Abstract
Background
Parasitic infections are among the most common diseases worldwide, and enterobiasis is a well-known type of parasitic infection in children. Given the existence of several reports on the prevalence of Enterobius vermicularis in different provinces of Iran and the heterogeneity of the reported prevalence data, this study aims to investigate the overall prevalence of Enterobius vermicularis among children in Iran through a systematic review and meta-analysis.
Methods
This systematic review and meta-analysis study involved a comprehensive search of several databases, including PubMed, ScienceDirect, SID, and Google Scholar, focusing on cross-sectional studies that examined the prevalence of Enterobius vermicularis infection in Iranian children. The identified studies were entered into the EndNote software for review. The quality of observational studies was evaluated using the STROBE checklist. The information extracted from the studies was entered into the Comprehensive Meta-analysis (CMA, Version 2) software. Heterogeneity among the studies was analyzed using the I2 test, and publication bias was assessed using the Egger test and funnel plot.
Results
A total of 51 studies, with a sample size of 46,070 children, were included in the review. Using the random effects method, the overall prevalence of Enterobius vermicularis among children in Iran was determined to be 6.7% (95%CI: 5.2–8.6). The review of the factors affecting study heterogeneity and sample size indicated that as sample size increased, the prevalence of Enterobius vermicularis among children in Iran also increased (p = 0.578). Additionally, with an increase in the year of conducting the studies, the prevalence of Enterobius vermicularis among children in Iran decreased (p < 0.05).
Conclusion
The findings of this study show a relatively high prevalence of Enterobius vermicularis among children in Iran. We recommend health policymakers recognize the significance of this issue and take necessary measures to reduce the incidence of this infectious agent in children, implementing more effective preventive measures through mass media and educational campaigns.
Keywords: Enterobius vermicularis, Enterobiasis, Pinworm, Children
Graphical abstract
1. Background
Enterobius vermicularis, a species of parasitic worms belonging to the Oxyurida order, is responsible for causing enterobiasis (Fallah et al., 2022). These worms primarily inhabit the colon and migrate towards the anus, where they release approximately 10,000 eggs, facilitating the transmission of infectious eggs to other individuals, including self-reinfection, due to eggs' adhesive properties that cause irritation and itching in the anal region (Fallah et al., 2022; Fouladvand et al., 2018).
Enterobius vermicularis eggs can lead to infection through direct, indirect, and retro-infection transmission methods (Fallah et al., 2022; Haghi, 2013). In direct transmission, the eggs are transferred to food, drinks, or the mouth via contaminated hands, eventually reaching the ileocecal area and causing disease. However, their presence has been observed throughout the digestive tract, from the stomach to the anus (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013). The eggs are resistant to stomach acids and relatively resistant. To become infective, they require a temperature of 35 degrees Celsius for 4–7 h, while temperatures below 22 degrees Celsius induce dormancy (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013). Enterobius vermicularis infection manifests as anal itching, which can lead to sleep disturbances, restlessness, itching in the anal or vaginal area, irritability, teeth grinding, occasional stomach pain, and nausea (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013).
In indirect transmission, individuals at risk come into the infected person's clothing. Retroinfection, also known as double transmission, occurs when the remaining eggs around the anus re-infect the same individual (Haghi, 2013; Najafi et al., 2020). The severity of the disease in individuals is classified into two categories, severe and mild, based on the number of Enterobiasis vermicularis present in the body. In the mild type, fewer eggs are transmitted, usually through inhalation (Haghi, 2013). Due to their lightweight, the eggs can remain suspended in the air and infect individuals through inhalation or ingestion via the mouth or nose (Haghi, 2013; Najafi et al., 2020). Although practising proper hygiene, such as handwashing with soap, is generally recommended to prevent infectious diseases, it does not destroy Enterobiasis vermicularis eggs. However, exposure to sunlight and ultraviolet rays can destroy the eggs (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013; Najafi et al., 2020).
Parasitic infections, including enterobiasis, are prevalent worldwide (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013; Najafi et al., 2020; Rahim, 2013). It is estimated that approximately 400 million people globally are affected by enterobiasis, with earlier sources suggesting around one billion people being affected (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013; Najafi et al., 2020; Rahim, 2013). The prevalence of this infection is typically higher in temperate climates. Additionally, the level of personal hygiene in a society plays a significant role in the prevalence of enterobiasis. Developed societies with high literacy rates, awareness, industrialization, and the use of chemical fertilizers instead of human waste, generally experience lower prevalence rates compared to developing or less developed countries (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013; Najafi et al., 2020; Rahim, 2013).
In a developed country like the United States of America, which had a population of around 300 million in 2006, approximately 20–42 million people, or 7–14% of the population, were estimated to be affected by enterobiasis. In countries like Thailand or Sudan, much higher percentages, such as 38.5% and 37%, have been observed (Haghi, 2013; Najafi et al., 2020; Rahim, 2013).
Iran, a developing country with an infection rate of 17.2%, is at risk of a widespread epidemic of enterobiasis due to its geographical location and diverse climate. The prevalence of Enterobius vermicularis is higher in children compared to adults. Symptoms such as restlessness, loss of appetite, bedwetting, anal itching, teeth grinding, nightmares, stomach discomfort, nausea, and growth disorders cause significant suffering in children, leading to reduced productivity (Najafi et al., 2020; Rahim, 2013). The nocturnal migration of female worms for oviposition disrupts sleep, resulting in fatigue, difficulty concentrating and academic failure (Fallah et al., 2022; Fouladvand et al., 2018; Haghi, 2013; Najafi et al., 2020; Rahim, 2013).
