Highlights
-
•
Aqueous-based pipetting assessment can mask competency in common clinical matrices.
-
•
Challenging pipetting event which highlights imprecision/bias in clinical matrices.
-
•
Event can be optimized to suit specific assays and repeated to assess performance.
Keywords: Practical training, Pipetting, Imprecision, Bias, Mass spectrometry
Abstract
Introduction
Engaging pipetting events were developed to assess and challenge technicians’ practical sample handling using matrices common to the clinical laboratory. As correct pipetting stands as a prerequisite for accurate clinical laboratory testing, this helped to understand sources of imprecision and bias attributed to the underlying step of aspirating and dispensing patient samples and internal standard in clinical LC-MS/MS assays while highlighting the importance for the clinical laboratory to evaluate this source of variability on an on-going basis and mitigate its impact.
Methods
The events involved pipetting water, methanol, serum, and whole blood. Gravimetric analysis was used to determine the exact volumetric delivery of each matrix using two different techniques. Imprecision and bias were calculated based on the volume derived from the mass and density of each matrix, using literature values for each matrix type.
Results
Low imprecision and bias were observed when pipetting water, as in common commercial pipetting assessment programs. Significantly increased imprecision and bias were observed in more applicable matrices (i.e., serum, whole blood, and methanol), indicating that water-based pipetting proficiency assessment leads to a false sense of technical ability. Additionally, the events within illuminated areas for training, leading to improved imprecision and bias. It was shown that pre-rinsing (aspirating and dispensing matrix three times to coat the tip) improved bias, particularly for delivery of methanol and whole blood.
Conclusions
Precise and accurate pipetting within the clinical laboratory should not be taken for granted, nor implicitly inferred from proficiency assessment using aqueous solutions. The engaging and collegial events fostered training opportunities. Assay-specific patient sample delivery considerations (pipets and matrices) can inform the practicality of these events – the Pipetting Olympics – and drive improvements within the laboratory.
1. Introduction
Within clinical laboratory testing, there are numerous pre-analytical variables that can adversely affect the final result of a patient sample [1], [2]. These variables start at the patient and typically end once the specimen is received in the laboratory. However, laboratory personnel training, particularly specimen pipetting, is a logical source of pre-analytic variance which should be continually assessed and errors mitigated by the laboratory. Moreover, the College of American Pathologists general laboratory checklist requires personnel training and competency be demonstrated prior to any patient testing without providing prescriptive guidance [3]. Many laboratories, including ours, utilize commercially available instruments and solutions (e.g., PCS® Pipette Calibration System, Artel, Westbrook, US) to assess laboratory personnel pipetting proficiency; however, these commercial solutions are inconsistent with the entire suite of pipets used within the laboratory and only evaluate the delivery of aqueous solution containing dye [4]. This proficiency program assumes that all pipetting steps in the clinical laboratory are equivalent. In contrast, this paper recommends an in-lab “Olympic Event”, which can be adapted to fit many clinical laboratory workflows using matrix appropriate tests in a collegial and competitive manner.
In clinical mass spectrometry (MS), precise and accurate pipetting enables confident conclusions through test development, validation, and, ultimately, patient sample analysis. The use of appropriately selected stable labeled internal standards (IS) effectively controls downstream assay imprecision after IS addition; however, it does not correct for patient sample pipetting imprecision. Often IS is prepared in an organic (or organic/aqueous hybrid) matrix, and without assessment, the process assumes that addition of the IS is precise. There are also instances where pipetting accuracy is important, particularly when different matrix types and fluidic properties exist between calibrators, QC’s and patient specimens. This paper describes practical assessment tools we have used to engage and challenge laboratory personnel. Review of the data obtained in this study was used to provide an understanding of the sources of variability attributable to imprecision and bias of patient sample and IS addition steps and subsequently improve assay protocols. These events, colloquially referred to as the “Pipetting Olympics”, can be held during onboarding new hires, semi-annual competency assessments, and/or as part of Medical Laboratory Professionals Week.
