Abstract
Background
Chest compressions (CC) are the cornerstone of cardiopulmonary resuscitation (CPR). But CC are also known to cause injuries, specifically rib fractures. The effects of such fractures have not been examined yet. This study aimed to investigate hemodynamic effects of rib fractures during mechanical CPR in a porcine model of cardiac arrest (CA).
Methods
We conducted a retrospective hemodynamic study in 31 pigs that underwent mechanical CC. Animals were divided into three groups based on the location of rib fractures: No Broken Ribs group (n = 11), Left Broken Ribs group (n = 13), and Right Broken Ribs group (n = 7). Hemodynamic measurements were taken at 10 seconds before and 10, 30, and 60 seconds after rib fractures.
Results
Baseline hemodynamic parameters did not differ between the three groups. Systolic aortic pressure was overall higher in the Left Broken Ribs group than in the No Broken Ribs group at 10, 30, and 60 seconds after rib fracture (p = 0.02, 0.01, and 0.006, respectively). The Left Broken Ribs group had a significantly higher right atrial pressure compared to the No Broken Rib group after rib fracture (p = 0.02, 0.01, and 0.03, respectively). There was no significant difference for any parameter for the Right Broken Ribs group, when compared to the No Broken Ribs group.
Conclusion
An increase in main hemodynamic parameters was observed after left rib fractures while right broken ribs were not associated with any change in hemodynamic parameters. Reporting fractures and their location seems worthwhile for future experimental studies.
Keywords: Cardiac arrest, Rib fractures, Resuscitation, Chest compressions
Introduction
There are approximately 340,000 cardiac arrests (CA) annually in the United States, with a poor overall survival rate to hospital discharge of around 9%.1 Chest compressions (CC) are essential for blood flow,2 allowing partial restoration of cerebral and coronary perfusion, increasing ROSC rates and survival.3, 4, 5
The American Heart Association emphasizes the importance of high-quality CPR, with their guidelines recommending providing CC “of adequate rate and depth, avoiding leaning on the chest between compressions”.2 The recommended compression rate for adults is between 100 and 120 compressions per minute with a depth of at least 2 inches.2 Over the years, it has been shown that manual as well as mechanical compressions are a source of injuries during CPR, and the foremost cause of rib fractures.6, 7, 8
Rib and sternal fractures are the most common complication after CPR,6, 7, 8 with an incidence ranging between 10–96%, irrespective of whether patients survive.8, 9, 10, 11 The risk of rib fractures further increases with mechanical CPR.9, 11, 12. Although rib fractures have not been shown to be fatal, their impact on resuscitation outcomes is still disputed.13, 14 In animal experiments, CC are mainly performed by a mechanical piston or any mechanical device for the sake of reproducibility, and broken ribs are common.15, 16, 17
To the best of our knowledge, the hemodynamic effects of rib fractures during CPR have not yet been evaluated. The aim of this study was to investigate the hemodynamic effects of rib fractures during automated mechanical CPR in a porcine model of cardiac arrest.
Material and methods
The studies were all approved by the Institutional Animal Care Committees of the Minneapolis Medical Research Foundation of Hennepin County Medical Center and the University of Minnesota. All animal care complied with the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals.18
Design and animal selection
Over the span of three years, our group performed a number of porcine cardiac arrest studies,19, 20 using female farm bred Yorkshire swine treated with CC. To conduct this retrospective analysis, we pooled the animal populations of these studies and assessed the hemodynamic effects of rib fractures occurring during CPR, and before any administration of pharmacologic agents. According to the location of the majority of the pigs’ rib fractures, the animals were classified as either predominantly Left Rib Fracture pigs or predominantly Right Rib Fracture pigs. The animals without any rib fractures served as the control group. After each study was concluded, an autopsy was performed to confirm the side with predominant rib fractures and their number. If the animal had bilateral rib fractures, it was included according to the predominant side of fractures. The analysed population was derived from two rounds of selection.
Initial selection
During an initial cohort selection, pigs with visual thoracic deformation were included in the Broken Ribs groups. The time of a rib fracture was determined by a study personnel who was assigned to watch the chest wall during CPR. During direct observation of CPR, if the chest wall acutely angulated in any direction without movement of the animal, and this deviation persisted during subsequent compressions, this was marked as the time of rib fracture. The majority of pigs (n = 25, 81%) were selected this way. Fracture side was confirmed during autopsy.
Additional selection
It was observed that the initial cohort of animals contained only three right-sided rib fractures. Thus, an additional selection of animals was conducted to supplement the predominantly left-sided rib fracture group. However, these studies did not have a chest wall observer assigned. Instead, rib fracture timing was determined by an acute change in the calculated chest wall compliance (calculated as Force per unit Distance, sampling rate of 250 Hz). As ribs fracture and the chest wall deforms, it is observed that the force exerted on the CPR piston acutely drops, despite minimal change in the distance that the piston compresses. This method of fracture timing was used to select an additional six pigs (n = 4 Right Rib Fracture pigs and n = 2 Left Rib Fracture pigs). Fracture side was confirmed during autopsy.
