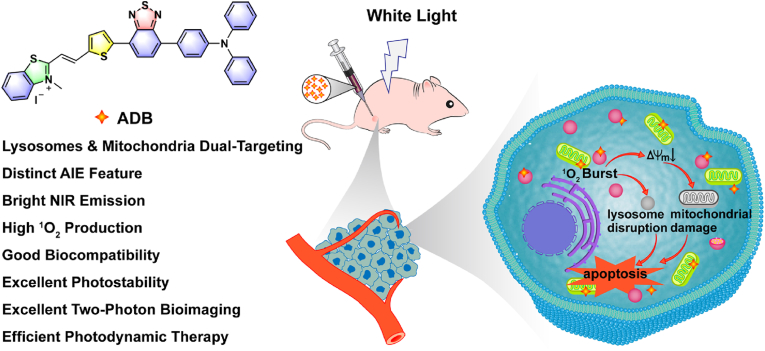
Abstract
Development of lysosomes and mitochondria dual-targeting photosensitizer with the virtues of near-infrared (NIR) emission, highly efficient reactive oxygen generation, good phototoxicity and biocompatibility is highly desirable in the field of imaging-guided photodynamic therapy (PDT) for cancer. Herein, a new positively charged amphiphilic organic compound (2-(2-(5-(7-(4-(diphenylamino)phenyl)benzo[c][1,2,5]thiadiazol-4-yl)thiophen-2-yl)vinyl)-3-methylbenzo[d]thiazol-3-ium iodide) (ADB) based on a D-A-π-A structure is designed and comprehensively investigated. ADB demonstrates special lysosomes and mitochondria dual-organelles targeting, bright NIR aggregation-induced emission (AIE) at 736 nm, high singlet oxygen (1O2) quantum yield (0.442), as well as good biocompatibility and photostability. In addition, ADB can act as a two-photon imaging agent for the elaborate observation of living cells and blood vessel networks of tissues. Upon light irradiation, obvious decrease of mitochondrial membrane potential (MMP), abnormal mitochondria morphology, as well as phagocytotic vesicles and lysosomal disruption in cells are observed, which further induce cell apoptosis and resulting in enhanced antitumor activity for cancer treatment. In vivo experiments reveal that ADB can inhibit tumor growth efficiently upon light exposure. These findings demonstrate that this dual-organelles targeted ADB has great potential for clinical imaging-guided photodynamic therapy, and this work provides a new avenue for the development of multi-organelles targeted photosensitizers for highly efficient cancer treatment.
Keywords: Photosensitizer, Aggregation-induced emission, Dual-organelles targeting, Near-infrared, Photodynamic therapy
Graphical abstract
Highlights
-
•
ADB possesses positively charged amphiphilic D-A-π-A structure.
-
•
ADB shows special lysosomes and mitochondria dual-organelles targeting capability.
-
•
ADB can act as a two-photon bioimaging agent.
-
•
ADB can produce 1O2 efficiently and effectively suppress tumor growth.
-
•
ADB demonstrates great potential for imaging-guided photodynamic cancer therapy.
1. Introduction
Malignant tumor has increasingly become a public problem for human health [[1], [2], [3]]. Photodynamic therapy, emerging as an enthusiastic method for cancer treatment, has attracted intensive research interest owing to the merits of noninvasiveness, high spatiotemporal selectivity, minimal drug resistance, negligible adverse effect and easy combination with other therapeutics, as comparing with the conventional strategies such as surgery, chemotherapy and radiotherapy [[3], [4], [5], [6], [7], [8]]. Photosensitizers play a critical role in transferring light energy to molecular oxygen to generate reactive oxygen species (such as 1O2), which then leading to the apoptosis, necrosis and autophagy of tumor cells [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. Especially, photosensitizers integrating with fluorescence imaging capability are of great interest for they can realize diagnosis and treatment simultaneously [[14], [15], [16], [17], [18]]. Some traditional photosensitizers, including phthalocyanine, porphyrin or chlorin derivatives, have been developed and applied clinically or preclinically for PDT [2,5,7,[18], [19], [20]]. However, the poor singlet oxygen production efficiency, photobleaching, low photostability, and limited fluorescence emission in aqueous media caused by aggregation-caused quenching (ACQ) effect, have greatly limited their applications [16,[19], [20], [21], [22]].
The discovery of AIE phenomenon in 2001 by Tang provides an excellent choice for the solution of ACQ problem [23]. AIE fluorophores with twisted conformation and reduced intermolecular π-π stacking exhibit weak luminescence in solutions but bright fluorescence in aggregate states via the mechanism of restriction of intramolecular motion [24,25]. A variety of AIE luminogens have been developed and found huge potential in sensing, bioimaging and disease theranostics [[26], [27], [28], [29], [30], [31], [32], [33]]. Investigations have shown that AIE luminogens not only emit strong fluorescence in aggregate state, but also possessing the virtues of sensitive photosensitizing and high singlet oxygen production [29,30,[34], [35], [36]]. Moreover, elaborately-designed AIE luminogens could exhibit good photostability, excellent biocompatibility and two-photon fluorescence imaging, demonstrating immense potential as photosensitizers for PDT [8,22,28,[37], [38], [39], [40]].
Due to the short lifetime (<40 ns) and small diffusion radius (<20 nm) of ROS [37,41,42], the accumulation of photosensitizers in subcellular organelles is usually necessary to enhance PDT efficiency. Mitochondria, known as the powerhouse of eukaryotic cells, play significant roles in energy supply, biosynthesis, signaling and cell differentiation [8,37,43]. Mitochondria are promising target sites of 1O2 for its function in regulating cellular redox status. Lysosomes are monolayered digestive compartments responsible for degradation of biomacromolecules, and also play vital roles in mediating fundamental life activities such as intracellular transportation, metabolic adaptation, cell apoptosis and necrosis [8,39,44]. The extracellular nanoparticles are usually captured by lysosomes under the endocytosis pathway [45]. Tang and Yoon et al. have designed a number of D-A structured mitochondria- or lysosomes-targeted photosensitizers and found that they could significantly promote cancer ablation [39,46,47]. However, these photosensitizers could only target single organelle. It is considered that AIE fluorophores which can simultaneously accumulate in mitochondria and lysosomes would greatly enhance the precise cancer therapy [8,[48], [49], [50]]. So far, the dual-organelles targeting AIEgen, as well as its anti-cancer mechanism, has been rarely reported. Therefore, the development of mitochondria and lysosomes dual-organelles targeting photosensitizer is highly desirable for photodynamic therapy.
In this study, a new amphiphilic organic NIR photosensitizer ADB with a D-A-π-A structure was developed. This cationic AIE fluorophore was rationally designed by adopting triphenylamine as the electron donor and 2,1,3-benzothiadiazole and 2,3-dimethylbenzo[d]thiazol-3-ium iodide groups as combined electron acceptors. ADB exhibited bright NIR emission at 736 nm with a Stokes shift of 203 nm, high 1O2 generation efficiency, as well as good biocompatibility and photostability. In this structure, the lipophilic segment facilitated the insertion of ADB into the mitochondrial membrane, and the electrostatic interaction between ADB and mitochondria was enhanced by the cationic part, enabling the mitochondrial targeting of the AIEgen ADB. At the same time, lysosomes, known as the “waste disposal station” in the cell, are single membrane-enclosed vesicles composed of a 7–10 nm phospholipid bilayer, the cationic characteristics of ADB may facilitate the endocytosis of the nanoparticles into lysosomes, resulting in the formation of phagocytotic vesicles as well as lysosomal disruption, which will then trigger the release of hydrolases into cytoplasm, leading to a series of responses that are associated with cell death [45]. Therefore, in addition to the impressive optical properties, ADB exhibited interesting mitochondria and lysosomes dual-organelles targeting capability. In vitro and in vivo experiments revealed that ADB facilitate high quality cell and tissue two-photon bioimaging, and effectively inhibit tumor growth upon white light irradiation (Scheme 1). These studies demonstrated that ADB would become a potential photosensitizer for clinical two-photon bioimaging and targeted photodynamic therapy. Furthermore, our work provides new insight for the design and construction of multi-organelles targeting organic photosensitizers for phototheranostics.
Scheme 1.
Schematic illustration of the chemical structure of ADB and its application for lysosomes- and mitochondria-targeted photodynamic cancer therapy.
2. Experimental section
2.1. Materials
4-(Diphenylamino)phenylboronic acid, 4,7-dibromo-2,1,3-benzothiadiazole, 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene-2-carbaldehyde, 2-methyl-benzo[d]thiazole and tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) were purchased from Bide Pharmatech Ltd. ABDA was obtained from Beijing MREDA Technology Co., Ltd. The widely used commercial photosensitizer RB was supplied by Shanghai Yuanye Bio-Technology Co., Ltd. Fetal bovine serum (FBS) was obtained from Wuhan Procell Life Science&Technology Co., Ltd. Dulbecco's modified eagle medium (DMEM), phosphate buffered saline (PBS), penicillin-streptomycin and trypsin-EDTA solution were purchased from Gibco (Thermo Fisher, US). DAPI, Hoechst 33342 and a Live (Calcein-AM)/Dead (propidium iodide, PI) viability/cytotoxicity kit were purchased from Shanghai BioScience Co., Ltd. DCFH-DA, Lyso-Tracker Green, Tunel Apoptosis Assay Kit and JC-1 were purchased from Beyotime. MitoTracker Orange was supplied by Nanjing EnoGene Biotechnology Co., Ltd. Acridine Orange Detection Kit was obtained from Beijing Leagene Biotechnology Co., Ltd. All reagents and solvents were obtained commercially and used without further purification unless otherwise noted.
