Abstract
Objective
During cardiac arrest, current guidelines recommend attempting intravenous access first and to consider intraosseous access if intravenous access is unsuccessful or impossible. However, these recommendations are only based on very low-certainty evidence. Therefore, the “Intravenous vs Intraosseous Vascular Access During Out-of-Hospital Cardiac Arrest“ (IVIO) trial aims to determine whether there is a difference in patient outcomes depending on the type of vascular access attempted during out-of-hospital cardiac arrest. This current article describes the clinical IVIO trial.
Methods
The IVIO trial is an investigator-initiated, randomised trial of intravenous vs. intraosseous vascular access during adult non-traumatic out-of-hospital cardiac arrest in Denmark. The intervention will consist of minimum two attempts (if unsuccessful on the first attempt) to successfully establish intravenous or intraosseous vascular access during cardiac arrest. The intraosseous group will be further randomised to the humeral or tibial site. The primary outcome is sustained return of spontaneous circulation and key secondary outcomes include survival and survival with a favourable neurological outcome at 30 days. A total of 1,470 patients will be included.
Results
The trial started in March 2022 and the last patient is anticipated to be included in the spring of 2024. The primary results will be reported after 90-day follow-up and are anticipated in mid-2024.
Conclusion
The current article describes the design of the Danish IVIO trial. The findings of this trial will help inform future guidelines for selecting the optimal vascular access route during out-of-hospital cardiac arrest.
Keywords: Out-of-hospital cardiac arrest, Intravenous, Intraosseous, Vascular access, Randomised trial
Introduction
Out-of-hospital cardiac arrest (OHCA) occurs in an estimated 4 million people each year globally of which approximately 5,000 happen in Denmark.1, 2 OHCA is a detrimental condition with an extremely low survival rates with only approximately 14% being alive after 30 days in Denmark.1, 3 Of those with a non-shockable rhythm, which accounts for more than 80% of all OHCA, less than 10% are alive after 30 days, and in contrast to those with a shockable rhythm, survival has not improved substantially over the last decade.1, 3
The treatment of OHCA consists of basic and advanced life support, where current guidelines support the use of pharmacological treatments during cardiac arrest.4, 5 This include amiodarone/lidocaine and adrenaline for patients with a refractory shockable rhythm, and adrenaline for patients with a non-shockable rhythm.4, 5 Adrenaline triples the rate of return of spontaneous circulation (ROSC) and increases overall 30-day survival by approximately one-third, while amiodarone and lidocaine increase short-term outcomes, albeit with uncertain effect on long-term survival.6, 7 Given the favourable role of administered drugs during cardiac arrest, the type and location of vascular access may be of importance to patient outcomes. Current guidelines recommend to attempt intravenous access first and to consider intraosseous access if intravenous access is unsuccessful or not possible.4, 5 However, this recommendation is based on very low-certainty evidence.8 Therefore, the “Intravenous vs. Intraosseous Vascular Access During Out-of-Hospital Cardiac Arrest (IVIO)” randomised clinical trial aims to determine whether there is a difference in patient outcomes depending on the type of vascular access (intravenous or intraosseous) attempted during OHCA.
Methods
Protocol
The full trial protocol is provided in the Supplemental Material, and all previous versions are available on the trial’s website.9 The trial and the protocol was developed in accordance with the International Conference on Harmonization (ICH) guidelines10, 11, 12 and the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement.13, 14 The trial was prospectively registered at the EU Clinical Trials Register (EudraCT Number: 2021-005922-82) on 30 October 2021, updated to EU Clinical Trials number: 2022-500744-38-00 on 4 July 2022, and ClinicalTrials.gov (Identifier: NCT05205031) on 24 January 2022.
Design
The IVIO trial is an investigator-initiated, randomised, parallel group, patient and follow-up outcome assessor-blinded, superiority trial of intravenous vs. intraosseous vascular access during adult OHCA. The intraosseous group will be further randomised to humeral or tibial access.
Setting
Thís nationwide trial will be conducted in the pre-hospital setting in Denmark, where emergency medical technician-manned ambulances can attempt and establish intravenous vascular access, while three distinct units can attempt and establish both intravenous and intraosseous vascular access during OHCA: physician-manned vehicles, paramedic-manned ambulances or vehicles, and helicopter emergency medical services (HEMS). HEMS will not participate in the trial. OHCA patients that receive their initial vascular access by HEMS or an emergency medical technician-manned ambulance will not be eligible. A list of participating centres is provided in the full protocol in the Supplemental Material.
