Abstract
Arbuscular mycorrhizal fungi (AMF) are ubiquitous in soil and form nutritional symbioses with ~80% of vascular plant species, which significantly impact global carbon (C) and nitrogen (N) biogeochemical cycles. Roots of plant individuals are interconnected by AMF hyphae to form common AM networks (CAMNs), which provide pathways for the transfer of C and N from one plant to another, promoting plant coexistence and biodiversity. Despite that stable isotope methodologies (13C, 14C and 15N tracer techniques) have demonstrated CAMNs are an important pathway for the translocation of both C and N, the functioning of CAMNs in ecosystem C and N dynamics remains equivocal. This review systematically synthesizes both laboratory and field evidence in interplant C and N transfer through CAMNs generated through stable isotope methodologies and highlights perspectives on the system functionality of CAMNs with implications for plant coexistence, species diversity and community stability. One-way transfers from donor to recipient plants of 0.02-41% C and 0.04-80% N of recipient C and N have been observed, with the reverse fluxes generally less than 15% of donor C and N. Interplant C and N transfers have practical implications for plant performance, coexistence and biodiversity in both resource-limited and resource-unlimited habitats. Resource competition among coexisting individuals of the same or different species is undoubtedly modified by such C and N transfers. Studying interplant variability in these transfers with 13C and 15N tracer application and natural abundance measurements could address the eco physiological significance of such CAMNs in sustainable agricultural and natural ecosystems.
Keywords: 13C, 15N, carbon and nitrogen cycling, interplant nutrient exchange, plant coexistence, resource competition, resource share
Highlights
• Plants interconnected by arbuscular mycorrhizal (AM) fungi form common AM networks
• 13C and 15N labeling can trace the amount of AM-mediated interplant C and N transfers
• 0.02‒41% C transfers are from a donor to a receiver, but< 10% in the reverse route
• 0.04‒80% N transfers are from a donor to a receiver, but< 15% in the reverse route
• Interplant C and N transfers should enhance plant survival under nutrient-limitations
1. Introduction
1.1. Arbuscular mycorrhiza
Arbuscular mycorrhizas (AM) are formed between arbuscular mycorrhizal fungi (AMF) and roots of ~70% of ~391,000 higher plant species (Wang and Qiu, 2006; Smith and Read, 2008; Brundrett, 2009; Brundrett, 2017; Brundrett and Tedersoo, 2018). Currently 25 genera and ~338 fungal species belonging to the sub-Phylum Glomeromycota form AMF globally (Schüßler and Walker, 2010). AMF acquire soil nutrients, such as nitrogen (N), phosphorus (P) and other mineral nutrients, and transport them to their host plant in exchange for up to 20% of photosynthetically fixed carbon (C) (Smith and Read, 2008; Roth and Paszkowski, 2017). In an arbuscular mycorrhiza, the intraradical mycelium (IRM) often penetrates root cortical cells to form arbuscules, while the extraradical mycelium (ERM) extends into soil, far beyond the root zone. The ERM forages for N, P, potassium and other soil nutrients, and translocates them to the IRM, where they are exchanged for C from the host (Smith et al., 2009). The ERM is extensive enabling plant access to nutrient resources well beyond the root depletion zone (Li et al., 1991). In addition, several findings revealed that sources of carbon for mutualistic AMF include fatty acids exported from the host plants, as well as lipids and sugars (Pfeffer et al., 1999; Keymer et al., 2017; Jiang et al., 2017).
1.2. Common arbuscular mycorrhizal networks
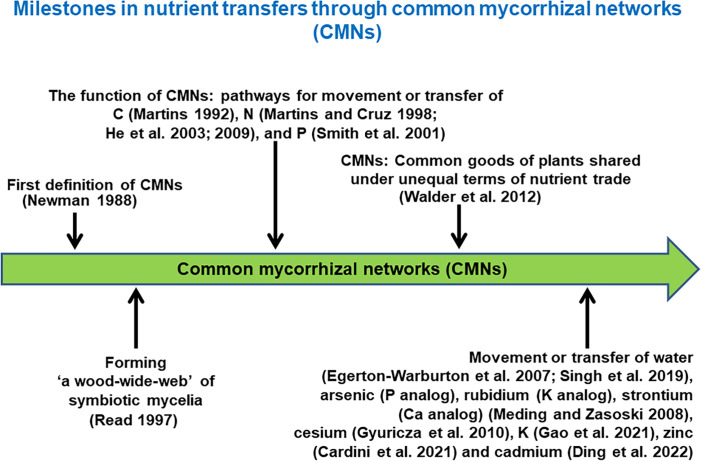
AMF are ubiquitous components of most soil ecosystems, where they grow through soil, colonize plant roots, and can form links between plants (Newman et al., 1992; Newman et al., 1994; He et al., 2003; He et al., 2009; Molina and Horton, 2015). The plants suppling AMF with labile carbon often grow close together, primarily in multiple species communities. Because AMF exhibit little host specificity (Smith and Read, 2008), and plant roots can thus be linked by a common AM network (CAMN) (Wipf et al., 2019). Such CAMNs, being formed among individual plants of the same species or genus, or from different genera or families (Ronsheim and Anderson, 2001; Southworth et al., 2005), are usually woven into an even larger network of fungi and roots in natural communities (Smith and Read, 2008; Wipf et al., 2019). In this way, plant species within CAMNs may be joined together as a functional guild and become pathways for movement or transfer of nutrients ( Figure 1 ), including C (Francis and Read, 1984; Martins, 1992; Martins, 1993; Watkins et al., 1996; Fitter et al., 1998; Mikkelsen et al., 2008; Voets et al., 2008; Walder et al., 2012), N (Hamel et al., 1991a; Hamel et al., 1991b; He et al., 2003; He et al., 2009; Frey and Schüepp, 1993; Rogers et al., 2001; Moyer-Henry et al., 2006), P (Tuffen et al., 2002; Smith and Smith, 2011; Merrild et al., 2013), arsenic (P analog, Meding and Zasoski, 2008), cadmium (Ding et al., 2022), K (Gao et al., 2021), cesium (Meding and Zasoski, 2008; Gyuricza et al., 2010), rubidium (K analogs) and strontium (Ca analog) (Meding and Zasoski, 2008, and zinc (Cardini et al., 2021). Water (Egerton-Warburton et al., 2007) and genetic material (Giovannetti et al., 2004) can also move within these networks. Movement of these materials can thus promote coexistence and biodiversity among plants (Read, 1991; Smith and Read, 2008).
Figure 1.
Milestones in nutrient transfers through common mycorrhizal networks (CMNs) (Note that the year of a reference pointing to the green arrow bar is not to scale).
Despite the considerable evidence of the functional role of CAMNs, they have not been directly visualized in natural ecosystems due to their cryptic, fragile, and microscopic nature (Newman et al., 1994; Ronsheim and Anderson, 2001; Southworth et al., 2005; Wipf et al., 2019). Plants invest photosynthetic products to feed their fungal partners, which, in return, provide mineral nutrients foraged in soil by their intricate hyphal networks (Bever et al., 2010). The Driver (AMF partners drive plant communities) and Passenger (AMF community dynamics follows changes in the host plant community) hypotheses were suggested to explain the mutual relationships of plant and AMF communities (Zobel and Öpik, 2014). Research into this complex system of plant-fungus interactions indicates that plants and fungi can choose their trading partners (Kiers et al., 2011; Walder et al., 2012).
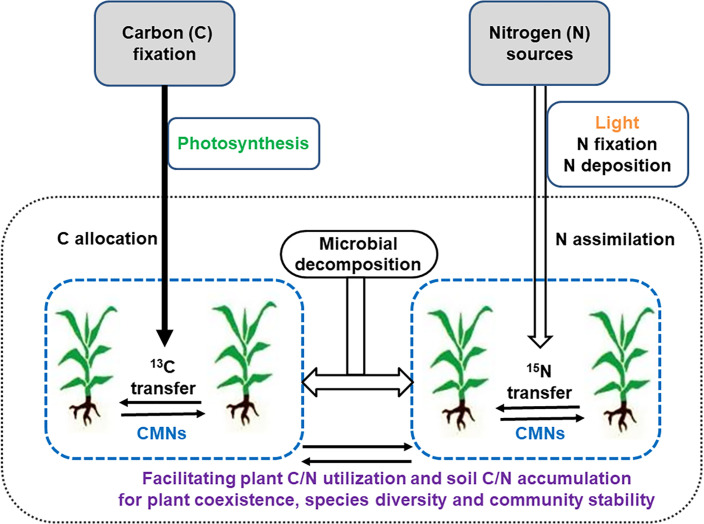
An understanding of the stoichiometry of C, N, or other nutrients mediated by CAMNs could better elucidate the potential roles of CAMNs in C and N functioning in plant-soil systems ( Figure 2 ), although at present CAMNs have not been directly visualized in natural ecosystems due to their fragile and microscopic nature. Application of high-throughput genome sequences or all sorts of omics and BONCAT-FACS (bioorthogonal non-canonical amino acid tagging + fluorescence-activated cell sorting, Couradeau et al., 2019) could have an in situ observation of these underground cryptic microorganisms. Meanwhile, the employment of other emerging technologies, such as cryo-scanning electron microscope (Cryo-SEM), DNA stable isotope probing (DNA-SIP), quantitative multi-isotope imaging mass spectrometry (MIMS), nanoscale secondary ion mass spectrometry (NanoSIMS), single-molecule electronic device and synchrotron radiation facility, could enable the mapping the interplant flow of 13C and 15N through CAMNs. Given this demonstrated autonomy and the key role that CAMNs play in interplant nutrient transfers and biodiversity in ecosystems, it is crucial to understand how nutrient resources (e.g., C, N, P, other elements, see Figure 1 ) are shared among plants through CAMNs. And whether there may be a mechanism between CAMNs and ecosystems by which a greater biodiversity is associated with a greater productivity.
Figure 2.
A conceptual framework of roles played by common mycorrhizal networks (CMNs) in regulating carbon (C) and nitrogen (N) flow or transfer within and between plants.
1.3. Application of isotopes of 13C and 15N labeling
The abundance level of stable isotopes is theoretically expressed as delta (δ) in parts per thousand or per mil (‰), which is calculated as δ13C or δ15N (‰) = [(RSample/RStandard) –1] × 1,000, where R is the 13C/12C or 15N/14N (atom%) ratio of the sample and standard, and “Vienna”-Pee Dee Belemnite (0.0112372) or atmospheric N2 (0.0036765) is their respective standard material. The 13C and 15N isotopic composition (also expressed as δ13C and δ15N signatures) of plant materials can provide information on (i) inputs of photosynthetic C or uptake of fertilizer N, (ii) plant N derived from N2 fixation by symbiotic microorganisms, (iii) C or N cycling and (iv) the sources of N available for host plant growth (Dawson et al., 2002). For instance, the δ13C or δ15N signatures in vegetation could reflect the relative availability of C sources to fungi and N sources to plants differing in isotopic composition (Querejeta et al., 2003). Here we examine the unique and common characteristics of CMN-mediated interplant C and N transfers that are demonstrated by 13C and 15N labeling (sometimes referred to as 13C and 15N enrichment) or variations in their isotopic composition for exploring the beneficial functionality of CMNs in sustaining managed and natural systems in a changing climate (Dawson et al., 2002; He et al., 2003; Querejeta et al., 2003; Moyer-Henry et al., 2006; He et al., 2009; Jalonen et al., 2009; Kurppa et al., 2010; Walder et al., 2012; Ren et al., 2013; Meng et al., 2015; Wang et al., 2016; Řezáčová et al., 2018; Wipf et al., 2019; Muneer et al., 2020a; Alaux et al., 2021; Avital et al., 2022; Reay et al., 2022).