2.2. Measurements and characterizations
The 1H NMR and 13C NMR spectra of all compounds were tested on a 400 MHz Brücker spectrometer. HRMS were performed with Waters Xevo G2-XS qtof Mass Spectrometer. The UV–vis spectra were captured by a Metash UV-6000 spectrophotometer. Fluorescence spectra and fluorescence lifetime were detected on a Transient Steady-state Fluorescence Spectrometer (Edinburgh, FLS 980). The absolute fluorescence quantum yield was determined by a hamamatsu absolute PL quantum yield spectrometer C11347. The dynamic light scattering (DLS) size distribution was determined by a Malvern Zetasizer Nano instrument. Transmission electron microscope (TEM) images were recorded by a JEM 2100 microscope. The CLSM images were acquired on Olympus FV3000 and ZEISS Axio Observer A1. The two-photon fluorescence images were collected by a FV1200MPE multi-photon laser scanning microscopy. The mitochondrial morphology was examined under a Hitachi H-7500 transmission electron microscope. In vivo imaging and in vitro organ fluorescence images were obtained by a PerkinElmer IVIS Lumina II pre-clinical imaging system. Photodynamic experiments were implemented by using a 400–700 nm xenon lamp (PL-X500D, Beijing Precise Technology Co., Ltd.).
2.3. Synthesis of 4-(7-Bromo-2,1,3-benzothiadiazol-4-yl)-N,N-diphenylbenzenamine (compound A1)
The synthetic method of A1 was similar as we reported previously [28]. In a 100 mL Schlenk tube, 4,7-dibromo-2,1,3-benzothiadiazole (2.92 g, 10 mmol), 4-(diphenylamino)phenylboronic acid (2.89 g, 10 mmol), Pd(PPh3)4 (0.46 g, 0.4 mmol) and K2CO3 (3.31 g, 24 mmol) were dissolved in THF/water mixture (v/v = 50: 12). The reaction was carried out at 90 °C under the protection of nitrogen for 20 h. After cooling, deionized water was added to quench the reaction, then the mixture was extracted with dichloromethane (3 × 40 mL). The organic layer was dried with anhydrous magnesium sulfate, then the solvent was removed by vacuum evaporation. The crude product was purified by column chromatography (dichloromethane: petroleum = 1: 2), yielding pure A1 (2.37 g, 52%) as orange powder. 1H NMR (400 MHz, CD2Cl2, δ): 7.91 (d, J = 7.6 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.56 (d, J = 7.6 Hz, 1H), 7.31 (d, J = 7.6 Hz, 4H), 7.17 (d, J = 8.0 Hz, 6H), 7.09 (t, J = 7.3 Hz, 2H).
2.4. Synthesis of 5-[7-[4-(Diphenylamino)phenyl]-2,1,3-benzothiadiazol-4-yl]-2-thiophene- carboxaldehyde (compound A2)
Compound A2 was also prepared via the similar method of A1 as we reported previously [28]. In a 100 mL Schlenk tube, compound A1 (1.83 g, 4 mmol), 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophene-2-carbaldehyde (1.14 g, 4.8 mmol) and Pd(PPh3)4 (0.184 g, 0.16 mmol) were added to a mixture of THF (50 mL) and aqueous solution of K2CO3 (2 M, 12 mL). The mixture was stirred at 90 °C under an atmosphere of nitrogen for 20 h. After the mixture was cooled down, it was poured into deionized water to quench the reaction. The mixture was extracted with dichloromethane for three times, then the organic layer was dried over magnesium sulfate and the solvent was removed by vacuum evaporation. The crude sample was purified by column chromatography (dichloromethane: petroleum = 1: 4) to obtain compound A2 as red powder (1.13 g, 58%). 1H NMR (400 MHz,CD2Cl2, δ): 9.97 (s, 1H), 8.21 (s, 1H), 8.09 (d, J = 7.2 Hz, 1H), 7.93–7.87 (m, 3H), 7.77 (d, J = 7.2 Hz, 1H), 7.32 (t, J = 7.3 Hz, 4H), 7.18 (d, J = 7.6 Hz, 6H), 7.10 (t, J = 6.8 Hz, 2H).
2.5. Synthesis of ADB
2,3-dimethylbenzo[d]thiazol-3-ium iodide (0.35 g, 1.2 mmol) and compound A2 (0.49 g, 1 mmol) were dissolved in 20 mL of methanol/dichloromethane (v/v = 3: 1) mixed solvent, then 0.5 mmol of pyridine was added. The mixture was stirred at 68 °C for 17 h. After cooling to room temperature, the solvent was removed by vacuum evaporation and the resulting residue was washed several times with ether and methanol to obtain product ADB as black powder. (0.61 g, 80%). 1H NMR (400 MHz, DMSO-d6, δ): 8.42 (d, J = 15.5 Hz, 1H), 8.34 (dd, J = 16.0, 7.8 Hz, 2H), 8.26 (d, J = 3.7 Hz, 1H), 8.18 (d, J = 8.3 Hz, 1H), 8.05 (d, J = 3.6 Hz, 1H), 7.96 (d, J = 8.4 Hz, 2H), 7.91 (d, J = 7.6 Hz, 1H), 7.80 (t, J = 7.7 Hz, 1H), 7.72–7.68 (m, 2H), 7.38–7.35 (m, 4H), 7.10 (m, 8H), 4.32 (s, 3H). 13C NMR (100 MHz, DMSO-d6, δ): 171.44, 153.44, 152.28, 148.32, 147.17, 146.39, 142.42, 141.26, 141.05, 136.24, 133.38, 130.74, 130.23, 130.04, 129.80, 128.87, 128.68, 128.15, 127.82, 127.60, 125.23, 124.63, 124.32, 123.91, 122.27, 117.07, 112.02, 36.73. HRMS (ESI) m/z: [M]+ calcd for C38H27N4S3, 635.1392; found, 635.1394.
2.6. Evaluation of 1O2 generation
In order to detect the 1O2 generation ability of ADB, ABDA was used as an active oxygen detector. ADB solution in DMSO (1 mmol, 10 μL) and ABDA solution in DMSO (10 mmol, 14 μL) were added into 1.976 mL of water so that the final concentrations of ADB and ABDA were 5 μM and 70 μM, respectively. Then, the mixed solution was irradiated with white light (400–700 nm, 50 mW cm−2). RB and ABDA solution in absence of ADB were chosen as the control groups. The absorption spectra of the solutions were recorded by UV–vis spectrophotometer.
2.7. Measurement of 1O2 generation quantum yield
ABDA was used as an active oxygen indicator. Preparing the aqueous solution of RB and ADB and adjusting the concentrations so that the absorption maxima of both of them were 0.2 OD. Then the solutions were irradiated with white light (400–700 nm, 50 mW cm−2) for 10 min, and the absorbance of ABDA at 378 nm was recorded every 30 s. The 1O2 generation quantum yield of ADB in water was calculated by the following equation [51].
ΦRB is the ROS quantum yield of RB, which is 0.75 in water.
2.8. Cell culture
Cells were cultured in DMEM medium at 37 °C in a 5% CO2 atmosphere. The DMEM medium was supplemented with 10% FBS and 1% antibiotics (penicillin-streptomycin).
2.9. Cytotoxicity and phototoxicity assessment of ADB
MTT assay was used to detect the biocompatibility and toxicity of ADB. The HepG2, HeLa, MCF-7 and MCF-10 A cells were seeded in 96-well plates and cultured for 24 h. Then the medium was replaced by fresh DMEM containing different concentrations of ADB. After 24 h, the cells were washed with PBS for three times and co-incubated with MTT working solution for 4 h in the dark. Then the MTT solution was sucked out and the cells were stained with DMSO for 15 min. The absorbance of each well at 490 nm was detected with a microplate reader.
The phototoxicity of ADB was also evaluated by MTT assay. MCF-7 cells were incubated with DMEM containing different concentrations of ADB for 6 h. Then the cells were irradiated with white light (400–700 nm, 50 mW cm−2) for 10 min and cultured in the dark for 18 h, then the absorbance at 490 nm was measured by a microplate reader.
2.10. Confocal images
MCF-7 cells were seeded in 20 mm glass bottom cell culture dishes and cultured 24 h to grow until a confluence of around 80%. Then, the medium was removed and replaced by fresh DMEM containing ADB (4, 8, 12, 16, 20 μM). After treating for 6 h, the medium was removed and the cells were washed three times with PBS. Then cells were imaged by an Olympus FV3000 confocal laser scanning microscope.
2.11. Confocal colocalization experiments
MCF-7 or MCF-10 A cells (5 × 104 cells/well) were inoculated in glass bottom cell culture dishes at 37 °C in 5% CO2 atmosphere for 24 h. After removing the medium, ADB (20 μM) was added to incubate with MCF-7 or MCF-10 A cells for 1, 2, 4 and 8 h, respectively. After washing the cells by PBS, Lyso-Tracker Green (50 nM) and Mito-Tracker Orange (30 nM) were added and incubated for 15 min respectively. Finally, the residual probe was washed off with PBS for three times. Cells were imaged by an Olympus FV3000 confocal laser scanning microscope and the colocalization coefficient was calculated by Image J.