Preparatory phase
The surgical preparation, anesthesia, data monitoring, and recording procedures used in the studies performed during the inclusion period have been previously described.19, 20 Under aseptic surgical conditions, we initially sedated the pigs with intramuscular ketamine (10 mL of 100 mg/mL) followed by inhaled isoflurane (0.8–1.2%). Pigs were intubated with a size 7.0 endotracheal tube then ventilated with room air, using a ventilator (Narkomed, Telford, Pennsylvania) with a tidal volume of 10 ml/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mmHg and PaO2 around 80 mmHg (blood oxygen saturation >95%). Central aortic blood pressure was recorded continuously with a catheter (Mikro-Tip Transducer, Millar Instruments, Houston, Texas) placed in the descending thoracic aorta. A second Millar catheter was inserted in the right atrium via the right internal jugular vein to measure right atrial (RA) pressure. Animals received up to 1000 ml of normal saline solution after surgical preparation in order to maintain a mean right atrial pressure between 3–5 mmHg. Animals received an intravenous heparin bolus (100 units/kg). Hemodynamic data and telemetry were continuously monitored and recorded (BIOPAC MP 150, BIOPAC Systems, Inc., CA, USA). Coronary perfusion pressure (CPP) was calculated as the difference between aortic and right atrial diastolic pressures during the decompression phase of CPR. End-tidal carbon dioxide, tidal volume, and blood oxygen saturation were continuously measured (COSMO Plus, Novametrix Medical Systems, Wallingford, Connecticut). ROSC was defined using the Utstein guidelines for uniform reporting in animal research as maintenance of systolic pressure of ≥60 mm Hg for ≥10 consecutive minutes.21
Experimental protocol
Following the surgical preparation, ventricular fibrillation (VF) was induced by delivering a direct intra-cardiac electric current via a temporary pacing wire descending through the jugular vein, into the right ventricle. Standard-CPR (STD-CPR) or active compression and decompression-CPR (ACD-CPR) were performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark) as previously described.22, 23 During STD-CPR, uninterrupted chest compressions were performed at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter. After each compression, the chest wall was allowed to fully recoil passively. With ACD-CPR, after each compression, the chest was actively pulled upwards with a suction cup attached to the skin, supplying a decompression force of ∼20 lbs.22, 24 Simultaneous with ACD-CPR, an impedance threshold device (ITD, ResQPOD TM, Advanced Circulatory Systems, Roseville, MN) with a resistance of 16 mm Hg was attached to the endotracheal tube. Asynchronous positive pressure ventilations were delivered with room air (FiO2 of 0.21) with a manual resuscitator bag. The tidal volume was maintained at ∼10 mL/kg and the respiratory rate was 10 breaths/min.
Statistical analysis
Categorical variables are represented as counts with proportions and continuous variables are presented as mean ± standard deviations (SD). Categorical variables were compared with the chi-squared test and continuous variables were compared with the t-test and analysis of variance (ANOVA). The distribution of all continuous variables was checked with histograms before performing analyses. We then examined the effect of rib fractures (left-sided, right-sided, or no rib fractures) on CPP, systolic blood pressure (SBP), mean aortic pressure (MAP), RA pressure, and EtCO2. These hemodynamic variables were compared between the rib fracture groups at four stages: 10 seconds before (baseline) and 10, 30, and 60 seconds after rib fractures (or similar mean fracture time in the No Broken Ribs group). Mixed effects linear regression was used to compare the hemodynamic variables at those four stages. In this model, the hemodynamic variable was the dependent variable. The fixed covariates were rib fracture side, stage of the experiment (10 seconds before or 10 seconds, 30 seconds, or 60 seconds after the rib fracture), and type of CPR (active compression-decompression CPR with an impedance-threshold device or standard CPR). Random intercepts were used for pigs to account for the dependence of repeated measures. A linear rib fracture group experiment stage interaction term was used to determine whether the effect of the type of rib fracture had different responses on the hemodynamics at different stages of the experiment. If the margins plots suggested the presence of an interaction, the hemodynamic values were reported by each group at each stage. We reported comparisons of the mean hemodynamic values for all animals across the experiment stages to describe changes in the hemodynamic values across stages and, where the rib fracture group covariate was < 0.05 in the mixed effects model or if the interaction term was positive, between-group comparisons. These differences were also depicted visually in plots. All statistical tests were two-sided and the level of significance was set at 0.05 for all analyses. All statistical analyses were done in Stata MP 17 (STATA Corp, College Station, TX, U.S.A.).