Given that there have been several reports of the outbreak of Enterobius vermicularis in various provinces of Iran and the heterogeneous information available in published articles, this study aims to investigate the overall prevalence of Enterobius vermicularis among children in Iran through a systematic review and meta-analysis.
2. Methods
This systematic review and meta-analysis conducted a systematic search of selected databases. The articles retrieved were then screened, and studies meeting the predefined criteria were chosen by the authors. The relevant information from the selected studies was extracted, analyzed, and finally presented in accordance with the guidelines outlined in the PRISMA 2020 statement.
2.1. Search strategy
A systematic search was conducted across multiple databases, including PubMed, ScienceDirect, SID, and Google Scholar, to identify relevant articles for this study. The selection of keywords for the search was based on previously published primary studies and MESH Terms available in the PubMed database. The choice of keywords followed the PECO criteria, which included the studied population (children in Iran), exposure (Enterobius vermicularis worm), comparison (presence or absence of enterobiasis parasitic infection) and outcome (prevalence of enterobiasis parasitic infection caused by Enterobius vermicularis). The selected keywords were in English, while their Persian equivalents were used when searching in Persian databases. These keywords included comprised terms such as Enterobius, Enterobius vermicularis, Oxyuris vermicularis, Pinworms, Threadworms, and Children. Boolean search operators were used to combine these keywords effectively. The search was conducted without any time limitations, encompassing articles published until November 2022.
The search strategy of different databases is as follows:
((((((Enterobius[Title/Abstract]) OR (Enterobius vermicularis[Title/Abstract])) OR (Oxyuris vermicularis[Title/Abstract])) OR (enterobiasis[Title/Abstract])) OR (Pinworms[Title/Abstract])) OR Threadworms[Title/Abstract]) /Abstract])) AND (Child[Title/Abstract])) OR (Children[Title/Abstract])) AND (Iran[Title/Abstract]))))))))
2.2. Inclusion and exclusion criteria
This review focused on cross-sectional studies that specifically investigated the prevalence of Enterobius vermicularis among children in Iran. Studies such as case studies, case-control studies, cohort studies, clinical trials, systematic reviews, and meta-analyses were excluded from the analysis.
2.3. Study selection and data extraction
After collecting the identified studies using the Endnote software, the review process was initiated by the authors. The evaluations were conducted independently and in a blinded manner. Initially, two authors (EM and MH) assessed the title and abstract of each article based on the predefined inclusion criteria. In cases where there were discrepancies among the authors regarding the eligibility of an article, the final decision was made by a third party (MM).
2.4. Quality evaluation
The quality of observational studies was assessed using the STROBE checklist, which evaluates multiple aspects of study reporting. These aspects include the title, problem statement, study objectives, study type, statistical population, sampling method, data collection tool, and statistical analysis methods. The checklist comprises 32 items used to assess the quality of the studies. Based on these checklist items, each study was assigned a score ranging from 0 to 32. For this particular study, articles scoring 16 or above were considered as medium to good quality studies by the authors and included in the analysis.
2.5. Data analyses
The information extracted from the studies was entered into the Comprehensive Meta-analysis (CMA, Version 2) software. The heterogeneity among the studies was assessed using the I2 test, and the results were analyzed based on the heterogeneity determined by the random effects model. The presence of publication bias was investigated using the Egger test and Funnel plot. A meta-regression analysis was conducted to explore the factors contributing to the observed heterogeneity among the included studies.
3. Results
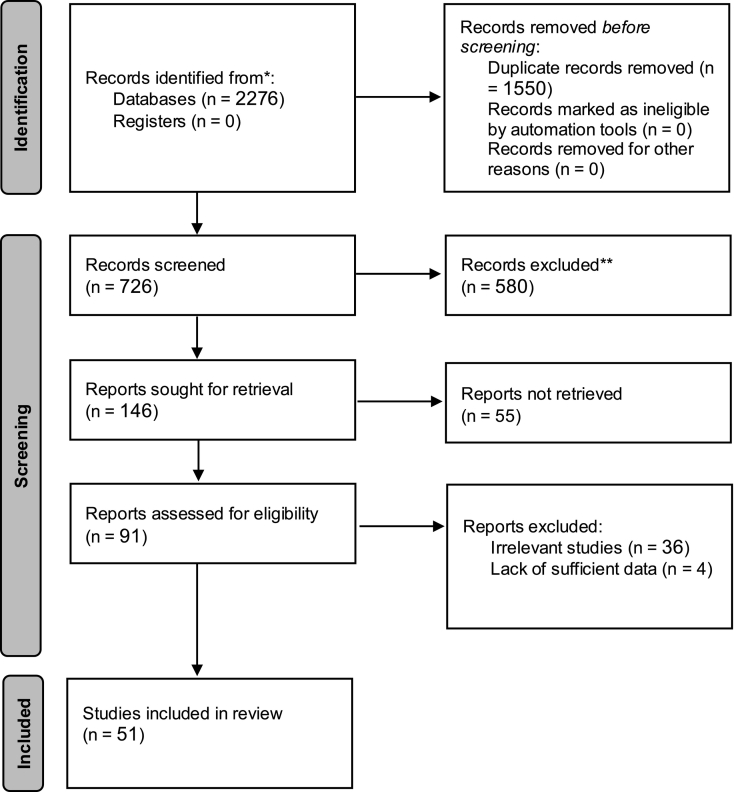
The search conducted across the analyzed databases yielded a total of 100 articles from PubMed, 134 articles from ScienceDirect, 1152 from Google Scholar, and 890 articles from SID. In total, 2276 articles were obtained from all the databases. After removing 1550 duplicate articles and further eliminating 580 articles based on the specified inclusion and exclusion criteria, 146 articles underwent a secondary evaluation. Finally, after excluding 36 articles unrelated to the subject and four articles lacking sufficient information, a total of 51 articles were included in the meta-analysis (Fig. 1 and Table 1).
