Abstract
Pulmonary chronic graft-versus-host disease (cGVHD) is a substantial cause of pulmonary morbidity and mortality post-haematopoietic stem cell transplantation (HSCT). Current spirometry-based monitoring strategies have significant limitations. Understanding the utility of novel peripheral airway function tests – multiple breath washout (MBW) and oscillometry – is critical in efforts to improve detection, facilitate earlier intervention and improve outcomes. In this scoping review, we identified 17 studies investigating MBW or oscillometry, or both, after allogenic HSCT. Despite small study numbers limiting the ability to draw firm conclusions, several themes were evident. Detectable peripheral airway abnormality in MBW occurred in a substantial proportion prior to HSCT. MBW indices post-HSCT were more frequently abnormal than spirometry when reporting group data and among those with extrapulmonary cGVHD and pulmonary cGVHD. Changes in MBW indices over time may be more indicative of pulmonary complications than absolute values at any given time point. Oscillometry indices were often normal at baseline, but more frequently abnormal in those who developed pulmonary cGVHD. Pooling currently available individual participant data across these studies may improve our ability to formally compare their respective sensitivity and specificity at specific time points and assess the trajectory of MBW and oscillometry indices over time.
Tweetable abstract
Multiple breath washout and oscillometry have the potential to improve diagnosis of pulmonary chronic graft-versus-host disease. While small study numbers limit firm conclusions, the data presented support further studies to address key knowledge gaps. https://bit.ly/3Ba3jge
Introduction
Haematopoietic stem cell transplantation (HSCT) is associated with substantial pulmonary morbidity and mortality [1]. The most significant morbidity is the pulmonary manifestation of chronic graft-versus-host disease (cGVHD). Histologically, this is characterised by narrowing or fibrotic obliteration of terminal bronchioles, termed bronchiolitis obliterans (BO) [2], resulting in obstructive airways disease. In adults, pulmonary cGVHD has an estimated incidence of 4–9% post-HSCT using current monitoring and diagnostic approaches, increasing to ≈14–24% in those with cGVHD in other organs [3–7]. It may be rapidly progressive and is associated with poor treatment response and significant mortality [8, 9], with 5-year survival of only 10% in some cohorts [8]. It is therefore critical to improve detection, facilitate earlier intervention and ultimately improve outcomes. Earlier and more precise diagnosis may be possible using the novel pulmonary peripheral airway function tests described in this review, namely multiple breath washout (MBW) and oscillometry.
Bronchiolitis obliterans syndrome
National Institutes of Health (NIH) criteria for pulmonary cGVHD post-HSCT are summarised in box 1. The gold standard diagnosis is histopathological, with a lung biopsy demonstrating BO, but the sensitivity of this invasive procedure is compromised by the patchy distribution of the disease process. To combat these limitations, a clinical surrogate termed bronchiolitis obliterans syndrome (BOS) was established [10]. Given that these were influenced by the BOS criteria originally developed for the setting of lung transplantation, the original International Heart and Lung Transplant Society criteria [11] are also shown in box 1. However, the NIH consensus criteria have several key limitations.
BOX 1.
National Institutes of Health 2015 criteria for chronic graft-versus-host disease of the lung following haematopoietic stem cell transplantation [10], diagnostic criteria for bronchiolitis obliterans syndrome (BOS), BOS stage 0p (BOS 0p) [15] and, as a reference, the International Heart and Lung Transplant Society (ISHLT) BOS grading system used in lung transplantation [11]
BOS:
|
BOS 0p:
|
ISHLT criteria for BOS in lung transplantation:
Values represent spirometry % of baseline.
|
Firstly, spirometry is well recognised to be insensitive for detection of changes occurring in peripheral airways where BO evolves. Spirometry primarily measures flow, reflecting changes in airway resistance. However, the contribution of peripheral airways to overall airway resistance measured at the mouth is only minimal. In animal studies, obstruction of 50% of peripheral airways only increased airway resistance by 10%, in comparison to a doubling effect when 50% of central airways were obstructed [12]. Detrimental changes in flow within peripheral airway regions may be compensated for by increased flow through adjacent unaffected airways [13]. Therefore, the BO process is well advanced once spirometry becomes abnormal [14]. To address this issue, an earlier “at-risk” stage of BOS was proposed [15], termed BOS stage 0p (BOS 0p; box 1), which was shown to be a risk factor for subsequent BOS development among adult HSCT recipients [15]. Secondly, whilst conventional pulmonary function tests (PFTs), such as spirometry and plethysmography, may detect pulmonary cGVHD earlier than clinical signs and symptoms alone [16], guidelines for surveillance are lacking, with screening protocols varying widely across centres [17]. A third factor limiting utility of spirometry, in paediatric subjects, is its poor feasibility in many young children who struggle with the co-ordinated effort-dependent breathing manoeuvres required [18]. Finally, the sensitivity of NIH criteria has been challenged: 30–50% of patients with biopsy-diagnosed BO did not meet NIH criteria in one retrospective analysis [19] and only 17% of 12 subjects thought to have pulmonary cGVHD fulfilled NIH criteria while alive in another prospective trial [7]. More sensitive and feasible tools to detect peripheral airway changes are therefore urgently required as recognised by multiple NIH working groups [20–22]. Two such tools are MBW and oscillometry.
MBW
MBW assesses gas mixing within the lungs, a process involving all airways [23]. The predominant region for gas mixing is the peripheral airways, which represents 95% of the lung volume [24] and defined (in adult lungs) as those with a diameter of ≤2 mm, corresponding to generation 8 and beyond. BO affects the peripheral airways in a heterogenous (or patchy) manner [2] resulting in uneven gas mixing or greater ventilation inhomogeneity (VI). The most commonly reported MBW outcome measure is the lung clearance index (LCI), which increases with greater VI. Additional insight into the underlying VI mechanisms can be gained from the progression of the concentration-normalised phase III slope (SnIII) through the test. Ventilation distribution occurs by convection and diffusion. Convection is the predominant mechanism in the conducting airway zone where linear gas flow velocity is relatively high. As the airways divide, the total cross-sectional area increases markedly and linear gas flow velocity decreases dramatically, until diffusion becomes the dominant mechanism in the very distal lung. At the entry of the acinus, the relative contributions of convection and diffusion to gas mixing become similar, forming a “diffusion–convection front”. The first mechanism that SnIII analysis provides insight into, convection-dependent inhomogeneity, reflects VI arising in the more proximal conducting airways and is quantified by the index Scond. The second, diffusion–convection-dependent inhomogeneity, arises more distally within the region of the entrance to the acinus and is quantified by the index Sacin [24]. Abnormalities in these indices allow assessment of the contribution of pathology in the proximal (increase in Scond) and distal (increase in Sacin) peripheral airways to the VI seen.
During MBW, an inert gas of interest is washed out of the lungs during tidal breathing. Inert gas options include those both resident within the lungs (e.g. nitrogen, N2, washed out with 100% oxygen, O2) or exogenous (e.g. sulphur hexafluoride, SF6, washed out with medical air after an initial wash-in phase). Results obtained with different gases are not interchangeable [25–27] and inert gas specific reference equations are recommended [28]. Due to its higher molar mass and slower speed of diffusion, the diffusion–convection front for SF6 sits more distal to that of N2. As such, in theory, SF6 may provide a more sensitive signal to detect abnormalities in disease processes affecting the distal lung preferentially. In the MBW studies discussed within this review article, we have indicated those where SF6 is the inert gas of interest. As a tidal breathing test MBW has high feasibility (≥80%) across infants and preschool-aged children [29–31]. Improved ability of MBW to detect peripheral airway dysfunction, in comparison to spirometry, has been demonstrated in a number of other disease conditions including cystic fibrosis and primary ciliary dyskinesia [32, 33]. Imaging techniques, such as hyperpolarised gas magnetic resonance imaging, can also provide information on ventilation distribution (and therefore peripheral airway function) and have the additional advantage of providing topographical information about the regions affected by disease. However, a discussion of the evidence for imaging-based techniques reflecting peripheral airway function is beyond the scope of this review.
Oscillometry
Oscillometry is another tidal breathing test with high feasibility across the paediatric age range [34, 35]. It measures lung mechanics, specifically impedance, by superimposing pressure waves onto normal breathing [36]. Airway impedance consists of two components, respiratory system resistance (Rrs) and reactance (Xrs). Resistance predominantly reflects airway calibre, with narrowing of the airways leading to greater resistance. Reactance predominantly reflects airway stiffness, but it may also reflect the degree of VI present [37]. By varying the frequencies contained within impulses applied to breathing, relative contributions of more central and peripheral airways may be assessed. Insight into peripheral airway function is gained from low-frequency impulses (typically 5–6 Hz) or change occurring across a range of frequencies using indices such as Rrs 5–19 Hz, the area under the reactance curve (termed AX) and the frequency at which Xrs is 0 (termed resonant frequency or Fres). Oscillometry has greater sensitivity than spirometry to detect peripheral airway dysfunction related to processes such as acute cellular rejection after lung transplantation and (intrauterine) smoke exposure [38–40]. As with MBW, differences in values of indices exists between oscillometry devices [41] and, as such, reference equations remain device specific.
Given their improved sensitivity to detect peripheral airway changes and feasibility in all age groups, MBW and oscillometry represent attractive tools to improve detection of pulmonary cGVHD [20], particularly in populations unable to adequately perform spirometry.
The objective of this scoping review was to identify and provide an overview of the studies to date that have explored the use of these tools in paediatric and adult HSCT populations. Through this, we aimed to identify important take-home messages and knowledge gaps to direct future research.
Methods
Literature was searched to identify all original observational research on the use of either MBW or oscillometry, or both, in patients after allogenic HSCT. There was no restriction on age of participants, nor on the underlying diagnosis for which HSCT was performed. MBW or oscillometry could be performed within 3 months prior to HSCT or at any time after HSCT. A comparator to MBW or oscillometry was not required.
A systematic literature search was performed on 11 March 2022 in Embase via OvidSP (1947–2022), Medline via Ovid (1946–2022), the Cochrane Library (including CENTRAL) from inception to 2022, clinicaltrials.gov, reference lists of all identified reports and conference proceedings from the past 10 years of the following societies: American Thoracic Society, European Respiratory Society, Australian and New Zealand Society of Respiratory Science, Thoracic Society of Australia and New Zealand, Asian Pacific Society of Respirology, European Society for Blood and Marrow Transplantation, and American Society for Transplantation and Cellular Therapy.
Full search terms used for the Medline search are listed in the supplementary appendix and were a combination of “haematopoietic stem cell transplantation” and either “multiple breath washout” or “oscillometry”, or terms similar to these. There was no language restriction. However, only published data, including in conference abstracts, were included in this scoping review.