1.4. Calculation of carbon and nitrogen transfer from a donor to a receiver plant
Estimates of C or N transfer from a donor to a receiver plant are based on the assumption that an equal proportion of applied and unapplied C or N are transferred. The percentage of total C or N transferred to the receiver (% Ntransfer) is then assessed from the ratio of applied C or N in the receiver and total applied C or N in the receiver and donor. Based mostly on the calculations from Giller et al. (1991); Ikram et al. (1994); Johansen and Jensen (1996), the following equations are commonly employed by almost all relevant studies to calculate C or N transfers.
| (1) |
where 13Ccontentplant or 15Ncontentplant = atom%13Cexcessplant or 15Nexcessplant ×
| (2) |
and atom%13Cexcessplant or atom%15Nexcessplant = atom%13Cplant or
| (3) |
The amount of C or N (mg plant−1) transferred from the donor (Ctransfer or Ntransfer) is calculated as:
| (4) |
The % of C or N in the receiver derived from transfer (% CDFT or % NDFT) is calculated as:
| (5) |
2. Carbon transfer between plants through common arbuscular mycorrhizal networks
2.1. Arbuscular mycorrhizal fungi and carbon
In the AM associations, C is the major flux from plant to fungus while P, and possibly N, are the primary fluxes from fungus to plant (Smith and Read, 2008). In general, 5–20% of plant assimilated net C was transferred between linked plants via the CAMNs (Pearson and Jakobsen, 1993). Using 13CO2 to label Hypochaeris radicata growing in Danish coastal grasslands and tracing that labeled C for one growing season, Lekberg et al. (2013) concluded that plants allocated C to AMF even at temperatures close to freezing and that fungal structures persisted in the roots during periods of little C-allocation. Plants could release 13C into rhizosphere soil through AM mycelia. These results suggest that the host plant maintained a supply of C to its AMF symbionts to ensure its own ability to obtain soil mineral nutrition from the AMF’s mycelia (Fitter et al., 1998; Lekberg et al., 2010). On the other hand, C transfer via an AM network does not allow resource sharing among linked plants (Robinson and Fitter, 1999). The mycocentric view is that fungal structures within roots are parts of extended mycelia through which fungi move C according to their own C demands, not those of their autotrophic hosts (Fitter et al., 1998).
Since the growth of AMF completely depends on supply of photosynthetically fixed C from their hosts, the C supply from the plant can be regarded as an infinitely large benefit for AMF fitness (Bago et al., 2000) ( Figure 2 ). From the perspective of the plant, the amount of C provided to the fungal symbiont represents the symbiotic costs (Konvalinková and Jansa, 2016). The dynamics of C exchange between plants and fungi in AM associations is conceptualized as a biological market, in which C sources are reciprocally exchanged in both directions, with preferential allocation to the partner offering the best rate of exchange (Dawson et al., 2002; Werner et al., 2014; Konvalinková and Jansa, 2016). Previous studies have suggested that there is an asymmetry in C-for-nutrient exchange between AMF and host plants. AMF acquire C from their hosts not only as carbohydrates but also as fatty acids (Pfeffer et al., 1999; Trépanier et al., 2005; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017). It is known that the extraradical hyphae and spores of AMF secrete a special glycoprotein, glomalin into the soil, defined as glomalin-related soil protein (GRSP) (Rillig, 2004). GRSP is released to cover the surface of soil organic matter and aggregates, and can store C in protein and carbohydrate subunits, forming a protective layer that avoids the loss of nutrients (e.g., C) in soil aggregates (Schindler et al., 2007; Wang et al., 2023).
AMF manage plant-soil interactions, suppling mineral nutrients to host plants while providing a conduit of C to soil microbial community. Field studies applying 13CO2 pulse labeling have demonstrated that AMF ERM provides a rapid and important pathway of C flux from plants to soil and the atmosphere (Johnson et al., 2002). By tracing in situ flows of photo-assimilated C of 13CO2-exposed wheat (Triticum aestivum) through AMF into root- and hyphae-associated soil microbial communities over an eight-hour period, Kaiser et al. (2015) found that intraradical AMF hyphae were significantly 13C-enriched compared to the root-cortex area, suggesting an efflux of photosynthate C from the plant to the mycorrhizosphere over time. In addition, they showed that 13C photosynthate was delivered to general bacteria and Gram-negative bacteria primarily through the AM pathway rather than directly through roots. These results suggest that AMF play a vital role in the translocation of new fixed plant C to soil microbes (Kaiser et al., 2015).
2.2. Interplant carbon transfer through common arbuscular mycorrhizal networks
In mixed-species communities connected through CAMNs, plant species benefit differently depending on the AMF species involved and plant coexistence can be significantly affected by these differences (Wagg et al., 2011; van der Heijden et al., 2015). Differences in the natural abundance of 13C between plants of the C3 and C4 photosynthetic pathways were used in several studies of AMF-linked plants to quantify C transfer. In one study, C transfer of AMF-linked Plantago lanceolata (C3) and Cynodon dactylon (C4) was quantified. This varied from 0 to 41% with median at approximately 5%for individual C. dactylon plants but was not determined for P. lanceolata individuals (Watkins et al., 1996). Walder et al. (2012) found that C3 flax (Linum usitatissimum) contributed 30% of the CAMN carbon but gained up to 90% of N from the CAMNs formed by R. intraradices, which highly facilitated its growth, while the CAMN-interconnected neighboring C4 sorghum (Sorgum bicolor) contributed 70% of CAMN carbon with little N but was barely affected growth. One possible mechanism affecting these results could be the exchange of “luxury goods” between plants and AMF symbionts (Kiers and van der Heijden, 2006). Consequently, resource trading through networks of plant-AMF assemblages could be weakly reciprocal, depending on the sink strength and exchange efficiency at the symbiotic interfaces, which should differ with different plant-fungus combinations (Helgason et al., 2007). Walder et al. (2012) demonstrated that plants transferred underground resources to each other through such a mycelial network, so that nutrients could be quickly transported between different plants.
Recent observations show that mycorrhizal fungi are important regulators of C dynamics because of slow decomposition of fungal residues (van der Heijden et al., 2015) and that C storage is increased in AM-dominated ecosystems (Averill et al., 2014; van der Heijden et al., 2015; Wurzburger et al., 2017). To quantify the involvement of AMF in the intraspecific transport of C between plants, Graves et al. (1997) fumigated a mycorrhizal Festuca ovina turf with 13C-depleted CO2 for one week and found that 41% of the newly fixed C that was exported belowground was subsequently transported to neighbouring F. ovina ( Table 1 ). Although Francis and Read (1984) showed that transfer of C between plants connected by AM mycelia occurred primarily by the direct hyphal pathway, levels of C in whole receiver plants (F. ovina) only reached to 0.058 ± 0.023% of that in donors (Plantago lanceolata). In contrast, by labeling plants with 14CO2 in the field, Lerat et al. (2002) reported that a direct 0.064 ± 0.049% transfer of 14C in the sugar maple (Acer saccharum) were from Erythronium americanum, while 14C was detected in 7 of 22 E. americanum roots from the sugar maple, with labeling in those 7 only 0.018 ± 0.021% that of sugar maple. Both the enrichment and natural abundance of 13C methods show one-way transfer of C between mycorrhizal plants to be 0 to 41%, in controlled or field conditions (He et al., 2003; He et al., 2009; Table 1 ). For instance, such C transfers have been detected between Allium cepa plants (Hirrel and Gerdemann, 1979), Festuca idahoensis and Centaurea maculosa (Carey et al., 2004), F. ovina and F. ovina (Graves et al., 1997), Lolium perenne and Plantago lanceolata (Martins, 1992), Oryza sativa and Citrullus lanatus (Carey et al., 2004), and Trifolium subterraneum and P. lanceolata (Nakano-Hylander and Olsson, 2007) ( Table 1 ). Most recently, by labelling 13CO2 to one of the four tree species growing in “community boxes” using natural forest soil as fungal inoculums, 6.4 to 29.0% C transfers were facilitated by shared AMF of R. fasciculatus and R. irregularis, with oak (Quercus calliprinos) being a better donor, while pistacia (Pistacia lentiscus) and cypress (Cupressus sempervirens) better recipients (Avital et al., 2022). They concluded that an asymmetric C exchange between co-existing plant species could contribute to forest resilience. However, the mechanism of C transfer and role of mycorrhizal hyphae in the direct transfer of C are not well established (Robinson and Fitter, 1999; Smith and Read, 2008). Therefore, more needs to be done to lay out the arguments for why and how CAMN transfer of C could contribute to the accumulation of C in ecosystems.
Table 1.
Transfer of C from one plant to another via CAMNs (see Section 1.4 for transfer calculations).
