Abstract
Klebsiella oxytoca can assimilate nitrate and nitrite by using enzymes encoded by the nasFEDCBA operon. Expression of the nasF operon is controlled by general nitrogen regulation (Ntr) via the NtrC transcription activator and by pathway-specific nitrate and nitrite induction via the NasR transcription antiterminator. This paper reports our analysis of nasR gene expression. We constructed strains bearing single-copy Φ(nasR-lacZ) operon fusions within the chromosomal rhaBAD-rhaSR locus. The expression of ΔrhaBS::[Φ(nasR-lacZ)] operon fusions was induced about 10-fold during nitrogen-limited growth. Induction was reduced in both ntrC and rpoN null mutants, indicating that Ntr control of nasR gene expression requires the NtrC and ςN (ς54) proteins. Sequence inspection of the nasR control region reveals an apparent ςN-dependent promoter but no apparent NtrC protein binding sites. Analysis of site-specific mutations coupled with primer extension analysis authenticated the ςN-dependent nasR promoter. Fusion constructs with only about 70 nucleotides (nt) upstream of the transcription initiation site exhibited patterns of β-galactosidase expression indistinguishable from Φ(nasR-lacZ) constructs with about 470 nt upstream. Expression was independent of the Nac protein, implying that NtrC is a direct activator of nasR transcription. Together, these results indicate that nasR gene expression does not require specific upstream NtrC-binding sequences, as previously noted for argT gene expression in Salmonella typhimurium (G. Schmitz, K. Nikaido, and G. F.-L. Ames, Mol. Gen. Genet. 215:107–117, 1988).
Klebsiella spp., members of the family Enterobacteriaceae, can use nitrate (NO3−) and nitrite (NO2−) as sole nitrogen sources during aerobic growth. Nitrate and nitrite are reduced to ammonium by assimilatory nitrate and nitrite reductases, respectively (reviewed in reference 31). The resulting ammonium is incorporated into central metabolism through the action of glutamine synthetase and glutamate synthase (reviewed in reference 49).
Molecular genetic analysis of K. oxytoca (pneumoniae) M5al has identified the nasFEDCBA operon required for nitrate and nitrite assimilation. The nasFED genes encode a periplasmic binding protein-dependent nitrate and nitrite transporter (64). The nasCA genes encode assimilatory nitrate reductase, and the nasB gene encodes assimilatory nitrite reductase (28, 29). The nasR gene, located immediately upstream of nasF, encodes a nitrate- and nitrite-responsive positive regulator for nasF operon expression (19). Expression of the nasF operon is controlled by general nitrogen regulation (Ntr) via the NtrC transcription activator (reviewed in reference 31) and by pathway-specific nitrate and nitrite induction via the NasR transcription antiterminator (8, 9, 30). The regulation of nasR gene expression has not previously been examined.
Ntr control in enterobacteria has been extensively studied (reviewed in references 34, 38, 44, and 48). Genes required for Ntr control include rpoN (also called ntrA and glnF), which encodes the sigma factor ςN (ς54); ntrC (also called glnG), which encodes the enhancer binding transcription activator NtrC; and ntrB (also called glnL), which encodes the protein kinase/phosphoprotein phosphatase (NtrB) that controls NtrC activity. Promoters recognized by ςN-RNA polymerase (EςN) contain GG and GC at 24 and 12 bp, respectively, upstream of the transcription initiation site (reviewed in reference 36). The activity of the NtrB protein is controlled in response to internal nitrogen such that the phosphorylation state of the NtrC protein is elevated during nitrogen-limited growth (22, 24). Phosphorylation stimulates transcription activation by NtrC (reviewed in references 34 and 48).
The DNA binding sites for phospho-NtrC are located 100 bp or more upstream of the transcription initiation site and thereby constitute upstream activation sequences (UAS) or enhancers. The best-studied examples are those for the Escherichia coli and Salmonella typhimurium glnA-ntrBC operon, encoding glutamine synthetase along with NtrB and NtrC, and the K. oxytoca (pneumoniae) nifLA operon, encoding the regulators of dinitrogen fixation (nif) gene expression (41, 45, 50, 63). Formation of a DNA loop facilitates contact between upstream-bound phospho-NtrC and EςN to activate transcription initiation (reviewed in references 34 and 48). Binding to the UAS or enhancer increases the local concentration of phospho-NtrC. Nevertheless, NtrC can significantly stimulate transcription, even for constructs in which the NtrC binding sites have been deleted (14, 46, 50, 53). Indeed, no upstream binding sites have been identified for NtrC-dependent activation of the S. typhimurium argT gene, encoding the periplasmic lysine-arginine-ornithine binding protein (52).
In enterobacteria, some Ntr-regulated operons are controlled only indirectly by phospho-NtrC. Rather, the Nac protein activates the expression of ς70-dependent operons such as hut (histidine utilization) and put (proline utilization) (10, 23, 32). Expression of the nac gene itself is activated by phospho-NtrC during nitrogen-limited growth (18, 54). However, nasF operon expression is independent of nac+ (19, 32).
In this paper, we describe a system for integrating single-copy constructs into the chromosomal rhaBAD-rhaSR locus of K. oxytoca M5al and illustrate its use in the analysis of Φ(nasR-lacZ) operon fusions. We demonstrate that nasR gene expression is subject to Ntr control, requires a ςN-dependent promoter, and is independent of nac+. Deletion analysis revealed that the regulatory elements for nasR expression lie within 71 bp of the transcription initiation site.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this work are listed in Table 1. Aerobacter aerogenes M5al was reclassified as K. pneumoniae (61). However, its phenotypic properties (such as a positive reaction in the indole test) place this strain in the species K. oxytoca. Genetic crosses were performed by bacteriophage P1 kc-mediated transduction (39, 60). Standard methods were used for restriction endonuclease digestion, ligation, and transformation of DNA (35).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| K. oxytoca M5al strains | ||
| UNF1801 | hsdR1 hisD2 ntrC36::Tn7 | 17 |
| UNF1831 | hsdR1 hisD2 ntrC50::Tn5 | 17 |
| UNF2651 | hsdR1 hisD2 rpoN::Km lac-2002 | 37 |
| VJSK838 | hsdR1 lacZ101::Tn10d(Tc) | Laboratory collection |
| VJSK1018 | hsdR1 nasD124::Ω-Cm | 29 |
| VJSK1190 | hsdR1 nasR131::Tn10d(Cm) | 19 |
| VJSK1603 | hsdR1 lacZ101::Tn10d(Tc) ntrC36::Tn7 | Laboratory collection |
| VJSK2216 | hsdR1 lacZ101::Tn10d(Tc) Δ(narKG)302 rpsL | 64 |
| Derivatives of strain VJSK2216 | ||
| VJSK2212 | Δ(nasFED)137 | 64 |
| VJSK2215 | Δ(nasCB)139 | This work |
| VJSK2504 | ΔrhaBS::lacZ | This work |
| VJSK2506 | ΔrhaBS::[Φ(nasR-lacZ) {−71/+71}] | This work |
| VJSK2507 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] | This work |
| VJSK2512 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] Δ(nasFED)137 | This work |
| VJSK2516 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] Δ(nasCB)139 | This work |
| VJSK2517 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] nasR131::Tn10d(Cm) | This work |
| VJSK2520 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] rpoN::Km | This work |
| VJSK2521 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] ntrC50::Tn5 | This work |
| VJSK2529 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] nasD124::Ω-Cm | This work |
| VJSK2531 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] (−24G→A) | This work |
| VJSK2541 | ΔrhaBS::[Φ(nasR-lacZ) {−14/+362}] | This work |
| VJSK2548 | ΔrhaBS::[Φ(nasR-lacZ) {−71/+362}] | This work |
| VJSK2588 | ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] (−13,−12GC→AT) | This work |
| K. aerogenes W70 strains | ||
| KC1043 | hutC515 | 54 |
| KC2000 | hutC515 nac-203::Tn5-131 | 54 |
| KC2473 | hutC515 nac-306::Tn5-tac1 | 54 |
| KC2582 | hutC515 nac-306::Tn5-tac1 ntrB45 ntrC5 | 54 |
| Plasmids | ||
| pALTER-1 | Aps Tcr (for site-specific mutagenesis) | 27 |
| pKAS46 | Apr Kmr, ori R6K rpsL+ (suicide vector) | 57 |
| pNM481 | Apr, ′lacZ lacY+ (for gene fusions) | 42 |
| pRS415 | Apr, lacZ+ lacY+ (for operon fusions) | 56 |
| pV16 | Apr, Φ(hutU-lacZ) | 47 |
| pVJS1370 | As pRS415 but Φ(nasF-lacZ) {−38/+277} | 30 |
| pVJS1376 | As pRS415 but Φ(nasF-lacZ) {−141/+277} | 30 |
| pVJS2009 | As pRS415 but Φ(nasR-lacZ) {−471/+910} | This work |
| pVJS2010 | As pRS415 but Φ(nasR-lacZ) {−64/+910} | This work |
| pVJS2354 | As pKAS46 but ΔrhaBS::′lacZ lacY+ | This work |
| pVJS2502 | Tcr, nasRFED; ∼5.7-kb insert in pALTER-1 (for gene fusions) | 64 |
| pVJS2520 | Tcr, nasCBA; ∼7.8-kb insert in pALTER-1 | 64 |
| pVJS2558 | Apr Kmr, Δ(nasCB); EcoRI-KpnI fragment in pKAS46 | This work |
| pVJS2595 | Tcr, {−469 to +71} of nasR in pALTER-1 | This work |
| pVJS2596 | As pVJS2354 but Φ(nasR-lacZ) {−71/+71} | This work |
| pVJS2597 | As pVJS2354 but Φ(nasR-lacZ) {−469 to +71} | This work |
| pVJS3002 | As pVJS2597 but −24G→A [Φ(nasR-lacZ)6] | This work |
| pVJS3004 | Apr, {−469 to +71} of nasR in pNM481 [Φ(nasR′-lacZ+)11] | This work |
| pVJS3006 | As pVJS2354 but Φ(nasR-lacZ) {−71 to +362} | This work |
| pVJS3009 | As pVJS2354 but Φ(nasR-lacZ) {−14 to +362} | This work |
| pVJS3029 | As pVJS3004 but −29,−28AT→GC [Φ(nasR′-lacZ+)13] | This work |
| pVJS3030 | As pVJS3004 but +36 to +38ATG→TAA [Φ(nasR′-lacZ+)12] | This work |
| pVJS3033 | As pVJS2597 but −13,−12GC→AT [Φ(nasR-lacZ)5] | This work |
Culture media.
