Abstract
N-acetylcysteine (NAC) is a cystine prodrug shown to reduce cocaine- and cue-primed reinstatement of cocaine-seeking behavior in preclinical studies. In this inpatient study, the effects of NAC maintenance versus placebo on cocaine-seeking behavior were examined during cocaine-primed and unprimed self-administration sessions among non-treatment-seeking, cocaine-dependent individuals. Twelve participants completed this double-blind, placebo-controlled, within-subject crossover study. Each participant was maintained for 1 week (Sat–Fri) on NAC (1200-mg TID; 3600 mg/day total) and 1 week on placebo (0-mg TID); medication order was randomized. A subset of participants underwent proton magnetic resonance spectroscopy scans (n = 8) on the third day of medication (Mon) to assess neurochemistry in the rostral anterior cingulate (rACC; voxel = 4.5 cm3). In four randomized sessions (Tue–Fri) each week, each participant could earn unit amounts of cocaine (10 mg, fixed) versus money ($0.50 vs. $1.50) on a choice, progressive ratio schedule after insufflating active versus placebo cocaine-priming doses (110 mg vs. 4 mg). Relative to the placebo priming dose, the active cocaine priming dose (110 mg) increased cocaine-seeking behavior (p = .003). NAC reduced cocaine-primed cocaine-seeking behavior compared with placebo levels (p = .044) but did not alter placebo-primed cocaine-seeking behavior. The larger money alternative ($1.50) suppressed cocaine-seeking behavior relative to the smaller money alternative ($0.50; p = .011). Compared with placebo levels, NAC significantly decreased rACC glutamate + glutamine levels (p = .035) and numerically decreased rACC glutamate levels (p = .085). These preliminary findings indicate that NAC suppresses cocaine-seeking behavior in some, but not all, experimental scenarios. Further, our findings suggest NAC may exert its therapeutic effects by modulating excitatory tone in the rACC.
Keywords: anterior cingulate cortex, cocaine, drug-seeking behavior, glutamate, N-acetylcysteine, relapse
1 |. INTRODUCTION
Chronic cocaine use poses serious public health, economic, and societal problems. At present, there are no Food and Drug Administration (FDA)-approved medications to treat cocaine use disorder (CUD). However, one medication that has received considerable recent interest is N-acetylcysteine (NAC). NAC is a cystine prodrug that binds to the cystine-glutamate antiporter located on glial cells and exchanges 1:1 with glutamate, thereby increasing extracellular glutamate levels. This mechanism aligns well with CUD neurobiological deficits. Preclinical studies indicate that chronic cocaine exposure significantly lowers basal extrasynaptic glutamate levels throughout corticostriatal brain regions, which are central to cocaine-seeking behavior.1–5 NAC has been shown to reverse this deficit by restoring cystine-glutamate exchange, normalizing glial glutamate transporter (GLT-1) activity6–8 and regulating presynaptic mGluR2/3 inhibition of neurotransmission.9,10 These glutamatergic effects are thought to underlie NAC’s therapeutic effects. NAC has been reliably shown to reduce cocaine-primed reinstatement of cocaine-seeking behavior in animal models.6,9,11,12 The extant literature indicates NAC does not decrease stable, nonprimed cocaine-seeking behavior,13 except when paired with punishment.14 The preclinical literature suggests NAC may be an effective therapeutic agent for prolonging abstinence, but not for abstinence initiation. That is, NAC has been shown to attenuate the reinforcing properties of cocaine but only after a period of cocaine abstinence, which can be as brief as 1 day.11,12
Clinical research has investigated NAC’s effects on cocaine craving/withdrawal symptoms, cocaine and cue reactivity, and outpatient cocaine use. In an open-label, 4-week outpatient study, NAC was well tolerated at doses up to 3600 mg/day.15 Subject retention was better for higher doses (2400 and 3600 mg/day) compared with 1200 mg/day.15 Among patients who completed the study, cocaine use and craving scores significantly decreased with NAC dosing; however, these encouraging findings must be tempered as placebo control and experimental blinding were not implemented.15 In two double-blind, placebo-controlled crossover studies among cocaine-dependent individuals, 2400 mg of NAC did not significantly reduce cocaine withdrawal symptoms and craving16 but did significantly decrease cocaine cue-induced craving, interest in cocaine, and cue viewing time.17 Results from a small pilot trial comparing the effect of 4-day NAC dosing (400-mg TID [1200 mg] or 800-mg TID [2400 mg]) on cocaine versus neutral cue reactivity and physiological and subjective responses to 20 mg/70 kg intravenous cocaine showed that NAC did not alter cocaine’s subjective or physiological effects but did significantly reduce cocaine priming-induced craving, compared with an earlier phase when NAC was not administered.11 A randomized clinical trial compared the effects of placebo, NAC 1200 mg/day, and NAC 2400 mg/day on outpatient cocaine use, cocaine craving, dropout, and medication adherence.18 NAC was not more effective than placebo for reducing cocaine use, craving, dropout or improving medication adherence.18 However, among patients who were cocaine abstinent in the week prior to starting NAC, the 2400 mg/day NAC dose significantly increased time-to-lapse compared with placebo, providing clinical evidence of NAC’s therapeutic efficacy for relapse prevention.18 Finally, Bolin et al.19 conducted a double-blind, placebo-controlled, within-subject crossover study to examine whether short-term maintenance on NAC 2400 mg/day versus placebo altered cocaine-cue attentional bias, and reinforcing, subjective, and physiological effects of intranasal cocaine (0, 30, and 60 mg).19 NAC reduced cocaine-cue attentional bias and subjective responses to intranasal cocaine and reduced cocaine choice but only among participants who received NAC first before placebo.19 In summary, clinical evidence indicates NAC can reduce cocaine cue salience, attenuate subjective responses to cocaine administration and cocaine cues, and prolongs abstinence among individuals who were cocaine abstinent prior to NAC dosing.
To our knowledge, only one clinical study has investigated NAC’s putative neurochemical mechanism of action, that is, modulation of glutamatergic tone, among cocaine-dependent individuals. In a randomized crossover, open-label proton magnetic resonance spectroscopy (1H MRS) study, Schmaal et al.20 reported that basal dorsal anterior cingulate (dACC) glutamate to total creatine ratio levels (GLU/tCr) among abstinent cocaine-dependent individuals (n = 8) were higher than healthy volunteers (n = 14). A single unblinded dose of 2400-mg NAC 1 h before 1H MRS significantly reduced dACC GLU/tCr levels among cocaine-dependent individuals compared with basal levels but did not alter GLU/tCr levels among controls. After NAC dosing, dACC GLU/tCr levels among cocaine-dependent individuals were not different from controls. The authors concluded that NAC “normalized” dACC GLU levels among cocaine-dependent individuals.20 Although that study provided the first clinical evidence of NAC’s effects on neurochemistry, only a single dose of NAC was administered; thus, effects of repeated NAC dosing remain unclear. Finally, Schmaal et al did not measure cocaine-seeking/self-administration behavior; thus, NAC’s effects on neurochemistry and cocaine self-administration have yet to be reported in the same individuals.
