Abstract
Chronic lung diseases result from alteration and/or destruction of lung tissue, inevitably causing decreased breathing capacity and quality of life for patients. While animal models have paved the way for our understanding of pathobiology and the development of therapeutic strategies for disease management, their translational capacity is limited. There is, therefore, a well-recognised need for innovative in vitro models to reflect chronic lung diseases, which will facilitate mechanism investigation and the advancement of new treatment strategies. In the last decades, lungs have been modelled in healthy and diseased conditions using precision-cut lung slices, organoids, extracellular matrix-derived hydrogels and lung-on-chip systems. These three-dimensional models together provide a wide spectrum of applicability and mimicry of the lung microenvironment. While each system has its own limitations, their advantages over traditional two-dimensional culture systems, or even over animal models, increases the value of in vitro models. Generating new and advanced models with increased translational capacity will not only benefit our understanding of the pathobiology of lung diseases but should also shorten the timelines required for discovery and generation of new therapeutics. This article summarises and provides an outline of the European Respiratory Society research seminar “Innovative 3D models for understanding mechanisms underlying lung diseases: powerful tools for translational research”, held in Lisbon, Portugal, in April 2022. Current in vitro models developed for recapitulating healthy and diseased lungs are outlined and discussed with respect to the challenges associated with them, efforts to develop best practices for model generation, characterisation and utilisation of models and state-of-the-art translational potential.
Tweetable abstract
3D models provide a wide spectrum of mimicry of the lung microenvironment. Novel models with increased translational capacity will benefit our understanding of the pathobiology of lung diseases and hasten the discovery and generation of new therapeutics. https://bit.ly/3MbbHlR
Introduction
Chronic lung diseases contribute significantly to the global burden of healthcare [1]. Asthma, chronic obstructive pulmonary disease (COPD), lung fibrosis, pulmonary hypertension and lung cancer are some common examples [1]. Structural alterations and cellular damage in these chronic diseases can be the result of (combinations of) prolonged exposure to environmental factors such as smoke (cigarette or other sources), air pollution and pathogens or genetic predisposition [2]. As a result, chronic diseases are often characterised by excessive mucus secretion [3, 4], reduced mucociliary clearance [3, 4] and aberrant remodelling of the airways, pulmonary vasculature and distal parenchymal tissue [5]. Subsequently, airflow limitation and alveolar tissue destruction results in the loss of lung function [3–5]. Localised repair and regeneration in the lung can be facilitated by resident progenitor cells [2, 6], but these progenitor cells can be functionally impaired in disease conditions [6]. Therefore, for most end-stage lung diseases, the only definitive treatment is lung transplantation [7].
Developing appropriate treatments for chronic lung diseases can be bolstered through thorough understanding of disease mechanisms. In spite of the abundance of and advancement in knowledge, the pathogenesis and progression of most chronic diseases remains unclear. Much knowledge has been obtained from rodent studies, which often do not completely recapitulate human diseases [8]. Traditional two-dimensional (2D) in vitro cell culture approaches have played a fundamental role in advancing current knowledge of cell behaviour and fate. However, these approaches lack a range of essential cell–cell and cell–extracellular matrix (ECM) interactions that have been shown to define cell signalling and function [9]. Bioengineering has promoted the development of innovative in vitro systems for modelling diseases in lung research [10]. Hence, three-dimensional (3D) models containing one or more matrix components and multiple cell types are becoming the sought-after standard for in vitro studies. These models support the growth of cells in all directions and allow for interaction with their surroundings. The use of models emulating human disease will not only aid in increased understanding of disease processes, but new models can also improve the drug development process and safety testing.
This review extensively describes the state of the art for in vitro models developed for ex vivo engineering in lung research as a summary and postscript of the presentations and discussions that took place during the European Respiratory Society (ERS) research seminar “Innovative 3D models for understanding mechanisms underlying lung diseases: powerful tools for translational research” in Lisbon, Portugal, in April 2022. A synopsis of various traditional and novel methods for characterisation of these models along with a comprehensive overview of the advantages and disadvantages of each model are presented. Furthermore, the translational potential of in vitro systems supplemented with potential challenges are reviewed.
Key terms
Click chemistry: Fast, simple and high-yielding reactions used to join molecular entities through a simple action like snapping things together.
Crosslinking: Formation of bonds between two molecular chains to link them together.
Cryopreservation: Storing of cells, tissues or organs by freezing in protective substances that enable the frozen object to be retrieved in a living state.
Decellularisation: Removal of cells and cellular content from any given tissue or organ.
Elastic modulus/stiffness: Measure of mechanical properties that defines the compressive elastic deformation of materials.
Extracellular matrix (ECM): A bioactive and dynamic three-dimensional network composed of proteins, glycosaminoglycans, glycoproteins and proteoglycans. It provides structure and support to the cells, being present in all tissues and organs.
Feeder (layer): A layer of cells that do not grow themselves but provide support for other cells.
Lyophilisation: Removal of frozen water from frozen tissues or organs under vacuum conditions.
Microenvironment: The local environment surrounding a cell, composed of the ECM, other cells and factors that dynamically interact with one another to affect the cell directly or indirectly.
Pluripotent stem cells (PSCs): Cells that have the capacity to self-renew and are able to differentiate into all different types of cells in the body.
Remodelling: Series of changes in the content and organisation of the components (of the ECM).
Resident progenitor cells: Self-renewing cells resident in a tissue that are activated during injury to participate in the repair process through differentiating into tissue-specific cell types.
Thermosensitive: Responsive to changes in temperature.
Viscoelastic (stress) relaxation: Measure of the decrease in the accumulated load (stress) in the structure through both viscous (fluid) and elastic (solid) manners.
Generation of (3D) innovative in vitro lung models
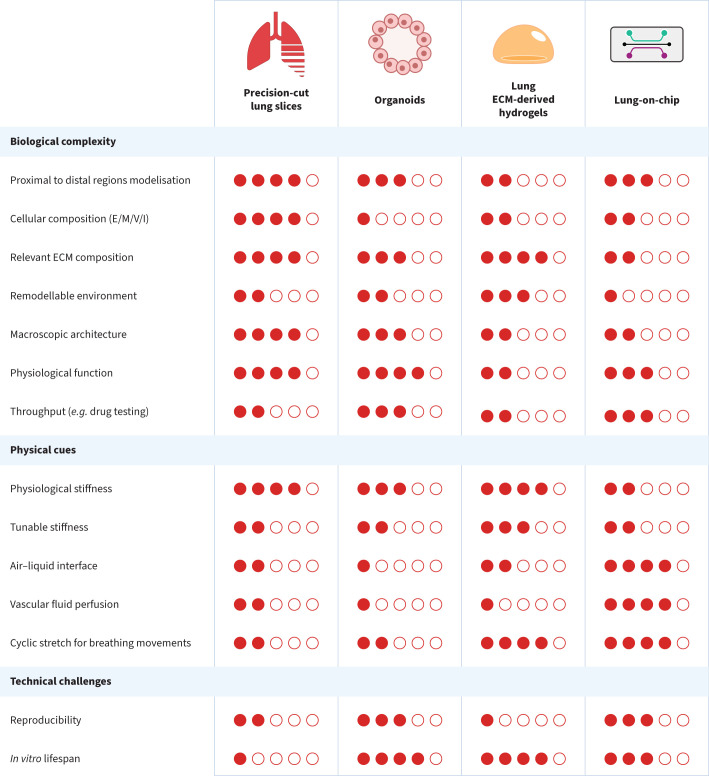
Currently, translation of respiratory therapies from bench to bedside remains limited in part due to the lack of relevant in vitro and in vivo models. The need for more accurate and physiologically representative models of the lung is thus clearly established. Various approaches to develop new models range from models exclusively using the source material of the lung, like precision-cut lung slices (PCLS), to fully engineered environments, like the lung-on-chip (LOC). This article will focus on disease modelling in four main types of in vitro lung models: PCLS, organoids, lung ECM-derived hydrogels and LOC. Each of these models has their strengths and weaknesses, as presented in figure 1.
FIGURE 1.
A general comparison of in vitro models used for modelling the lung: precision-cut lung slices, organoids, lung extracellular matrix (ECM)-derived hydrogels and lung-on-chip. The proportion of filled dots reflects the score of the model to fulfil the stated criterion, from least representative (one filled dot) to a complete lung in vitro (five filled dots). Lung ECM-derived hydrogel comparisons were based on cell-seeded hydrogels. Reported scores reflect the opinion of the authors and the meeting attendees rather than an objective scoring. E/M/V/I: epithelial cells/mesenchymal cells/vascular cells/immune cells.
The common ground in the above-mentioned models is the development of a cellular environment away from stiff and non-physiological plastic culture dishes, proposing instead a transition towards softer materials, with the possibility of including ECM proteins and/or reproducing the structural arrangement and biochemical/biomechanical properties of the lung. To recapitulate the lung microenvironment, several different factors are important. The main factors are biomechanics (mechanics of breathing and stiffness), lung ECM (structure and composition) and lung cell composition (cell types and cell source). Other factors that may be added include but are not limited to an immune component, hypoxic and normoxic regions and blood flow (perfusion).
These in vitro models can be used to investigate the influence of individual mechanical parameters and immune cell interactions on disease origin and progression, along with screening for druggable targets and developing therapies. Animal models remain an indispensable tool for research because in vitro models cannot yet mimic the complex structures of tissues and systemic effects. However, there is an evolving role for these preclinical systems for modelling in vivo conditions and screening therapeutics to reduce the burden on in vivo systems.
Each model has different advantages and disadvantages, and the choice of model is highly dependent on the research question to be addressed and the availability of source material. This review section will highlight some of the innovative in vitro lung models and their recent developments.
Precision-cut lung slices
PCLS are a unique translational ex vivo model of peripheral lung tissue that have applicability for many different research approaches including drug discovery and toxicity screenings [11, 12]. The slice lung model was first introduced as a pharmacological model by Dandurand et al. [13]. Unlike tissue fragments obtained by cutting or shredding explanted tumour resections [14], PCLS are well-defined models with standardised protocols for preparation and use in experiments. Upon collection, the lungs are filled via the trachea in animals or bronchi/bronchioles in human lung explants with a low melting agarose solution (0.75–3%) and then cut into slices of 200–500 μm thickness [15, 16]. The slices consist of distal airways and vessels with their intact lung architecture, maintaining cell–cell interactions and ECM.
It is possible to sequentially study various lung cell populations in airways and vessels of different sizes and anatomical locations from a healthy or diseased lung, thus optimally using available resources. Such an approach provides opportunities for specialised screening of the responses of alveoli, bronchioles, vessels and surrounding ECM. Calcium fluxes and ciliary beat frequency can be followed and visualised in the small airways [16, 17], with smaller airways being known to respond to a greater extent to bronchoconstrictors than larger airways [15, 18].