Eligibility criteria
Patients will be included based on the following criteria: OHCA (defined as an unconscious patient with abnormal breathing, and a loss of pulses requiring chest compressions where the prehospital system is activated), age ≥18 years, and indication for intravenous or intraosseous vascular access during cardiac arrest. Exclusion criteria are blunt trauma, penetrating trauma, or burn injury suspected to be the cause of the cardiac arrest, prior enrolment in the trial, and a working intravenous or intraosseous vascular access already in place at the time of randomisation.
Interventions
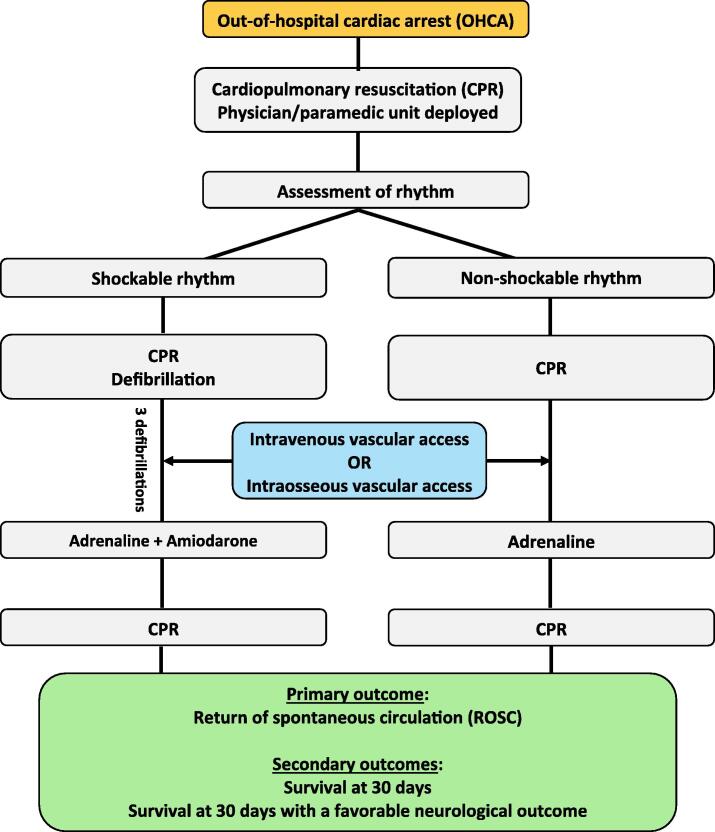
The intervention will consist of attempts to successfully establish an intravenous or intraosseous vascular access during the cardiac arrest, and this will happen as soon as possible after the patient has been randomised by opening an opaque envelope. Fig. 1 shows the trial flow chart. The pre-hospital clinician will be required to attempt the intervention twice at a minimum, if the first attempt is unsuccessful. An attempt is defined by skin penetration with the peripheral venous catheter or the intraosseous needle, and a successfully established peripheral venous or intraosseous access will be assessed by clinical judgement. Protocol deviations will be monitored and registered.
Fig. 1.
Trial flow chart.
Allocation
Patients will be randomised in a 1:1 ratio to either intravenous or intraosseous vascular access. The intraosseous group will be further randomised to the humeral or tibial site in a 1:1 ratio. The full allocation ratio will therefore be 2:1:1 for intravenous access, tibial intraosseous access, and humeral intraosseous access. Envelopes containing the allocation will be opened on site of the cardiac arrest when the patient is deemed to fulfil all inclusion criteria and none of the exclusion criteria, thus ensuring allocation concealment.
Blinding
Due to the nature of the intervention, the pre-hospital clinicians performing the intervention cannot be blinded. Patients along with any legally designated representatives will be blinded. As it is common to note vascular access during cardiac arrest in the patient’s medical records, clinicians involved in post-resuscitative care will not necessarily be blinded either. For the same reason, investigators involved in data entry will also not be blinded meaning that outcomes such as ROSC and mortality are entered without blinding. However, there is little – if any – subjectivity in evaluating these outcomes and blinding is therefore likely to be of minor importance.15, 16, 17 Outcome assessors performing the follow-up interviews will be blinded.
Outcomes
The primary outcome will be sustained ROSC, defined as palpable pulses or other signs of circulation without the need for chest compression for at least 20 minutes.
Key secondary outcomes include survival and neurological outcome at 30 days. Neurological outcome will be assessed with the modified Rankin Scale (mRS); scores 0–6 will be presented as counts and percentages, while the outcome will be dichotomised as favourable (mRS 0–3) vs. unfavourable (mRS 4–6).