2. Equipment and events (i.e. Materials and methods)
The goals of the Pipetting Olympics events are to assess the imprecision and bias of the critical liquid delivery steps of standard clinical MS analytical workflows (e.g., patient sample and IS addition) using representative matrices and solvents for a given lab technician. Relevant matrices are pipetted onto an analytical balance as six replicates, with determination of the random error associated with pipetting each matrix and solvent calculated as the coefficient of variability (CV %). Systematic error is determined as bias (%) based on the dispensed mass using published values for fluid density (which were additionally confirmed within the laboratory). Technicians can be judged based on their performance relative to objective metrics for imprecision and bias, but also against one another as a collegial team-building exercise.
Regardless of the matrix, a high-precision analytical balance must be used. Certified weights should be used to verify its accuracy. Additionally, calibrated pipettes are required; the volume range, or fixed volume, of the pipette should be carefully selected for the volume to be delivered. Each laboratory should evaluate the applicability of the following study design and consider making modifications as needed.
In the Olympic pipetting competition, we report here, a volume of 20 µL (∼20 mg for water) was pipetted with a Thermo Electron Finnpipette 5–50 μL adjustable pipette (Thermo Fisher; Waltham, MA) and 200 µL SureOne™ Pipet Tips (Fisher Scientific; Hampton, NH). Individual mass measurements were performed using an analytical balance (±0.1 mg or ∼0.5% rounding error, Sartorious; Göttingen, Germany). The four matrices evaluated were water, methanol, pooled EDTA whole blood, and pooled serum. Pooled EDTA whole blood and serum were created from de-identified discarded specimens. Density values, derived from literature, were used for all bias calculations in this paper (0.997 g/mL for water [5], 1.024 g/mL for serum [6], 0.790 g/mL for methanol [7], and 1.055 g/mL for whole blood [6]). The density of each matrix used in this study was confirmed via Class A volumetric delivery of 1 mL as six replicates at 22 °C. The confirmation yielded densities of 0.999 g/mL for water (−0.2% bias versus literature), 1.011 g/mL for serum (−1.3% bias), 0.7759 g/mL for methanol (−1.8% bias), and 1.016 g/mL for whole blood (−3.7% bias). Notably, CV calculations were all <1.0%. Admittedly, these values do vary from the published values above; modifying the protocol to use larger Class A volumetric pipettes or flasks could mitigate or reduce wetting/residual error associated with the 1 mL Class A volumetric pipette used in this study. Nevertheless, published values were used herein and are a pragmatic choice, as labs may not be able to pool sufficient volume for all matrix types to perform density calculations and will rely on published data.
Pipetting of each matrix was treated as an individual event within our Pipetting Olympics. Materials were allowed to equilibrate to room temperature (20–24°C) prior to use. For each event, six replicates of test solutions were aspirated and then dispensed onto the analytical balance. After each aspirate and dispense action, the mass was recorded by the technician and then the balance was tared for the next replicate. Admittedly, self-recording mass measurements could lead technicians to tailor their technique for more precise results; modifying the process where a second individual records mass measurements could alleviate this possibility. A new pipette tip was used for each replicate. Technicians were instructed to forward pipette (i.e., aspirate/dispense target volume with a separate blowout step to empty the pipette tip) with a single aspirate and dispense action for the first round of events and to touch any hanging droplet to the collection vessel after dispensing. After compiling results for the first set of events, each technician was asked to repeat the events while pre-rinsing the pipette tip three times (i.e., fully aspirate and completely dispense to coat the inside of the tip) prior to the final dispense onto the balance. Again, a new pipette tip was used and pre-rinsed for each replicate.
The imprecision (CV) was calculated for each technician in each of the four events (i.e., matrices tested). Additionally, bias was calculated as the ratio of the average volume dispensed (mass corrected by density) to the target volume (i.e., 20 µL). As part of the events, medals were awarded in the categories of imprecision (random error) and bias (systematic error).