Defined, complex, and indicator media for genetic manipulations were used as described previously (35). Nitrogen-free medium contained 0.2% (wt/vol) glucose, 1% (wt/vol) sodium citrate, 0.74% (wt/vol) sodium phosphate (pH 8), and 1 mM MgSO4 (28). This medium was supplemented with additional nitrogen sources (5 mM NaNO3, NaNO2, or NH4Cl) as indicated to test nitrogen utilization phenotypes. Alanyl-glutamine (5 mM) was added to all solid media used for cultivation of rpoN (Gln−) and ntrC strains (11).
Selection for Klebsiella transformants carrying bla-containing plasmids was accomplished with combination of carbenicillin and ampicillin at 800 and 60 μg/ml, respectively (33). E. coli transformants were selected on 200 μg of ampicillin per ml. Chloramphenicol was used at 50 and 25 μg/ml for selecting K. oxytoca and E. coli transformants, respectively. Kanamycin was used at 100 and 75 μg/ml for selecting K. oxytoca and E. coli transformants, respectively. Tetracycline was used at 20 μg/ml for selecting E. coli transformants. Streptomycin was used at 500 μg/ml for selecting K. oxytoca Smr segregants.
Defined medium to grow cultures for β-galactosidase assays was buffered with 3-(N-morpholino) propanesulfonic acid (MOPS) as previously described (58). The initial pH of this medium was adjusted with NaOH to 8.0. Glucose (40 mM) was used as the sole carbon source. The nitrogen sources NaNO3, NaNO2, NH4Cl, and l-glutamine were each added to 5 mM as indicated. Arginine, hypoxanthine, thiamine, and uracil were added to stimulate the growth of rpoN strains (26). For induction of tac-nac expression, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added very early in the exponential phase (about 15 Klett units [54]).
Culture conditions.
Cultures for β-galactosidase assays were grown at 30°C to minimize deamidation of glutamine (4). Culture densities were monitored with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, N.Y.) equipped with a no. 66 (red) filter. Cultures were aerated at 240 rpm in 10 ml of medium in 125-ml sidearm flasks. Cultures in the mid-exponential phase (about 40 Klett units) were harvested, chilled on ice, and washed with saline. Cell pellets were stored overnight at −20°C, prior to assay for β-galactosidase activity.
β-Galactosidase assays.
β-Galactosidase assays were done at room temperature, approximately 21°C. Cell pellets were suspended in 4 ml of Z buffer (39) and stored on ice. β-Galactosidase activity was measured in CHCl3-sodium dodecyl sulfate-permeabilized cells by monitoring the hydrolysis of o-nitrophenyl-β-d-galactopyranoside. Activities are expressed in terms of cell density (absorbance at 600 nm), using the formula of Miller (39). All reported values are averages from at least three independent experiments.
DNA sequencing.
Double-stranded templates were sequenced on a model 373A stretch DNA sequencer by using dye terminator chemistry and AmpliTaq-FS DNA polymerase (Perkin Elmer/Applied Biosystems Division, Foster City, Calif.). Templates were prepared by using QIAprep spin plasmid kits (Qiagen Inc., Chatsworth, Calif.). DNA sequences were analyzed with programs from DNASTAR Inc. (Madison, Wis.), and database searches were performed with the BLAST programs (1) accessed through the National Center for Biotechnology Information.
DNA oligonucleotides.
In the following sequence, nucleotide substitutions for site-specific mutagenesis are underlined, added nucleotide tails are in lower case, and introduced restriction endonuclease sites are in boldface. Sequences are presented from 5′ to 3′. The nucleotide sequences of oligonucleotides used for site-specific mutagenesis were −24G→A, TCCTTCTATAAGACACGGTTATTGC; −13/−12GC→AT, TCCTTCTATAAGGCACGGTTATTATTTGGCTGAAGTATAAAGCGTTAA; −29/−28 AT→GC, TAAGGACTCCTTCTGCAAGGCACGGTTATT; +36/+38ATG→TAA, GGAGAGGGGTATGAATAATTAAGCGGGCAATACGCCTGAGGT. The nucleotide sequences of oligonucleotides used for construction of lacZ fusions were RZF1 (fusion starting from −469), ttagtcggatccGCTTTCAGCTGGCATTGT; RZF2 (fusion starting from −71), taactggatcCCGTTCGCGATAATCACAAT; RZF3 (fusion ending at +71), tggctgtctagaAAACCAGTCGACCACCTCAG; RZF4 (fusion starting from −14), taactggatccTGCTTGGCTGAAGTATAAA; and RZF5 (fusion ending at +362), tggctgtctagaCGCAAGCTGGGGCAGATA. The nucleotide sequences of oligonucleotides used for colony PCR analysis were C1, CCTGACGCGGGCGCATTTACA; B2, GTCCGGCTCATCGCTGTTCACG; RB1, AATTTCATTTTCAGGATTAGG; and LZ1, GCGAATGACCTTGAGTTTGTC. The nucleotide sequences of oligonucleotides used for loop deletion mutagenesis were nasC Δ(95–343), GAAACGGTTACCGCGGTGGATATGCATCCGCTGGACCGCCACTACCGT; and nasB Δ(86–713), CGCCTGAGCGAATCGGTCGCCAGCATGCATGCCGACCTGTTCGCCAGCGAT. The nucleotide sequence of the oligonucleotide used for primer extension was TGCCTGCAGGTCGACTCTAGAAAACCAGT.
Site-specific mutagenesis.
Oligonucleotide-directed site-specific mutagenesis was performed by ampicillin selection as described previously (27, 35). The DNA sequence of each mutant insert was determined to confirm the mutational alterations and to ensure that no spurious changes were introduced.
PCR.
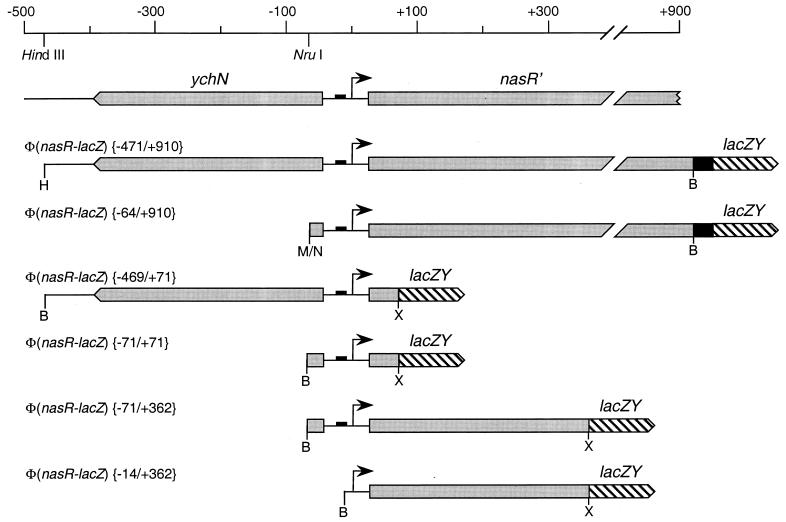
PCR-generated DNA fragments from the nasR control region were amplified (51) from plasmid pVJS2502. BamHI and XbaI sites were introduced into the upstream and downstream primers, respectively, for subsequent cloning to construct Φ(nasR-lacZ) fusions. Different pairs of primers resulted in different fusions; for example, primers RZF1 and RZF3 were used to construct the Φ(nasR-lacZ) {−469/+71} fusion, whereas primers RZF2 and RZF3 were used to construct the Φ(nasR-lacZ) {−71/+71} fusion (Fig. 1). (Fusions are designated by their upstream and downstream junctions relative to the transcription initiation site [Fig. 1].)
