol. 1997;23:853–859. doi: 10.1046/j.1365-2958.1997.2771641.x. [DOI] [PubMed] [Google Scholar]
- 6.Busby S, Kolb A. The CAP modulon. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Austin, Tex: Landes Co.; 1996. pp. 255–279. [Google Scholar]
- 7.Dahl J L, Wei B Y, Kadner R J. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J Biol Chem. 1997;272:1910–1919. doi: 10.1074/jbc.272.3.1910. [DOI] [PubMed] [Google Scholar]
- 8.Dombroski A. ς factors: purification and DNA binding. Methods Enzymol. 1996;273:134–144. doi: 10.1016/s0076-6879(96)73013-6. [DOI] [PubMed] [Google Scholar]
- 9.Dombroski A J, Walter W A, Record M T, Jr, Siegele D A, Gross C A. Polypeptides containing highly conserved regions of transcription initiation factor ς70 exhibit specificity of binding to promoter DNA. Cell. 1992;70:501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- 10.Ebright R H. Transcription activation at class I CAP-dependent promoters. Mol Microbiol. 1993;8:797–802. doi: 10.1111/j.1365-2958.1993.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 11.Friedrich M J, Kadner R J. Nucleotide sequence of the uhp region of Escherichia coli. J Bacteriol. 1987;169:3556–3563. doi: 10.1128/jb.169.8.3556-3563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the α subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 13.Galas D, Schmitz A. DNase footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978;5:3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaston K, Bell A, Kolb A, Buc H, Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990;62:733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Gross C, Lonetto M, Losick R. Bacterial sigma factors. In: McKnight S, Yamamoto K, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 16.Hayward R S, Igarashi K, Ishihama A. Functional specialization within the alpha-subunit of Escherichia coli RNA polymerase. J Mol Biol. 1991;221:23–29. doi: 10.1016/0022-2836(91)80197-3. [DOI] [PubMed] [Google Scholar]
- 17.Jair K-W, Fawcett W P, Fujita N, Ishihama A, Wolf R E., Jr Ambidextrous transcriptional activation by SoxS: requirement for the C-terminal domain of the RNA polymerase alpha subunit in a subset of Escherichia coli superoxide-inducible genes. Mol Microbiol. 1996;19:307–317. doi: 10.1046/j.1365-2958.1996.368893.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase α subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 19.Jin R, Sharif K A, Krakow J S. Evidence for contact between the cyclic AMP receptor protein and the ς70 subunit of Escherichia coli RNA polymerase. J Biol Chem. 1995;270:19213–19216. doi: 10.1074/jbc.270.33.19213. [DOI] [PubMed] [Google Scholar]
- 20.Kadner R J. Expression of the Uhp sugar-phosphate transport system of Escherichia coli. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 263–274. [Google Scholar]
- 21.Kim S-K, Makino K, Amemura M, Nakata A, Shinagawa H. Mutational analysis of the role of the first helix of region 4.2 of the ς70 subunit of Escherichia coli RNA polymerase in transcriptional activation by activator protein PhoB. Mol Gen Genet. 1995;248:1–8. doi: 10.1007/BF02456607. [DOI] [PubMed] [Google Scholar]
- 22.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 23.Landini P, Bown J A, Volkert M R, Busby S J W. Ada protein-RNA polymerase ς subunit interaction and α subunit-promoter DNA interaction are necessary at different steps in transcription initiation at the Escherichia coli ada and aidB promoters. J Biol Chem. 1998;273:13307–13312. doi: 10.1074/jbc.273.21.13307. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Moyle H, Susskind M M. Target of the transcriptional activation function of phage λ cl protein. Science. 1994;263:75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- 25.Lonetto M A, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 26.Merkel T J. Thesis. Charlottesville: University of Virginia; 1992. [Google Scholar]