Results
Thirty-one animals were included between March 2013 and November 2016. Mean weight was 39.7 ± 3.3 kg in the Left Broken Ribs group, 40.9 ± 2.4 kg in the Right Broken Ribs group, and 38.0 ± 2.8 kg in the No Broken Ribs group (p = 0.25). Broken ribs were observed during CPR in 20 pigs at 107 ± 11 sec after the initiation of CPR (13 with left rib-cage deformation and 7 with right rib-cage deformation). The mean number of broken ribs on the side with the majority of fractures was 4 ± 0.7 in the predominantly Left Broken Ribs group and 4.1 ± 1.1 in the predominantly Right Broken Ribs group. Hemodynamic parameters were not statically different at baseline between the three groups (Table 1).
Table 1.
Baseline characteristics.
| Parameter |
No Broken Ribs (N = 11) |
Left Broken Ribs (N = 13) |
Right Broken Ribs (N = 7) |
p value |
|---|---|---|---|---|
| Weight (kg) | 38.0 ± 2.7 | 39.7 ± 3.3 | 40.9 ± 2.4 | 0.25 |
| CPR Method: Standard | 4 | 4 | 1 | 0.59 |
| CPR Method: ITD-ACD | 7 | 9 | 6 | |
| Number of Fractured Ribs (n) | 0 | 4.0 ± 0.7 | 4.1 ± 1.1 | <0.0001 |
| Time to fracture (seconds) | 0 | 92.4 ± 47.9 | 130.3 ± 39.8 | <0.0001 |
| ROSC | 2 | 13 | 5 | 0.10 |
| Baseline Hemodynamics (Before Ribs Were Fractured) | ||||
| CPP (mmHg) | 10.8 ± 6.1 | 16.1 ± 8.6 | 18.4 ± 4.6 | 0.07 |
| Systolic Aortic Pressure (mmHg) | 43.6 ± 12.8 | 51.6 ± 20.1 | 46.7 ± 8.0 | 0.46 |
| Mean Aortic Pressure (mmHg) | 24.2 ± 6.7 | 31.0 ± 10.2 | 28.2 ± 4.2 | 0.14 |
| Right Atrial Pressure (mmHg) | 21.5 ± 10.0 | 22.1 ± 5.5 | 19.5 ± 5.8 | 0.76 |
| End Tidal CO2 (mmHg) | 31.6 ± 6.8 | 30.4 ± 15.1 | 26.0 ± 3.3 | 0.63 |
CPR: cardiopulmonary resuscitation, ACD/ITD: active compression/decompression and impedance threshold device, CPP: coronary perfusion pressure.
Categorical variables were compared by the chi-squared test. Continuous variables were compared by the analysis of variance (ANOVA) test.
Systolic aortic pressure
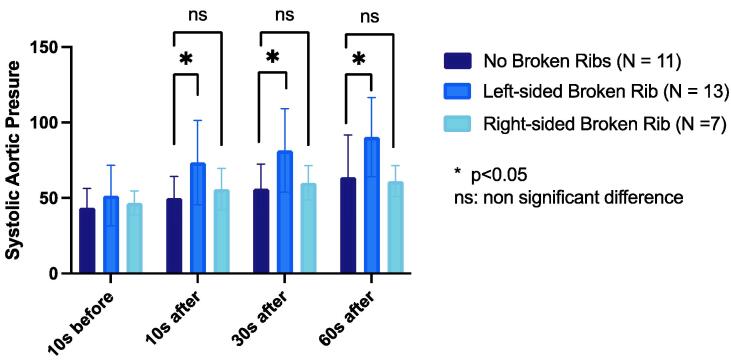
The No Broken Ribs group did not exhibit any significant difference in SBP between the baseline measure and 10 seconds (p = 0.19) but exhibited an increase in mean SBP at 30 seconds and 60 seconds timepoints (p = 0.01 and <0.0001, respectively). The Left Broken Ribs group had a significantly higher mean SBP than the No Broken Ribs group at 10 seconds, 30 seconds, and 60 seconds after the rib fracture (p = 0.02, 0.01, and 0.006, respectively). The Right Broken Ribs group did not exhibit any significant difference in mean SBP compared to No Broken Ribs group at 10 seconds, 30 seconds, and 60 seconds after the rib fracture (p = 0.74, 0.93, and 0.47, respectively). (Fig. 1).
Fig. 1.
Comparison of systolic aortic pressure (mmHg) in each group at four timepoints.