| Donor Species A |
Recipient Species B |
Linkage direction* |
Inoculum involved | Substance transferred |
Ctransfer % | Reference |
|---|---|---|---|---|---|---|
| Acer saccharum | Erythronium americanum | A → B | Field soil | 14C | 0.06 | Lerat et al., 2002 |
| Achillea millefolium | Centaurea maculosa | A ↔ B | Field soil | — | — | Zabinski et al., 2002 |
| Allium cepa | A. cepa | A → B | Claroideoglomus etunicatum | — | — | Hirrel and Gerdemann, 1979 |
| Calamagrostis epigejos | C. epigejos | A ↔ B |
Funneliformis mosseae
C. claroideum |
— | — | Malcova et al., 2001 |
| Ceratonia siliqua | Ce. siliqua | A → B |
R. fasciculatus, R. irregularis |
13CO2 | 6.35 | Avital et al., 2022 |
| Ce. siliqua | Cupressus sempervirens | A → B |
R. fasciculatus, R. irregularis |
13CO2 | 4.41 | Avital et al., 2022 |
| Ce. siliqua | Pistacia lentiscus | A → B |
R. fasciculatus, R. irregularis |
13CO2 | 29.01 | Avital et al., 2022 |
| Daucus carota | D. carota | A → B | Rhizophagus intraradices | 13C / 14C -glucose, | — | Pfeffer et al., 2004 |
| D. carota | D. carota | A → B |
R. intraradices, R. irregularis |
13CO2 | — | Lekberg et al., 2010 |
| Digitalis purpurea | Dactylis glomerata | A ↔ B | Field soil | — | — | Newman et al., 1994 |
| E. americanum | A. saccharum | A → B | Field soil | 14C | — | Lerat et al., 2002 |
| Festuca idahoensis | Centaurea maculosa | A → B | Field soil | — | — | Zabinski et al., 2002 |
| Festuca idahoensis | Centaurea maculosa | A → B | Field soil | 12C | 15.00 | Carey et al., 2004 |
| Festuca ovina | Briza media | A → B | Septoglomus constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Centaurea nigra | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Centaurium erythraea | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | F. ovina | A → B | AM root segments | 13C | 41.00 | Graves et al., 1997 |
| F. ovina | F. ovina | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Hieracium pilosella | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Leontodon hispidus | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Plantago lanceolata | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Poa pratensis | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| F. ovina | Scabiosa columbaria | A → B | S. constrictum | 14C | — | Grime et al., 1987 |
| Flaveria bidentis | Setaria viridis | A Δ B | R. intraradices | 13C | 2.54‒2.67 | Chen et al., 2021 |
| Flaveria bidentis | Eclipta prostrata | A Δ B | R. intraradices | 13C | 3.26‒3.37 | Chen et al., 2021 |
| Juglans nigra | Zea mays | A → B | Fileld soil and root | 13C | — | van Tuinen et al., 2020 |
|
Lolium perenne
(Full light) |
Pl. lanceolata
(Full light) |
A → B | AM root segments | 14C | 0.09 | Martins, 1992 |
|
L. perenne
(Dark) |
Pl. lanceolata
(Dark) |
A → B | AM root segments | 14C | 0.27 | Martins, 1992 |
| Lolium perenne | L. perenne | A → B | AM root segments | 14C | — | Martins, 1993 |
| Lotus corniculatus | Lotus corniculatus | A ↔ B | Fileld soil and roots | 14C | 1.20 | Waters and Borowicz, 1994 |
| Medicago truncatula | M. truncatula | A → B | R. intraradices | 13C | — | Voets et al., 2008 |
| Oryza sativa | O. sativa | A → B | F. mosseae | 14C | — | Ren et al., 2013 |
| O. sativa | Citrullus lanatus | A → B | F. mosseae | 14C | — | Ren et al., 2013 |
| Plantago lanceolata | Cynodon dactylon | A → B | AM root segments | δ13C | 10.00 | Watkins et al., 1996 |
| Pl. lanceolata | C. dactylon | A → B | F. mosseae | δ13C | — | Fitter et al., 1998 |
| Pl. lanceolata |
F. ovina
(Full light) |
A → B |
C. caledonium or Field root pieces |
14C | 0.02 | Francis and Read, 1984 |
| Pl. lanceolata |
F. ovina
(Half light) |
A → B |
C. caledonium or Field root pieces |
14C | 0.05 | Francis and Read, 1984 |
| Pl. lanceolata |
F. ovina
(Dark) |
A → B |
C. caledonium or Field root pieces |
14C | 0.11 | Francis and Read, 1984 |
| Pl. sativum cv. Frisson and P2 | Triticum × Secale | A → B | AMF inoculum | 13C-glucose | 1.08 | Hupe et al., 2021 |
| Quercus calliprinos | Cu. sempervirens | A → B |
R. fasciculatus, R. irregularis |
13CO2 | 15.09 | Avital et al., 2022 |
| Q. calliprinos | Pi. lentiscus | A → B |
R. fasciculatus, R. irregularis |
13CO2 | 27.12 | Avital et al., 2022 |
| Trifolium subterraneum | Pl. lanceolata | A → B | R. intraradices | Organic C | — | Nakano-Hylander and Olsson, 2007 |
*Symbols indicate the direction of nutrients transferred: → unidirectionally, Δ bi-directionally, ↔ either direction. Updated from He et al. (2003; He et al., 2009).
3. Nitrogen transfer between plants through common arbuscular mycorrhizal networks
3.1. Arbuscular mycorrhizal fungi and nitrogen
In contrast to C, AMF were previously thought to play no roles in organic N acquisition for their host plant (Read, 1991). In ecosystems where decomposition and nitrification processes are favored, although both poorly mobile ammonium (NH4 +) and highly mobile nitrate (NO3 −, most available in non-waterlogging habitats, compared to NH4 +) are the principal plant-available N forms, the enhancement of plant N acquisition by AMF may be small (Read, 1991). However, studies have shown that AMF can acquire N from both inorganic and organic N sources and transfer some of this N to their host plant (Johnson et al., 2002; Leigh et al., 2008; Hodge and Fitter, 2010; Hodge and Storer, 2015; Jansa et al., 2019). Since N is a key limiting nutrient in terrestrial ecosystems, if AMF enhance inorganic, organic or unspecified N uptake, this could improve host plant fitness (Hodge and Storer, 2015). In 15N labelling studies, inorganic (Johansen et al., 1993; Tobar et al., 1994a; Tobar et al., 1994b; Hawkins et al., 2000) and organic (Hawkins et al., 2000) N uptake by host plants is positively correlated to AMF colonization rates. In a recent 15N natural abundance study, 30% of total N in maize was AMF-mediated when maize grew within 5 m of N2-fixing Faidherbia albida (Dierks et al., 2021). Generally, inorganic N absorbed by the fungal ERM could be incorporated into amino acids and then transported to the fungal IRM (Johansen et al., 1996).
In most natural and productivity ecosystems most nutrient inputs to soils are as organic materials. These materials vary widely in their physical and chemical complexity, nutrient quality and quantity, and source (Read and Perez-Moreno, 2003). Single 15N-labelled organic materials have been used to trace the flow of N in the soil-plant system, showing that plants, or their mycorrhizal symbionts, acquired N in intact glycine (Näsholm et al., 1998). Subsequently, Leigh et al. (2008) demonstrated that R. intraradices could increase the N concentration of its host plant, apparently by taking up N from a decomposing patch of organic matter. AMF can obtain substantial amounts of N from decomposing organic materials, thereby enhancing plant fitness (Hodge and Fitter, 2010). However, in the absence of other microbes, there is so far no experimental evidence for any quantitative acquisition of N by AMF hyphae from organic sources (Jansa et al., 2019). On the other hand, there is partly equivocal evidence from experiments using quantum dot technology indicating that organic N could be taken up via AMF hyphae and that uptake of N in the form of certain simple amino acids was enhanced in mycorrhizal compared with non-mycorrhizal plant roots (Whiteside et al., 2012). A surprising finding, revealed through feeding 13C-acetate or 15N-Arginine to the ERM, showed that N is transported from fungus to plant as NH4 +, not amino acid (Fellbaum et al., 2012). A possible mechanism to explain these observations is that arginine delivered to the fungal IRM was broken down and the NH4 + was then released to the symbiotic interface and transferred from fungus to plant ((Parniske, 2008). The external hyphae of AM fungi can directly take up 15NH4 + or 15NO3 –, reduce nitrate to NH4 +, and then assimilate NH4 + into the pool of free amino acids. Understanding the link between CAMN transfer of nutrient and accumulation of C and N in ecosystems is crucial to clarify potential C-for-N trades between symbionts (Hodge and Storer, 2015; Thirkell et al., 2016). Indeed, interplant nutrient exchanges could play a vital ecological role in promoting plant coexistence in ecosystems under future climatic and anthropogenic pressures with profound relevance to restoration of plant communities ( Figure 2 ).
3.2. Interplant nitrogen transfer through common arbuscular mycorrhizal networks
Changes in plant δ15N values may reflect N inputs, N outputs and N isotope fractionation processes in ecosystems (Dawson et al., 2002). Use of natural 15N variability to investigate the role of mycorrhizae in N transfer is increasing (Dawson et al., 2002; He et al., 2003; Moyer-Henry et al., 2006; He et al., 2009). Earlier studies showed one-way AM-mediated N transfer from N2-fixing soybean (Glycine max) to non-N2-fixing maize (Zea mays) (van Kessel et al., 1985) and from N2-fixing Trifolium pratense to non-N2-fixing Lolium perenne (Haystead et al., 1988), indicating that such N transfers could be important for non-N2-fixing plants under N-limited conditions. Transfers of both NH4 + and NO3 – between N2-fixing and non-N2-fixing plants were mediated by CMNs (Frey and Schüepp, 1993; Johansen et al., 1996; Moyer-Henry et al., 2006). Likewise, He et al. (2006) found that N moved quickly between AM and EM mycorrhizal plant individuals in a California oak woodland, indicating a CMN-mediated nutrient distribution mechanism between plants, including the release and recapture of N from the rhizosphere soil. By foliar 15NO3 labeling to quantify N transfer between non-N2-fixation plants (Eucalyptus marginata, ectomycorrhizal (EM) species; Melaleuca preissiana, AM/EM species; Verticordia nitens AM species), Teste et al. (2015) demonstrate that plants with cluster-roots (Banksia menziesii, non-mycorrhizal species) or ectomycorrhizal plants were more 15N enriched than with AM-only plants. Nitrogen transfer was relatively high (4% of the donor plant N) among these non-N2-fixation plants with contrasting N-acquisition strategies. Montesinos-Navarro et al. (2016) found that CAMNs mediated N transfer between facilitated plants, suggesting that nutrient transfer through CAMNs might be a potential mechanism allowing persistent benefits for their adult facilitated plants. The potential pathways for CAMNs-mediated N transfer could be (1) direct transfer of N via connecting hyphae across the symbiotic interface, (2) increased root surface area and a reduced distance for nutrient diffusion, and (3) increased assimilation or exudation of N in the AM-colonized plants (Haystead et al., 1988; Trannin et al., 2000; He et al., 2003; He et al., 2009).
Both 15N enrichment studies and natural abundance measurements showed a one-way movement of 0 to 80% of receiver N from N2-fixing mycorrhizal to non-N2-fixing mycorrhizal plants, under controlled or field conditions (He et al., 2003; He et al., 2009). For instance, one-way transfer through CAMNs of ~0.09‒80% of plant N was reported in a white clover (Trifolium repens) ‒ ryegrass (Lolium perenne) system with F. mosseae (Haystead et al., 1988), a berseem (Trifolium alexandrinum) ‒ maize (Zea mays) or pea (Pisum sativum) ‒ barley (Hordeum vulgare) system with R. intraradices (Frey and Schüepp, 1993; Johansen and Jensen, 1996), a soybean (Glycine max) ‒ semen cassiae (Senna obtusifolia) and a peanut (Arachis hypogaea) ‒ prickly sida (Sida spinosa) or sicklepod (Senna obtusifolia) (Moyer-Henry et al., 2006), a Cinnamomum camphora ‒ C. camphora system with Claroideoglomus etunicatum (He et al., 2019), a Vachellia seyal ‒ Sporobolus robustus system with R. irregularis ( Table 2 ). In addition, N can also be transferred from non-N2-fixing mycorrhizal plants to N2-fixing mycorrhizal plants via a CAMN, although N transfer is generally less than 10% of plant N budgets (Johansen and Jensen, 1996; Li et al., 2009). For instance, the respective N transfer was 4.2% ‒ 9.9% of plant N budgets from the donor mung bean (Vigna radiata) to its associated receiver rice (Oryza sativa) or from the rice (O. sativa) to mung bean (Li et al., 2009). AM-mediated N transfer can be from N2-fixing to non-N2-fixing plants or from non-N2-fixing to N2-fixing plants, indicating bi-directional transfer. Recently, 15N labeling demonstrated that CMNs increased 15N enrichment of Trifolium pratense, but did not affect its biomass production, when the holoparasite Cuscuta australis was absent (Yuan YG. et al., 2021). In contrast, both 15N enrichment and biomass production in T. pratense plants were increased by CAMNs when the holoparasite was present. These results indicated that CAMNs could preferentially distribute more N to a non-parasitized neighboring T. pratense, while resulting in negative feedback on the growth of the parasite C. australis (Yuan YG. et al., 2021).