2.12. Two-photon cell and tissue imaging experiments
MCF-7 cells in logarithmic growth stage were seeded in glass bottom cell culture dishes for 24 h. Then the medium was replaced by fresh DMEM containing ADB (20 μM). After incubation for 6 h, the medium was removed and the cells were washed three times with PBS. The fluorescence intensity of the cells under different excitation wavelengths (740–880 nm) were measured by a FV1200MPE multi-photon laser scanning microscopy.
For In vivo tissue fluorescence imaging, ADB (3 mg kg−1) was injected into BALB/c nude mice via tail vein. After anesthetizing the mice by 10% pentobarbital, the enterocoelia was opened and the mesenteric vessels were fixed with a small animal organ fixation adsorber. Then the fluorescence imaging of mesenteric vessels was observed with a FV1200MPE multi-photon laser scanning microscopy (λex = 800 nm).
2.13. Detection of intracellular ROS
MCF-7 cells (5 × 104 cells/well) in the logarithmic growth stage were seeded in glass bottom cell culture dishes for 24 h. Subsequently, ADB (20 μM) was added to the cell dishes and incubated for 6 h. Then the cells were irradiated with white light (400–700 nm, 50 mW cm−2) for 10 min. After washing by PBS for three times, the cells were cocultured with DCFH-DA (10 μM) in the dark for 50 min, then a ZEISS Axio Observer A1 microscope was used to record the intracellular fluorescence.
2.14. Live/dead cell staining assay
MCF-7 cells were incubated with ADB (20 μM) for 6 h. After washing with PBS, the cells were exposed to white light (400–700 nm, 50 mW cm−2) for 10 min. Then the cells were cultured in the dark for 1 h. The medium was sucked out, followed by the addition of fresh DMEM containing Calcein AM and PI. After staining for 15 min, the survival of the cells was observed by an Olympus FV3000 confocal laser scanning microscope.
2.15. Mitochondria membrane potential (MMP) analysis
MCF-7 cells were cultured in the confocal culture dishes at 37 °C under an atmosphere containing 5% CO2 for 24 h. MCF-7 cells were then washed by PBS and incubated with DMEM containing ADB (20 μM) for 6 h. After irradiating with white light (400–700 nm, 50 mW cm−2) for 10 min and then cultured in dark for 1 h, cells were washed with PBS and treated with 1 mL DMEM containing JC-1 probe (10 μM) for 20 min. Finally, the cells were washed with PBS three times and imaged by the FV 3000 confocal laser scanning microscope.
2.16. Mitochondrial damage evidenced by Bio-TEM
MCF-7 cells were incubated with ADB (20 μM) for 6 h. Then the cells were irradiated with white light (400–700 nm, 50 mW cm−2) for 10 min. 1 h later, the cells were washed with PBS and digested by trypsin solution without EDTA, then the cells were fixed with 2.5% glutaraldehyde in PBS buffer. After staining at room temperature for 1 h and incubated overnight at 4 °C, glutaraldehyde was removed and the cells were washed with PBS. Sections were postfixed with 2% osmium, rinsed, dehydrated and embedded in durcupan resin. Ultrathin sections were cut with a diamond knife, stained with lead citrate and examined under a Hitachi H-7500 transmission electron microscope.
2.17. Lysosome disruption analysis
MCF-7 cells (5 × 104 cells/well) were seeded in glass bottom cell culture dishes for 24 h. Subsequently, ADB (20 μM) was incubated with cells for 6 h. Then the cells were irradiated with white light (400–700 nm, 50 mW cm−2) for 10 min and cultured in the dark for 1 h. After washing by PBS for three times, the cells were stained with acridine orange (AO) for 15 min. Then an Olympus FV3000 confocal laser scanning microscope was used to observe the intracellular fluorescence (green channel: excited at 488 nm, collected at 500–540 nm; red channel: excited at 561 nm, collected at 570–620 nm).
2.18. Tumor-bearing mouse model
All animals were provided by the Laboratory Animal Center of Southern Medical University with certificates (approval number: No.44002100032650). All animal experiments were carried out according to the procedures approved by the Care and Use of Laboratory Animals of Southern Medical University and strictly conformed to the guidelines formulated by Southern Medical University Experimental Animal Ethics Committee.
MCF-7 tumor-bearing mouse model: MCF-7 cells (2 × 106 cells in 0.1 mL of PBS) were injected subcutaneously into the right flank of the BALB/c nude mice (5 weeks, female) to establish tumor model. When the tumor volume developed to nearly 100 mm3, the mice were allowed for in vivo imaging and PDT experiments.
2.19. In vivo imaging
When the tumor volume reached about 100 mm3, MCF-7 tumor-bearing mouse was intratumorally injected with PBS containing ADB (3 mg kg−1). A multispectral small animal imaging system was used to observe the in vivo fluorescence signals at different time after injection (10 min, 1 h, 3 h, 12 h, 24 h, 48 h, 72 h). The excitation wavelength was 465 nm, and the fluorescence was collected at 810 nm. 72 h later, the mouse was sacrificed and the organs (heart, liver, spleen, lung, kidney and tumor) were taken to observe the fluorescence intensity.
2.20. Assessment of in vivo PDT effect
When the tumor volumes reached about 100 mm3, 16 mice were randomly divided into four groups (n = 4) for different treatments: (1) saline; (2) saline + light; (3) ADB and (4) ADB + light. In Group 1 and 3, the mice were fed in the dark after intratumoral injection of 50 μL saline or ADB (3 mg kg−1), respectively. The mice in Group 2 and 4 were intratumorally injected with 50 μL saline or ADB (3 mg kg−1), respectively, and then the tumor sites of the mice were irradiated with white light (400–700 nm, 100 mW cm−2) for 10 min at day 0, 3, 6, 9. The body weight and tumor volume of the mice in each group were measured and recorded every two days (volume = width2 × length/2) to evaluate the in vivo PDT effect.
2.21. Hematological and histological examinations
After complete treatment for 14 days, the mice were sacrificed, and the blood, tumors and main organs (heart, liver, spleen, lung and kidney) were collected for analysis. The tumors and organs were immersed in 4% paraformaldehyde solution for fixation, then dehydrated, paraffin embedded, and sectioned. The sections were stained with hematoxylin & eosin (H&E) for histological examinations, and the tumor sections were also analyzed by TUNEL staining assay. The blood samples were dropped into tubes containing Anticoagulant Sodium Citrate Solution, then the samples were shaken up, and further used for whole blood cell analysis.
2.22. Statistical analysis
The values of the bar charts were expressed as mean ± standard deviation. Graphic drawing was drawn on Origin 8 software. The statistical significance of differences between groups was performed by using ordinary one-way ANOVA method on the GraphPad Prism 8.0.1 software. A value of P < 0.05 was considered significant and were indicated with asterisks: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001,∗∗∗∗P < 0.0001.
3. Results and discussion
3.1. Design and optical characterization of ADB
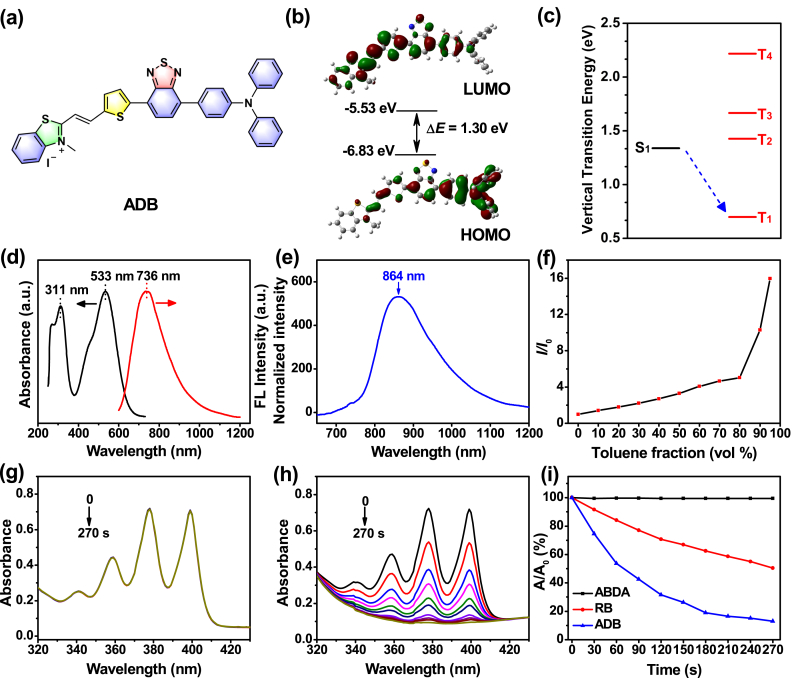
Encouraged by the excellent light-harvesting ability and power-conversion efficiency of organic sensitizers based on D-A-π-A structure in solar energy conversion studies [52], the concept of combined acceptor has been introduced into the construction of PDT photosensitizers, which demonstrated obvious potential in improving the absorption and emission of the photosensitizers [6,28]. In this contribution, a rigid molecule, namely ADB (Fig. 1a), based on a D-A-π-A skeleton was designed and synthesized with the expectation to endow it with AIE characteristics, two-photon absorption, long-wavelength absorption and 1O2 generation upon irradiation. Triphenylamine was chosen as an electron donor, benzothiazole as an auxiliary electron acceptor, and 2,3-dimethylbenzo[d]thiazol-3-ium iodide as a second acceptor, which was very conducive to render the photosensitizer with red-shifted absorption and good water solubility. In addition, a thiophene bridge was adopted to connect the two acceptors. The synthetic route of ADB was shown in Scheme S1. Intermediate A1 was prepared by the typical Pd-catalyzed Suzuki coupling reaction between 4,7-dibromo-2,1,3-benzothiadiazole and 4-(diphenylamino)phenylboronic acid. Then thiophene-2-carbaldehyde group was subsequently linked to A1 to yield A2 via the similar method. Finally, ADB was obtained through Knoevenagel condensation between A2 and 2,3-dimethylbenzo[d]thiazol-3-ium iodide using pyridine as the catalyst. The detailed synthetic route, NMR and high-resolution mass spectra of ADB and the intermediates can be found in the Supplementary Material (Scheme S1, Figs. S1–S5).