Mean aortic pressure
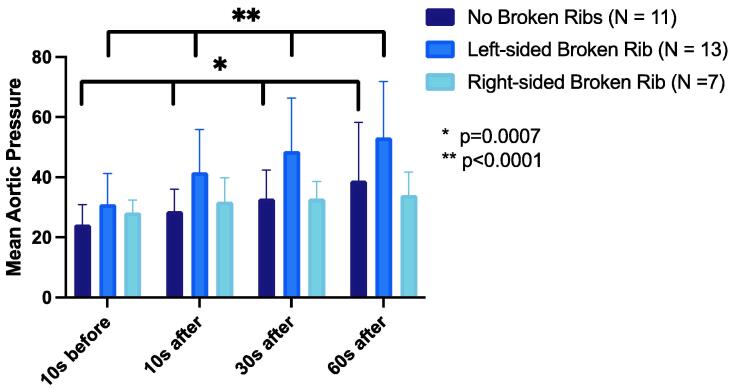
There was a progressive increase in mean MAP after the fracture in the overall cohort (p for trend <0.0001). The mean MAP increased in the No Broken Ribs group and the Left Broken Ribs group (p trend = 0.0007 and <0.0001, respectively) but not in the Right Broken Ribs group (p = 0.61). There was no difference in the mean MAP between the No Broken Ribs group and the Left Broken Ribs group or the Right Broken Ribs group (p = 0.18 and 0.69, respectively; Fig. 2).
Fig. 2.
Comparison of mean aortic pressure (mmHg) in each group at four timepoints.
Right atrial pressure
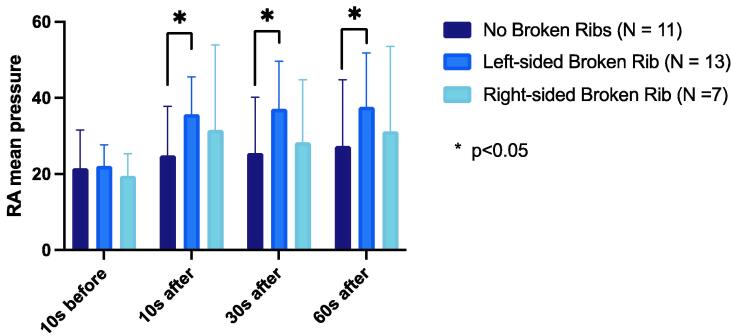
The No Broken Ribs group did not exhibit any significant difference in mean RAP between the baseline measure and 10 seconds, 30 seconds, and 60 seconds timepoints (p = 0.28, 0.21, and 0.07, respectively). The Left Broken Ribs group had a significantly higher mean RAP than the No Broken Ribs group at 10 seconds, 30 seconds, and 60 seconds after the rib fracture (p = 0.02, 0.01, and 0.03, respectively). The Right Broken Ribs group did not exhibit any significant difference in mean RAP compared to the No Broken Ribs group at 10 seconds, 30 seconds, and 60 seconds after the rib fracture (p = 0.10, 0.35, and 0.26, respectively) (Fig. 3).
Fig. 3.
Comparison of right atrial mean pressure (mmHg) in each group at four timepoints.
Coronary perfusion pressure
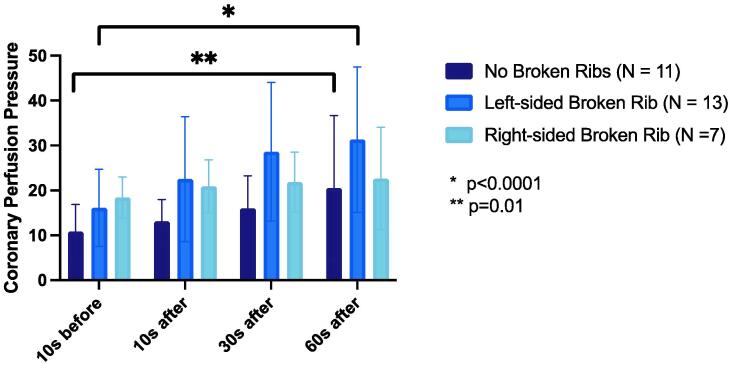
There was a progressive increase in mean CPP between the 10 seconds before and 60 seconds after rib fracture timepoints in the overall cohort (p for trend = 0.049).
The mean CPP increased in the No Broken Ribs group and the Left Broken Ribs group (p trend = 0.01 and <0.0001, respectively) but not the Right Broken Ribs group (p = 0.73). There was no difference in the mean CPP between No Broken Ribs group and the Left Broken Ribs group or Right Broken Ribs group (both p = 0.24) (Fig. 4).
Fig. 4.
Comparison of coronary perfusion pressure (mmHg) in each group at four timepoints.
