Abstract
In this work, the phage-encoded proteins involved in site-specific excision of the prophage genome of the temperate lactococcal bacteriophage TP901-1 were identified. The phage integrase is required for the process, and a low but significant frequency of excision is observed when the integrase is the only phage protein present. However, 100% excision is observed when the phage protein Orf7 is provided as well as the integrase. Thus, Orf7 is the TP901-1 excisionase, and it is the first excisionase identified that is used during excisive recombination catalyzed by an integrase belonging to the family of extended resolvases. Orf7 is a basic protein of 64 amino acids, and the corresponding gene (orf7) is the third gene in the early lytic operon. This location of an excisionase gene of a temperate bacteriophage has never been described before. The experiments are based on in vivo excision of specifically designed excision vectors carrying the TP901-1 attP site which are integrated into attB on the chromosome of Lactococcus lactis. Excision of the vectors was investigated in the presence of different TP901-1 genes. In order to detect very low frequencies of excision, a method for positive selection of loss of genetic material based upon the upp gene (encoding uracil phosphoribosyltransferase) was designed, since upp mutants are resistant to fluorouracil. By using this system, frequencies of excision on the order of 10−5 per cell could easily be measured. The described selection principle may be of general use for many organisms and also for types of deletion events other than excision.
During the establishment of lysogeny, many temperate bacteriophages integrate their genomes site specifically into the bacterial host chromosome by recombination between the attachment sites attB and attP, located on the bacterial and phage genomes, respectively. This leads to the formation of the hybrid attachment sites attL and attR at the junctions between the phage and bacterial genomes. At a later stage, induction of the phage will lead to recombination between these sites and excision of the integrated phage genome. Both integration and excision require the phage-encoded integrase, but for efficient excision an additional phage-encoded protein, the excisionase, is required. The excisionase counteracts the integration process and should therefore be expressed only during excision. This coordination of the expression of the integrase and excisionase proteins is obtained by different mechanisms in different phages. In Escherichia coli phage λ, the gene encoding the excisionase, xis, is located upstream of and partially overlapping the int gene, which encodes the integrase. In the situation where only the integrase is required, transcription of int is initiated at a promoter located within xis, and when both the integrase and excisionase are required, transcription is initiated from a promoter upstream of xis (7).
In bacteriophage P2 of E. coli, the two major early promoters, pC and pE, are divergently located (9, 16). The protein product of the first gene downstream of pC is the phage repressor C, and the first gene downstream of pE encodes the Cox protein, which both is the phage excisionase and is involved in regulation of expression from pC and pE (26, 32). Cox is thus a very important protein in the choice between the lytic and lysogenic responses of P2. Regulation of the activities of pC and pE by C and Cox results in only one of the promoters being active at a time. The gene encoding the integrase of P2 is located further downstream of pC, and thus expression of the integrase without concomitant expression of the excisionase is obtained when pC is active and pE is repressed. However, for excision of the prophage genome during induction of the prophage, both the integrase and Cox are required. It has been suggested that in this case pE is active, leading to expression of Cox, while at the same time transcription of the integrase gene is initiated from a minor promoter upstream of the integrase gene (33).
The lactococcal temperate bacteriophage TP901-1 is the subject of investigation in the present study. Like that of P2, the TP901-1 genome contains two major early promoters, termed pL (for lytic) and pR (for repression) (20). By analogy to P2, pL corresponds to pE. The first gene downstream of pL, orf5, encodes a protein which has been demonstrated to be involved in regulation of the activities of pR and pL (20) and thus bears some resemblance to Cox of P2. Similarly, pR corresponds to pC, since the protein encoded by the first gene downstream of pR is Orf4, which is required for repression of both pL and pR during lysogeny (20).
The gene encoding the TP901-1 integrase, Orf1, is located further downstream of pR, followed by the attP site. Most of the integrases of the temperate bacteriophages infecting lactic acid bacteria which have been identified are related to the integrase of the E. coli bacteriophage λ (for example, see references 2, 15, 30 and 31), suggesting that the mechanism of recombination is similar to that of the λ integrase. In contrast, the integrase of temperate lactococcal bacteriophage TP901-1 belongs to a family of recombinases which has been termed the extended resolvases (6). The N-terminal parts of the proteins of this family show homology to the catalytic domains of the resolvases and invertases of site-specific recombinases, suggesting that the proteins perform recombination by a similar mechanism. However, instead of the short DNA-binding domain present in the C-terminal part of the resolvases and invertases, the extended resolvases contain an extension of about 300 amino acids or more.
This is the first report on the protein requirements for excisive recombination for a phage encoding an integrase belonging to this protein family. It is also the first identification of the excisionase gene of a temperate bacteriophage of lactic acid bacteria. The investigations have been performed by a new method for in vivo determination of the frequency of excision, based on excision vectors containing two marker genes, enabling selection both for the presence (erm) and the absence (upp) of the vector. By this method we have shown that the excisionase of TP901-1 is Orf7, encoded by the third gene in the early lytic operon. TP901-1 is the first temperate bacteriophage described in which the gene encoding the excisionase is located at this position on the phage genome.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Lactococcal strains were grown without stirring at 30°C in M17 broth (29) supplemented with 0.5% (wt/vol) glucose (GM17). Selection for 5-fluorouracil (FU)-resistant cells was performed in GSA medium, which was prepared by supplementing the defined medium SA (13) with 1% (wt/vol) glucose. FU was added to a final concentration of 10 μg/ml, erythromycin (ERM) was added to 2 μg/ml, and chloramphenicol (CAM) was added to 5 μg/ml. E. coli cells were propagated at 37°C with stirring in Luria-Bertani broth (24), and ERM was added to a final concentration of 150 μg/ml, CAM was added to 25 μg/ml, and ampicillin was added to 100 μg/ml. To prepare plates, all media were solidified by adding 1.5% Bacto Agar.