The present study investigated four gaps in the literature. First, this study extended the Schmaal et al. study by investigating rACC neurochemistry after repeated NAC dosing under double-blind and placebo-controlled conditions. Second, this is the first study in humans to measure NAC’s effects on neurochemistry and cocaine-seeking behavior in the same participants. Third, we evaluated NAC’s effects on cocaine-seeking behavior under cocaine-primed and unprimed conditions, that is, translation of robust preclinical findings. Fourth, we evaluated NAC’s effects on cocaine-seeking behavior in combination with a human laboratory analogue of contingency management, that is, by modifying the alternative choice money amount in a cocaine versus money choice progressive ratio paradigm.21 Contingency management is well established as an effective behavioral strategy for promoting cocaine abstinence22–24 and may augment NAC’s efficacy. Using a double-blind, placebo-controlled, randomized-crossover design, we evaluated whether maintenance on a high oral dose of NAC (3600 mg/day) decreases cocaine-seeking behavior using a cocaine versus money choice progressive ratio paradigm (e.g., Greenwald et al25) among cocaine-dependent individuals during a 16-day continuous, monitored inpatient stay. Specifically, we tested whether NAC alters cocaine seeking under cocaine-primed and unprimed conditions and whether NAC’s effects on cocaine seeking interact with alternative money amount. Finally, using single-voxel 1H MRS, we measured rostral ACC glutamate (GLU) and GLX (glutamate + glutamine) levels after 3 days of NAC versus placebo during recent cocaine abstinence. The rostral ACC was selected because previous research indicates cocaine users exhibit structural, functional, and neurochemical impairments in the rACC.26–28 Further, the rACC is implicated in response inhibition: a cognitive process shown to be impaired among cocaine users29 and one that may be rescued by NAC dosing.30 We hypothesized the following: (1) cocaine priming would increase cocaine-seeking behavior, (2) NAC would reduce cocaine-primed cocaine seeking, (3) the higher money amount alternative would reduce cocaine seeking, (4) NAC would augment the effect of the higher money amount alternative to further reduce cocaine seeking, and (5) NAC would decrease GLU and GLX levels in the rACC.
2 |. METHODS
2.1 |. Participant recruiting and selection
The local Institutional Review Board approved this study, which was conducted according to the Declaration of Helsinki. Volunteers aged 18–55 years, regardless of racial/ethnic background, were recruited by newspaper advertisements and word-of-mouth referral and were not seeking substance use disorder treatment. All volunteers provided informed consent for screening and separate consent to participate in the study.
All candidates received routine medical (history and physical) exam with standard laboratory tests (complete blood chemistry, urinalysis, urine pregnancy test for females, tuberculin screening), 12-lead ECG, and psychiatric interview (SCID-IV31). Table S1 presents all inclusion and exclusion criteria. Fifty-three candidates were screened, and 36 were excluded. Of the 17 individuals who enrolled, three were lost to contact prior to the cocaine reinforcement qualifying session. Experimental cocaine did not function as a reinforcer for two participants during a cocaine reinforcement screen, and thus, they were excluded (defined below).
The Cocaine Purchasing and Use Patterns (CPUP) semistructured interview was used to characterize past-month sources and amounts of income, drug purchasing and consumption, and price- and availability-related factors (e.g., number of dealers, time, and distance costs) related to these behaviors.32
A single-session cocaine reinforcement screen served as the final selection criterion.21 Participants insufflated Drug A (4 or 110 mg) at 9:00 a.m. and Drug B (110 or 4 mg) at 11:00 a.m., in counterbalanced order. Subjective and physiological effects were measured at −0.5 h (baseline), and 0.25-, 0.5-, 0.75-, 1.0-, and 1.5-h postdrug administration. An 11-trial Drug A versus Drug B choice progressive ratio (PR) task was conducted from 1:00 to 4:00 p.m. during which participants could earn 10 mg and/or 0 mg units (max earned dose = 110 mg), with response requirements for each option increasing independently and exponentially across the 11 choice trials (100, 250, 505, 915, 1530, 2400, 3575, 5105, 7040, 9430, and 12325). Cocaine reinforcement was defined as occurring when cocaine (10-mg unit dose) maintained global preference (earned ≥6 of 11 trials) and the participant earned cocaine ≥2 more trials than placebo. Two subjects did not meet these criteria and were excluded.
2.2 |. Experimental design
For cocaine-seeking behavior, there were three factors: Medication Dose (NAC 3600 mg vs. 0 mg) × Priming Dose (Cocaine 110 mg vs. 4 mg [placebo]) × Money Alternative ($1.50 vs. $0.50). For 1H MRS metabolites, Medication Dose was the sole factor. For subjective and physiological response measures, there were three factors: Medication Dose (NAC 3600 mg vs. 0 mg) × Priming Dose (Cocaine 110 mg vs. 4 mg) × Session Time (prepriming vs. postpriming dose).
2.3 |. Protocol timeline
Each participant lived on a residential unit for 16 consecutive nights. The participant was admitted Thursday afternoon (procedural training) and, after passing the cocaine reinforcement qualifying session (Friday), maintained on a medication (NAC or placebo) for the first experimental week (Saturday–Friday), then crossed over to the other medication condition for the second week (Saturday–Friday), and was discharged Saturday morning after the final Friday session. Importantly, NAC dosing was initiated during experimental cocaine abstinence (Saturday–Monday), consistent with the preclinical literature.11,12 1H MRS scans were acquired on Monday of each week. Thus, for participants who received NAC first, there was a 3-day washout period (Friday–Monday) before 1H MRS, and a 4-day washout period (Friday–Tuesday) before behavioral testing, to prevent possible carry-over effects (e.g., Bolin et al19). Participants were transported to/from the residential unit and laboratory under staff observation. Daily urinalysis ensured abstinence from unsanctioned drug use. Participants earned $40 per night on the residential unit.
2.4 |. Drug administration
Cocaine HCl powder (Research Triangle Institute, NC) was prepared in 110-mg constant-volume doses. Placebo contained 106-mg lactose and 4-mg cocaine. Response-contingent doses contained the earned cocaine dose with the remainder being lactose. The cocaine/lactose mixture was placed in a small plastic cup, and the participant insufflated the powder through a short plastic straw, while the research assistant observed.
NAC 600-mg capsules (Swanson Health Products, Fargo, ND) were placed inside opaque size-00 capsules with lactose filler. Each placebo dose (0 mg) consisted of lactose placed inside these same-colored opaque size-00 capsules. NAC or placebo doses were self-administered orally three times daily at 7:00 a.m., 3:00 p.m., and 11:00 p.m. under staff supervision.
2.5 |. Experimental sessions
2.5.1 |. Cocaine/money choice
Participants completed eight choice sessions (four per week), in randomized order, involving all medication-dose, cocaine priming-dose, and money-alternative combinations. Cigarette smoking was prohibited starting 1-h prior to each session. At 9:20 a.m., 10 min prior to the 3-h choice task (9:30 a.m. to 12:30 p.m.), the participant insufflated 110-mg powder that contained a placebo (4 mg) or active (110 mg) cocaine dose. The drug code referring to the active cocaine dose from the reinforcement-screening session (Drug A or Drug B, based on each participant’s random assignment) was used to label the active dose for choice sessions, and participants were told they could work for unit amounts of the same drug they preferred in the qualifying session.