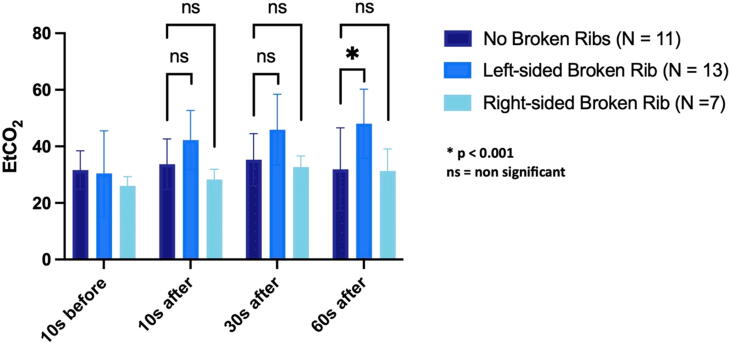
End tidal carbon dioxide (EtCO2)
The No Broken Ribs group did not exhibit any significant difference in mean EtCO2 between the baseline measure and 10 seconds, 30 seconds, and 60 seconds timepoints (p = 0.39, 0.13, and 0.87, respectively). The Left Broken Ribs group had a significantly higher mean EtCO2 than the No Broken Ribs group at 60 seconds after the rib fracture (p = 0.006), but not at the other stages (p = 0.32 at 10 seconds after rib fracture and p = 0.15 at 30 seconds after rib fracture). The Right Broken Ribs group did not exhibit any significant difference in mean EtCO2 compared to the No Broken Ribs group at 10 seconds, 30 seconds, and 60 seconds after the rib fracture (p = 0.18, 0.42, and 0.67, respectively) (Fig. 5).
Fig. 5.
Comparison of end-tidal CO2 in each group at four timepoints.
Discussion
In this cohort study of 31 swine, we observed an increase in systolic blood pressure, right atrial pressure and EtCO2 in the left rib fractures group compared to the group of swine without rib fractures. Hemodynamic or ventilatory parameters did not seem to be affected by right rib fractures in our study.
Most studies reporting injuries during CPR are observational, comparing injury patterns between mechanical and manual chest compressions and the potential effects on overall survival. Mechanical chest compression devices have been associated with elevated injury rates, including rib fractures, but also with improved 30-day survival for in-hospital cardiac arrest.9, 11, 25, 26, 27 On the other hand, no study has shown a clear benefit of mechanical CPR for out-of-hospital cardiac arrest (OHCA).28, 29
Comparing mechanical and manual CPR in swine, Liao et al. reported significantly higher CPP and improved survival in the mechanical CPR group.30 Although there were more rib fractures in the manual group, the mechanical group suffered more left-sided rib fractures, whereas the manual group exhibited fractures on both sides.30 These left-sided rib fractures might have been the reason for the elevation in CPP during CPR and thus in survival, as higher CPP is associated with improved rates of ROSC during CPR.31, 32
Animal studies have shown that by shifting the compression site to the left, a more direct compression of the left ventricle was achieved, thus increasing aortic pressure, CPP, EtCO2, and rates of ROSC in swine.33, 34 Also, in a recent porcine CA model, cerebral blood velocity measurements were significantly greater when the left ventricle was directly compressed during CPR by checking the left ventricle’s position by ultrasound rather than compression in the standard position.33 Therefore, left-sided rib fractures observed in our experiment may account for a more direct compression of the left ventricle, and thus elevated perfusion pressures.
Also, by directly compressing the left ventricle, once the ribs are broken on the left side, the descending aorta, located posterior to the heart might also be compressed, potentially leading to an aortic counter pulsation effect. This may, in turn, increase blood flow to the coronary arteries and the brain during diastole and lowering afterload in systole, mimicking the effects of a resuscitative endovascular balloon occlusion of the aorta (REBOA).35, 36, 37
Human and swine chests are very different despite having a similar vascular anatomy. In pigs, the ventricles are surrounded by lung tissue and are centrally located in the thoracic cavity and the thorax is laterally compressed.30, 38, 39 Chest compressions will mainly lead to increased intrathoracic pressure, thus favoring blood flow (thoracic pump theory).40 In contrast, in humans the thorax is dorsoventrally compressed and flat with the right ventricle just below the sternum. Chest compressions will cause direct compression of the heart against the spine, generating blood flow (cardiac pump theory).41, 42, 43, 44 By breaking the ribs on the left side during CPR in swine, the chest’s anatomy is changed, and CC might compress the heart more directly. The increase in hemodynamic parameters measured in the Left Rib Fracture group could reflect an increased cardiac pump effect. On the other hand, right rib fractures could further crush the right heart, thus increasing right atrial pressure and prevent venous return leading to lower cardiac output and CPP.45 Although left rib fractures occurred significantly earlier than right rib fractures, the difference is probably not clinically significant (92.4 ± 47.9 vs 130.3 ± 39.8 seconds) given that the baseline hemodynamics were similar and the same time benchmarks were used to examine hemodynamic parameters.