In some instances, the thickness of the slices may be a concern because the readouts and functionality can differ depending on the thickness. Therefore, it is important to describe slice thickness, area of the slices and lumen size of airways and pulmonary vessels when reporting generated data. Moreover, cutting and embedding the lung tissues in agarose can trigger repair and regenerative responses that can subsequently affect further experiments. Thorough washing and resting periods prior to experimental procedures can considerably reduce the effect of the processing steps. The viable culture period (5–28 days) is dependent on the type of analysis to be performed and should always be specified to enable replication of the studies. Cryopreservation of PCLS is a way forward to maximise the number of slices that can be used per lung and the possibility of sharing valuable material; however, current protocols under development for cryopreservation need further improvement to enhance PCLS viability and functionality upon revival.
PCLS can also be modified to mimic disease with different approaches and protocols either by treating the animal prior to harvesting of the lung or by directly treating the PCLS. Examples of applications of such models include allergic asthma [19, 20], emphysema [21, 22], acute respiratory distress syndrome [23] and fibrosis [24, 25]. PCLS can also be decellularised and used as lung scaffolds with native ECM architecture for 3D cell culture studies with and without cyclic stretch [26–28].
Material availability and viable culture duration limit the use of PCLS; however, these models provide unmatched resemblance of the physiological architecture and multicellularity of the lung. These models represent the status of the lung at a given time of harvest, thus providing excellent tools to study different stages of disease progression. Overcoming the current restrictions, combined with facilitating immune cell recruitment, would aid in unlocking the true potential of PCLS.
Organoids
Organoid cultures are defined as 3D cultures grown from pluripotent stem cells (PSCs) or adult progenitor cells that self-organise to form structures histologically similar to human organs [29, 30]. For a 3D culture to be considered an organoid, it must have the capacity to self-renew and it must replicate some function specific to the mimicked organ (such as the mucociliary function in the case of bronchial organoids) [29, 30]. In contrast to organoids, spheroids are typically cell aggregates grown scaffold-free from a single or a mix of multiple cell types and have lower complexity while recapitulating native tissue [31]. While organoids modelling bronchial epithelium have been rapidly instituted, those for alveoli have been more challenging to establish [32]. Early efforts employed flow cytometry sorting of murine alveolar type II epithelial cells co-cultured with mesenchymal cells [33]. To date, the minimal composition of lung organoids built from adult progenitor cells comprises bronchial epithelial cells that are embedded in a 3D ECM-based hydrogel with a complex combination of growth factors [34]. This gives rise to spherical organoids, often filled with secretions, that are classified into tracheospheres [35], bronchospheres [36] or alveolospheres [33, 37], depending on the source of epithelial cells. More recently, isolation of epithelial cells (often with selection of epithelial cell adhesion molecule-positive cells via flow cytometry [38] or magnetic bead-based selection [39]), coupled with culture with or without mitomycin-restricted feeder fibroblasts has been described for alveolar organoid culture [40, 41]. Moreover, generating alveolar organoids without the presence of a feeder cell population has also recently become possible [42, 43].
Research using organoids has progressed exponentially during the last decade, and results obtained have been reviewed in detail elsewhere [44, 45]. Relatively simple to obtain, human airway organoids derived from epithelial cells and adult stem cells have been successfully used to study important processes such as respiratory virus infections, including respiratory syncytial virus [46] and influenza viruses [47]. Donor-specific organoids can be generated from progenitor cells purified from human lung tissue to model the healthy state and various respiratory diseases (table 1). In contrast, PSC-derived lung organoids rely on complex manipulation of developmental signalling pathways to form a lung bud organoid that can develop into branching airways with primitive alveoli [48]. PSC-based, in particular induced PSC (iPSC)-based, organoids have great potential thanks to their ability to combine multiple cell types such as epithelial cells, smooth muscle cells and fibroblasts [49]. However, owing to their intrinsic immature nature, generating disease models is not straightforward, except for monogenic disorders such as cystic fibrosis [50]. Lung organoids have rapidly shown their value for respiratory research thanks to their physiologically relevant properties, the possibility to recreate and understand lung development, and their regeneration mechanisms. Organoid amplification [46] and cryopreservation of organoids opens a world of possibilities for the establishment of biobanks to facilitate greater availability of progenitors for organoid development for biomedical research and personalised medicine [51].
TABLE 1.
Overview of the use of the different 3D in vitro systems to model healthy and diseased human lungs
| PCLS | Organoids# | Lung ECM-derived hydrogels ¶ | Lung-on-chip | |
| Healthy | Both alveolar and airway: Bronchoconstriction assay [18, 85] Response to drugs or industrial chemicals [86] Long-term (14 days) [87, 88] Indacaterol efficacy [89] |
Airway: From adult stem cells [90, 91] From PSCs [49, 92] With bronchial epithelial cells, lung fibroblasts and endothelial cells [93] Alveolar: From PSCs [94–96] Both alveolar and airway: Human fetal lung-derived organoids [97] |
Both alveolar and airway: Human lung ECM hydrogels and comparison to native tissue [54] |
Airway: With epithelial cells only [98] Epithelial–vascular interface [99] With fibroblast compartment [100] Epithelial–ECM–smooth muscle interface [101] Epithelial–ECM–vascular interface [102] Cell extravasation [103] Alveolar: Alveolar–capillary interface [70] Alveolar acini with immune compartment [104] With micro-diaphragm [76] With inverse opal gelatine hydrogel [105] With biological and stretchable membrane [79] With fibroblast compartment [75] |
| Asthma | Airway only: Effect of soluble guanylate cyclase on bronchodilation [106] Asthma mediators [107] β2-agonist efficacy modulated by steroids [108] Rhinovirus infection: increased IL-13, IL-25 and thymic stromal lymphopoietin [109] |
Airway only: Notch2 role in goblet cell metaplasia [110] |
Airway only: IL-13-induced asthma [99] IL-13 and viral-induced exacerbations [111] |
|
| COPD | Alveolar only: CS model±viral exacerbations FZD4 inhibitor efficiency in COPD patient PCLS [112] Wnt/β-catenin signal activation efficiency [22] |
Alveolar only: COPD patient-derived epithelial cell organoids [113] Emphysematous alveolar epithelial cells [42] |
Both alveolar and airway: COPD lung ECM hydrogels and comparison to native tissue [54] |
Airway: With COPD epithelial cells, poly-I:C induced [99] Cigarette smoke induced [73, 114] Alveolar: Cigarette smoke induced [105] With emphysematous cells [42] |
| Cystic fibrosis | Airway: From patient-derived adult stem cells [46] From patient-derived PSCs [50, 115] From multipotent human foregut stem cells with cystic fibrosis transmembrane conductance regulator mutation [116] Not categorised: Nasospheres for drug testing [117, 118] |
Both alveolar and airway: With epithelial cells from CF patients [100] |
||
| Lung fibrosis | Alveolar only: IPF model with cytokines [24] From IPF patients [25] |
Alveolar only: Hermansky–Pudlak syndrome [48] Hermansky–Pudlak syndrome mutation in embryonic stem cell-derived organoids [119] |
Both alveolar and airway: IPF lung ECM hydrogels and comparison to native tissue [54] |
Both alveolar and airway: With IPF fibroblasts [100] Alveolar: With epithelial cells (wound-healing and stretch) [120] |
| Cancer | Not categorised: Kras mutation model [121] Non-small-cell lung cancer [122] T-cell infiltration [123] |
Not categorised: From patient-derived adult stem cells [46] Patient-derived organoids: Non-small-cell [124] Adenocarcinoma [125] Five different subtypes [126] Co-cultured with endogenous tumour-infiltrating lymphocytes [127] |
Not categorised: Non-small-cell lung cancer [128, 129] Mesothelioma [128] Safety studies [130] |
Only the studies using human-origin cells and ECM were included in the table. PCLS: precision-cut lung slice; ECM: extracellular matrix; PSC: pluripotent stem cell; iPSC: induced pluripotent stem cell; IL: interleukin; COPD: chronic obstructive pulmonary disease; CS: cigarette smoke; poly-I:C: polyinosinic:polycytidylic acid; IPF: idiopathic pulmonary fibrosis. #: studies using the following terminology were included under the “organoids” section: spheroids, tracheosphere, bronchosphere, alveolosphere, nasosphere and organoids; ¶: studies aiming at building lung tissues for transplantation were not included in this table.
Lung extracellular matrix-derived hydrogels
Hydrogels are hydrophilic polymers physically or chemically crosslinked to form a 3D network [52]. Hydrogels can hold copious amounts of fluids while maintaining their structural integrity and represent the hydrated nature of physiological ECM. Individual ECM components or derivatives of them such as collagen, gelatine, fibronectin, fibrin and hyaluronic acid have frequently been used to form hydrogels to mimic the ECM and provide bioactive components in cell culture [53]. However, they do not fully recapitulate the physiological complexity of the ECM and its macrostructure. Organ-derived ECM recapitulates the tissue-specific biochemical and biophysical complexity of the native tissue [54]. Thus, cell-seeded lung ECM-derived hydrogels have emerged as an important in vitro system to model the lung microenvironment. The first lung ECM-derived hydrogel was reported in 2016 by Pouliot et al. [55] using porcine lung ECM, which was followed by human lung-derived ECM [54, 56] for establishing such models. Lung tissue can be decellularised using different methods, including chemical detergents, freezing and thawing, sonication, enzymatic digestion [53, 57] and recently developed apoptosis-associated approaches [58], followed by lyophilisation and milling into fine power [54] or homogenising using mortar and pestle [53]. The decellularised ECM is solubilised using pepsin in acidic conditions with constant agitation for 24–72 h [53]. Pepsin preserves the ultrastructure of collagens by cleaving collagen triple helices only at their telopeptide bonds [53], although the digestion time has been shown to affect hydrogel properties and morphology and metabolic activity of cells seeded onto the hydrogels [53, 59]. The pH of the solution is neutralised, followed by buffering with PBS to generate a self-assembling and thermosensitive ECM pre-gel that forms a hydrogel at 37°C [54]. Ultrasonic cavitation has also been used to solubilise milled ECM of porcine trachea [60] as an alternative to pepsin digestion, which also solves the challenge of leftover pepsin in the lung ECM-derived hydrogels. Despite these rigorous treatments, ECM-derived hydrogels retain most of the native composition and reflect mechanical properties of the lung ECM both in healthy and diseased states [54]. A recent report illustrates organ-specific elasticity, viscoelastic relaxation and gelling properties of ECM-derived hydrogels [56]. These studies highlight the importance of mimicking the underlying architectural and mechanical properties of the ECM that are disease- and organ-specific and can thus differentially regulate cellular responses. To this end, multiple studies have demonstrated alterations in cellular morphology or phenotype [61, 62], differentiation [63, 64] and gene and protein expression [61, 65] in cells cultured on or harvested from within ECM-derived hydrogels. Further investigation into individual biomechanical properties in studies using novel tools such as click chemistry for modulating elastic modulus [66], ECM-derived and synthetic material hybrid hydrogels to modulate stiffness [67], or applying fibre crosslinking to native lung ECM-derived hydrogels to increase stiffness and decrease viscoelastic stress relaxation [68] have provided novel insights into the role of the lung microenvironment in driving disease processes.