Several additional outcomes are also collected, with a full list provided in the Supplemental Material. These include survival, neurological outcome, and quality of life at 30, 90, 180 days, and one year after the cardiac arrest. Health-related quality of life will be assessed by the EQ-5D-5L questionnaire,18 which is supported by the AHA19 as well as the COSCA-initiative.20 Specific adverse events are also collected. The outcomes adhere to the “Core Outcome Set for Cardiac Arrest” (COSCA) guidelines.20
Sample size
The sample size is based on the primary outcome of sustained ROSC to detect group differences between intravenous and intraosseous access, and the trial is therefore not powered to detect differences within the intraosseous subgroup (humeral vs. tibial).
In our setting, we have previously found a sustained ROSC-rate of 27% in an adult non-traumatic OHCA population who received at least one dose of adrenaline.21 The original sample size was calculated assuming an overall ROSC-rate of 27% with 31.5% in the beneficial intervention group and 22.5% in the harmful intervention group (relative treatment effect of 40%). Based on a chi-squared test and an alpha of 0.05, a sample size of 762 patients (i.e., 381 in each group) was needed to obtain 80% power.
The sample size was updated and increased in October 2022, after additional funding made expansion to additional sites possible. The updated sample size was calculated with the same assumptions of the overall ROSC-rate, but assuming ROSC-rates with 30.5% in the beneficial intervention group and 23.5% in the harmful intervention group enabling us to detect an increased relative treatment effect of 30%. Based on a chi-squared test and an alpha of 0.05, a sample size of 1,262 patients (i.e., 631 in each group) was needed to obtain 80% power.
Based on data from the pre-planned interim analysis after the first 400 included patients, the overall proportion with the primary outcome of sustained ROSC was 34%, which was higher than anticipated (an overall sustained ROSC rate of 27% as noted above). Given the new estimate and a fast inclusion rate, we re-estimated and increased the sample size on 14 June 2023. Assuming ROSC rates of 30% and 38% in the two groups (corresponding to a relative treatment effect of approximately 27%), and 90% power, 1,470 patients are needed. The final sample size is therefore 1,470 patients.
Statistical analysis plan
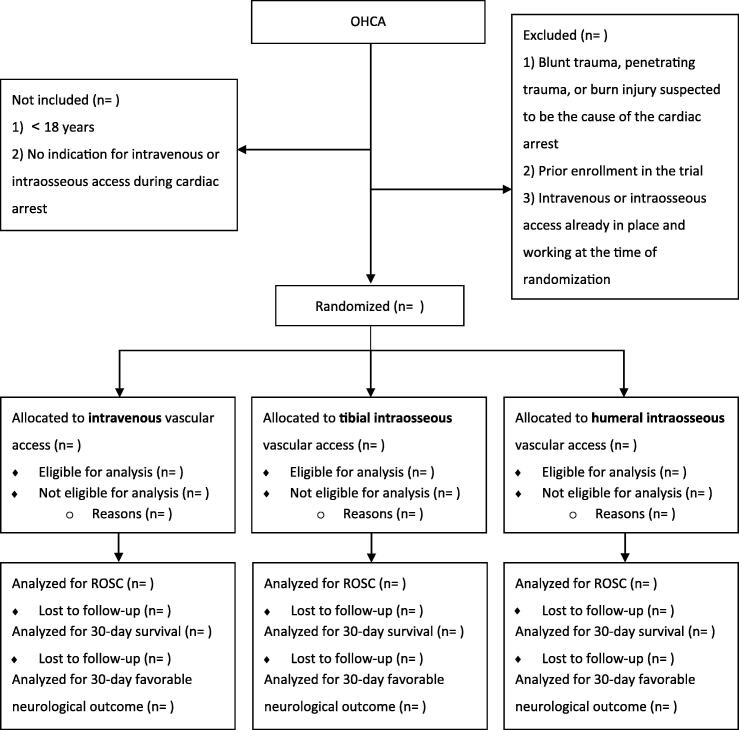
A detailed statistical analysis plan is provided in the protocol provided in the Supplemental Material.9 The reporting will adhere to the Consolidated Standards of Reporting Trials (CONSORT)-guidelines.22, 23 Fig. 2 shows the proposed CONSORT flow diagram.
Fig. 2.
Draft of CONSORT flow diagram OHCA: Out-of-hospital cardiac arrest, ROSC: Return of spontaneous circulation.
All analyses will, unless noted otherwise, be conducted on a modified intention-to-treat basis including all randomised patients that met all inclusion criteria as well as no known exclusion criteria at the time of randomisation. The patient is considered randomised once the randomisation envelope has been opened. Patients will be analysed according to their assigned group.
The primary and secondary outcomes (binary variables) will be presented as counts and proportions in each group. Results will be reported as both risk ratios and risk differences. P-values will be obtained from Fisher’s Exact test.