Fig. 1.
Identification of studies via databases and registers.
Table 1.
Extracted data from analyzed studies.
| Row | Authors | year of publication | Location | age | Sample size | Prevalence |
|---|---|---|---|---|---|---|
| 1 | Fallah (Fallah et al., 2022) | 2021 | Hamadan | – | 688 | 12.5 |
| 12 | Foladvand (Fouladvand et al., 2018) | 2018 | Bushehr | – | 203 | 3.1 |
| 27 | Najafi (Najafi et al., 2020) | 2020 | Tehran | 2–6 | 399 | 22.1 |
| 2 | Zami (Zamini et al., 2016) | 2002 | Marivan | 1–6 | 338 | 41.1 |
| 3 | Fani(Fani et al., 2003) | 2002 | Gonabad | 0–6 | 328 | 15.8 |
| 4 | Moghimi (Moghimi and Sharifi, 2002) | 2002 | Yasouj | – | 300 | 9 |
| 5 | Taherkhani (Taherkhani and Sardarian, 2005) | 2005 | Hamadan | 8.1 | 776 | 20 |
| 6 | Yaghoubi (Yaghoubi, 2016) | 2016 | Gilan | – | 599 | 4.01 |
| 7 | Moshfea (Moshfea and Sharifi, 2000) | 2000 | Yasouj | – | 612 | 23.2 |
| 8 | Hazrati Tapeh (Hazratitape et al., 2007) | 2006 | Urmia | 1–6 | 393 | 4.5 |
| 9 | Hazrati Tapeh (Hazrati Tapeh et al., 2003) | 2002 | Urmia | 1–6 | 830 | 35.4 |
| 10 | Abbasi Fard (Abbasi Fard et al., 2004) | 2004 | Zahedan | – | 415 | 4.7 |
| 11 | Abbasi Fard (Abbasi Fard et al., 2004) | 2004 | Zahedan | – | 438 | 15.5 |
| 13 | Daryani (Daryani et al., 2004) | 2003 | Ardabil | – | 400 | 3.1 |
| 14 | Mouszadeh (Moosazadeh et al., 2017) | 2017 | Iran | – | 11,676 | 17.2 |
| 15 | Atashnafas (Atash Nafas et al., 2007) | 2007 | Semnan | – | 688 | 17.2 |
| 16 | Abedi (Abedi et al., 2004) | 2004 | Isfahan | – | 451 | 0.4 |
| 17 | Rahimi (Rahimi et al., 2015) | 2015 | Shahroud | – | 261 | 2.3 |
| 18 | Sharif (Sharif and ziaie hezar garibi H., 2000) | 2000 | Sari | 2–5 | 811 | 2.1 |
| 19 | Mousaviani (Mosaviani and Sc, 2006) | 2006 | Tehran | 1–6 | 217 | 29.5 |
| 20 | Amiri (Amiri et al., 2016) | 2016 | Babol | – | 351 | 26.4 |
| 21 | Sharifi Maud (Sharifi Moud et al., 2000) | 2000 | Zahedan | – | 126 | 22.2 |
| 22 | Afrakhteh (Afrakhteh et al., 2016) | 2016 | Amol | – | 384 | 31.8 |
| 23 | Kalantari (Kalantari and Mobadi, 2000) | 2000 | Babol | – | 462 | 7.1 |
| 24 | Azad (Soheili Azad et al., 2005) | 2005 | Tehran (Rabat karim) | – | 368 | 33.6 |
| 25 | Baghai (Baghaei et al., 2001) | 2001 | Isfahan (Mobarake) | under 12 years old | 555 | 9.3 |
| 26 | Taheri (Taheri et al., 2004) | 2003 | Birhand | 6 | 650 | 16.1 |
| 28 | Kosha (Koosha, 2006) | 2006 | Tehran | – | 154 | 9 |
| 29 | Nowrozi (Norouzi et al., 2016) | 2016 | Zanjan | – | 1548 | 2.7 |