Lung ECM-derived hydrogels are an innovative and powerful tool that can be manipulated. Through the addition of cyclic stretch to mimic breathing, this model has the potential to further mimic the lung microenvironment in vivo [62, 69], thus being one of the models that most closely represents physiological conditions. A challenge yet to be addressed effectively is the capacity to mimic the physiological architecture of peripheral lung tissue. Through the employment of this model system, the outcomes of cell–ECM interactions in the lung are slowly becoming evident.
Lung-on-chip
LOC devices are microphysiological systems that model part of the lung and make it possible to combine physiological flow, mechanical stretching, multi-compartment co-culture, drug/particle exposure and ECM material in an in vitro system. The LOC models began with 2D or 2.5D systems; however, they have now advanced to multicellular and multidimensional 3D systems. Microfluidic devices offer many possibilities that have recently been used to develop different LOC devices and some relevant LOC devices are highlighted here. The first LOC with physiological flow and mechanical stretch was developed by the Ingber group [70]. This alveolus-on-chip, with lung epithelial cells and endothelial cells, was perfused with neutrophils to mimic an infection, and later expanded to initiate COVID-19 research on the chip [71, 72]. Diseases like asthma and COPD have been modelled in LOC mimicking small airways [73, 74]. In addition, a stromal layer can be included, combining LOC technology with hydrogel culture [75]. LOC technology has evolved further and a LOC with a stretch regimen that is closer to physiological stretch was recently developed. This platform uses a micro-diaphragm that stretches the cell culture membrane in three dimensions [76, 77]. It can be used with immortalised primary alveolar cells for nanoparticle exposure and lipopolysaccharide challenge [78]. To create a more in vivo-like environment, the polymer membrane of the LOC was replaced by a collagen-elastin membrane spread by surface tension on an ultra-thin grid with holes the size of alveoli, enabling the creation of an array of alveoli [79]. Lung microvasculature and vascular diseases are also modelled with microfluidics. For example, side-effects of the idiopathic pulmonary fibrosis (IPF) drug nintedanib [80], effects of physiological stretch on the endothelium [81], and combined effects of hypoxia and cyclic stretch [82] have been investigated.
A challenge with LOC experiments is the incorporation of primary material that presents wide heterogeneity. This has been tackled by generating type II alveolar epithelial cell organoids to enable expansion of primary lung alveolar cells in hydrogels before culture in a LOC [42]. Another challenge is the complicated fabrication of LOC devices, which often requires highly specialised equipment, although a recent study showed that it is possible to develop a LOC to study ventilation-induced injury solely by using a simple 3D printer and syringe pump [83]. In spite of these challenges, LOC as a technology has been successfully commercialised by multiple startups, e.g. Emulate [71], AlveoliX [78] and Mimetas [84], which support research on a wide range of human (lung) diseases. Often, these companies offer customisation of chips, allowing easy technology transfer and adaptation of LOC systems, thus enabling rapid uptake of LOC without the limitation of requiring development and manufacture in-house by various research groups. However, the scalability and costs of these systems remain an important challenge. The LOC field is still young and exciting with new developments continuously occurring. Overall, LOC technology is promising for modelling lung diseases and drug efficacy/safety screening with the advantage of being able to model the complexity of the different compartments of the lung in one system.
Perspectives and future directions
A summary of recent applications of the use of PCLS, organoids, ECM-derived hydrogels and LOC as in vitro systems for lung disease modelling is presented in table 1. Excitingly, the development of lung in vitro models continues to rapidly advance. In the future, there is the potential for different models highlighted in this perspective to be combined to create an optimal in vitro model that can use the advantages of different models in concert. For example, by combining LOC technology with porcine lung ECM hydrogels, a physiomimetic acute respiratory distress syndrome model incorporating primary rat mesenchymal stromal cells and type II alveolar epithelial cells has recently been developed [62]. Furthermore, these in vitro models can be improved using techniques such as the 3D bioprinting that is rapidly offering several new opportunities, e.g. bioinks with bioactive components and tuneable mechanical properties [131, 132]. The cellular and ECM diversity in the lungs and an increase in the use of human material in research calls for the use of spatio-specific material for defined research questions. An indispensable feature of animal models that is yet to be recapitulated in in vitro models is the systemic responses that arise from a functioning immune system. The inclusion of immune cells in in vitro models developed for testing novel cellular and molecular interventions will aid in advancing knowledge about local and systemic immune responses associated with lung diseases [133]. Furthermore, development of advanced in vitro models will reduce the dependency on animal models for scientific research. However, to reach this point, adequate characterisation of in vitro models is necessary, which will be discussed in the next section.
Challenges associated with 3D in vitro models
In vitro models including PCLS, organoids, lung ECM-derived hydrogels and LOC are powerful methods in the quest to decipher the in vivo status of lung diseases at the cellular and ECM level. A comprehensive overview of the challenges associated with the above-mentioned in vitro models is presented in table 2. In addition to the challenges specific for each model, there are overarching challenges for in vitro models in general. As research using such models progresses, the materials used, methods applied and outcomes tested also diversify. As one of the primary outcomes of the ERS 2022 research seminar “Innovative 3D models for understanding mechanisms underlying lung diseases: powerful tools for translational research”, the participants were invited to contribute to a discussion on defining best practices when using these models and resources to improve reporting and reproducibility of data generated with these models. The main take-home message from these discussions was the need for clear and detailed methodology sections when reporting outcomes and open communication. Additionally, several other important issues were highlighted.
TABLE 2.
Main challenges associated with the four different in vitro culture systems for modelling lung environment in health and disease
| PCLS | Organoids | Lung ECM-derived hydrogels | Lung-on-chip |
| Patient-to-patient variations | Heterogeneity in number and sizes (due to intra- and inter-patient variations and various culture modalities) | Patient-to-patient variations influencing ECM hydrogel properties | Challenges associated with incorporation of cells (only for closed lung-on-chip systems) |
| Heterogeneity in the diseased regions, resulting in intra- and inter-slice differences | Closed architecture with limited access to the lumen, which is filled with liquid instead of air | Heterogeneity in the diseased regions and in decellularisation methods | Restriction of the morphogenesis to predefined geometry |
| Absence of easy air–liquid interface setting | Presence of necrotic cores | Macroscopic architecture differing from native lung | Non-permissive environment that cannot be remodelled |
| Need for fresh starting material that limits lifespan of various cells and the tissue in vitro | Difficult to obtain fully differentiated lung cell types | Pepsin removal currently not possible | Non-physiological stiffness of the device |
| Snapshot of the cell populations in the tissue and absence of access to infiltrating cells | Lack of vascularisation and difficult to introduce perfusion or immune cells | Incorporation of cells is challenging | Challenges associated with translation of observations from microscale to in vivo systems |
| Difficult to preserve arteriole morphology and function | Limited inclusion of mechanical forces associated with breathing | Limited inclusion of mechanical forces associated with breathing | For non-commercialised lung-on-chips: rather long production time of the microfluidic devices with a small throughput |
| Cellular behaviour likely impacted by processing and agarose embedding | Limitations in hydrogel types to develop organoid culture systems | Lack of vascularisation and difficult to introduce perfusion |
PCLS: precision-cut lung slice; ECM: extracellular matrix.
Best practices for handling patient samples: All participants were appreciative of the opportunities and importance of using precious human samples; however, the limitations of this practice were also recognised. The variability in the results caused by sample processing can be addressed by suggesting optimal windows of time between explanting of tissues and processing, and clarity of methodologies for processing when working with human material [134]. Extensive information about each protocol used by investigators in the materials and methods sections would improve the potential for accurate comparisons to be made between different studies. Primary lung tissue material is generally seen as the optimal source for cell and ECM material, but access to such primary tissue is limited and not equally distributed between research centres. Obtaining ethical board permissions to use the sparse human-sourced material is a challenge [135], although the establishment of local exchange networks has aided in tackling this. However, a European network currently does not exist, which limits optimal distribution of primary samples. Moreover, limited-to-no access to “true” healthy lung samples hinders how we can model truly “normal” tissues, resulting in potential bias in the data generated. Extensive descriptions of the details of obtaining and processing animal-sourced materials used in combination with patient-derived material for in vitro models would stimulate cumulative research.
Best practices for (description of) the methods applied: Different in vitro models require different methods for production. The development of innovative and novel techniques is encouraged by the interdisciplinary nature of lung disease research, which is supported by the fields of cell biology, pulmonology, biomaterials and bioengineering. As a result, various research groups are developing different methods for generating in vitro models depending on available resources and applicability to the research questions explored. This, however, results in diversity and hence variability in the outcomes obtained from in vitro models, making comparison between different studies challenging. As we attempt to build on existing knowledge, the lack of unified approaches renders our efforts more complex. Agreeing on minimum parameters to define the steps required to create each version of these models, preparing such guidelines for the community and encouraging clear communication are important starting points for tackling this conundrum [136].
Best practices for measuring and reporting end-points: Different research questions require generating and measuring different outcomes. Characterisation of end-points can be performed with different methodologies that can provide information that speaks towards similar end-points. While the separate measurements will ideally provide data that can be informative for comparisons, differences in the sample processing and individual experimental setups are likely to influence the outcomes, generating difficulties when it comes to making subsequent comparisons between different studies. Defining certain standards or establishing recommendations for reporting of specific details where variances may occur that influence the outcomes (e.g. number of cells used, exact media composition, timing for all culture steps of experiment) would greatly benefit the field. Such an approach will enable the field to move forward because it would bring different studies performed at different locations and/or times to a comparable basis.
Establishing best practices for the materials used, the methods applied for generating in vitro models and the end-points tested will be a difficult task. However, this newly advancing topic would strongly benefit from starting discussions regarding best practices for above-mentioned criteria. Enforcing “gold standards” to be met with each model system prematurely would potentially result in missing development opportunities represented by the many innovative pioneering studies. It must also be noted that these standards might vary based on the research question. One way to start such discussions is creating awareness of the need to follow best practices by providing detailed information for other influential elements of academic research. Engaging editors and journals regarding the development of good practices for the field would be an ideal starting point. The development of agreements such as minimum information about a microarray experiment (MIAME) [137], Animal Research: Reporting of in vivo Experiments (ARRIVE) [138] and the US Food and Drug Administration Modernization Act 2.0 [139] provide guidelines intended to improve quality and reproducibility of scientific reports involving microarrays, animal research and alternative methods used to test drug safety and effectiveness. These agreements are advanced to ensure unified evaluation and interpretation of results and better reproducibility of experiments, moving towards new standards for certifying new methodologies for drug development. Such initiatives could subsequently be moved forward by engaging with reviewers about what to look out for when presented with manuscripts reporting such methodologies, as well as providing information for the research community. Together with a scientific community working harmoniously, reviewers and editors of journals paying attention and looking to apply such standards would greatly benefit the advancements made in generating and using innovative in vitro models for translational research [140, 141].