During the choice PR task, the participant could earn fixed amounts of cocaine (10-mg unit dose) or money ($1.50 or $0.50, varied across sessions); the participant was also informed she/he was not obligated to respond at all. Cocaine-priming doses and money amounts were orthogonal factors, nested within medication dose (NAC or placebo) maintenance week. Response requirements on cocaine and money options were identical to the reinforcement-qualifying session. Maximum earned cocaine dose was 110 mg (10-mg units × 11 possible choices), and maximum earned money amount per condition was $16.50 ($1.50 per choice) or $5.50 ($0.50 per choice). The participant was told there was no advantage to completing the choice task before the 3-h time limit, as she/he could not receive the earned cocaine dose before the 3-h time limit and money earnings were deferred until end of participation.
2.5.2 |. Subjective and physiological effects
Vital signs (respiration rate, oxygen saturation, heart rate, and blood pressure) and subjective effects (see below) were assessed 15 min before and 10 min after the cocaine/placebo priming dose (just before the choice task), 3 h later just after the choice task (before earned cocaine self-administration), and, for safety, monitored for 2.5 h after earned cocaine self-administration.
Craving was measured with the 45-item, 7-point Likert format, Cocaine Craving Questionnaire (CCQ).33 CCQ subscales (Desire to Use Cocaine, Intention and Planning to Use Cocaine, Anticipation of Positive Outcome, Anticipation of Relief from Withdrawal or Dysphoria, and Lack of Control Over Use) were analyzed separately.
Eight visual analog scale (VAS) drug-effect ratings were obtained: Any Effect, High, Liking, Good Drug Effect, Bad Drug Effect, Stimulated, Sedated, and Want to Take Drug Again. Each VAS rating was on a scale from 0 (not at all) to 100 (extremely).
Psychostimulant effects were assessed with the 21-item, 5-point Likert format, Stimulant-Sensitive Adjective Rating Scale.34
2.5.3 |. Brain chemistry
Eight participants (one excluded for MRI contraindications; three excluded due to scanner unavailability) were scanned each Monday (third day of medication maintenance dose and cocaine abstinence) on a 3T Tesla Siemens Verio system. Cigarette smoking was prohibited starting 1 h prior to each scan. Anatomical T1-weighted images were acquired using the magnetization prepared rapid gradient echo sequence (TR = 2.2 s, TE = 3 ms, TI = 799 ms, flip angle = 13°, field-of-view [FOV] = 256 × 256 × 160 mm, 256 × 1 mm thick axial slices, matrix = 176 × 256, 1 mm3 pixel resolution). A voxel (2.0 × 1.5 × 1.5 cm; 4.5 cm3) was positioned in the rACC (Figure 3A). Anatomical landmarks and each participant’s prior scan guided voxel placement during the second scan. Water suppressed single-voxel 1H MRS spectra were acquired (eight measurements; 16 averages/measurement) using point resolved spectroscopy sequence (TE = 22 ms, TR = 4.0 s, data points = 2048, bandwidth = 2 kHz, no apodization) from each voxel location, followed by unsuppressed water spectra acquisition (one measurement of two averages; TR = 10 s).
FIGURE 3.
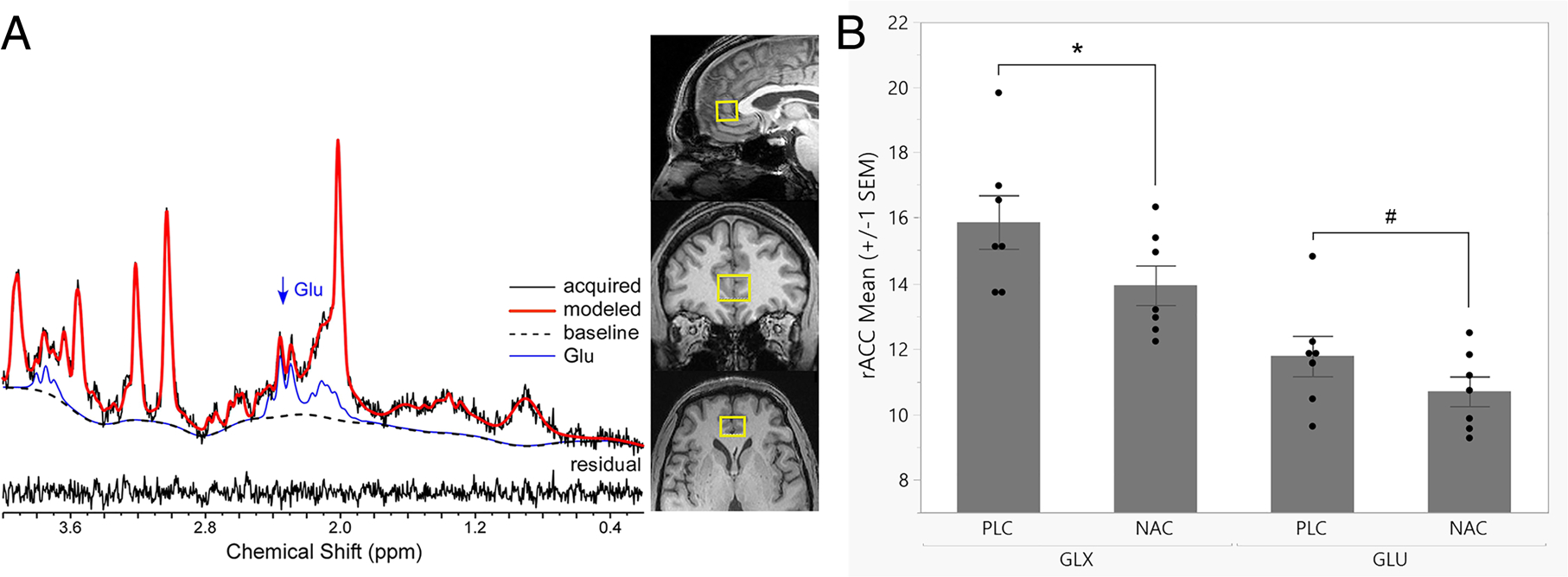
(A) A representative 1H MRS spectrum with LCModel fit and orthonormal slices of the rostral anterior cingulate cortex voxel (rACC; 4.5 cm3) are depicted. (B) Mean concentrations of rACC glutamate (GLU) and GLX (glutamate + glutamine) are depicted. Relative to placebo levels, NAC significantly reduced GLX levels by 11.5%, F(1,6) = 7.36, *p = .035, partial η2 = 0.55, very large effect; and numerically reduced GLU levels by 8.2%, F(1,6) = 4.23, #p = .085, partial η2 = 0.41, large effect
Postprocessing and quantification steps were 100% automated. For each 1H MRS scan, the eight spectra were phase-, shift-, and eddy current corrected before averaging and quantification using LCModel version 6.3 with a simulated basis set.35 T1-weighted images were B1-field bias-corrected, brain extracted, and segmented into partial volume maps of gray and white matter and cerebrospinal fluid using FreeSurfer and FSL tools.36,37 Voxel tissue composition and appropriate relaxation values, along with other correction factors, were used to quantify absolute metabolite values expressed as institutional units.38 Voxel replacement accuracy was confirmed by nonsignificant differences in gray/white matter and cerebrospinal fluid tissue fractions between scans 1 and 2. One participant exhibited poor data quality (possibly due to motion) and was excluded from analyses.
2.6 |. Data analyses
All variable distributions were evaluated for normality (skewness and kurtosis statistics) and corrected as needed (e.g., winsorization and log-10 transformations) prior to outcome analyses.