Titles and abstracts of records were screened by one reviewer (N.S.) on eligibility criteria. Full texts of potentially eligible studies were reviewed by one reviewer (N.S.). Any uncertainty regarding eligibility was discussed and resolved through consensus with another reviewer (P.R.). If multiple conference abstracts or publications reported on the same cohort, all abstracts and publications were utilised in order to extract all available information.
The following data was extracted by one reviewer (N.S.):
Publication/abstract details: authors, year of publication, country of origin, aim or purpose of study, sample size.
Demographics of the study population (including age and sex), time post-HSCT of PFTs, presence of (pulmonary) cGVHD or its precursor BOS 0p.
Conventional lung function tests: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, forced expiratory flow at 25–75% (FEF25–75) or at 50% (FEF50) of FVC and transfer factor for carbon monoxide (DLCO).
MBW: inert gas used, LCI, Sacin and Scond.
Oscillometry: Rrs and Xrs at 5 Hz or equivalent, AX and Fres.
A risk of bias assessment was not performed for the purpose of this scoping review. Methodological differences between studies were explored. Results were synthesised qualitatively for MBW and oscillometry separately.
Results and discussion
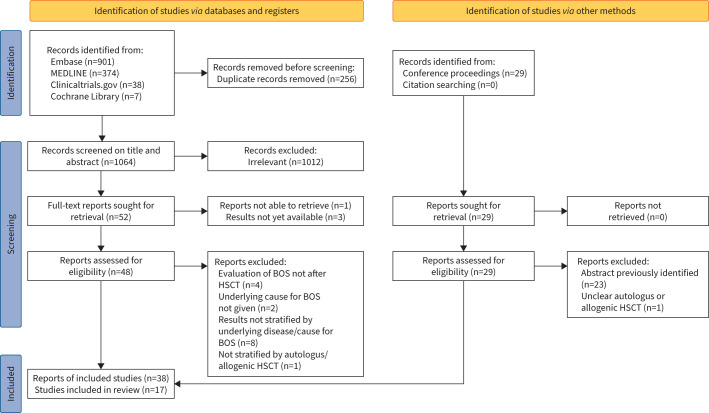
We identified 1320 records through the databases and registers, as well as 29 conference abstracts (figure 1). After removal of duplicates (n=256) and exclusion of ineligible reports (n=1055), 17 studies (38 reports) were included in this review. Studies were performed in Europe (n=10), Australia (n=6) and Canada (n=2) on a total of 790 subjects (256 paediatric subjects and 534 adults). BOS was diagnosed in 43 children and 145 adults.
FIGURE 1.
Flow diagram of the systematic literature review. BOS: bronchiolitis obliterans syndrome; HSCT: haematopoietic stem cell transplantation.
Methodological differences
Study characteristics are summarised in table 1. All but three studies (14/17, 82%) reported spirometry outcomes, 14/17 (82%) described MBW outcomes and 8/17 (47%) described oscillometry outcomes. Both MBW and oscillometry were performed concurrently in three adult cohorts and two paediatric cohorts (5/17, 29%).
TABLE 1.
Characteristics of the 17 studies included in this scoping review
| Cohort | Study design | Study population | Comparison group | n with cGVHD (%) | n with BOS (%) | Timing of PFTs | MBW inert gas used | ||
| n | Sex (% male) | Age | |||||||
| Paediatric subjects | |||||||||
| Uhlving et al. [42, 72] | Cross-sectional | 64 | 58 | Mean age at HSCT: 7.8 years | 64 age- and sex-matched healthy controls | 11 (17) current of prior cGHVD | 1 (2) prior BOS | Mean 7 years (range: 3.5–10.4) after HSCT | N2 |
| Rayment et al. [46, 73] | Cross-sectional | 26 | 65 | Median age at testing: 11.1 years | NA | NA | 12 (46.2), of which six quiescent | Between 100 days and 5 years after HSCT | N2 |
| Schindera et al. [43] and Usemann et al. [74–76] | Cross-sectional | 5 HSCT recipients (out of 46 adult childhood cancer survivors) | 52 (of entire cohort) | Median age at testing: 30 years (entire cohort) | NA | NA | NA | Median 20 years after diagnosis (entire cohort) | N2 |
| Jayasuriya et al. [47, 60, 71, 77, 78] | Cross-sectional/longitudinal | 30, MBW cross-sectional data available for 24 and longitudinal for 17; oscillometry available for 5 | 67 | Median age at testing: 14.3 years | NA | NA | NA | Median 4.8 years after HSCT and a median 1.2 years following initial test | SF6 and He |
| Kavouridou et al. [56] | Longitudinal | 110, data available for 62 | NA | Mean age at time of HSCT: 9.6 years (entire cohort) | NA | NA | 4 (6) | Baseline and 1 year after HSCT | SF6 |
| Uhlving et al. [57] and Jensen et al. [79] | Longitudinal | 28 | 68 | Median age at time of HSCT: 11.1 years | NA | 8 (29), two of which without pulmonary involvement | 6 (21) | Baseline, 3, 6, 9 and 12 months after HSCT | SF6 |
| Wong et al. [54], Hardaker et al. [80–82] and Robinson et al. [83] | Longitudinal | 24 | 71 | Mean age at HSCT: 10.2 years | NA | 4 (17) with cGVHD without pulmonary involvement | 3 (13) | Baseline, monthly in the first year after HSCT and 6-monthly in the 2 years thereafter | N2 |
| Walther et al. [55] | Longitudinal | 14 | 64 | Mean age at HSCT: 6 years | NA | 14 (100) | 14 (100) | Baseline and between 1 and 13.6 years after HSCT; timing of MBW not given | SF6 |
| Khalid et al. [58] | Case report with longitudinal measurement | 3 | 100 | Median age at HSCT: 1 year | NA | 2 (67) with cGVHD in other organ systems | 3 (100) | Variable, no baseline MBW | NA |
| Adult subjects | |||||||||
| Nyilas et al. [44] and Baumeler et al. [84, 85] | Cross-sectional | 225 | 57 | Mean age at testing: 52.8 years | NA | 48 (21.3) with cGVHD without pulmonary involvement | 64 (28.4) with BOS and 23 (10.2) with BO | Median 5.4 years after HSCT | N2 |
| De Giacomi et al. [45, 86, 87] | Cross-sectional | 78 | NA | NA | 41 healthy controls 8 patients with COPD |
NA | 38 (49) | Unknown | N2 |
| Lahzami et al. [48, 88] and Pechey et al. [89] | Cross-sectional/longitudinal | 33, with longitudinal data available for 22 | 67 | Mean age at time of testing: 47 years | NA | 18 (55) | 8 (24) | Median 12 months after HSCT (range: 3–73 months) and median 10 months thereafter | N2 |
| Blin et al. [52] | Longitudinal | 68 | 69 | Median age at time of testing: 50 years | NA | 26 (38) cGVHD at start of testing | 9 (13) with BOS diagnosed after start of study | 3-monthly for 2 years starting at median 217 days after HSCT | – |
| Htun et al. [50, 90] | Longitudinal | 65 | 54 | Mean age at HSCT: 50.3 years | NA | NA | NA | Baseline, 3-monthly during the first year post-HSCT or monthly if FEV1 declined by 10% or more | N2 |
| Barisione et al. 2012 [53, 91] | Longitudinal | 26 | NA | NA | NA | NA | 0 | 2 weeks pre- and 2 months post-HSCT | – |
| Schoeffel et al. [49, 59] | Longitudinal | 23 | NA | NA | NA | NA | 3 (13) | Baseline, 100 days and 12 months post-HSCT, with 24 months post-HSCT testing available for one subject | N2 |
| Rutting et al. [51, 92] | Longitudinal | 16 | NA | NA | 20 COPD and 25 asthma patients, and 12 healthy controls | NA | NA | Routine clinic visits | – |
BO: bronchiolitis obliterans; BOS: bronchiolitis obliterans syndrome; cGVHD: chronic graft-versus-host disease; FEV1: forced expiratory volume in the 1 s; HSCT: haematopoietic stem cell transplantation; MBW: multiple breath washout; NA: not available; PFT: pulmonary function test.
Methodology differed considerably across all included studies (both paediatric and adult cohorts). Five of 17 cohorts were cross-sectional [42–46], while two further studies had a cross-sectional design with follow-up data available for a subset of participants [47, 48]. Nine of 17 were longitudinal [49–57] and one case report with longitudinal data was available [58]. In addition, PFT timing relative to HSCT was inconsistent. Follow-up post-HSCT ranged from 1 month to 20 years, with only 6/17 (35%) including baseline measurement. Furthermore, while all MBW tests of the adult population were performed using N2 [45, 48–50], among the paediatric population this was evenly distributed between N2 [42, 43, 46, 54] and SF6 [47, 55–57], with the inert gas not specified in the case report [58]. Clinical stability at time of testing was required in 6/17 studies (35%) [42, 44, 47, 48, 51, 53], but unclear in the remainder of studies. A healthy control group was incorporated within the study design in only 2/17 studies [42, 45].
The majority of cohorts were small in size, with 9/17 (53%) reporting on <30 HSCT recipients. In contrast, the largest study by Nyilas et al. [44] included a cohort of 225 adult HSCT recipients.
Only 9/17 studies (53%) had a published manuscript available [42–44, 46, 48, 53, 55, 57, 58], with the remaining eight being described in conference abstracts.
Sex distribution was available for 12/17 cohorts (71%) [42–44, 46–48, 50, 52, 54, 55, 57, 58].
Age at HSCT or at time of PFT was available for all but 4/17 cohorts [45, 49, 51, 53].
12/17 studies provided number of subjects with pulmonary cGVHD [42, 44–46, 48, 49, 52, 54–58], whilst only eight studies provided number of subjects with extrapulmonary cGVHD [42, 44, 48, 52, 54, 55, 57, 58].
Among those studies reporting pulmonary cGVHD status, 4/17 (24%) did not provide the definition used [45, 49, 56, 58], four used NIH criteria available at the time [42, 48, 54, 57] and four used a modification of NIH criteria by adding criteria relating to presence of respiratory symptoms or cGVHD in other organ systems [44, 52, 55]. In one study, pulmonary cGVHD diagnosis was defined based on respiratory signs or symptoms and compatible computed tomography scan in the absence of an alternative cause [46].
Reference equations used to obtain percent predicted spirometry values, if obtained, were often not stated [45, 50, 52, 54, 55, 58–60] and varied among the remaining studies. Four of 17 [43, 44, 46, 57] used Global Lung Function Initiative (GLI) equations [61], one [42] used all-age reference equations, which were the precursor to GLI equations [62], and one [48] used European Coal and Steel Community equations [63]. This was also a challenge for both MBW and oscillometry where formal GLI reference equations are not yet available, although some groups have published reference equations using similar statistical approaches to those used by the GLI [64]. Across studies, different reference equations were chosen by investigators. Thresholds to define abnormality also varied between studies.
Lastly, aggregate values, whether absolute or z-scores, for the various PFTs performed were often not reported, with counts and relative frequency of PFT abnormality provided instead.