| 30 | Shahabi (Shahabi, 2000) | 2000 | Tehran (Shahriar) | – | 854 | 1.5 |
| 31 | Rafiei (Rafiei et al., 2000) | 2000 | Tehran (Shahre ray) | – | 1902 | 3 |
| 32 | Mohammadzadeh (Mohammedzadeh et al., 1990) | 1990 | Tehran | – | 1155 | 0.08 |
| 33 | Mohammadzadeh (Mohammedzadeh et al., 1990) | 1990 | Tehran | – | 254 | 0.4 |
| 34 | Mohammadzadeh (Mohammedzadeh et al., 1990) | 1990 | Tehran | – | 1060 | 0.85 |
| 35 | Mohammadzadeh (Mohammedzadeh et al., 1990) | 1990 | Tehran | – | 399 | 648 |
| 36 | Ali Talari (Talari et al., 1998) | 1997 | Kashan | – | 859 | 35.7 |
| 37 | Ali Talari (Talari et al., 1998) | 1997 | Kashan | – | 362 | 20.7 |
| 38 | Eslami Rad (Eslami Rad et al., 1999) | 1999 | Arak | – | 394 | 1.5 |
| 39 | Hazrati tapeh (Hazrati Tappeh et al., 2010) | 2015 | Urmia | – | 405 | 10.6 |
| 40 | Ghahramanloo (Ghahramanlou et al., 2001) | 2001 | Babol | – | 3429 | 0.7 |
| 41 | Sharifi (Sharifi Sarasiabi et al., 2001) | 2001 | Bandar Abbas | – | 1369 | 1.5 |
| 42 | Badparva (Badparva et al., 2009) | 2009 | Lorestan | – | 598 | 33.8 |
| 43 | Farajzadeh (Farajzadeh and Foroughameri, 2003) | 2003 | Birjand | 1–6 | 355 | 14.9 |
| 44 | Ahmad Rajabi (Ahmad-Rajab et al., 2003) | 2003 | Bam | <7 | 370 | 15.9 |
| 45 | Haji Alyani (Haji Aliani et al., 2014) | 2014 | Karaj | 1–6 | 904 | 1.8 |
| 46 | Khademi (Khademi and Arman, 2009) | 2010 | BandarAbbas | <8 | 534 | 0.9 |
| 47 | Dawami (Davami et al., 2008) | 2008 | Jahrom | 7–15 | 410 | 0.4 |
| 48 | Mohammadvand (Mahmoudvand et al., 2020) | 2005 | Lorestan | 2–15 | 366 | 6.8 |
| 49 | Bahadori (Ranjbar-Bahadori et al., 2005) | 2005 | GhaemShahr | <9 | 2145 | 3.9 |
| 50 | Torki (Turki et al., 2017) | 2017 | BandarAbbas | – | 1465 | 0.1 |
| 51 | Daryani (Daryani et al., 2012) | 2021 | Sari | 7–14 | 1100 | 2.2 |
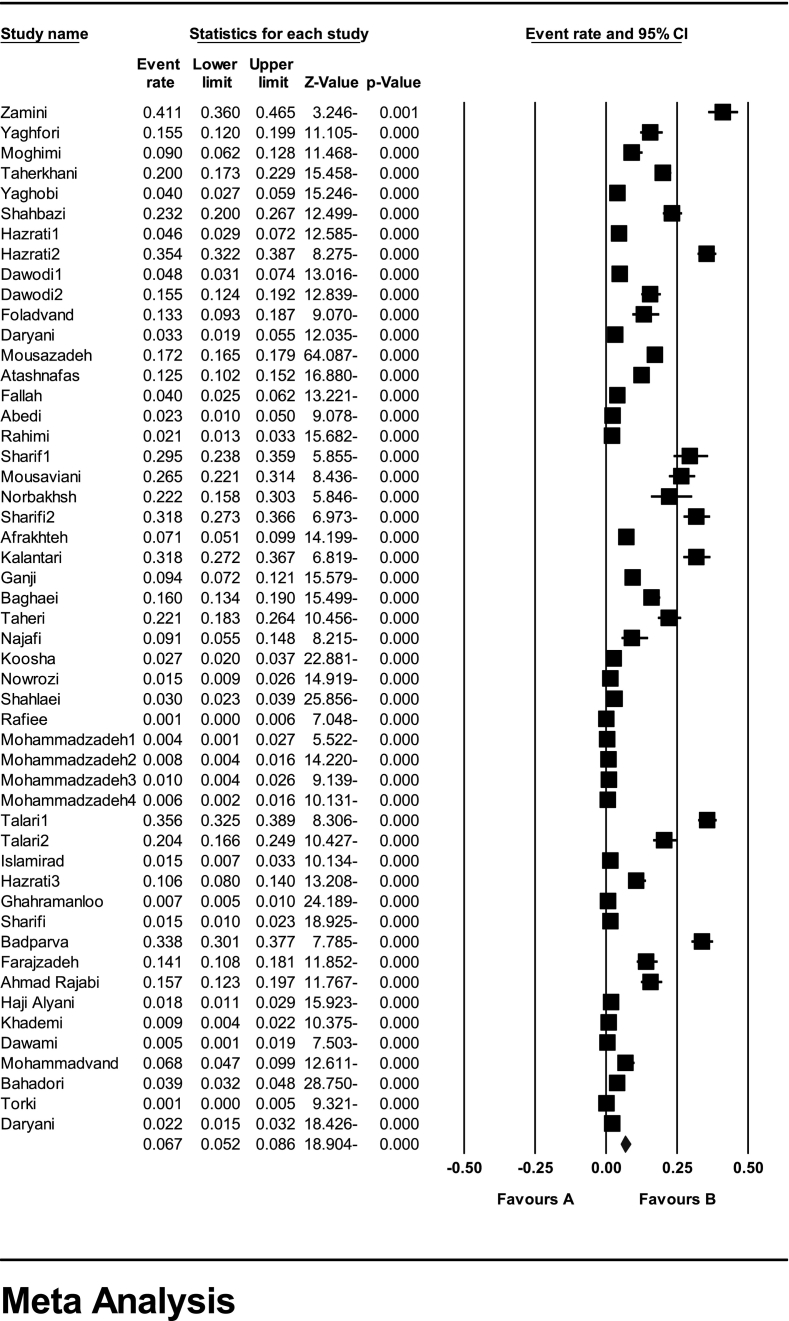
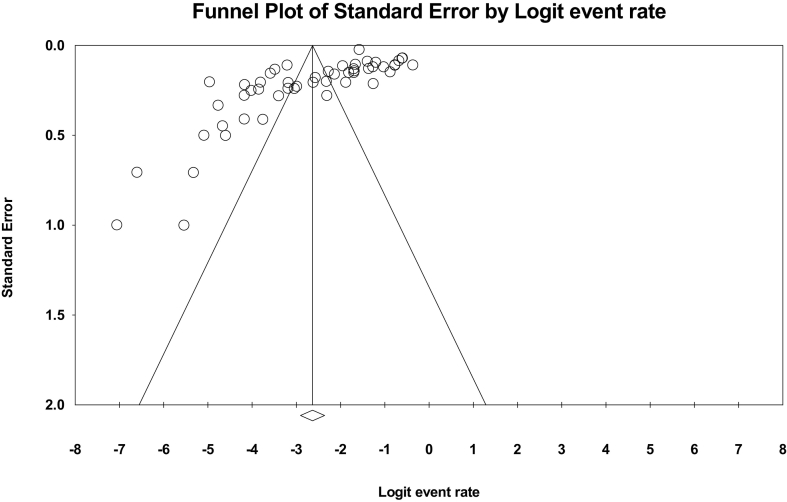
In the systematic review of 51 studies involving a total of 46,070 children, the I2 heterogeneity test showed high heterogeneity (I2: 98.2). Based on this, the random effects method was used to analyze the results. The meta-analysis indicated a prevalence of Enterobius vermicularis in 6.7% (95% CI: 5.2–8.6) of children in Iran (Fig. 2). Furthermore, the presence of publication bias in the studies was assessed using the Egger test, indicating such bias's existence (p-value = 0.0003) (Fig. 3). As a result, caution should be exercised when interpreting the reported prevalence based on the meta-analysis.