Construction of plasmids.
The plasmids used in this study are listed in Table 1. The upp gene was amplified from pJM300 (22) by using primers pupp2-1 and pupp2-2 (Table 2) and cloned in pGEM-3Zf(+), giving rise to pAB204. The upp gene was not sequenced, but it encodes a functional product. Plasmid pAB211 contains the upp gene of pAB204 cloned as an XhoI-HindIII fragment in pIC-19R (21). By inserting upp from pAB211 on a BglII-PstI fragment at the BglII-PstI sites in pTRKL2 (25), pAB227 was constructed. Subsequently, excision vector pAB112 was obtained by inserting a 1.1-kb SmaI fragment with the upp gene of pAB227 in SmaI in pBF12, which is an E. coli vector containing a 333-bp TP901-1 attP fragment and the erm cassette (3). Plasmid pAB174b was obtained by introducing the upp gene on an XbaI-ApaI fragment in pLB44 (6). In this case the upp gene was amplified by PCR with pJM300 as the template and primers pupp1-1 and pupp1-2. Again, the PCR fragment was not sequenced, but the upp gene encodes a functional product. Excision vector pAB174a was obtained from pAB174b by digesting with BamHI, inactivating the enzyme and religating, and subsequently identifying a plasmid in which erm was inverted relative to pAB174b.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Origin | Coordinates of TP901-1 fragment(s)a | Antibiotic resistance | Reference or source |
|---|---|---|---|---|
| Strains | ||||
| L. lactis subsp. cremoris | ||||
| JM342 | MG1363 Δupp tdk | 23 | ||
| AB112 | JM342::pAB112 | −104–228 | Ermr | This study |
| AB174a | JM342::pAB174a | −98–1910, 2521–2708 | Ermr | This study |
| E. coli DH5α | ø80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 | Lab strain | ||
| Plasmids | ||||
| pCI3340 | Shuttle vector for E. coli and L. lactis | Camr | 10 | |
| pCI372 | Shuttle vector for E. coli and L. lactis | Camr | 10 | |
| pTRKL2 | Shuttle vector for E. coli and L. lactis | Ermr | 25 | |
| pAK80 | Shuttle vector for E. coli and L. lactis | Ermr | 12 | |
| pGEM-3Zf(+) | E. coli vector | Ampr | Promega | |
| pGEM-7Zf(+) | E. coli vector | Ampr | Promega | |
| pIC-19R | E. coli vector | Ampr | 21 | |
| pACYC184 | E. coli vector | Camr | New England Biolabs | |
| pBF12 | pMOSblue-T::333-bp TP901-1 attP | −104–228 | Ampr, Ermr | 3 |
| pPM92 | pIC-19R::TP901-1 orf4–9 | 2621–5476 | Ampr | 20 |
| pPM115 | pCI3340::TP901-1 orf2–9 | 1287–5476 | Camr | 17 |
| pPM129 | pAK80::TP901-1 orf4–7 | 2621–4672 | Ermr | 18 |
| pJM300 | pUN121::upp | Ampr, Tetr | 22 | |
| pAJ37 | pCI372::TP901-1 attP, orf1–2, orf4–6d | −98–4475d | Camr | This study |
| pAJ39 | pCI372::TP901-1 attP, orf1, orf3–6e | −98–4475e | Camr | This study |
| pAJ41 | pCI372::TP901-1 attP, orf2–6f | −98–4475f | Camr | This study |
| pAJ43 | pCI372::TP901-1 attP, orf1–6 | −98–4475 | Camr | This study |
| pAJ95 | pAK80::TP901-1 pR-pL | 3142–3332 | Ermr | 14 |
| pAB35 | pCI372::TP901-1 attP, orf1, orf4–6 | −98–1910, 2521–4475 | Camr | This study |
| pAB112 | pMOSblue-T::333-bp TP901-1 attP, upp | −104–228 | Ampr, Ermr | This study |
| pAB174a | pGEM-7Zf(+)::TP901-1 attP, orf1,b upp | −98–1910, 2521–2708 | Ampr, Ermr | This study |
| pAB174b | pGEM-7Zf(+)::TP901-1 attP, orf1,cupp | −98–1910, 2521–2708 | Ampr, Ermr | This study |
| pAB204 | pGEM-3ZF(+)::upp | Ampr | This study | |
| pAB211 | pIC-19R::upp | Ampr | This study | |
| pAB221 | pCI3340::TP901-1 orf2–5 | 1287–3900 | Camr | This study |
| pAB223 | pCI3340::TP901-1 orf4–5 | 2621–3900 | Camr | This study |
| pAB227 | pTRKL2::upp | Ermr | This study | |