Given the varying methodology used, including timing of PFTs after HSCT and inert gas use, pooling or quantitatively comparing aggregate data was not possible for this scoping review based on the data available in the reports. Consequently, available evidence for MBW and oscillometry utility among HSCT recipients was described qualitatively.
MBW
The findings of studies evaluating MBW among HSCT recipients are summarised in table 2. Of the nine paediatric studies, five included preschool-aged children [46, 47, 54, 57, 58], and one further study included subjects transplanted at a preschool age, although timing of MBW testing relative to HSCT was not reported [55]. MBW was evaluated in five adult cohorts.
TABLE 2.
Summary of findings in 13 individual studies reporting on multiple breath washout (MBW) after haematopoietic stem cell transplant (HSCT)
| Cohort | Summary of findings | |
| Spirometry, other conventional PFTs | MBW | |
| Paediatric subjects | ||
| Uhlving et al. [42, 72] | Pulmonary function after HSCT (mean (sd; % abnormal)) was significantly different from healthy controls: FEV1 −0.56 z-score (1.40; 9%), FEV1/FVC 0.33 z-score (0.93; 0%) | Pulmonary function after HSCT (mean (sd; % abnormal)) was significantly different from healthy controls: LCI 7.23 (0.85; 34%), Sacin 0.080 (0.041; 25%) and Scond 0.029 (0.012; 52%) Scond was, after adjustment, correlated with cGVHD |
| Rayment et al. [46, 73] | Overall: median FEV1 was 80.7% pred (IQR: 72.2–92.8%) and median FEV1/FVC was 85% (IQR: 82–90%) No cGVHD: median FEV1/FVC 0.88 (IQR: 0.82–0.90) Extrapulmonary cGVHD: median FEV1/FVC 0.86 (IQR: 0.85–0.90) Pulmonary cGVHD: median FEV1/FVC 0.71 (IQR: 0.46–0.80) |
Overall: median LCI was 7.8 (IQR: 7.1–9.6) with 31% being over 9.0 No cGVHD: median LCI 7.7 (IQR: 7.1–8.0) Extrapulmonary cGVHD: median LCI 7.5 (IQR: 6.9–7.6) Pulmonary cGVHD: median LCI 11.8 (IQR: 9.6–18.7) Area under ROC curve for LCI to identify pulmonary cGVHD was 0.97 (95% CI 0.80–0.99) with a threshold of 9.0 giving a sensitivity of 100%, specificity of 90% and PPV of 75% |
| Schindera et al. [43] and Usemann et al. [74–76] | Spirometry was available for 4/5 subjects: FEV1 and FVC were abnormal in one subject | MBW was available for 4/5 subjects: Sacin was abnormal in all subjects and LCI was abnormal in two |
| Jayasuriya et al. [47, 60, 71, 77, 78] | Cross-sectional: FEV1 was abnormal in 21%, FEF25–75 in 17% Follow-up: no significant change in spirometry z-score (FEV1 median change 0.16 (range: −0.77–0.71)) |
Cross-sectional: MBW abnormal in 18 subjects. Median (range; % abnormal) Scond 0.034 (0.004–0.089; 75%), LCI 7.52 (5.03–23.59; 58%) and Sacin 0.105 (0.056–0.463; 33%) Follow-up: no significant change in LCI z-score (median 0.18 (range: −3.93–2.05)) |
| Kavouridou et al. [56] | NA | Mean±sd LCI pre-HSCT: 7.09±0.8; mean±sd LCI post-HSCT: 7.47±1.41 Mean±sd LCI pre-HSCT in BOS subjects: 6.60±0.16; mean±sd LCI post-HSCT in BOS subjects: 9.93±2.79 All four BOS subjects had ≥1 unit LCI increase at 1 year post-HSCT, no subjects with <1 unit increase developed BOS |
| Uhlving et al. [57] and Jensen et al. [79] | Baseline: FEV1 abnormal in 13% and DLCO in 70% 1-year follow-up: FEV1 and FVC at baseline were associated with FEV1 and FVC at 12 months FEV1 at 3 months and FVC at all time points were lower than at baseline GVHD: FEV1 significantly lower in GVHD than non-GVHD subjects BOS/BO: all patients with abnormal FEV1 at 3 months developed BOS |
Baseline: LCI abnormal in 48% 1-year follow-up: LCI at baseline and 3 months was not associated with LCI at 12 months; LCI did not change over time GVHD: LCI was significantly higher in GVHD than non-GVHD subjects BOS/BO: abnormal LCI at baseline and 3 months was not associated with development of BOS; absolute median (range) LCI in BOS: 7.9 (6.4–11.8) at baseline, 7.9 (7.4–14.0) at 3 months, 8.4 (6.3–13.0) at 6 months, 8.4 (6.8–13.0) at 9 months and 8.8 (7.7–14.7) at 12 months |
| Wong et al. [54], Hardaker et al. [80–82] and Robinson et al. [83] | cGHVD was associated with significant deterioration in FEV1 and FEF25–75 compared to those without cGVHD At 3 years post-HSCT, BOS 0p subjects (n=8) had FEV1 worse than subjects unaffected by BOS 0p/BOS that approached significance |
cGVHD was associated with significant deterioration in LCI and Sacin compared to those without cGVHD BOS was associated with significant abnormality in LCI, Scond and Sacin; MBW abnormality was detected at first available test in one BOS subject and 26 and 207 days prior to BOS diagnosis in the remaining two At 3 years post-HSCT, BOS 0p subjects (n=8) had significantly worsened LCI and Sacin, as compared to subjects unaffected by BOS 0p/BOS |
| Walther et al. [55] | Baseline: pulmonary function was normal in all 14 subjects Respiratory failure (n=3): FEV1 53.4, 36.5 and 23.9% pred |
Respiratory failure (n=3): LCI not available Persistent BOS (n=5): LCI 12.59, 14.69, 14.90, 19.30 and 20.90 Recovered BOS (n=6): LCI 6.51, 7.79 and 8.02 |
| Khalid et al. [58] | Case 1: baseline unavailable, reduced airflow at time of BOS diagnosis (7 months after HSCT), obstructive pattern on spirometry and hyperinflation on body plethysmography 2 years after repeat HSCT Case 2: spirometry normal at baseline, obstructive pattern 7 months post-HSCT at time of BO diagnosis, which remained stable for the following 2 years Case 3: unavailable |
Case 1: at 2 years post-HSCT, LCI was greatly increased (at approximately 18) and phase III-slope abnormality was present MBW abnormality worsened after another approximately 4 months; LCI and phase-III slope indices normalised after bilateral lung transplantation Case 2: MBW at 3.5 years post-HSCT was abnormal with LCI approximately 13, which subsequently deteriorated to 22.0 2 years later (at which point spirometry was relatively stable) Case 3: LCI at 1 month post-HSCT was 11.4, increasing to a maximum of approximately 12.5, then decreasing to approximately 9 with treatment |
| Adult subjects | ||
| Nyilas et al. [44] and Baumeler et al. [84, 85] | Overall mean pulmonary function (sd, % abnormal): FEV1 91% pred (20.6; 36%), FEV1/FVC 72% pred (10.5; 36%) cGVHD: FEV1 94.4% pred (18.3; 21%), FEV1/FVC 78.4 (5.3; 0%) BOS: FEV1 89% pred (18; 28%), FEV1/FVC 63% pred (7; 100%) BO: FEV1 71% pred (18; 74%), FEV1/FVC 65% pred (13; 57%) |
Overall mean pulmonary function (sd, % abnormal): LCI 9.6 (2.8; 74%), Sacin 0.17 (0.14; 59%) and Scond 0.029 (0.02; 4%) cGVHD: LCI 9.1 (2.2; 76%), Sacin 0.14 (0.1; 57%) and Scond 0.02 (0.02; 0%) BOS: LCI 9.7 (2.2; 85%), Sacin 0.17 (0.1; 68%) and Scond 0.03 (0.02; 6%) BO: LCI and Sacin were significantly more abnormal in this group than among subjects in the other groups; LCI 11.4 (3.3; 96%), Sacin 0.3 (0.2; 82%) and Scond 0.04 (0.03; 14%) |
| De Giacomi et al. [45, 86, 87] | NA | There was evidence of worsened Sacin and Scond as compared to healthy controls regardless of the presence of chronic GVHD in other organ systems in subjects without BO In subjects with BO Sacin was 551% (sd 360) and worse than in controls (p<0.001); Sacin with a 321% cut-off had an 89% sensitivity and 93% specificity in identifying patients with BO |
| Lahzami et al. [48, 88] and Pechey et al. [89] | Initial testing (mean±sd or median (range)): FEV1 was 87±20% pred, FEV1/FVC 80 (45–89) FEV1 was independently associated with time post-HSCT Follow-up: no significant change in spirometry outcomes |
Initial testing (mean±sd or median (range)): LCI 12.1 (8.0–22.2), Sacin 0.24 (0.08–1.72) and Scond 0.07±0.03; Sacin was independently associated with time post-HSCT; Sacin was correlated with chronic GVHD grade Follow-up: LCI significantly increased from mean±sd 11.6±2.5 to 13.5±3.5 and Sacin increased from median 0.28 (range: 0.06–0.69) to 0.41 (0.08–1.07); only change in Sacin correlated with change in GVHD grade |
| Htun et al. [50, 90] | Baseline: mean±sd FEV1 101±15% pred, FEV1/FVC 79±6.6 Follow-up: FEV1 did not change |
Baseline: mean±sd LCI 8.6±1.1, Sacin 0.10±0.05 and Scond 0.03±0.02 Follow-up: Sacin significantly increased by 42% and was associated with age and time since HSCT; LCI and Scond did not change |
| Schoeffel et al. [49, 59] | Baseline: mean±sd FEV1 was 105±13% pred, FEV1/FVC was normal Follow-up: FVC declined |
Baseline: Sacin and Scond were abnormal in 43% Follow-up: there were no changes in LCI, Sacin or Scond BOS subjects: Sacin and Scond were abnormal at time of BOS diagnosis; respiratory function at the visit prior to diagnosis was not different from baseline |
BO: bronchiolitis obliterans; BOS: bronchiolitis obliterans syndrome; BOS 0p: BOS stage 0p; cGVHD: chronic graft-versus-host disease; DLCO: diffusing capacity of the lung for carbon monoxide; FEF25–75: forced expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in the 1 s; FVC: forced vital capacity; GVHD: graft-versus-host disease; IQR: interquartile range; LCI: lung clearance index; NA: not available; PFT: pulmonary function test; PPV: positive predictive value; ROC: receiver operating characteristic; Sacin: index of acinar ventilation heterogeneity; Scond: index of ventilatory inhomogeneity in the conducting airways.