Fig. 2.
Forest plot of the prevalence of Enterobius vermicularis among children in Iran based on the random-effects method.
Fig. 3.
Funnel plot of the distribution bias in the reviewed studies.
The investigation of factors influencing the heterogeneity of the studies and the effect of sample size on this heterogeneity revealed that as the sample size increased, the prevalence of Enterobius vermicularis among children in Iran also increased (p-value = 0.578) (Fig. 4). Additionally, an inverse relationship was observed between the prevalence of Enterobius vermicularis among children in Iran and the years of the conducted studies, indicating a decrease in prevalence over time (p < 0.05) (Fig. 5).
Fig. 4.
Meta-regression of the effect of sample size on the prevalence of Enterobius vermicularis in children.
Fig. 5.
Meta-regression of the impact of the year of conducting studies on the majority of Enterobius vermicularis in children.
4. Discussion
According to the findings of this study, the prevalence of Enterobius vermicularis among children in Iran is reported as 6.7%. A previous study by Mouszadeh et al. in 2017 reported a higher prevalence of 17.2%. It is believed that the increase in the overall health level of society will lead to a decrease in the prevalence of this disease in the country (Moosazadeh et al., 2017).
Studies conducted in different regions have reported different prevalence rates for this parasite. For instance, a study in China by Li and colleagues in 2019 found an infection rate of 0.3% among the tested population of 45,427 individuals (Li et al., 2019). In India, a study by Latha Ragunathan and colleagues reported an infection rate of 1.9% among the participants (Ragunathan et al., 2010). Studies conducted in European cities such as Estonia and Slovakia reported 22.8% and 3.5% prevalence rates, respectively (Remm and Remm, 2008; Dudlová et al., 2018).
In Mexico, a study with a sample of 277 people found an infection rate of 18.3% among the participants (de la Luz et al., 2019). Another study conducted in Argentina reported a prevalence rate of 28.4% among the 303 participants (Rivero et al., 2018). In Ethiopia, a study by Tadege and colleagues found a % infection rate of 1% among the collected 600 samples (Tadege et al., 2022). In Egypt, a study by Elmonir et al. reported an infection rate of 8.6% among a total of 996 collected samples (Elmonir et al., 2021). Another report by Chia et al. found that 12.1% of individuals in the Marshall Islands had enterobiasis out of 346 samples collected (Fan et al., 2021).
Enterobius vermicularis is the only parasite from the Oxyurida family known to infect humans and cause various diseases. The presence of this worm in the human body directly leads to clinical symptoms of enterobiasis. This can result in fatigue, lack of concentration, reduced efficiency, and increased susceptibility to other diseases in children. Enterobius vermicularis is the only parasite from the Oxyurida family known to infect humans and cause various diseases. The presence of this worm in the human body directly leads to clinical symptoms of enterobiasis. This can result in fatigue, lack of concentration, reduced efficiency, and increased susceptibility to other diseases in children. Treating this infectious agent and the diseases it causes can impose significant costs on the healthcare sector (Rivero et al., 2018; Tadege et al., 2022; Elmonir et al., 2021; Fan et al., 2021).
Since this infection does not require an intermediate host, its transmission can occur rapidly. Neglecting personal and social hygiene contributes to its widespread occurrence and incurs substantial healthcare costs for treating the disease and its associated complications (Fouladvand et al., 2018). Factors such as the educational level and occupation of parents, place of residence, number of family members, and the social and economic status of the family, along with government policies, are believed to play influential roles in the spread of this infectious agent in society (de la Luz et al., 2019; Rivero et al., 2018; Tadege et al., 2022; Elmonir et al., 2021; Fan et al., 2021).
Implementing public education programs for children in kindergartens and schools, screening suspicious samples, prompt treatment and providing advice and health tips to parents can significantly reduce the likelihood of outbreaks in the community.
4.1. Limitation
The present study has several limitations, the most significant being publication bias in the reviewed studies. This bias affects the reliability of the results, and therefore caution is advised when interpreting them.
This study has comprehensively collected data from studies until 2022. By updating the results and using meta-regression analysis, the impact of two factors, namely sample size and year of publication, on the observed heterogeneity of 98.2% has been investigated. This approach enhances the reliability of the results and provides valuable insights for healthcare policymakers.