3. Podium ceremonies (i.e. Results)
Unsurprisingly, pipetting water yielded imprecision of <1% CV for all technicians; however, Technician 2 edged out Technician 1 for the gold medal with 0.49% imprecision (Fig. 1A). Pre-rinsing pipette tips did alter the battle for gold, with Technician 1 capturing the gold medal in the water event, Technician 2 obtaining the silver medal, and Technician 4 on the podium with bronze (Fig. 1A). In the water event, average imprecision and bias for all technicians, regardless of pipetting technique, fell between the pipette manufacturer’s performance specifications of the pipette at 25 µL (imprecision ≤ 0.5% and bias ≤ 1.0%) and ISO 8655 specifications (imprecision ≤ 0.8% and bias ≤ 2.0%).
Fig. 1.
Imprecision results for all Pipetting Olympic events. There was minimal difference in imprecision between no pre-rinse pipette tip (solid fill) and pre-rinse pipette tip (dashed fill) for most technicians and events. However, noteworthy reductions in imprecision were observed when pre-wetting was deployed in the methanol event.
In the second pipetting event, the technicians were challenged with serum. This matrix yielded imprecision higher than that of water and indicated that matrices common to the clinical laboratory are more challenging to pipette. Nevertheless, Technician 1 took the gold medal, demonstrating a CV of 1.7% (Fig. 1B). Technicians 3 and 2 finished with silver and bronze demonstrating 2.7% and 3.7% imprecision, respectively. In the pre-rinsed pipette tip round of the serum event, Technicians 2–4 demonstrated little to no impact from pre-rinsing the pipette tip whereas Technician 1 demonstrated an increase in imprecision from 1.7% to 3.2% (Fig. 1B). Whole blood can be a more challenging matrix to pipette in the clinical laboratory due to its viscosity.
That said, the three technicians who obtained medals in the whole blood event each pipetted with CVs ≤ 2.5% (Fig. 1C), which was an impressive feat. The whole blood event using pre-rinsed pipette tips demonstrated equivalent imprecision for Technicians 1 and 2, while Technicians 3 and 4 generated higher imprecision than single aspirate/dispense pipetting (Fig. 1C). It should be noted that Technicians 3 and 4 had never attempted precise and accurate low-volume pipetting of whole blood prior to these events. This lack of experience offered a training opportunity for the two individuals (e.g., slow aspiration and dispense speeds), whereas these skills were habitual to Technicians 1 and 2.
The final event was delivering 20 µL of methanol. In this event, Technician 2 obtained the gold medal by demonstrating an imprecision of 1.9% for six replicates (Fig. 1D). It should also be noted that the methanol event demonstrated the highest imprecision among the four events at 7.4%. Additionally, pre-rinsing pipette tips during the methanol event yielded a reduction in imprecision for Technicians 1–3 and a slight increase for Technician 4 (2.3% to 3.2%). As a result, Technician 1 missed the podium in the single aspirate/dispense event, but achieved a silver medal finish in the pre-rinse event with an imprecision of 2.4% (Fig. 1D).
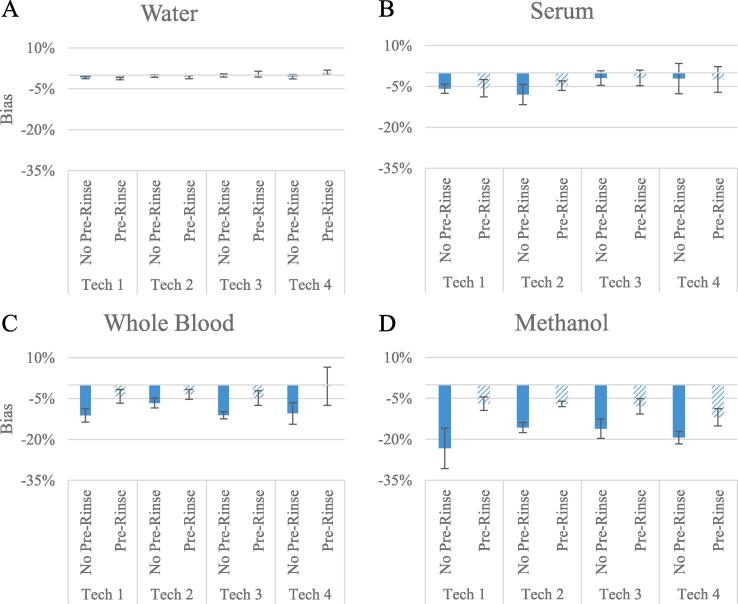