Cross-sectional data
Baseline MBW
Only four studies clearly described baseline MBW data within their cohorts [50, 56, 57, 59]. In one paediatric cohort (n=62), mean SF6-based LCI was within the normal range [56]. By contrast, in the second paediatric cohort, almost half (48%, n=11/23) had an abnormal SF6-based LCI at baseline [57]. The majority had mild LCI abnormality (z-scores 2–4), with more significant abnormality in three subjects (z-scores 5–10). In this same cohort, FEV1 was abnormal in only 13% whilst DLCO was abnormal in 70%. Among the two studies with adult subjects, mean spirometry indices were within the normal range [50, 59], with mean LCI reported as abnormal in one study (n=65) [50], and both Scond and Sacin abnormal in almost half (43%) in the other (n=23) [59]. LCI at baseline was not reported in this latter conference abstract [59].
The rate of baseline MBW abnormality varied across the identified studies. Authors felt that baseline impairment may reflect underlying disease processes (e.g. malignant disease) or treatment related to this [57, 59]. It is assumed that MBW measurement occurred prior to conditioning for HSCT, although this was not explicitly stated and may be a further contributing factor if not the case. Uhlving et al. [57] described that only a subset had other comorbidities, such as prior infection, and the degree to which adult studies were affected by this is unclear. Rates of prior smoking or vaping was not clearly described in these cohorts [59]. Factors associated with baseline MBW abnormality were not formally explored by any of these studies.
Take-home message: baseline MBW abnormality may be encountered in a substantial proportion of both children and adults, reinforcing the importance of its measurement at that time point. Future work should target better understanding of the contributing factors for baseline MBW abnormality, including, but not limited to, underlying disease process, prior treatment (e.g. chemotherapy), infection and smoking/vaping status.
Rates of MBW abnormality after HSCT
In a single cross-sectional study of 64 paediatric subjects 7 years after HSCT, MBW indices (LCI, Scond and Sacin) were all significantly more abnormal in HSCT recipients compared to 64 age- and sex-matched healthy controls [42]. Summary outcome measures for PFTs were inconsistently reported (i.e. mean versus median) and difficult to compare. However, among paediatric studies where spirometry data was reported, proportions of patients with abnormal spirometry at any time post-HSCT ranged from 9 to 25% of subjects for FEV1 [42, 43, 57, 60], 0% for FEV1/FVC [42] and 17% for FEF25–75 [60]. In contrast, MBW indices were abnormal in a greater proportion of subjects: 31–58% for LCI [42, 43, 46, 60], 52–75% for Scond [42, 60] and 25–100% for Sacin [42, 43, 60]. It is worth noting that abnormality in this context was inconsistently defined (e.g. z-score exceeding ±1.64 [43] versus z-score exceeding ±1.96 [42]).
A similar pattern was observed among adult studies. Three studies reported data at 1 year after HSCT [48, 50, 59], with mean FEV1 and FEV1/FVC within the normal range, whilst mean LCI, Sacin and Scond were abnormal. The same pattern was observed in a separate cohort 5 years after HSCT (n=225), with a higher proportion of MBW abnormality observed (74.3% for LCI, 58.6% for Sacin and 4.2% for Scond) than spirometry (36% for both FEV1 and FEV1/FVC) [44].
Studies looking at MBW outcomes following HSCT in adults and children uniformly show high rates of peripheral airways dysfunction in this population. It is unclear to what degree selection bias during recruitment may have affected results (e.g. respiratory clinic versus general HSCT clinic). The majority of studies that specified recruitment, recruited from the entire HSCT population at their centre [44, 46, 48, 50, 60], which in some instances included all HSCT recipients in the specific country [42, 57]. Clinical stability at time of testing (e.g. absence of infection) was not always stated.
Take-home message: there is a consistent suggestion within both paediatric and adult studies that cross-sectional MBW values are more frequently abnormal than spirometry post-HSCT, occurring in all nine studies that evaluated this. However, rates of abnormality varied across studies. The ability to compare studies was limited by the fact that abnormality (or equations used to define abnormality) was not consistently defined.
MBW abnormality in BOS subjects
Seven of nine studies evaluating MBW among the paediatric population described the number of pulmonary cGVHD subjects. Of these seven studies, only two (29%) clearly described MBW indices at the same visit as BOS diagnosis [54, 57] and, in both studies, consistently demonstrated significantly abnormal MBW values, with LCI abnormal in eight of nine subjects (89%) [54, 57]. Wong et al. [54] also described marked increases in Scond and Sacin in their three BOS subjects. LCI and Sacin were also significantly higher in patients with BOS 0p (versus those without BOS 0p) [54]. Rayment et al. [46], while not specifically testing at time of diagnosis, reported significantly higher LCI among subjects with a history of pulmonary cGVHD (versus those without or with extrapulmonary cGVHD only). FEV1/FVC was also significantly lower in this subgroup, with no difference in FEV1 [46]. In this cohort, an LCI of ≥9.0 provided the highest sensitivity (100%) and specificity (90%) to identify pulmonary cGVHD, with ≈1 in 3 enrolled subjects (n=8/26) having an LCI above this threshold [46]. Multiple other authors described abnormal MBW indices among BOS subjects (21 subjects in total) [55, 56, 58]. Finally, cross-sectional data from 14 paediatric BOS subjects classified as either “recovered” or “persistent” BOS reported much lower LCI values in those considered to have recovered; LCI was <8.0 in all recovered subjects versus LCI values between 12.6–20.9 with “persistent” BOS [55]. The timing of MBW relative to BOS diagnosis and treatment was not reported.
In adult subjects (n=78), De Giacomi et al. [45] reported good sensitivity (89%) and specificity (93%) for Sacin at discriminating those with and without pulmonary cGVHD, although neither timing of PFTs nor method of pulmonary cGVHD diagnosis were described.
While other studies in adults combined subjects diagnosed either clinically or through biopsy into one single group, of the ≈1 in 3 subjects with pulmonary cGVHD (n=87/225), Nyilas et al. [44] separated adult BOS (n=64) and BO subjects (n=23) into two distinct categories and compared these with subjects without cGVHD (n=79), with extrapulmonary cGVHD (n=48) and those with other pulmonary complications (n=11). LCI and Sacin were significantly more abnormal in histologically diagnosed BO subjects versus the remaining subjects, which included those with clinically diagnosed BOS and those without airway obstruction (defined as FEV1/FVC ≥0.70) [44].
Take-home message: across all studies, MBW indices were abnormal in cohorts of subjects with pulmonary cGVHD, including in those where testing was performed at time of diagnosis. The high sensitivity and specificity reported in two studies is encouraging, noting that specificity reduced at lower LCI thresholds in one study, implying that milder abnormality in LCI may either represent other pathological processes or earlier pulmonary cGVHD that does not fulfil conventional diagnostic criteria. Interestingly, MBW indices were more abnormal in histologically proven BO versus BOS.
MBW abnormality in subjects with cGVHD
Cross-sectionally, in two paediatric study cohorts where patients with pulmonary and extrapulmonary cGVHD were included in the overall cGVHD subgroup [42, 57], subjects with cGVHD had significantly higher LCI and lower FEV1 values than subjects without cGVHD [42, 57]. Scond, but not LCI or Sacin, was independently associated with cGVHD in the one cohort (n=64) of the two that investigated this association [42]. Two adult studies evaluating the subgroup with cGVHD were identified. In a small cohort (n=33), only Sacin correlated with cGVHD grade [48], where cGVHD could be both extrapulmonary or pulmonary. Nyilas et al. [44] specifically examined an extrapulmonary cGVHD subgroup (n=48) in their adult study at a median 5 years after HSCT and described more frequent abnormal MBW indices (76% for LCI and 57% for Sacin) compared to spirometry (21% abnormal FEV1), with the MBW abnormality less marked than observed in subjects with BO/BOS. Subjects with apparent extrapulmonary cGVHD may in fact have a subclinical pulmonary component that does not fulfil formal spirometry-dependent NIH criteria.
Take-home message: although those with and without pulmonary cGVHD are often grouped together, MBW indices appear to be abnormal in a significant proportion of subjects with cGVHD, irrespective of whether pulmonary cGVHD has been diagnosed.
Changes observed in longitudinal data
Change in MBW indices over time among all HSCT recipients
Paediatric longitudinal data is limited to three studies. In one larger cohort (n=62), no significant change in mean SF6-based LCI from baseline to 1 year after HSCT was observed [56]. This cohort included three subjects who developed pulmonary cGVHD within that first year and one who developed the complication after 3 years. Similarly, in a smaller cohort (n=28), average SF6-based LCI z-score did not change over the course of the first year following HSCT as compared to baseline [57]. Five children in this cohort were diagnosed with pre-HSCT pulmonary comorbidities and six children developed pulmonary cGVHD within the first year post-HSCT. Lastly, in a cohort of 17 subjects, LCI z-score did not significantly change between the first (median 4.8 years after HSCT) and second (1.2 years later) time point of assessment [47]. Respiratory symptoms as measured through the Liverpool Respiratory Questionnaire remained stable over this time in the overall cohort, but further clinical data for individual subjects, particularly the development of pulmonary cGVHD, is unavailable. Wong et al. [54] did not report changes occurring in MBW indices for the entire cohort in their longitudinal study.
The change in MBW indices over time after HSCT was evaluated in three adult cohorts. Compared to baseline (i.e. pre-HSCT), FEV1, LCI and Scond values did not significantly worsen over the first year in two cohorts [49, 50, 59]. In the smaller of these two cohorts, Sacin did not change in 23 subjects, two of whom developed BOS within this first year [59]. In contrast, Sacin increased significantly by 42% in a larger cohort of 65 subjects, for which clinical data was unavailable [50]. Beyond the first year post-HSCT, at a median 10 months later (i.e. 22 months after HSCT), Lahzami et al. [48] found that both LCI and Sacin significantly worsened in 22 subjects, whereas FEV1 did not. Clinical data for these subjects within this time period was unavailable. Within their total cohort of 33 subjects, eight of whom developed BOS prior to testing (median 12 months (range: 3–73 months) post-HSCT), both FEV1 and Sacin were independently associated with time post-HSCT, after adjusting for age and smoking status.
It appeared that, within these small cohorts, the average LCI remained relatively stable in the first year in the overall population of HSCT recipients, whereas Sacin may more commonly deteriorate within this timeframe. This may represent early development of pulmonary cGVHD in the most peripheral airways, detectable by a greater number of MBW indices beyond the first year. In those surviving the initial years, it may be that greater pulmonary stability is present. It would be important in future work to assess the trajectory of MBW indices for individual subjects, to define the minimally important difference for MBW indices and report the number and characteristics of subjects with a clinically important change in MBW indices, rather than average values for the overall cohort only, as these provide limited information.
Take-home message: change in MBW indices over time in the overall post-HSCT population has been studied in few relatively small cohorts only. These data suggest that deterioration may occur and be detectable with the right index as early as the first year. Sacin may be the most sensitive index to detect this. It is currently unclear to what extent peripheral lung function deteriorates over the first 5 years following HSCT, the time during which subjects are at greatest risk of developing pulmonary cGVHD.