Table 2.
Transfer of N from one plant to another via CAMNs (see Section 1.4 for transfer calculations).
| Donor Species A |
Recipient Species B |
Linkage direction* |
Inoculum involved | Substance transferred |
Ntransfer % | Reference |
|---|---|---|---|---|---|---|
|
Andropogon gerardii
Arachis hypogaea |
An. gerardii
Sida spinosa |
A → B A → B |
Seven AM fungi Field soil with roots |
15NH4+15NO3
−
14NH4 + |
27.00 30.00 |
Weremijewicz et al., 2016
Moyer-Henry et al., 2006 |
| Ar. hypogaea | Senna obtusifolia | A → B | Field soil with roots | 14NH4 + | 80.00 | Moyer-Henry et al., 2006 |
| Bromus hordeaceus | Vitis vinifera | A → B | Field soil with roots | 15NH4 + | 24.80 | Cheng and Baumgartner, 2004 |
|
Cleistogene squarrosa
Cl. squarrosa |
Cl. squarrosa
Leymus chinensis |
A → B A → B |
Field soil with roots Root zone soil from A |
15NH4
+
15NH4 + |
45.70‒55.30 16.00‒61.00 |
Muneer et al., 2020a; Muneer et al., 2020b
Muneer et al., 2022 |
|
Cinnamomum
camphora |
Ci. camphora | A → B | Claroideoglomus etunicatum | 15NH4 + | 0.09 | He et al., 2019 |
| Ci. camphora | Bidens pilosa | A → B | C.etunicatum | 15NH4 + | 0.22 | He et al., 2019 |
| Ci. camphora | Broussonetia papyrifera | A → B | C. etunicatum | 15NH4 + | 0.19 | He et al., 2019 |
| Cupressus goveniana | Cu. goveniana | A → B | Pygmy forest soil | 15NH4 + | 0.99 | Rains and Bledsoe, 2007 |
|
Eucalyptus marginata
Flaveria bidentis |
Verticordia nitens
Setaria viridis |
A ↔ B A Δ B |
Nursery soil and potting media Rhizophagus intraradices |
15NO3
−
15NH4 + |
2.90‒4.40 0.98‒2.14 |
Teste et al., 2015
Chen et al., 2021 |
| Flaveria bidentis | Eclipta prostrata | A Δ B | R. intraradices | 15NH4 + | 2.99‒4.29 | Chen et al., 2021 |
|
Leymus chinensis
L. chinensis |
L. chinensis
Cl. squarrosa |
A → B A → B |
Field soil with roots Root zone soil from A |
15NH4
+
15NH4 |
21.50‒64.90 3.98‒5.98 |
Muneer et al., 2020a; Muneer et al.2020b
Muneer et al., 2022 |
| Gliricidia sepium | Dichantium aristatum | A → B | Field soil with roots | 15NO3 − | 0.70‒2.50 | Jalonen et al., 2009 |
| Glycine max | G. max (non-nodulated) | A → B | Field soil with roots | 14NH4 + | 48.00 | Moyer-Henry et al., 2006 |
| G. max | Senna. obtusifolia | A → B | Field soil with roots | 14NH4 + | 80.00 | Moyer-Henry et al., 2006 |
| G. max | Sorghum bicolor | A → B | F. mosseae | 14NH4 + | 22.50 | He, 2002 |
| G. max | Zea mays | A → B | 3 Glomus species | 15NH4 + | ~5.00 | Hamel et al., 1991a; Hamel et al., 1991b |
| G. max | Z. mays | A → B | Field soil with roots | 15NH4 + | 3.00 | Eissenstat, 1990 |
| G. max | Z. mays | A → B | R. irregularis | 15NH4 + | 11.40 | Wang et al., 2016 |
| G. max | Z. mays | A → B | R. fascculatus | 15NH4 + | — | van Kessel et al., 1985 |
| G. max | Z. mays | A → B | Funneliformis mosseae | 15NH4 + | 6.08 | Meng et al., 2015 |
| Gliricidia sepium | Theobroma cacao | A → B | Field soil with roots | 15NH4 + | 0.40–0.85 | Kurppa et al., 2010 |
| Hordeum vulgare (barley) | Pisum sativum | A → B | R. intraradices | 15NH4 + | 4.00 | Eissenstat, 1990 |
| Inga edulis | T. cacao | A → B | Field soil with roots | 15NH4 + | 0.55–0.88 | Kurppa et al., 2010 |
| Kummerowa striata | Solidago canadensis | A → B |
Acaulospora scrobiculata
Gigaspora margarita F. geosporum |
15NO3 − | — | Awaydul et al., 2019 |
|
Medicago polymorpha
Melaleuca preissiana |
Vitis vinifera
V. nitens |
A → B A ↔ B |
Field soil with roots Nursery soil and potting media |
15NH4
+
15NO3 − |
5.50 2.90‒4.40 |
Cheng and Baumgartner, 2004
Teste et al., 2015 |
|
Oryza sativa
(Rice) |
Vigna radiate
(Peanut) |
A → B | Claroideoglomus caledonium | 15NH4 + | 1.40‒4.40 | Li et al., 2009 |
| Phaseolus vulgaris | Zea mays | A → B | F. mosseae | 15NH4 + | 0.32 | Giller et al., 1991 |
|
Pisum sativum
(pea) |
Cichorium intybus | A → B | R. irregularis + F. mosseae | 15NH4 15NO3 − | 52.50 | Ingraffia et al., 2021 |
| Linum usitatissimum | A → B | 13.40 | ||||
| Triticum durum | A → B | 34.00 | ||||
| Pi. sativum | Hordeum vulgare (barley) | A Δ B | R. intraradices | 15NH4+15NO3 − | 15.00 | Johansen and Jensen, 1996 |
| Pi. sativum cv. Frisson and P2 | Triticum × Secale | A → B | AMF inoculum | 15N-urea | 0.67 | Hupe et al., 2021 |
| Plantago lanceolata | Pl. lanceolata | A ↔ B | Filed soil | 15NH4 + | 0.70 | Eissenstat, 1990 |
| Pl. lanceolata | Pl. lanceolata | A ↔ B | Glomus hoi | 15N organic patch | — | Hodge and Fitter, 2010 |
| Pl. lanceolata | Pl. lanceolata | A ↔ B | F. mosseae | 15N organic patch | — | Hodge and Fitter, 2010 |
| Pueraria phaseoloides | Hevea brasiliensis | A → B | C. clarum | 15NO3 − | 0.04‒0.20 | Ikram et al., 1994 |
| Sorghum bicolor | Glycine max | A → B | F. mosseae | 15NH4 + | 28.50 | He, 2002 |
| Sesbania virgata | Eucalyptus grandis | A → B |
G. macrocarpum, G. etunicatum Entrophospora colombiana |
15NH4 + | 0.06‒0.08 | Rodrigues et al., 2003 |
| Trifolium alexandrinum | Malus domestica | A → B | R. intraradices | 15NH4 + | 4.70 | Frey and Schüepp, 1993 |
| Trifolium repens | Citrus sinensis Osbeck | A → B | R. intraradices | 15NH4 + | 1.40‒1.70 | Fang et al., 2021 |
|
T. repens
T. repens |
Lolium perenne
l. perenne |
A ↔ B |
F. mosseae
R. irregularis) |
15NH4
+
15-urea |
4.20–5.00 2.00–3.00 |
Haystead et al., 1988
Reay et al., 2022 |
| Vachellia seyal | Sporobolus robustus | A → B | R. irregularis | 15NH4 + | 13.90 | Fall et al., 2022 |
| Verticordia nitens | Melaleuca preissiana | A ↔ B | Nursery soil and potting media | 15NO3 − | 2.90–4.40 | Teste et al., 2015 |
| Vicia faba | Triticum durum | A → B | 8 AMF species | 15NH4 15NO3 | 2.00‒2.70 | Ingraffia et al., 2019 |
| V. faba | Triticum turgidum | A → B | R. irregularis | 15NH4 + | 32 | Wahbi et al., 2016 |
| V. faba | T. turgidum | A → B | R. irregularis | 15NH4 + | 50 | Wahbi et al., 2016 |
|
Vigna radiate
(Peanut) |
Oryza sativa
(Rice) |
A → B | C. caledonium | 15NH4 + | 4.20‒9.90 | Li et al., 2009 |
| Vigna unguiculata | Z. mays | A → B | C. etunicatum | 15NH4 + | 21.20 | Martins and Cruz, 1998 |
|
Z. mays
Z. mays |
M. sativa
T. alexandrinum |
A → B A → B |
Field study R. intraradices |
15N-urea 15NH4 + |
7-10 0.10 |
Zhang et al., 2019
Frey and Schüepp, 1993 |
*Symbols indicate the direction of nutrients transferred: → unidirectionally, Δ bi-directionally, ↔ either direction. Updated from He et al. (2003; He et al., 2009).
4. Conclusions and future perspectives
A range of 0.02 to 41% (C) and 0.04 to 80% (N) of one-way transfer have been observed from donor to recipient plants through the determination of 13C and 15N signatures ( Tables 1 , 2 ). Interplant C and N transfers can affect not only the growth and competition between donor and recipient plants but also ecosystem stability. For example, Weremijewicz et al. (2016) observed that Andropogon gerardii plants in intact CMNs under sunlight acquired 9% of their N, but shaded plants (~35% photosynthetically active radiation) acquired only 1% N, from their conspecific neighbors. They suggested that AM fungi in CAMNs preferentially provide N to conspecific hosts of with fixed C or presenting the strongest sinks, thus potentially expanding asymmetric underground competition. Castro-Delgado et al. (2020) showed that the mycelium could transfer diverse compounds and signals among plants that would modify plant behavior in favor of protection of the whole network. In general, stable isotope tracing has provided an effective way to study the exchange of mineral nutrients between plants through CAMNs. Although 13C and 15N labeling techniques have demonstrated that CAMNs are an important pathway for the translocation of both C and N, the functioning of CAMNs in ecosystem C and N dynamics remains equivocal. To make an explicit link between nutrient transfer in CAMNs and nutrient cycling in ecosystems new approaches are needed. For example, a combination of high-throughput genome sequence techniques with model-based assessments could further identify the extent of CAMNs in interplant C and N translocation in natural and managed ecosystems (Orwin et al., 2011; Zhou et al., 2021). The following issues about the physiological and ecological functions of AMF or CAMNs should be addressed.
1. Can 13C and 15N natural abundance, like 13C and 15N external labeling, be employed to detect C and N transfer? Study have shown that plant δ13C signatures could reflect the δ13C of the C sources of associated fungi and δ15N signatures could reflect the δ15N of N sources to plants (Querejeta et al., 2003). However, the reliability of using 15N natural abundance to estimate AMF-mediated N transfer has been recently questioned (Choi et al., 2020; Jach-Smith and Jackson, 2020).