Characterisation of in vitro models
Today, the use of complex 3D systems has made it possible to undertake translational studies in models that more closely mimic the in vivo situation. State-of-the-art characterisation of these 3D in vitro models is therefore important. The characterisation of experimental 3D models such as PCLS, organoids, (cell-seeded) ECM-derived hydrogels and decellularised lung matrices, and LOC presents different challenges, with appropriate ways for characterising biological and physical aspects within the models dependent on the research question being addressed. These research questions might include different conditions, including but not limited to basal levels, challenged and stimulated. Here we present a non-exhaustive summary of recent advances in the characterisation of these in vitro models.
Imaging
Imaging is a major methodology used for characterising the macroscopic architecture and spatial localisation of specific proteins or cells, as well as cell–cell and cell–ECM interactions [142]. Light sheet fluorescence microscopy (LSFM) is an emerging technique for imaging larger 3D samples with high spatiotemporal resolution [143]. LSFM has been used extensively for organoid development studies, where visualisation is essential for understanding the cellular complexity [144] and airway development [145]. LSFM, however, requires fixation of the sample and optical clearing, which is a challenge when working with 3D structures with different thickness, e.g. PCLS. This is due to the difficulty with optical clearing in thicker PCLS that render blurry images at ≥100 µm depth. Scanning electron microscopy has also been used to characterise cellular structures in PCLS [146], lung organoids [46, 147] and LOC [105] and fibre structure of empty lung ECM-derived hydrogels [56]. Second harmonic generation microscopy [148, 149] has greatly facilitated the characterisation of ECM components in a PCLS model [146]; however, it has yet to be applied to other model systems for characterising cells and the microenvironment. The imaging technique employed depends on several properties of the particular in vitro model, thus limiting the applicability of different microscopic approaches to certain 3D models. Some important considerations while choosing an imaging technique for an in vitro model include the thickness and size of the sample, the effect on live cells and fixation methods.
Protein and gene level characterisations
Recent advances in sensitivity and resolution of mass spectrometry allow for deeper characterisation of all 3D models [26, 150] and have been commonly used for the characterisation of ECM composition, ECM remodelling and cell phenotyping [151]. Isotope-labelled amino acids that tag chemical, metabolic and enzymatic processes have been used to differentiate, in combination with mass spectrometry, between de novo ECM synthesis and native ECM proteins in decellularised PCLS [26, 152]. While mass spectrometry analysis on regional and disease-specific decellularised lung ECM has been recently performed [153], employing such analysis on empty and cell-seeded lung ECM-derived hydrogels is yet to be completed. Parallel to mass spectrometry, Raman spectroscopy is a label-free method that allows investigation of biochemical composition of ECM-based models such as PCLS, decellularised PCLS and hydrogels [154]. Regarding gene level characterisation, RNA-sequencing approaches have been successfully applied to PCLS [155], organoid [46] and LOC systems [156]. Single-cell RNA-sequencing, a method based on capturing the mRNA content of each cell at single-cell resolution, has been recently applied to lung organoid samples [157]; however, it has yet to be implemented on the remaining systems. Metabolomics approaches have also been applied to PCLS [146] and iPSC-derived epithelial progenitor cells [158]; however, no application of these approaches to other model systems has been reported.
Mechanical characterisation
Lung diseases are often characterised by an alteration in physiological mechanical properties of the ECM, and thus of the lung tissue as a whole. These changes in turn affect cellular behaviour. Importantly, the macroscopic stiffness of a tissue is rarely the same as the local microscopic stiffness which is sensed by cells through their cell surface focal adhesions [159]. Mechanical properties of in vitro models (mainly hydrogel based) can be manipulated to mimic disease conditions and characterised using various methods including rheometry [131], low-load compression testing [54, 68, 160] and atomic force microscopy (AFM) [61]. On a cellular and thus a micrometre scale, spatial elastic modulus can be measured using Brillouin microscopy. Brillouin light scattering allows for live measurements of viscoelastic properties over the surface of the sample and can be applied on bioprinted cells and 3D in vitro models [161, 162]. Innovative use of 4D traction force microscopy and an open-source MATLAB software package is an emerging method to measure and calculate traction forces exerted by cells in 3D hydrogel cultures over time [163]. On a microscopic scale, AFM has been commonly used to measure both stiffness and viscoelastic properties, which allows for a local assessment of the material properties [164]. Cells in the lungs experience dynamic forces and are under constant cyclic stretching, which is exaggerated in patients being mechanically ventilated [165]. Additionally, cells also receive biomechanical cues from the local microenvironment [166, 167]. A novel approach using AFM was highlighted as a way to measure microrheology of tissue exposed to different levels of stretch [168].
All of these methodologies have the potential to be applied in the examination of PCLS, organoids, cultures with ECM-derived hydrogels and decellularised lung matrices, and LOC. Generating such information will further our understanding of how altered mechanical conditions in lung disease impact cellular behaviour in the 3D environment [169].
Translational potential of in vitro models
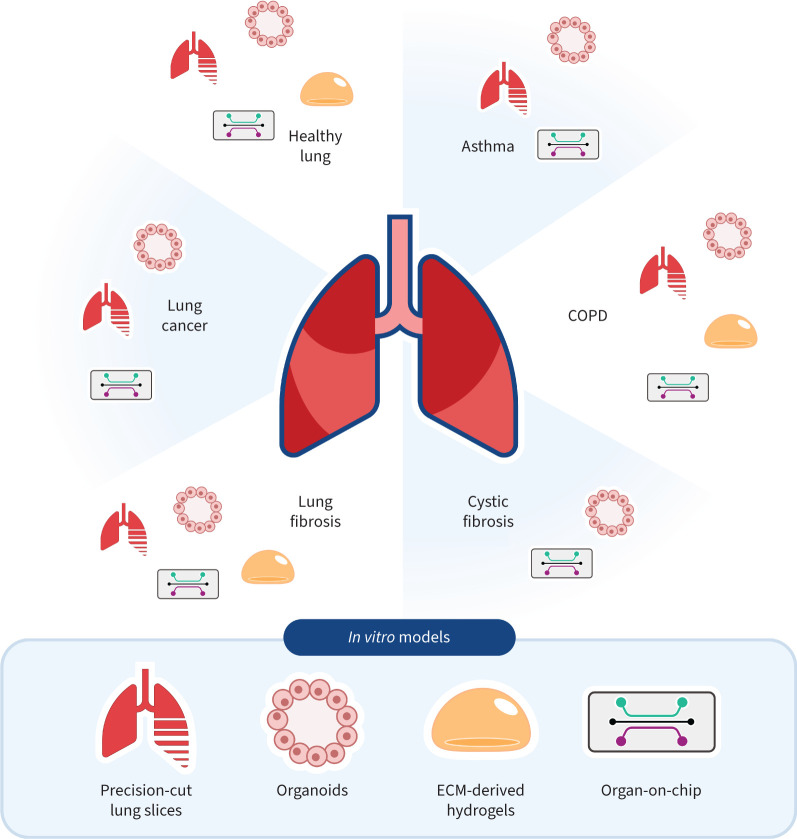
The high number of failures of new drugs for respiratory diseases in clinical trials might be related to limitations in preclinical studies [170, 171]. Many of these trials fail in phase 3; while the therapy is not toxic, its efficacy cannot be demonstrated. One of the main problems with the preclinical models that are currently used is the lack of attention given to the physical aspects that are characteristic of the lung and (spatial) organisation of different cells [172, 173]. Because 3D models can be implemented with human cells, they can more closely resemble human physiology, which has resulted in a growing number of studies using 3D models to study lung development and lung disease pathogenesis. Furthermore, these models have potential as screening tools for pharmacological drugs being developed to treat pathologies such as lung fibrosis and other rare lung diseases. Figure 2 illustrates currently available models for most common lung diseases while highlighting the models that have yet to be developed for these diseases.
FIGURE 2.
Summary of the state-of-the-art status of different models used for modelling of different diseases. COPD: chronic obstructive pulmonary disease; ECM: extracellular matrix.
Mechanopharmacology is emerging as one of the key fields associated with the development of 3D models and harvesting their potential in translational research, because it investigates the effects of mechanics in dictating the efficacy of drugs and vice versa. Pioneering studies on healthy and asthmatic subjects [174, 175] highlighted the difference in the mechanical properties of the airways and the relation with strains produced by deep inhalations [176–178]. In IPF, transforming growth factor-β (TGF-β) signalling [179] is involved in an aberrant feedback loop, because the composition of ECM [180] and stiffening of the microenvironment activates TGF-β, which further promotes production and crosslinking of fibrillar collagen [181]. Experiments performed with IPF fibroblasts cultured on either stiff tissue culture plastic, in CytoSoft plates (2 kPa) or in soft spheroids (0.4 kPa) showed that softer materials induced expression of cycloxygenase-2 (COX-2), suggesting that higher stiffness reduced COX-2 expression while activating TGF-β [182].
Human airway organoids have been explored with respect to their translational potential for cell therapy [90]. However, dissociating such organoids into single-cell suspensions for subsequent therapeutic application remains a challenge for advancing their use in cell therapy, as well as the development of platforms for high-throughput drug screening. As an example, in the rare disease primary ciliary dyskinesia [183], cultures of basal epithelial cells from patients have been performed in 96-well plates for the screening of different drugs from a reduced starting cell number [184]. Methods to promote the expansion and differentiation of epithelial cells within 3D organoids, only requiring low cell numbers, provide novel opportunities to investigate how these cells behave in health and disease [185].
The translational capacity of preclinical models has recently been highlighted as a discussion point. Because 92% of the preclinical trials fail for new lung cancer treatments, it is clear that there is a need to increase the preclinical “confidence” [186]. Therefore, new approach methodologies (NAMs) are needed [187]. The main steps that must be considered when developing NAMs are 1) a detailed methodological description; 2) consideration of the diseased organ anatomy and control of the cell/cell ratio; 3) overcoming the uncertainty due to interspecies differences, by using human cells and no animal-derived proteins, and including the microbiome; and 4) exposure conditions and time points [188, 189]. Primarily driven by cancer research, these lessons can be applied to develop the innovative 3D in vitro models described here to investigate disease pathogenesis, progression and therapeutic targets for chronic lung diseases.