Rate and amount of MBW change in pulmonary cGVHD and cGVHD subjects
The presence of abnormal MBW values prior to HSCT in subjects who subsequently developed pulmonary cGVHD was inconsistent across populations. SF6-based LCI was abnormal at baseline in two of four BOS subjects with baseline values available among a total of six BOS subjects in one paediatric cohort [57], whereas average SF6-based LCI (individual values not provided) was normal in four BOS subjects in another paediatric cohort [56]. Despite a high rate of baseline LCI abnormality, Uhlving et al. [57] did not find any predictive value of pre-HSCT LCI or in the 3 months post-HSCT LCI value. It may be that the trajectory of MBW indices is of greater importance; Kavouridou et al. [56] reported that, among 62 children, all subjects with an increase in LCI of ≥1 unit from baseline to 1 year post-HSCT either developed BOS or experienced repeated airways infections. In contrast, none of the subjects in this cohort with an increase <1 unit developed BOS.
Within one paediatric cohort (n=24), in the three BOS subjects, MBW indices deteriorated earlier than the time point at which NIH diagnostic criteria were met in the two subjects where MBW data was available prior to diagnosis [54]. In the remaining subject, BOS was diagnosed at the first follow-up point post-HSCT, so an MBW deterioration may have been missed. In adults, Schoeffel et al. [49] did not observe abnormal MBW values at test occasions immediately prior to BOS diagnosis for their three BOS subjects. However, this may reflect a much longer interval between that prior test occasion and BOS diagnosis (range 265–365 days) compared to the monthly testing in the protocol of Wong et al. [54]. As BOS may potentially develop in a short timeframe, more frequent testing may be required to assess whether MBW demonstrates abnormality earlier than spirometry in this population.
In one paediatric cohort (n=24), both LCI and Sacin deteriorated significantly more among cGVHD subjects than those without cGVHD over the first 1–3 years post-HSCT [54], although it is unclear whether this subgroup included those with pulmonary cGVHD. In an adult cohort (n=22), change in Sacin over 10 months was associated with change in cGVHD grade [48].
Take-home message: while baseline MBW may not be predictive of BOS development, earlier deterioration in MBW indices may occur and in fact be predictive of subsequent BOS development. Rate of decline in those with cGVHD may be greater than in subjects without cGVHD.
Oscillometry
The findings of the eight studies evaluating oscillometry among HSCT recipients are summarised in table 3. Two studies were performed in children, with six studies evaluating adult subjects.
TABLE 3.
Summary of findings in eight individual studies reporting on oscillometry after haematopoietic stem cell transplant (HSCT)
| Cohort | Summary of findings | |
| Spirometry | Oscillometry | |
| Paediatric subjects | ||
| Jayasuriya et al. [47, 60, 71, 77, 78] | FEV1 was abnormal in 5, FEF25–75 in 4 | Median Rrs at 6 Hz 6.73 (range: 3.05–8.00) and Xrs −2.10 (range: −1.13–2.71) |
| Wong et al. [54], Hardaker et al. [80–82] and Robinson et al. [83] | cGHVD was associated with significant deterioration in FEV1 and FEF25–75 compared to those without cGVHD At 3 years post-HSCT, BOS 0p subjects (n=8) had FEV1 worse than subjects unaffected by BOS 0p/BOS that approached significance |
cGVHD: significant increases in Xrs at 5 Hz, AX and Fres as compared to subjects without cGVHD BOS was associated with significant abnormality in Xrs at 5 Hz, AX and Fres; oscillometry abnormality was not detected prior to BOS diagnosis BOS 0p (n=8) subjects at 3 years post-HSCT significantly worse Xrs at 5 Hz as compared to subjects unaffected by BOS 0p/BOS and this approached significance for Fres |
| Adult subjects | ||
| De Giacomi et al. [45, 86, 87] | NA | Subjects with BO: mean±sd Xrs at 5 Hz was −1.84±2.26 and significantly worse than in controls (p=0.023) |
| Blin et al. [52] | NA | Xrs at 5 Hz, AX, Fres and R5-R20 were significantly worsened at time of BOS diagnosis as compared to first test; these indices did not change significantly earlier than FEV1 |
| Lahzami et al. [48, 88] and Pechey et al. [89] | Initial testing (mean±sd or median (range)): FEV1 was 87±20% pred, FEV1/FVC 80 (45–89); FEV1 was independently associated with time post-HSCT Follow-up: no significant change in spirometry outcomes |
Mean±sd or median (range): Rrs at 6 Hz 118±31% pred and Xrs at 6 Hz 153 (9–997)% pred Follow-up: median (range) Rrs was 103% pred (73–259) and Xrs was 172% pred (47–788) There was no significant change from initial testing |
| Barisione et al. 2012 [53, 91] | Baseline: spirometry and oscillometry were within normal range; mean±sd FEV1 was 3.67±0.74 L, FEV1/FVC was 0.81±0.06 Follow-up: there was no significant change in FEV1 or FEV1/FVC, which were mean±sd 3.54±0.75 L and mean±sd 0.81±0.15, respectively |
Baseline: oscillometry was within normal range, Xrs at 5 Hz was −0.50±0.29 Follow-up: Xrs at 5 Hz became less negative (p=0.01) and was mean±sd −0.43±0.28 |
| Schoeffel et al. [49, 59] | Baseline: mean±sd FEV1 was 105±13% pred, FEV1/FVC was normal Follow-up: FVC declined |
Baseline: Rrs and Xrs at 6 Hz were normal in 91% Follow-up: no changes in Rrs and Xrs at 6 Hz BOS subjects: Rrs and Xrs at 6 Hz were all abnormal at time of BOS diagnosis; respiratory function at the visit prior to diagnosis was not different from baseline |
| Rutting et al. [51, 92] | Mean±sd FEV1/FVC ratio was 0.78±0.07 | The median (IQR) within-session sd for Rrs and Xrs were 0.19 (0.10–0.40) and 0.11 (0.04–0.15), respectively; the median (IQR) between-visit sd for Rrs and Xrs were 0.26 (0.17–0.39) and 0.16 (0.10–0.26), respectively; within- and between-visit variability was greater than among healthy controls, but less than among COPD and asthma patients; worse FEV1/FVC was correlated with increased within- and between-visit variability; no absolute values of resistance or reactance are provided |
AX: area under the reactance curve; BO: bronchiolitis obliterans; BOS: bronchiolitis obliterans syndrome; BOS 0p: BOS stage 0p; cGVHD: chronic graft-versus-host disease; FEF25–75: forced expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in the 1 s; Fres: resonant frequency; FVC: forced vital capacity; IQR: interquartile range; NA: not available; R5-R20: frequency dependence of resistance; Rrs: respiratory system resistance; Xrs: respiratory system reactance.
Cross-sectional data
Baseline oscillometry
One study evaluating oscillometry in children prior to HSCT was identified describing normal baseline values, but this study included subjects receiving autologous or allogeneic HSCT and, therefore, this study did not meet our inclusion criteria [65]. There were no other paediatric studies describing baseline oscillometry. Among adults, baseline oscillometry appeared to be normal in the majority of subjects. Schoeffel et al. [59] reported normal Rrs and Xrs at 6 Hz in 21 of 23 subjects (91%). Barisione et al. [53] reported mean Rrs at 5 Hz to be within the normal range for 26 adult subjects, but did not report the proportion with abnormality.
Rates of abnormality after HSCT
Across the whole cohort, at a median 1 year after HSCT, Lahzami et al. [48] found that oscillometry indices were not associated with time post-HSCT among 33 adult subjects. Xrs correlated with percent predicted FEV1. In a cross-sectional comparison of 78 adult HSCT recipients at an unknown time after HSCT and 41 healthy controls, De Giacomi et al. [45] reported significantly higher Rrs 5–20 Hz, Rrs and Xrs at 5 Hz in HSCT subjects versus healthy controls.
Oscillometry abnormality in pulmonary cGVHD subjects
One study of 24 paediatric subjects found that among children diagnosed with BOS 0p (n=8), Xrs was significantly worse at 3 years after HSCT than among those without a deterioration in FEV1 over time (i.e. <10% change from baseline) [54]. Blin et al. [52] suggested that this abnormality may be present at the time of BOS 0p diagnosis; they reported significantly worse Rrs 5–20 Hz, Xrs at 5 Hz, AX and Fres in nine of 68 adult subjects who developed BOS (defined using modified criteria to include a 10% decline in FEV1, equivalent to BOS 0p), compared to baseline values. Schoeffel et al. [49] reported highly variable changes in Rrs and Xrs from baseline to time of BOS diagnosis among three subjects with BOS; Rrs increased 1–44% relative to baseline, whilst Xrs became more negative by 21–846%.
Take-home message: Oscillometry appears to be within normal range in the majority at baseline and, while limited information is available, appears generally to remain normal following HSCT. However, among those with diagnosed BOS, oscillometry indices are frequently abnormal and abnormality may be detectable in BOS 0p.
Longitudinal data
Change in oscillometry indices over time among all HSCT recipients
Whilst longitudinal oscillometry data was discussed in one paediatric study, changes occurring across the entire cohort were not reported for the 3-year period of follow-up [54]. Stability in Rrs and Xrs was reported in 22 adults, initially assessed a median 1 year after HSCT, over a median duration of 10 months afterwards [48]. Similarly, stability was observed at 100 days and 12 months after HSCT compared to baseline in a separate cohort of 23 adult subjects [59].
In contrast, Barisione et al. [53]evaluated 26 adult subjects without BOS and found that Rrs at 5 Hz significantly decreased and Xrs at 5 Hz significantly increased (i.e. became less negative) at 2 months after HSCT compared to baseline. Changes over time need to be interpreted within the context of known variability. Specific to this setting, Rutting et al. [51] demonstrated that among 16 HSCT recipients without airflow obstruction as measured by spirometry, within- and between-visit variability was greater than among healthy controls. The subsequent full publication of this work, however, included 23 clinically stable HSCT recipients, and in this population, between-visit variability of oscillometry indices was similar to the healthy control group. Within-visit varaibility was not reported [66].
Rate and amount of oscillometry change in pulmonary cGVHD and cGVHD subjects
In the Wong et al. [54] paediatric cohort there were two subjects for whom measurements were available prior to the visit at which BOS was diagnosed. Both demonstrated oscillometric abnormality earlier than meeting NIH criteria. A deterioration of oscillometry prior to BOS development was not observed among the three of 23 adult subjects developing BOS in the Schoeffel et al. [49] cohort. In the Blin et al. [52] cohort (n=68), with measurements performed every 3 months, the oscillometry deterioration did not occur at an earlier time than spirometric deterioration in their BOS 0p subjects.