FIG. 1.
Φ(nasR-lacZ) fusion constructs (drawn to scale). The ςN-dependent promoter is indicated by a small solid box, and the transcription initiation site is indicated by an arrow. Upstream and downstream endpoints for each construct are indicated in brackets. The lacZY genes are not to scale. Restriction sites: B, BamHI; H, HindIII; M/N, SmaI-NruI fusion; X, XbaI.
PCR was performed by using Vent DNA polymerase (New England Biolabs, Inc., Beverly, Mass.). The final PCR mixture (100 μl) contained 4 ng of plasmid DNA, 1× ThermoPol reaction buffer, 200 μM deoxynucleoside triphosphates, 1 mM each oligonucleotide primer, and 2.5 U of Vent DNA polymerase. The program of reactions was exactly as described previously (64). PCR products were purified with the Wizard PCR Preps DNA purification system (Promega Corp., Madison, Wis.) and then cloned into plasmid pALTER-1. The DNA sequence of the entire insert was determined to confirm that no spurious changes were introduced.
Electrotransformation.
A K. oxytoca culture (50 ml) was grown at 37°C in a 250-ml sidearm flask and harvested in the early exponential phase (about 25 to 30 Klett units). The cell pellet was washed three times with 1 M glycerol and then resuspended in 200 μl of 1 M glycerol. A 2- to 5-μl volume of plasmid DNA (100 ng/μl) was mixed with 40 μl of glycerol-washed cells in a 1.5-ml microcentrifuge tube and incubated on ice for 5 min. This mixture was pipetted into a precooled 0.2-cm electroporation cuvette and electroporated at 2.5 kV with an E. coli Pulser electrotransformation apparatus (Bio-Rad, Hercules, Calif.). After the pulse, 1 ml of TY broth (0.8% tryptone, 0.5% yeast extract, 0.5% NaCl) was immediately added to the cuvette, and the cell suspension was quickly transferred into a 1.5-ml microcentrifuge tube and incubated at 30°C for 1 h prior to plating on selective medium.
Construction of plasmid-borne Φ(nasR-lacZ) and Φ(nasF-lacZ) operon fusions.
Plasmid pRS415 (56), with the pBR322 replication origin, served as the vector. The downstream junction was the BamHI site near the end of MudK #7 (29), which is inserted into codon 304 of nasR. The upstream cloning site was the HindIII site distal to ychN, located 471 nucleotides (nt) upstream of the nasR transcription initiation site (Fig. 1). Recloning into a polylinker plasmid placed this HindIII site adjacent to an EcoRI site, which was used for cloning into plasmid pRS415. This formed the Φ(nasR-lacZ) {−471/+910} operon fusion (Fig. 1). A second plasmid was constructed by joining the NruI site, just upstream of nasR, to the SmaI cloning site in plasmid pRS415. This formed the Φ(nasR-lacZ) {−64/+911} operon fusion.
The Φ(nasF-lacZ) fusions (30) were constructed similarly, except that the downstream junction was the BamHI site near the end of MudK #34, which is inserted into codon 53 of nasF.
Construction of plasmid-borne Φ(nasR′-′lacZ) gene (translational) fusions.
Plasmid pNM481 (42), with the pBR322 replication origin, served as the vector. PCR-generated nasR inserts, described above, were cloned into plasmid pALTER-1 for sequence analysis and for site-specific mutagenesis. This cloning placed the downstream XbaI site next to the polylinker HindIII site. Therefore, these inserts were cloned as BamHI-HindIII fragments into plasmid pNM481.
Construction of the rha integration plasmid.
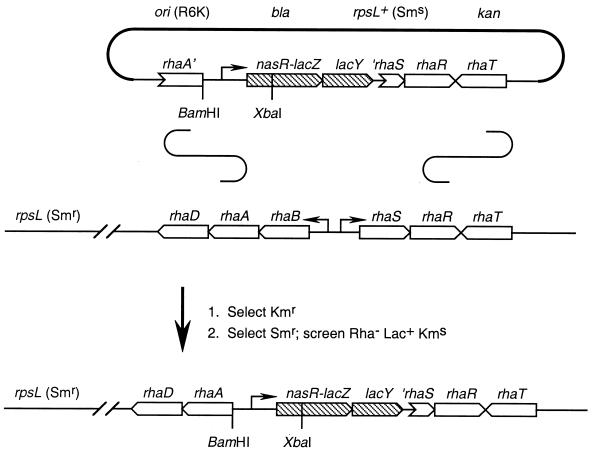
The vector pVJS2354, used for integrating lacZ operon fusion constructs into the chromosomal rhaBAD-rhaSR locus, is based on the allelic-exchange vector pKAS46 (57). Plasmid pKAS46 contains the R6K replication origin and therefore requires the π protein (product of the pir gene) for replication. It also contains the rpsL+ gene, allowing for the selection (in an rpsL strain background) of plasmid-free segregants as Smr colonies (57). We have previously used plasmid pKAS46 to construct several allelic-replacement strains of K. oxytoca (64).
Construction of plasmid pVJS2354 proceeded in several steps. First, we cloned and identified the rha (rhamnose utilization) locus of K. oxytoca M5al. The in vivo cloning method of Groisman and Casadaban (21) was used essentially as described previously (28, 35). An E. coli rha::Tn10 hsdR recipient was used to identify Rha+ clones. Next, we identified an approximately 7-kb HindIII subclone that complemented E. coli rhaB, rhaA, rhaD, rhaS, and rhaR mutants (kindly provided by Susan Egan, University of Kansas, Lawrence, Kans.). End sequencing of this and further subclones was used to localize the individual rha genes by comparison to the E. coli rha sequence (43). Next, an approximately 5-kb BamHI fragment containing rhaBA-rhaSR-rhaT was made blunt ended and cloned into EcoRI-EcoRV-digested, blunt-ended plasmid pKAS46. A rha-internal EcoRI fragment of approximately 2.1 kb was deleted, removing all of rhaB and most of rhaS. The two BamHI sites in the pKAS46 backbone were made blunt ended and religated, removing the 1.7-kb mob site (derived from plasmid RP4). Finally, a 5.1-kb PCR fragment containing the molecular cloning site, the RNase III-processing site, and the promoterless lacZY operon from vector λTXF97 (59) was cloned into the EcoRI site, resulting in plasmid pVJS2354.
Construction of chromosomal ΔrhaBS::[Φ(nasR-lacZ)] operon fusions.
Derivatives of plasmid pVJS2354 containing different nasR upstream regions were constructed by cloning the PCR-generated nasR inserts described above. Plasmids were electrotransformed into strain VJSK2216 or derivatives with selection for Kmr. Integrants (Kmr Sms Rha+) were streaked on MacConkey-rhamnose-streptomycin agar, and segregants (Kms Smr Rha−) were colony purified. The veracity of the resulting ΔrhaBS::[Φ(nasR-lacZ)] allelic replacements was confirmed by colony PCR analysis with primers RB1 (rhaB-rhaA intergenic region) and LZ1 (lacZ) as previously described (64).
Construction of the Δ(nasCB) deletion mutant.
Loop deletion mutagenesis and allelic exchange of the Δ(nasCB)139 deletion into the chromosome of K. oxytoca were performed essentially as described previously (64). The deletion Δ(nasCB)139 was constructed on plasmid pVJS2520 by adding two oligonucleotides (for ΔnasC, deleting codons 95 through 343, and for ΔnasB, deleting codons 86 through 713) to a single mutagenesis reaction. The double-deletion ΔnasC and ΔnasB was isolated and subjected to NsiI reduction to generate Δ(nasCB)139. All deletions are in frame. Oligonucleotide primers C1 and B2 were used for colony PCR to confirm the veracity of the allelic exchanges as described previously (64).
Primer extension.
Total RNA was isolated from K. oxytoca VJSK2507 by using the RNeasy method (Qiagen Inc.). Cultures were grown in 20 ml of 10 mM MOPS-glutamine medium in a 250-ml sidearm flask. The cultures were harvested in the latter part of the exponential phase (approximately 60 Klett units).
To determine the 5′ end of nasR RNA, a 29-bp oligonucleotide complementary to the fusion junction was first end labeled with [γ-32P]ATP (Amersham Life Science Inc., Arlington Heights, Ill.) by using T4 polynucleotide kinase (New England Biolabs, Inc., Beverly, Mass.) and then annealed to approximately 40 μg of isolated RNA. Primer extension reactions with avian myeloblastosis virus reverse transcriptase (Promega Corp.) were performed by the method of Kingston (25). The DNA sequence ladder was generated by using plasmid pVJS2595 as the DNA template and the same oligonucleotide as primer.
Nucleotide sequence accession number.
The DNA sequence reported in this paper has been deposited in the GenBank nucleotide sequence database under accession no. L27824.
RESULTS
The nasR upstream sequence.
We previously reported the sequence of the nasR structural gene (19). For this study, the DNA sequence of the nasR upstream region was determined on both strands. A 354-bp hypothetical ychN gene lies in the opposite orientation to the nasR gene (Fig. 2). The ychN initiation codon is located 68 bp upstream of the assigned nasR initiation codon (see below). The deduced amino acid sequence of the K. oxytoca YchN protein is 88% identical to that of the E. coli YchN protein (5). The function of the YchN protein is unknown.