The number and side of broken ribs are rarely, if ever, reported, in swine studies exploring hemodynamic effects of different interventions, such as drugs, ventilation devices, and animal position, among others. However, we have shown that these factors could be associated with significant changes in hemodynamic parameters. This could partly explain the lack of successful transition from animal trials to human trials that has been described.46, 47, 48, 49, 50 The number and side of broken ribs during animal experimentation should be stated in future publications.
Limitations
This was an observational study, using a convenience sample, with no sample size calculation, from different experiments over three years. Due to the nature of the study, it was neither randomized nor blinded. The number of animals was very limited, and a larger sample size would be needed to confirm our data. Multiple observers were assigned to assess fractures during CPR. Also, the selection methods differed over the three years (visual deformation vs. compliance data) but to the best of our knowledge it should not interfere with hemodynamic parameters after rib fractures. In addition, pigs that received ACD-CPR and STD-CPR were both included in the cohort. The ACD-CPR technique involves changes in CPR depth compared to STD-CPR that could itself alter hemodynamics. However, the proportion of ACD-CPR animals was comparable between the groups with the most significant differences (69% of Left Rib Fracture pigs; 64% of No Rib Fracture pigs). We also adjusted for the type of CPR in our statistical analyses to account for this. Finally, human torsos have a very different shape and broken ribs might not affect hemodynamic parameters in the same way.
Conclusions
Ribs fractures on the left side of the chest improved systolic aortic pressure and EtCO2 compared to no broken ribs or right sided broken ribs. Accounting for rib-fractures and their location is important in improving the quality of CPR. An argument could be made for the inclusion of rib fracture data in preclinical studies involving swine models of CPR, as rib fractures could be a source of significant hemodynamic variation between study groups.
Funding Statement
This study received no specific funding.
CRediT authorship contribution statement
Deborah Jaeger: Conceptualization, Formal analysis, Writing – original draft. Rajat Kalra: Methodology, Formal analysis, Writing – original draft. Pierre Sebastian: Conceptualization, Data curation, Writing – review & editing. Christopher Gaisendrees: Writing – review & editing. Marinos Kosmopoulos: Writing – review & editing. Guillaume Debaty: Conceptualization, Data curation, Writing – review & editing. Tahar Chouihed: Validation, Writing – review & editing. Jason Bartos: Validation, Writing – review & editing. Demetris Yannopoulos: Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics—2022 Update: a Report From the American Heart Association. Circulation. 2022:145. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 3.Kilgannon J.H., Kirchhoff M., Pierce L., Aunchman N., Trzeciak S., Roberts B.W. Association between chest compression rates and clinical outcomes following in-hospital cardiac arrest at an academic tertiary hospital. Resuscitation. 2017;110:154–161. doi: 10.1016/j.resuscitation.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idris A.H., Guffey D., Pepe P.E., et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43:840–848. doi: 10.1097/CCM.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 5.Idris A.H., Guffey D., Aufderheide T.P., et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125:3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleem S., Sonkin R., Sagy I., et al. Traumatic Injuries Following Mechanical versus Manual Chest Compression. Open Access Emerg Med OAEM. 2022;14:557–562. doi: 10.2147/OAEM.S374785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olds K., Byard R.W., Langlois N.E.I. Injuries associated with resuscitation - An overview. J Forensic Leg Med. 2015;33:39–43. doi: 10.1016/j.jflm.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kralj E., Podbregar M., Kejžar N., Balažic J. Frequency and number of resuscitation related rib and sternum fractures are higher than generally considered. Resuscitation. 2015;93:136–141. doi: 10.1016/j.resuscitation.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Smekal D., Lindgren E., Sandler H., Johansson J., Rubertsson S. CPR-related injuries after manual or mechanical chest compressions with the LUCASTM device: a multicentre study of victims after unsuccessful resuscitation. Resuscitation. 2014;85:1708–1712. doi: 10.1016/j.resuscitation.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Karasek J., Blankova A., Doubková A., et al. The comparison of cardiopulmonary resuscitation-related trauma: Mechanical versus manual chest compressions. Forensic Sci Int. 2021;323 doi: 10.1016/j.forsciint.2021.110812. [DOI] [PubMed] [Google Scholar]