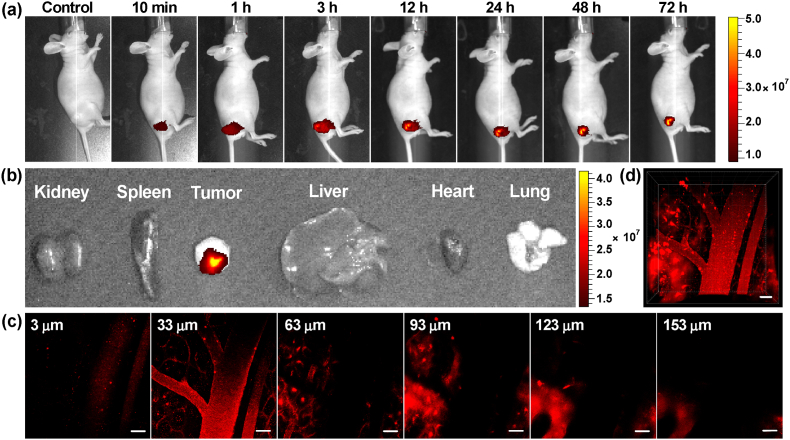
Fig. 1.
(a) Chemical structure of ADB. The (b) HOMO and LUMO distributions and (c) ΔEST value of ADB obtained by DFT/B3LYP/6-31G(d,p) Gaussian calculations. (d) Absorption and emission spectra of ADB in DMSO. (e) Normalized emission spectrum of ADB solid powder. (f) Fluorescence intensity ratios (I/I0) of ADB in toluene/DMSO mixture with different fts at the maximum emission wavelength; I0 is the fluorescence intensity of ADB in DMSO. Degradation of (g) ABDA and (h) ABDA with ADB under light irradiation (400–700 nm, 50 mW cm−2). (i) Decomposition of ABDA under the presence of ADB or RB upon irradiation, where A0 and A are the absorbance of ABDA at 378 nm before and after irradiation, respectively. [ADB or RB] = 5 μM; [ABDA] = 70 μM.
UV–vis absorption and fluorescence spectrophotometer were used to investigate the optical properties of ADB. ADB had a good solubility in DMSO, CH2Cl2, CHCl3 and other organic solvents. The absorption and emission peaks of ADB in different solvents were different (Fig. S6), and the values of which were summerized in Table S1. It was clear that ADB gave two absorption bands in the range of 300–340 nm and 470–580 nm in the solvents, which were derived from the π–π∗ transition of the conjugated backbone structure and intramolecular charge transfer (ICT) between the D–A units [36,38,[52], [53], [54], [51]], respectively. The ICT transition was further confirmed by the significantly solvent-dependent emission spectra as shown in Fig. S6b. The emission peak of ADB red-shifted obviously with the increasing of solvent polarity. For example, the maximum emission appeared at 659 nm in ethyl acetate, while it centered at 736 nm in DMSO, belonging to the near-infrared I region (Fig. 1d). In solid state, the emission maximum of ADB further red-shifted to 864 nm (Fig. 1e). Near-infrared emission has a large penetration depth, which can effectively avoid the interference of biological background fluorescence. At the same time, the Stokes shift of ADB in DMSO exceeded 200 nm, which could reduce the mutual interference between absorption and emission spectra, and improve the signal-to-noise ratio for biological imaging. The fluorescence lifetime of ADB solid powders and the cyclic voltammogram of ADB film were listed in Figs. S7 and S8, respectively. To investigate the AIE feature of ADB, its fluorescence spectra in toluene/DMSO mixture with different toluene fractions (ft) were measured (Fig. 1f and Fig. S9). The fluorescence of ADB in DMSO was very weak because of the active intramolecular rotations in solution [55,56]. However, the fluorescence intensity of the mixture increased obviously with the increase of the ratio of toluene/DMSO. When the toluene fraction reached 95%, the fluorescence intensity was about 16 times higher than that in pure DMSO, revealing a typical AIE characteristics of ADB. The hydrodynamic diameter of ADB in water measured by dynamic light scattering (DLS) was in accordance with the result detected by TEM (Fig. S10), which confirmed the nanoparticle formation in aqueous solution. The absolute fluorescence quantum yields of ADB in DMSO solution ([ADB] = 10 μM), aggregate (ft = 90%) and solid state were measured and the values were 6.9, 19.4 and 0.8% (excited at 500 nm), respectively. Comparing with other AIE-active fluorophores, ADB has bright emission in the near-infrared region [6,28,36]. The photostability of ADB was also analyzed. The fluorescence intensity of ADB only decreased by about 5.65% upon light (200 mW cm−2) irradiation for 10 min, and the absorption profile remained almost unchanged, indicating that ADB had a good photostability (Fig. S11).
3.2. 1O2 generation property of ADB
To evaluate the 1O2 generation efficiency of ADB, the classical dye 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) was used as an active oxygen indicator. As illustrated in Fig. 1g and h, the UV–vis absorption of ABDA solution without ADB remained almost unchanged after irradiation (400–700 nm, 50 mW cm−2) for 10 min. Whereas, in the presence of ADB, the absorption of ABDA solution at 378 nm decreased gradually with the increasing of irradiation time. After illumination for 270 s, the absorbance of ABDA decreased by 86.92%, which was almost difficult to detect. These results indicated that most ABDA was consumed by the ROS generated by ADB. Meanwhile, rose Bengal (RB) was selected as a reference. Under the same conditions, the absorption of ABDA at 378 nm only decreased by 49.65% after irradiation for 270 s (Fig. S12). Compared with RB, ADB could produce ROS more efficiently, and its 1O2 generation quantum yield was calculated to be 0.442 according to the method that have been reported previously [28,57,58]. These results proved that ADB could generate ROS effectively and could be used as a photosensitizer in photodynamic therapy.
Intersystem crossing from the lowest singlet excited state (S1) to the lowest triplet excited state (T1) plays a significant role in the generation of 1O2. To get a further insight into the molecular property of ADB, the theoretical calculation based on DFT/B3LYP/6-31G method was conducted. As could be seen in Fig. 1b, the lowest unoccupied molecular orbital (LUMO) of ADB was mainly distributed on the benzothiazole and thiophene units, while the highest occupied molecular orbital (HOMO) was mainly localized on the triphenylamine unit, which suggesting effective intramolecular charge transfer in ADB. The HOMO and LUMO were about −6.83 and −5.53 eV, respectively, and the energy gap of ADB was 1.30 eV. The relatively narrow band gap could result in red-shifted absorption and lower ΔEST, which was mainly attributed to the stronger electron push-pull effect in the D-A-π-A structure. The ΔEST value of ADB was calculated to be 0.6402 eV (Fig. 1c), indicating effective intersystem crossing from S1 to T1 state and highly efficient 1O2 generation [[59], [60], [61]].
3.3. Fluorescence imaging in vitro
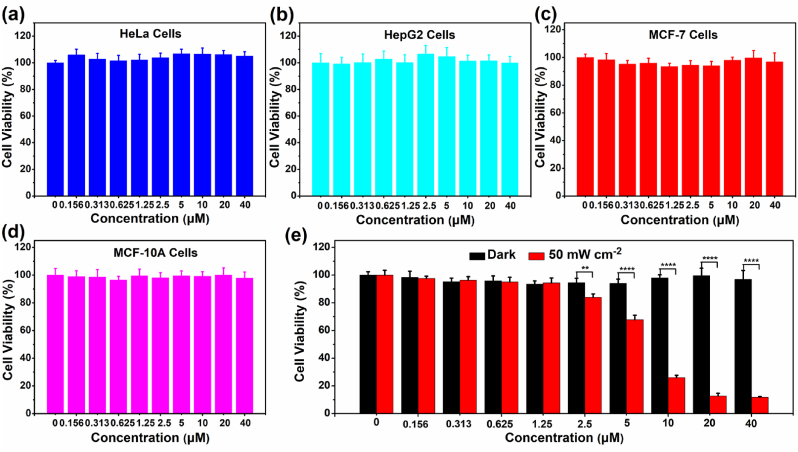
The near-infrared emission, good photostability, large Stokes shift and efficient 1O2 generation of ADB encouraged us to further investigate its bioapplications. Biocompatibility was one of the deciding factors to determine whether the compound was suitable for the ensuing biological applications. So, the in vitro cytotoxicity of ADB towards HeLa, HepG2, MCF-7 and MCF-10 A cells was firstly measured by using the MTT method. As presented in Fig. 2, the cells could still retain >90% viability after incubation with 40 μM ADB for 24 h, proving that ADB has low toxicity and good biocompatibility to cells, which opened the door for further biological applications.