Three sensitivity analyses will be performed. Firstly, we will estimate the risk ratio with 95% confidence intervals while adjusting for strong prognostic factors including age, whether the cardiac arrest was witnessed, whether early cardiopulmonary resuscitation (CPR) was initiated or not (i.e., bystander CPR or EMS-witnessed arrest), and the initial rhythm.24, 25, 26, 27 Secondly, we will exclude patients who were randomised but did not receive any attempt at intravascular access (i.e., at least one attempt at any modality of vascular access during the cardiac arrest). Thirdly, per-protocol analyses will be conducted, including patients who received attempts with the allocated modality.
Subgroup analyses will be performed on both the absolute and relative scale. The analyses will include five pre-defined subgroup analyses for the primary and key secondary outcomes according to 1) initial rhythm (shockable vs. non-shockable), 2) whether or not the OHCA was witnessed by a bystander, 3) whether or not the OHCA was witnessed by the ambulance staff, 4) whether or not bystander CPR was performed, and 5) sex.
Secondary Bayesian analyses will be performed for the primary and key secondary outcomes in order to aid interpretation of the results.28 Given the limited evidence on vascular access during cardiac arrest, we will primarily use noninformative prior probability distributions and the results obtained from the trial to obtain posterior probability distributions for risk ratios. Sceptical, neutral, and optimistic prior probability distributions will also be used consistent with a recent trial by our group.21.
Interim analyses will be conducted by an independent data-monitoring committee at four predetermined milestones (50, 200, 400, and 800 enrolled patients, respectively). There are no formal stopping criteria for efficacy, futility, or safety.
Data collection and follow-up
Data on trial ID, number of attempts with the allocated intervention, type of vascular access with initial success, time of initial successful vascular access, time of first adrenaline during cardiac arrest, displacement or other problems with the vascular access until hospital arrival, and the primary outcome ROSC will be obtained from the pre-hospital team through a trial-specific case report form. Additional data will be manually obtained from the electronic medical records by trained research staff. A centrally-located, trained, and blinded researcher will assess mRS and health-related quality of life using a standardised telephone interview, which ensures good reliability and validity.29, 30, 31, 32 Details of the included variables and their definitions are provided in the Data Dictionary on the trial website.9
Clinical treatment
The clinical management of the included patients (other than the interventions) will be at the discretion of the treating pre-hospital and in-hospital teams to test the interventions in a real-life clinical scenario. In general, management will adhere to the intra- and post-cardiac arrest guidelines provided by the European Resuscitation Council33 and the Danish Resuscitation Council34, but no specific treatments will be prohibited or mandated and further treatment will not be monitored. There will be no general restrictions on patient entry into other clinical trials, although this will be evaluated on a case-by-case basis.35
Ethical considerations and consent
A detailed description of the ethical considerations is provided in the protocol located in the Supplemental Material. The trial was approved by the regional ethics committee (case number: 1-10-72-347-21) on 13 January 2022 and the Danish Medicine Agency (EudraCT Number: 2021-005922-82) on 30 October 2021. The trial was initiated 1 March 2022. On 1 February 2023, the trial transitioned to the European Regulation No. 536/2014.
For patients who survive to hospital admission, but remain unable to provide consent, written consent from a legally designated representative is obtained as soon as possible. If the patient regains consciousness, written consent is obtained from the patient.
Data sharing
Six months after the publication of the last results, all de-identified individual patient data will be made available for data sharing.36 Procedures, including re-coding of key variables, will be put in place to allow for complete de-identification of the data.
Discussion
The current article, along with the full protocol available in the Supplemental Material, describes the design of the IVIO trial. The objective of this trial is to compare the effect of intravenous vs. intraosseous access during OHCA on patient outcomes.
Although the effectiveness of drugs during cardiac arrest has been challenged,37 recent randomised clinical trials have yielded evidence in favour of using both adrenaline and amiodarone/lidocaine.6, 7 Given the favourable role of drugs in the management of cardiac arrest, the type and location of vascular access could potentially impact patient outcomes for several reasons. Firstly, time to successful vascular access could have an impact as earlier drug administration may lead to increased survival.7, 38 Secondly, there are substantial hemodynamic changes during cardiac arrest, resulting in plasma concentrations of endogenous adrenaline increase up to 300-fold during cardiac arrest39 which increases blood flow to the heart and brain40, 41, 42 while constricting blood to visceral organs40, 42, 43 and the peripheral circulation. Furthermore, the venous return during closed-chest compressions may primarily come via the superior caval system.44 Therefore, the anatomical placement of the vascular access may be important.