While imprecision has been the main focus of the podium ceremonies thus far, medals for bias were next to be awarded. The mean absolute bias in the water no pre-rinse event was −0.4% (Fig. 2A). Technician 3 won the gold medal, with Technicians 2 and 4 winning silver and bronze, demonstrating −0.3% and −0.5% biases, respectively. Although bias when pipetting water was <±1.2% regardless of technique or technician, this was not representative of the bias observed in other matrices even though water (an aqueous solution containing a dye) is often used to ascertain pipetting competency. For example, technician 3 took gold in both water and serum no pre-rinse events with biases ≤±1.9%, yet exhibited biases ≥±10.4% when pipetting whole blood and methanol. In the serum no pre-rinse event, the mean absolute bias was −4.5% (Fig. 2B). Technicians 3, 4, and 1 earned gold, silver, and bronze in this event with biases of −1.9%, −2.1%, and −5.8%, respectively. The mean absolute bias in the no pre-rinse event for whole blood was −9.9% (Fig. 2C). In this event, it was Technician 2 who dominated and took gold with a −6.6% bias; Technicians 4 and 3 placed for silver and bronze demonstrating −10.4% and −11.1% biases, respectively. The methanol no pre-rinse event yielded the largest mean absolute bias at −18.6% (Fig. 2D). Technicians 2, 3, and 4 were awarded gold, silver and bronze in this event with biases of −15.7%, −16.1%, and −19.4%, respectively.
Fig. 2.
Inaccuracy results for all Pipetting Olympic events. Minimal bias was observed when pipetting water regardless of pipetting technique deployed. The largest impact of pre-rinsing (dashed lines) in reducing the bias of pipetting was observed in the Whole Blood and Methanol events. Error bars represent one standard deviation of the observed bias. The improvements through pre-rinsing of these two matrices could prove beneficial when pipetting critical reagents (i.e. internal standard or calibrators) in mass spectrometry assays.
In the water and serum events, the impact of pre-rinsing proved to have a negligible impact as all demonstrated <1% relative bias between pipetting techniques. Indeed, there was minimal change on the podium in the water pre-rinse event as Technician 3 maintained the gold medal and Technicians 2 and 4 swapped places for silver and bronze. In the serum pre-rinse event, the only change came by way of the battle for bronze where Technician 2 overtook Technician 1 with −4.7% bias. In contrast to water and serum, mean absolute bias when pipetting blood was reduced markedly from −9.9% to −3.2% across the four technicians when changing from the single aspirate/dispense (no pre-rinse) technique to the pre-rinsing technique. All podium positions changed as a result of the pre-rinsed whole blood pipetting event and Technician 4 earned their first and only gold medal of the games with a −0.5% bias. The pre-rinse methanol pipetting event continued to shuffle the podium standings compared to the no pre-rinse event. Most notably, Technician 1 missed out on a podium position in the whole blood event without pre-rinsing with a −23.3% bias, yet managed a gold medal in the whole blood pre-rinse event with a −7.0% bias. However, overall improvements were noticed by using the pre-rinsing technique as average absolute bias was decreased from −18.6% to −8.5% when pipetting methanol for all technicians (Fig. 2D).
At the conclusion of our Pipetting Olympics, all technicians earned medals and podium positions across the four events (Table 1).
Table 1.
Final medal count across all events and technicians.
 |
4. Closing ceremony (i.e. Discussion)
Our findings clearly show that an aqueous solution (water containing dye) is not an appropriate matrix for generalized competency assessment. While low imprecision and bias were observed when pipetting water, significant increases in imprecision and bias were observed when pipetting serum, whole blood, and methanol (i.e., clinically relevant matrices). As such, competency assessments should be tailored by each laboratory with respect to liquid types and volumes.