| pAB235 | pCI372::TP901-1 attP, orf1 | −98–1910, 2521–2708 | Camr | This study |
| pAB241 | pCI3340::TP901-1 orf4–7 | 2621–4672 | Camr | This study |
| pAB243 | pCI3340::TP901-1 orf4–5, orf7 | 2621–3900, 4353–4672 | Camr | This study |
| pAB244 | pCI3340::TP901-1 orf7 | 4353–4672 | Camr | This study |
| pAB245 | pCI3340::TP901-1 pR-pL, orf7 | 3142–3332, 4353–4672 | Camr | This study |
| pLB28 | pGEM-7Zf(+)::TP901-1 attP, orf2–3 | −98–2708f | Ampr | 6 |
| pLB29 | pGEM-7Zf(+)::TP901-1 attP, orf1–3 | −98–2708 | Ampr | 6 |
| pLB44 | pGEM-7Zf(+)::TP901-1 attP, orf1c | −98–1910, 2521–2708 | Ampr | 6 |
| pLB55 | pCI372::TP901-1 attP, orf1–2d | −98–2708d | Camr | This study |
| pLB56 | pCI372::TP901-1 attP, orf1, orf3e | −98–2708e | Camr | This study |
| pLB57 | pCI372::TP901-1 attP, orf2–3f | −98–2708f | Camr | This study |
| pLB58 | pCI372::TP901-1 attP, orf1–3 | −98–2708 | Camr | This study |
| pLB61 | pGEM-7Zf(+)::TP901-1 orf1 | 25–1910 | Ampr | 3 |
| pLB71 | pGEM-7Zf(+)::TP901-1 attP, orf1–2d | −98–2708d | Ampr | 6 |
| pLB72 | pGEM-7Zf(+)::TP901-1 attP, orf1, orf3e | −98–2708e | Ampr | 6 |
| pLB81 | pACYC184::TP901-1 orf1 | 25–1910 | Camr | This study |
Coordinates of the TP901-1 fragment(s), according to the numbering system introduced previously (19), where first base pair in the core region is assigned position 1 and the last base pair of the core is assigned position 5. Since the sequence of the entire phage genome is not known, we have assigned the bases on the other side of the core from orf1 negative numbers. Positive coordinates thus correspond to the sequence reported under EMBL accession no. Y14232. Negative coordinates −1 to −104 correspond to bases 2727 to 2831 in the sequence reported under EMBL accession no. X85213.
The erm gene is oriented divergently from orf1.
The erm gene is oriented in the same direction as orf1.
Contains an amber stop mutation in orf3.
Contains an amber stop mutation in orf2.
Contains an amber stop mutation in orf1.
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| pupp1-1 | CCCCGAATTCATCGATCCTAGCTCCATTTTTAA TCAAG |
| pupp1-2 | CCCCTCTAGATAAGGAATGATAACCAGTTC |
| pupp2-1 | CCCCGAATTCATCGATCCTAGCTCCATTTTTAA TCAAG |
| pupp2-2 | CCCCATCGATAAGGAATGATAACCAGTTC |
| 1211 | GTAAAACGACGGCCAGT |
| orf8PstI | AAAACTGCAGGGTCAGCAAGAATCGCAC |
| pAK80 | TCCTTTCAAAGTTACCC |
| pAK80erm | TTTCAACTGCCTGGCAC |
| pattB-R1 | AAATGCACTGTTTGAGTC |
| pattBR-L1 | GATGATTGTCTTATTGTA |
| pattP-L1 | GATGAACTATGGGACCAAA |
| PB2 | GCTGCTTAAAGCTAAGATT |
| pattBL | CTACTGCTGCTTCACCAG |
| BI-POB1inv | GTATGCAGCGATGTCGTTACCC |
Plasmids pLB58, pLB57, pLB56, and pLB55 were constructed by cloning the 2.8-kb EcoRI-NsiI TP901-1 fragments of pLB29, pLB28, pLB72, and pLB71 (6), respectively, at the EcoRI-PstI sites of pCI372. The TP901-1 fragment of pLB58 contains orf1, orf2, and orf3 as well as attP. The TP901-1 fragments of pLB57, pLB56, and pLB55 are equivalent to pLB58 except for amber stop codons and XbaI sites introduced in orf1, orf2, and orf3, respectively. For details concerning the mutations, see reference 6. Plasmids pAJ43, pAJ41, pAJ39, and pAJ37 were constructed by inserting the 1.7-kb TP901-1 EcoRI fragment 7 containing orf4 to -6 (19) in the EcoRI sites of pLB58, pLB57, pLB56, and pLB55, respectively, in the same orientation as in TP901-1. A 6.1-kb XbaI-BglII fragment of pAJ37 was ligated to a 3.5-kb XbaI-BglII fragment of pAJ39, giving rise to pAB35. Plasmid pAB235 was obtained by digesting pAB35 with EcoRI and religating the 8.3-kb fragment, leading to deletion of a 1.7-kb EcoRI fragment carrying orf4 to -6.