Both intravenous and intraosseous access have advantages and disadvantages. When a drug is administered into a healthy circulation through an upper limb intravenous catheter, clinical effects are noted as early as 20 seconds after injection.45 The disadvantage of peripheral venous catheter placement during OHCA lies in the initial success rate, where first attempt success rate seems much lower than both the tibial and the humeral access.46 While intraosseous access requires identification of anatomic landmarks, it does not – like the peripheral venous access – require placement of a proximal tourniquet nor identification of a blood-filled peripheral vein. Various anatomical locations are available for intraosseous access and significant differences are described in both first attempt success rate of tibial vs. humeral intraosseous access (95% vs. 71%) as well as mean time to initial success (4.6 vs. 7.0 minutes after arrival on site).46 Furthermore, the tibial access was found to displace less often than the humeral (5% vs. 20%), but not significantly different to peripheral venous access (5% vs. 6%).46 There are several factors that could explain these differences. During CPR, increased movement and crowding in the upper area of the patient may favour the tibial site for accessibility compared to both upper limb intravenous access and the humeral site. While potential initial peripheral vasoconstriction, followed by vasodilation, complicates intravenous access, a thick layer of overlying soft tissue may complicate the humeral site in contrast to the tibial site, which may be easier to recognise given the flat and larger surface area.
If the selected drug route does not enable rapid drug delivery to the central circulation, drug administration may not be clinically beneficial. Animal models have consistently found that tibial drug administration during cardiac arrest leads to slower peaks and lower levels of plasma concentration than both humeral47, 48, 49 and peripheral venous drug administration47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, whereas the humeral drug administration reaches slightly lower plasma concentrations than peripheral venous drug administration.47, 48, 49, 58, 59, 60.
The International Liaison Committee on Resuscitation has highlighted the uncertainty regarding the optimal vascular access route during cardiac arrest and an urgent need for randomised clinical trials.8 This is based on a 2020-systematic review, identifying six observational studies comparing peripheral venous to intraosseous vascular access during adult OHCA.61 Two of these studies were deemed to have critical risks of bias primarily due to concerns of confounding, and the remaining four were pooled for meta-analyses.62, 63, 64, 65 Although intravenous access was associated with higher rates of ROSC and mid-term survival (e.g., at hospital discharge), the findings of the observational studies should be interpreted very carefully as the included studies were deemed to have a serious risks of bias mainly due to concerns about confounding and selection bias.61 There are several factors that – if not corrected for – could be sources of confounding, e.g., preferred use of intraosseous access in the obese or those with severe hypovolemia. Another important source of bias is “resuscitation time bias” as described in detail elsewhere.66.
Two randomised clinical trials comparing different vascular accesses have been published.46, 67 One only addressed procedure-related characteristics as described earlier,46 while the other evaluated the effect of implementing intraosseous access in a pre-hospital system.67 In the latter cluster-randomised trial, patients were randomised to intravenous access only or intravenous plus intraosseous access if initial intravenous attempts were unsuccessful. Although the intravenous plus intraosseous group demonstrated a higher success rate in achieving vascular access, there were no statistical differences in ROSC-rates. However, several limitations may challenge the generalisability of these findings, including intraosseous access being used as a “rescue therapy”. There are currently no published randomised clinical trials directly testing intravenous vs. intraosseous vascular access during cardiac arrest while also reporting ROSC or survival.61.
Therefore, to address this evidence gap, the IVIO trial was initiated. The findings of this and other similar trials will help inform future guidelines for selecting the optimal vascular access route during OHCA. The trial was started in March 2022 and the last patient is anticipated to be included in the spring of 2024. The primary results will be reported after 90-day follow-up and are therefore anticipated in mid-2024.