Only one study evaluated repeated oscillometry measurements in cGVHD subjects. In a small study of 24 children, compared to pre-HSCT values, both Fres and AX decreased significantly more after HSCT among subjects with cGVHD (n=4) than those without cGVHD (n=20) [54]. It was not reported whether the cGVHD group included those with pulmonary cGVHD or only those with extrapulmonary cGVHD. Data informing minimal clinically important difference is emerging for oscillometry indices. Within- and between-session variability has been described for both health and disease [67–70], including initial adult data specific to this setting of allogeneic HSCT [66].
Take-home message: limited data is available regarding change in oscillometric indices over time. Indices may remain stable in the overall post-HSCT population, but may deteriorate among subjects developing pulmonary cGVHD and potentially those with extrapulmonary cGVHD. The timing of deterioration is presently unclear but may be prior to NIH diagnosis.
Direct comparison of MBW to oscillometry within studies
Only five studies performed MBW and oscillometry concurrently. In the paediatric population, Wong et al. [54] found significant abnormality in both peripheral function tests among subjects with cGVHD, BOS and BOS 0p, whereas Jayasuriya et al. [71] found abnormality in both among five post-HSCT subjects with pulmonary cGVHD. Comparison of the magnitude of abnormality or utility of these tests was not performed in either abstract. In the adult population, at baseline, MBW indices appeared more frequently abnormal than oscillometry indices among one cohort of 23 subjects [59]. BO subjects in another cohort (n=38) demonstrated abnormal indices for both MBW and oscillometry [45]. However, Lahzami et al. [48] concluded that there may be limited utility for oscillometry in this population as, while abnormal, indices were not correlated with time after HSCT or clinical parameters. Conversely, MBW indices and, given its association with time after HSCT and cGVHD grade, particularly Sacin, were found to be promising for pulmonary monitoring after HSCT [48].
Conclusion
As demonstrated in this scoping review, due to the small sample size in the majority of studies, firm conclusions regarding the utility of MBW and oscillometry after HSCT cannot be drawn. Given substantial methodological and reporting differences between studies, it was not possible to perform a meta-analysis based on aggregate data. However, we found that MBW demonstrated a peripheral airway abnormality in a substantial proportion of patients prior to HSCT, although the proportions varied between studies. In both children and adults, MBW indices post-HSCT were more frequently abnormal than spirometry when reporting group data and among those with pulmonary cGVHD (typically defined as BOS). Data regarding the change in MBW indices over time was sparse but may be more indicative of pulmonary complications than absolute values at any given time point. Oscillometry indices were often normal at baseline, but frequently abnormal in those who develop pulmonary cGVHD. Fewer data were available regarding the rates of deterioration of oscillometry indices among the post-HSCT population overall. Given the fact that diagnosis based on current NIH criteria is often made when obstructive airways disease has already progressed substantially and the considerable morbidity and mortality associated with pulmonary cGVHD, peripheral airway function tests such as MBW and oscillometry hold promise for early identification of peripheral airway dysfunction in HSCT recipients.
Implications for future research
Important research questions to be answered are summarised in table 4 and may guide the design of both retrospective and prospective studies. Pooling currently available individual participant data across these various studies may improve our ability to formally compare their respective sensitivity and specificity at specific time points (e.g. diagnosis and 1, 2 and 3 years post-HSCT) and assess the trajectory of MBW and oscillometry indices over time. Prospective multicentre studies are urgently required to clarify whether these peripheral airway function tools identify pulmonary cGVHD better and earlier than spirometry, and whether earlier intervention based on this monitoring approach improves overall outcomes in this at-risk population.
TABLE 4.
Take-home messages and questions for future research regarding peripheral pulmonary function tests after haematopoietic stem cell transplantation (HSCT)
| Take-home messages | Questions for future work |
| MBW | |
| 1) MBW at baseline | |
| Baseline MBW abnormality may be encountered in a substantial proportion of both children and adults, reinforcing the importance of its measurement at that time point. Future work should target better understanding of the contributing factors for this, including, but not limited to, the underlying disease process, prior treatment (e.g. chemotherapy), infection and smoking/vaping status. | • What proportion of paediatric and adult subjects have abnormality at baseline in MBW, in comparison to spirometry? • What are the risk factors for baseline abnormality in MBW and how do they compare to those for spirometry? Could they be related to oncological treatment, conditioning regimen (although all MBW most likely performed prior to conditioning) or nontreatment related insults that occurred prior to transplantation? • Are any of these risk factors modifiable? • Does the presence of peripheral airway abnormality at baseline put patients at risk of developing pulmonary cGVHD (or is the development of pulmonary cGVHD independent of prior pulmonary status)? • Does the presence of baseline peripheral airway abnormality put patients who develop pulmonary cGVHD at higher risk of adverse outcomes? |
| 2) Rate of MBW abnormality after HSCT | |
| There is a consistent suggestion within the studies to date in both paediatric and adult cohorts, that cross-sectional MBW values are more frequently abnormal than spirometry post-HSCT, occurring in all nine studies that evaluated this. Rates of abnormality were relatively variable between studies. | • How does selection bias affect the rates of abnormality reported? • Is abnormality in MBW indices associated with increased risk of current/subsequent pulmonary cGVHD? • What is the clinical relevance of a single abnormal value in an MBW index post-HSCT and is this affected by the magnitude of abnormality? Investigation of what drives the abnormality and how specific it is for pulmonary cGVHD is important in future work. |
| 3) Rate of MBW abnormality after HSCT in BOS subjects | |
| Across all studies, MBW indices were abnormal in cohorts of subjects with pulmonary cGVHD, including in those where testing was performed at the time of diagnosis. The strong sensitivity and specificity reported in two studies was encouraging, noting that specificity reduced at lower LCI thresholds in one study, implying that milder abnormality in LCI may either represent other pathological processes or earlier pulmonary cGVHD that does not fulfil conventional diagnostic criteria. Interestingly, MBW indices were more abnormal in histologically proved BO versus BOS. | • Does incorporating MBW into standardised criteria improve our ability to diagnose BO/BOS? • What is the optimal threshold for pulmonary cGVHD diagnosis? • What is the effect of treatment for pulmonary cGVHD on MBW indices? • What is/are the underlying disease process(es) leading to peripheral pulmonary function abnormality? |
| 4) Rate of MBW abnormality after HSCT in cGVHD subjects | |
| Although those with and without pulmonary cGVHD are often grouped together, MBW indices appear to be abnormal in a significant proportion of subjects with cGVHD. Additionally, the results of the one study specifically investigating whether those without apparent extrapulmonary cGVHD had a detectable level of pulmonary function impairment suggests this is the case. | • What proportion of subjects with cGVHD have evidence of pulmonary involvement based on MBW that does not fulfil current NIH criteria? • How specific is MBW in that setting and to what degree do non-cGVHD pulmonary complications also elevate MBW? |
| 5) Changes in MBW indices over time in post-HSCT population | |
| The change in MBW indices over time in the overall post-HSCT population has been studied in small cohorts only. These data suggest that deterioration may occur and be detectable with the right index as early as the first year. Sacin may be the most sensitive index to detect this. It is currently unclear to which extent the peripheral lung function deteriorates over the first 5 years following HSCT, the time during which subjects are at greatest risk of developing pulmonary cGVHD. | • What is the natural trajectory of MBW indices among the overall post-HSCT population over the first 5 years following HSCT (the time during which subjects are at greatest risk of developing pulmonary cGVHD)? • Is Sacin the most sensitive index to detect pulmonary cGVHD? • What is the prognostic value of a change in MBW indices? |
| 6) Changes in MBW indices over time in BOS and cGVHD subjects | |
| While it appears that baseline MBW may not be predictive of BOS development, earlier deterioration in MBW indices may occur and in fact be predictive of subsequent BOS development. Rate of decline in those with cGVHD appears greater than in subjects without cGVHD. | • What is a clinically important change (or MCID) in MBW indices in this population? • Does a clinically important change in MBW indices occur prior to a change in spirometry or the fulfilment of NIH BOS diagnostic criteria? • How frequently should MBW monitoring occur post-HSCT? • Among patients with extrapulmonary cGVHD, is a deterioration of MBW indices associated with subsequent NIH BOS diagnosis? |
| Oscillometry | |
| 1) Cross-sectional measurements of oscillometry prior to and following HSCT | |
| Oscillometry appears to be within normal range in the majority at baseline and, while limited information is available, appears generally to remain normal following HSCT. However, among those who develop BOS, oscillometry indices are frequently abnormal and abnormality may be detectable in BOS 0p. | • What proportion of paediatric and adult subjects have abnormality at baseline in oscillometry, in comparison to spirometry and MBW? What are risk factors for baseline oscillometric abnormality? • Is abnormality in oscillometric indices associated with increased risk of current/subsequent pulmonary cGVHD? |
| 2) Change in oscillometry indices over time | |
| Limited data is available regarding change in oscillometric indices over time. Indices may not deteriorate in the overall post-HSCT population, but likely do among subjects developing pulmonary cGVHD and potentially those with extrapulmonary cGVHD. The timing of this deterioration is presently unclear but may be prior to NIH diagnosis. | • What is the trajectory of oscillometry after HSCT in the overall population? • What is a clinically important change (or MCID) in oscillometric indices after HSCT? • Do oscillometric indices change prior to spirometric and/or MBW indices among subjects who subsequently develop BOS according to NIH criteria? |
BO: bronchiolitis obliterans; BOS: bronchiolitis obliterans syndrome; BOS 0p: BOS stage 0p; cGVHD: chronic graft-versus-host disease; LCI: lung clearance index; MBW: multiple breath washout; MCID: minimal clinically important difference; NIH: National Institutes of Health; Sacin: index of acinar ventilation heterogeneity.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0251-2022.SUPPLEMENT (159.5KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: N. Sonneveld has nothing to disclose.
Conflict of interest: J.H. Rayment has nothing to disclose.
Conflict of interest: J. Usemann has nothing to disclose.
Conflict of interest: K.G. Nielsen has nothing to disclose.
Conflict of interest: P.D. Robinson has nothing to disclose.