2. In what form are C and N transferred through CAMNs? Amino acids, lipids, or carbohydrates for C, amino acids or ammonium for N? Does a pollen development encompass a mechanism that is shared with CAMNs symbiosis? What may the two phenomena have in common (Nouri and Reinhardt, 2015)? Can fluorescent nanoscale semiconductors or quantum dots (Whiteside et al., 2012) be combined with 13C and 15N labelling to trace the transfer of organic nutrients through CAMNs (Govindarajulu et al., 2005; Parniske, 2008)?
3. Can network theory and computer modeling (Southworth et al., 2005; Wipf et al., 2019) simulate the direction and distribution of interplant C and N transfer facilitated by CMNs and thus predict both positive and negative effects of CMNs in natural and managed systems (Alaux et al., 2021)?
4. 15N labeling showed that AMF could not directly decompose organic matter, but the interaction between AMF and other decomposers enhanced organic matter decomposition and hence the absorption of N by AMF (Hodge and Fitter, 2010). However, how can CAMNs regulate the process of C and N translocation and absorption between AMF mycelia and host plants? In addition, a coupled concurrent C and N movement through CAMNs has not been reported.
5. What determines the net effect of CAMN-mediated interplant nutrient transfer on plant C assimilation and N metabolism? Does the transferred C and N affect the performance or fitness of the donor, receiver, or both? What is the ecological significance of CAMN mediated nutrient transfers in natural and managed ecosystems? Whether AMF-mediated interplant C and N transferred is agronomically important to managed ecosystems, including agroforestry, forestry, croplands, and grasslands, is debated (Rillig et al., 2019; Ryan et al., 2019). How do modern agricultural practices, such as long-term organic farming, no-till, or fertigation affect the establishment and performance of CMNs and subsequent effects on fertilizer use efficiency, crop agronomic characters and productivity?
6. How the abundance and function of soil bacterial and other fungal communities could be manipulated and promoted through a CAMN-mediated interplant C and N transfers (Bonfante and Anca, 2009; Yuan MTM. et al., 2021)? Is plant C investment in AM fungal growth related to soil N acquisition within a CAMN? How is the N for C trade between mycorrhizal symbionts regulated if plants are linked through a CAMN? What determines the magnitude and direction of such C and N transfer within the same or different plant species in mono-species or mixed-species systems, particularly along their complete plant growth and development cycle? How exogenous and endogenous factors can interplay with CAMNs, and how a nutrient can impinge on AM symbiotic signaling and also on a later cellular program in host plants (Nouri et al., 2014).
7. Irrespective of photosynthetic capabilities or N2-fixation characteristics of plant species, what the phylogenetic and functional diversity of plant species can benefit from nutrient transfer through CAMNs? These species would be in a diverse range as C3, C4, C3-C4, CAM and parasitic plants. Are there interactions between AM and EM networks on C and N transfers since some plants do have dual AM/EM associations (Wang and Qiu, 2006; Teste et al., 2015)? How can technical problems be overcome in demonstrating unequivocally that a C or N transfer directly occurs through CMNs rather than indirectly through root exudates or soils (Zhang et al., 2019; Fall et al., 2022; Reay et al., 2022)?
8. How will drivers of global environment change including elevated CO2 concentration, N deposition, drought and temperature affect interplant C and N transfer through CAMNs? Each can have substantial impacts on the direction and magnitude of such C and N transfers and ultimately on resource sharing or competition (Fellbaum et al., 2014; Řezáčová et al., 2018; Mickan et al., 2021). To answer these issues, it is important to keep in mind that mycorrhizal symbiotic benefits are interactively formed between plants and fungi under specific habitats and soil properties.
Author contributions
Conceptualization, XH. Methodology, XH and SL. Data analysis, XL and YL. Original draft, XL and XH. Review and editing, XH, XL and SL. Funding acquisition, XH and XL. All authors contributed to the article and approved the submitted version.
Acknowledgments
For valuable comments on the manuscript before submission, we thank Dr. Julie Deslippe at Victoria University of Wellington, New Zealand and Dr. Erik Hobbie at University of New Hampshire University, USA.
Funding Statement
This work received financial support from the Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education), Chongqing Key Laboratory of Plant Resource Conservation and Germplasm Innovation, National Base of International S&T Collaboration on Water Environmental Monitoring and Simulation in the Three Gorges Reservoir Region and Centre of Excellence for Soil Biology at Southwest University to XH, and the Scientific Research Foundation of Wuhan Institute of Technology to XL (22QD65). Researches in the authors’ laboratory are jointly supported by projects from the Key Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education), Chongqing Key Laboratory of Plant Resource Conservation and Germplasm Innovation, National Base of International S&T Collaboration on Water Environmental Monitoring and Simulation in the Three Gorges Reservoir Region and Centre of Excellence for Soil Biology at Southwest University to XH, and the Scientific Research Foundation of Wuhan Institute of Technology to XL (22QD65).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Alaux P. L., Zhang Y. Q., Gilbert L., Johnson D. (2021). Can common mycorrhizal fungal networks be managed to enhance ecosystem functionality? Plants People Planet 3, 433‒444. doi: 10.1002/ppp3.10178 [DOI] [Google Scholar]
- Averill C., Turner B. L., Finzi A. C. (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543‒545. doi: 10.1038/nature12901 [DOI] [PubMed] [Google Scholar]
- Avital S., Rog I., Livne-Luzon S., Cahanovitc R., Klein T. (2022). Asymmetric belowground carbon transfer in a diverse tree community. Mol. Ecol. 31, 3481‒95. doi: 10.1111/mec.16477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaydul A., Zhu W. Y., Yuan Y. G., Xiao J., Hu H., Chen X., et al. (2019). Common mycorrhizal networks influence the distribution of mineral nutrients between an invasive plant, Solidago canadensis, and a native plant, Kummerowa striata . Mycorrhiza 29, 29–38. doi: 10.1007/s00572-018-0873-5 [DOI] [PubMed] [Google Scholar]
- Bago B., Pfeffer P. E., Shachar-Hill Y. (2000). Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 124, 949–957. doi: 10.1104/pp.124.3.949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever J. D., Dickie I. A., Facelli E., Facelli J. M., Klironomos J., Moora M., et al. (2010). Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 25, 468–478. doi: 10.1016/j.tree.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P., Anca I. A. (2009). Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu. Rev. Microbiol. 63, 363–383. doi: 10.1146/annurev.micro.091208.073504 [DOI] [PubMed] [Google Scholar]
- Brundrett M. C. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320, 37‒77. doi: 10.1007/s11104-008-9877-9 [DOI] [Google Scholar]
- Brundrett M. C. (2017). “Global diversity and importance of mycorrhizal and nonmycorrhizal plants,” in Biogeography of mycorrhizal symbiosis. Ed. Tedersoo L. (Switzerland: Springer, Cham; ), 533‒556. doi: 10.1007/978-3-319-56363-3_21 [DOI] [Google Scholar]
- Brundrett M. C., Tedersoo L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. doi: 10.1111/nph.14976 [DOI] [PubMed] [Google Scholar]
- Cardini A., Pellegrino E., Declerck S., Calonne-Salmon M., Mazzolai B., Ercoli L. (2021). Direct transfer of zinc between plants is channelled by common mycorrhizal network of arbuscular mycorrhizal fungi and evidenced by changes in expression of zinc transporter genes in fungus and plant. Environ. Microbiol. 23, 5883–5900. doi: 10.1111/1462-2920.15542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey E. V., Marler M. J., Callaway R. M. (2004). Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecol. 172, 133‒141. doi: 10.1023/B:VEGE.0000026031.14086.f1 [DOI] [Google Scholar]
- Castro-Delgado A. L., Elizondo-Mesen S., Valladares-Cruz Y., Rivera-Mendez W. (2020). Wood wide web: communication through the mycorrhizal network. Tecnol Marcha 33, 114‒125. doi: 10.18845/tm.v33i4.4601 [DOI] [Google Scholar]
- Chen X., Li Q., Wang L. T., Meng Y. L., Jiao S. N., Yin J. L., et al. (2021). Nitrogen uptake, not transfer of carbon and nitrogen by CMN, explains the effect of AMF on the competitive interactions between Flaveria bidentis and native species. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.625519 [DOI] [Google Scholar]
- Cheng X. M., Baumgartner K. (2004). Arbuscular mycorrhizal fungi-mediated nitrogen transfer from vineyard cover crops to grapevines. Biol. Fertil Soils 40, 406–412. doi: 10.1007/s00374-004-0797-4 [DOI] [Google Scholar]
- Choi W. J., Chang S. X., Jin-Hyeob Kwak J. H. (2020). Comment on inorganic n addition replaces n supplied to switchgrass (Panicum virgatum) by arbuscular mycorrhizal fungi. Ecol. Appl. 31, e2270. doi: 10.1002/eap.2270 [DOI] [PubMed] [Google Scholar]
- Couradeau E., Sasse J., Goudeau D., Nath N., Hazen T. C., Bowen B. P., et al. (2019). Probing the active fraction of soil microbiomes using BONCAT-FACS. Nat. Commun. 10, 2770. doi: 10.1038/s41467-019-10542-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. E., Mambelli S., Plamboeck A. H., Templer P. H., Tu K. P. (2002). Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 33, 507–559. doi: 10.1146/annurev.ecolsys.33.020602.095451 [DOI] [Google Scholar]
- Dierks J., Blaser-Hart W. J., Gamper H. A., Six J. (2021). Mycorrhizal fungi-mediated uptake of tree-derived nitrogen by maize in smallholder farms. Nat. Sustain 5, 64–70. doi: 10.1038/s41893-021-00791-7 [DOI] [Google Scholar]
- Ding C. H., Zhao Y., Zhang Q. R., Lin Y. B., Xue R. R., Chen C. Y., et al. (2022). Cadmium transfer between maize and soybean plants via common mycorrhizal networks. Ecotox Environ. Safe 232, 113273. doi: 10.1016/j.ecoenv.2022.113273 [DOI] [PubMed] [Google Scholar]