Conclusions
In vitro models with 3D structures are powerful tools that provide variability and versatility for both basic and translational research in lung diseases. PCLS, organoids, lung ECM-derived hydrogels and LOC systems present a wide spectrum of tune-ability and biomimicry. Each of these models have advantages and limitations, and selection and application of the models for different studies requires a thorough understanding of their strengths and disadvantages. The future of these models, moving towards increased complexity to better represent the lung physiological conditions, will be built upon refinement of the currently developed model versions, by defining best practices for the materials used, the characterisation methods applied, and how the reported readouts can be compared. Similarly, combinations of these models to create increasingly tailored models for specific research questions would also increase the lung mimicking capacity of the models. By improving the currently existing models, generating new and innovative alternatives, and developing creative combinations of these systems, the translational capacity of in vitro research for lung preclinical studies will be greatly enhanced. The path to achieve more applicable and accepted models lies in open communication, clear descriptions of all materials/components used and methods applied, and educating all parts of the scientific community. It is not unlikely that in the near future advanced in vitro models will replace several animal models that do not accurately reflect the diseased microenvironment of some lung diseases.
Points for future research
Exploring opportunities for combining elements from precision-cut lung slices, organoids, lung extracellular matrix-derived hydrogels and lung-on-chip models to expand 3D models for mimicking lung homeostasis and pathogenesis will advance the field.
Establishing best practices for reporting the materials used, methods applied for generation of in vitro models and the end-points tested, based on the research question and model used, would greatly benefit the field. Thorough and cross-platform characterisations of different in vitro models have great potential in furthering our understanding of how altered biochemical and mechanical conditions in lung disease affect cellular behaviour in the 3D environment.
New approach methodologies for in vitro models are required to increase the preclinical confidence in these models.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflict of interest: M. Nizamoglu reports grants from Boehringer Ingelheim; outside the submitted work. M.M. Joglekar reports support for the present manuscript from Graduate School of Medical Sciences, University of Groningen, The Netherlands. C.R. Almeida reports grants from Fundação para a Ciência e a Tecnologia; consulting fees from Fundação para a Ciência e a Tecnologia; and travel support from Fundação para a Ciência e a Tecnologiam, outside the submitted work. A-K. Larsson Callerfelt reports grants from Swedish Heart Lung Foundation and from EMPIR 18HLT02 AeroTox project; outside the submitted work. The EMPIR programme is co-financed by the Participating States and from the European Union's Horizon 2020 research and innovation programme. I. Dupin reports grants from Fondation Bordeaux Université, the Agence Nationale de la Recherche (ANR-21-CE18-0001-01); and a patent granted for “New compositions and methods of treating and/or preventing chronic obstructive pulmonary disease” (EP 3050574) and patent pending for “New compositions and methods of treating COVID-19 disease” (EP20173595.8); outside the submitted work. O.T. Guenat reports grants from Swiss National Science Foundation (no. 185365), Eurostars (H2020) (project no. 12977 Aim4Doc), ITN (H2020) (project no. 812954, EUROoC); patents WO2015032889 and WO2018096054 (lung-on-chip), licensed to AlveoliX AG; and is a minority shareholder in AlveoliX AG, Switzerland and AlveoliX Technologies AG, Switzerland; outside the submitted work. P. Henrot reports grants from Bordeaux University Hospital, Fondation Bordeaux Université; lecture honoraria from Rhumatos journal; travel support from Chiesi; and receipt of equipment from Avad; outside the submitted work. L. van Os reports grants from European Union's Horizon 2020 research and innovation programme under grant agreement no. 812954 (EUROoC ITN project), and University of Bern, outside the submitted work. J.K. Burgess reports support for the present manuscript from Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) Aspasia-premie subsidienummer 015.013.010. In addition, J.K. Burgess reports grants from Boehringer Ingelheim, outside the submitted work, and is the Assembly Chair of the Respiratory Structure and Function Assembly within the American Thoracic Society and a board member of the Netherlands Respiratory Society. All other authors have nothing to disclose.
Support statement: M.M. Joglekar is funded by the Graduate School of Medical Sciences of the University of Groningen. M. Nizamoglu and J.K. Burgess receive unrestricted research funds from Boehringer Ingelheim. J.K. Burgess also acknowledges support from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) (Aspasia 015.013.010). A-K. Larsson Callerfelt acknowledges support from the Swedish Heart Lung Foundation (grant 20210382). L. van Os and O.T. Guenat thank the H2020 ITN network EUROoC, project no. 812954 for its financial support. R. Farre acknowledges partial support from the Spanish Ministry of Science and Innovation (PID2020-113910RB-I00-AEI/10.13039/501100011033). J. Otero acknowledges partial support from the Spanish Ministry of Science and Innovation (PGC2018-097323-A-I00). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Labaki WW, Han MK. Chronic respiratory diseases: a global view. Lancet Respir Med 2020; 8: 531–533. doi: 10.1016/S2213-2600(20)30157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melo-Narváez MC, Stegmayr J, Wagner DE, et al. Lung regeneration: implications of the diseased niche and ageing. Eur Respir Rev 2020; 29: 200222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med (Lausanne) 2020; 7: 191. doi: 10.3389/fmed.2020.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou-Suckow Z, Duerr J, Hagner M, et al. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res 2017; 367: 537–550. doi: 10.1007/s00441-016-2562-z [DOI] [PubMed] [Google Scholar]
- 5.Khedoe PPPSJ, Wu X, Gosens R, et al. Repairing damaged lungs using regenerative therapy. Curr Opin Pharmacol 2021; 59: 85–94. doi: 10.1016/j.coph.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen TH, Calle EA, Niklason LE. Strategies for lung regeneration. Materials Today 2011; 14: 196–201. doi: 10.1016/S1369-7021(11)70114-6 [DOI] [Google Scholar]
- 7.van der Mark SC, Hoek RAS, Hellemons ME. Developments in lung transplantation over the past decade. Eur Respir Rev 2020; 29: 190132. doi: 10.1183/16000617.0132-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AJ, Spence JR. In vitro models to study human lung development, disease and homeostasis. Physiology 2017; 32: 246–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess JK, Harmsen MC. Chronic lung diseases: entangled in extracellular matrix. Eur Respir Rev 2022; 31: 210202. doi: 10.1183/16000617.0202-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira A, Müller M, Costa PF, et al. Advanced in vitro lung models for drug and toxicity screening: the promising role of induced pluripotent stem cells. Advanced Biology 2021; 6: 2101139. doi: 10.1002/adbi.202101139 [DOI] [PubMed] [Google Scholar]
- 11.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther 2011; 24: 452–465. doi: 10.1016/j.pupt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G, Betts C, Cunoosamy DM, et al. Use of precision cut lung slices as a translational model for the study of lung biology. Respir Res 2019; 20: 162. doi: 10.1186/s12931-019-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandurand RJ, Wang CG, Phillips NC, et al. Responsiveness of individual airways to methacholine in adult rat lung explants. J Appl Physiol (1985) 1993; 75: 364–372. doi: 10.1152/jappl.1993.75.1.364 [DOI] [PubMed] [Google Scholar]
- 14.Voabil P, de Bruijn M, Roelofsen LM, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat Med 2021; 27: 1250–1261. doi: 10.1038/s41591-021-01398-3 [DOI] [PubMed] [Google Scholar]
- 15.Martin C, Uhlig S, Ullrich V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J 1996; 9: 2479–2487. doi: 10.1183/09031936.96.09122479 [DOI] [PubMed] [Google Scholar]
- 16.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 2002; 119: 187–198. doi: 10.1085/jgp.119.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmotte P, Sanderson MJ. Ciliary beat frequency is maintained at a maximal rate in the small airways of mouse lung slices. Am J Respir Cell Mol Biol 2006; 35: 110–117. doi: 10.1165/rcmb.2005-0417OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohlsen A, Martin C, Vollmer E, et al. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J 2003; 21: 1024–1032. doi: 10.1183/09031936.03.00027502 [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006; 27: 143–150. doi: 10.1183/09031936.06.00114004 [DOI] [PubMed] [Google Scholar]
- 20.Donovan C, Bailey SR, Tran J, et al. Rosiglitazone elicits in vitro relaxation in airways and precision cut lung slices from a mouse model of chronic allergic airways disease. Am J Physiol Lung Cell Mol Physiol 2015; 309: L1219–L1228. doi: 10.1152/ajplung.00156.2015 [DOI] [PubMed] [Google Scholar]
- 21.Van Dijk EM, Culha S, Menzen MH, et al. Elastase-induced parenchymal disruption and airway hyper responsiveness in mouse precision cut lung slices: toward an ex vivo COPD model. Front Physiol 2016; 7: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhl FE, Vierkotten S, Wagner DE, et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J 2015; 46: 1150–1166. doi: 10.1183/09031936.00183214 [DOI] [PubMed] [Google Scholar]
- 23.Nonaka PN, Falcones B, Farre R, et al. Biophysically preconditioning mesenchymal stem cells improves treatment of ventilator-induced lung injury. Arch Bronconeumol 2020; 56: 179–181. doi: 10.1016/j.arbres.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 24.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, et al. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 2017; 312: L896–L902. doi: 10.1152/ajplung.00084.2017 [DOI] [PubMed] [Google Scholar]
- 25.Lofdahl A, Wenglen C, Rydell-Tormanen K, et al. Effects of 5-hydroxytryptamine class 2 receptor antagonists on bronchoconstriction and pulmonary remodeling processes. Am J Pathol 2018; 188: 1113–1119. doi: 10.1016/j.ajpath.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 26.Rosmark O, Ahrman E, Muller C, et al. Quantifying extracellular matrix turnover in human lung scaffold cultures. Sci Rep 2018; 8: 5409. doi: 10.1038/s41598-018-23702-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosmark O, Ibanez-Fonseca A, Thorsson J, et al. A tunable physiomimetic stretch system evaluated with precision cut lung slices and recellularized human lung scaffolds. Front Bioeng Biotechnol 2022; 10: 995460. doi: 10.3389/fbioe.2022.995460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondonedo JR, Bartolak-Suki E, Bou Jawde S, et al. A high-throughput system for cyclic stretching of precision-cut lung slices during acute cigarette smoke extract exposure. Front Physiol 2020; 11: 566. doi: 10.3389/fphys.2020.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clevers H. Modeling development and disease with organoids. Cell 2016; 165: 1586–1597. doi: 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- 30.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014; 345: 1247125. doi: 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- 31.Gunti S, Hoke ATK, Vu KP Jr, et al. Organoid and spheroid tumor models: techniques and applications. Cancers (Basel) 2021; 13: 874. doi: 10.3390/cancers13040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009; 106: 12771–12775. doi: 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–3036. doi: 10.1172/JCI68782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Wu Q, Sun X, et al. Organoids as a powerful model for respiratory diseases. Stem Cells Int 2020; 2020: 5847876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynds RE, Butler CR, Janes SM, et al. Expansion of human airway basal stem cells and their differentiation as 3D tracheospheres. Methods Mol Biol 2016; 1576: 43–53. doi: 10.1007/7651_2016_5 [DOI] [PubMed] [Google Scholar]