References
- 1.Gower WA, Collaco JM, Mogayzel PJ,Jr. Pulmonary dysfunction in pediatric hematopoietic stem cell transplant patients: non-infectious and long-term complications. Pediatr Blood Cancer 2007; 49: 225–233. doi: 10.1002/pbc.21060 [DOI] [PubMed] [Google Scholar]
- 2.Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol 1995; 26: 668–675. doi: 10.1016/0046-8177(95)90174-4 [DOI] [PubMed] [Google Scholar]
- 3.Gower WA, Collaco JM, Mogayzel PJ,Jr. Lung function and late pulmonary complications among survivors of hematopoietic stem cell transplantation during childhood. Paediatr Respir Rev 2010; 11: 115–122. doi: 10.1016/j.prrv.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17: 1072–1078. doi: 10.1016/j.bbmt.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland HK, Wingard JR, Beschorner WE, et al. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood 1988; 72: 621–627. doi: 10.1182/blood.V72.2.621.621 [DOI] [PubMed] [Google Scholar]
- 6.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003; 168: 208–214. doi: 10.1164/rccm.200212-1468OC [DOI] [PubMed] [Google Scholar]
- 7.Cuvelier GDE, Nemecek ER, Wahlstrom JT, et al. Benefits and challenges with diagnosing chronic and late acute GVHD in children using the NIH consensus criteria. Blood 2019; 134: 304–316. doi: 10.1182/blood.2019000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudek AZ, Mahaseth H, DeFor TE, et al. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003; 9: 657–666. doi: 10.1016/S1083-8791(03)00242-8 [DOI] [PubMed] [Google Scholar]
- 9.Marras TK, Chan CK, Lipton JH, et al. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplant 2004; 33: 509–517. doi: 10.1038/sj.bmt.1704377 [DOI] [PubMed] [Google Scholar]
- 10.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21: 389–401.e1. doi: 10.1016/j.bbmt.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 2014; 44: 1479–1503. doi: 10.1183/09031936.00107514 [DOI] [PubMed] [Google Scholar]
- 12.Brown R, Woolcock AJ, Vincent NJ, et al. Physiological effects of experimental airway obstruction with beads. J Appl Physiol 1969; 27: 328–335. doi: 10.1152/jappl.1969.27.3.328 [DOI] [PubMed] [Google Scholar]
- 13.McNamara JJ, Castile RG, Glass GM, et al. Heterogeneous lung emptying during forced expiration. J Appl Physiol 1987; 63: 1648–1657. doi: 10.1152/jappl.1987.63.4.1648 [DOI] [PubMed] [Google Scholar]
- 14.Bergeron A. Late-onset noninfectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Clin Chest Med 2017; 38: 249–262. doi: 10.1016/j.ccm.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 15.Abedin S, Yanik GA, Braun T, et al. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol Blood Marrow Transplant 2015; 21: 1127–1131. doi: 10.1016/j.bbmt.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon J-S, Chun YH, Lee JW, et al. Value of screening spirometry for early diagnosis of bronchiolitis obliterans syndrome in children after allogeneic hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2015; 37: e462-7. doi: 10.1097/MPH.0000000000000421 [DOI] [PubMed] [Google Scholar]
- 17.Shanthikumar S, Gower WA, Abts M, et al. Pulmonary surveillance in pediatric hematopoietic stem cell transplant: a multinational multidisciplinary survey. Cancer Rep 2021; 5: e1501. doi: 10.1002/cnr2.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurora P, Stocks J, Oliver C, et al. Quality control for spirometry in preschool children with and without lung disease. Am J Respir Crit Care Med 2004; 169: 1152–1159. doi: 10.1164/rccm.200310-1453OC [DOI] [PubMed] [Google Scholar]
- 19.Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19: 973–980. doi: 10.1016/j.bbmt.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamburro RF, Cooke KR, Davies SM, et al. Pulmonary complications of pediatric hematopoietic cell transplantation. A National Institutes of Health workshop summary. Ann Am Thorac Soc 2021; 18: 381–394. doi: 10.1513/AnnalsATS.202001-006OT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitko CL, Pidala J, Schoemans HM, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group report. Transplant Cell Ther 2021; 27: 545–557. doi: 10.1016/j.jtct.2021.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff D, Radojcic V, Lafyatis R, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 highly morbid forms report. Transplant Cell Ther 2021; 27: 817–835. doi: 10.1016/j.jtct.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson PD, Goldman MD, Gustafsson PM. Inert gas washout: theoretical background and clinical utility in respiratory disease. Respiration 2009; 78: 339–355. doi: 10.1159/000225373 [DOI] [PubMed] [Google Scholar]
- 24.Verbanck S, Schuermans D, Van Muylem A, et al. Ventilation distribution during histamine provocation. J Appl Physiol 1997; 83: 1907–1916. doi: 10.1152/jappl.1997.83.6.1907 [DOI] [PubMed] [Google Scholar]
- 25.Bayfield KJ, Horsley A, Alton E, et al. Simultaneous sulfur hexafluoride and nitrogen multiple-breath washout (MBW) to examine inherent differences in MBW outcomes. ERJ Open Res 2019; 5: 00234-2018. doi: 10.1183/23120541.00234-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahl M, Joachim C, Wielpütz MO, et al. Comparison of lung clearance index determined by washout of N2 and SF6 in infants and preschool children with cystic fibrosis. J Cyst Fibros 2019; 18: 399–406. doi: 10.1016/j.jcf.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Jensen R, Stanojevic S, Gibney K, et al. Multiple breath nitrogen washout: a feasible alternative to mass spectrometry. PLoS One 2013; 8: e56868. doi: 10.1371/journal.pone.0056868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson PD, Latzin P, Verbanck S, et al. ERS/ATS consensus statement for inert gas washout measurement using multiple and single breath tests. Eur Respir J 2013; 41: 507–522. doi: 10.1183/09031936.00069712 [DOI] [PubMed] [Google Scholar]
- 29.Stahl M, Joachim C, Blessing K, et al. Multiple breath washout is feasible in the clinical setting and detects abnormal lung function in infants and young children with cystic fibrosis. Respiration 2014; 87: 357–363. doi: 10.1159/000357075 [DOI] [PubMed] [Google Scholar]
- 30.Stahl M, Joachim C, Kirsch I, et al. Multicentre feasibility of multiple-breath washout in preschool children with cystic fibrosis and other lung diseases. ERJ Open Res 2020; 6: 00408-2020. doi: 10.1183/23120541.00408-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratjen F, Davis SD, Stanojevic S, et al. Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2019; 7: 802–809. doi: 10.1016/S2213-2600(19)30187-0 [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson PM, De Jong PA, Tiddens HA, et al. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 2008; 63: 129–134. doi: 10.1136/thx.2007.077784 [DOI] [PubMed] [Google Scholar]
- 33.Kinghorn B, McNamara S, Genatossio A, et al. Comparison of multiple breath washout and spirometry in children with primary ciliary dyskinesia and cystic fibrosis and healthy controls. Ann Am Thorac Soc 2020; 17: 1085–1093. doi: 10.1513/AnnalsATS.201905-375OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björn L, Erik M, Per T, et al. Agreement between spirometry and impulse oscillometry for lung function assessment in 6-year-old children born extremely preterm and at term. Pediatr Pulmonol 2020; 55: 2745–2753. doi: 10.1002/ppul.24976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AlBlooshi A, AlKalbani A, Narchi H, et al. Respiratory function in healthy Emirati children using forced oscillations. Pediatr Pulmonol 2018; 53: 936–941. doi: 10.1002/ppul.23985 [DOI] [PubMed] [Google Scholar]
- 36.DuBois AB, Brody AW, Lewis DH, et al. Oscillation mechanics of lungs and chest in man. J Appl Physiol 1956; 8: 587–594. doi: 10.1152/jappl.1956.8.6.587 [DOI] [PubMed] [Google Scholar]
- 37.Eddy RL, Westcott A, Maksym GN, et al. Oscillometry and pulmonary magnetic resonance imaging in asthma and COPD. Physiol Rep 2019; 7: e13955. doi: 10.14814/phy2.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho E, Wu JKY, Birriel DC, et al. Airway oscillometry detects spirometric-silent episodes of acute cellular rejection. Am J Respir Crit Care Med 2020; 201: 1536–1544. doi: 10.1164/rccm.201908-1539OC [DOI] [PubMed] [Google Scholar]
- 39.Kattan M, Bacharier LB, O'Connor GT, et al. Spirometry and impulse oscillometry in preschool children: acceptability and relationship to maternal smoking in pregnancy. J Allergy Clin Immunol Pract 2018; 6: 1596–603.e6. doi: 10.1016/j.jaip.2017.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faria AC, Lopes AJ, Jansen JM, et al. Evaluating the forced oscillation technique in the detection of early smoking-induced respiratory changes. Biomed Eng Online 2009; 8: 22. doi: 10.1186/1475-925X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dandurand RJ, Lavoie JP, Lands LC, et al. Oscillometry harmonisation study G. Comparison of oscillometry devices using active mechanical test loads. ERJ Open Res 2019; 5: 00160-2019. doi: 10.1183/23120541.00160-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlving HH, Mathiesen S, Buchvald F, et al. Small airways dysfunction in long-term survivors of pediatric stem cell transplantation. Pediatr Pulmonol 2015; 50: 704–712. doi: 10.1002/ppul.23058 [DOI] [PubMed] [Google Scholar]
- 43.Schindera C, Usemann J, Zuercher SJ, et al. Pulmonary dysfunction after treatment for childhood cancer comparing multiple-breath washout with spirometry. Ann Am Thorac Soc 2021; 18: 281–289. doi: 10.1513/AnnalsATS.202003-211OC [DOI] [PubMed] [Google Scholar]
- 44.Nyilas S, Baumeler L, Tamm M, et al. Inert gas washout in bronchiolitis obliterans following hematopoietic cell transplantation. Chest 2018; 154: 157–168. doi: 10.1016/j.chest.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 45.De Giacomi F, Lupo-Stanghellini MT, Orsini A, et al. Acinar ventilation heterogeneity (Sacin) assessed by nitrogen multiple breath washout is an accurate and sensitive marker of bronchiolitis obliterans (BO). Eur Respir J 2014; 44: Suppl. 58, P3320. [Google Scholar]
- 46.Rayment JH, Sandoval RA, Roden JP, et al. Multiple breath washout testing to identify pulmonary chronic graft versus host disease in children after hematopoietic stem cell transplantation. Transplant Cell Ther 2022; 328: e1-328.e7. doi: 10.1016/j.jtct.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Jayasuriya R, King G, Selvadurai H, et al. Peripheral airway function over time in children post bone marrow transplantation. Eur Respir J 2016; 48: Suppl. 60, PA365. doi: 10.1183/13993003.congress-2016.PA365 [DOI] [Google Scholar]