Funding
Funding for the trial is provided by the Novo Nordisk Foundation, Aarhus University, TrygFonden, the Independent Research Fund Denmark, and Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond through The Danish Heart Foundation. The funding agencies will have no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
CRediT authorship contribution statement
Carsten Meilandt: Investigation, Writing – original draft, Writing – review & editing. Mikael Fink Vallentin: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Project administration. Kristian Blumensaadt Winther: Investigation, Writing – review & editing. Allan Bach: Investigation, Writing – review & editing. Thomas H. Dissing: Investigation, Writing – review & editing. Steffen Christensen: Investigation, Writing – review & editing. Christian Juhl Terkelsen: Investigation, Writing – review & editing. Thomas Lass Klitgaard: Investigation, Writing – review & editing. Søren Mikkelsen: Investigation, Writing – review & editing. Fredrik Folke: Investigation, Writing – review & editing. Asger Granfeldt: Conceptualization, Methodology, Investigation, Writing – review & editing. Lars W. Andersen: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100428.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dansk Hjertestopregister. Rapport for Dansk Hjertestopregister 2021 [Danish]. (Accessed 15 May 2023, at https://hjertestopregister.dk/wp-content/uploads/2022/10/Dansk-Hjertestopregister-aarsrapport-2021.pdf)
- 2.Berdowski J., Berg R.A., Tijssen J.G., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Møller Hansen S, Ringgren KB, Wissenberg M, et al. Hjertestop uden for Hospital i Danmark - Sammenfatning of resultater fra Dansk Hjertestopsregister 2001-2016 [Danish]. (Accessed 8 May 2023, at http://genoplivning.dk/wp-content/uploads/2018/04/Dansk_Hjertestopregister2016.pdf)
- 4.Panchal A.R., Bartos J.A., Cabanas J.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 5.Soar J., Böttiger B.W., Carli P., et al. European Resuscitation Council guidelines 2021: adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Perkins G.D., Ji C., Deakin C.D., et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018 doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 7.Kudenchuk P.J., Brown S.P., Daya M., et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 8.Berg K.M., Soar J., Andersen L.W., et al. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:S92–S139. doi: 10.1161/CIR.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 9.Website for IVIO-trial. (Accessed 05 May 2023, at https://www.ivio.dk/)
- 10.ICH Harmonised Tripartite Guideline. Integrated Addendum To ICH E6(R1): Guideline For Good Clinical Practice E6(R2). (Accessed 08 May 2023, at https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf)
- 11.ICH Harmonised Tripartite Guideline. General Considerations for Clinical Trials E8. (Accessed 08 May 2023, at https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ich-guideline-e8-r1-general-considerations-clinical-studies_en.pdf)
- 12.ICH Harnomised Tripartite Guidelines. Statistical Principles for Clinical Trials E9. (Accessed 08 May 2023, at https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-9-statistical-principles-clinical-trials-step-5_en.pdf)
- 13.Chan A.W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A.W., Tetzlaff J.M., Gotzsche P.C., et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthon C.T., Granholm A., Perner A., Laake J.H., Moller M.H. No firm evidence that lack of blinding affects estimates of mortality in randomized clinical trials of intensive care interventions: a systematic review and meta-analysis. J Clin Epidemiol. 2018;100:71–81. doi: 10.1016/j.jclinepi.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Moustgaard H., Clayton G.L., Jones H.E., et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ. 2020;368 doi: 10.1136/bmj.l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin G.L., Trioux T., Gaudry S., Tubach F., Hajage D., Dechartres A. Association between lack of blinding and mortality results in critical care randomized controlled trials: a meta-epidemiological study. Crit Care Med. 2021;49:1800–1811. doi: 10.1097/ccm.0000000000005065. [DOI] [PubMed] [Google Scholar]
- 18.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker L.B., Aufderheide T.P., Geocadin R.G., et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124:2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haywood K., Whitehead L., Nadkarni V.M., et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Circulation. 2018;137:e783–e801. doi: 10.1161/CIR.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 21.Vallentin M.F., Granfeldt A., Meilandt C., et al. Effect of intravenous or intraosseous calcium vs saline on return of spontaneous circulation in adults with out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2021;326:2268–2276. doi: 10.1001/jama.2021.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz K.F., Altman D.G., Moher D., Group C CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Hopewell S., Schulz K.F., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernando S.M., Tran A., Cheng W., et al. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ. 2019;367 doi: 10.1136/bmj.l6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez A.V., Steyerberg E.W., Habbema J.D. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol. 2004;57:454–460. doi: 10.1016/j.jclinepi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Kahan B.C., Jairath V., Dore C.J., Morris T.P. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139. doi: 10.1186/1745-6215-15-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahan B.C. Accounting for centre-effects in multicentre trials with a binary outcome - when, why, and how? BMC Med Res Methodol. 2014;14:20. doi: 10.1186/1471-2288-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegelhalter D.J., Freedman L.S., Parmar M.K.B. Bayesian approaches to randomized trials. J R Statist Soc, Ser A. 1994;157:357–416. [Google Scholar]