- Egerton-Warburton L. M., Querejeta J. I., Allen M. F. (2007). Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J. Exp. Bot. 58, 3484–3484. doi: 10.1093/jxb/erm009 [DOI] [PubMed] [Google Scholar]
- Eissenstat D. M. A. (1990). A comparison of phosphorus and nitrogen transfer between plants of different phosphorus status. Oecologia 82, 342–347. doi: 10.1007/BF00317481 [DOI] [PubMed] [Google Scholar]
- Fall F., Ndoye D., Galiana A., Diouf D., Ba A. M. (2022). Arbuscular mycorrhizal fungi-mediated biologically fixed n transfer from Vachellia seyal to Sporobolus robustus . Symbiosis 86, 205–214. doi: 10.1007/s13199-022-00833-4 [DOI] [Google Scholar]
- Fang L. F., He X. H., Zhang X. L., Yang Y. H., Liu R., Shi S. M., et al. (2021). A small amount of nitrogen transfer from legume cover crop to citrus seedling via common arbuscular mycorrhizal networks. Agronomy 11, 32. doi: 10.3390/agronomy11010032 [DOI] [Google Scholar]
- Fellbaum C. R., Gachomo E. W., Beesetty Y., Choudhari S., Strahan G. D., Pfeffer P. E., et al. (2012). Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. P Natl. Acad. Sci. U.S.A. 109, 2666–2671. doi: 10.1073/pnas.1118650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellbaum C. R., Mensah J. A., Cloos A. J., Strahan G. D., Pfeffer P. E., Kiers E. T., et al. (2014). Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol. 203, 646–656. doi: 10.1111/nph.12827 [DOI] [PubMed] [Google Scholar]
- Fitter A. H., Graves J. D., Watkins N. K., Robinson D., Scrimgeour C. (1998). Carbon transfer between plants and its control in networks of arbuscular mycorrhizas. Funct. Ecol. 12, 406–412. doi: 10.1046/j.1365-2435.1998.00206.x [DOI] [Google Scholar]
- Francis R., Read D. J. (1984). Direct transfer of carbon between plants connected by vesicular arbuscular mycorrhizal mycelium. Nature 307, 53–56. doi: 10.1038/307053a0 [DOI] [Google Scholar]
- Frey B., Schüepp H. (1993). A role of vesicular-arbuscular (VA) mycorrhizal fungi in facilitating interplant nitrogen transfer. Soil Biol. Biochem. 25, 651–658. doi: 10.1016/0038-0717(93)90104-J [DOI] [Google Scholar]
- Gao D. M., Pan X. J., Rahman M. K. U., Zhou X. G., Wu F. Z. (2021). Common mycorrhizal networks benefit to the asymmetric interspecific facilitation via K exchange in an agricultural intercropping system. Biol. Fertil. Soils 57, 959–971. doi: 10.1007/s00374-021-01561-5 [DOI] [Google Scholar]
- Giller K. E., Ormesher J., Awah F. M. (1991). Nitrogen transfer from Phaseolus bean to intercropped maize measured using 15N-enrichment and 15N-isotope dilution methods. Soil Biol. Biochem. 23, 339–346. doi: 10.1016/0038-0717(91)90189-Q [DOI] [Google Scholar]
- Giovannetti M., Sbrana C., Avio L., Strani P. (2004). Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytol. 164, 175–181. doi: 10.1111/j.1469-8137.2004.01145.x [DOI] [PubMed] [Google Scholar]
- Govindarajulu M., Pfeffer P., Jin H., Abubaker J., Douds D. D., Allen J. W., et al. (2005). Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435, 819–823. doi: 10.1038/nature03610 [DOI] [PubMed] [Google Scholar]
- Graves J. D., Watkins N. K., Fitter A. H., Robinson D., Scrimgeour C. (1997). Intraspecific transfer of carbon between plants linked by a common mycorrhizal network. Plant Soil 192, 153‒159. doi: 10.1023/A:1004257812555 [DOI] [Google Scholar]
- Grime J. P., Mackey J. M. L., Hillier S. H., Read D. J. (1987). Floristic diversity in a model system using experimental microcosms. Nature 328, 420–422. doi: 10.1038/328420a0 [DOI] [Google Scholar]
- Gyuricza V., Thiry Y., Wannijn J., Declerck S., de Boulois H. D. (2010). Radiocesium transfer between Medicago truncatula plants via a common mycorrhizal network. Environ. Microbiol. 12, 2180–2189. doi: 10.1111/j.1462-2920.2009.02118.x [DOI] [PubMed] [Google Scholar]
- Hamel C., Barrantes-Cartin U., Furlan V., Smith D. L. (1991. a). Endomycorrhizal fungi in nitrogen transfer from soybean to maize. Plant Soil 138, 33–40. doi: 10.1007/BF00011805 [DOI] [Google Scholar]
- Hamel C., Nesser C., Barrantes-Cartin U., Smith D. L. (1991. b). Endomycorrhizal fungal species mediate 15N transfer from soybean to maize in non-fumigated soil. Plant Soil 138, 41–47. doi: 10.1007/BF00011806 [DOI] [Google Scholar]
- Hawkins H. J., Johansen A., George E. (2000). Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226, 275–285. doi: 10.1023/A:1026500810385 [DOI] [Google Scholar]
- Haystead A., Malajczuk N., Grove T. S. (1988). Underground transfer of nitrogen between pasture plants infected with vesicular arbuscular mycorrhizal fungi. New Phytol. 108, 417–423. doi: 10.1111/j.1469-8137.1988.tb04182.x [DOI] [Google Scholar]
- He X. H. (2002). Nitrogen exchange between plants through common mycorrhizal networks (Brisbane, Australia: The University of Queensland; ). [Google Scholar]
- He X. H., Bledsoe C. S., Zasoski R. J., Southworth D., Horwath W. R. (2006). Rapid nitrogen transfer from ectomycorrhizal pines to adjacent ectomycorrhizal and arbuscular mycorrhizal plants in a California oak woodland. New Phytol. 170, 143–151. doi: 10.1111/j.1469-8137.2006.01648.x [DOI] [PubMed] [Google Scholar]
- He Y. J., Cornelissen J. H. C., Wang P. P., Dong M., Ou J. (2019). Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environ. Sci. pollut. R 26, 8828–8837. doi: 10.1007/s11356-019-04385-x [DOI] [PubMed] [Google Scholar]
- He X. H., Critchley C., Bledsoe C. (2003). Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit. Rev. Plant Sci. 22, 531–567. doi: 10.1080/713608315 [DOI] [Google Scholar]
- He X. H., Xu M. G., Qiu G. Y., Zhou J. B. (2009). Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2, 107–118. doi: 10.1093/jpe/rtp015 [DOI] [Google Scholar]
- Helgason T., Merryweather J. W., Young J. P. W., Fitter A. H. (2007). Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community. J. Ecol. 95, 623‒630. doi: 10.1111/j.1365-2745.2007.01239.x [DOI] [Google Scholar]
- Hirrel M. A., Gerdemann J. W. (1979). Enhanced carbon transfer between onions infected with a vesicular-arbuscular mycorrhizal fungus. New Phytol. 83, 731–738. doi: 10.1111/j.1469-8137.1979.tb02303.x [DOI] [Google Scholar]
- Hodge A., Fitter A. H. (2010). Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for n cycling. P Natl. Acad. Sci. U.S.A. 107, 13754–13759. doi: 10.1073/pnas.1005874107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A., Storer K. (2015). Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386, 1–19. doi: 10.1007/s11104-014-2162-1 [DOI] [Google Scholar]
- Hupe A., Naether F., Haase T., Bruns C., Hess J., Dyckmans J., et al. (2021). Evidence of considerable c and n transfer from peas to cereals via direct root contact but not via mycorrhiza. Sci. Rep. 11, 11424. doi: 10.1038/s41598-021-90436-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram A., Jensen E. S., Jakobsen I. (1994). No significant transfer of n and p from Pueraria phaseoloides to Hevea brasiliensis via hyphal links of arbuscular mycorrhiza. Soil Biol. Biochem. 26, 1541–1547. doi: 10.1016/0038-0717(94)90096-5 [DOI] [Google Scholar]
- Ingraffia R., Amato G., Frenda A. S., Giambalvo D. (2019). Impacts of arbuscular mycorrhizal fungi on nutrient uptake, N2 fixation, n transfer, and growth in a wheat/faba bean intercropping system. PloS One 14, e0213672. doi: 10.1371/journal.pone.0213672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraffia R., Giambalvo D., Frenda A. S., Roma E., Ruisi P., Amato G. (2021). Mycorrhizae differentially influence the transfer of nitrogen among associated plants and their competitive relationships. Appl. Soil Ecol. 168, 104127. doi: 10.1016/j.apsoil.2021.104127 [DOI] [Google Scholar]
- Jach-Smith L. C., Jackson R. D. (2020). Inorganic n addition replaces n supplied to switchgrass (Panicum virgatum) by arbuscular mycorrhizal fungi. Ecol. Appl. 30, e2047. doi: 10.1002/eap.2047 [DOI] [PubMed] [Google Scholar]
- Jalonen R., Nyqren P., Sierra J. (2009). Transfer of nitrogen from a tropical legume tree to an associated fodder grass via root exudation and common mycelial networks. Plant Cell Environ. 32, 1366–1376. doi: 10.1111/j.1365-3040.2009.02004.x [DOI] [PubMed] [Google Scholar]
- Jansa J., Forczek S. T., Rozmos M., Puschel D., Bukovska P., Hrselova H. (2019). Arbuscular mycorrhiza and soil organic nitrogen: network of players and interactions. Chem. Biol. Technol. Agric. 6, 10. doi: 10.1186/s40538-019-0147-2 [DOI] [Google Scholar]
- Jiang Y. N., Wang W. X., Xie Q. J., Liu N., Liu L. X., Wang D. P., et al. (2017). Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356, 1172‒1175. doi: 10.1126/science.aam9970 [DOI] [PubMed] [Google Scholar]
- Johansen A., Finlay R. D., Olsson P. A. (1996). Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices . New Phytol. 133, 705–712. doi: 10.1111/j.1469-8137.1996.tb01939.x [DOI] [Google Scholar]
- Johansen A., Jakobsen I., Jensen E. S. (1993). External hyphae of vesicular-arbuscular mycorrhizal fungi associated with trifolium subterraneum l. III. hyphal transport of 32P and 15N. New Phytol. 124, 61–68. doi: 10.1111/j.1469-8137.1993.tb03797.x [DOI] [Google Scholar]
- Johansen A., Jensen E. S. (1996). Transfer of n and p from intact or decomposing roots of pea to barley interconnected by an arbuscular mycorrhizal fungus. Soil Biol. Biochem. 28, 73–81. doi: 10.1016/0038-0717(95)00117-4 [DOI] [Google Scholar]
- Johnson D., Leake J. R., Ostle N., Ineson P., Read D. J. (2002). In situ (CO2)-C-13 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol. 153, 327–334. doi: 10.1046/j.0028-646X.2001.00316.x [DOI] [Google Scholar]
- Kaiser C., Kilburn M. R., Clode P. L., Fuchslueger L., Koranda M., Cliff J. B., et al. (2015). Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol. 205, 1537‒1551. doi: 10.1111/nph.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keymer A., Pimprikar P., Wewer V., Huber C., Brands M., Bucerius S., et al. (2017). Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 6, e29107. doi: 10.7554/eLife.29107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers E. T., Duhamel M., Beesetty Y., Mensah J. A., Franken O., Verbruggen E., et al. (2011). Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333, 880–882. doi: 10.1126/science.1208473 [DOI] [PubMed] [Google Scholar]