FIG. 2.
Nucleotide sequence around the nasR control region. Numbering is with respect to the transcription initiation site (+1). A ςN promoter consensus sequence (34) is shown below the nasR promoter sequence (R = purine, Y = pyrimidine, and W = A or T). The deletion endpoints of lacZ fusion constructs are indicated (see also Fig. 1). Site-specific mutational changes are shown above the sequence. The Shine-Dalgarno regions for the nasR and ychN genes are underlined. Hypothetical nasR initiation codons (encoding Met-1, Met-4, and Val-11) are also underlined; see the text for details. The ychN gene is in the opposite orientation from the nasR gene. Deduced amino acid sequences are shown in standard single-letter code.
A presumptive ςN-dependent promoter lies just upstream of the nasR initiation codon (Fig. 2). However, potential sites for binding phospho-NtrC protein (half-site consensus TGCACCA; reviewed in reference 34) are not evident (Fig. 2). Below we present experimental evidence that confirms the identity of this promoter and that also permits us to assign the transcription initiation site to nucleotide position +1 (Fig. 2). All nucleotide coordinates described in this paper are therefore in relation to position +1.
Analysis of plasmid-borne Φ(nasR-lacZ) operon fusions.
Finding the presumptive ςN-dependent promoter sequence suggested that nasR gene expression is controlled by Ntr. To evaluate this possibility, we constructed the Φ(nasR-lacZ) {−471/+910} operon (transcriptional) fusion containing DNA from 471 nt upstream to 910 nt downstream of the transcription initiation site (Fig. 1; see Materials and Methods). We also constructed a derivative containing DNA from only 64 nt upstream to 910 nt downstream, with the goal of determining if phospho-NtrC binding sites lie upstream of position −64. Both plasmids were introduced into ntr+, ntrC null, and rpoN null strains of K. oxytoca. Cultures were grown in defined medium supplemented with the nitrogen sources glutamine (nitrogen limiting) or glutamine plus ammonium (nitrogen excess), and assayed for β-galactosidase specific activity.
Expression of the Φ(nasR-lacZ) {−471/+910} operon fusion was induced between 5- and 20-fold by nitrogen limitation, and this induction required ntrC+ (Table 2) (15). Surprisingly, expression of the Φ(nasR-lacZ) {−64/+910} fusion was indistinguishable from expression of the Φ(nasR-lacZ) {−471/+910} fusion. Expression of both fusions required ntrC+ (Table 2) and rpoN+ (15), as expected.
TABLE 2.
Expression of plasmid-borne Φ(nasR-lacZ) operon fusions
| Plasmida | Genotypeb | β-Galactosidase sp actc with:
|
|||
|---|---|---|---|---|---|
|
ntrC+
|
ntrC::Tn7
|
||||
| NH4+, Gln | Gln | NH4+, Gln | Gln | ||
| pVJS2009 | Φ(nasR-lacZ) {−471/+910} | 200 | 3,590 | 260 | 420 |
| pVJS2010 | Φ(nasR-lacZ) {−64/+910} | 190 | 4,200 | 210 | 550 |
Plasmids in strain VJSK838 (ntrC+) or VJSK1603 (ntrC36::Tn7).
See Fig. 1 for structures of Φ(nasR-lacZ) operon fusions.
Determined as described in Materials and Methods and expressed in arbitary (Miller) units. Cultures were aerated in media containing the indicated nitrogen sources as described in Materials and Methods.
As a control, we also measured β-galactosidase specific activity expressed from two previously characterized plasmid-borne Φ(nasF-lacZ) operon fusions (30). The Φ(nasF-lacZ) {−141/+277} fusion contains the upstream phospho-NtrC binding sites, whereas these sites are deleted from the Φ(nasF-lacZ) {−38/+277} fusion. As expected, the construct containing 141 bp upstream of the transcription initiation site directed an approximately 10-fold induction in β-galactosidase activity in response to nitrogen limitation whereas the construct containing only 38 bp upstream directed only a 3-fold increase. Again, induction required both ntrC+ and rpoN+ (15).
These results indicated that nasR gene expression is subject to Ntr control and that sequences upstream of position −64 are dispensable for this regulation. However, β-galactosidase activities measured from plasmid-borne fusions exhibited significant differences in independent experiments, perhaps due in part to variations in plasmid copy number. Therefore, we elected to conduct further analysis with chromosomal monocopy Φ(nasR-lacZ) operon fusion constructs.
System for construction of monocopy lacZ operon fusions.
Our laboratory routinely uses bacteriophage λ specialized transduction for constructing single-copy lacZ operon fusions in Escherichia coli (56). However, we have been unsuccessful in our efforts to adapt bacteriophage λ for use with K. oxytoca M5al. We therefore elected to follow the strategy of Shimotsu and Henner (55), who used the amyE gene of Bacillus subtilis as a target for integration, via homologous recombination, of fusion constructs into the chromosome. Two considerations guided our scheme. The first was to use as the target a locus involved in sugar catabolism, so that we could readily screen colonies arising from allelic replacements by virtue of their fermentation-negative phenotype. The second was to embed the fusion constructs between divergently transcribed genes to help insulate them from exogenous promoters (16). The ara and rha operons of enterobacteria satisfy both of these criteria. K. oxytoca M5al is Ara− Rha+, and so we cloned and analyzed its rhaBAD-rhaSR-rhaT locus as described in Materials and Methods.
The suicide integration vector pVJS2354 contains a promoterless lacZY operon (59) between the divergent rhaA and rhaR genes (Fig. 3). It also carries the rpsL+ (Sms) allele, in addition to Kmr and Apr markers. In the absence of the pir gene encoding the replication protein π, Kmr transformants arise only from integration of the plasmid into the chromosome. The rha locus homology ensures that most integrations will occur at rha (Fig. 3). Integration by recombination within rhaR yields an intact rhaBAD-rhaSR locus (Rha+ phenotype), whereas integration by recombination within rhaA serves to separate rhaD from the upstream rhaB promoter (43). Thus, integration within rhaA is expected to yield the Rha− phenotype (Fig. 3). In practice, however, we have observed that virtually all integrants are Rha+.
FIG. 3.
Strategy for single-copy transplantation of Φ(nasR-lacZ) fusion constructs into the K. oxytoca chromosome. See Materials and Methods for details. The Φ(nasR-lacZ) operon fusion constructs, derivatives of plasmid pVJS2354, span the divergent rhaB and rhaS genes. Conventional integration-excision allelic exchange (not depicted) results in transfer of the construct to the chromosome, replacing the resident rha+ locus.
Segregants lose the integrated plasmid by homologous recombination between the tandemly repeated rha sequences. They were selected as Smr colonies, because loss of the dominant rpsL+ (Sms) allele unmasked the phenotype conferred by the chromosomal rpsL (Smr) allele (57). Authentic segregants were also Kms (Fig. 3). The desired strains, in which the Φ(nasR-lacZ) construct had replaced the rhaB and rhaS genes, were thus readily identified as Smr Rha− colonies (see Materials and Methods). Candidates were tested for the Lac+ and Kms phenotype, and then two independent isolates were subjected to colony PCR analysis to verify the structure of the ΔrhaBS::[Φ(nasR-lacZ)] allelic replacement as described in Materials and Methods (data not shown).
Analysis of monocopy ΔrhaBS::[Φ(nasR-lacZ)] operon fusions.
We constructed four different ΔrhaBS::Φ[(nasR-lacZ)] operon fusions (Fig. 1), with different amounts of upstream and downstream sequence, in order to localize the nasR regulatory elements. We monitored the expression of these fusions by measuring β-galactosidase activities from cultures grown in defined medium with limiting or excess nitrogen. The results are shown in Table 3.
TABLE 3.
Expression of chromosomal ΔrhaBS::[Φ(nasR-lacZ)] operon fusions
| Straina | Genotypeb | β-Galactosidase sp actc with:
|
Ratio of sp act values | |
|---|---|---|---|---|
| NH4+, Gln | Gln | |||
| VJSK2504 | lacZd | 5 | 9 | 1.8 |
| VJSK2507 | Φ(nasR-lacZ) {−469/+71} | 7 | 67 | 9.6 |
| VJSK2506 | Φ(nasR-lacZ) {−71/+71} | 6 | 65 | 11 |
| VJSK2548 | Φ(nasR-lacZ) {−71/+362} | 17 | 150 | 8.8 |
| VJSK2541 | Φ(nasR-lacZ) {−14/+362} | 16 | 29 | 1.8 |
Derivatives of strain VJSK2216 (Table 1).
See Fig. 1 for structures of Φ(nasR-lacZ) operon fusions.
Determined as described in Materials and Methods and expressed in arbitary (Miller) units. Cultures were aerated in media containing the indicated nitrogen sources as described in Materials and Methods.
Allelic replacement with plasmid pVJS2354 only, with no cloned insert, to monitor background lacZ expression.