Plasmid pLB81 contains the 1.9 XhoI-SphI fragment of pLB61 with TP901-1 orf1 (3) inserted at the XhoI-SphI sites in pACYC184. Plasmid pPM115 is a pCI3340 derivative with the 4.2-kb TP901-1 EcoRV fragment 4 (5) inserted at the EcoRV site. The 2.6-kb HindIII fragment of pPM115 containing TP901-1 orf2 to -5 was inserted at the HindIII site in pCI3340 to obtain pAB221. This plasmid was digested with XhoI, and the 7.0-kb fragment was religated, resulting in pAB223. Plasmid pPM129 is a pAK80 derivative containing a 2.0-kb TP901-1 fragment obtained by PCR amplification with pPM92 as the template and primers 1211 and orf8PstI. The PCR fragment was digested with PstI and inserted in the PstI site of pAK80. The amplified fragment was not sequenced. The 2.1-kb XhoI-PstI TP901-1 fragment of pPM129 containing orf4 to -7 was inserted at the XhoI-PstI sites in pCI3340 to give pAB241. Plasmid pAB243 was constructed by digesting pAB241 with HindIII and religating the 7.3-kb fragment. To obtain pAB244, the 6.0-kb EcoRV-HindIII fragment of pAB243, containing orf7 but no other TP901-1 open reading frames, was religated. Plasmid pAJ95 contains a 220-bp TP901-1 fragment with pL and pR inserted at the BamHI site in pAK80 (14). The 220-bp fragment of pAJ95 was amplified by PCR with primers pAK80 and pAK80 erm and digested with HindIII. By ligation to the 6-kb EcoRV-HindIII fragment of pAB243, pAB245 was obtained; this plasmid contains orf7 as the only TP901-1 open reading frame, positioned directly after pL. The sequence of the 220-bp promoter fragment in pAB245 was verified.
DNA preparation.
Plasmid DNA was isolated from lactococcal and E. coli cells by the alkaline lysis technique (27). Lactococcal cells were treated with lysozyme at a final concentration of 20 mg/ml for 20 min at 37°C, with shaking to promote lysis, before the addition of NaOH. When required, the plasmid DNA was further purified by applying the DNA on Qiagen columns as recommended by the supplier (Qiagen Ltd., Hilden, Germany). Chromosomal DNA from lactococcal cells was prepared as described for E. coli (27), except that the cells were frozen for 30 min at −80°C after harvesting, thawed to room temperature, and treated with lysozyme at a final concentration of 20 mg/ml for 30 min at 37°C to promote lysis.
Recombinant DNA techniques.
DNA manipulations were performed by standard techniques (27). Restriction nuclease enzymes, T4 DNA ligase, and corresponding buffers were supplied by Pharmacia Biotech or New England Biolabs; all enzymes were used as recommended by the supplier. The Ampli taq DNA polymerase was used for PCR amplification of DNA fragments, with reaction conditions as recommended by the supplier (Perkin-Elmer Cetus). DNA sequences were determined as described previously (28), with modifications according to the instructions with the Thermo Sequenase Radiolabeled terminator cycle sequencing kit (Amersham Life Science). Oligonucleotides were supplied by T-A-G-Copenhagen, Copenhagen, Denmark, or by Pharmacia Biotech, Allerød, Denmark.
Transformation of E. coli and Lactococcus lactis.
Plasmid DNA was introduced into E. coli cells by making the cells competent with CaCl2 and transforming as described previously (27). Lactococcal cells were made electrocompetent and transformed by electroporation as described previously (11).
Integration of excision vectors into attB of JM342.
To obtain strain AB112, L. lactis subsp. cremoris JM342 (23) was transformed with 0.05 μg of pAB112 DNA and 0.05 μg of pLB81 DNA and plated on plates containing ERM. To ensure that pLB81 had been lost, leading to loss of the cat gene, the cells were tested on CAM plates. To obtain strain AB174a, JM342 was transformed with 0.1 μg of pAB174a DNA and plated on plates containing ERM. For both excision vectors, site-specific integration was verified by PCR with chromosomal DNA, demonstrating that the attB site had been lost and that attL and attR sites had been created (data not shown). The primers used for amplification of attB were pattB-R1 and pattBR-L1, the primers for attL were pattB-R1 and pattP-L1, and the primers for attR were pattBR-L1 and PB2 (Table 2).
Determination of the frequency of excision.
To determine the effect of the open reading frames of a protein donor plasmid on the frequency of excision of an excision vector, the protein donor was introduced in the L. lactis subsp. cremoris JM342 strains containing the excision vectors integrated on the chromosome (strains AB112 and AB174a). After transformation, the cells were plated on plates containing CAM, to select for the incoming protein donor plasmid, but not ERM, allowing loss of the excision vector. Approximately 2,000 transformants were plated for each experiment. After growth at 30°C overnight, the colonies were pooled by being washed off the plates with 1 ml of 0.9% NaCl and were subsequently diluted in 0.9% NaCl. Appropriate dilutions were plated on GSA plates containing either only CAM (to determine the number of cells) or both FU and CAM (to determine the number of cells which had lost the excision vector and thus had become resistant to FU). When erm was used as the marker gene, FU was replaced by ERM in the GSA plates. Each experiment was repeated independently at least three times.
To investigate whether excision did take place, chromosomal DNA was prepared from the relevant FUr strains and used as the template in PCRs. The primers used were pattBL and BI-POB1inv, which amplify the attB region (Table 2).
RESULTS
Excision vectors.
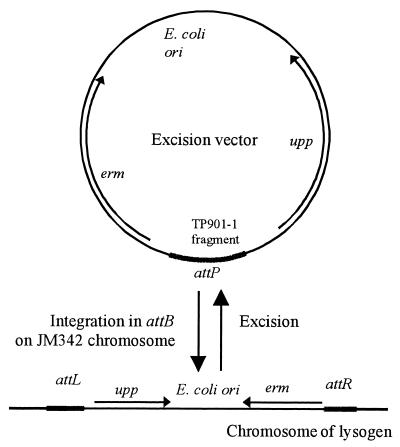
To investigate the effect of selected TP901-1-encoded proteins on excision, specific excision vectors which enable accurate determinations of low frequencies of excision were designed. These vectors contain an E. coli origin of replication, the erm gene, and a region of the TP901-1 genome, including at least attP (Fig. 1). In the presence of the TP901-1 integrase, site-specific integration of these vectors into the attB site on the host chromosome can be obtained, as described for other attP-containing vectors (3).