5. Conclusion
The present study shows a relatively high prevalence of Enterobius vermicularis among children in Iran. We urge health policymakers to recognize the significance of this issue and take necessary measures to reduce the occurrence of this infectious agent in children. Implementing effective educational programs and preventive measures through mass media channels is important to address this public health concern more efficiently.
Ethics approval and consent to participate
Ethics approval was received from the ethics committee of the Deputy Vice Chancellor of Research and Technology Gerash University of Medical Sciences (IR.GERUMS.REC.1401.012). All methods were performed in accordance with the ethical standards as laid down in the Declaration of Helsinki. The present study was a review, and informed consent was not required.
Consent for publication
Not applicable.
Funding
By Deputy for Research and Technology, Gerash University of Medical Sciences (IR) (401000018). This deputy has no role in the study process.
Authors contributions
MM and NS contributed to the design and MM statistical analysis and participated in most of the study steps. MM, EM, MmH, AA, AK, MvH, and SHSH prepared the manuscript. All authors have read and approved the content of the manuscript.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study is the result of research project No. 401000018, approved by the Student Research Committee of Gerash University of Medical Sciences. We would like to thank the esteemed officials of the centre for the financial affords of this study.
Data availability
Datasets are available through the corresponding author upon reasonable request.
References
- Abbasi Fard S., Yazdanshenas M., Motayerzadeh S., Abbasi Fard A. Intestinal parasitic infections in Zahedan daycare units. Zahedan J. Res. Med. Scie. (Tabib-E-Shargh) 2004;6(2):123–128. [Google Scholar]
- Abedi S., Eezadi Sh., Davari B. Prevalence of Oxyuriasis in kindergartens of Isfahan, Iran. Hormozgan Med. J. 2004;8(1):63–66. [Google Scholar]
- Afrakhteh N., Marhaba Z., Mahdavi S.A., Garoosian S., Mirnezhad R., Vakili M.E., Shahraj H.A., Javadian B., Rezaei R., Moosazadeh M. Prevalence of Enterobius vermicularis amongst kindergartens and preschool children in Mazandaran Province, North of Iran. J. Parasit. Dis. 2016;40(4):1332–1336. doi: 10.1007/s12639-015-0683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad-Rajab R., Varzandeh F., Arab M., Abbaszadeh A. Prevalence of intestinal parasites infections in the day care centers of bam. JRUMS. 2003;2(2):102–111. [Google Scholar]
- Amiri S.A., Rahimi M.T., Mahdavi S.A., Moosazadeh M., Ramzani O., Koshk A.F., Rosbehan R., Siyadatpanah S.A. Prevalence of Enterobius vermicularis infection among preschool children, Babol, North of Iran. J. Parasit. Dis. 2016;40(4):1558–1562. doi: 10.1007/s12639-015-0727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atash Nafas E., Raheb Ghorbani, Peyvandi S., Imani E. Prevalence of Oxyuriasis and some related factors in kindergarten and primary school children in urban areas of Semnan Province. Koomesh. 2007;9(1):67–74. [Google Scholar]
- Badparva E., Fallahi S., Aminizadeh H., Ebrahim zadeh F. Prevalence of Enterobious vermicolaris in the primary school students of Kouhdasht rural regions in the academic year of 2007-2008. Iran S. Med. J. 2009;12(1):75–80. [Google Scholar]
- Baghaei M., Daneshvar Farzanegan P., Mirlouhi M., Mahmoudi M. Intestinal parasitic prevalence in rural area children Mobarakeh-Isfahan -1997. J. Res. Med. Scie. (JRMS) 2001;6(2):104–107. [Google Scholar]
- Daryani A., Etehad Gh H., Abyar B. Enterobius Vermicularis infection among children going to daycare Centers in Ardabil. J. Ardabil Univ. Med. Sci. (Jaums) 2004;3(2):18–22. [Google Scholar]
- Daryani A., Sharif M., Nasrolahei M., Khalilian A., Mohammadi A., Barzegar G. Epidemiological survey of the prevalence of intestinal parasites among schoolchildren in Sari, northern Iran. Trans. R. Soc. Trop. Med. Hyg. 2012 Aug;106(8):455–459. doi: 10.1016/j.trstmh.2012.05.010. Epub 2012 Jun 15. PMID: 22703897. [DOI] [PubMed] [Google Scholar]
- Davami M.H., Roohi R., Sadeghi A.R. The prevalence of intestinal parasitic infections among 7-15-year-old children in Jahrom, Iran during 2006-7. jmj. 2008;6(1 and 2):49–55. [Google Scholar]
- de la Luz Galván-Ramírez M., Madriz-Elisondo A.L., Ramírez C.G.T., de Jesús Romero Rameño J., Carrasco D.A. De la O., MAC López. Enteroparasitism and risk factors associated with clinical manifestations in children and adults of Jalisco state in Western Mexico. Osong Pub. Health Res. Perspect. 2019;10(1):39–48. doi: 10.24171/j.phrp.2019.10.1.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudlová A., Juriš P., Jarčuška P., Vasilková Z., Vargová V., Sumková M., Krčméry V. The incidence of pinworm (Enterobius vermicularis) in preschool and school aged children in Eastern Slovakia. Helminthologia. 2018;55(4):275–280. doi: 10.2478/helm-2018-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmonir W., Elaadli H., Amer A., El-Sharkawy H., Bessat M., Mahmoud S.F., Atta M.S., El-Tras W.F. Prevalence of intestinal parasitic infections and their associated risk factors among preschool and school children in Egypt. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0258037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami Rad Z., Mosayyebi M., Khazaei M.R. An investigation on the prevalence of intestinal parasites in Arak City. J. Arak Univ. Med Sci. 1999;2(8):1–4. [Google Scholar]