- 29.Quinn T.J., Lees K.R., Hardemark H.G., Dawson J., Walters M.R. Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials. Stroke. 2007;38:2257–2261. doi: 10.1161/STROKEAHA.106.480723. [DOI] [PubMed] [Google Scholar]
- 30.Wilson J.T., Hareendran A., Grant M., et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 31.Wilson J.T., Hareendran A., Hendry A., Potter J., Bone I., Muir K.W. Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36:777–781. doi: 10.1161/01.STR.0000157596.13234.95. [DOI] [PubMed] [Google Scholar]
- 32.McPhail S., Lane P., Russell T., et al. Telephone reliability of the Frenchay Activity Index and EQ-5D amongst older adults. Health Qual Life Outcomes. 2009;7:48. doi: 10.1186/1477-7525-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monsieurs K.G., Nolan J.P., Bossaert L.L., et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation. 2015;95:1–80. doi: 10.1016/j.resuscitation.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Danish Resuscitation Council. Advanced resusciation [Danish] (Accessed 08 May 2023, at https://genoplivning.dk/avancerede-guides/)
- 35.Nichol G., Powell J.L., Emerson S. On coenrollment in clinical resuscitation studies: review and experience from randomized trials. Resuscitation. 2010;81:792–795. doi: 10.1016/j.resuscitation.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Taichman D.B., Sahni P., Pinborg A., et al. Data sharing statements for clinical trials - a requirement of the International Committee of Medical Journal Editors. N Engl J Med. 2017;376:2277–2279. doi: 10.1056/NEJMe1705439. [DOI] [PubMed] [Google Scholar]
- 37.Olasveengen T.M., Sunde K., Brunborg C., Thowsen J., Steen P.A., Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 38.Perkins G.D., Kenna C., Ji C., et al. The influence of time to adrenaline administration in the Paramedic 2 randomised controlled trial. Intens Care Med. 2020;46:426–436. doi: 10.1007/s00134-019-05836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wortsman J., Frank S., Cryer P.E. Adrenomedullary response to maximal stress in humans. Am J Med. 1984;77:779–784. doi: 10.1016/0002-9343(84)90512-6. [DOI] [PubMed] [Google Scholar]
- 40.Holmes H., Babbs C.F., Voorhees W., Tacker W., De Garavilla B. Influence of adrenergic drugs upon vital organ perfusion during CPR. Crit Care Med. 1980 doi: 10.1097/00003246-198003000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Michael J.R., Guerci A., Koehler R., et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69:822–835. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 42.Lindner K.H., Ahnefeld F.W., Bowdler I.M. Comparison of different doses of epinephrine on myocardial perfusion and resuscitation success during cardiopulmonary resuscitation in a pig model. Am J Emergency Med. 1991;9:27–31. doi: 10.1016/0735-6757(91)90008-8. [DOI] [PubMed] [Google Scholar]
- 43.Voelckel W.G., Lindner K.H., Wenzel V., et al. Effects of vasopressin and epinephrine on splanchnic blood flow and renal function during and after cardiopulmonary resuscitation in pigs. Crit Care Med. 2000;28:1083–1088. doi: 10.1097/00003246-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Dalsey W.C., Barsan W.G., Joyce S.M., Hedges J.R., Lukes S.J., Doan L.A. Comparison of superior vena caval and inferior vena caval access using a radioisotope technique during normal perfusion and cardiopulmonary resuscitation. Ann Emergency Med. 1984;13:881–884. doi: 10.1016/s0196-0644(84)80661-7. [DOI] [PubMed] [Google Scholar]
- 45.Woerlee G.M. Kinetics and dynamics of intravenous anesthetics. Springer Science & Business Media; 2007. Physiological Aspects of Drug Kinetics and Dynamics; pp. 23–54. chap 2. [Google Scholar]
- 46.Reades R., Studnek J.R., Vandeventer S., Garrett J. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: a randomized controlled trial. Ann Emerg Med. 2011;58:509–516. doi: 10.1016/j.annemergmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Beaumont L.D., Baragchizadeh A., Johnson C., Johnson D. Effects of tibial and humerus intraosseous administration of epinephrine in a cardiac arrest swine model. Am J Disaster Med. 2016;11:243–251. doi: 10.5055/ajdm.2016.0246. [DOI] [PubMed] [Google Scholar]
- 48.Adams T.S., Blouin D., Johnson D. Effects of tibial and humerus intraosseous and intravenous vasopressin in porcine cardiac arrest model. Am J Disaster Med. 2016;11:211–218. doi: 10.5055/ajdm.2016.0241. [DOI] [PubMed] [Google Scholar]