Three Φ(nasR-lacZ) constructs (−469/+71, −71/+71, and −71/+362) exhibited indistinguishable patterns of β-galactosidase synthesis: each was induced about 10-fold by nitrogen limitation (Table 3). This delimits the region extending from positions −71 to +71 as containing all necessary information for Ntr-controlled nasR expression. The fourth Φ(nasR-lacZ) construct (−14/+362) was expressed at a low unregulated level, indicating that the promoter is deleted from this cloned insert, as expected (see also below). Both ntrC+ and rpoN+ were required for Ntr-regulated expression of the monocopy constructs, Φ(nasR-lacZ) {−469/+71} (Table 4) and Φ(nasR-lacZ) {−71/+71} (data not shown), congruent with results from the analogous plasmid-borne fusions (Table 2).
TABLE 4.
Effects of regulatory mutations on expression of a chromosomal Φ(nasR-lacZ) operon fusion
| Straina | Genotypeb | β-Galactosidase sp actc with:
|
Ratio of sp act values | |
|---|---|---|---|---|
| NH4+, Gln | Gln | |||
| VJSK2507 | Wild type | 7 | 67 | 9.6 |
| VJSK2520 | rpoN::Km | 15 | 22 | 1.5 |
| VJSK2521 | ntrC::Tn5 | 11 | 21 | 1.9 |
| VJSK2588 | −13/−12GC→AT | 13 | 22 | 1.7 |
| VJSK2531 | −24G→A | 8 | 14 | 1.8 |
Derivatives of strain VJSK2216 (Table 1).
All strains carry ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] (Fig. 1).
Determined as described in Materials and Methods and expressed in arbitary (Miller) units. Cultures were aerated in media containing the indicated nitrogen sources as described in Materials and Methods.
Transcription initiation.
To confirm the −24/−12 element as the ςN-dependent nasR promoter, we constructed two different site-specific mutants: one with G at position −24 changed to A, and one with GC at positions −13 and −12 changed to AT (Fig. 2). We constructed ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] operon fusions bearing these alterations and monitored their expression by measuring β-galactosidase activities from cultures grown in defined medium with limiting or excess nitrogen. The results are shown in Table 4. The changes at −24 and at −13/−12 both virtually abolished expression. These results establish the −24/−12 sequence identified by sequence inspection as the nasR promoter.
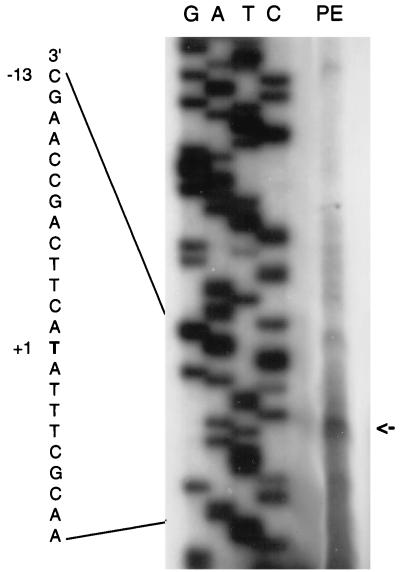
We sought to evaluate this assignment independently by using primer extension to determine the 5′ end of nasR mRNA. We isolated total RNA from strain VJSK2507, which contains the ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] operon fusion. Cultures were grown aerobically in defined medium with limiting nitrogen. The products of reverse transcriptase extension from a primer complementary to sequence spanning the nasR-lacZ fusion junction were resolved by gel electrophoresis and visualized by radioautography (Fig. 4). The significant background resulted from the overexposure required to detect the signal from a single copy of a weakly expressed gene. Nevertheless, the predominant band corresponded to a 92-bp cDNA (Fig. 4). This band aligns with a T in the sequence ladder that corresponds to the A residue at position +1 in the nasR coding strand, appropriately spaced from the −24/−12 promoter (Fig. 2; see above).
FIG. 4.
Primer extension analysis of nasR mRNA (see Materials and Methods for details). Total RNA was isolated from K. oxytoca VJSK2507. The assigned transcription start site (+1) and part of the ςN-dependent promoter sequence (−13) are labeled. The lanes marked G, A, T, and C show the corresponding dideoxynucleotide chain termination sequencing reactions. The lane marked PE shows the primer extension product, indicated by an arrow.
Nac protein is not required for nasR gene expression.
Work with K. aerogenes has demonstrated that expression of certain nitrogen-regulated operons, such as hut and put, requires the nac gene product in addition to a functional Ntr system (reviewed in reference 3). The nac gene, which is under positive regulation by Ntr, encodes a LysR family regulator that couples Ntr regulation to target ς70-dependent operons. Although the results summarized above establish that the nasR gene is expressed from a ςN-dependent promoter, the absence of essential regulatory sites upstream of position −71 (i.e., an enhancer) directed us to evaluate an alternative hypothesis, namely, that the Nac protein mediates Ntr control of nasR gene expression.
We do not have a nac mutant of K. oxytoca M5al, and so we used strains of the close relative K. aerogenes W70. The K. aerogenes nasF operon control region is very similar to that of K. oxytoca (9), and a K. oxytoca Φ(nasC-lacZ) operon fusion is expressed normally in K. aerogenes (19), demonstrating that K. aerogenes contains nasR+. Thus, K. aerogenes provides an appropriate surrogate strain for examining the Nac dependence of K. oxytoca nasR gene expression.
Plasmid pVJS3004, carrying the Φ(nasR-lacZ) {−469/+71} gene (translational) fusion, was used to monitor nasR gene expression. Plasmid pV16, which contains a Φ(hutU-lacZ) operon fusion, provided a control for Nac function (47). We monitored the expression of these fusions by measuring β-galactosidase activities from cultures grown in defined medium with limiting or excess nitrogen. The results are shown in Table 5.
TABLE 5.
Effects of nac on expression of plasmid-borne Φ(nasR-lacZ) and Φ(hutU-lacZ) operon fusions
| Genotypea | Fusionb | β-Galactosidase sp actc with:
|
|||
|---|---|---|---|---|---|
| No IPTG
|
IPTG added
|
||||
| NH4+, Gln | Gln | NH4+, Gln | Gln | ||
| nac+ | nasR | <1 | 230 | —d | — |
| nasR −29/−28AT→GC | 4 | 290 | — | — | |
| hutU | 260 | 9,510 | — | — | |
| nac::Tn5 | nasR | <1 | 670 | — | — |
| nasR −29/−28AT→GC | <1 | 460 | — | — | |
| hutU | 240 | 1,340 | — | — | |
| tac-nac+ | nasR | <1 | 230 | 8 | 140 |
| nasR −29/−28AT→GC | <1 | 300 | 9 | 150 | |
| hutU | 340 | 3,270 | 6,830 | 6,230 | |
| tac-nac+ ntrC | nasR | <1 | <1 | 6 | 3 |
| nasR −29/−28AT→GC | <1 | <1 | 8 | 9 | |
| hutU | 460 | 1,090 | 3,610 | 3,250 | |
K. aerogenes W70 strains (Table 1).
Φ(nasR-lacZ) {−469/+71} gene fusion on plasmids pVJS3004 (wild type) and pVJS3029 (−29/−28AT→G), and Φ(hutU-lacZ) operon fusion on plasmid pV16 (Table 1).
Determined as described in Materials and Methods and expressed in arbitary (Miller) units. Cultures were aerated in media containing the indicated nitrogen sources as described in Materials and Methods.
—, not determined.
In the nac+ K. aerogenes strain KC1043, both nasR and hutU gene expression were strongly induced by nitrogen limitation (Table 5). In the nac null strain KC2000 grown with limiting nitrogen, nasR gene expression was slightly increased whereas hutU expression was significantly decreased as expected. This result demonstrates that the Nac protein is not essential for activating nasR gene expression. Further analysis was performed with a nac::Tn5 tac strain, in which nac gene expression is controlled by the IPTG-inducible tac promoter (54). In this strain cultured with IPTG, Φ(hutU-lacZ) expression was indifferent to the nitrogen source whereas Φ(nasR-lacZ) expression remained nitrogen responsive (Table 5). Finally, an ntrC null allele abolished Φ(nasR-lacZ) expression in the nac::Tn5 tac strain, whereas Φ(hutU-lacZ) expression was little affected. These results establish that nitrogen-regulated nasR gene expression is independent of the Nac protein.
Mutational analysis indicates that the sequence ATA-N9-TAT, centered at −64, is an important element of Nac DNA binding sites (10, 20, 47). That sequence is also present in the nasR control region, centered at −22 and thereby overlapping the promoter sequence (Fig. 2). We therefore constructed a site-specific mutant changing AT at positions −29 and −28 to GC, thereby significantly damaging the upstream ATA element while leaving the promoter elements intact (Fig. 2). However, this double change had no discernible effect on Φ(nasR-lacZ) expression (Table 5). This result reinforces the above conclusion, i.e., that nasR gene expression is Nac independent.
Translation initiation.