- Fallah M., Parsaei M., Soleymani E., Jamshizad A., Azimi A. Investigation of the prevalence of Enterobius vermicularisInfection and risk factors among kindergartens in Hamadan, west of Iran, in 2019. Avicenna J. Clin. Med. 2022;28(4):253–259. [Google Scholar]
- Fan C.K., Sonko P., Lee Y.L., Yin A.W., Chuang T.W., Kios R., Wang Y.T., Chou C.M., Hsu S.L., Wu M.S., Lin J.W., Tu C.Y. Epidemiologic study of Enterobius vermicularis infection among schoolchildren in the republic of Marshall Islands. J. Trop. Med. 2021;2021 doi: 10.1155/2021/6273954. 6273954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani M.J., Minoian M.H., Motalebi M. The survey of Enterobiusis prevalence in nursery homes and Kgs of Gonabad City with considering their demographic data in 1381. Ofogh-E-Danesh. 2003;8(2):1–6. [Google Scholar]
- Farajzadeh Z., Foroughameri G. Study of enterobiasis prevalence in 1-6 years old children of day - care centers in Birjand city. J. Birjand Univ. Med. Sci. 2003;10(2):9–15. [Google Scholar]
- Fouladvand M.A., Heydari A., Barazesh A. Prevalence of Enterobius vermicularis in Primary School Children of Bushehr, Iran in 2011. Iran S. Med J. 2018;21(2):125–133. [Google Scholar]
- Ghahramanlou M., Hassanjani Roshan M.R., Haji Ahmadi M. Prevalence of intestinal parasites in primary school children (Eastern Bandpay, Babol, 1999) J. Babol Univ. Med. Sci. (JBUMS) 2001;3(2):47–51. [Google Scholar]
- Haghi Seyyed Mousa Motevalli. Prevalence of Enterobius vermicularis infection among kindergartens in Mazandaran Province. J. Mazand Univ. Med. Sci. 2013;23(Supple 1):241–247. [Google Scholar]
- Haji Aliani F., Einipor S., Abadi A., Tahvildar Bidrouni F. Consideration of intestinal parasite in daycare center children in Karaj City in 2012. aumj. 2014;3(4):239–252. [Google Scholar]
- Hazrati Tapeh Kh., Salari Sh., Elmasi R., Mohammadzadeh H. Prevalence of children infected by Oxyuris in Orumiyeh kindergartens and the ways to control that. Sci. J. Kurdistan Univ. Med. Sci. 2003;7(2):29–36. [Google Scholar]
- Hazrati Tappeh K., Mohammadzadeh H., Khashaveh S., Rezapour B. Prevalence of intestinal parasitic infections among primary school students in Barandooz-Chay rural region of Urmia, 2007. Stud. Med. Sci. 2010;21(3):237–242. [Google Scholar]
- Hazratitape Kh., Salari Sh., Alavi S., Tankhahahi B. Spreading of Oxyuris and effective factors on that in Urmia kindergartens. Stud. Med. Sci. (J. Urmia Univ. Med. Sci.) 2007;17(4):273–277. [Google Scholar]
- Kalantari N., Mobadi I. Enteral parasitic contamination in Babol daycare centre, 1997. JBUMS. 2000;2(5):57–60. [Google Scholar]
- Sharifi Sarasiabi K.H., Madani A.B.D. Alhossein, Zare S.H. Prevalence of intestinal parasites in primary school pupils of Bandar Abbas. Hormozgan Med. J. 2001;5(4):25–30. [Google Scholar]
- Khademi Seyedah Zahra, Arman Mitra. Prevalence of intestinal parasites in children under 8 years of age in kindergartens and schools in Bandar Abbas. Infect. Trop. Dis. J. 2009;15(51):31. [Google Scholar]
- Koosha S. The effect of two educational methods to prevent re-infection of students with intestinal parasites in Tehran. Pajohandeh Mag. (Res. Bull. Med. Sci.) 2006;11(3):183–186. [Google Scholar]
- Li S.S., Wang L., Li A.H. Surveillance of soil-transmitted nematode infections in Zhenjiang City from 2006 to 2018. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2019;32(1):83–86. doi: 10.16250/j.32.1374.2019137. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand H., Badparva E., Khalaf A.K., Niazi M., Khatami M., Nazer M.R. Prevalence and associated risk factors of intestinal helminthic infections in children from Lorestan province, Western Iran. Parasite Epidemiol. Control. 2020 Jan 9;9 doi: 10.1016/j.parepi.2020.e00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi M., Sharifi A. Prevalence of intestinal parasites in preschool children of Yasuj (1380-1381) Armaghan Danesh. 2002;7(26):41–44. [Google Scholar]
- Mohammedzadeh H., Moubedi I., Mahmoudi M., Begham Kiya E., Dalimi A., Halakui K. Determining the prevalence of intestinal parasitic infections in elementary students of Tehran (second phase) Res. Technol. Deputy (Cent. Off.) 1990;9(1):182. [Google Scholar]
- Moosazadeh M., Abedi G., Afshari M., Sa Mahdavi, Farshidi F., Kheradmand E. Prevalence of Enterobius Vermicularis among children in Iran: a systematic review and Meta-analysis. Osong Pub. Health Res. Perspect. 2017;8(2):108–115. doi: 10.24171/j.phrp.2017.8.2.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaviani Z., Sc M. Contamination with Oxyure and Giardia in children of kindergartens and welfare Centers in Tehran. Avicenna J. Nurs. Midwifery Care. 2006;14(1):40–50. [Google Scholar]