- 49.Burgert J.M., Johnson A.D., O'Sullivan J.C., et al. Pharmacokinetic effects of endotracheal, intraosseous, and intravenous epinephrine in a swine model of traumatic cardiac arrest. Am J Emergency Med. 2019;37:2043–2050. doi: 10.1016/j.ajem.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 50.James Burgert C.R.N.A.M., Desai M., Michael Schlicher R., et al. Comparison of tibial intraosseous, sternal intraosseous, and intravenous routes of administration on pharmacokinetics of epinephrine during cardiac arrest: a pilot study. AANA J. 2012;80:S6. [PubMed] [Google Scholar]
- 51.Wong M.R., Reggio M.J., Morocho F.R., et al. Effects of intraosseous epinephrine in a cardiac arrest swine model. J Surg Res. 2016;201:327–333. doi: 10.1016/j.jss.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Fulkerson J., Lowe R., Anderson T., Moore H., Craig W., Johnson D. Effects of intraosseous tibial vs. intravenous vasopressin in a hypovolemic cardiac arrest model. Western J Emergency Med. 2016;17:222. doi: 10.5811/westjem.2015.12.28825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson D., Giles K., Acuna A., Saenz C., Bentley M., Budinich C. Effects of tibial intraosseous and IV administration of vasopressin on kinetics and survivability in cardiac arrest. Am J Emergency Med. 2016;34:429–432. doi: 10.1016/j.ajem.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Mara O'Sullivan M., Long A., Michelle Johnson D., Blouin D., Johnson A.D., Burgert J.M. Comparison of the effects of sternal and tibial intraosseous administered resuscitative drugs on return of spontaneous circulation in a swine model of cardiac arrest. Am J Disaster Med. 2016;11:175–182. doi: 10.5055/ajdm.2016.0237. [DOI] [PubMed] [Google Scholar]
- 55.Hampton K., Wang E., Argame J.I., Bateman T., Craig W., Johnson D. The effects of tibial intraosseous versus intravenous amiodarone administration in a hypovolemic cardiac arrest procine model. Am J Disaster Med. 2016;11:253–260. doi: 10.5055/ajdm.2016.0247. [DOI] [PubMed] [Google Scholar]
- 56.Burgert J.M., Martinez A., O'Sullivan M., Blouin D., Long A., Johnson A.D. Sternal route more effective than tibial route for intraosseous amiodarone administration in a swine model of ventricular fibrillation. Prehospital Emergency Care. 2018;22:266–275. doi: 10.1080/10903127.2017.1358782. [DOI] [PubMed] [Google Scholar]
- 57.Yauger Y.J., Johnson M.D., Mark J., et al. Tibial intraosseous administration of epinephrine is effective in restoring return of spontaneous circulation in a pediatric normovolemic but not hypovolemic cardiac arrest model. Pediatric Emergency Care. 2020 doi: 10.1097/PEC.0000000000002127. [DOI] [PubMed] [Google Scholar]
- 58.Holloway C., Jurina C., Orszag C., et al. Effects of humerus intraosseous versus intravenous amiodarone administration in a hypovolemic porcine model. Am J Disaster Med. 2016;11:261–269. doi: 10.5055/ajdm.2016.0248. [DOI] [PubMed] [Google Scholar]
- 59.Burgert J.M., Johnson A.D., Garcia-Blanco J., Fulton L.V., Loughren M.J. The resuscitative and pharmacokinetic effects of humeral intraosseous vasopressin in a swine model of ventricular fibrillation. Prehospital and disaster medicine. 2017;32:305–310. doi: 10.1017/S1049023X17000140. [DOI] [PubMed] [Google Scholar]
- 60.Neill M.J., Burgert J.M., Blouin D., et al. Effects of humeral intraosseous epinephrine in a pediatric hypovolemic cardiac arrest porcine model. Trauma Surgery Acute Care Open. 2020;5 doi: 10.1136/tsaco-2019-000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Granfeldt A., Avis S.R., Lind P.C., et al. Intravenous vs. intraosseous administration of drugs during cardiac arrest: a systematic review. Resuscitation. 2020;149:150–157. doi: 10.1016/j.resuscitation.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Feinstein B.A., Stubbs B.A., Rea T., Kudenchuk P.J. Intraosseous compared to intravenous drug resuscitation in out-of-hospital cardiac arrest. Resuscitation. 2017;117:91–96. doi: 10.1016/j.resuscitation.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 63.Kawano T., Grunau B., Scheuermeyer F.X., et al. Intraosseous vascular access is associated with lower survival and neurologic recovery among patients with out-of-hospital cardiac arrest. Ann Emergency Med. 2018;71:588–596. doi: 10.1016/j.annemergmed.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Mody P., Brown S.P., Kudenchuk P.J., et al. Intraosseous versus intravenous access in patients with out-of-hospital cardiac arrest: insights from the resuscitation outcomes consortium continuous chest compression trial. Resuscitation. 2019;134:69–75. doi: 10.1016/j.resuscitation.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y., Zhu J., Liu Z., et al. Intravenous versus intraosseous adrenaline administration in out-of-hospital cardiac arrest: a retrospective cohort study. Resuscitation. 2020;149:209–216. doi: 10.1016/j.resuscitation.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Andersen L.W., Grossestreuer A.V., Donnino M.W. “Resuscitation time bias” – a unique challenge for observational cardiac arrest research. Resuscitation. 2018;125:79–82. doi: 10.1016/j.resuscitation.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan B.K.K., Chin Y.X., Koh Z.X., et al. Clinical evaluation of intravenous alone versus intravenous or intraosseous access for treatment of out-of-hospital cardiac arrest. Resuscitation. 2021;159:129–136. doi: 10.1016/j.resuscitation.2020.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.