- Kiers E. T., van der Heijden M. G. A. (2006). Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology 87, 1627‒1636. doi: 10.1890/0012-9658(2006)87[1627:msitam]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Konvalinková T., Jansa J. (2016). Lights off for arbuscular mycorrhiza: on its symbiotic functioning under light deprivation. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurppa M., Leblanc H. A., Nygren P. (2010). Detection of nitrogen transfer from N2-fixing shade trees to cacao saplings in 15N labelled soil: ecological and experimental considerations. Agrofor Syst. 80, 223–239. doi: 10.1007/s10457-010-9327-6 [DOI] [Google Scholar]
- Leigh J., Hodge A., Fitter A. H. (2008). Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 181, 199–207. doi: 10.1111/j.1469-8137.2008.02630.x [DOI] [PubMed] [Google Scholar]
- Lekberg Y., Hammer E. C., Olsson P. A. (2010). Plants as resource islands and storage units–adopting the mycocentric view of arbuscular mycorrhizal networks. FEMS Microbiol. Ecol. 74, 336–345. doi: 10.1111/j.1574-6941.2010.00956.x [DOI] [PubMed] [Google Scholar]
- Lekberg Y., Rosendahl S., Michelsen A., Olsson P. A. (2013). Seasonal carbon allocation to arbuscular mycorrhizal fungi assessed by microscopic examination, stable isotope probing and fatty acid analysis. Plant Soil 368, 547–555. doi: 10.1007/s11104-012-1534-7 [DOI] [Google Scholar]
- Lerat S., Gauci R., Catford J. G., Vierheilig H., Piche Y., Lapointe L. (2002). 14C transfer between the spring ephemeral Erythronium americanum and sugar maple saplings via arbuscular mycorrhizal fungi in natural stands. Oecologia 132, 181–187. doi: 10.1007/s00442-002-0958-9 [DOI] [PubMed] [Google Scholar]
- Li X. L., Marschner H., George E. (1991). Acquisition of phosphorus and copper by VA mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136, 49–57. doi: 10.1007/BF02465219 [DOI] [Google Scholar]
- Li Y. F., Ran W., Zhang R. P., Sun S. B., Xu G. H. (2009). Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil 315, 285–296. doi: 10.1007/s11104-008-9751-9 [DOI] [Google Scholar]
- Luginbuehl L. H., Menard G. N., Kurup S., van Erp H., Radhakrishnan G. V., Breakspear A., et al. (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356, 1175–1178. doi: 10.1126/science.aan0081 [DOI] [PubMed] [Google Scholar]
- Malcova R., Albrechtova J., Vosatka M. (2001). The role of the extraradical mycelium network of arbuscular mycorrhizal fungi on the establishment and growth of Calamagrostis epigejos in industrial waste substrates. Appl. Soil Ecol. 18, 129‒142. doi: 10.1016/S0929-1393(01)00156-1 [DOI] [Google Scholar]
- Martins M. A. (1992). The role of the external mycelial network of vesicular-arbuscular mycorrhizal fungi: a study of carbon transfer between plants interconnected by a common mycelium. Mycorrhiza 2, 69–73. doi: 10.1007/BF00203252 [DOI] [Google Scholar]
- Martins M. A. (1993). The role of the external mycelium of vesicular-arbuscular mycorrhizal fungi in the carbon transfer process between plants. Mycol Res. 97, 807–810. doi: 10.1016/S0953-7562(09)81155-6 [DOI] [Google Scholar]
- Martins M. A., Cruz A. F. (1998). The role of the external mycelial network of arbuscular mycorrhizal fungi: III. a study of nitrogen transfer between plants interconnected by a common mycelium. Rev. Microbiol. 29, 289‒294. doi: 10.1590/S0001-37141998000400011 [DOI] [Google Scholar]
- Meding S. M., Zasoski R. J. (2008). Hyphal-mediated transfer of nitrate, arsenic, cesium, rubidium, and strontium between arbuscular mycorrhizal forbs and grasses from a California oak woodland. Soil Biol. Biochem. 40, 126‒134. doi: 10.1016/j.soilbio.2007.07.019 [DOI] [Google Scholar]
- Meng L. B., Zhang A. Y., Wang F., Han X. G., Wang D. J., Li S. M. (2015). Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrild M. P., Ambus P., Rosendahl S., Jakobsen I. (2013). Common arbuscular mycorrhizal networks amplify competition for phosphorus between seedlings and established plants. New Phytol. 200, 229–240. doi: 10.1111/nph.12351 [DOI] [PubMed] [Google Scholar]
- Mickan B. S., Hart M., Solaiman Z. M., Renton M., Siddique K. H. M., Jenkins S. N., et al. (2021). Arbuscular mycorrhizal fungus-mediated interspecific nutritional competition of a pasture legume and grass under drought-stress. Rhizosphere 18, 100349. doi: 10.1016/j.rhisph.2021.100349 [DOI] [Google Scholar]
- Mikkelsen B. L., Rosendahl S., Jakobsen I. (2008). Underground resource allocation between individual networks of mycorrhizal fungi. New Phytol. 180, 890–898. doi: 10.1111/j.1469-8137.2008.02623.x [DOI] [PubMed] [Google Scholar]
- Molina R., Horton T. (2015). “Mycorrhiza specificity: its role in the development and function of common mycelial networks,” in Mycorrhizal networks. Ed. Horton T. (Dordrecht, Netherlands: Springer; ), 1‒39. doi: 10.1007/978-94-017-7395-9_1 [DOI] [Google Scholar]
- Montesinos-Navarro A., Verdu M., Querejetac J. I., Sortibrán L., Valiente-Banuet A. (2016). Soil fungi promote nitrogen transfer among plants involved in long-lasting facilitative interactions. Perspect. Plant Ecol. 18, 45–51. doi: 10.1016/j.ppees.2016.01.004 [DOI] [Google Scholar]
- Moyer-Henry K. A., Burton J. W., Israel D. W., Rufty T. W. (2006). Nitrogen transfer between plants: a 15N natural abundance study with crop and weed species. Plant Soil 282, 7–20. doi: 10.1007/s11104-005-3081-y [DOI] [Google Scholar]
- Muneer M. A., Chen X. H., Munir M. Z., Nisa Z. U., Saddique M. A. B., Mehmood S., et al. (2022). Interplant transfer of nitrogen between C3 and C4 plants through common mycorrhizal networks under different nitrogen availability. J. Plant Ecol. 16, rtac058. doi: 10.1093/jpe/rtac058 [DOI] [Google Scholar]
- Muneer M. A., Wang P., Zaib-un-Nisa, CC Li, Ji B. M. (2020. a). Potential role of common mycorrhizal networks in improving plant growth and soil physicochemical properties under varying nitrogen levels in a grassland ecosystem. Glob Ecol. Conserv. 24, e01352. doi: 10.1016/j.gecco.2020.e01352 [DOI] [Google Scholar]
- Muneer M. A., Wang P., Zhang J., Li Y. M., Munir M. Z., Ji B. M. (2020. b). Formation of common mycorrhizal networks significantly affects plant biomass and soil properties of the neighboring plants under various nitrogen levels. Microorganisms 8, 230. doi: 10.3390/microorganisms8020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano-Hylander A., Olsson P. A. (2007). Carbon allocation in mycelia of arbuscular mycorrhizal fungi during colonisation of plant seedlings. Soil Biol. Biochem. 39, 1450–1458. doi: 10.1016/j.soilbio.2006.12.031 [DOI] [Google Scholar]
- Näsholm T., Ekblad A., Nordin A., Giesler R., Högberg M., Högberg P. (1998). Boreal Forest plants take up organic nitrogen. Nature 392, 914–916. doi: 10.1038/31921 [DOI] [Google Scholar]
- Newman E. I., Devoy A. L. N., Basen N. J., Fowles K. J. (1994). Plant species that can be linked by VA mycorrhizal fungi. New Phytol. 126, 691–693. doi: 10.1111/j.1469-8137.1994.tb02963.x [DOI] [Google Scholar]
- Newman E. I., Eason W. R., Eissenstat D. M., Ramos M. I. R. F. (1992). Interaction between plants: the role of mycorrhizae. Mycorrhiza 1, 47–53. doi: 10.1007/BF00206135 [DOI] [Google Scholar]
- Nouri E., Breuillin-Sessoms F., Feller U., Reinhardt D. (2014). Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida . PloS One 9, e90841. doi: 10.1371/journal.pone.0090841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri E., Reinhardt D. (2015). Flowers and mycorrhizal roots–closer than we think? Trends Plant Sci. 20, 344–350. doi: 10.1016/j.tplants.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Orwin K. H., Kirschbaum M. U., St John M. G., Dickie I. A. (2011). Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol. Lett. 14, 493‒502. doi: 10.1111/j.1461-0248.2011.01611.x [DOI] [PubMed] [Google Scholar]
- Parniske M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbiosis. Nat. Rev. Microbiol. 6, 763‒775. doi: 10.1038/nrmicro1987 [DOI] [PubMed] [Google Scholar]
- Pearson J. N., Jakobsen I. (1993). Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 124, 481–488. doi: 10.1111/j.1469-8137.1993.tb03839.x [DOI] [Google Scholar]
- Pfeffer P. E., Douds D. D., Becard G., Shachar-Hill Y. (1999). Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 120, 587‒598. doi: 10.1104/pp.120.2.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. E., Douds D. D., Bücking H., Schwartz D. P., Shachar-Hill Y. (2004). The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis. New Phytol. 163, 617–627. doi: 10.1111/j.1469-8137.2004.01152.x [DOI] [PubMed] [Google Scholar]
- Querejeta J. I., Barea J. M., Allen M. F., Caravaca F., Roldan A. (2003). Differential response of δ13C and water use efficiency to arbuscular mycorrhizal infection in two aridland woody plant species. Oecologia 135, 510–515. doi: 10.1007/s00442-003-1209-4 [DOI] [PubMed] [Google Scholar]
- Rains K. C., Bledsoe C. S. (2007). Rapid uptake of 15N-ammonium and glycine 13C, 15N by arbuscular and ericoid mycorrhizal plants native to a Northern California coastal pygmy forest. Soil. Biol. Biochem. 39, 1078–1086. doi: 10.1016/j.soilbio.2006.11.019 [DOI] [Google Scholar]
- Read D. J. (1991). Mycorrhizas in ecosystems. Experientia 47, 376–391. doi: 10.1007/BF01972080 [DOI] [Google Scholar]
- Read D. J., Perez-Moreno J. (2003). Mycorrhizas and nutrient cycling in ecosystems - a journey towards relevance? New Phytol. 157, 475–492. doi: 10.1046/j.1469-8137.2003.00704.x [DOI] [PubMed] [Google Scholar]
- Reay M. K., Pears K. A., Kuhl A., Evershed R. P., Murray P. J., Cardenas L. M., et al. (2022). Mechanisms of nitrogen transfer in a model clover-ryegrass pasture: a 15N-tracer approach. Plant Soil 480, 369–389. doi: 10.1007/s11104-022-05585-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L. X., Lou Y. S., Zhang N., Zhu X. D., Hao W. Y., Sun S. B., et al. (2013). Role of arbuscular mycorrhizal network in carbon and phosphorus transfer between plants. Biol. Fert Soils 49, 3‒11. doi: 10.1007/s00374-012-0689-y [DOI] [Google Scholar]