Previous sequence analysis revealed the presumed nasR translation initiation region, as indicated in Fig. 2, where the presumed Shine-Dalgarno region and two potential initiation codons (encoding Met-1 and Met-4, as shown) are underlined (19). However, we had no direct evidence to show that nasR translation initiates at one of these ATG codons rather than further downstream (for example, the underlined GTG codon for Val-11). We wished to more precisely localize the start of nasR translation, in order to provide a context for evaluating transcriptional regulatory signals.
To determine if either ATG codon serves in initiating translation, we first constructed a plasmid-borne Φ(nasR′-′lacZ) {−469/+71} gene (translational) fusion (Fig. 1; see Materials and Methods), in which the synthesis of the active NasR-LacZ fusion protein is directed by nasR translation initiation signals. The cloned insert results in the in-frame fusion of the nasR codon for Phe-15 to lacZ codon 9. (Ten additional codons derived from polylinker sequence were introduced at the fusion junction.) We also constructed a site-specific mutant in which the second ATG codon, at nucleotides 36 to 38, was changed to ochre (TAA; Fig. 2). Both plasmids were introduced into K. oxytoca, and β-galactosidase specific activity was measured after growth in defined medium with limiting nitrogen. Whereas the wild-type fusion expressed about 230 Miller units, activity from the ochre fusion was negligible (about 1.5 Miller units).
The high activity expressed from the wild-type plasmid establishes the nasR translation initiation site as being located upstream of nucleotides 69 to 71 (codon 15). The very low activity expressed from the ochre (nt 36 to 38) mutant shows that translation initiates either at this second ATG or further upstream. Based on spacing from the Shine-Dalgarno sequence, we assign the first ATG (+27 to +29) as the likely nasR initiation codon. Irrespective of which ATG is the initiator, the short untranslated leader region seemingly presents an insufficient target for either antitermination or translational autoregulation by NasR protein.
Effects of nitrate and nitrite on nasR gene expression.
The NasR protein is a nitrate- and nitrite-responsive regulator of nasF operon transcription antitermination. To determine whether nasR gene expression is autoregulated, we examined the effects of nitrate and nitrite on expression of the ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] operon fusion. Indeed, nasR gene expression was decreased by about threefold during growth with nitrate and nitrite (Table 6). A nasR null allele abolished this nitrate- and nitrite-dependent inhibition while having no effect on Ntr-dependent ammonium inhibition (Table 6). On their face, these results could suggest that the NasR protein is a weak negative regulator of nasR gene expression.
TABLE 6.
Effects of nitrate and nitrite on expression of a chromosomal Φ(nasR-lacZ) operon fusion
| Straina | Genotypeb | β-Galactosidase sp actc with:
|
|||
|---|---|---|---|---|---|
| Gln | NO3−, Gln | NO2−, Gln | NH4+, Gln | ||
| VJSK2507 | nas+ | 67 | 22 | 28 | 7 |
| VJSK2517 | nasR131::Tn10d(Cm) | 77 | 140 | 96 | 13 |
| VJSK2512 | Δ(nasFED)135 | 70 | 140 | 32 | 8 |
| VJSK2516 | Δ(nasCB)139 | 61 | 135 | 106 | 5 |
| VJSK2529 | nasD124::Ω-Cm | 67 | 150 | 101 | 6 |
Derivatives of strain VJSK2216 (Table 1).
All strains carry ΔrhaBS::[Φ(nasR-lacZ) {−469/+71}] (Fig. 1).
Determined as described in Materials and Methods and expressed in arbitary (Miller) units. Cultures were aerated in media containing the indicated nitrogen sources as described in Materials and Methods.
An alternative hypothesis is that the decreased nasR gene expression was due to assimilation of nitrate and nitrite into ammonium, thereby diminishing the magnitude of Ntr activation. To test this idea, we introduced mutational alterations that block various steps of nitrate assimilation. The Δ(nasFED) deletion eliminates the nitrate and nitrite uptake system, without affecting the expression of nasCBA genes encoding assimilatory nitrate and nitrite reductases (64). The strain carrying this deletion exhibited nitrate-insensitive nasR gene expression, although nitrite inhibition was unaffected (Table 6). In fact, nitrite is efficiently transported by a second, uncharacterized pathway in nasFED mutants (under the growth conditions used [64]), and so this result indicates that nitrate must be transported to exert its inhibitory effect on nasR gene expression.
The Δ(nasCB) deletion eliminates assimilatory nitrate and nitrite reductase activities while leaving the nasFED-encoded transport system intact. The strain carrying this deletion exhibited nitrate- and nitrite-insensitive nasR gene expression (Table 6), indicating that nitrate and nitrite must be converted to ammonium to exert their inhibitory effect on nasR gene expression. Finally, the nasD::Ω insertion eliminates both nitrate transport (through inactivation of the nasD gene [64]) as well as assimilatory nitrate and nitrite reductase activities (through strong polarity on nasCBA expression [29]). Again, nitrate- and nitrite-dependent inhibition of nasR gene expression was abolished in this strain. Together, these results indicate not that NasR is a negative regulator of nasR gene expression but, rather, that nitrate and nitrite conversion to ammonium influences Ntr regulation sufficiently to measurably decrease nasR gene expression. [We do not understand why nitrate caused an approximately twofold increase in Φ(nasR-lacZ) expression in the various nas mutants (Table 6).]
DISCUSSION
K. oxytoca (pneumoniae) M5al is a genetically amenable enterobacterium that has been widely used for studies of dinitrogen fixation and Ntr (60). Previous studies have shown that nasF (nitrate assimilation) operon expression is subject to dual regulation: phospho-NtrC-dependent activation of a ςN-dependent promoter, and NasR-dependent nitrate- and nitrite-responsive transcription antitermination (8, 9, 30). We report here our analysis of nasR gene regulation. We found that nasR gene expression was modulated about 10-fold by Ntr. This regulation required a ςN-dependent promoter and the ntrC+ gene and was independent of the nac+ gene. However, deletion of upstream sequences had no discernible effect on ntrC+-dependent stimulation of Φ(nasR-lacZ) expression. Thus, like the S. typhimurium argT promoter (2, 52), the nasR promoter may not require an upstream binding site for full-level NtrC activation.
The nasR promoter.
Sequence inspection identified the critical elements of a ςN-dependent promoter — GG and GC — upstream of the nasR coding region (Fig. 2). Site-specific changes at −24 and at −12 plus −13 both virtually abolished Φ(nasR-lacZ) expression, as did introduction of an rpoN null allele (Table 4; rpoN encodes ςN). A deletion to −14 likewise eliminated Φ(nasR-lacZ) expression (Table 3), demonstrating that no other promoters lie between −14 and +362 (Fig. 1). Primer extension analysis provided independent support for these conclusions (Fig. 4). Therefore, nasR gene expression is controlled by a single ςN-dependent promoter.
Although the −24 GG and −12 GC elements are critical for ςN-dependent promoter function, nucleotides surrounding the −12 sequence also contribute to promoter function and activation (reviewed in references 34 and 36). One example comes from study of the NifA-activated K. oxytoca nifH promoter. Transcription from a mutant promoter, changed from the wild-type CCCTGC to TTTTGC, is much less dependent on NifA binding to the UAS, perhaps due to increased affinity for ςN (6, 7). Note that the corresponding region of the nasR promoter, TATTGC, is also T rich (Fig. 2). Nevertheless, it is not yet possible to predict reliably the ςN-dependent promoter function from sequence inspection alone (62).
The Nac protein does not control nasR expression.
One mechanism for controlling nitrogen-regulated gene expression involves the Nac protein, which is known to activate only ς70-dependent promoters (3). However, Φ(nasR-lacZ) expression was indifferent to nac+ in K. aerogenes W70 (Table 5). Site-specific mutational analysis further demonstrated that a sequence (ATA-N9-TAT [Fig. 2]) that might be construed as a Nac protein binding site (10, 20, 47) was irrelevant for nasR gene control (Table 5). Therefore, Ntr control of nasR gene expression does not involve the Nac protein.
How does phospho-NtrC protein activate nasR gene expression?
The only other known mechanism for nitrogen-regulated gene expression in enterobacteria involves phospho-NtrC protein, which activates transcription initiation at ςN-dependent promoters (reviewed in references 34, 38, 44, and 48). For most of these, including the well-studied glnA, nifL, and glnH promoters, activation is stimulated by phospho-NtrC binding to upstream sites, the proximal boundaries of which are located 70 to 100 bp upstream of the −24 GG element.
Characterized NtrC binding sites consist of 17-bp sequences, containing an inverted pair of 7-bp half-sites, that are similar to the consensus sequence TGCACCA-N3-TGGTGCA (reviewed in reference 34). The glnA, nifL, and glnH upstream control regions each contain at least two such 17-mers, and only one of the two nifL 17-mers has as many as 6 of 14 mismatches from this consensus. Inspection of the nasR upstream region to position −300 failed to reveal even a single 17-mer with fewer than 7 of 14 mismatches (Fig. 2). Furthermore, deletions to positions −64 and −71 had no influence on Φ(nasR-lacZ) expression (Tables 2 and 3). Thus, any upstream NtrC binding site must have its distal boundary no more than 39 bp upstream of the −24 GG element (Fig. 2); the proximal boundary of such a 17-mer would be at position −47. Such a location is unprecedented. Additionally, any NtrC binding sites in this region would overlap with the beginning of the ychN gene (Fig. 2), which, unlike the nasRFEDCBA region, is conserved in E. coli K-12 (5). Finally, any downstream NtrC binding site must have its distal boundary upstream of +71. Nonetheless, nasR gene expression was fully dependent upon ntrC+, even in fusion constructs with only 64 or 71 nt upstream of the transcription initiation site (Table 2 and data not shown). (The residual twofold nitrogen regulation in ntrC null strains is an unexplained peculiarity of Klebsiella spp. [32].)