- Moshfea A., Sharifi A. Prevalence of intestinal parasites in primary school students of Yasuj. Armaghan Danesh. 2000;5(17–18):1–9. [Google Scholar]
- Najafi E., Rafiei Sefiddashti R., Rampisheh Z., Amni F., Hadighi R. Prevalence of Enterobius vermicularis infection in 2-6 years old children in affiliate kindergartens of Iran University of Medical Sciences, Tehran, Iran, 2018-2019. Razi J. Med. Sci. 2020;27(3):159–167. [Google Scholar]
- Norouzi R., Nourian A.A., Hanilo A., Kamali K. Prevalence of intestinal parasites among primary school students in Zanjan City (2013) J. Adv. Med. Biomed. Res. 2016;24(102):121–130. [Google Scholar]
- Rafiei M., Torkaman M., Sharbatdar Alaei M. Asymptomatic giardiasis in school children in Rey city. Tehran Univ. Med. J. 2000;58(1):82–86. [Google Scholar]
- Ragunathan L., Kalivaradhan S.K., Ramadass S., Nagaraj M., Ramesh K. Helminthic infections in school children in Puducherry, South India. J. Microbiol. Immunol. Infect. 2010;43(3):228–232. doi: 10.1016/S1684-1182(10)60036-9. [DOI] [PubMed] [Google Scholar]
- Rahim H. Frequency of Giardia lamblia and Enterobius vermicularis infection in Shahroud kindergartens. J. Ardabil Univ. Med. Sci. 2013;15(1):7–14. [Google Scholar]
- Rahimi H., Dehghani M., Norouzi P., Fazli M. Frequency of Giardia lamblia and Enterobius Vermicularis infections in Shahroud kindergartens, 2013. J. Ardabil Univ. Med. Sci. (Jaums) 2015;15(1):7–14. [Google Scholar]
- Ranjbar-Bahadori Sh., Dastorian A.R., Heidari B. Prevalence of intestinal parasites in Ghaemshahr in 2004. Med. Sci. 2005;15(3):151–155. [Google Scholar]
- Remm M., Remm K. Case-based estimation of the risk of enterobiasis. Artif. Intell. Med. 2008;43(3):167–177. doi: 10.1016/j.artmed.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Rivero M.R., De Angelo C., Nuñez P., Salas M., Liang S. Intestinal parasitism and nutritional status among indigenous children from the Argentinian Atlantic Forest: determinants of enteroparasites infections in minority populations. Acta Trop. 2018;187:248–256. doi: 10.1016/j.actatropica.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Shahabi S. Epidemiologic survey of intestinal parasites in school children of Shahriar in 1993. Res. Med. 2000;24(2):133–139. [Google Scholar]
- Sharif M., ziaie hezar garibi H. Study the rate of oxyuris vermicularis and vulvitis in children of 2-5 years of age in sari township kindergarten in 1378. J. Mazandaran Univ. Med. Sci. 2000;10(27):59–65. [Google Scholar]
- Sharifi Moud B., et al. Determining the frequency of Oxyurida infection in Zahedan schools in 1379 with scotch tape. Zahedan J. Res. Med. Sci. 2000;2(3–4):71–75. [Google Scholar]
- Soheili Azad A., Nourjah N., Shahbazi F. Relationship between parasite infection and malnutrition in Robat Karim elementary school students. RJMS. 2005;12(45):87–96. [Google Scholar]
- Tadege B., Mekonnen Z., Dana D., Tiruneh A., Sharew B., Dereje E., Loha E., Ayana M., Levecke B. Assessment of the nail contamination with soil-transmitted helminths in schoolchildren in Jimma Town, Ethiopia. PLoS One. 2022;17(6) doi: 10.1371/journal.pone.0268792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri F., Fesharekinia A., Saadatjoo S.A. Study of prevalence of intestinal parasitic infection among 6-year-old children in Birjand. J. Isfahan Med. School (IUMS) 2004;21(71):35–39. [Google Scholar]
- Taherkhani H., Sardarian Kh. Enterobiasis in children of schools in Hamadan City. Iran. J. Infect. Dis. Trop. Med. 2005;10(30):49–53. [Google Scholar]
- Talari S.A., Arbabi M., Vali G.R. Prevalence of Oxyuriasis among children in Kashan mountainous and desert regions. Feyz. 1998;1(4):49–54. [Google Scholar]
- Turki H., Hamedi Y., Heidari-Hengami M., Najafi-Asl M., Rafati S., Sharifi-Sarasiabi K. Prevalence of parasitic intestinal infection among primary school children in southern Iran. J. Parasit. Dis. 2017 Sep;41(3):659–665. doi: 10.1007/s12639-016-0862-6. Epub 2016 Nov 23. PMID: 28848255; PMCID: PMC5555908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghoubi K. Shahid Beheshti University of Medical Sciences School of Pharmacy Library. 2016. Investigating the prevalence of intestinal parasites among primary school students in Astara city in Gilan province and comparing the therapeutic effect of Mebendazole and Metronidazole in patients with Giardiasis.http://dsp.sbmu.ac.ir/handle/123456789/46223 Available at: [Google Scholar]
- Zamini G., Khadem-Irfan M.B., Karimi M.N., Faridi A. Prevalence of Entrobius vermicularis (pinworm) infection and its relationship with clinical manifestations of oxyurosis, in children between 1 and 6 years of age in Oraman region of Marivan. SJKU. 2016;21(3):26–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available through the corresponding author upon reasonable request.