FIG. 1.
Excision vectors before and after integration into attB on the lactococcal chromosome. Arrows, upp and erm genes; heavy black lines, attachment sites.
After establishment of a lactococcal strain containing the excision vector integrated in attB, the frequency of excision of the vector can be monitored as follows. The vector contains the upp gene (Fig. 1), encoding uracil phosphoribosyltransferase, an enzyme of the pyrimidine salvage pathway which catalyzes the conversion of uracil to UMP, a precursor for the formation of dUMP. When uracil is replaced by FU, the toxic fluoro-dUMP is produced, and a lactococcal upp mutant is thus resistant to 0.3 μg of FU per ml (22). However, to facilitate the selection procedure in the excision experiments, the double mutant strain JM342 was chosen as the host (23). JM342 is a Δupp tdk derivative of L. lactis subsp. cremoris MG1363 (8). The gene product of tdk catalyzes a step in an alternative pathway for the conversion of uracil to dUMP, and JM342 is therefore resistant to 10 μg of FU per ml. Integration of an excision vector containing upp in JM342 leads to sensitivity to FU and resistance to ERM. A cell having lost the integrated vector can therefore be selected for during the excision experiments by plating on GSA plates containing 10 μg of FU per ml; this can be further verified by testing for ERM sensitivity.
The integrase of TP901-1 is required for excision.
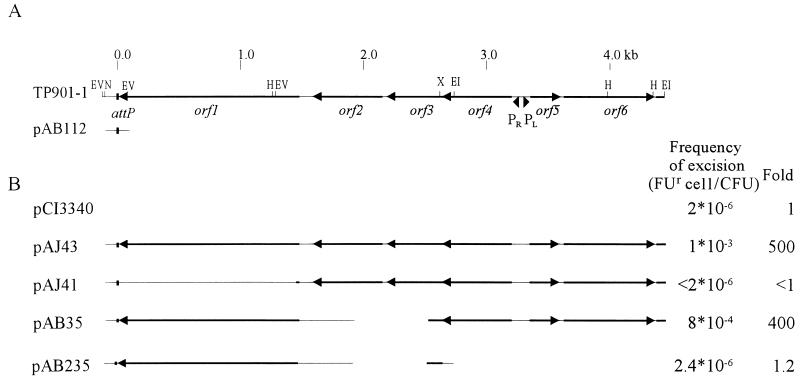
The simplest excision vector (pAB112) used in this study carries a 333-bp TP901-1 fragment with attP (excision system I) (Fig. 2). This excision vector is integrated site specifically into the attB site of the recipient chromosome by donation of the integrase in trans, using cotransformation with an E. coli vector containing the orf1 gene (pLB81). Even though the orf1-containing plasmid is lost during growth of the recipient, enough integrase is produced to allow for integration of the excision vector, and the resulting strain is termed AB112.
FIG. 2.
Excision system I. (A) The relevant region of the TP901-1 genome is shown at the top, with selected restriction sites indicated (EV, EcoRV; N, NsiI; H, HindIII; X, XhoI; EI, EcoRI). The numbering of the base pairs shown at the top corresponds to the numbering described in footnote a of Table 1. DNA is shown as a thin line; open reading frames are shown as black arrows. The TP901-1 integrase is encoded by orf1. The attP site is depicted as a black box. The region of the TP901-1 genome inserted in excision vector pAB112 is indicated. pAB112 is integrated into attB in JM342 to obtain strain AB112. (B) Regions of the TP901-1 genome present in the protein donor plasmids and frequencies of FUr cells obtained when these plasmids are introduced in AB112.
To investigate the involvement of phage-encoded proteins in excision, derivatives of shuttle vectors pCI372 and pCI3340 carrying different TP901-1 genes were introduced into AB112, and the frequency of occurrence of FUr cells was measured. In a number of these protein donor plasmids, expression of the phage genes is controlled by the original phage promoters pL and pR and the TP901-1 genes orf4 and orf5 are present (Fig. 2; see also Fig. 3). After transformation of a lactococcal cell with a plasmid containing pL, pR, orf4, and orf5, either (i) pL is open and pR is repressed or (ii) pL is repressed and pR is slightly derepressed. These phenotypes are stable during prolonged periods of growth, and this feature has been termed clonal variation (20). Since the frequency of excision is likely to be influenced by the level of expression of the proteins involved in the process, this clonal variation probably results in different rates of excision in the two types of cells. Therefore, the frequency of excision is determined for a large number of cells, right from the point of introduction of the protein donor plasmid into the cell (as described in Materials and Methods).
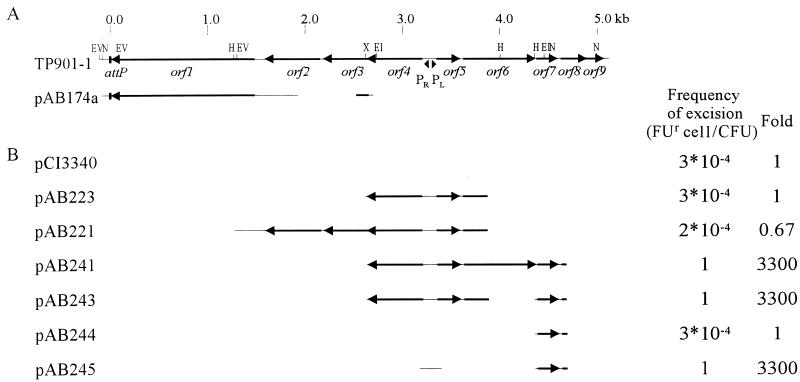
FIG. 3.