Fig. 2.
Cell viability of (a) HeLa, (b) HepG2, (c) MCF-7 and (d) MCF-10 A cells treated with different concentrations of ADB using the MTT method. (e) Cell viability of MCF-7 cells incubated with ADB upon white light irradiation (400–700 nm, 50 mW cm−2) for 10 min ∗∗, P < 0.01; ∗∗∗∗, P < 0.0001.
To investigate its in vitro cellular imaging ability, ADB was incubated with MCF-7 cells for 6 h, and then observed with a confocal laser scanning microscope (CLSM). As shown in Fig. 3a, ADB could effectively accumulate in the cytoplasm and form a relatively uniform distribution around the nucleus, exhibiting bright red fluorescence signals. In addition, the fluorescence intensity in MCF-7 cells obviously enhanced with the increase of the concentration of ADB (Fig. 3b). This result proved that ADB could be internalized by cells and emited red fluorescence in cells.
Fig. 3.
(a) CLSM images of MCF-7 cells incubated with ADB at different concentrations for 6 h. Scale bars: 30 μm. (c) Two-photon fluorescence images of MCF-7 cells incubated with ADB (20 μM) upon excitation at different wavelengths. Scale bars: 20 μm. (b) and (d) Fluorescence intensities gathered from MCF-7 cells in (a) and (c) respectively. (e) CLSM images of MCF-7 cells incubated with ADB (20 μM) for 1, 2, 4 and 8 h, followed by co-staining with Lyso-Tracker Green and Mito-Tracker Orange, respectively. R1, Pearson's correlation coefficient of ADB merged with Lyso-Tracker Green; R2, Pearson's correlation coefficient of ADB merged with Mito-Tracker Orange. Scale bars: 20 μm.
The two-photon imaging capability of ADB was further studied. The MCF-7 cells were incubated with ADB for 6 h and observed with a multi-photon laser scanning microscope. The excitation wavelength was adjusted in the range of 740–880 nm, and the fluorescence images were taken every 20 nm. It can be seen from Fig. 3c that the intracellular fluorescence gradually increased with the increase of excitation wavelength, and the intracellular fluorescence was the brightest when excited at 800 nm, then the fluorescence gradually faded, which was further confirmed by calculation of the average fluorescence intensity of the cells (Fig. 3d). The photostability of ADB upon continuous one-photon and two-photon laser excitation was also evaluated, as shown in Fig. S13, the intracellular fluorescence kept almost constant under laser irradiation for 9 min. These results indicated that ADB had excellent two-photon imaging potential and excellent photostability.
To examine the organelle targeting specificity of ADB, the fluorescence colocalization experiment was performed. MCF-7 cells were cocultured with ADB for 1, 2, 4 and 8 h, followed by incubation with Lyso-Tracker Green and Mito-Tracker Orange for 15 min respectively. Then the fluorescence distributions of these probes in the cells were observed with a CLSM. As shown in Fig. 3e, the NIR fluorescence of ADB almost completely overlapped with the green fluorescence of Lyso-Tracker Green, and the Pearson's correlation coefficients were 0.7639, 0.8596, 0.8778 and 0.8886 for lysosomes of MCF-7 cells cocultured with ADB for 1, 2, 4 and 8 h respectively. At the same time, the fluorescence signals of Mito-Tracker Orange and ADB also overlapped fairly good with Pearson's coefficients of 0.4341, 0.6112, 0.7199 and 0.8139 for mitochondria of MCF-7 cells cocultured with ADB for 1, 2, 4 and 8 h respectively. It was noticed that the AIEgen ADB entered into lysosomes more rapidly than into mitochondria in the initial 2 h. Lysosomes were well known as the “waste disposal station” in the cell, so it was reasonable that they would capture ADB as extraneous particles rapidly when they entered cells. Meanwhile, the lipophilic and electrostatic interaction between ADB and mitochondria would facilitate the selective accumulation of ADB in the mitochondria, as confirmed by the Pearson's coefficients after 2 h. The details of the Quantitation analysis of the fluorescence intensities in MCF-7 cells were shown in Fig. S14 and were consistent with the cell imaging results. Fig. S15 further revealed the dual-specificity of ADB towards MCF-10 A normal cells after incubating for a period of time. These investigations distinctly demonstrated that ADB had high affinity for both lysosomes and mitochondria. This interesting mitochondria and lysosomes dual-organelles targeting capability would be very conducive to the photodynamic therapy of tumors.
3.4. In vitro PDT therapy using ADB
In the previous experiments, it was verified that ADB had bright NIR fluorescence, good biocompatibility, excellent bioimaging capability, specific dual-organelles targeting and high 1O2 generation efficiency, which encouraged us to further explore its application potential in photodynamic therapy.
MTT method was used to detect the photodynamic effect of ADB on MCF-7 cells. As presented in Fig. 2e, after exposure to white light (400–700 nm, 50 mW cm−2) for 10 min in the presence of 10 μM ADB, the survival rate of the cells was significantly reduced to 26.05%. When increasing the concentration of ADB to 20 μM, the survival rate of the cells was further reduced to 12.74%, and the IC50 value was 6.6 μM. As a comparison, the cell viability of MCF-7 cells under dark remained above 90% at the same concentration of ADB. These results suggested that ADB has an excellent effect in photodynamic therapy.
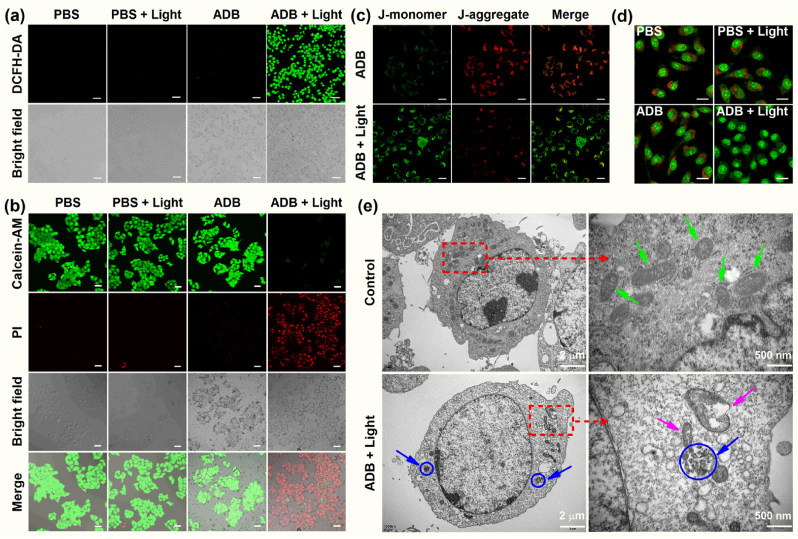
In order to verify that ADB promotes cell apoptosis by producing 1O2 in cells, 2,7-dichlorofluorescein diacetate (DCFH-DA) was utilized as an 1O2 indicator. As shown in Fig. 4a, MCF-7 cells incubated with ADB exhibited bright green fluorescence after irradiation upon white light (400–700 nm, 50 mW cm−2) for 10 min, confirming efficient generation of 1O2. Whereas there was no discernible green fluorescence in ADB or light alone treated MCF-7 cells. It could be screened that ADB greatly enhanced the production of 1O2 under irradiation, which may become an efficient photosensitizer for photodynamic therapy.
Fig. 4.
(a) Confocal images of 1O2 generation in MCF-7 cells stained with ADB (20 μM) using DCFH-DA as an indicator upon white light irradiation (400–700 nm, 50 mW cm−2). Scale bars: 100 μm. (b) Confocal images of MCF-7 cells stained with ADB (20 μM) using Calcein-AM/PI as the fluorescence probe upon irradiation (400–700 nm, 50 mW cm−2). Scale bars: 50 μm. (c) MMP analysis of MCF-7 cells using JC-1 mitochondrial membrane probe. Scale bars: 20 μm. (d) Lysosome disruption analysis of MCF-7 cells after different treatments (green channel: λex: 488 nm and λem: 500–540 nm; red channel: λex: 561 nm and λem: 570–620 nm). Scale bars: 15 μm. (e) The mitochondrial ultrastructure observed by bio-TEM.
To further verify the PDT effect of ADB, a Calcein-AM/PI kit was used to detect live/dead cells. Fig. 4b showed that upon white light (400–700 nm, 50 mW cm−2) irradiation for 10 min, MCF-7 cells cultured with ADB exhibited bright red fluorescence in the PI channel, while there was no green fluorescence could be observed in the AM channel, indicating that the cells were almost all dead. At the same time, for the cells treated by ADB or light alone, there was almost no red fluorescence in the PI channel whereas strong green fluorescence in the AM channel was observed, proving that the cells were still alive. These results further demonstrated that ADB had an outstanding PDT effect.