2.6.1 |. Cocaine seeking
Three-factor (2 Medication Doses [NAC vs. Placebo] × 2 Priming Doses [110 mg vs. 4 mg Cocaine] × 2 Money Alternative [$1.50 vs. $0.50]) repeated measures analyses of variance (rmANOVAs) were used to analyze total cocaine choices and cocaine breakpoint (separately).
2.6.2 |. Cocaine priming
Three-factor rmANOVAs (Medication Dose × Priming Dose × Session Time) were used to evaluate subjective and physiological responses to placebo versus cocaine priming doses.
2.6.3 |. Brain chemistry
The effect of NAC (vs. Placebo) on glutamate (GLU), glutamate plus glutamine (GLX), and other metabolite levels was analyzed via rmANOVAs.
The threshold for statistical significance for rmANOVA main effects was p ≤ .05 and partial η2 effect sizes were interpreted as follows: “small” ≤0.09, 0.10 ≤ “moderate” ≤0.24, 0.25 ≤ “large” ≤0.49, and “very large” ≥ 0.50. Bonferroni-corrected thresholds were adopted within each set of analyses to avoid false positives from multiple comparisons. Descriptive statistics are presented as mean ± 1 standard deviation (SD) in the text and mean ± 1 standard error of the mean in figures, unless otherwise noted.
3 |. RESULTS
3.1 |. Participant characteristics
The 12 participants who completed the behavioral procedures were 48.1 ± 4.5 years old and reported lifetime cocaine-use duration of 20.7 ± 6.7 years (Table 1). Eleven smoked cocaine, whereas one injected. All reported smoking tobacco cigarettes, averaging 5.3 ± 2.5 daily.
TABLE 1.
Participant characteristics
| Subj. | Med First | Scan | Demographics |
Lifetime and recent pattern of cocaine use |
Other past-month substance use |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | Sex | Age | Duration cocaine | Route | # quit attempts | CPUP purchase time | CPUP $ spent cocaine | # past 30d cocaine any use | # past 30d alcohol any use | # past 30d marijuana any use | |||
| 1 | NAC | No | AA | M | 54 | 28 | SM | 10 | 45 | 500 | 8 | 0 | 0 |
| 2 | NAC | (Yes)a | AA | M | 51 | 23 | SM | 10 | 30 | 1200 | 4 | 25 | 0 |
| 3 | NAC | Yes | AA | M | 47 | 22 | SM | 0 | 10 | 240 | 12 | 12 | 12 |
| 4 | NAC | Yes | C | M | 47 | 17 | SM | 2 | 30 | 500 | 13 | 0 | 0 |
| 5 | NAC | Yes | AA | M | 46 | 27 | IV | 3 | 40 | 500 | 6 | 6 | 2 |
| 6 | PLA | No | Multi | M | 55 | 5 | SM | 2 | 5 | 120 | 9 | 12 | 0 |
| 7 | PLA | Yes | AA | M | 40 | 22 | SM | 2 | 1 | 2000 | 28 | 0 | 25 |
| 8 | PLA | No | AA | F | 45 | 20 | SM | 50 | 60 | 80 | 4 | 8 | 3 |
| 9 | NAC | Yes | AA | M | 45 | 15 | SM | 10 | 20 | 600 | 20 | 8 | 1 |
| 10 | PLA | Yes | AA | M | 53 | 25 | SM | 10 | 20 | 200 | 3 | 7 | 7 |
| 11 | NAC | No | AA | M | 50 | 28 | SM | 3 | 5 | 80 | 16 | 30 | 28 |
| 12 | PLA | Yes | AA | M | 44 | 16 | SM | 4 | 10 | 280 | 15 | 30 | 0 |
| % or M | 58% | 67% | 83% | 92% | 48.1 (4.5) | 20.7 (6.7) | 92% | 8.8 (13.5) | 23.0 (18.4) | $525.0 ($559.7) | 11.1 (7.7) | 11.5 (11.0) | 7.1 (10.3) |
| (±1 SD) median | NAC | Yes | AA | M | 47 | 22 | SM | 3.5 | 20 | $390 | 10.5 | 8 | 1.5 |
Note. Patterns of cocaine use are described in terms of self-reported duration of regular use (years), current primary route of use (smoked [SM] or intravenous [IV]), and number of lifetime quit attempts.
Abbreviations: AA, African-American; C, Caucasian; Multi, multi-racial; M, Male; F, Female; NAC, N-acetylcysteine; PLA, placebo; SD, standard deviation.
H MRS data quality was poor subject #2 and thus excluded from analyses of the imaging data. See text for further details on participant characteristics.
Using median values from the CPUP, participants reported past-month total income of $1057, spending a total of $390 on cocaine. Participants had easy access to cocaine from their suppliers: eight walked and three drove to get cocaine, and one had it delivered. Participants reported a median of 3.5 weekly cocaine purchases, subjective cocaine purity of 50%, round-trip purchase time of 20 min, and purchase amount per episode of $40.
3.2 |. Cocaine-seeking behavior
3.2.1 |. Cocaine breakpoints
Three-factor rmANOVA indicated significant cocaine breakpoint effects for Cocaine-Priming, Money Amount Alternative, and Medication Dose by Cocaine-Priming interaction (ps < .05), but a nonsignificant Medication Dose effect, F(1,11) = 2.84, p = .12, partial η2 = 0.21, moderate-to-large effect (Figure 1; Table S2. Relative to placebo-priming, cocaine-priming significantly increased cocaine breakpoint, F (1,11) = 14.11, p = .003, partial η2 = 0.56, very large effect. Relative to $0.50, the $1.50 money alternative significantly decreased cocaine breakpoint, F(1,11) = 9.35, p = .011, partial η2 = 0.46, large effect. Finally, relative to the placebo dose, NAC attenuated the effect of cocaine-priming on cocaine breakpoint, F(1,11) = 5.19, p = .044, partial η2 = 0.32, large effect.
FIGURE 1.