- 36.Sprott RF, Ritzmann F, Langer F, et al. Flagellin shifts 3D bronchospheres towards mucus hyperproduction. Respir Res 2020; 21: 222. doi: 10.1186/s12931-020-01486-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loebel C, Weiner AI, Eiken MK, et al. Microstructured hydrogels to guide self-assembly and function of lung alveolospheres. Adv Mater 2022; 34: e2202992. doi: 10.1002/adma.202202992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Ng-Blichfeldt JP, Ota C, et al. Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells 2020; 38: 1467–1478. doi: 10.1002/stem.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng-Blichfeldt JP, Schrik A, Kortekaas RK, et al. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 2018; 36: 461–474. doi: 10.1016/j.ebiom.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, van Dijk EM, Ng-Blichfeldt JP, et al. Mesenchymal WNT-5A/5B signaling represses lung alveolar epithelial progenitors. Cells 2019; 8: 1147. doi: 10.3390/cells8101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kathiriya JJ, Wang C, Zhou M, et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells. Nat Cell Biol 2022; 24: 10–23. doi: 10.1038/s41556-021-00809-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Riet S, van Schadewijk A, Khedoe P, et al. Organoid-based expansion of patient-derived primary alveolar type 2 cells for establishment of alveolus epithelial lung-chip cultures. Am J Physiol Lung Cell Mol Physiol 2022; 322: L526–L538. doi: 10.1152/ajplung.00153.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim JH, An GH, Kim JY, et al. Human pluripotent stem-cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov 2021; 7: 48. doi: 10.1038/s41420-021-00439-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol 2016; 18: 246–254. doi: 10.1038/ncb3312 [DOI] [PubMed] [Google Scholar]
- 45.Corro C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol 2020; 319: C151–C165. doi: 10.1152/ajpcell.00120.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, et al. Long-term expanding human airway organoids for disease modeling. EMBO J 2019; 38: e100300. doi: 10.15252/embj.2018100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J, Li C, Sachs N, et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci USA 2018; 115: 6822–6827. doi: 10.1073/pnas.1806308115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YW, Huang SX, de Carvalho A, et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 2017; 19: 542–549. doi: 10.1038/ncb3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dye BR, Dedhia PH, Miller AJ, et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife 2016; 5: e19732. doi: 10.7554/eLife.19732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCauley KB, Hawkins F, Serra M, et al. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 2017; 20: 844–857. doi: 10.1016/j.stem.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dekkers JF, Berkers G, Kruisselbrink E, et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 2016; 8: 344ra384. doi: 10.1126/scitranslmed.aad8278 [DOI] [PubMed] [Google Scholar]
- 52.Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels 2017; 3: 6. doi: 10.3390/gels3010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saldin LT, Cramer MC, Velankar SS, et al. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater 2017; 49: 1–15. doi: 10.1016/j.actbio.2016.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Hilster RHJ, Sharma PK, Jonker MR, et al. Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am J Physiol Lung Cell Mol Physiol 2020; 318: L698–L704. doi: 10.1152/ajplung.00451.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pouliot RA, Link PA, Mikhaiel NS, et al. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J Biomed Mater Res A 2016; 104: 1922–1935. doi: 10.1002/jbm.a.35726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Garcia FD, de Hilster RHJ, Sharma PK, et al. Architecture and composition dictate viscoelastic properties of organ-derived extracellular matrix hydrogels. Polymers 2021; 13: 3113. doi: 10.3390/polym13183113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heath DE. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen Eng Transl Med 2019; 5: 155–166. doi: 10.1007/s40883-018-0080-0 [DOI] [Google Scholar]
- 58.Song YH, Maynes MA, Hlavac N, et al. Development of novel apoptosis-assisted lung tissue decellularization methods. Biomater Sci 2021; 9: 3485–3498. doi: 10.1039/D1BM00032B [DOI] [PubMed] [Google Scholar]
- 59.Pouliot RA, Young BM, Link PA, et al. Porcine lung-derived extracellular matrix hydrogel properties are dependent on pepsin digestion time. Tissue Eng Part C Methods 2020; 26: 332–346. doi: 10.1089/ten.tec.2020.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussey GS, Nascari DG, Saldin LT, et al. Ultrasonic cavitation to prepare ECM hydrogels. Acta Biomater 2020; 108: 77–86. doi: 10.1016/j.actbio.2020.03.036 [DOI] [PubMed] [Google Scholar]
- 61.Falcones B, Sanz-Fraile H, Marhuenda E, et al. Bioprintable lung extracellular matrix hydrogel scaffolds for 3D culture of mesenchymal stromal cells. Polymers (Basel) 2021; 13: 2350. doi: 10.3390/polym13142350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marhuenda E, Villarino A, Narciso ML, et al. Lung extracellular matrix hydrogels enhance preservation of type II phenotype in primary alveolar epithelial cells. Int J Mol Sci 2022; 23: 4888. doi: 10.3390/ijms23094888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beachley V, Ma G, Papadimitriou C, et al. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J Biomed Mater Res A 2018; 106: 147–159. doi: 10.1002/jbm.a.36218 [DOI] [PubMed] [Google Scholar]
- 64.Ravindra A, D'Angelo W, Zhang L, et al. Human bronchial epithelial cell growth on homologous versus heterologous tissue extracellular matrix. J Surg Res 2021; 263: 215–223. doi: 10.1016/j.jss.2021.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saldin LT, Klimak M, Hill RC, et al. The effect of normal, metaplastic, and neoplastic esophageal extracellular matrix upon macrophage activation. J Immunol Regen Med 2021; 13: 100037. doi: 10.1016/j.regen.2020.100037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petrou CL, D'Ovidio TJ, Bolukbas DA, et al. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J Mater Chem B 2020; 8: 6814–6826. doi: 10.1039/D0TB00613K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saleh KS, Hewawasam R, Serbed P, et al. Engineering hybrid-hydrogels comprised of healthy or diseased decellularized extracellular matrix to study pulmonary fibrosis. Cell Mol Bioeng 2022; 15: 505–519. doi: 10.1007/s12195-022-00726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nizamoglu M, de Hilster RHJ, Zhao F, et al. An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition. Acta Biomater 2022; 147: 50–62. doi: 10.1016/j.actbio.2022.05.031 [DOI] [PubMed] [Google Scholar]
- 69.Falcones B, Soderlund Z, Ibanez-Fonseca A, et al. hLMSC secretome affects macrophage activity differentially depending on lung-mimetic environments. Cells 2022; 11: 1866. doi: 10.3390/cells11121866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010; 328: 1662–1668. doi: 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Si L, Bai H, Rodas M, et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat Biomed Eng 2021; 5: 815–829. doi: 10.1038/s41551-021-00718-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thacker VV, Sharma K, Dhar N, et al. Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model. EMBO Rep 2021; 22: e52744–e52744. doi: 10.15252/embr.202152744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benam KH, Novak R, Nawroth J, et al. Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung Chip. Cell Systems 2016; 3: 456–466. doi: 10.1016/j.cels.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 74.Hajipouran Benam K, Novak R, Villenave R, et al. Human small airway-on-a-chip: a novel microphysiological system to model lung inflammation, accelerate drug development and enable inhalational toxico-analysis. Eur Respir J 2016; 48: Suppl. 60, OA4542. [Google Scholar]
- 75.Varone A, Nguyen JK, Leng L, et al. A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it. Biomaterials 2021; 275: 120957. doi: 10.1016/j.biomaterials.2021.120957 [DOI] [PubMed] [Google Scholar]
- 76.Stucki AO, Stucki JD, Hall SR, et al. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015; 15: 1302–1310. doi: 10.1039/C4LC01252F [DOI] [PubMed] [Google Scholar]
- 77.Stucki JD, Hobi N, Galimov A, et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci Rep 2018; 8: 14359. doi: 10.1038/s41598-018-32523-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sengupta A, Roldan N, Kiener M, et al. A new immortalized human alveolar epithelial cell model to study lung injury and toxicity on a breathing lung-on-chip system. Front Toxicol 2022; 4: 840606. doi: 10.3389/ftox.2022.840606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zamprogno P, Wüthrich S, Achenbach S, et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun Biol 2021; 4: 168–168. doi: 10.1038/s42003-021-01695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeinali S, Bichsel CA, Hobi N, et al. Human microvasculature-on-a chip: anti-neovasculogenic effect of nintedanib in vitro. Angiogenesis 2018; 21: 861–871. doi: 10.1007/s10456-018-9631-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeinali S, Thompson EK, Gerhardt H, et al. Remodeling of an in vitro microvessel exposed to cyclic mechanical stretch. APL Bioeng 2021; 5: 026102–026102. doi: 10.1063/5.0010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campillo N, Jorba I, Schaedel L, et al. A novel chip for cyclic stretch and intermittent hypoxia cell exposures mimicking obstructive sleep apnea. Front Physiol 2016; 7: 319. doi: 10.3389/fphys.2016.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tas S, Rehnberg E, Bölükbas DA, et al. 3D printed lung on a chip device with a stretchable nanofibrous membrane for modeling ventilator induced lung injury. BioRivx 2021; preprint [ 10.1101/2021.07.02.450873]. [DOI]