Excision system II. (A) A region of the TP901-1 genome is shown at the top, with relevant restriction sites indicated (EV, EcoRV; N, NsiI; H, HindIII; X, XhoI; EI, EcoRI). The numbering of the base pairs shown at the top corresponds to the numbering described in footnote a of Table 1. DNA is shown as a thin line; open reading frames are shown as black arrows. The TP901-1 integrase is encoded by orf1. The attP site is depicted as a black box. The region of the TP901-1 genome inserted in excision vector pAB174a is indicated. pAB174a is integrated into attB in JM342 to obtain strain AB174a. (B) Regions of the TP901-1 genome present in the protein donor plasmids and frequencies of FUr cells obtained when these plasmids are introduced in AB174a.
First, the stability of the integrated plasmid in AB112 was tested in the presence of the vector pCI3340, containing no phage genes. Only very few FUr cells were obtained, and these were still resistant to ERM, showing that the resistance of these cells to FU was caused by a mutation in upp. The observed frequency of FUr cells (2 × 10−6/CFU) thus corresponds to the spontaneous mutation rate of the upp gene. In the presence of pAJ43, the frequency of occurrence of FUr cells increased 500-fold, showing that excision is stimulated by the presence of orf1 to orf6 (Fig. 2). To investigate whether the integrase is needed for excision, an amber stop codon was introduced into orf1. This mutation completely abolished excision (pAJ41 in Fig. 2), showing that the integrase is required for excision of TP901-1.
In some temperate bacteriophages the excisionase gene is located immediately upstream of the integrase gene. Therefore, TP901-1 orf2 or orf3 could be expected to encode the excisionase. However, when nonsense mutations were introduced into these genes separately (data not shown) or when both genes were inactivated by a deletion (pAB35), no reduction in the frequency of excision was observed. Thus, neither Orf2 nor Orf3 is involved in excision of TP901-1.
Plasmid pAB235 contains the same deletion in orf2 and orf3 as pAB35, but in addition, a fragment upstream of orf3, including orf4, pR, pL, orf5, and orf6, is deleted. When this plasmid was introduced in AB112, the frequency of occurrence of FUr cells was slightly higher than the upp mutation rate (Fig. 2). Some of the FUr colonies obtained were found to be Erms, confirming that excision did take place at a low frequency. This indicates that the integrase of TP901-1 can catalyze a low but significant level of excision without other phage-encoded proteins present and that even though the pR promoter is not present upstream of orf1 in pAB235, sufficient amounts of the integrase are present to ensure a low frequency of excision.
Identification of the excisionase.
Since the amount of the TP901-1 integrase produced from the phage fragment in pAB235 is sufficient to enable a low frequency of excision, we constructed a second excision system (system II) (Fig. 3), in which the excision vector (pAB174a) contains the same TP901-1 fragment as pAB235. With this excision system, protein donor plasmids not containing orf1 can be used. pAB174a was integrated into attB in JM342, and the strain was termed AB174a. Surprisingly, an excision frequency of 3 × 10−4 FUr cell/CFU was observed in AB174a when the only phage protein present was Orf1, encoded by the integrated excision vector (Fig. 3). This frequency is 125-fold greater than the excision frequency obtained with pAB235 as protein donor in system I. The reason for the much higher excision frequency obtained with AB174a could be that in system I the integrase is donated in trans, whereas in system II it is donated in cis. Furthermore, the excision frequency obtained with pCI3340 as protein donor in system II is 150-fold higher than the mutation rate of the upp gene, and from this it is concluded that the TP901-1 integrase alone is sufficient to obtain a significant frequency of excision.
Plasmid derivatives of pCI3340 containing different early-expressed phage genes were tested for the ability to further increase the frequency of excision of the excision vector in AB174a. When orf4 and orf5 were introduced (pAB223) no effect on the frequency of excision was found (Fig. 3), indicating that neither Orf4 nor Orf5 is required for excision. When orf2 and orf3 were also included (pAB221), again no increase in excision was found, in accordance with the conclusion drawn from the experiments using system I. However, when a plasmid containing orf4, orf5, orf6, and orf7 (pAB241) was introduced, all cells became resistant to FU (Fig. 3); that is, the excision frequency was 1 FUr cell/CFU. To obtain a more precise evaluation of the frequency of excision, erm was used as a selection marker, since this allows selection for cells in which excision has not taken place. By this procedure, a frequency of 1.2 × 10−1 Ermr cell/CFU was obtained, showing that excision takes place in 88% of the cells.