These observations mimic those made by Schmitz et al., who found that Ntr control of argT gene expression operates through a ςN-dependent promoter (52). Further analysis demonstrated that sequences within 44 bp of the transcription initiation site are sufficient to confer essentially wild-type Ntr control — about fivefold — on expression of Φ(argT-galK) operon fusions. Furthermore, NtrC protein failed to protect argT control region DNA from DNase I digestion under conditions where the glnA, ntrBC, and dhuA NtrC binding sites were well protected (2). Together, these results indicate that argT gene expression is independent of upstream or downstream NtrC binding sites.
Although stimulatory, upstream binding sites are not essential for phospho-NtrC activation of the glnA or nifLA promoters (53). Expression of single- or low-copy Φ(glnA-lacZ) operon fusion constructs during nitrogen-limited growth is reduced by 10-fold or less upon deletion of the upstream binding sites, whereas introduction of an ntrC null allele reduces expression by about 100-fold (12, 50). Likewise, Φ(nifL-lacZ) expression is reduced but not eliminated upon deletion of the upstream binding sites (14, 40). Thus, significant phospho-NtrC-dependent transcription activation can occur even without specific DNA binding sites (46).
Schneider et al. suggested that “minimal” Ntr-controlled promoters with no NtrC binding sites (such as the argT promoter) may allow differentially regulated gene expression, perhaps in response to different nitrogen sources (53). Presumably, phospho-NtrC activation of these promoters results from nonspecific binding to DNA (46). It would be tempting to speculate that the nucleotide sequences of these minimal promoters provide sufficient activation in the absence of activator binding sites (see above). However, the S. typhimurium argT and K. oxytoca nasR promoters exhibit little sequence similarity aside from the conserved −24 and −12 elements. Evaluating this and other ideas will require further investigation.
Ntr control of nitrate assimilation.
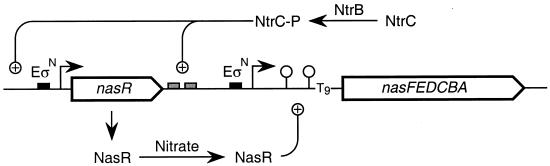
Manifestation of the nitrate assimilation phenotype involves at least two targets for Ntr control (Fig. 5). The first target is expression of the nasF structural gene operon, which is controlled by phospho-NtrC binding to a conventional upstream sequence (30). The second target is expression of the nasR regulatory gene, also apparently controlled by phospho-NtrC albeit in an unconventional manner. Thus, growth under nitrogen-sufficient conditions serves to dampen nasF operon expression both directly (by decreasing Ntr activation of the nasF promoter) and indirectly (by decreasing synthesis of the NasR regulatory protein) (Fig. 5).
FIG. 5.
Control of nitrate assimilation gene expression (not to scale). The promoters for the nasR gene and the nasF operon, indicated by solid rectangles, are recognized by ςN-RNA polymerase (EςN). Transcription initiation sites are indicated by arrows. Phospho-NtrC protein binding sites upstream of the nasF operon are indicated by shaded rectangles. NtrC is phosphorylated by NtrB protein in response to nitrogen limitation. The nasF operon leader region contains two RNA stem-loop structures, the distal one of which is a factor-independent terminator that includes a polythymidine tract (T9). NasR protein binds to leader RNA to effect transcription antitermination.
Why should nasR gene expression be controlled by Ntr? One idea is that elevation of NasR protein levels during nitrogen-limited growth serves to sensitize the response of the organism to even relatively low levels of nitrate, which is a preferred alternative nitrogen source. A second idea derives from the notion that NasR protein can effect a significant level of transcription antitermination in the nasF operon leader region even in the absence of nitrate or nitrite (9). For example, introduction of a nasR null allele results in a fivefold decrease of nasF operon expression during nitrogen-limited growth in the absence of nitrate or nitrite (19). Thus, Ntr control of NasR synthesis might provide an additional means of further reducing the basal level of nasF operon expression.
The control of nitrate assimilation (Fig. 5) shares some general features with control of dinitrogen fixation in K. oxytoca M5al. In both cases, expression of pathway-specific regulatory genes (nasR and nifLA, respectively) is directly controlled by Ntr. The activities of these regulatory proteins are themselves controlled by physiological cues (nitrate and nitrite for NasR; oxygen and fixed nitrogen for NifL-NifA). However, the correspondence is inexact. Expression of the nif structural genes is directly activated by the NifA protein and is not known to be regulated by phospho-NtrC under ordinary physiological conditions (reviewed in reference 13). By contrast, expression of the nasF structural gene operon appears to be subject directly to dual control by both NasR and phospho-NtrC (Fig. 5).
Effect of nitrate and nitrite on nasR gene expression.
Both nitrate and nitrite decreased Φ(nasR-lacZ) expression only in nasR+ strains (Table 6). However, this diminution required both the transport and reduction of nitrate and nitrite. Thus, the observed inhibition of Φ(nasR-lacZ) expression is due to decreased Ntr activation in response to formation of ammonium from nitrate. Furthermore, the short distance (26 bp) between the transcription initiation site and the probable nasR initiation codon is insufficient to contain a regulated transcription terminator, which is the target of NasR action in the nasF operon. Thus, we conclude that nasR gene expression is not subject to transcriptional autoregulation.
ACKNOWLEDGMENTS
Catherine Dunn (University of California, Davis) performed experiments to confirm and extend the results summarized in Table 2. Robert Bender, Alexander Ninfa, and an anonymous reviewer provided invaluable constructive critique of an early version of the manuscript. We are obliged to Mike Merrick for sharing many useful K. oxytoca M5al strains. We thank Karen Skorupski for providing her allelic exchange vector in advance of publication, Thomas Linn for the λTXF97 transcriptional fusion vector, Brian Janes and Robert Bender for the K. aerogenes nac strains, and Susan Egan for E. coli rha mutants. Automated DNA sequence analyses were performed by the Cornell University Biotechnology Program central services group.
This study was supported by U.S. Department of Energy grant 91ER20027 from the Division of Energy Biosciences and by Hatch project CA-D*-MIC-6572-H from the California Agricultural Experiment Station.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ames G F-L, Nikaido K. Nitrogen regulation in Salmonella typhimurium. Identification of an ntrC protein-binding site and definition of a consensus binding sequence. EMBO J. 1985;4:539–547. doi: 10.1002/j.1460-2075.1985.tb03662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender R A. The role of the NAC protein in the nitrogen regulation of Klebsiella aerogenes. Mol Microbiol. 1991;5:2575–2580. doi: 10.1111/j.1365-2958.1991.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 4.Bender R A, Janssen K A, Resnick A D, Blumenberg M, Foor F, Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977;129:1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Buck M, Cannon W. Mutations in the RNA polymerase recognition sequence of the Klebsiella pneumoniae nifH promoter permitting transcriptional activation in the absence of NifA binding to upstream activator sequences. Nucleic Acids Res. 1989;17:2597–2612. doi: 10.1093/nar/17.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon W, Claverie-Martin F, Austin S, Buck M. Core RNA polymerase assists binding of the transcription factor ς54 to promoter DNA. Mol Microbiol. 1993;8:287–298. doi: 10.1111/j.1365-2958.1993.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 8.Chai W, Stewart V. NasR, a novel RNA-binding protein, mediates nitrate-responsive transcription antitermination of the Klebsiella oxytoca M5al nasF operon leader in vitro. J Mol Biol. 1998;283:339–351. doi: 10.1006/jmbi.1998.2105. [DOI] [PubMed] [Google Scholar]
- 9.Chai W, Stewart V. RNA sequence requirements for NasR-mediated, nitrate-responsive transcription antitermination of the Klebsiella oxytoca M5al nasF operon leader. J Mol Biol. 1999;292:203–216. doi: 10.1006/jmbi.1999.3084. [DOI] [PubMed] [Google Scholar]
- 10.Chen, L.-M., G. T. J., R. A. Bender, S. Swift, and S. Maloy. 1998. Genetic analysis, using P22 challenge phage, of the nitrogen activator protein DNA-binding site in the Klebsiella aerogenes put operon. J. Bacteriol. 180:571–577. [DOI] [PMC free article] [PubMed]
- 11.Christie A, Butler M. Growth and metabolism of a murine hybridoma in cultures containing glutamine-based dipeptides. Focus (Gibco BRL) 1994;16:9–13. [Google Scholar]
- 12.Dixon R. Tandem promoters determine regulation of the Klebsiella pneumoniae glutamine synthetase (glnA) gene. Nucleic Acids Res. 1984;12:7811–7830. doi: 10.1093/nar/12.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon R A. The genetic complexity of nitrogen fixation. J Gen Microbiol. 1984;130:2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- 14.Drummond M, Clements J, Merrick M, Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983;301:302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, C. A., and V. Stewart. Unpublished observations.