- 84.Jung O, Tung YT, Sim E, et al. Development of human-derived, three-dimensional respiratory epithelial tissue constructs with perfusable microvasculature on a high-throughput microfluidics screening platform. Biofabrication 2022; 14: 025012. doi: 10.1088/1758-5090/ac32a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banerjee A, Trivedi CM, Damera G Jr, et al. Trichostatin A abrogates airway constriction, but not inflammation, in murine and human asthma models. Am J Respir Cell Mol Biol 2012; 46: 132–138. doi: 10.1165/rcmb.2010-0276OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauenstein L, Switalla S, Prenzler F, et al. Assessment of immunotoxicity induced by chemicals in human precision-cut lung slices (PCLS). Toxicol In Vitro 2014; 28: 588–599. doi: 10.1016/j.tiv.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 87.Neuhaus V, Schaudien D, Golovina T, et al. Assessment of long-term cultivated human precision-cut lung slices as an ex vivo system for evaluation of chronic cytotoxicity and functionality. J Occup Med Toxicol 2017; 12: 13. doi: 10.1186/s12995-017-0158-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Temann A, Golovina T, Neuhaus V, et al. Evaluation of inflammatory and immune responses in long-term cultured human precision-cut lung slices. Hum Vaccin Immunother 2017; 13: 351–358. doi: 10.1080/21645515.2017.1264794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sturton RG, Trifilieff A, Nicholson AG, et al. Pharmacological characterization of indacaterol, a novel once daily inhaled β2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther 2008; 324: 270–275. doi: 10.1124/jpet.107.129296 [DOI] [PubMed] [Google Scholar]
- 90.Butler CR, Hynds RE, Gowers KH, et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 2016; 194: 156–168. doi: 10.1164/rccm.201507-1414OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hild M, Jaffe AB. Production of 3D airway organoids from primary human airway basal cells and their use in high-throughput screening. Curr Protoc Cell Biol 2016; 37: IE.9.1–IE.9.15. [DOI] [PubMed] [Google Scholar]
- 92.Konishi S, Gotoh S, Tateishi K, et al. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports 2016; 6: 18–25. doi: 10.1016/j.stemcr.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan Q, Choi KM, Sicard D, et al. Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 2017; 113: 118–132. doi: 10.1016/j.biomaterials.2016.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacob A, Morley M, Hawkins F, et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 2017; 21: 472–488. doi: 10.1016/j.stem.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018; 555: 251–255. doi: 10.1038/nature25786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller AJ, Dye BR, Ferrer-Torres D, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 2019; 14: 518–540. doi: 10.1038/s41596-018-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nikolić MZ, Caritg O, Jeng Q, et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. eLife 2017; 6: e26575. doi: 10.7554/eLife.26575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bol L, Galas J-C, Hillaireau H, et al. A microdevice for parallelized pulmonary permeability studies. Biomed Microdevices 2013; 16: 277–285. doi: 10.1007/s10544-013-9831-3 [DOI] [PubMed] [Google Scholar]
- 99.Benam KH, Villenave R, Lucchesi C, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods 2016; 13: 151–157. doi: 10.1038/nmeth.3697 [DOI] [PubMed] [Google Scholar]
- 100.Mejias JC, Nelson MR, Liseth O, et al. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip 2020; 20: 3601–3611. doi: 10.1039/D0LC00644K [DOI] [PubMed] [Google Scholar]
- 101.Humayun M, Chow CW, Young EWK. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip 2018; 18: 1298–1309. doi: 10.1039/C7LC01357D [DOI] [PubMed] [Google Scholar]
- 102.Barkal LJ, Procknow CL, Alvarez-Garcia YR, et al. Microbial volatile communication in human organotypic lung models. Nat Commun 2017; 8: 1770. doi: 10.1038/s41467-017-01985-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Punde TH, Wu W-H, Lien P-C, et al. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integr Biol (Camb) 2014; 7: 162–169. doi: 10.1039/c4ib00239c [DOI] [PubMed] [Google Scholar]
- 104.Artzy-Schnirman A, Zidan H, Elias-Kirma S, et al. Capturing the onset of bacterial pulmonary infection in acini-on-chips. Adv Biosyst 2019; 3: e1900026. doi: 10.1002/adbi.201900026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang D, Liu T, Liao J, et al. Reversed-engineered human alveolar lung-on-a-chip model. Proc Natl Acad Sci USA 2021; 118: e2016146118. doi: 10.1073/pnas.2016146118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghosh A, Koziol-White CJ, Asosingh K, et al. Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma. Proc Natl Acad Sci USA 2016; 113: E2355–E2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ressmeyer AR, Larsson AK, Vollmer E, et al. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J 2006; 28: 603–611. doi: 10.1183/09031936.06.00004206 [DOI] [PubMed] [Google Scholar]
- 108.Cooper PR, Panettieri RA,Jr. Steroids completely reverse albuterol-induced β2-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol 2008; 122: 734–740. doi: 10.1016/j.jaci.2008.07.040 [DOI] [PubMed] [Google Scholar]
- 109.Kennedy JL, Koziol-White CJ, Jeffus S, et al. Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J Allergy Clin Immunol 2018; 141: 1887–1890. doi: 10.1016/j.jaci.2017.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danahay H, Pessotti Angelica D, Coote J, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep 2015; 10: 239–252. doi: 10.1016/j.celrep.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 111.Nawroth JC, Lucchesi C, Cheng D, et al. A microengineered airway lung chip models key features of viral-induced exacerbation of asthma. Am J Respir Cell Mol 2020; 63: 591–600. doi: 10.1165/rcmb.2020-0010MA [DOI] [PubMed] [Google Scholar]
- 112.Skronska-Wasek W, Mutze K, Baarsma HA, et al. Reduced frizzled receptor 4 expression prevents WNT/β-catenin-driven alveolar lung repair in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 172–185. doi: 10.1164/rccm.201605-0904OC [DOI] [PubMed] [Google Scholar]
- 113.Kruk DMLW, Wisman M, Noordhoek JA, et al. Paracrine regulation of alveolar epithelial damage and repair responses by human lung-resident mesenchymal stromal cells. Cells 2021; 10: 2860. doi: 10.3390/cells10112860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benam KH, Novak R, Ferrante TC, et al. Biomimetic smoking robot for in vitro inhalation exposure compatible with microfluidic organ chips. Nat Protoc 2020; 15: 183–206. doi: 10.1038/s41596-019-0230-y [DOI] [PubMed] [Google Scholar]
- 115.Hirai H, Liang X, Sun Y, et al. The sodium/glucose cotransporters as potential therapeutic targets for CF lung diseases revealed by human lung organoid swelling assay. Mol Ther Methods Clin Dev 2022; 24: 11–19. doi: 10.1016/j.omtm.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hannan NR, Sampaziotis F, Segeritz CP, et al. Generation of distal airway epithelium from multipotent human foregut stem cells. Stem Cells Dev 2015; 24: 1680–1690. doi: 10.1089/scd.2014.0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calucho M, Gartner S, Barranco P, et al. Validation of nasospheroids to assay CFTR functionality and modulator responses in cystic fibrosis. Sci Rep 2021; 11: 15511. doi: 10.1038/s41598-021-94798-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sette G, Lo Cicero S, Blacona G, et al. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient-derived nasal epithelial conditionally reprogrammed stem cells. Eur Respir J 2021; 58: 2100908. doi: 10.1183/13993003.00908-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strikoudis A, Cieślak A, Loffredo L, et al. Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 2019; 27: 3709–3723. doi: 10.1016/j.celrep.2019.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Felder M, Trueeb B, Stucki AO, et al. Impaired wound healing of alveolar lung epithelial cells in a breathing lung-on-a-chip. Front Bioeng Biotechnol 2019; 7: 3. doi: 10.3389/fbioe.2019.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Rijt SH, Bölükbas DA, Argyo C, et al. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015; 9: 2377–2389. doi: 10.1021/nn5070343 [DOI] [PubMed] [Google Scholar]
- 122.Dong M, Philippi C, Loretz B, et al. Tissue slice model of human lung cancer to investigate telomerase inhibition by nanoparticle delivery of antisense 2′-O-methyl-RNA. Int J Pharm 2011; 419: 33–42. doi: 10.1016/j.ijpharm.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 123.Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012; 122: 899–910. doi: 10.1172/JCI45817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi R, Radulovich N, Ng C, et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res 2019; 26: 1162–1174. doi: 10.1158/1078-0432.CCR-19-1376 [DOI] [PubMed] [Google Scholar]
- 125.Li Z, Qian Y, Li W, et al. Human lung adenocarcinoma-derived organoid models for drug screening. iScience 2020; 23: 101411. doi: 10.1016/j.isci.2020.101411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun 2019; 10: 3991. doi: 10.1038/s41467-019-11867-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell 2018; 175: 1972–1988. doi: 10.1016/j.cell.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruppen J, Wildhaber FD, Strub C, et al. Towards personalized medicine: chemosensitivity assays of patient lung cancer cell spheroids in a perfused microfluidic platform. Lab Chip 2015; 15: 3076–3085. doi: 10.1039/C5LC00454C [DOI] [PubMed] [Google Scholar]
- 129.Yang X, Li K, Zhang X, et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 2018; 18: 486–495. doi: 10.1039/C7LC01224A [DOI] [PubMed] [Google Scholar]
- 130.Kerns SJ, Belgur C, Petropolis D, et al. Human immunocompetent organ-on-chip platforms allow safety profiling of tumor-targeted T-cell bispecific antibodies. eLife 2021; 10: e67106. doi: 10.7554/eLife.67106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Santis MM, Alsafadi HN, Tas S, et al. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv Mater 2020; 33: 2005476. doi: 10.1002/adma.202005476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dabaghi M, Carpio MB, Moran-Mirabal JM, et al. 3D (bio)printing of lungs: past, present, and future. Eur Respir J 2023; 61: 2200417. doi: 10.1183/13993003.00417-2022 [DOI] [PubMed] [Google Scholar]
- 133.Campillo N, Falcones B, Otero J, et al. Differential oxygenation in tumor microenvironment modulates macrophage and cancer cell crosstalk: novel experimental setting and proof of concept. Front Oncol 2019; 9: 43. doi: 10.3389/fonc.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Redrup MJ, Igarashi H, Schaefgen J, et al. Sample management: recommendation for best practices and harmonization from the Global Bioanalysis Consortium Harmonization Team. AAPS J 2016; 18: 290–293. doi: 10.1208/s12248-016-9869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sabroe I, Dockrell DH, Vogel SN, et al. Identifying and hurdling obstacles to translational research. Nat Rev Immunol 2007; 7: 77–82. doi: 10.1038/nri1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Curtis MJ, Alexander S, Cirino G, et al. Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 2018; 175: 987–993. doi: 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brazma A, Hingamp P, Quackenbush J, et al. Minimum information about a microarray experiment (MIAME) – toward standards for microarray data. Nat Genet 2001; 29: 365–371. doi: 10.1038/ng1201-365 [DOI] [PubMed] [Google Scholar]
- 138.Percie Du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMC Vet Res 2020; 16: 242. doi: 10.1186/s12917-020-02451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.FDA Modernization Act 2.0, S.5002, 117th Congress. 2022. Available from: www.congress.gov/bill/117th-congress/senate-bill/5002.