Since orf4 and orf5 have previously been found not to have any effect on excision, it was then investigated whether the high frequency of excision with pAB241 was due to orf6 or orf7 by introducing a deletion into orf6, resulting in pAB243. This plasmid also resulted in a frequency of excision of 1 FU cell/CFU when upp was used as the marker gene (Fig. 3). By using erm as marker, a frequency of 5.1 × 10−2 Ermr cell/CFU was obtained, showing that excision takes place in 94.9% of the cells. Thus, the results strongly indicate that the high level of excision obtained with pAB241 is caused by Orf7. To establish whether the effect is solely due to the presence of Orf7, a plasmid (pAB245) containing orf7 transcribed from the pL promoter was constructed. Introduction of pAB245 in AB174a also led to 1 FUr cell/CFU, and with erm as the marker it led to 2.6 × 10−4 Ermr cell/CFU; thus, excision takes place in 99.97% of the cells. To verify that the increased excision frequency is due to the expression of orf7, pAB244 was constructed. This plasmid is similar to pAB245, except that no promoter region is present upstream of orf7. The introduction of pAB244 in AB174a did not lead to an increase in excision, demonstrating that transcription of orf7 is necessary for the observed increase. In conclusion, Orf7 and the integrase are the only phage-encoded proteins required for efficient excision of the TP901-1 prophage, and Orf7 is thus the TP901-1 excisionase.
DISCUSSION
New method for determination of frequencies of excision.
In order to be able to monitor genetic events occurring at low frequencies, a positive selection procedure is required. The upp gene allows for positive selection for loss of genetic material encompassing the gene, and a upp mutation confers resistance to FU in many organisms. The general outline of the procedure used in the present study is therefore expected to be functional in many different organisms and can be used for monitoring the loss of any specific part of the genetic material, provided that a functional upp gene has been inserted and that an organism with a proper genetic background is used. As shown in the present paper, in L. lactis, a tdk upp host strain is a good choice, since it is resistant to high levels of FU, thus making the selection for FU resistant colonies very clean. By using the present experimental procedures, excision events occurring with frequencies just above 2 × 10−6 per cell can be measured.
Phage TP901-1-encoded proteins involved in excision.
In all cases where the protein requirements for excision of a prophage have been examined, the phage-encoded integrase catalyzes excision as well as integration. However, in these phages the integrase belongs to the λ integrase family of recombinases. Since the TP901-1 integrase belongs to the extended resolvases, the protein requirement for excision of the TP901-1 prophage might be different. However, in the work reported here it was demonstrated that the TP901-1 integrase is required for excision as it is in other temperate bacteriophages.
It was furthermore shown that excision can occur, at a frequency of 0.2% of the maximal excision frequency obtained, when the integrase of TP901-1 is the only phage-encoded protein present (pCI3340 in system II [Fig. 3]). However, the ability to catalyze excision without other phage proteins present is not unique for the TP901-1 integrase. The integrase of E. coli phage λ can perform excisive recombination in vivo at a frequency of 0.2 to 4.5% of the frequency obtained with the excisionase present (1). Also, the λ integrase-type integrases of bacteriophages ø13 and ø42 of Staphylococcus aureus can catalyze excision alone (4). The frequency is dependent on the amount of integrase present, with the maximal frequencies observed being 0.5 and 1.5% of full excision, respectively.
However, maximal excision of a TP901-1 based excision vector is obtained only when the phage-carried orf7 is present in the cell, downstream of a promoter. Thus, it is concluded that Orf7 is the TP901-1 excisionase. Orf7 is a small basic protein (7.5 kDa, 64 amino acids, pI 9.80). No significant amino acid homology between TP901-1 Orf7 and other known excisionases or between Orf7 and other proteins in the database is observed. This is in accordance with the observation that phage excisionases are often small, basic proteins which have little sequence homology.
Novel location of a phage excisionase.
The excisionase of TP901-1 is encoded by the third gene downstream of the pL promoter (19). This position of an excisionase gene is unique among temperate phages. In the other cases where the position of this gene is known, it is positioned either in the vicinity of int (exemplified by λ) or as the first gene of the early lytic operon (exemplified by P2).
However, the overall genetic organization of the early-expressed region of TP901-1 seems to be somewhat like that described for P2 (26, 32), in that one of the two operons transcribed by the major early promoters encodes the factors required for establishment and maintenance of lysogeny, while the other encodes factors involved in the lytic life cycle. In TP901-1 the lysogenic operon is transcribed from pR, and the first gene of the operon encodes the phage repressor (Orf4), which represses the transcription of the early lytic operon during the maintenance of lysogeny (17, 20). The last gene of the lysogenic operon encodes the integrase, and this gene is followed by the attP site (6). The early lytic operon of TP901-1 is transcribed from the pL promoter (19). The first gene of this operon encodes a protein (Orf5) which is involved in regulation of the activity of the early promoters (20) but not in excisive recombination (this work). In contrast, in P2 the protein product of the first gene of the early lytic operon is at the same time the phage excisionase and a regulator protein affecting the expression from the early promoters.
ACKNOWLEDGMENTS
We thank Annette Johansen for constructing the plasmids pAJ43, pAJ41, pAJ39, and pAJ37 and for providing pAJ95 prior to publication. We thank Peter L. Madsen for pPM92, pPM115, and pPM129. We also thank Todd R. Klaenhammer for the gift of pTRKL2 and Gerald Fitzgerald for pCI3340 and pCI372. We sincerely appreciate the expert technical assistance of Lotte Bredahl and Lise Sørensen.
This work was supported by the FØTEK program through The Center of Advanced Food Studies and by grants from the EC STARLAB program (BIO4-CT96-0402) and the Carlsberg Foundation.