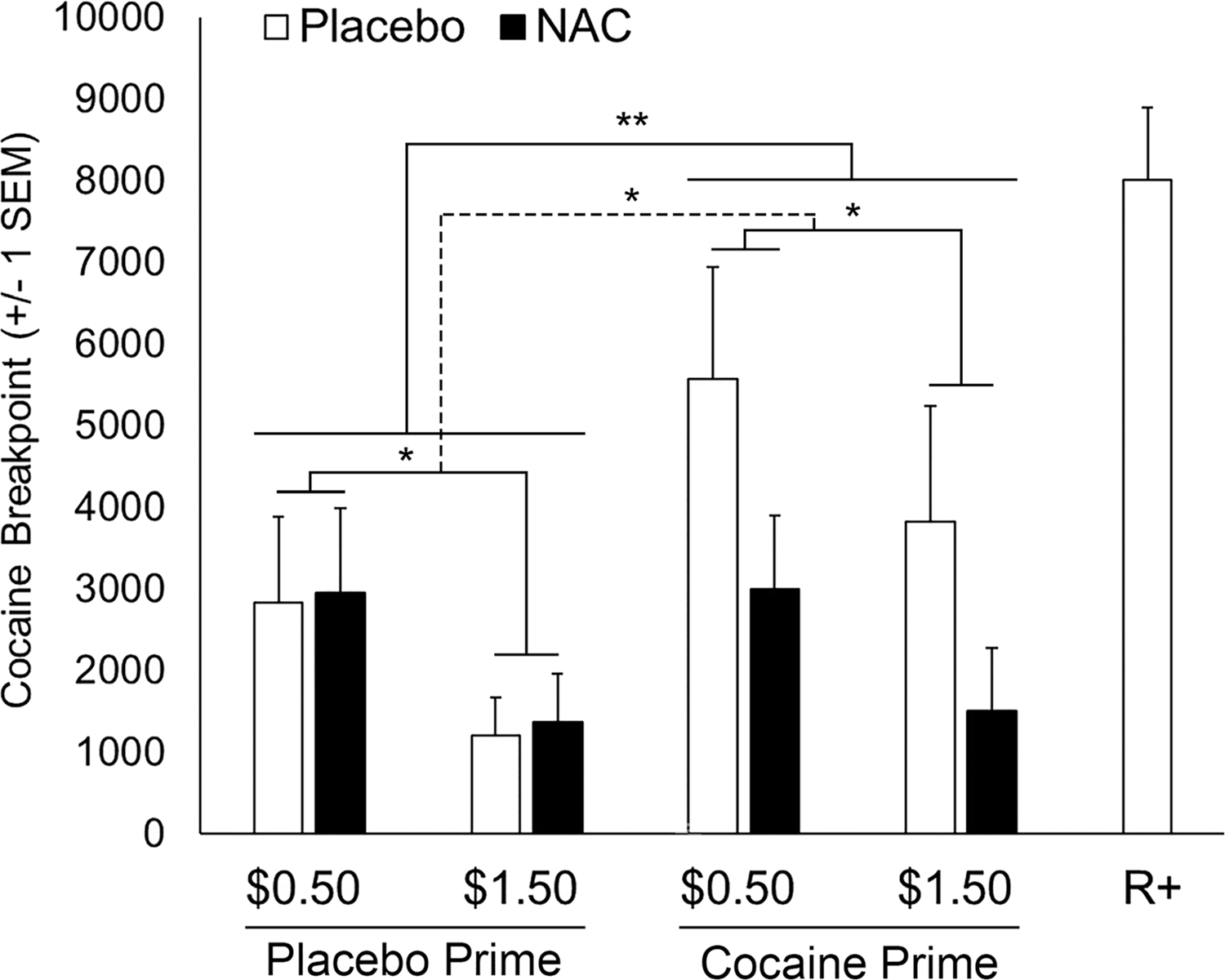
The effects of cocaine- versus placebo-priming, money-alternative amount ($0.50 vs. $1.50 per choice), and NAC versus placebo on mean (±1 SEM) cocaine breakpoint values (10 mg unit dose) are depicted. Relative to placebo-priming, cocaine-priming significantly increased cocaine breakpoint, F(1,11) = 14.11, **p = .003, partial η2 = 0.56, very large effect (**solid line). Relative to the $0.50 money alternative, the $1.50 money alternative significantly decreased cocaine breakpoint for placebo- and cocaine-priming, F(1,11) = 9.35, *p = .011, partial η2 = 0.46, large effect (*solid lines). Relative to the placebo dose, NAC (3600 mg/day) attenuated the effect of cocaine-priming on cocaine breakpoint, F(1,11) = 5.19, *p = .044, partial η2 = 0.32, large effect (*dashed line). For comparison, the rightmost bar indicates cocaine (10-mg unit dose vs. placebo [no money] choice) breakpoint during the reinforcement screening session (R+) prior to medication exposure
3.2.2 |. Cocaine choices
Consistent with the cocaine breakpoint findings, three-factor rmANOVA exhibited significant effects for Cocaine-Priming, Money Amount Alternative, and Medication Dose by Cocaine-Priming interaction, but a non-significant Medication Dose effect, F(1,11) = 2.60, p = .14, partial η2 = 0.19, moderate-to-large effect (Figure S1). Relative to the placebo-priming dose, the cocaine-priming dose significantly increased total number of cocaine choices, F(1,11) = 10.53, p = .008, partial η2 = 0.49, large effect (not shown). Relative to $0.50, the $1.50 money alternative significantly decreased total cocaine choices, F (1,11) = 13.20, p = .004, partial η2 = 0.55, very large effect. Finally, relative to the placebo dose, NAC attenuated the effect of cocaine-priming on total number of cocaine choices, F(1,11) = 4.71, p = .05, partial η2 = 0.30, large effect.
3.2.3 |. Naturalistic and experimental cocaine-seeking behavior are correlated
Pearson correlations indicated that CPUP cocaine purchasing time was significantly positively correlated with cocaine breakpoint and cocaine choices in seven of the eight experimental conditions (rs = 0.58–0.94, ps < .05; all except for the placebo-medication, cocaine-primed, and $0.50 money alternative condition). Three of eight experimental conditions remained significant at the Bonferroni-corrected threshold (p < .0063) for both cocaine breakpoint and cocaine choices (rs = 0.74–0.94; ps < .0063; Figure 2) and accounted for 55.4%–87.4% of the variance. The extent to which individuals “worked” to buy cocaine outside of the laboratory (i.e., purchase travel time) correlated with the extent to which individuals “worked” to earn cocaine in this laboratory paradigm (i.e., cocaine breakpoint) and thus support the external validity of our cocaine-seeking findings.
FIGURE 2.
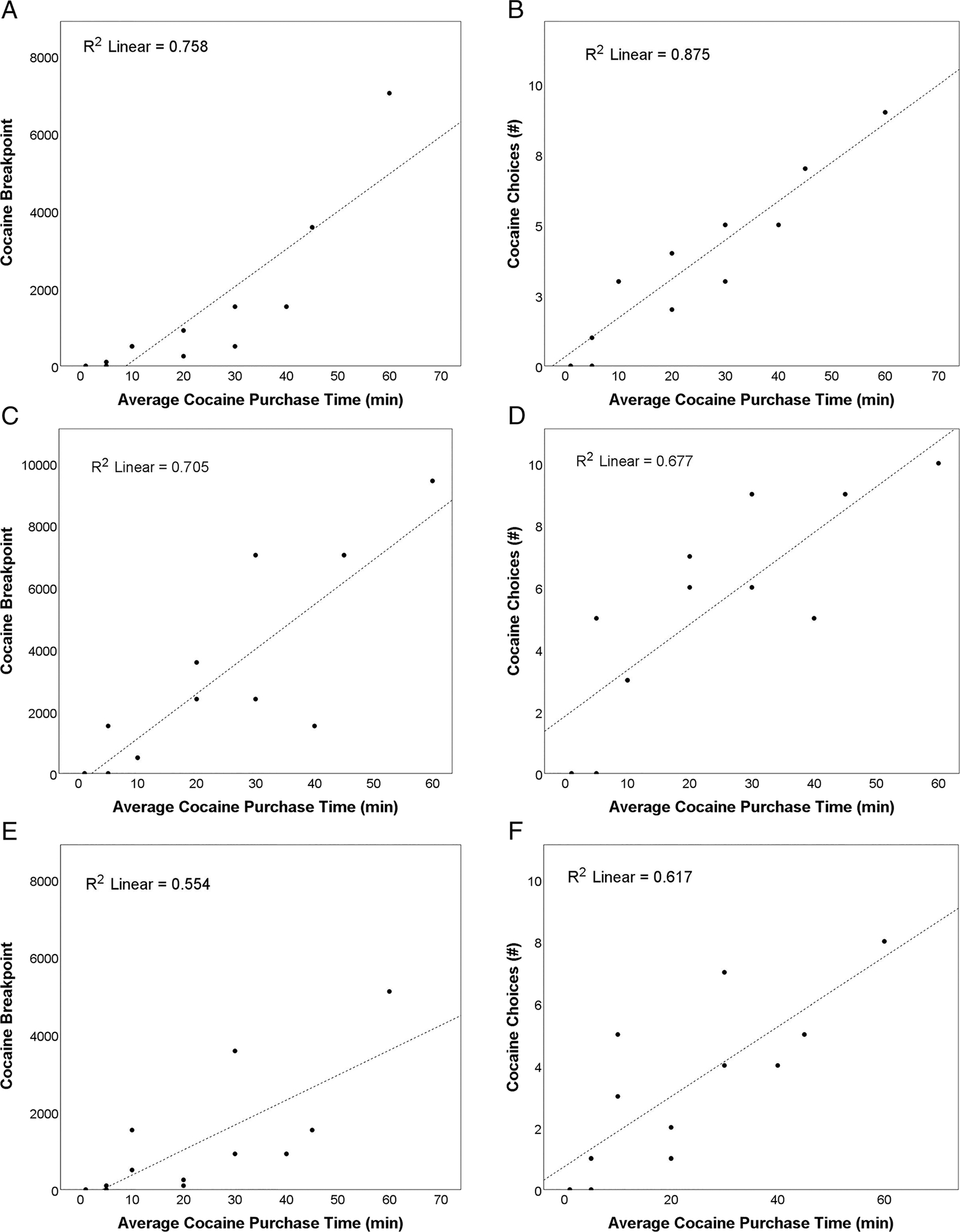