- 11.Viniol S., Thomas R.P., Gombert S., König A.M., Betz S., Mahnken A.H. Comparison of different resuscitation methods with regard to injury patterns in cardiac arrest survivors based on computer tomography. Eur J Radiol. 2020;131 doi: 10.1016/j.ejrad.2020.109244. [DOI] [PubMed] [Google Scholar]
- 12.Gaisendrees C., Gerfer S., Ivanov B., et al. Outcomes after mechanical versus manual chest compressions in eCPR patients. Expert Rev Med Devices. 2021;18:1023–1028. doi: 10.1080/17434440.2021.1970528. [DOI] [PubMed] [Google Scholar]
- 13.Karasek J., Slezak J., Stefela R., et al. CPR-related injuries after non-traumatic out-of-hospital cardiac arrest: Survivors versus non-survivors. Resuscitation. 2022;171:90–95. doi: 10.1016/j.resuscitation.2021.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Prins J.T.H., Van Lieshout E.M.M., Van Wijck S.F.M., et al. Chest wall injuries due to cardiopulmonary resuscitation and the effect on in-hospital outcomes in survivors of out-of-hospital cardiac arrest. J Trauma Acute Care Surg. 2021;91:966–975. doi: 10.1097/TA.0000000000003379. [DOI] [PubMed] [Google Scholar]
- 15.Rezoagli E., Magliocca A., Grieco D.L., Bellani G., Ristagno G. Impact of lung structure on airway opening index during mechanical versus manual chest compressions in a porcine model of cardiac arrest. Respir Physiol Neurobiol. 2022;296 doi: 10.1016/j.resp.2021.103807. [DOI] [PubMed] [Google Scholar]
- 16.Magliocca A., Olivari D., De Giorgio D., et al. LUCAS Versus Manual Chest Compression During Ambulance Transport: A Hemodynamic Study in a Porcine Model of Cardiac Arrest. J Am Heart Assoc. 2019;8:e011189. doi: 10.1161/JAHA.118.011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruemmler R., Stein J., Duenges B., Renz M., Hartmann E.K. Standardized post-resuscitation damage assessment of two mechanical chest compression devices: a prospective randomized large animal trial. Scand J Trauma Resusc Emerg Med. 2021;29:79. doi: 10.1186/s13049-021-00892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academies Press; 1996. 10.17226/5140. [DOI] [PubMed]
- 19.Debaty G., Metzger A., Rees J., et al. Enhanced perfusion during advanced life support improves survival with favorable neurologic function in a porcine model of refractory cardiac arrest. Crit Care Med. 2015;43:1087–1095. doi: 10.1097/CCM.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debaty G., Matsuura T.R., Bartos J.A., et al. Sodium nitroprusside-enhanced cardiopulmonary resuscitation facilitates intra-arrest therapeutic hypothermia in a porcine model of prolonged ventricular fibrillation. Crit Care Med. 2015;43:849–855. doi: 10.1097/CCM.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Idris A.H., Becker L.B., Ornato J.P., et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33:69–84. doi: 10.1016/s0300-9572(96)01055-6. [DOI] [PubMed] [Google Scholar]
- 22.Lurie K.G., Coffeen P., Shultz J., McKnite S., Detloff B., Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 23.Debaty G., Lurie K., Metzger A., et al. Reperfusion injury protection during Basic Life Support improves circulation and survival outcomes in a porcine model of prolonged cardiac arrest. Resuscitation. 2016;105:29–35. doi: 10.1016/j.resuscitation.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Lindner K.H., Pfenninger E.G., Lurie K.G., Schürmann W., Lindner I.M., Ahnefeld F.W. Effects of active compression-decompression resuscitation on myocardial and cerebral blood flow in pigs. Circulation. 1993;88:1254–1263. doi: 10.1161/01.cir.88.3.1254. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y., Sun T., Yuan D., et al. Safety of mechanical and manual chest compressions in cardiac arrest patients: A systematic review and meta-analysis. Resuscitation. 2021;169:124–135. doi: 10.1016/j.resuscitation.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Chun M.J., Zhang Y., Toraih E.A., McGrew P.R. Iatrogenic Injuries in Manual and Mechanical Cardiopulmonary Resuscitation. Am Surg. 2021 doi: 10.1177/00031348211047507. [DOI] [PubMed] [Google Scholar]
- 27.Couper K., Yeung J., Nicholson T., Quinn T., Lall R., Perkins G.D. Mechanical chest compression devices at in-hospital cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2016;103:24–31. doi: 10.1016/j.resuscitation.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang P.L., Brooks S.C. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD007260.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu N., Chen Q., Jiang Z., et al. A meta-analysis of the resuscitative effects of mechanical and manual chest compression in out-of-hospital cardiac arrest patients. Crit Care. 2019;23:100. doi: 10.1186/s13054-019-2389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Q., Sjöberg T., Paskevicius A., Wohlfart B., Steen S. Manual versus mechanical cardiopulmonary resuscitation. An experimental study in pigs. BMC Cardiovasc Disord. 