- 140.McGrath JC, Curtis MJ. BJP is changing its requirements for scientific papers to increase transparency. Br J Pharmacol 2015; 172: 2671–2674. doi: 10.1111/bph.12954 [DOI] [Google Scholar]
- 141.Reproducibility: let's get it right from the start. Nat Commun 2018; 9: 3716. doi: 10.1038/s41467-018-06012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu AP, Chaudhuri O, Parekh SH. New advances in probing cell-extracellular matrix interactions. Integr Biol (Camb) 2017; 9: 383–405. doi: 10.1039/C6IB00251J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stelzer EHK, Strobl F, Chang B-J, et al. Light sheet fluorescence microscopy. Nat Rev Methods Primers 2021; 1: 73. [Google Scholar]
- 144.Dekkers JF, Alieva M, Wellens LM, et al. High-resolution 3D imaging of fixed and cleared organoids. Nat Protoc 2019; 14: 1756–1771. doi: 10.1038/s41596-019-0160-8 [DOI] [PubMed] [Google Scholar]
- 145.Salwig I, Spitznagel B, Wiesnet M, et al. Imaging lung regeneration by light sheet microscopy. Histochem Cell Biol 2021; 155: 271–277. doi: 10.1007/s00418-020-01903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khan MM, Poeckel D, Halavatyi A, et al. An integrated multiomic and quantitative label-free microscopy-based approach to study pro-fibrotic signalling in ex vivo human precision-cut lung slices. Eur Respir J 2021; 58: 2000221. doi: 10.1183/13993003.00221-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vazquez-Armendariz AI, Heiner M, El Agha E, et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J 2020; 39: e103476. doi: 10.15252/embj.2019103476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen X, Nadiarynkh O, Plotnikov S, et al. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 2012; 7: 654–669. doi: 10.1038/nprot.2012.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abraham T, Hirota J, Elliot M, et al. How multiphoton and harmonic generation microscopy methods are useful in understanding lung structure and related diseases. Microsc Microanal 2017; 17: 278–279. doi: 10.1017/S1431927611002261 [DOI] [Google Scholar]
- 150.Rozanova S, Barkovits K, Nikolov M, et al. Quantitative mass spectrometry-based proteomics: an overview. In: Marcus K, Eisenacher M, Sitek B, eds. Quantitative Methods in Proteomics. New York, NY, Springer US, 2021; pp. 85–116. [DOI] [PubMed] [Google Scholar]
- 151.Marzi J, Brauchle EM, Schenke-Layland K, et al. Non-invasive functional molecular phenotyping of human smooth muscle cells utilized in cardiovascular tissue engineering. Acta Biomater 2019; 89: 193–205. doi: 10.1016/j.actbio.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 152.Elowsson Rendin L, Lofdahl A, Ahrman E, et al. Matrisome properties of scaffolds direct fibroblasts in idiopathic pulmonary fibrosis. Int J Mol Sci 2019; 20: 4013. doi: 10.3390/ijms20164013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoffman ET, Uhl FE, Asarian L, et al. Regional and disease specific human lung extracellular matrix composition. Biomaterials 2023; 293: 121960. doi: 10.1016/j.biomaterials.2022.121960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bergholt MS, Serio A, Albro MB. Raman spectroscopy: guiding light for the extracellular matrix. Front Bioeng Biotechnol 2019; 7: 303. doi: 10.3389/fbioe.2019.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stegmayr J, Alsafadi HN, Langwinski W, et al. Isolation of high-yield and -quality RNA from human precision-cut lung slices for RNA-sequencing and computational integration with larger patient cohorts. Am J Physiol Lung Cell Mol Physiol 2021; 320: L232–L240. doi: 10.1152/ajplung.00401.2020 [DOI] [PubMed] [Google Scholar]
- 156.Bai Y, Krishnamoorthy N, Patel KR, et al. Cryopreserved human precision-cut lung slices as a bioassay for live tissue banking. A viability study of bronchodilation with bitter-taste receptor agonists. Am J Respir Cell Mol Biol 2016; 54: 656–663. doi: 10.1165/rcmb.2015-0290MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alysandratos KD, Garcia-de-Alba C, Yao C, et al. Culture impact on the transcriptomic programs of primary and iPSC-derived human alveolar type 2 cells. JCI Insight 2023; 8: e158937. doi: 10.1172/jci.insight.158937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leibel SL, Tseu I, Zhou A, et al. Metabolomic profiling of human pluripotent stem cell differentiation into lung progenitors. iScience 2022; 25: 103797. doi: 10.1016/j.isci.2022.103797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Uriarte JJ, Meirelles T, Gorbenko Del Blanco D, et al. Early impairment of lung mechanics in a murine model of Marfan syndrome. PLoS One 2016; 11: e0152124. doi: 10.1371/journal.pone.0152124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Migulina N, Tjin G, Faiz A, et al. Differential roles for lysyl oxidase (like) family members in chronic obstructive pulmonary disease; from gene and protein expression to function. FASEB J 2022; 36: e22374. doi: 10.1096/fj.202101553R [DOI] [PubMed] [Google Scholar]
- 161.Bevilacqua C, Hambura S, Wang L, et al. High-resolution line-scanning Brillouin microscopy for fast and low phototoxicity live-imaging of mechanical properties in biology. SPIE Proceedings 2021; 11645: 116450q. doi: 10.1117/12.2582722 [DOI] [Google Scholar]
- 162.Rad MA, Mahmodi H, Filipe EC, et al. Micromechanical characterisation of 3D bioprinted neural cell models using Brillouin microspectroscopy. Bioprinting 2022; 25: e00179. [Google Scholar]
- 163.Barrasa-Fano J, Shapeti A, Jorge-Peñas Á, et al. TFMLAB: A MATLAB toolbox for 4D traction force microscopy. SoftwareX 2021; 15: 100723. doi: 10.1016/j.softx.2021.100723 [DOI] [Google Scholar]
- 164.Alcaraz J, Otero J, Jorba I, et al. Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. Semin Cell Dev Biol 2018; 73: 71–81. doi: 10.1016/j.semcdb.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 165.Pugin J. Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit Care Med 2003; 31: Suppl. 4, S200–S206. [DOI] [PubMed] [Google Scholar]
- 166.Burgstaller G, Oehrle B, Gerckens M, et al. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 2017; 50: 1601805. doi: 10.1183/13993003.01805-2016 [DOI] [PubMed] [Google Scholar]
- 167.Nizamoglu M, Burgess JK. The multi-faceted extracellular matrix: unlocking its secrets for understanding the perpetuation of lung fibrosis. Curr Tissue Microenviron Rep 2022; 2: 53–71. doi: 10.1007/s43152-021-00031-2 [DOI] [Google Scholar]
- 168.Junior C, Narciso M, Marhuenda E, et al. Baseline stiffness modulates the non-linear response to stretch of the extracellular matrix in pulmonary fibrosis. Int J Mol Sci 2021; 22: 12928. doi: 10.3390/ijms222312928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reimelt AM, Vasilescu DM, Beare R, et al. 3D image analysis of the alveolar shape in human lungs. Eur Respir J 2021; 58: Suppl. 65, PA1876. [Google Scholar]
- 170.Barnes PJ, Bonini S, Seeger W, et al. Barriers to new drug development in respiratory disease. Eur Respir J 2015; 45: 1197–1207. doi: 10.1183/09031936.00007915 [DOI] [PubMed] [Google Scholar]
- 171.Yamaguchi S, Kaneko M, Narukawa M. Approval success rates of drug candidates based on target, action, modality, application, and their combinations. Clin Transl Sci 2021; 14: 1113–1122. doi: 10.1111/cts.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 2014; 13: 419–431. doi: 10.1038/nrd4309 [DOI] [PubMed] [Google Scholar]
- 173.Sgalla G, Lerede M, Richeldi L. Emerging drugs for the treatment of idiopathic pulmonary fibrosis: 2020 phase II clinical trials. Expert Opin Emerg Drugs 2021; 26: 93–101. doi: 10.1080/14728214.2021.1931119 [DOI] [PubMed] [Google Scholar]
- 174.Wilson JW, Li X, Pain MC. The lack of distensibility of asthmatic airways. Am Rev Respir Dis 1993; 148: 806–809. doi: 10.1164/ajrccm/148.3.806 [DOI] [PubMed] [Google Scholar]
- 175.Ward JE, Harris T, Bamford T, et al. Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur Respir J 2008; 32: 362–371. doi: 10.1183/09031936.00119307 [DOI] [PubMed] [Google Scholar]
- 176.Chapman DG, King GG, Berend N, et al. Avoiding deep inspirations increases the maximal response to methacholine without altering sensitivity in non-asthmatics. Respir Physiol Neurobiol 2010; 173: 157–163. doi: 10.1016/j.resp.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 177.Tschumperlin DJ, Dai G, Maly IV, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature 2004; 429: 83–86. doi: 10.1038/nature02543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park J-A, Tschumperlin DJ. Chronic intermittent mechanical stress increases MUC5AC protein expression. Am J Respir Cell Mol 2009; 41: 459–466. doi: 10.1165/rcmb.2008-0195OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stewart AG, Thomas B, Koff J. TGF-β: master regulator of inflammation and fibrosis. Respirology 2018; 23: 1096–1097. doi: 10.1111/resp.13415 [DOI] [PubMed] [Google Scholar]
- 180.Liu G, Cooley MA, Jarnicki AG, et al. Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis. JCI Insight 2019; 5: e124529. doi: 10.1172/jci.insight.124529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jones MG, Andriotis OG, Roberts JJ, et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. eLife 2018; 7: e36354. doi: 10.7554/eLife.36354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Berhan A, Harris T, Jaffar J, et al. Cellular microenvironment stiffness regulates eicosanoid production and signaling pathways. Am J Respir Cell Mol Biol 2020; 63: 819–830. doi: 10.1165/rcmb.2020-0227OC [DOI] [PubMed] [Google Scholar]
- 183.Wallmeier J, Nielsen KG, Kuehni CE, et al. Motile ciliopathies. Nat Rev Dis Primers 2020; 6: 77. doi: 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- 184.Lee DDH, Cardinale D, Nigro E, et al. Higher throughput drug screening for rare respiratory diseases: readthrough therapy in primary ciliary dyskinesia. Eur Respir J 2021; 58: 2000455. doi: 10.1183/13993003.00455-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hynds RE, Bonfanti P, Janes SM. Regenerating human epithelia with cultured stem cells: feeder cells, organoids and beyond. EMBO Mol Med 2017; 10: 139–150. doi: 10.15252/emmm.201708213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pan E, Bogumil D, Cortessis V, et al. A systematic review of the efficacy of preclinical models of lung cancer drugs. Front Oncol 2020; 10: 591. doi: 10.3389/fonc.2020.00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 2010; 9: 203–214. doi: 10.1038/nrd3078 [DOI] [PubMed] [Google Scholar]
- 188.Movia D, Prina-Mello A. Preclinical development of orally inhaled drugs (OIDs) – are animal models predictive or shall we move towards in vitro non-animal models? Animals 2020; 10: 1259. doi: 10.3390/ani10081259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Movia D, Bruni-Favier S, Prina-Mello A. In vitro alternatives to acute inhalation toxicity studies in animal models – a perspective. Front Bioeng Biotechnol 2020; 8: 549. doi: 10.3389/fbioe.2020.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]