Accuracy of pipetting is of critical importance when matrix types differ for calibrators and patient samples/controls, as it can result in quantitative error if not controlled for and corrected [8], [9]. Additionally, the use of less frequent calibration protocols (i.e., historical calibration) relies on accurate delivery of the calibrators/patient samples and IS between technicians [10]. As demonstrated herein, differences in bias were observed between pipetting blood-based matrices and methanol. However, through refinement of technique, there was a broad reduction in bias across the technicians.
The Olympic events can be optimized to evaluate multiple pipetting techniques, matching the exact volume and matrix/matrices pipetted in the assay. Challenge technicians’ choice of appropriate pipette size by supplying multiple pipette options, all of which have the ability to pipette the desired volume. If pipetting low volumes or challenging matrices, consider the pipette (positive or air displacement, fixed or adjustable volume [11]) and alternate techniques (e.g., reverse pipetting techniques where one would aspirate the target volume plus excess volume and dispense the target volume only without a secondary blowout of the pipette tip). Additionally, include all matrices of key reagents at the appropriate volume (e.g., IS, buffers, etc.). We encourage the reader to consider this study and repeat the Pipetting Olympics with a new set of events in your laboratory. The results can be used to inform standard operating procedures. The nature of the events provides a collegial, but competitive, structure that encourages self-improvement. This should translate into higher quality lab results we, clinical laboratory workers, provide to clinicians for patient care.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Patricia Holland, Whitney Brandon, Matthew Chappell, and Meghan Bradley for their contributions to generation of the data.
Disclosures
Mr. Crawford, Dr. Shuford and Dr. Grant receive salary from Labcorp as well as have stock ownership.
References
- 1.Ashakiran S., Sumati M.E., Murthy N.K. A study of pre-analytical variables in clinical biochemistry laboratory. Clin. Biochem. 2011;44(10-11):944–945. doi: 10.1016/j.clinbiochem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Becan-McBride K, Behúlová D, Bowen RA, Church S, Delanghe J, et al. Preanalytical quality improvement: in quality we trust. Clin Chem Lab Med. 2013; 51:229-41. [DOI] [PubMed]
- 3.College of American Pathologist, Laboratory General Checklist. October 24th, 2022.
- 4.Kitchener R.L., Hentz N.G. Artel; Westbrook, ME: 2019. Single channel pipette calibration and operator competency assessment using a dual-dye ratiometric photometry system. https://pubs.acs.org/doi/full/10.1021/acsguide.40303 (accessed 2022-08-03) [Google Scholar]
- 5.Lide D.R., editor. vol. 85. CRC Press; 2004. (CRC handbook of chemistry and physics). [Google Scholar]
- 6.Sniegoski L.T., Moody J.R. Determination of serum and blood densities. Anal. Chem. 1979;51(9):1577–1578. doi: 10.1021/ac50045a052. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin RD. Methanol Thermodynamic Properties from 176 to 673 K at pressures to 700 Bar. J. Phys. Chem. Ref. Data 1987; 16:779.
- 8.de Jager A.D., Warner J.V., Henman M., Ferguson W., Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine – An Australian prespective. J. Chrom. B. 2012;897:22–31. doi: 10.1016/j.jchromb.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Staeheli S.N., Poetzsch M., Kraemer T., Steuer A.E. Develoment and validation of a dynamic range-extended LC-MS/MS multi-analyte method for 11 different postmortem matrices for redistribustion studies applying solvent calibration and additional 13C isotope monitoring. Anal. Bioanal. Chem. 2015;407:8681–8712. doi: 10.1007/s00216-015-9023-5. [DOI] [PubMed] [Google Scholar]
- 10.Cheng W.L., Markus C., Lim C.Y., Tan R.Z., Sethi S.K., Loh T.P. Clinical practices in clinical mass spectrometry: review and recommendations. Ann. Lab. Med. 2023;43:5–18. doi: 10.3343/alm.2023.43.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster C., Habler K., Vogeser M. Effect of gravimetric correction and type of pipettes used in sample preparation on the precision of LC-MS/MS-based analyses. Clin. Biochem. 2021;91:63–66. doi: 10.1016/j.clinbiochem.2021.01.017. [DOI] [PubMed] [Google Scholar]