2010;10:53. doi: 10.1186/1471-2261-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paradis N.A., Martin G.B., Rivers E.P., et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 32.Reynolds J.C., Salcido D.D., Menegazzi J.J. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehospital Emerg Care Off J Natl Assoc EMS Physicians Natl Assoc State EMS Dir. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall R.A., Morton J.S., Luchkanych A.M.S., et al. Left ventricle chest compression improves ETCO2, blood pressure, and cerebral blood velocity in a swine model of cardiac arrest and cardiopulmonary resuscitation. Resusc Plus. 2022;12 doi: 10.1016/j.resplu.2022.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson K.L., Castaneda M.G., Boudreau S.M., Sharon D.J., Bebarta V.S. Left Ventricular Compressions Improve Hemodynamics in a Swine Model of Out-of-Hospital Cardiac Arrest. Prehosp Emerg Care. 2017;21:272–280. doi: 10.1080/10903127.2016.1241328. [DOI] [PubMed] [Google Scholar]
- 35.Tiba M.H., McCracken B.M., Cummings B.C., et al. Use of resuscitative balloon occlusion of the aorta in a swine model of prolonged cardiac arrest. Resuscitation. 2019;140:106–112. doi: 10.1016/j.resuscitation.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang D.-H., Lee D.K., Jo Y.H., Park S.M., Oh Y.T., Im C.W. Resuscitative endovascular occlusion of the aorta (REBOA) as a mechanical method for increasing the coronary perfusion pressure in non-traumatic out-of-hospital cardiac arrest patients. Resuscitation. 2022;179:277–284. doi: 10.1016/j.resuscitation.2022.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Olsen M.H., Olesen N.D., Karlsson M., et al. Randomized blinded trial of automated REBOA during CPR in a porcine model of cardiac arrest. Resuscitation. 2021;160:39–48. doi: 10.1016/j.resuscitation.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Steen S., Liao Q., Pierre L., Paskevicius A., Sjöberg T. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation. 2002;55:285–299. doi: 10.1016/s0300-9572(02)00271-x. [DOI] [PubMed] [Google Scholar]
- 39.Lelovas P.P., Kostomitsopoulos N.G., Xanthos T.T. A comparative anatomic and physiologic overview of the porcine heart. J Am Assoc Lab Anim Sci JAALAS. 2014;53:432–438. [PMC free article] [PubMed] [Google Scholar]
- 40.Rudikoff M.T., Maughan W.L., Effron M., Freund P., Weisfeldt M.L. Mechanisms of blood flow during cardiopulmonary resuscitation. Circulation. 1980;61:345–352. doi: 10.1161/01.cir.61.2.345. [DOI] [PubMed] [Google Scholar]
- 41.Rich S., Wix H.L., Shapiro E.P. Clinical assessment of heart chamber size and valve motion during cardiopulmonary resuscitation by two-dimensional echocardiography. Am Heart J. 1981;102:368–373. doi: 10.1016/0002-8703(81)90311-2. [DOI] [PubMed] [Google Scholar]
- 42.Maier G.W., Tyson G.S., Olsen C.O., et al. The physiology of external cardiac massage: high-impulse cardiopulmonary resuscitation. Circulation. 1984;70:86–101. doi: 10.1161/01.CIR.70.1.86. [DOI] [PubMed] [Google Scholar]
- 43.Ewy G.A. The mechanism of blood flow during chest compressions for cardiac arrest is probably influenced by the patient’s chest configuration. Acute Med Surg. 2018;5:236–240. doi: 10.1002/ams2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouwenhoven W.B. Closed-chess Cardiac Massage. JAMA. 1960;173:1064. doi: 10.1001/jama.1960.03020280004002. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg M.T., Olsen J.-A., Eriksen M., et al. Haemodynamic outcomes during piston-based mechanical CPR with or without active decompression in a porcine model of cardiac arrest. Scand J Trauma Resusc Emerg Med. 2018;26:31. doi: 10.1186/s13049-018-0496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kern K.B., Hilwig R.W., Berg R.A., Sanders A.B., Ewy G.A. Importance of Continuous Chest Compressions During Cardiopulmonary Resuscitation: Improved Outcome During a Simulated Single Lay-Rescuer Scenario. Circulation. 2002;105:645–649. doi: 10.1161/hc0502.102963. [DOI] [PubMed] [Google Scholar]
- 47.Nichol G., Leroux B., Wang H., et al. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015;373:2203–2214. doi: 10.1056/NEJMoa1509139. [DOI] [PubMed] [Google Scholar]
- 48.Little C.M., Marietta M.H., Peng K., et al. Vasopressin alone or with epinephrine may be superior to epinephrine in a clinically relevant porcine model of pulseless electrical activity cardiac arrest. Am J Emerg Med. 2006;24:810–814. doi: 10.1016/j.ajem.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Wenzel V., Krismer A.C., Arntz H.R., et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- 50.Fabian-Jessing B.K., Vallentin M.F., Secher N., et al. Animal models of cardiac arrest: A systematic review of bias and reporting. Resuscitation. 2018;125:16–21. doi: 10.1016/j.resuscitation.2018.01.047. [DOI] [PubMed] [Google Scholar]