REFERENCES
- 1.Abremski K, Gottesman S. Site-specific recombination. Xis-independent excisive recombination of bacteriophage lambda. J Mol Biol. 1981;153:67–78. doi: 10.1016/0022-2836(81)90527-1. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J D, Davidson B E, Hillier A J. Sequence analysis of the Lactococcus lactis temperate bacteriophage BK5-T and demonstration that the phage DNA has cohesive ends. Appl Environ Microbiol. 1995;61:4089–4098. doi: 10.1128/aem.61.11.4089-4098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brøndsted L, Hammer K. Use of integration elements encoded by the temperate lactococcal bacteriophage TP901-1 to obtain chromosomal single-copy transcriptional fusions in Lactococcus lactis. Appl Environ Microbiol. 1999;65:752–758. doi: 10.1128/aem.65.2.752-758.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll D, Kehoe M A, Cavanagh D, Coleman D C. Novel organization of the site-specific integration and excision recombination functions of the Staphylococcus aureus serotype F virulence-converting phages ø13 and ø42. Mol Microbiol. 1995;16:877–893. doi: 10.1111/j.1365-2958.1995.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen B, Johnsen M G, Stenby E, Vogensen F K, Hammer K. Characterization of the lactococcal temperate phage TP901-1 and its site-specific integration. J Bacteriol. 1994;176:1069–1076. doi: 10.1128/jb.176.4.1069-1076.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiansen B, Brøndsted L, Vogensen F K, Hammer K. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901-1. J Bacteriol. 1996;178:5164–5173. doi: 10.1128/jb.178.17.5164-5173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echols H, Guarneros G. Control of integration and excision. In: Hendrix R W, Roberts J W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. pp. 75–92. [Google Scholar]
- 8.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggård-Ljungquist E, Kockum K, Bertani L E. DNA sequences of bacteriophage P2 early genes cox and B and their regulatory sites. Mol Gen Genet. 1987;208:52–56. doi: 10.1007/BF00330421. [DOI] [PubMed] [Google Scholar]
- 10.Hayes F, Daly C, Fitzgerald G F. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl Environ Microbiol. 1990;56:202–209. doi: 10.1128/aem.56.1.202-209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israelsen H, Madsen S M, Vrang A, Hansen E B, Johansen E. Cloning and partial characterization of regulated promoters from Lactococcus lactis Tn917-lacZ integrants with the new promoter probe vector, pAK80. Appl Environ Microbiol. 1995;61:2540–2547. doi: 10.1128/aem.61.7.2540-2547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen P R, Hammer K. Minimal requirements for exponential growth of Lactococcus lactis. Appl Environ Microbiol. 1993;59:4363–4366. doi: 10.1128/aem.59.12.4363-4366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen, A. H. Unpublished data.
- 15.Lillehaug D, Birkeland N-K. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage øLC3 and construction of integration-negative øLC3 mutants. J Bacteriol. 1993;175:1745–1755. doi: 10.1128/jb.175.6.1745-1755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljungquist E, Kockum K, Bertani L E. DNA sequences of the repressor gene and operator region of bacteriophage P2. Proc Natl Acad Sci USA. 1984;81:3988–3992. doi: 10.1073/pnas.81.13.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen P L. Transcription of the lactococcal temperate phage TP901-1. Ph.D. thesis. Copenhagen, Denmark: University of Copenhagen; 1996. [Google Scholar]
- 18.Madsen, P. L. Unpublished data.
- 19.Madsen P L, Hammer K. Temporal transcription of the lactococcal temperate phage TP901-1 and DNA sequence of the early promoter region. Microbiology. 1998;144:2203–2215. doi: 10.1099/00221287-144-8-2203. [DOI] [PubMed] [Google Scholar]
- 20.Madsen P L, Johansen A H, Hammer K, Brøndsted L. Genetic switch regulating activity of early promoters of the temperate lactococcal bacteriophage TP901-1 J. 1999. Bacteriol., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh J L, Erfle M, Wykes E J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 22.Martinussen J, Hammer K. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J Bacteriol. 1994;176:6457–6463. doi: 10.1128/jb.176.21.6457-6463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinussen J, Hammer K. Powerful methods to establish chromosomal markers in Lactococcus lactis: an analysis of pyrimidine salvage pathway mutants obtained by positive selections. Microbiology. 1995;141:1883–1890. doi: 10.1099/13500872-141-8-1883. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.O’Sullivan D J, Klaenhammer T R. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 26.Saha S, Haggård-Ljungquist E, Nordström K. The cox protein of bacteriophage P2 inhibits the formation of the repressor protein and autoregulates the early operon. EMBO J. 1987;6:3191–3199. doi: 10.1002/j.1460-2075.1987.tb02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Guchte M, Daly C, Fitzgerald G F, Arendt E K. Identification of int and attP on the genome of lactococcal bacteriophage Tuc2009 and their use for site-specific plasmid integration in the chromosome of Tuc2009-resistant Lactococcus lactis MG1363. Appl Environ Microbiol. 1994;60:2324–2329. doi: 10.1128/aem.60.7.2324-2329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Sinderen D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage rlt. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu A, Haggård-Ljungquist E. The Cox protein is a modulator of directionality in bacteriophage P2 site-specific recombination. J Bacteriol. 1993;175:7848–7855. doi: 10.1128/jb.175.24.7848-7855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu A, Barreiro V, Haggård-Ljungquist E. Regulation of int gene expression in bacteriophage P2. J Virol. 1994;68:4220–4226. doi: 10.1128/jvi.68.7.4220-4226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]