Significant Pearson correlations (Bonferroni-corrected threshold; p < .0063) between average cocaine purchase time (minute) and cocaine-seeking behavior (cocaine breakpoint depicted in the left column; total cocaine choices depicted in the right column) under different experimental conditions are denoted: (A) and (B) NAC dosing, placebo-prime, and $1.50 money alternative (r = 0.87, p < .001 and r = 0.94, p < .001; respectively); (C) and (D) NAC dosing, cocaine-prime, and $0.50 money alternative (r = 0.82, p = .001 and r = 0.84, p = .001, respectively); and (E) and (F) placebo dosing, placebo-prime, and $1.50 money alternative (r = 0.79, p = .002 and r = 0.74, p = .006, respectively)
3.3 |. Brain chemistry
3.3.1 |. rACC 1H MRS
Spectra quality and LCModel fit was very good (SNR = 18, CRLB% GLU = 4.5%, and CRLB% GLN = 14.6%) and did not differ by medication (NAC vs. placebo; Table S3). Relative to placebo levels, rACC GLX levels were significantly lower by 11.5%, F(1,6) = 7.36, p = .035 (partial η2 = 0.55; very large effect; GLX: 15.86 ± 0.81 vs. 13.94 ± 0.60), and rACC GLU levels were lower by 8.2%, during NAC dosing, F (1,6) = 4.23, p = .085 (partial η2 = 0.41; large effect; GLU: 11.77 ± 0.61 vs. 10.70 ± 0.45; Figure 3). No other metabolites approached significance (ps > .30).
3.4 |. Subjective and physiological effects
3.4.1 |. Vital signs
Relative to session baseline, cocaine-priming significantly increased heart rate and systolic and diastolic blood pressure more than placebo-priming (Priming × Time interactions; all ps < .001; Bonferroni-corrected threshold: p < .01; Figure 4) but did not affect oxygen saturation. NAC decreased oxygen saturation to a statistically (but not clinically) significant degree relative to placebo levels, F(1,11) = 9.52, p < .01 (Figure 4). NAC did not alter cocaine-priming effects on any physiological measures.
FIGURE 4.
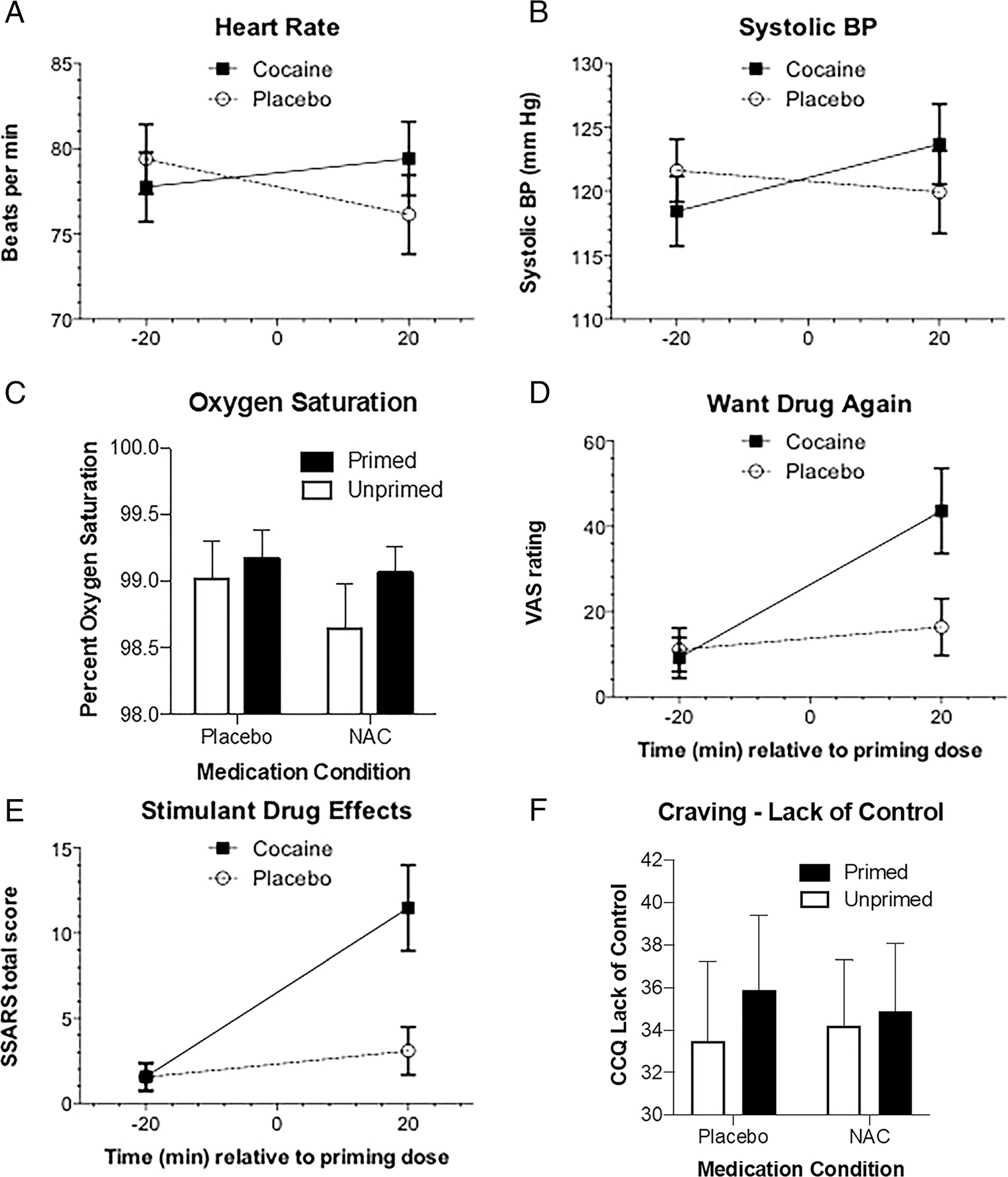
Physiological and subjective effects of placebo- versus cocaine-priming are depicted. Findings indicate significant cocaine-priming effects (ps < .01) on (A) heart rate; (B) systolic blood pressure (BP); (C) oxygen saturation; (D) want drug again; and (E) Stimulant-Sensitive Adjective Rating Scale (SSARS) total score. Further, a significant NAC by priming interaction, F(1,11) = 10.30, p < .01, indicated that NAC attenuated the cocaine-priming effect on (F) “Lack of Control Over Use” score
3.4.2 |. Subjective drug effects
Relative to placebo-priming, cocaine-priming significantly increased VAS ratings of “good drug effect,” “want drug again,” “liking,” “high,” and “stimulated” (all ps < .01; Bonferroni-corrected threshold: p < .012; Figure 4). Stimulant-Sensitive Adjective Rating Scale scores also significantly increased after cocaine-priming (p = .001). The only significant interaction of NAC × Priming indicated that NAC attenuated the cocaine-priming effect on CCQ “Lack of Control Over Use” scores, F(1,11) = 10.30, p < .01; Bonferroni-corrected threshold: p < .01. A complete description of these data is provided in Table S4.