- 16.Elliott T. A method for constructing single-copy lac fusions in Salmonella typhimurium and its application to the hemA-prfA operon. J Bacteriol. 1992;174:245–253. doi: 10.1128/jb.174.1.245-253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espin G, Alvarez-Morales A, Merrick M. Complementation analysis of glnA-linked mutations which affect nitrogen fixation in Klebsiella pneumoniae. Mol Gen Genet. 1981;184:213–217. doi: 10.1007/BF00272907. [DOI] [PubMed] [Google Scholar]
- 18.Feng J L, Goss T J, Bender R A, Ninfa A J. Activation of transcription initiation from the nac promoter of Klebsiella aerogenes. J Bacteriol. 1995;177:5523–5534. doi: 10.1128/jb.177.19.5523-5534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman B S, Lin J T, Stewart V. Identification and structure of the nasR gene encoding a nitrate- and nitrite-responsive positive regulator of nasFEDCBA (nitrate assimilation) operon expression in Klebsiella pneumoniae M5al. J Bacteriol. 1994;176:5077–5085. doi: 10.1128/jb.176.16.5077-5085.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goss T J, Bender R A. The nitrogen assimilation control protein, Nac, is a DNA binding transcription activator in Klebsiella aerogenes. J Bacteriol. 1995;177:3546–3555. doi: 10.1128/jb.177.12.3546-3555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groisman E A, Casadaban M J. Mini-Mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986;168:357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda T P, Shauger A E, Kustu S. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 23.Janes B, Bender R A. Alanine catabolism in Klebsiella aerogenes: molecular characterization of the dadAB operon and its regulation by the nitrogen assimilation control protein. J Bacteriol. 1998;180:563–570. doi: 10.1128/jb.180.3.563-570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang P, Ninfa A J. Regulation of autophosphorylation of Escherichia coli nitrogen regulator II by the PII signal transduction protein. J Bacteriol. 1999;181:1906–1911. doi: 10.1128/jb.181.6.1906-1911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingston R E. Primer extension. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. Boston, Mass: Wiley Interscience; 1987. pp. 4.8.1–4.8.3. [Google Scholar]
- 26.Kustu S, Burton D, Garcia E, McCarter L, McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci USA. 1979;76:4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis M K, Thompson D V. Efficient site directed in vitro mutagenesis using ampicillin selection. Nucleic Acids Res. 1990;18:3439–3443. doi: 10.1093/nar/18.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J T, Goldman B S, Stewart V. Structures of genes nasA and nasB, encoding assimilatory nitrate and nitrite reductases in Klebsiella pneumoniae M5al. J Bacteriol. 1993;175:2370–2378. doi: 10.1128/jb.175.8.2370-2378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J T, Goldman B S, Stewart V. The nasFEDCBA operon for nitrate and nitrite assimilation in Klebsiella pneumoniae M5al. J Bacteriol. 1994;176:2551–2559. doi: 10.1128/jb.176.9.2551-2559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J T, Stewart V. Nitrate and nitrite-mediated transcription antitermination control of nasF (nitrate assimilation) operon expression in Klebsiella pneumoniae M5al. J Mol Biol. 1996;256:423–435. doi: 10.1006/jmbi.1996.0098. [DOI] [PubMed] [Google Scholar]
- 31.Lin J T, Stewart V. Nitrate assimilation by bacteria. Adv Microb Physiol. 1998;38:1–30. doi: 10.1016/s0065-2911(08)60014-4. [DOI] [PubMed] [Google Scholar]
- 32.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacNeil D, Zhu J, Brill W J. Regulation of nitrogen fixation in Klebsiella pneumoniae: isolation and characterization of strains with nif-lac fusions. J Bacteriol. 1981;145:348–357. doi: 10.1128/jb.145.1.348-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magasanik B. Regulation of nitrogen utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1344–1356. [Google Scholar]
- 35.Maloy S R, Stewart V, Taylor R K. Genetic analysis of pathogenic bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. [Google Scholar]
- 36.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 37.Merrick M J, Coppard J R. Mutations in genes downstream of the rpoN gene (encoding ς54) of Klebsiella pneumoniae affect expression from ς54-dependent promoters. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 38.Merrick M J, Edwards R A. Nitrogen control in bacteria. Microbiol Rev. 1995;59:604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 40.Minchin S D, Austin S, Dixon R A. The role of activator binding sites in transcriptional control of the divergently transcribed nifF and nifLA promoters from Klebsiella pneumoniae. Mol Microbiol. 1988;2:433–442. doi: 10.1111/j.1365-2958.1988.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 41.Minchin S D, Austin S, Dixon R A. Transcriptional activation of the Klebsiella pneumoniae nifLA promoter by NTRC is face-of-the-helix dependent and the activator stabilizes the interaction of sigma 54-RNA polymerase with the promoter. EMBO J. 1989;8:3491–3499. doi: 10.1002/j.1460-2075.1989.tb08514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minton N P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984;31:269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- 43.Moralejo P, Egan S M, Hidalgo E, Aguilar J. Sequencing and characterization of a gene cluster encoding the enzymes for l-rhamnose metabolism in Escherichia coli. J Bacteriol. 1993;175:5585–5594. doi: 10.1128/jb.175.17.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ninfa A J, Atkinson M R, Kamberov E S, Feng J, Ninfa E G. Control of nitrogen assimilation by the NRI-NRII two-component system of enteric bacteria. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 67–88. [Google Scholar]
- 45.Ninfa A J, Reitzer L J, Magasanik B. Initiation of transcription at the bacterial glnAP2 promoter by purified E. coli components is facilitated by enhancers. Cell. 1987;50:1039–1046. doi: 10.1016/0092-8674(87)90170-x. [DOI] [PubMed] [Google Scholar]
- 46.North A K, Kustu S. Mutant forms of the enhancer-binding protein NtrC can activate transcription from solution. J Mol Biol. 1997;267:17–36. doi: 10.1006/jmbi.1996.0838. [DOI] [PubMed] [Google Scholar]
- 47.Pomposiello P J, Janes B, Bender R A. Two roles for the DNA recognition site of the Klebsiella aerogenes nitrogen assimilation control protein. J Bacteriol. 1998;180:578–585. doi: 10.1128/jb.180.3.578-585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter S C, North A K, Kustu S. Mechanism of transcriptional activation by NtrC. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 147–158. [Google Scholar]
- 49.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 391–407. [Google Scholar]
- 50.Reitzer L J, Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986;45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 51.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz G, Nikaido K, Ames G F-L. Regulation of a transport operon promoter in Salmonella typhimurium: identification of sites essential for nitrogen regulation. Mol Gen Genet. 1988;215:107–117. doi: 10.1007/BF00331311. [DOI] [PubMed] [Google Scholar]
- 53.Schneider B L, Shiau S P, Reitzer L J. Role of multiple environmental stimuli in control of transcription from a nitrogen-regulated promoter in Escherichia coli with weak or no activator-binding sites. J Bacteriol. 1991;173:6355–6363. doi: 10.1128/jb.173.20.6355-6363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwacha A, Bender R A. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J Bacteriol. 1993;175:2116–2124. doi: 10.1128/jb.175.7.2116-2124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimotsu H, Henner D J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43:85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- 56.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 57.Skorupski K, Taylor R T. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 58.Stewart V, Parales J., Jr Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J Bacteriol. 1988;170:1589–1597. doi: 10.1128/jb.170.4.1589-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St. Pierre R, Linn T. A refined vector system for in vitro construction of single-copy transcriptional or translational fusions of lacZ. Gene. 1996;169:65–68. doi: 10.1016/0378-1119(95)00787-3. [DOI] [PubMed] [Google Scholar]
- 60.Streicher S, Gurney E, Valentine R C. The nitrogen-fixation genes. Nature. 1972;239:495–499. doi: 10.1038/239495a0. [DOI] [PubMed] [Google Scholar]
- 61.Streicher S L, Shanmugam K T, Ausubel F, Morandi C, Goldberg R B. Regulation of nitrogen fixation in Klebsiella pneumoniae: evidence for a role of glutamine synthetase as a regulator of nitrogenase synthesis. J Bacteriol. 1974;120:815–821. doi: 10.1128/jb.120.2.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Gralla J D. Multiple in vivo roles for the −12 region elements of sigma 54 promoters. J Bacteriol. 1998;180:5626–5631. doi: 10.1128/jb.180.21.5626-5631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong P-K, Popham D, Keener J, Kustu S. In vitro transcription of the nitrogen fixation regulatory operon nifLA of Klebsiella pneumoniae. J Bacteriol. 1987;169:2876–2880. doi: 10.1128/jb.169.6.2876-2880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q, Stewart V. NasFED proteins mediate assimilatory nitrate and nitrite transport in Klebsiella oxytoca (pneumoniae) M5al. J Bacteriol. 1998;180:1311–1322. doi: 10.1128/jb.180.5.1311-1322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]