To further investigate the mechanism responsible for the organelle targeting-mediated PDT enhancement of ADB, the fluorescent probe JC-1 was adopted as an indicator to monitor the changes of mitochondrial membrane potential. As presented in Fig. 4c, for the cells without light irradiation, the green fluorescence was weak while the red fluorescence was relatively strong. However, upon light irradiation, the green fluorescence became stronger and the red fluorescence became weaker. The quantitation showed that there was a 2.6-fold increase in the intensity ratio of green-to-red (Fig. S16), indicating the decrease of MMP. Furthermore, biological transmission electron microscopy (bio-TEM) was conducted to observe the mitochondrial ultrastructure. As presented in Fig. 4e, without white light irradiation, the mitochondria with clear double membrane were well distributed in cytoplasm (green arrow). However, upon light irradiation, the mitochondria in MCF-7 cells treated with ADB showed obvious swollen, cavitation and crista fragmentation (purple arrow). Lysosome disruption analysis was undertaken by using Acridine Orange as indicator to monitor the integrity of the lysosomes. As presented in Fig. 4d, the red and green fluorescence in the PBS, PBS + light and ADB Groups meant that the lysosomes were intact, while in the ADB + light Group, the red fluorescence disappeared, indicating that the cell lysosomes were damaged by the 1O2 burst. Furthermore, after careful observation of the bio-TEM images in Fig. 4e, it was amazing to find that there existed really some phagocytotic vesicles in the cells of the ADB + light Group, which were indicated by blue circles and arrows. There were obviously a number of nanoparticles in the phagocytotic vesicles, which providing direct evidence of the endocytosis of ADB nanoparticles by lysosomes, and perfectly indicating the lysosomes-targeting of ADB.
Taken together, these results clearly demonstrated the lysosomes and mitochondria dual-targeting of ADB nanoparticles, both of the critical organelles were damaged by the 1O2 generated upon light irradiation, which further caused cell death and greatly enhanced the PDT effect.
3.5. Fluorescence imaging in vivo
Next, the use of ADB for in vivo fluorescence imaging of tumor-bearing mouse intratumorally injected with ADB (3 mg kg−1) was performed, and the fluorescence signals at different post-injection time were recorded by a multispectral small animal imaging system. As could be seen from Fig. 5a, ADB was gradually absorbed by the tumor after injection, and the fluorescence intensified increasingly with time. After 3 h, ADB was diffused to the whole tumor, and the bright NIR fluorescence lightened the tumor site very clearly. Then the fluorescence intensity slightly weakened with a prolongation of time, but still remained at a relatively high level. The bright NIR fluorescence could still be seen at the tumor site after 72 h, proving that ADB had an effective tumor retention property. Quantification of the average fluorescence intensity of the images in Fig. 5a was shown in Fig. S17. After injection of ADB for 72 h, the mouse was sacrificed and the main organs and tumor were taken for ex vivo fluorescence imaging (Fig. 5b). The fluorescence signal could only be observed in the excised tumor and there was no NIR fluorescence in other organs. To further verify the metabolic capability of ADB, the in vivo fluorescence imaging was carried out again and the observation time was prolonged to 7 days. As shown in Fig. S18, the fluorescence intensity obviously diminished after 96 h, and it was nearly disappeared after 7 days. These results proved that ADB could selectively accumulate at tumor and retain in mouse body for an appropriate time, which would be very conducive to the following in vivo photodynamic therapy.
Fig. 5.
(a) In vivo fluorescence imaging of tumor-bearing nude mice at different time points after intratumorally injected with ADB. (b) Ex vivo fluorescence images of the internal organs of mice after injection of ADB for 72 h. (c) Two-photon confocal images of mouse mesenteric vessels stained with ADB at a penetration depth from 3 to 153 μm. (d) Reconstructed 3D image of mouse mesenteric vessels stained with ADB. Scale bars: 50 μm, λex: 800 nm.
Considering the virtues of deeper penetration, high contrast and longer excitation wavelength of two-photon imaging, and simultaneously inspired by the excellent imaging capability of ADB in cells, in vivo two-photon tissue depth and time series fluorescence imaging of the mesenteric vessels of nude mouse was performed on a multi-photon laser scanning microscope. Because of its rich blood vessels and large blood flow, the mesenteric vessel was an ideal place for imaging. As shown in Fig. 5c and d, the mesenteric vessel network was clearly visualized and the imaging depth could reach 153 μm. Simultaneously, the time and 3D series vascular images in mesentery revealed that the blood flow could be dynamically observed (see SM videos). These results demonstrated that ADB had great potential in bioimaging and real-time tumor and organ monitoring.
3.6. ADB-mediated PDT therapy in vivo
The PDT performance of ADB in vivo was further investigated by establishing tumor-bearing mice model. The MCF-7 cells were subcutaneously inoculated to the lateral part of the right lower limb of the mouse. When the tumor volumes reached about 100 mm3, the mice were randomly divided into four groups for different treatments. Group 1: saline; Group 2: saline + light; Group 3: ADB and Group 4: ADB + light. After being subjected to different treatments, the body weights and tumor volumes of these mice were monitored (Fig. 6). It was noticed that the tumor volumes of the mice in Groups 1–3 gradually increased, and could reach about 8 times of magnitude to the initial volume after 14 days. In comparison, the tumor growth of the mice in Group 4 was effectively inhibited during the whole treatment period, and showing statistical difference to the control groups. The average tumor lengths, widths and volumes of the mice in Groups 1–4 in different days were listed in Table S2 in the Supplementary Material. In addition, no significant difference in the body weight of the mice in Groups 1–4 was observed (Fig. S19). TUNEL analysis of tumor tissues (Fig. S20) further showed that there were large areas of apoptotic cells with condensed nuclei for the sample treated with ADB and white light irradiation (Group 4). These observations revealed that compound ADB could efficiently generate ROS upon irradiation and effectively suppress tumor growth, light exposure or ADB had no adverse effects on the growth of mice, which was further validated by the following pathological experiments.
Fig. 6.
(a) Photographs of living mice upon treatment for 14 days. (b) Photographs of tumors collected after different treatments. (c) Tumor growth curves of the mice during the process of different treatments. (d) Weights of dissected tumor after 14 days of treatments. ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.0001.
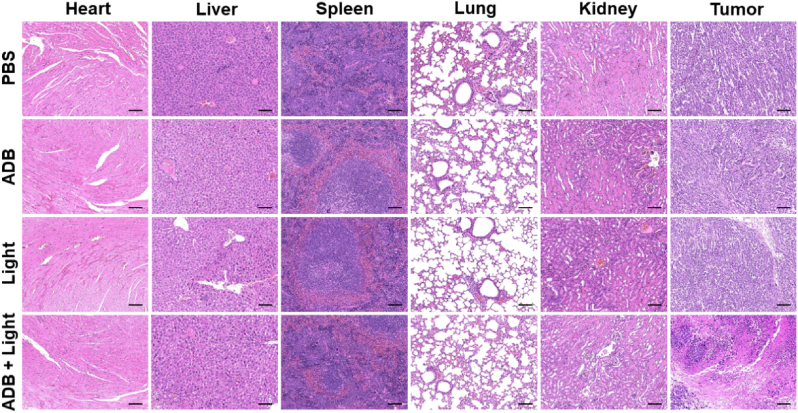
H&E staining was carried out to assess the photoinduced damages to the main tissues and organs, and simultaneously evaluate the biocompatibility of ADB in vivo. As presented in Fig. 7, no obvious pathological abnormalities and damages were observed in the main organs (including heart, liver, spleen, lung and kidney) of the treated mice in Groups 1–4. However, it was noticed that in comparison with the other three groups, the H&E staining of the tumor slice after ADB + light treatment exhibited severe necrosis. The whole blood analyses revealed that there was no significant difference in the blood routine parameters (WBC (white blood cell), RBC (red blood cell), HGB (hemoglobin), HCT (hematocrit), MCV (mean corpuscular volume), MCH (mean corpuscular hemoglobin)) and hepatic and renal function markers (ALT (alanine aminotransferase), AST (aspartate aminotransferase), BUN (blood urea nitrogen) and CR (creatinine)) (Fig. S21). These results altogether demonstrated the great potential for ADB as an efficient photosensitizer for in vivo PDT, and confirmed the negligible toxicity and desirable biocompatibility of ADB, which were crucial for a photodynamic agent.
Fig. 7.
H&E staining images of major organs of mice from Groups 1–4 subjected to different treatments. Scale bars: 100 μm.
4. Conclusion
In summary, a lysosomes and mitochondria dual-organelles targeting organic photosensitizer, ADB, which possessed amphiphilic D-A-π-A structure, was rationally designed and synthesized. This positively charged AIE fluorophore exhibited good biocompatibility, high photostability, bright NIR emission and excellent two-photon bioimaging capability. In vitro investigations revealed that ADB nanoparticles could locate precisely at both lysosomes and mitochondria, and presented significant antitumor activity through efficient generation of reactive oxygen species which induced the damage of critical organelles upon light irradiation. More importantly, the imaging-guided in vivo PDT applied on MCF-7 tumor-bearing mice successfully suppressed tumor growth upon light exposure, demonstrating the great potential of using ADB for bioimaging and clinical imaging-guided photodynamic therapy applications. Moreover, this research provided a new sight for the design of multi-organelles targeted and more biocompatible NIR photosensitizers for phototheranostics.