4 |. DISCUSSION
This well-controlled inpatient human laboratory study evaluated the effects of NAC versus placebo on cocaine-seeking behavior, rACC neurochemistry, and subjective and physiological effects of cocaine self-administration. There were four primary significant findings: (1) cocaine-priming increased cocaine-seeking behavior, (2) NAC attenuated cocaine-primed increases in cocaine seeking, (3) the higher money amount alternative suppressed cocaine seeking, and (4) NAC reduced rACC GLX levels. Here, we report the first clinical evidence that NAC can suppress cocaine-primed cocaine-seeking behavior and replicate prior research indicating NAC modulates rACC neurochemistry in cocaine users. Finally, we report the first evidence of NAC’s therapeutic effects on cocaine-seeking behavior in parallel with its neurochemical effects, that is, modulation of rACC excitatory tone, which is theorized to underlie NAC’s therapeutic efficacy.
Animal research indicates that NAC reliably attenuates reinstatement of cocaine-seeking behavior after cue- and cocaine-priming challenges.6,9,11,12 The present study translated robust preclinical findings by evaluating NAC effects on cocaine seeking under cocaine-primed and unprimed conditions in cocaine-dependent individuals. A critical feature of the study design was the onset of NAC maintenance during a 3-day period of experimental cocaine abstinence, similar to preclinical reinstatement models. In the absence of NAC, cocaine-priming significantly increased cocaine seeking by 47% and 48% for the $0.50 and $1.50 money amounts, respectively, compared with placebo-priming. This finding is analogous to cocaine-induced reinstatement in preclinical models and replicates prior human laboratory research.39 NAC significantly attenuated cocaine-primed cocaine seeking on both behavioral metrics (cocaine breakpoint and total number of cocaine choices) with a large effect size. Relative to placebo levels, NAC reduced the total number of cocaine choices after cocaine-priming by 30%, on average. This is additional evidence that NAC may be an effective therapeutic agent for suppressing cocaine-seeking behavior, building on preclinical data.6,9,11,12 Interestingly, NAC did not attenuate physiological responses to the cocaine-priming dose (i.e., blood pressure and heart rate), suggesting that NAC’s behavioral effects may be driven by neurochemical/cognitive changes. Our data indicate NAC did not alter placebo-primed cocaine seeking at either money alternative amount. It is important not to overinterpret a null finding, especially in a pilot study. However, this is consistent with the extant literature that indicates that NAC does not alter stable, cocaine-reinforced responding in preclinical research,13 nor outpatient cocaine use among actively using patients,18 suggesting NAC may not be effective for abstinence initiation. Finally, NAC attenuated cocaine-primed increases in subjective “Loss of Control Over Use.” NAC may reduce cocaine’s acute disinhibiting effects, which may account for NAC’s effective attenuation of cocaine-primed cocaine seeking. In summary, we provide the first clinical evidence that NAC suppresses cocaine-primed cocaine-seeking behavior. Our findings contribute to the growing literature that NAC has greater therapeutic promise for prolonging cocaine abstinence, that is, relapse prevention, than abstinence initiation.
One prior study has reported NAC’s effect on neurochemistry among cocaine-dependent individuals. Using single-voxel 1H MRS, Schmaal and colleagues found a single oral dose of 2400 mg NAC “normalized” dACC GLU/tCr ratio levels among abstinent cocaine users, compared with controls.20 We extended that initial study in three important ways: (1) rather than a single NAC acute dose, NAC’s effect on glutamate neurochemistry was evaluated after 3 days of repeated NAC dosing, to simulate the onset of a therapeutic regimen, (2) placebo control was implemented, and (3) cocaine-seeking behavior was measured in parallel. Our findings indicate that repeated NAC dosing significantly reduced rACC GLX levels by 12% and numerically reduced GLU levels by 8% (both large effect sizes). While the brain region and calculated endpoint are different than Schmaal et al., the consistency of NAC’s effect on neurochemistry is noteworthy. Taken together with Schmaal et al., NAC can reduce ACC glutamate + glutamine levels among cocaine users and may restore glutamatergic homeostasis.20 1H MRS does not distinguish glutamate pools (neuronal vs. glial vs. extracellular), but the predominant source of the 1H MRS glutamate signal is intracellular.40 Based on preclinical findings,9,10 we speculate NAC’s neurochemical effect is driven by increased inhibitory tone via mGluR2/3 signaling. Prior research guides interpretation of these neurochemical findings. rACC glutamate levels have been linked to impulsivity,20,41 cocaine cue-reactivity,42–44 and years of cocaine use.28 We found that NAC attenuated cocaine’s subjective disinhibiting effect, whereas prior research indicated that NAC improved inhibitory control30 and reduced cocaine cue-induced craving16,17 and cocaine attentional bias.19 NAC’s effect on cocaine-seeking behavior may be mediated by changes in any (or all) of these processes via modulation of rACC excitatory tone.
Contingency management is a robust behavioral intervention for initiating cocaine abstinence.22–24 In this study, we implemented a contingency management analogue adapted for the money versus cocaine choice progressive ratio paradigm. Specifically, on half of the experimental testing days, the alternative choice money amount was three times greater than other days ($1.50 vs. $0.50). Results indicated the higher alternative money amount significantly suppressed cocaine seeking by ~32% across all experimental conditions (large effect sizes), consistent with prior research.39 However, NAC did not augment the suppressing effect of the higher money amount alternative on cocaine-seeking behavior. We stress that these findings should be strictly interpreted within the context of money versus drug choice paradigms and should not be extended to contingency management interventions in general.