- 48.Lahzami S, Schoeffel RE, Pechey V, et al. Small airways function declines after allogeneic haematopoietic stem cell transplantation. Eur Respir J 2011; 38: 1180–1188. doi: 10.1183/09031936.00018311 [DOI] [PubMed] [Google Scholar]
- 49.Schoeffel R, Htun C, Taylor J, et al. Airway function in patients with bronchiolitis obliterans following allogeneic hematopoietic stem cell transplantation (ALLO-HSCT). Respirology 2014; 19: 11. doi: 10.1111/resp.12416_4 [DOI] [Google Scholar]
- 50.Htun C, Schoeffel R, Lahzami S, et al. Changes in peripheral airway function at 12 months post allogeneic haematopoietic stem cell transplantation (ALLO-HSCT). Respirology 2017; 22: Suppl. 2, 173. [Google Scholar]
- 51.Rutting S, Roche N, Wallis R, et al. Long-term variability in oscillatory impedance in stable obstructive airways diseases. Am J Respir Crit Care Med 2020; 201: A2982. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2982 [DOI] [PubMed] [Google Scholar]
- 52.Blin E, Perez T, Ramon P, et al. Impulse oscillometry for the diagnosis of bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2020; 201: A2990. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2990 [DOI] [Google Scholar]
- 53.Barisione G, Pompilio PP, Bacigalupo A, et al. Airway distensibility with lung inflation after allogeneic haematopoietic stem-cell transplantation. Respir Physiol Neurobiol 2012; 184: 80–85. doi: 10.1016/j.resp.2012.07.021 [DOI] [PubMed] [Google Scholar]
- 54.Wong A, Hardaker KM, Jayasuriya G, et al. Longitudinal utility of peripheral airway function tests in pulmonary graft versus host disease in pediatric bone marrow transplant patients. Am J Respir Crit Care Med 2019; 207: A5460. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A5460 [DOI] [Google Scholar]
- 55.Walther S, Rettinger E, Maurer HM, et al. Long-term pulmonary function testing in pediatric bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Pediatr Pulmonol 2020; 55: 1725–1735. doi: 10.1002/ppul.24801 [DOI] [PubMed] [Google Scholar]
- 56.Kavouridou C, Mellgren K, Lindblad A, et al. Multiple breath washout can facilitate the diagnosis of lung graft-versus-host disease in children after allogeneic hematopoietic stem cell transplantation. Eur Respir J 2016; 48: Suppl. 60, OA4554. doi: 10.1183/13993003.congress-2016.OA4554 [DOI] [Google Scholar]
- 57.Uhlving HH, Skov L, Buchvald F, et al. Lung clearance index for early detection of pulmonary complications after allo-HSCT in children. Pediatr Pulmonol 2019; 54: 1029–1038. doi: 10.1002/ppul.24340 [DOI] [PubMed] [Google Scholar]
- 58.Khalid Y, Mellgren K, Fasth A, et al. Obliterative bronchiolitis after stem cell transplantation. Inert gas washout makes early diagnosis of this serious complication possible. Lakartidningen 2007; 104: 3373–3376. [PubMed] [Google Scholar]
- 59.Schoeffel RE, Taylor J, Lazamir S, et al. Respiratory function remains stable up to one year following haematopoetic stem cell transplantation (allo-HSCT). Respirology 2012; 17: 8. [Google Scholar]
- 60.Jayasuriya G, King G, Selvadurai H, et al. Peripheral airway abnormalities are common in children post bone marrow transplantation. Eur Respir J 2015; 46: Suppl. 59, PA1269. doi: 10.1183/13993003.congress-2015.PA1269 [DOI] [Google Scholar]
- 61.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 2008; 177: 253–260. doi: 10.1164/rccm.200708-1248OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 6: 5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 64.Hagiwara S, Mochizuki H, Muramatsu R, et al. Reference values for Japanese children's respiratory resistance using the LMS method. Allergol Int 2014; 63: 113–119. doi: 10.2332/allergolint.13-OA-0591 [DOI] [PubMed] [Google Scholar]
- 65.Shackleton C, Ramachandran S, Fraser C, et al. Respiratory impedance is normal in children prior to transplantion for childhood cancers. Respirology 2012; 17: 8. [Google Scholar]
- 66.Rutting S, Badal T, Wallis R, et al. Long-term variability of oscillatory impedance in stable obstructive airways disease. Eur Respir J 2021; 58: 2004318. doi: 10.1183/13993003.04318-2020 [DOI] [PubMed] [Google Scholar]
- 67.Harkness LM, Patel K, Sanai F, et al. Within-session variability as quality control for oscillometry in health and disease. ERJ Open Res 2021; 7: 00074-2021. doi: 10.1183/23120541.00074-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timmins SC, Coatsworth N, Palnitkar G, et al. Day-to-day variability of oscillatory impedance and spirometry in asthma and COPD. Respir Physiol Neurobiol 2013; 185: 416–424. doi: 10.1016/j.resp.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 69.Robinson PD, Turner M, Brown NJ, et al. Procedures to improve the repeatability of forced oscillation measurements in school-aged children. Respir Physiol Neurobiol 2011; 177: 199–206. doi: 10.1016/j.resp.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 70.Gonem S, Corkill S, Singapuri A, et al. Between-visit variability of small airway obstruction markers in patients with asthma. Eur Respir J 2014; 44: 242–244. doi: 10.1183/09031936.00001814 [DOI] [PubMed] [Google Scholar]
- 71.Jayasuriya G, Robinson P, King G, et al. Small airways function in children post bone marrow transplant (BMT). Respirology 2014; 19: 77. [Google Scholar]
- 72.Uhlving HH, Mathiesen S, Buchvald F, et al. Peripheral airway dysfunction following allogeneic haematopoietic stem cell transplantation in childhood. Bone Marrow Transplant 2013; 48: Suppl. 2: S72–S458. 10.1038/bmt.2013.23 [DOI] [Google Scholar]
- 73.Rayment J, Sandoval R, Roden J, et al. Multiple breath washout testing after paediatric blood and marrow transplantation. Eur Respir J 2019; 54: Suppl. 63, PA2638. doi: 10.1183/13993003.congress-2019.PA2638 [DOI] [Google Scholar]
- 74.Usemann J, Schindera C, Zuercher S, et al. Pulmonary dysfunction after treatment for childhood cancer – comparing multiple-breath washout with spirometry. Eur Respir J 2020; 56: Suppl. 64, 148. doi: 10.1183/13993003.congress-2020.148 [DOI] [PubMed] [Google Scholar]
- 75.Usemann J, Schindera C, Zuercher S, et al. Pulmonary dysfunction after treatment for childhood cancer–comparing multiple-breath washout with spirometry. Respiration 2020; 99: 734. [DOI] [PubMed] [Google Scholar]
- 76.Usemann J, Schindera C, Kuehni C, et al. Pulmonary dysfunction after treatment for childhood cancer – a report from the SURfit study. Atemwegs- und Lungenkrankheiten 2020; 46: 89. [Google Scholar]
- 77.Jayasuriya G, King G, Selvadurai H, et al. Peripheral airway abnormalities are present in children post bone marrow transplant (BMT). Respirology 2015; 20: 92. [Google Scholar]
- 78.Jayasuriya G, King G, Selvaduari H, et al. Natural history of peripheral airway function in children post bone marrow transplantation. Respirology 2016; 21: 182. [Google Scholar]
- 79.Jensen LS, Uhlving HH, Buchvald F, et al. Role of lung clearance index in early detection of pulmonary complications and prediction of pulmonary outcomes following allo-HSCT in children. Bone Marrow Transplant 2019; 53: 720. [Google Scholar]
- 80.Hardaker K, Jayasuriya G, Gabriel M, et al. Changes in peripheral airway function after pulmonary graft vs host disease diagnosis in a longitudinal study of paediatric bone marrow transplant patients. Respirology 2016; 21: 183. [Google Scholar]
- 81.Hardaker K, Wong A, Jayasuriya G, et al. Longitudinal utility of peripheral airway function tests in pulmonary graft vs. host disease in paediatric bone marrow transplant patients. Respirology 2019; 24: Suppl. 1, 181. [Google Scholar]
- 82.Hardaker KM, Wong A, Jayasuriya G, et al. Longitudinal utility of peripheral airway function tests in pulmonary graft vs. host disease in pediatric bone marrow transplant patients. Biology Blood Marrow Transplant 2019; 25: Suppl., S247–S248. doi: 10.1016/j.bbmt.2018.12.247 [DOI] [Google Scholar]
- 83.Robinson PD, Hardaker KM, Jayasuriya G, et al. Changes in peripheral airway function with pulmonary graft vs. host disease in a longitudinal study of paediatric bone marrow transplant patients. Am J Respir Crit Care Med 2016; 193: A6357. doi: 10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6357 [DOI] [Google Scholar]
- 84.Baumeler L, Nyilas S, Halter J, et al. Nitrogen multiple breath washout for diagnosis of bronchiolitis obliterans in patients after allogeneic stem cell transplantation. Kardiovaskulare Medizin 2016; 19: Suppl. 26, 97S–98S. [Google Scholar]
- 85.Baumeler L, Nyilas S, Halter J, et al. Nitrogen multiple breath washout for diagnosis of bronchiolitis obliterans in patients after allogeneic stem cell transplantation. Respiration 2016; 91: 447–448. [Google Scholar]
- 86.De Giacomi F, Lupo-Stanghellini MT, Orsini A, et al. Ventilation heterogeneity of peripheral airways (Sacin*VT) assessed by nitrogen multiple breath washout (N2-MBW) is an accurate and sensitive marker of bronchiolitis obliterans following haematopoietic stem cell transplantation recipients. Blood 2013; 122: 4599. doi: 10.1182/blood.V122.21.4599.4599 [DOI] [Google Scholar]
- 87.De Giacomi F, Orsini A, Guggiari E, et al. Ventilation heterogeneity of peripheral airways (Sacin*Vt) assessed by nitrogen multiple breath washout (N2-MBW) is an accurate and sensitive marker of bronchiolitis obliterans–the San Raffaele experience in HSCT setting. Bone Marrow Transplant 2014; 49: Suppl. 1, S271–S272. [Google Scholar]
- 88.Lahzami S, Schoeffel RE, Pechey V, et al. Small airways function in allogeneic hematopoietic stem cell transplant recipients worsens over time and is correlated with change in chronic GVHD grade. Eur Respir J 2011; 38: Suppl. 55, p3509. [DOI] [PubMed] [Google Scholar]
- 89.Pechey V, Lahzami S, Schoeffel R, et al. Peripheral airway function declines following allogeneic transplantation and is associated with the development of chronic GVHD. Biology Blood Marrow Transplant 2010; 16: Suppl. 2, S225–S226. doi: 10.1016/j.bbmt.2009.12.218 [DOI] [Google Scholar]
- 90.Htun C, Schoeffel R, Lahzami S, et al. Peripheral airway function at 12 months post allogeneic haematopoietic stem cell transplantation (allo-HSCT). Eur Respir J 2016; 48: Suppl. 60, PA2237. doi: 10.1183/13993003.congress-2016.PA2237 [DOI] [Google Scholar]
- 91.Barisione G, Pompilio PP, Bacigalupo A, et al. Airway distensibility with lung inflation following allogeneic haematopoietic stemcell transplantation (HSCT). Eur Respir J 2012; 40: Suppl. 56, P860. [DOI] [PubMed] [Google Scholar]
- 92.Rutting S, Wallis R, Sanai F, et al. Long-term variability in oscillatory impedance in stable obstructive airways diseases. Respirology 2020; 25: 216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0251-2022.SUPPLEMENT (159.5KB, pdf)