Credit author statement
Shaozhen Wang: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Yunhui Liao: Investigation, Methodology, Software. Zhaoji Wu: Investigation, Software. Yihong Peng: Investigation. Yuchen Liu: Investigation. Yinghua Chen: Methodology, Resources. Longquan Shao: Resources, Funding acquisition, Methodology, Validation, Writing – review. Zhijie Zeng: Project administration. Yanshan Liu: Conceptualization, Methodology, Resources, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Guangdong Natural Science Foundation (2018A030313976), the Open Fund of the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology), China, 2022-skllmd-03, and National Natural Science Foundation of China (52072167). The authors are very grateful to Ting Guo and Lei Ying (South China University of Technology) for their selfless help during the experiment process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2023.100721.
Contributor Information
Longquan Shao, Email: shaolongquan@smu.edu.cn.
Zhijie Zeng, Email: zzjie@smu.edu.cn.
Yanshan Liu, Email: liuys9@smu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global cancer statistics GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2020;71(2021):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Li X.S., Lovell J.F., Yoon J., Chen X.Y. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17:657–673. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 3.Fan W.P., Huang P., Chen X.Y. Overcoming the achilles' heel of photodynamic therapy. Chem. Soc. Rev. 2016;45:6488–6519. doi: 10.1039/c6cs00616g. [DOI] [PubMed] [Google Scholar]
- 4.Yaqoob M.D., Xu L., Li C.F., Leong M.M.L., Xu D.D. Targeting mitochondria for cancer photodynamic therapy. Photodiagn. Photodyn. 2022;38 doi: 10.1016/j.pdpdt.2022.102830. [DOI] [PubMed] [Google Scholar]
- 5.Ethirajan M., Chen Y., Joshi P., Pandey R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 6.Wu W.B., Mao D., Xu S.D., Panahandeh-Fard M., Duan Y.K., Hu F., Kong D.L., Liu B. Precise molecular engineering of photosensitizers with aggregation-induced emission over 800 nm for photodynamic therapy. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 7.Lan M.H., Zhao S.J., Liu W.M., Lee C.S., Zhang W.J., Wang P.F. Photosensitizers for photodynamic therapy. Adv. Healthcare Mater. 2019;8 doi: 10.1002/adhm.201900132. [DOI] [PubMed] [Google Scholar]
- 8.Wang R., Li X.S., Yoon J. Organelle-targeted photosensitizers for precision photodynamic therapy. ACS Appl. Mater. Interfaces. 2021;13:19543–19571. doi: 10.1021/acsami.1c02019. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z.J., Song J.B., Nie L.M., Chen X.Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016;45:6597–6626. doi: 10.1039/c6cs00271d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim C.K., Heo J., Shin S., Jeong K., Seo Y.H., Jang W.D., Park C.R., Park S.Y., Kim S., Kwon I.C. Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Lett. 2013;334:176–187. doi: 10.1016/j.canlet.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y.J., Huang W.D., Tan X.Y., Wang J.H., Zhao Y.F., Hu J.B., Wang S.H. A mitochondria-targeted dual-functional aggregation-induced emission luminogen for intracellular mitochondrial imaging and photodynamic therapy. Biomater. Sci. 2021;9:1232–1236. doi: 10.1039/d0bm02099k. [DOI] [PubMed] [Google Scholar]
- 12.Dai J., Wu X., Ding S.Y., Lou X.D., Xia F., Wang S.X., Hong Y.N. Aggregation-induced emission photosensitizers: from molecular design to photodynamic therapy. J. Med. Chem. 2020;63:1996–2012. doi: 10.1021/acs.jmedchem.9b02014. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Jiang C.S., Longo J.P.F., Azevedo R.B., Zhang H., Muehlmann L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B. 2018;8:137–146. doi: 10.1016/j.apsb.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao M., Yu F.B., Lv C.J., Choo J., Chen L.X. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017;46:2237–2271. doi: 10.1039/c6cs00908e. [DOI] [PubMed] [Google Scholar]
- 15.Hernot S., van Manen L., Debie P., Mieog J.S.D., Vahrmeijer A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20:e354–e367. doi: 10.1016/S1470-2045(19)30317-1. [DOI] [PubMed] [Google Scholar]
- 16.Feng G.X., Liu B. Aggregation-induced emission (AIE) dots: emerging theranostic nanolights. Acc. Chem. Res. 2018;51:1404–1414. doi: 10.1021/acs.accounts.8b00060. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C.L., Kwok R.T.K., Lam J.W.Y., Tang B.Z. Aggregation-induced emission: a trailblazing journey to the field of biomedicine. ACS Appl. Bio Mater. 2018;1:1768–1786. doi: 10.1021/acsabm.8b00600. [DOI] [PubMed] [Google Scholar]
- 18.Lovell J.F., Liu T.W.B., Chen J., Zheng G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010;110:2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 19.Correia J.H., Rodrigues J.A., Pimenta S., Dong T., Yang Z.C. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13:1332. doi: 10.3390/pharmaceutics13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X.Z., Liu J.P., Fan J.L., Chao H., Peng X.J. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021;50:4185–4219. doi: 10.1039/d0cs00173b. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z.J., Yu L., Wang Y.H., Wang C.L., Mu Q.C., Liu X.J., Yu M., Wang K.-N., Yao G.Y., Yu Z.Q. Dynamic adjust of non-radiative and radiative attenuation of AIE molecules reinforces NIR-II imaging mediated photothermal therapy and immunotherapy. Adv. Sci. 2022;9 doi: 10.1002/advs.202104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu G.L., Zhang R.Y., Gu Y., Wang J.G., Ma C., Kwok R.T.K., Lam J.W.Y., Sung H.H.Y., Williams I.D., Wong K.S., Yu X.Q., Tang B.Z. Highly photostable two-photon NIR AIEgens with tunable organelle specificity and deep tissue penetration. Biomaterials. 2019;208:72–82. doi: 10.1016/j.biomaterials.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Luo J., Xie Z., Lam J.W.Y., Cheng L., Chen H., Qiu C., Kwok H.S., Zhan X., Liu Y., Zhu D., Tang B.Z. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001;18:1740–1741. doi: 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]
- 24.Hong Y.N., Lam J.W.Y., Tang B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011;40:5361–5388. doi: 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y.C., Lam J.W.Y., Kwok R.T.K., Liu B., Tang B.Z. Aggregation-induced emission: fundamental understanding and future developments. Mater. Horiz. 2019;6:428–433. [Google Scholar]
- 26.Cai X.L., Liu B. Aggregation-induced emission: recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020;59 doi: 10.1002/anie.202000845. 9868−9886. [DOI] [PubMed] [Google Scholar]
- 27.Lai Y.Y., Huang J.L., Wu S.G., Zhu Q.H., Liu Y.S. Aggregation-induced emission and reversible mechanofluorochromic characteristics of tetra-substituted tetrahydropyrimidine derivatives. Dyes Pigments. 2019;166:8–14. [Google Scholar]
- 28.Liao Y.H., Wang R.L., Wang S.Z., Xie Y.F., Chen H.H., Huang R.J., Shao L.Q., Zhu Q.H., Liu Y.S. Highly efficient multifunctional organic photosensitizer with aggregation-induced emission for in vivo bioimaging and photodynamic therapy. ACS Appl. Mater. Interfaces. 2021;13 doi: 10.1021/acsami.1c17476. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y.Y., Feng G.X., Qin W., Tang B.Z., Liu B. Targeted and image-guided photodynamic cancer therapy based on organic nanoparticles with aggregation-induced emission characteristics. Chem. Commun. 2014;50 doi: 10.1039/c4cc02767a. 8757−8760. [DOI] [PubMed] [Google Scholar]
- 30.Gu X.G., Kwok R.T.K., Lam J.W.Y., Tang B.Z. AIEgens for biological process monitoring and disease theranostics. Biomaterials. 2017;146:115–135. doi: 10.1016/j.biomaterials.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Yan D.Y., Wang M., Wu Q., Niu N., Li M., Song R.X., Rao J., Kang M.M., Zhang Z.J., Zhou F.F., Wang D., Tang B.Z. Multimodal imaging-guided photothermal immunotherapy based on a versatile NIR-II aggregation-induced emission luminogen. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202202614. [DOI] [PubMed] [Google Scholar]
- 32.Yang M., Zeng Z., Lam J.W.Y., Fan J., Pu K., Tang B.Z. State-of-the-art self-luminescence: a win-win situation. Chem. Soc. Rev. 2022;51:8815–8831. doi: 10.1039/d2cs00228k. [DOI] [PubMed] [Google Scholar]