Limitations of this study deserve note. First, sample size was relatively small, suggesting caution in generalizing these findings. Nonetheless, we observed significant NAC-induced reduction of cocaine-primed cocaine seeking, consistent with preclinical data. Second, 1H MRS data of acceptable quality were only available for seven of 12 participants who completed behavioral choice procedures. Brain chemistry measures were considered secondary so as not to rate-limit enrollment and evaluation of the primary behavioral outcomes. Although 1H MRS data are consistent with prior findings20 and exhibited large effect sizes, future studies are needed to confirm these findings and investigate brain–behavior relationships, which were underpowered herein. Third, we note NAC may exhibit other (possibly related) neurobiological mechanisms of action not examined in this study, for example, modulation of dopamine release in the striatum.13
In conclusion, this study offers translational experimental evidence regarding the effect of NAC on cocaine-seeking/self-administration behavior in the human laboratory setting. Our findings indicate NAC reduced cocaine seeking following an active cocaine priming dose and reduced rACC glutamate + glutamine levels. This is the first report of NAC’s putative neurochemical mechanism of action, that is, modulation of rACC excitatory tone, measured in parallel with its therapeutic effect on cocaine-seeking behavior. Finally, we report that a higher alternative choice money amount suppressed cocaine-seeking behavior, analogous to continency management, but did not exhibit additive effects when combined with NAC dosing in our human laboratory paradigm. Taken together with prior research, NAC may be an effective intervention for “short-circuiting” lapses and prolonging cocaine abstinence but may not be effective for abstinence initiation. CUD is a complex problem that will likely require multiple strategies. Future research is needed to investigate the therapeutic efficacy of NAC (or NAC-amide, which has better bioavailability than NAC) in combination with an effective intervention for initiating abstinence, such as contingency management.
Supplementary Material
ACKNOWLEDGMENTS
NIH grant R01 DA026861 to M.K.G. from the National Institute on Drug Abuse (under the American Recovery and Reinvestment Act), NIH grants F31 DA040369 and K99 DA048125 to E.A.W., Helene Lycaki/Joe Young, Sr., funds from the State of Michigan, and the Detroit Wayne Mental Health Authority supported this research. These agencies had no roles in the design, data collection, and analysis of, or decision to publish findings from, this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was registered as NCT01392092 (http://clinicaltrials.gov/ct2/show/NCT01392092). The authors thank Ken Bates for recruitment, and Debra Kish, Joi Moore, Elorie Eggleston, Katherine Mattison, and Lisa Sulkowski for data collection and management, and nurse Gwen DeWalt and Dr. Carl Christensen for medical monitoring.
Funding information
National Institute on Drug Abuse, Grant/Award Numbers: DA026861, DA040369, DA048125, K99 DA048125 to E.A.W., NIH grants F31 DA040369, NIH grant R01 DA026861 to M.K.G.; Detroit Wayne Mental Health Authority
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
All authors declare no ethical or financial conflict of interest with respect to the content of this work.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Cornish J, Duffy P, Kalivas P. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93(4):1359–1367. [DOI] [PubMed] [Google Scholar]
- 2.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89–RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. [DOI] [PubMed] [Google Scholar]
- 4.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187(1):35–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–749. [DOI] [PubMed] [Google Scholar]
- 7.Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW. Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol. 2015;20(2):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25(27):6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendyam S, Mohan A, Kalivas P, Nair S. Computational model of extracellular glutamate in the nucleus accumbens incorporates neuroadaptations by chronic cocaine. Neuroscience. 2009;158(4):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337(2):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauzo RM, Kimmel HL, Howell LL. The cystine–glutamate transporter enhancer N-acetyl-L-cysteine attenuates cocaine-induced changes in striatal dopamine but not self-administration in squirrel monkeys. Pharmacol Biochem Behav. 2012;101:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducret E, Puaud M, Lacoste J, et al. N-acetylcysteine facilitates self-imposed abstinence after escalation of cocaine intake. Biol Psychiatry. 2016;80(3):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389–394. [DOI] [PubMed] [Google Scholar]
- 16.LaRowe SD, Mardikian P, Malcolm R, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164(7):1115–1117. [DOI] [PubMed] [Google Scholar]
- 18.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolin BL, Alcorn JL III, Lile JA, et al. N-Acetylcysteine reduces cocaine-cue attentional bias and differentially alters cocaine self-administration based on dosing order. Drug Alcohol Depend. 2017;178:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwald MK, Ledgerwood DM, Lundahl LH, Steinmiller CL. Effect of experimental analogs of contingency management treatment on cocaine seeking behavior. Drug Alcohol Depend. 2014;139:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51(7):568–576. [DOI] [PubMed] [Google Scholar]
- 23.Higgins ST, Delaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148(9):1218–1224. [DOI] [PubMed] [Google Scholar]
- 24.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. [DOI] [PubMed] [Google Scholar]
- 25.Greenwald MK, Lundahl LH, Steinmiller CL. Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology. 2010;35(13):2624–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C-sR, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33(8):1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moeller SJ, Konova AB, Parvaz MA, et al. Functional, structural, and emotional correlates of impaired insight in cocaine addiction. JAMA Psychiat. 2014b;71(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang S, Salmeron BJ, Ross TJ, Xi Z-X, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users—a 1H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res Neuroimaging. 2009;174:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moeller SJ, Froböse MI, Konova AB, et al. Common and distinct neural correlates of inhibitory dysregulation: stroop fMRI study of cocaine addiction and intermittent explosive disorder. J Psychiatr Res. 2014a;58:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte MH, Kaag AM, Boendermaker WJ, van den Brink W, Goudriaan AE, Wiers RW. The effect of N-acetylcysteine and working memory training on neural mechanisms of working memory and cue reactivity in regular cocaine users. Psychiatry Res Neuroimaging. 2019;287:56–59. [DOI] [PubMed] [Google Scholar]
- 31.First M, Gibbon M, Spitzer R, Williams J. Structured Clinical Interview for DSM-IVAxis I Disorders* Research Version, SCID-I, Version 2.0 February, 1996, Final version. New York: Biometrics Research; 1996. [Google Scholar]
- 32.Greenwald MK, Steinmiller CL. Cocaine behavioral economics: from the naturalistic environment to the controlled laboratory setting. Drug Alcohol Depend. 2014;141:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. [DOI] [PubMed] [Google Scholar]
- 34.Rush CR, Baker RW, Wright K. Acute physiological and behavioral effects of oral cocaine in humans: a dose-response analysis. Drug Alcohol Depend. 1999;55(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 35.Provencher S LCModel. Version 6.3. 2008. [Google Scholar]
- 36.Dale AM, Fischl B, Sereno MI . Cortical surface-based analysis: I. segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 38.Stanley JA, Drost DJ, Williamson PC, Terry Thompson R. The use of a priori knowledge to quantify short echo in vivo 1H MR spectra. Magn Reson Med. 1995;34(1):17–24. [DOI] [PubMed] [Google Scholar]
- 39.Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: effects of dose, alternative reinforcement, and priming. Psychopharmacology (Berl) 2004;172(3):316–323. [DOI] [PubMed] [Google Scholar]
- 40.Erecinska M, Silver IA. Metabolism and role of glutamate in mamma- lian brain. Prog Neurobiol. 1990;35(4):245–296. [DOI] [PubMed] [Google Scholar]
- 41.Hoerst M, Weber-Fahr W, Tunc-Skarka N, et al. Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch Gen Psychiatry. 2010;67(9):946–954. [DOI] [PubMed] [Google Scholar]
- 42.Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. [DOI] [PubMed] [Google Scholar]
- 43.Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav. 2013;38(2):1509–1517. [DOI] [PubMed] [Google Scholar]
- 44.Ray S, Haney M, Hanson C, Biswal B, Hanson SJ. Modeling causal relationship between brain regions within the drug-cue processing network in chronic cocaine smokers. Neuropsychopharmacology. 2015;40(13):2960–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