- 27.Merkel T J, Dahl J L, Ebright R H, Kadner R J. Transcription activation at the Escherichia coli uhpT promoter by the catabolite gene activator protein. J Bacteriol. 1995;177:1712–1718. doi: 10.1128/jb.177.7.1712-1718.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkel T J, Nelson D M, Brauer C L, Kadner R J. Promoter elements required for positive control of transcription of the Escherichia coli uhpT gene. J Bacteriol. 1992;174:2763–2770. doi: 10.1128/jb.174.9.2763-2770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olekhnovich I N, Dahl J L, Kadner R J. Separate contributions of UhpA and CAP to activation of transcription of the uhpT promoter of Escherichia coli. J Mol Biol. 1999;292:973–986. doi: 10.1006/jmbi.1999.3127. [DOI] [PubMed] [Google Scholar]
- 31.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 32.Roy S, Garges S, Adhya S. Activation and repression of transcription by differential contact: two sides of a coin. J Biol Chem. 1998;273:14059–14062. doi: 10.1074/jbc.273.23.14059. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Savery N J, Lloyd G S, Kainz M, Gaal T, Ross W, Ebright R H, Gourse R L, Busby S J W. Transcription activation at class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase α subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schauer A T, Cheng S-W C, Zheng C, St. Pierre L, Alessi D, Hidayetoglu D L, Costantino N, Court D L, Friedman D I. The alpha subunit of RNA polymerase and transcription antitermination. Mol Microbiol. 1996;21:839–851. doi: 10.1046/j.1365-2958.1996.451409.x. [DOI] [PubMed] [Google Scholar]
- 36.Shattuck-Eidens D M, Kadner R J. Molecular cloning of the uhp region and evidence for a positive activator for expression of the hexose phosphate transport system of Escherichia coli. J Bacteriol. 1983;155:1060–1070. doi: 10.1128/jb.155.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 38.Slauch J M, Russo F D, Silhavy T J. Suppressor mutations in rpoA suggest that ompR controls transcription by direct interaction with the α subunit of RNA polymerase. J Bacteriol. 1991;173:7501–7510. doi: 10.1128/jb.173.23.7501-7510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Kim Y, Severinov K, Goldfarb A, Ebright R H. Escherichia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant α, β, β′, and ς subunits. Methods Enzymol. 1996;273:130–134. doi: 10.1016/s0076-6879(96)73012-4. [DOI] [PubMed] [Google Scholar]
- 40.Tang H, Severinov K, Goldfarb A, Ebright R H. Rapid RNA polymerase genetics: one-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 1995;92:4902–4906. doi: 10.1073/pnas.92.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang H, Severinov K, Goldfarb A, Fenyo D, Chait B, Ebright R H. Location, structure, and function of the target of a transcriptional activator protein. Genes Dev. 1994;8:3058–3067. doi: 10.1101/gad.8.24.3058. [DOI] [PubMed] [Google Scholar]
- 42.Ushida C, Aiba H. Helical phase-dependent action of CRP: effect of the distance between the CRP site and the −35 region on promoter activity. Nucleic Acids Res. 1990;18:6325–6330. doi: 10.1093/nar/18.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weston L A, Kadner R J. Identification of the Uhp polypeptides and evidence for their role in exogenous induction of the sugar-phosphate transport system of Escherichia coli. J Bacteriol. 1987;169:3546–3555. doi: 10.1128/jb.169.8.3546-3555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weston L A, Kadner R J. Role of uhp genes in expression of the Escherichia coli sugar-phosphate transport system. J Bacteriol. 1988;170:3375–3383. doi: 10.1128/jb.170.8.3375-3383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zalenskaya K, Lee J Y, Gujuluva C N, Shin Y K, Slutsky M, Goldfarb A. Recombinant RNA-polymerase-inducible overexpression, purification and assembly of Escherichia coli rpo gene products. Gene. 1990;89:7–12. doi: 10.1016/0378-1119(90)90199-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Gunasekera A, Ebright Y, Ebright R H. Derivatives of CAP having no solvent-accessible cysteine residues, or having a unique solvent-accessible cysteine residue at amino acid 2 of the helix-turn-helix motif. J Biomol Struct Dyn. 1991;9:463–473. doi: 10.1080/07391102.1991.10507929. [DOI] [PubMed] [Google Scholar]
- 47.Zou C, Fujita N, Igarashi K, Ishihama A. Mapping the cAMP receptor protein contact site on the α subunit of Escherichia coli RNA polymerase. Mol Microbiol. 1992;6:2599–2605. doi: 10.1111/j.1365-2958.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]