- Řezáčová V., Zemková L., Beskid O., Püschel D., Konvalinková T., Hujslová M., et al. (2018). Little cross-feeding of the mycorrhizal networks shared between C3-Panicum bisulcatum and C4-Panicum maximum under different temperature regimes. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig M. C. (2004). Arbuscular mycorrhizae glomalin and soil aggregation. Can. J. Soil Sci. 84, 355–363. doi: 10.4141/S04-003 [DOI] [Google Scholar]
- Rillig M. C., Aguilar-Trigueros C. A., Camenzind T., Cavagnaro T. R., Degrune F., Hohmann P., et al. (2019). Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 222, 1171–1175. doi: 10.1111/nph.15602 [DOI] [PubMed] [Google Scholar]
- Robinson D., Fitter A. (1999). The magnitude and control of carbon transfer between plants linked by a common mycorrhizal network. J. Exp. Bot. 50, 9–13. doi: 10.1093/jxb/50.330.9 [DOI] [Google Scholar]
- Rodrigues L. A., Martins M. A., Salomao M. S. M. B. (2003). Use of mycorrhizas and rhizobium in intercropping system of Eucalyptus and Sesbania. I: growth, uptake and transfer of nitrogen between plants. Rev. Bras. Cienc Solo 27, 583–591. doi: 10.1590/S0100-06832003000400002 [DOI] [Google Scholar]
- Rogers J. B., Laidlaw A. S., Christie P. (2001). The role of arbuscular mycorrhizal fungi in the transfer of nutrients between white clover and perennial ryegrass. Chemosphere 42, 153–159. doi: 10.1016/S0045-6535(00)00120-X [DOI] [PubMed] [Google Scholar]
- Ronsheim M. L., Anderson S. E. (2001). Population level specificity in the plant-mycorrhizae association alters intraspecific interactions among neighbouring plants. Oecologia 128, 77–84. doi: 10.1007/s004420000625 [DOI] [PubMed] [Google Scholar]
- Roth R., Paszkowski U. (2017). Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 39, 50–56. doi: 10.1016/j.pbi.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Ryan M. H., Graham J. H., Morton J. B., Kirkegaard J. A. (2019). Research must use a systems agronomy approach if management of the arbuscular mycorrhizal symbiosis is to contribute to sustainable intensification. New Phytol. 222, 1176–1178. doi: 10.1111/nph.15600 [DOI] [PubMed] [Google Scholar]
- Schindler F. V., Mercer E. J., Rice J. A. (2007). Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol. Biochem. 39, 320–329. doi: 10.1016/j.soilbio.2006.08.017 [DOI] [Google Scholar]
- Schüßler A., Walker C. (2010). “The glomeromycota,” in A species list with new families and new genera. (California: CreateSpace Independent Publishing Platform: ), 1–58. Available at: http://www.amf-phylogeny.com. [Google Scholar]
- Smith F. A., Grace E. J., Smith S. E. (2009). More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 182, 347–358. doi: 10.1111/j.1469-8137.2008.02753.x [DOI] [PubMed] [Google Scholar]
- Smith S. E., Read D. J. (2008). Mycorrhizal symbiosis. 3rd edn (London: Academic Press; ), 1‒100. [Google Scholar]
- Smith S. E., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 62, 227–250. doi: 10.1146/annurev-arplant-042110-103846 [DOI] [PubMed] [Google Scholar]
- Southworth D., He X. H., Swenson W. S., Bledsoe C. S., Horwath W. R. (2005). Application of network theory to potential mycorrhizal networks. Mycorrhiza 15, 589–595. doi: 10.1007/s00572-005-0368-z [DOI] [PubMed] [Google Scholar]
- Teste F. P., Veneklaas E. J., Dixon K. W., Lambers H. (2015). Is nitrogen transfer among plants enhanced by contrasting nutrient-acquisition strategies? Plant Cell Environ. 38, 50–60. doi: 10.1111/pce.12367 [DOI] [PubMed] [Google Scholar]
- Thirkell J. D., Cameron D. D., Hodge A. (2016). Resolving the “nitrogen paradox” of arbuscular mycorrhizas: fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 39, 1683–1690. doi: 10.1111/pce.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobar R. M., Azcón R., Barea J. M. (1994. a). The improvement of plant n acquisition from an ammonium-treated, drought stressed soil by the fungal symbiont in arbuscular mycorrhizae. Mycorrhiza 4, 105–108. doi: 10.1007/BF00203769 [DOI] [Google Scholar]
- Tobar R., Azcón R., Barea J. M. (1994. b). Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol. 126, 119–122. doi: 10.1111/j.1469-8137.1994.tb07536.x [DOI] [Google Scholar]
- Trannin W. S., Urquiaga S., Guerra G., Ibijbijen J., Cadisch G. (2000). Interspecies competition and n transfer in a tropical grass-legume mixture. Biol. Fertil Soils 32, 441–448. doi: 10.1007/s003740000271 [DOI] [Google Scholar]
- Trépanier M., Bécard G., Moutoglis P., Willemot C., GagnéS, TJ A., et al. (2005). Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl. Environ. Microb. 71, 5341–5347. doi: 10.1128/AEM.71.9.5341-5347.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffen F., Eason W. R., Scullion J. (2002). The effect of earthworms and arbuscular mycorrhizal fungi on growth of and 32P transfer between Allium porrum plants. Soil Biol. Biochem. 34, 1027–1036. doi: 10.1016/S0038-0717(02)00036-6 [DOI] [Google Scholar]
- van der Heijden M. G. A., Martin F. M., Selosse M. A., Sanders I. R. (2015). Mycorrhizal ecology and evolution: the past, the present and the future. New Phytol. 205, 1406‒1423. doi: 10.1111/nph.13288 [DOI] [PubMed] [Google Scholar]
- van Kessel C., Singleton P. W., Hoben H. J. (1985). Enhanced n transfer from a soybean to maize by vesicular-arbuscular mycorrhizal (VAM). Plant Physiol. 79, 562–563. doi: 10.1104/pp.79.2.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuinen D., Tranchand E., Hirissou F., Wipf D., Courty P. E. (2020). Carbon partitioning in a walnut-maize agroforestry system through arbuscular mycorrhizal fungi. Rhizosphere 15, 100230. doi: 10.1016/j.rhisph.2020.100230 [DOI] [Google Scholar]
- Voets L., Goubau I., Olsson P. A., Merckx R., Declerck S. (2008). Absence of carbon transfer between Medicago truncatula plants linked by a mycorrhizal network, demonstrated in an experimental microcosm. FEMS Microbiol. Ecol. 65, 350–360. doi: 10.1111/j.1574-6941.2008.00503.x [DOI] [PubMed] [Google Scholar]
- Wagg C., Jansa J., Stadler M., Schmid B., van der Heijden M. G. A. (2011). Mycorrhizal fungal identity and diversity relaxes plant-plant competition. Ecology 92, 1303–1313. doi: 10.1890/10-1915.1 [DOI] [PubMed] [Google Scholar]
- Wahbi S., Maghraoui T., Hafidi M., Sanguin H., Oufdou K., Prin Y., et al. (2016). Enhanced transfer of biologically fixed n from faba bean to intercropped wheat through mycorrhizal symbiosis. Appl. Soil Ecol. 107, 91‒98. doi: 10.1016/j.apsoil.2016.05.008 [DOI] [Google Scholar]
- Walder F., Niemann H., Natarajan M., Lehmann M. F., Boller T., Wiemken A. (2012). Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol. 159, 789–797. doi: 10.1104/pp.112.195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J., He X. H., Meng L. L., Zou Y. N., Wu Q. S. (2023). Extraradical mycorrhizal hyphae promote soil carbon sequestration through difficultly extractable glomalin−related soil protein in response to soil water stress. Microb. Ecol. doi: 10.1007/s00248-022-02153-y [DOI] [PubMed] [Google Scholar]
- Wang B., Qiu Y. L. (2006). Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299‒363. doi: 10.1007/s00572-005-0033-6 [DOI] [PubMed] [Google Scholar]
- Wang G., Sheng L., Zhao D., Sheng J., Wang X., Liao H. (2016). Allocation of nitrogen and carbon is regulated by nodulation and mycorrhizal networks in soybean/maize intercropping system. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. R., Borowicz V. A. (1994). Effect of clipping, benomyl and genet on 14C transfer between mycorrhizal plants. Oikos 71, 246–252. doi: 10.2307/3546272 [DOI] [Google Scholar]
- Watkins N. K., Fitter A. H., Graves J. D., Robinson D. (1996). Carbon transfer between C3 and C4 plants linked by a common mycorrhizal network, quantified using stable carbon isotopes. Soil Biol. Biochem. 28, 471–477. doi: 10.1016/0038-0717(95)00189-1 [DOI] [Google Scholar]
- Weremijewicz J., Sternberg L. D. L. O., Janos D. P. (2016). Common mycorrhizal networks amplify competition by preferential mineral nutrient allocation to large host plants. New Phytol. 212, 461‒471. doi: 10.1111/nph.14041 [DOI] [PubMed] [Google Scholar]
- Werner G. D. A., Strassmann J. E., Ivens A. B. F., Engelmoer D. J. P., Verbruggen E., Queller D. C., et al. (2014). Evolution of microbial markets. P Natl. Acad. Sci. U.S.A. 111, 1237–1244. doi: 10.1073/pnas.1315980111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside M. D., Digman M. A., Gratton E., Treseder K. K. (2012). Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest. Soil Biol. Biochem. 55, 7–13. doi: 10.1016/j.soilbio.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf D., Krajinski F., Courty P. E. (2019). Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol. 223, 1127–1142. doi: 10.1111/nph.15775 [DOI] [PubMed] [Google Scholar]
- Wurzburger N., Brookshire E. N. J., McCormack M. L., Lankau R. A. (2017). Mycorrhizal fungi as drivers and modulators of terrestrial ecosystem processes. New Phytol. 213, 996–999. doi: 10.1111/nph.14409 [DOI] [PubMed] [Google Scholar]
- Yuan M. T. M., Kakouridis A., Starr E., Nguyen N., Shi S. J., Pett-Ridge J., et al. (2021). Fungal-bacterial cooccurrence patterns differ between arbuscular mycorrhizal fungi and nonmycorrhizal fungi across soil niches. mBio 12, e03509–e03520. doi: 10.1128/mBio.03509-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. G., van Kleunen M., Li J. M. (2021). A parasite indirectly affects nutrient distribution by common mycorrhizal networks between host and neighboring plants. Ecology 102, e03339. doi: 10.1002/ecy.3339 [DOI] [PubMed] [Google Scholar]
- Zabinski C. A., Quinn L., Callaway R. M. (2002). Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct. Ecol. 16, 758‒765. doi: 10.1046/j.1365-2435.2002.00676.x [DOI] [Google Scholar]
- Zhang H. L., Wang X. Y., Gao Y. Z., Sun B. R. (2019). Short-term n transfer from alfalfa to maize is dependent more on arbuscular mycorrhizal fungi than root exudates in n deficient soil. Plant Soil 446, 23‒41. doi: 10.1007/s11104-019-04333-1 [DOI] [Google Scholar]
- Zhou X. Q., Li J. Y., Tang N. W., Xie H. Y., Fan X. N., Chen H., et al. (2021). Genome-wide analysis of nutrient signaling pathways conserved in arbuscular mycorrhizal fungi. Microorganisms 9, 1557. doi: 10.3390/microorganisms9081557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel M., Öpik M. (2014). Plant and arbuscular mycorrhizal fungal (AMF) communities–which drives which? J. Vegetation Sci. 25, 1133‒1140. doi: 10.1111/jvs.12191 [DOI] [Google Scholar]