- 33.Zou H., Zhang J., Wu C., He B., Hu Y., Sung H.H.Y., Kwok R.T.K., Lam J.W.Y., Zheng L., Tang B.Z. Making aggregation-induced emission luminogen more valuable by gold: enhancing anticancer efficacy by suppressing thioredoxin reductase activity. ACS Nano. 2021;15 doi: 10.1021/acsnano.1c02882. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Feng G., Tang B.Z., Liu B. Recent advances of AIE light-up probes for photodynamic therapy. Chem. Sci. 2021;12:6488–6506. doi: 10.1039/d1sc00045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin G.R., Feng G.X., Qin W., Tang B.Z., Liu B., Li K. Multifunctional organic nanoparticles with aggregation-induced emission (AIE) characteristics for targeted photodynamic therapy and RNA interference therapy. Chem. Commun. 2016;52:2752–2755. doi: 10.1039/c5cc07818k. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L.P., Che W.L., Yang Z.Y., Liu X.M., Liu S., Xie Z.G., Zhu D.X., Su Z.M., Tang B.Z., Bryce M.R. Bright red aggregation-induced emission nanoparticles for multifunctional applications in cancer therapy. Chem. Sci. 2020;11:2369–2374. doi: 10.1039/c9sc06310b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuang W.H., Yang L., Ma B.X., Kong Q.S., Li G.C., Wang Y.B., Tang B.Z. Multifunctional two-photon AIE luminogens for highly mitochondria-specific bioimaging and efficient photodynamic therapy. ACS Appl. Mater. Interfaces. 2019;11:20715–20724. doi: 10.1021/acsami.9b04813. [DOI] [PubMed] [Google Scholar]
- 38.He Z.Y., Gao Y.T., Zhang H.M., Xue Y., Meng F.L., Luo L. Mitochondrion-anchored photosensitizer with near infrared-I aggregation-induced emission for near infrared-II two-photon photodynamic therapy. Adv. Healthcare Mater. 2021;10 doi: 10.1002/adhm.202101056. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y.P., Zhang D., He G.H., Liu C., Tu Y.N., Li X., Zhang Q.B., Wu X., Liu R.Y. A lysosomal targeted NIR photosensitizer for photodynamic therapy and two-photon fluorescence imaging. J. Mater. Chem. B. 2021;9:1009–1017. doi: 10.1039/d0tb02692a. [DOI] [PubMed] [Google Scholar]
- 40.Gu B., Wu W.B., Xu G.X., Feng G.X., Yin F., Chong P.H.J., Qu J.L., Yong K.-T., Liu B. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics. Adv. Mater. 2017;29 doi: 10.1002/adma.201701076. [DOI] [PubMed] [Google Scholar]
- 41.Kim S., Tachikawa T., Fujitsuka M., Majima T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014;136:11707–11715. doi: 10.1021/ja504279r. [DOI] [PubMed] [Google Scholar]
- 42.Feng G.X., Qin W., Hu Q.L., Tang B.Z., Liu B. Cellular and mitochondrial dual-targeted organic dots with aggregation-induced emission characteristics for image-guided photodynamic therapy. Adv. Healthcare Mater. 2015;4:2667–2676. doi: 10.1002/adhm.201500431. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y.Y., Zhang G.X., Zhao R., Zhang D.Q. Aggregation-induced emission luminogens for mitochondria-targeted cancer therapy. ChemMedChem. 2020;15:2220–2227. doi: 10.1002/cmdc.202000632. [DOI] [PubMed] [Google Scholar]
- 44.Appelqvist H., Waster P., Kagedal K., Ollinger K. The lysosome: from waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013;5:214–226. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- 45.Yang J., Griffin A., Qiang Z., Ren J. Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology. Signal Transduct. Targeted Ther. 2022;7:379. doi: 10.1038/s41392-022-01243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T.F., Zhang J.Y., Wang F.B., Cao H., Zhu D.M., Chen X.Y., Xu C.H., Yang X.Q., Huang W.B., Wang Z.Y., Wang J.F., He Z.K., Zheng Z., Y Lam J.W., Tang B.Z. Mitochondria-targeting phototheranostics by aggregation-induced NIR-II emission luminogens: modulating intramolecular motion by electron acceptor engineering for multi-modal synergistic therapy. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 47.Li H.D., Lu Y., Chung J., Han J.J., Kim H., Yao Q.C., Kim G., Wu X.F., Long S.R., Peng X.J., Yoon J.Y. Activation of apoptosis by rationally constructing NIR amphiphilic AIEgens: surmounting the shackle of mitochondrial membrane potential for amplified tumor ablation. Chem. Sci. 2021;12:10522–10531. doi: 10.1039/d1sc02227j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X.H., Li Y.X., Li S.W., Gao M., Ren L., Tang B.Z. Mitochondria- and lysosomes-targeted synergistic chemo-photodynamic therapy associated with self-monitoring by dual light-up fluorescence. Adv. Funct. Mater. 2018;28 [Google Scholar]
- 49.Chai C.X., Zhou T., Zhu J.F., Tang Y., Xiong J., Min X.B., Qin Q., Li M., Zhao N., Wan C.D. Multiple light-activated photodynamic therapy of tetraphenylethylene derivative with AIE characteristics for hepatocellular carcinoma via dual-organelles targeting. Pharmaceutics. 2022;14:459. doi: 10.3390/pharmaceutics14020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D.H., Chen L.J., Zhao X., Yan X.P. Rational design of a dual organelle-targeted photosensitizer with dual-color emission for photodynamic therapy and cell death self-reporting. Dyes Pigments. 2022;203 [Google Scholar]
- 51.Galer P., Korošec R.C., Vidmar M., Šket B. Crystal structures and emission properties of the BF2 complex 1-phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: multiple chromisms, aggregation- or crystallization-induced emission, and the self-assembly effect. J. Am. Chem. Soc. 2014;136:7383–7394. doi: 10.1021/ja501977a. [DOI] [PubMed] [Google Scholar]
- 52.Guo F.L., Li Z.Q., Liu X.P., Zhou L., Kong F.T., Chen W.C., Dai S.Y. Metal-free sensitizers containing hydantoin acceptor as high performance anchoring group for dye-sensitized solar cells. Adv. Funct. Mater. 2016;26:5733–5740. [Google Scholar]
- 53.Zhang Z.Q., Wu Z., Sun J.B., Yao B.Q., Xue P.C., Lu R. β-Iminoenolate boron complex with terminal triphenylamine exhibiting polymorphism and mechanofluorochromism. J. Mater. Chem. C. 2016;4:2854–2861. [Google Scholar]
- 54.Chen M., Nie H., Song B., Li L.Z., Sun J.Z., Qin A.J., Tang B.Z. Triphenylamine-functionalized tetraphenylpyrazine: facile preparation and multifaceted functionalities. J. Mater. Chem. C. 2016;4:2901–2908. [Google Scholar]
- 55.Chen K.Q., He P., Wang Z.M., Tang B.Z. A feasible strategy of fabricating type I photosensitizer for photodynamic therapy in cancer cells and pathogens. ACS Nano. 2021;15:7735–7743. doi: 10.1021/acsnano.1c01577. [DOI] [PubMed] [Google Scholar]
- 56.Mei J., Hong Y.N., Lam J.W.Y., Qin A.J., Tang Y.H., Tang B.Z. Aggregation-induced emission: the whole is more brilliant than the parts. Adv. Mater. 2014;26:5429–5479. doi: 10.1002/adma.201401356. [DOI] [PubMed] [Google Scholar]
- 57.Zou J.L., Lu H.G., Zhao X.W., Li W., Guan Y., Zheng Y.D., Zhang L.J., Gao H. A multi-functional fluorescent probe with aggregation-induced emission characteristics: mitochondrial imaging, photodynamic therapy and visualizing therapeutic process in zebrafish model. Dyes Pigments. 2018;151:45–53. [Google Scholar]
- 58.Xu S.D., Yuan Y.Y., Cai X.L., Zhang C.J., Hu F., Liang J., Zhang G.X., Zhang D.Q., Liu B. Tuning the singlet-triplet energy gap: a unique approach to efficient photosensitizers with aggregation-induced emission (AIE) characteristics. Chem. Sci. 2015;6:5824–5830. doi: 10.1039/c5sc01733e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma H.J., Zhao C., Meng H.B., Li R.X., Mao L.C., Hu D.N., Tian M., Yuan J.Y., Wei Y. Multifunctional organic fluorescent probe with aggregation-induced emission characteristics: ultrafast tumor monitoring, two-photon imaging, and image-guide photodynamic therapy. ACS Appl. Mater. Interfaces. 2021;13 doi: 10.1021/acsami.0c21309. [DOI] [PubMed] [Google Scholar]
- 60.Gu X.G., Zhang X.Y., Ma H.L., Jia S.R., Zhang P.F., Zhao Y.J., Liu Q., Wang J.G., Zheng X.Y., Lam J.W.Y., Ding D., Tang B.Z. Corannulene-incorporated AIE nanodots with highly suppressed nonradiative decay for boosted cancer phototheranostics in vivo. Adv. Mater. 2018;30 doi: 10.1002/adma.201801065. [DOI] [PubMed] [Google Scholar]
- 61.Miao X.F., Hu W.B., He T.C., Tao H.J., Wang Q., Chen R.F., Jin L., Zhao H., Lu X.M., Fan Q.L., Huang W. Deciphering the intersystem crossing in near-infrared BODIPY photosensitizers for highly efficient photodynamic therapy. Chem. Sci. 2019;10:3096–3102. doi: 10.1039/c8sc04840a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.